UV-C and Nanomaterial-Based Approaches for Sulfite-Free Wine Preservation: Effects on Polyphenol Profile and Microbiological Quality
Abstract
1. Introduction
2. Results
2.1. Physical and Chemical Characterization of Iron Oxide–Silica/Titanium Oxide Core–Shell Nanocomposite
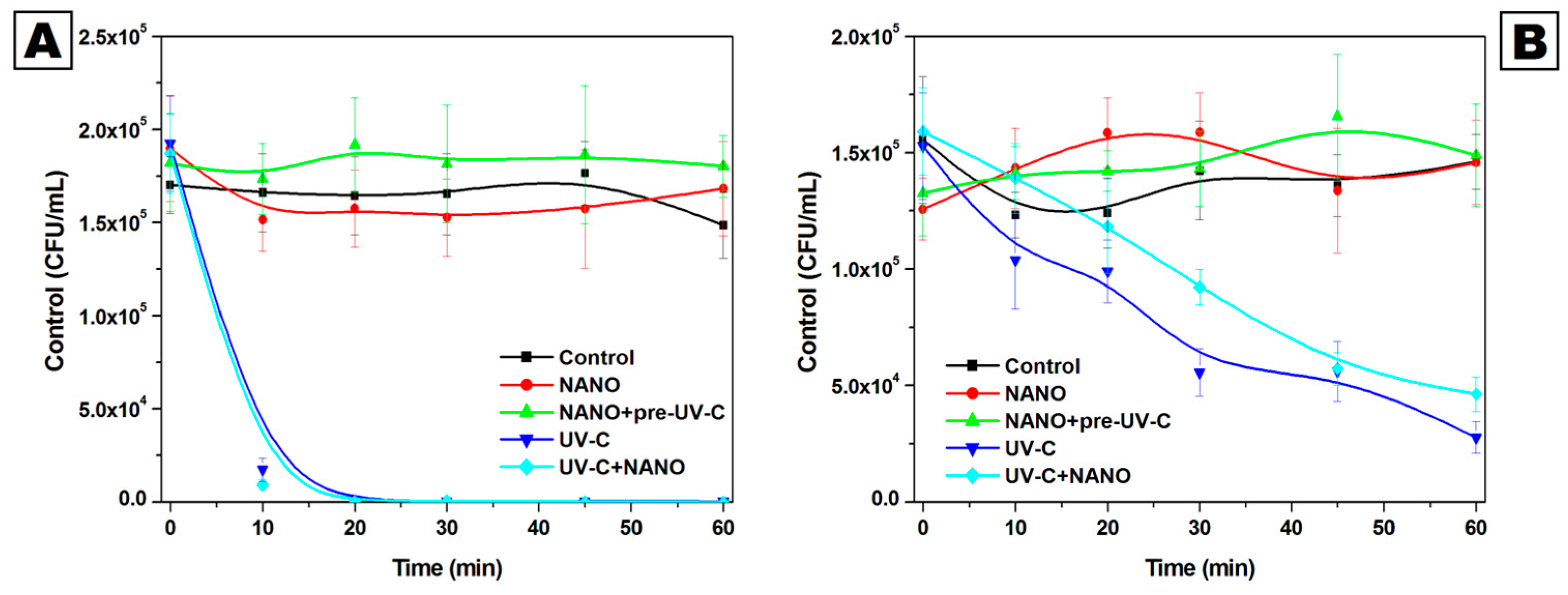
2.2. Effectiveness of Iron Oxide–Silica/Titanium Oxide Core–Shell Nanocomposite in Reduction in Saccharomyces cerevisiae Counts Under UV-C
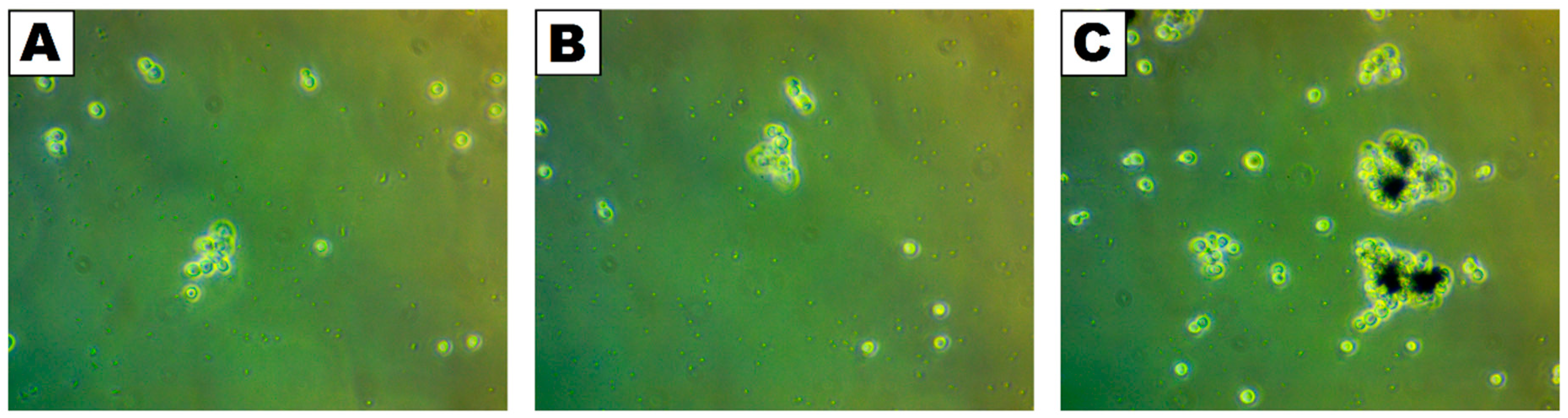
2.3. Microscopic Analysis of Interaction Between Yeasts Cells and Iron Oxide–Silica/Titanium Oxide Core–Shell Nanocomposite
2.4. General Parameters and Polyphenol Compounds in Wines After Treatment with UV-C and Hurdle Technology
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Reagents and Standards
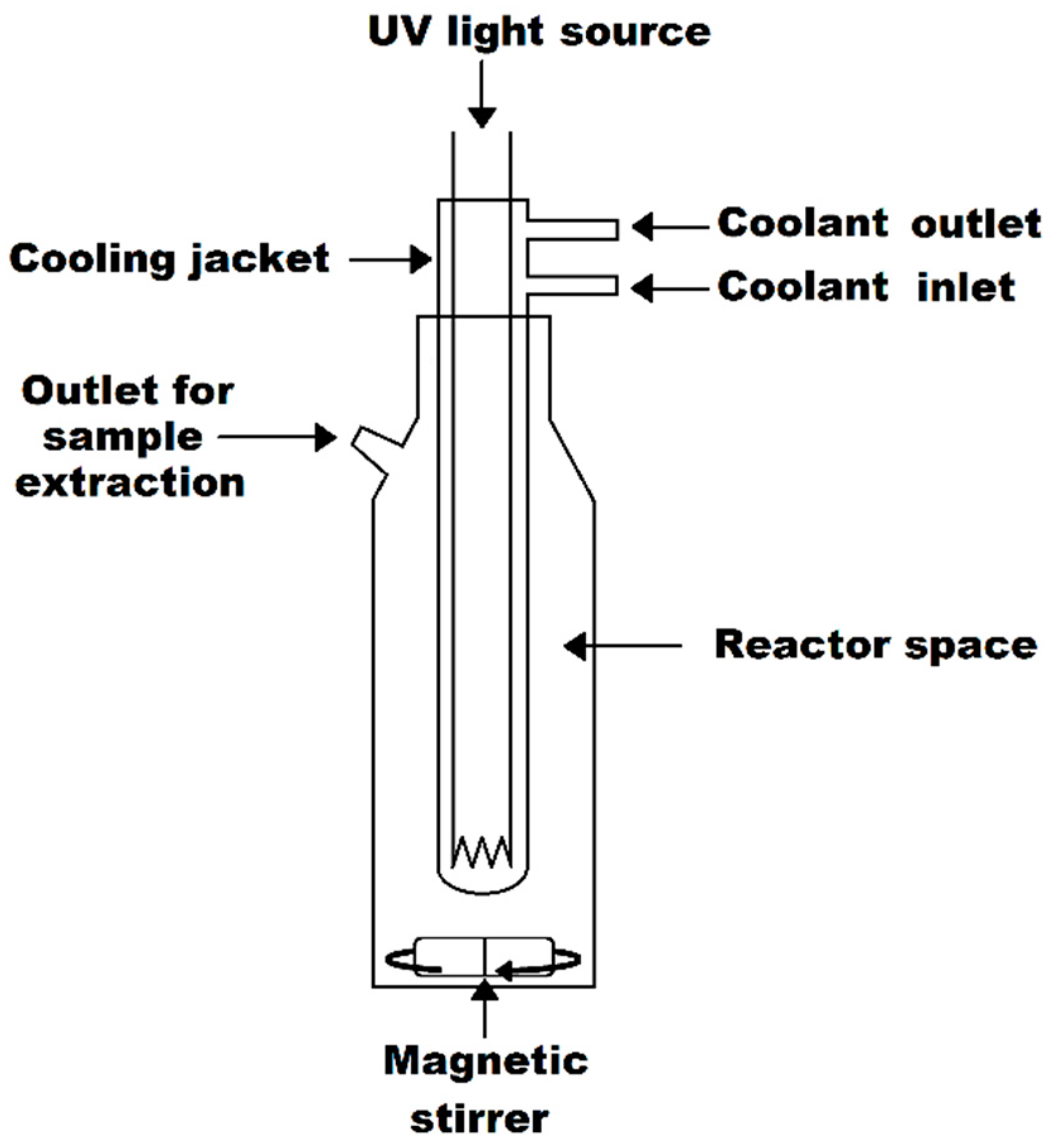
4.3. Synthesis and Analyses of Iron Oxide–Silica/Titanium Oxide Core–Shell Nanocomposite
4.4. Preparation of Saccharomyces cerevisiae Cultures
4.5. Post-Treatment Analyses of Samples
4.5.1. Saccharomyces cerevisiae Counts
4.5.2. Basic Oenological Parameter Determination
4.5.3. Polyphenol Determination with the UPLC-PDA-MS/MS Method
5. Statistics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buja, L.M. The History, Science, and Art of Wine and the Case for Health Benefits: Perspectives of an Oenophilic Cardiovascular Pathologist. Cardiovasc. Pathol. 2022, 60, 107446. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, D.; McGovern, P.E.; Hartl, D.L.; Mortimer, R.; Polsinelli, M. Evidence for S. Cerevisiae Fermentation in Ancient Wine. J. Mol. Evol. 2003, 57, S226–S232. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Portillo, M.C. Strategies for Microbiological Control of the Alcoholic Fermentation in Wines by Exploiting the Microbial Terroir Complexity: A Mini-Review. Int. J. Food Microbiol. 2022, 367, 109592. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring Wine Yeast for the New Millennium: Novel Approaches to the Ancient Art of Winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Junqua, R.; Vinsonneau, E.; Ghidossi, R. Microbial Stabilization of Grape Musts and Wines Using Coiled UV-C Reactor. OENO One 2020, 54, 109–121. [Google Scholar] [CrossRef]
- Vernhet, A. Red Wine Clarification and Stabilization. In Red Wine Technology, 1st ed.; Morata, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–251. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and Physical Methodologies for the Replacement/Reduction of Sulfur Dioxide Use during Winemaking: Review of Their Potentialities and Limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in wines: Rational use and possible alternatives. In Red Wine Technology, 1st ed.; Morata, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–321. [Google Scholar] [CrossRef]
- Pilard, E.; Harrouard, J.; Miot-Sertier, C.; Marullo, P.; Albertin, W.; Ghidossi, R. Wine Yeast Species Show Strong Inter- and Intra-Specific Variability in Their Sensitivity to Ultraviolet Radiation. Food Microbiol. 2021, 100, 103864. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.D.; Toledo, M.C.F.; Vicente, E. Sulfite Content in Some Brazilian Wines: Analytical Determination and Estimate of Dietary Exposure. Eur. Food Res. Technol. 2009, 229, 383–389. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the Efficiency of Sulphur Dioxide Replacements in Wine: A Parameter Review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, R.; Zhang, T.; Wang, B.; Li, N.; Sun, Y.; Ma, H.; Zhang, Q.; Zhang, J.; Liu, Y. Study on the Inactivation and Reactivation Mechanism of Pathogenic Bacteria in Aquaculture by UVC-LED. Front. Mar. Sci. 2023, 10, 1139713. [Google Scholar] [CrossRef]
- Keyser, M.; Műller, I.A.; Cilliers, F.P.; Nel, W.; Gouws, P.A. Ultraviolet Radiation as a Non-Thermal Treatment for the Inactivation of Microorganisms in Fruit Juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 348–354. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet Radiation: An Interesting Technology to Preserve Quality and Safety of Milk and Dairy Foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. UVC Radiation for Food Safety: An Emerging Technology for the Microbial Disinfection of Food Products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- Guerrero-Beltr·n, J.A.; Barbosa-C·novas, G.V. Advantages and Limitations on Processing Foods by UV Light. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of Ultraviolet Light Assisted Titanium Dioxide Photocatalysis for Food Safety: A Review. Innov. Food Sci. Emerg. Technol. 2016, 38, 105–115. [Google Scholar] [CrossRef]
- Gabriel, A.A. Combinations of Selected Physical and Chemical Hurdles to Inactivate Escherichia Coli O157:H7 in Apple and Orange Juices. Food Control 2015, 50, 722–728. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of Sonication and Ultraviolet-C Treatment as a Hurdle Concept on Quality Attributes of Chokanan Mango (Mangifera Indica L.) Juice. Food Sci. Technol. Int. 2015, 21, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Wu, S.-M.; Lin, Y.-H.; Wu, Y.-H.; Huang, B.-C.; Huang, H.-W.; Wang, C.-Y. High Pressure Processing-Based Hurdle Strategy for Microbial Shelf Life of Packed Food in the Cold Chain. Food Packag. Shelf Life 2022, 34, 100983. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Dumas, E.; Ismail, A.; Karam, L. Essential Oils and Their Active Components Applied as: Free, Encapsulated and in Hurdle Technology to Fight Microbial Contaminations. A Review. Heliyon 2022, 8, e12472. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent Advances in TiO2 -Based Photocatalysis. J. Mater. Chem. A Mater. 2014, 2, 12642. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Ferreira, L.F.; Silva, V.C. Magnetic Materials for Photocatalytic Applications—A Review. J. Sol-Gel Sci. Technol. 2020, 96, 1–14. [Google Scholar] [CrossRef]
- Rizzo, L. Inactivation and Injury of Total Coliform Bacteria after Primary Disinfection of Drinking Water by TiO2 Photocatalysis. J. Hazard. Mater. 2009, 165, 48–51. [Google Scholar] [CrossRef]
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Guo, M.; Wang, X. Preparation and Properties of a Nano TiO2/Fe3O4 Composite Superparamagnetic Photocatalyst. Rare Met. 2009, 28, 423–427. [Google Scholar] [CrossRef]
- Ningsih, L.A.; Yoshida, M.; Sakai, A.; Andrew Lin, K.-Y.; Wu, K.C.W.; Catherine, H.N.; Ahamad, T.; Hu, C. Ag-Modified TiO2/SiO2/Fe3O4 Sphere with Core-Shell Structure for Photo-Assisted Reduction of 4-Nitrophenol. Environ. Res. 2022, 214, 113690. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Julkapli, N.M. Modified Iron Oxide Nanomaterials: Functionalization and Application. J. Magn. Magn. Mater. 2016, 416, 117–133. [Google Scholar] [CrossRef]
- Mijowska, K.; Cendrowski, K.; Grygorcewicz, B.; Oszmiański, J.; Nawrotek, P.; Ochmian, I.; Zielińska, B. Preliminary Study on the Influence of UV-C Irradiation on Microorganism Viability and Polyphenol Compounds Content during Winemaking of ‘Regent’ Red Grape Cultivar. Pol. J. Chem. Technol. 2017, 19, 130–137. [Google Scholar] [CrossRef]
- Pachnowska, K.; Cendrowski, K.; Stachurska, X.; Nawrotek, P.; Augustyniak, A.; Mijowska, E. Potential Use of Silica Nanoparticles for the Microbial Stabilisation of Wine: An In Vitro Study Using Oenococcus Oeni as a Model. Foods 2020, 9, 1338. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, X.; Cendrowski, K.; Pachnowska, K.; Piegat, A.; Mijowska, E.; Nawrotek, P. Nanoparticles Influence Lytic Phage T4-like Performance In Vitro. Int. J. Mol. Sci. 2022, 23, 7179. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Cendrowski, K.; Grygorcewicz, B.; Jabłońska, J.; Nawrotek, P.; Trukawka, M.; Mijowska, E.; Popowska, M. The Response of Pseudomonas Aeruginosa PAO1 to UV-Activated Titanium Dioxide/Silica Nanotubes. Int. J. Mol. Sci. 2020, 21, 7748. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Barylak, M.; Mijowska, E. Investigating the Interaction Between Streptomyces Sp. and Titania/Silica Nanospheres. Water Air Soil Pollut. 2016, 227, 230. [Google Scholar] [CrossRef]
- Cendrowski, K.; Pachnowska, K.; Augustyniak, A.; Wierzbicka, J.; Pratnicki, F.; Kucharski, P.; Kukułka, W.; Mijowska, E. The Impact of Environmental Water on the Potential Application of Core—shell Titania—silica Nanospheres as Photocatalysts. Nanotechnology 2021, 32, 315703. [Google Scholar] [CrossRef]
- Fredericks, I.N.; du Toit, M.; Krügel, M. Efficacy of Ultraviolet Radiation as an Alternative Technology to Inactivate Microorganisms in Grape Juices and Wines. Food Microbiol. 2011, 28, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Gutiérrez, O.; López-Díaz, A.; Palou, E.; López-Malo, A.; Ramírez-Corona, N. Characterization and Effectiveness of Short-Wave Ultraviolet Irradiation Reactors Operating in Continuous Recirculation Mode to Inactivate Saccharomyces Cerevisiae in Grape Juice. J. Food Eng. 2019, 241, 88–96. [Google Scholar] [CrossRef]
- Hirt, B.; Fiege, J.; Cvetkova, S.; Gräf, V.; Scharfenberger-Schmeer, M.; Durner, D.; Stahl, M. Comparison and Prediction of UV-C Inactivation Kinetics of S. Cerevisiae in Model Wine Systems Dependent on Flow Type and Absorbance. LWT 2022, 169, 114062. [Google Scholar] [CrossRef]
- Chai, C.; Lee, J.; Lee, Y.; Na, S.; Park, J. A Combination of TiO2–UV Photocatalysis and High Hydrostatic Pressure to Inactivate Bacillus Cereus in Freshly Squeezed Angelica Keiskei Juice. LWT-Food Sci. Technol. 2014, 55, 104–109. [Google Scholar] [CrossRef]
- Shahbaz, H.M.; Yoo, S.; Seo, B.; Ghafoor, K.; Kim, J.U.; Lee, D.-U.; Park, J. Combination of TiO2-UV Photocatalysis and High Hydrostatic Pressure to Inactivate Bacterial Pathogens and Yeast in Commercial Apple Juice. Food Bioprocess Techol. 2016, 9, 182–190. [Google Scholar] [CrossRef]
- Ramesh, T.; Yaparatne, S.; Tripp, C.P.; Nayak, B.; Amirbahman, A. Ultraviolet Light-Assisted Photocatalytic Disinfection of Escherichia Coli and Its Effects on the Quality Attributes of White Grape Juice. Food Bioprocess Technol. 2018, 11, 2242–2252. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Sequential Non-Saccharomyces and Saccharomyces Cerevisiae Fermentations to Reduce the Alcohol Content in Wine. Fermentation 2020, 6, 60. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef]
- Varela, C.; Dry, P.R.; Kutyna, D.R.; Francis, I.L.; Henschke, P.A.; Curtin, C.D.; Chambers, P.J. Strategies for Reducing Alcohol Concentration in Wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Pala, Ç.U.; Toklucu, A.K. Effects of UV-C Light Processing on Some Quality Characteristics of Grape Juices. Food Bioprocess Techol. 2013, 6, 719–725. [Google Scholar] [CrossRef]
- Diesler, K.; Golombek, P.; Kromm, L.; Scharfenberger-Schmeer, M.; Durner, D.; Schmarr, H.-G.; Stahl, M.R.; Briviba, K.; Fischer, U. UV-C Treatment of Grape Must: Microbial Inactivation, Toxicological Considerations and Influence on Chemical and Sensory Properties of White Wine. Innov. Food Sci. Emerg. Technol. 2019, 52, 291–304. [Google Scholar] [CrossRef]
- Furtado, P.; Figueiredo, P.; Chaves das Neves, H.; Pina, F. Photochemical and Thermal Degradation of Anthocyanidins. J. Photochem. Photobiol. A Chem. 1993, 75, 113–118. [Google Scholar] [CrossRef]
- Pala, Ç.U.; Toklucu, A.K. Effect of UV-C Light on Anthocyanin Content and Other Quality Parameters of Pomegranate Juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar] [CrossRef]
- Luchian, E.C.; Codreanu, M.; Scutarașu, C.E.; Colibaba, C.; Cotea, V. Impact of Nanomaterials on Wine Quality: A Focus of Siliceous, Aluminosiliceous, and Carbon-Based Nanomaterials on the Phenolic Fraction of Wine. In Exploring Natural Phenolic Compounds-Recent Progress and Practical Applications; Gouvinhas, I., Novo Barros, A., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Li, P.; Cheng, L. The Shaded Side of Apple Fruit Becomes More Sensitive to Photoinhibition with Fruit Development. Physiol. Plant 2008, 134, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.O.; Gould, K.S. Anthocyanins in Leaves: Light Attenuators or Antioxidants? Funct. Plant Biol. 2003, 30, 865. [Google Scholar] [CrossRef] [PubMed]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in Vegetative Tissues: A Proposed Unified Function in Photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, T.J.; Zheng, J.; Huang, X.D.; Yu, Z.C.; Peng, C.L.; Chow, W.S. Anthocyanins Function as a Light Attenuator to Compensate for Insufficient Photoprotection Mediated by Nonphotochemical Quenching in Young Leaves of Acmena Acuminatissima in Winter. Photosynthetica 2018, 56, 445–454. [Google Scholar] [CrossRef]
- Guo, J.; Wang, M.-H. Ultraviolet A-Specific Induction of Anthocyanin Biosynthesis and PAL Expression in Tomato (Solanum Lycopersicum L.). Plant Growth Regul. 2010, 62, 1–8. [Google Scholar] [CrossRef]
- Modesti, M.; Macaluso, M.; Taglieri, I.; Bellincontro, A.; Sanmartin, C. Ozone and Bioactive Compounds in Grapes and Wine. Foods 2021, 10, 2934. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Puertas, B.; Fernández, M.I.; Piñeiro, Z.; Cantos-Villar, E. UVC-Treated Skin-Contact Effect on Both White Wine Quality and Resveratrol Content. Food Res. Int. 2010, 43, 2179–2185. [Google Scholar] [CrossRef]
- Cendrowski, K. Titania/Mesoporous Silica Nanotubes with Efficient Photocatalytic Properties. Pol. J. Chem. Technol. 2018, 20, 103–108. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis, 2024th ed.; OIV: Paris, France, 2024; Volume 1, Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis/annex-a-methods-of-analysis-of-wines-and-musts/section-3-chemical-analysis (accessed on 15 November 2024).
- Lachowicz-Wiśniewska, S.; Piwowarczyk, R.; Ochmian, I.; Kapusta, I.; Bernatek, M.; Piątek, J. Correlated Trophic and Bioactive Activities in the Parasite-Host Relationship—Phelipanche Purpurea vs. Achillea Arabica Case Study. Ind. Crops Prod. 2023, 204, 117379. [Google Scholar] [CrossRef]
- Błaszak, M.; Nowak, A.; Lachowicz, S.; Migdał, W.; Ochmian, I. E-Beam Irradiation and Ozonation as an Alternative to the Sulphuric Method of Wine Preservation. Molecules 2019, 24, 3406. [Google Scholar] [CrossRef] [PubMed]
- Vivas de Gaulejac, N.; Vivas, N.; Absalon, C.; Nonier, M.-F. Identification of Procyanidin A2 in Grape and Wine of Vitis vinifera L. cv: Merlot Noir and Cabernet Sauvignon. OENO One 2001, 35, 51. [Google Scholar] [CrossRef]
- Kapusta, I.; Cebulak, T.; Oszmiański, J. Characterization of Polish Wines Produced from the Interspecific Hybrid Grapes Grown in South-East Poland. Eur. Food Res. Technol. 2018, 244, 441–455. [Google Scholar] [CrossRef]
- Betés-Saura, C.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Phenolics in White Free Run Juices and Wines from Penedès by High-Performance Liquid Chromatography: Changes during Vinification. J. Agric. Food Chem. 1996, 44, 3040–3046. [Google Scholar] [CrossRef]

| Parameters | White Wine | Red Wine | ||||
|---|---|---|---|---|---|---|
| Control | UV-C | UV-C+NANO | Control | UV-C | UV-C+NANO | |
| Alcohol content | 12.13 b * | 12.13 b | 12.00 a | 13.13 b | 12.60 a | 12.63 a |
| Glucose + Fructose | 0.027 a | 0.030 a | 0.027 a | 0.027 a | 0.030 a | 0.027 a |
| Volatile acidity | 0.50 a | 0.51 b | 0.52 b | 0.51 a | 0.53 b | 0.50 a |
| Fixed acidity | 5.05 a | 5.04 ab | 5.08 b | 4.50 b | 4.44 a | 4.43 a |
| pH | 3.42 a | 3.42 a | 3.42 a | 3.69 a | 3.70 a | 3.71 a |
| L-Malic acid | 0.100 a | 0.097 a | 0.110 a | 0.040 a | 0.040 a | 0.037 a |
| Parameters [mg/L] | White Wine | Red Wine | ||||
|---|---|---|---|---|---|---|
| Control | UV-C | UV-C+NANO | Control | UV-C | UV-C+NANO | |
| Anthocyanins | - | - | - | 197.4 c * | 152.2 a | 167.1 b |
| C3G5G ** | - | - | - | 4.37 a | 5.68 b | 6.41 b |
| D3G | - | - | - | 16.93 b | 10.69 a | 11.15 a |
| P3G5G | - | - | - | 15.09 a | 17.05 a | 16.36 a |
| M3G5G | - | - | - | 36.62 a | 48.15 b | 56.81 c |
| P3G | - | - | - | 77.68 b | 32.79 a | 28.89 a |
| M3G | - | - | - | 46.75 ab | 37.35 a | 47.99 b |
| Flavonols | 1.34 a | 1.35 a | 1.53 a | 2.05 a | 2.05 a | 2.84 b |
| MY3R | 0.43 a | 0.42 a | 0.49 a | 0.80 a | 0.85 a | 1.18 b |
| MY3G | 0.06 a | 0.08 b | 0.06 a | 0.10 a | 0.10 a | 0.10 a |
| Q3GQ | 0.015 b | 0.010 b | 0.000 a | 0.010 a | 0.010 a | 0.055 b |
| I3G | 0.76 a | 0.76 a | 0.89 b | 1.05 a | 0.99 a | 1.38 b |
| Q3G | 0.065 a | 0.065 a | 0.075 a | 0.095 a | 0.105 a | 0.120 b |
| Q3R | 0.01 a | 0.01 a | 0.01 a | - | - | - |
| DQ3RH | - | - | 0.01 | - | - | 0.01 |
| Flavan-3-ols | 36.77 b | 28.47 a | 57.60 c | 65.30 b | 59.80 a | 61.52 a |
| PB1 | 10.36 b | 8.40 a | 11.86 b | 14.71 b | 13.03 a | 14.16 ab |
| PA1 | 8.58 b | 7.44 a | 10.78 c | 13.87 a | 14.92 a | 12.95 a |
| Ptr1 | 0.42 b | 0.29 a | 0.89 c | 1.29 b | 0.92 a | 1.17 ab |
| (+)-C | 0.97 b | 0.51 a | 2.26 c | 2.62 a | 2.49 a | 2.49 a |
| Ptr2 | 0.22 b | 0.13 a | 0.63 c | 0.88 b | 0.74 a | 0.84 ab |
| PB2 | 1.11 b | 0.63 a | 3.22 c | 4.67 b | 3.50 a | 4.11 b |
| PA2 | 3.85 b | 2.63 a | 8.61 c | 10.03 b | 8.63 a | 9.80 b |
| (-)-E | 3.20 b | 2.32 a | 6.60 c | 7.49 a | 7.35 a | 7.10 a |
| Ptr3 | 2.25 b | 1.61 a | 3.62 c | - | - | - |
| Ptr4 | 1.76 b | 0.85 a | 3.84 c | 4.39 b | 3.51 a | 4.05 b |
| PC1 | 3.56 ab | 3.40 a | 4.02 b | 3.89 b | 3.33 a | 3.49 a |
| PC2 | 0.52 b | 0.28 a | 1.29 c | 1.48 a | 1.39 a | 1.39 a |
| Stilbenes | 1.87 a | 1.92 a | 1.87 a | 3.43 b | 3.33 b | 2.92 a |
| tPi | 0.17 a | 0.16 a | 0.16 a | 0.22 a | 0.22 a | 0.29 b |
| cPi | 1.01 a | 1.02 a | 1.10 b | 1.54 a | 1.52 a | 2.06 b |
| tR | 0.13 a | 0.14 a | 0.12 a | 0.26 b | 0.30 c | 0.06 a |
| cR | 0.57 b | 0.62 b | 0.50 a | 1.42 b | 1.31 b | 0.51 a |
| Phenolic acids | 7.76 a | 8.39 b | 8.29 b | 10.00 a | 9.11 a | 8.86 a |
| GA | 4.61 a | 5.05 a | 4.99 a | 6.65 ab | 5.95 a | 7.72 b |
| PcA | 0.11 a | 0.09 a | 0.09 a | 0.15 a | 0.19 a | 0.15 a |
| CA | 2.25 a | 2.47 b | 2.46 b | 2.41 b | 2.21 b | 0.01 a |
| CoA | 0.66 a | 0.65 a | 0.62 a | 0.62 a | 0.59 a | - |
| CaA | 0.11 a | 0.10 a | 0.11 a | 0.12 a | 0.12 a | 0.72 b |
| pCuA | 0.01 a | 0.01 a | 0.01 a | 0.02 a | 0.02 a | 0.14 b |
| CuA | 0.03 a | 0.03 a | 0.03 a | 0.04 a | 0.04 a | 0.12 b |
| Total | 44.74 a | 40.11 a | 69.28 b | 278.2 c | 226.4 a | 243.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachnowska, K.; Kochel-Karakulska, J.; Augustyniak, A.; Obradović, V.; Ochmian, I.; Lachowicz-Wiśniewska, S.; Kapusta, I.; Maślana, K.; Mijowska, E.; Cendrowski, K. UV-C and Nanomaterial-Based Approaches for Sulfite-Free Wine Preservation: Effects on Polyphenol Profile and Microbiological Quality. Molecules 2025, 30, 221. https://doi.org/10.3390/molecules30020221
Pachnowska K, Kochel-Karakulska J, Augustyniak A, Obradović V, Ochmian I, Lachowicz-Wiśniewska S, Kapusta I, Maślana K, Mijowska E, Cendrowski K. UV-C and Nanomaterial-Based Approaches for Sulfite-Free Wine Preservation: Effects on Polyphenol Profile and Microbiological Quality. Molecules. 2025; 30(2):221. https://doi.org/10.3390/molecules30020221
Chicago/Turabian StylePachnowska, Kamila, Jolanta Kochel-Karakulska, Adrian Augustyniak, Valentina Obradović, Ireneusz Ochmian, Sabina Lachowicz-Wiśniewska, Ireneusz Kapusta, Klaudia Maślana, Ewa Mijowska, and Krzysztof Cendrowski. 2025. "UV-C and Nanomaterial-Based Approaches for Sulfite-Free Wine Preservation: Effects on Polyphenol Profile and Microbiological Quality" Molecules 30, no. 2: 221. https://doi.org/10.3390/molecules30020221
APA StylePachnowska, K., Kochel-Karakulska, J., Augustyniak, A., Obradović, V., Ochmian, I., Lachowicz-Wiśniewska, S., Kapusta, I., Maślana, K., Mijowska, E., & Cendrowski, K. (2025). UV-C and Nanomaterial-Based Approaches for Sulfite-Free Wine Preservation: Effects on Polyphenol Profile and Microbiological Quality. Molecules, 30(2), 221. https://doi.org/10.3390/molecules30020221










