Comprehensive Analysis of Trypoxylus dichotomus: Inorganic Elements, Amino Acids, and Bioactive Compounds and Their Anticancer, Antioxidant, and Neuroprotective Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of GC-MS Results
2.2. Analysis of Experimental Results for Total Flavonoid Content
2.3. Analysis of Experimental Results for Total Polyphenol Content
2.4. Analysis of Experimental Results for Total Triterpene Content
2.5. Analysis of Experimental Results for Total Polysaccharide Content
2.6. Experimental Results from Amino Acid Content Analysis
2.7. Analysis of Results of Inorganic Chemical Element Test
2.8. Analysis of Results of Anti-Tumor Activity Experiment
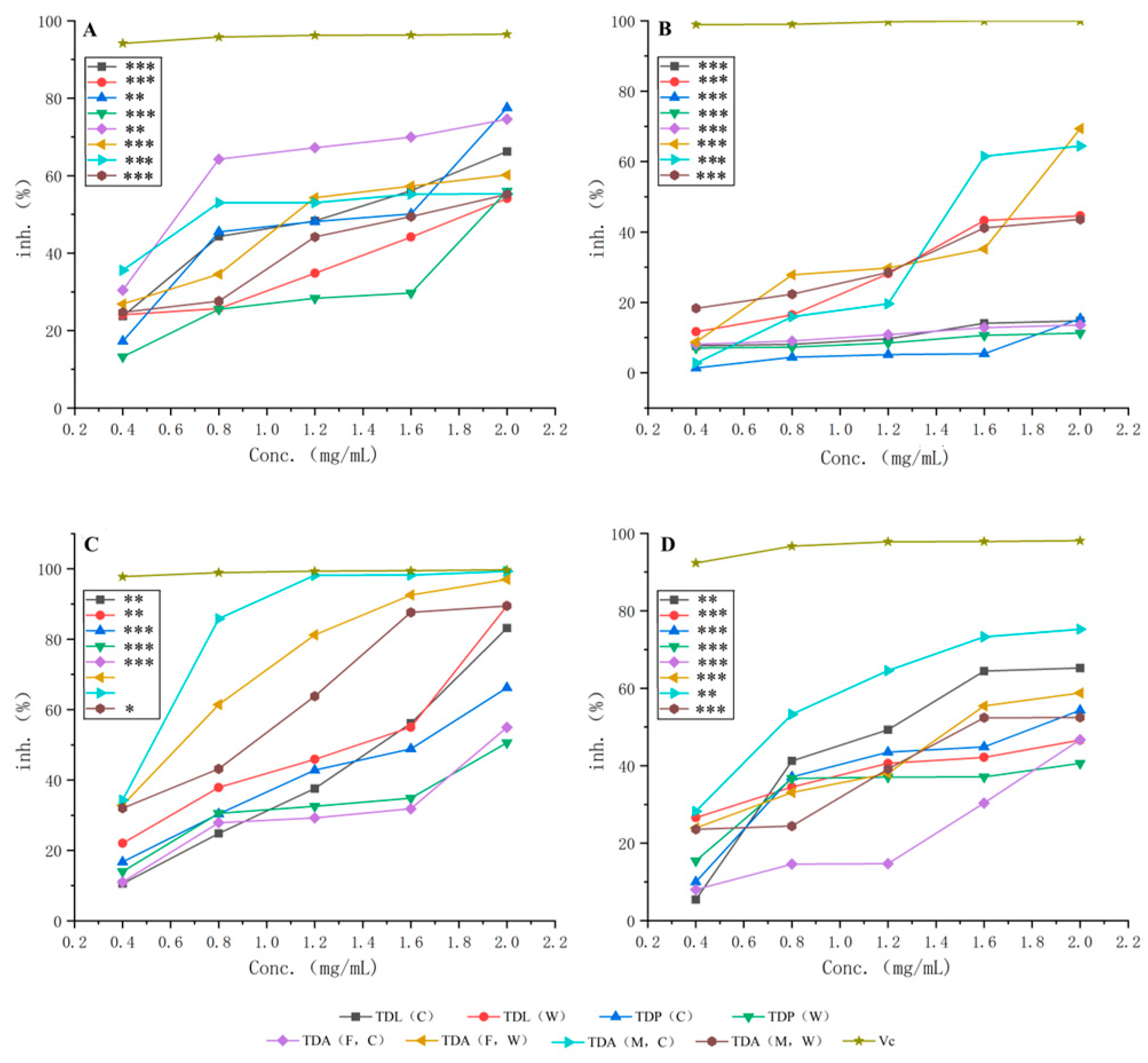
2.9. Analysis of Experimental Results on In Vitro Antioxidant Activity
2.10. Analysis of Neuro-Antioxidant Activity Detection Results
3. Experimental Section
3.1. Chemical and Reagents
3.2. Sample Grinding
3.3. Extraction Procedure
3.4. GC-MS Analysis
3.5. Total Flavonoid Content Determination
3.6. Total Polyphenol Content Determination
3.7. Total Triterpene Content Determination
3.8. Total Polysaccharide Content Determination
3.9. Amino Acid Content Determination
3.10. Inorganic Element Determination
3.11. In Vitro Anti-Tumor Activity Assay
3.12. DPPH Scavenging Rate Assay
3.13. ·OH Scavenging Rate Assay
3.14. ABTS+·Scavenging Rate Assay
3.15. ·O2− Scavenging Rate Assay
3.16. Neuro-Antioxidant Activity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bae, S.M.; Fan, M.; Choi, Y.-J.; Tang, Y.; Jeong, G.; Myung, K.; Kim, B.-G.; Kim, E.-K. Exploring the Role of a Novel Peptide from Allomyrina dichotoma Larvae in Ameliorating Lipid Metabolism in Obesity. Int. J. Mol. Sci. 2020, 21, 8537. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.; Na, Y.-E.; Kim, M.-H. Influence of rhinoceros beetle (Trypoxylus dichotomus septentrionalis) larvae and temperature on the soil bacterial community composition under laboratory conditions. Soil Biol. Biochem. 2017, 108, 27–35. [Google Scholar] [CrossRef]
- Kim, J.; Yun, E.Y.; Park, S.W.; Goo, T.W.; Seo, M. Allomyrina dichotoma Larvae Regulate Food Intake and Body Weight in High Fat Diet-Induced Obese Mice Through mTOR and Mapk Signaling Pathways. Nutrients 2016, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Bak, J.P.; Samad, N.B.; Jin, H.L.; Lee, B.R.; Lim, B.O. Antioxidant Activity of Mume Fructus Extract. J. Food Biochem. 2012, 36, 224–232. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Nakamura, K.; Saido-Sakanaka, H.; Asaoka, A.; Yamakawa, M.; Sameshima, T.; Motobu, M.; Hirota, H. Effect of modified oligopeptides from the beetle Allomyrina dichotoma on Escherichia coli infection in mice. J. Vet. Med. Sci. 2004, 66, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kui, Z.; Chen, J.; Kui, Y. Breeding of the medicinal insect unicorn fairy. Chin. Herb. Med. 2000, 31, 8. [Google Scholar]
- Ahamad, S.R.; Yaqoob, S.H.; Khan, A.; Shakeel, F. Metabolite profile and elemental determination of camel follicular fluid by GC-MS and ICP-MS. Trop. Anim. Health Prod. 2019, 51, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, Q.J.; Tang, C.H.; Liu, Y.F.; Ma, F.Y.; Zhang, X.Y.; Zhang, J.S. Triterpenes and Soluble Polysaccharide Changes in Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), During Fruiting Growth. Int. J. Med. Mushrooms 2018, 20, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ 2020, 8, e8354. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Alves, A.M.; Dias, T.; Hassimotto, N.M.A.; Naves, M.M.V. Ascorbic acid and phenolic contents, antioxidant capacity and flavonoids composition of Brazilian Savannah native fruits. Food Sci. Technol. 2017, 37, 564–569. [Google Scholar] [CrossRef]
- Jang, H.-G.; Heo, B.-G.; Park, Y.S.; Namiesnik, J.; Barasch, D.; Katrich, E.; Vearasilp, K.; Trakhtenberg, S.; Gorinstein, S. Chemical Composition, Antioxidant and Anticancer Effects of the Seeds and Leaves of Indigo (Polygonum tinctorium Ait.) Plant. Appl. Biochem. Biotechnol. 2012, 167, 1986–2004. [Google Scholar] [CrossRef]
- Alaklabi, A.; Arif, I.A.; Ahamed, A.; Kumar, R.S.; Idhayadhulla, A. Evaluation of antioxidant and anticancer activities of chemical constituents of the Saururus chinensis root extracts. Saudi J. Biol. Sci. 2018, 25, 1387–1392. [Google Scholar] [CrossRef]
- Liu, S.R.; Ke, B.R.; Zhang, W.R.; Liu, X.R.; Wu, X.P. Breeding of new Ganodernia lucidum strains simultaneously rich in polysaccharides and triterpenes by mating basidiospore-derived monokaryons of two commercial cultivars. Sci. Hortic. 2017, 216, 58–65. [Google Scholar] [CrossRef]
- de Andrades, E.O.; da Costa, J.M.A.R.; de Lima Neto, F.E.M.; de Araujo, A.R.; de Oliveira Silva Ribeiro, F.; Vasconcelos, A.G.; de Jesus Oliveira, A.C.; Sobrinho, J.L.S.; de Almeida, M.P.; Carvalho, A.P.; et al. Acetylated cashew gum and fucan for incorporation of lycopene rich extract from red guava (Psidium guajava L.) in nanostructured systems: Antioxidant and antitumor capacity. Int. J. Biol. Macromol. 2021, 191, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Xu, M.; Huang, Q.; Zhang, D.; Lin, Z.; Wang, Y.; Liu, Y. Nutrition and Flavor Evaluation of Amino Acids in Guangyuan Grey Chicken of Different Ages, Genders and Meat Cuts. Animals 2023, 13, 1235. [Google Scholar] [CrossRef]
- Agan, V.; Koyuncu, I.; Agan, F.Z.; Balos, M.M. Analysis of Content Profiles, Antioxidant and Anticancer Properties in Endemic Hypericum salsolifolium. Appl. Sci. 2023, 13, 7300. [Google Scholar] [CrossRef]
- Dong, S.F.; Zhu, Z.G. Determination of the content of inorganic elements in taponin tablet recipe. Spectrosc. Spectr. Anal. 2003, 23, 201–202. [Google Scholar]
- Suwanwong, Y.; Boonpangrak, S. Phytochemical contents, antioxidant activity, and anticancer activity of three common guava cultivars in Thailand. Eur. J. Integr. Med. 2021, 42, 7. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Tomczyk, M. A review of the traditional uses, phytochemistry, pharmacology, and clinical evidence for the use of the genus Alchemilla (Rosaceae). J. Ethnopharmacol. 2024, 320, 117439. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Al-Hajj, N.; Jaber, S. Myrtus communis L.: Essential oil chemical composition, total phenols and flavonoids contents, antimicrobial, antioxidant, anticancer, and α-amylase inhibitory activity. Chem. Biol. Technol. Agric. 2023, 10. [Google Scholar] [CrossRef]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, T.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Elkhatim, K.A.S.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y. Optimization of Extraction and Separation Method of Triterpenoids from Ganoderma lucidum Spore Powder. Acta Medica Mediterr. 2023, 39, 361–369. [Google Scholar]

| No. | Name | RT | Percentage | |||
|---|---|---|---|---|---|---|
| TDL | TDP | TDA (F) | TDA (M) | |||
| 1 | Dimethyl disulfide | 4.176 | — | — | 7.773 | 6.515 |
| 2 | 6,7-Dioxabicyclo[3.2.2]non-8-ene | 4.199 | — | 1.612 | — | — |
| 3 | 2,5-Dimethyl-pyrazine | 14.087 | — | — | 6.084 | — |
| 4 | 2-Methyl-butanoic acid | 16.192 | — | — | 1.188 | — |
| 5 | N-(2-Methylbutylidene)isobutylami | 17.348 | — | — | 1.518 | — |
| 6 | Benzaldehyde | 18.756 | 4.531 | 3.59 | 12.301 | 12.271 |
| 7 | Dimethyl trisulfide | 19.025 | — | 1.597 | 19.965 | 21.11 |
| 8 | Benzonitrile | 20.598 | — | — | 1.26 | — |
| 9 | 4,5-Dihydro-3-phenyl-6H-1,2,5-oxadiazine-6-thione | 20.661 | — | — | — | 0.879 |
| 10 | 1-Octen-3-ol | 20.81 | 5.895 | 11.732 | — | — |
| 11 | 3-Octanone | 21.136 | 4.953 | 10.97 | — | — |
| 12 | (2-Endo,5-exo)-1,7,7-trimethyl-bicyclo[2.2.1]heptane-2,5-diol | 21.308 | — | — | — | 1.525 |
| 13 | 3-Octanol | 21.897 | 11.742 | 31.765 | — | — |
| 14 | 2-Ethyl-6-methyl-pyrazine | 21.949 | — | — | 0.49 | 0.978 |
| 15 | 1,4-Dichloro-benzene | 22.223 | — | — | 2.423 | 4.517 |
| 16 | 2-Acetylthiazole | 23.03 | 1.813 | — | — | — |
| 17 | 2-Methyl-N-(2-methylbutylidene)-1-butanamine | 24.392 | — | — | 1.254 | 1.979 |
| 18 | Benzeneacetaldehyde | 24.541 | 1.401 | 8.546 | — | — |
| 19 | 3-Methyl-N-(3-methylbutylidene)-1-butanamine | 24.855 | — | — | — | 1.714 |
| 20 | 3,5-Bis(morpholinomethyl)-4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl | 25.222 | 0.924 | — | — | — |
| 21 | Acetophenone | 25.737 | 1.309 | 1.767 | 1.475 | 1.972 |
| 22 | 2-Ethyl-3,5-dimethyl-pyrazine | 26.561 | — | — | 0.368 | 0.586 |
| 23 | Tetramethyl-pyrazine | 26.852 | — | — | 3.493 | 4.387 |
| 24 | 4-Methyl-phenol | 27.018 | 4.064 | 3.484 | — | — |
| 25 | 2-Nonanone | 27.207 | — | — | 0.83 | 1.141 |
| 26 | Nonanal | 27.808 | 4.341 | 2.796 | — | — |
| 27 | Phenylethyl alcohol | 28.277 | — | 1.971 | — | — |
| 28 | 2,5-Bis[(trimethylsilyl)oxy]-benzaldehyde | 28.867 | 0.877 | 0.643 | 0.717 | 0.897 |
| 29 | Benzyl nitrile | 29.381 | — | — | 3.722 | 3.037 |
| 30 | N-(3-Methylbutyl)acetamide | 29.759 | — | — | 0.624 | 0.562 |
| 31 | 2-Methylisoborneol | 31.115 | 0.796 | — | — | — |
| 32 | Tricyclo[4.3.1.1(3,8)]undecan-1-ol | 31.327 | — | — | 0.902 | 0.825 |
| 33 | Nonanenitrile | 31.43 | — | — | 0.97 | — |
| 34 | 2-Hydroxy-4-methyl-benzaldehyde | 31.779 | — | — | 1.335 | 1.317 |
| 35 | 2-Decanone | 31.939 | 1.338 | — | 1.703 | 2.277 |
| 36 | Dodecane | 32.174 | — | — | 0.441 | — |
| 37 | 6-Heptyltetrahydro-2H-pyran-2-one | 32.202 | — | — | 0.395 | — |
| 38 | Pterin-6-carboxylic acid | 32.42 | — | — | — | 0.609 |
| 39 | Decanal | 32.563 | 1.699 | 1.536 | — | — |
| 40 | Dimethyl-tetrasulfide | 32.717 | — | — | 0.72 | |
| 41 | N-[5-Hydroxy-n-pentyl]-arachidonic amide | 32.74 | — | — | — | 0.559 |
| 42 | 5-Propyl-1,3-benzodioxole | 32.769 | 1.895 | — | — | — |
| 43 | 2-Azido-2,4,4,6,6-pentamethylheptane | 32.906 | — | — | 0.528 | — |
| 44 | Hexylresorcinol | 33.118 | — | 0.255 | — | — |
| 45 | Geranyl isovalerate | 33.301 | — | — | 0.642 | — |
| 46 | 3-Methoxy-2,4,6-trimethyl-cyclohex-2-enone | 33.61 | — | 0.603 | — | — |
| 47 | Benzenepropanol | 33.982 | 1.404 | 0.437 | — | — |
| 48 | 5,7-Dodecadiyn-1,12-diol | 34.182 | — | 0.358 | — | — |
| 49 | Pyrrolizin-1,7-dione-6-carboxylic acid methyl ester | 34.285 | — | — | 0.438 | — |
| 50 | 2-Myristynoyl pantetheine | 34.394 | — | — | 0.355 | — |
| 51 | 3-Ethyl-5-(2-ethylbutyl)-octadecane | 35.138 | — | — | 0.45 | 0.32 |
| 52 | (Z)-3-Decen-1-ol | 35.252 | — | 0.432 | — | — |
| 53 | (2-Isopropyl-phenoxy)silyloxy-silane | 35.452 | — | — | 2.304 | 0.428 |
| 54 | 2-Phenyl-l-p-toluenesulfonylaziridine | 35.464 | — | — | — | 1.755 |
| 55 | 3,3-Dimethyl-5-oxo-cyclohexanecarboxaldehyde | 35.67 | — | 0.406 | — | — |
| 56 | (E)-10-Heptadecen-8-ynoic acid methyl ester | 35.71 | — | — | — | 0.051 |
| 57 | 2-Trimethylsiloxy-6-hexadecenoic acid methyl ester | 35.825 | 0.833 | — | — | — |
| 58 | (E)-3-Decen-1-ol | 35.876 | — | 1.773 | — | — |
| 59 | 3-Trifluoroacetoxytetradecane | 35.99 | — | 1.023 | — | — |
| 60 | (Z)-7-Hexadecenal | 36.202 | — | 0.223 | — | — |
| 61 | 5-Acetyl-4,6,6-trimethylcyclohexa-2,4-dienone | 36.305 | — | — | — | 0.085 |
| 62 | Hexahydro-4,4,7a-trimethyl-2(3H)-benzofuranone | 36.54 | — | 0.105 | — | — |
| 63 | (7R,8R)-Ethyl-8-hydroxy-trans-bicyclo[4.3.0]-3-nonene-7-carboxylate | 36.557 | — | — | 1.081 | — |
| 64 | 1,2,3,4-Tetrahydro-5-methyl-naphthalene | 36.597 | 0.719 | — | — | — |
| 65 | 4-Methyl-3-heptanone | 36.74 | — | 0.153 | — | — |
| 66 | 2-Undecanone | 36.94 | 0.781 | 0.298 | 1.344 | 1.287 |
| 67 | Tridecane | 37.146 | — | 1.128 | 0.82 | 1.321 |
| 68 | 3-(2,5-Dimethylanilinomethyl)-5-(3-fluorobenzylidene)-2,4-thiazolidinedione | 37.221 | — | — | 0.644 | — |
| 69 | tert-Hexadecanethiol | 37.524 | — | — | 0.829 | — |
| 70 | Trichloroacetic acid-hexadecyl ester | 37.547 | — | — | — | 0.784 |
| 71 | Undecanal | 37.558 | 1.386 | — | — | — |
| 72 | 4-Hydroxy-4-methylhex-5-enoic acid-tert-butyl ester | 37.575 | — | 1.22 | — | — |
| 73 | 4-(3-Hydroxy-2,6,6-trimethylcyclohex-1-enyl)pent-3-en-2-one | 37.804 | — | — | 0.492 | — |
| 74 | 10-Methyl-tricyclo[4.3.1.1(2,5)]undec-3-en-10-ol | 37.827 | — | — | — | 0.227 |
| 75 | 1,2,3,4-Tetrahydro-2,7-dimethyl-naphthalene | 38.949 | 0.787 | — | — | — |
| 76 | Boldenone | 38.983 | — | — | — | 0.618 |
| 77 | 2,2′-Diethyl-1,1′-biphenyl | 39.572 | 0.694 | — | — | — |
| 78 | 3,7,11-Trimethyl-1-dodecanol | 39.658 | — | — | — | 0.389 |
| 79 | Geranyl isovalerate | 39.887 | — | — | — | 0.13 |
| 80 | 4,4,6-Trimethyl-6-phenyltetrahydro-1,3-oxazine-2-thione | 40.425 | — | — | — | 0.149 |
| 81 | Phytol | 40.556 | — | 0.299 | — | — |
| 82 | 3-Methyl-N-(2-phenylethylidene)-1-butanamine | 40.957 | — | — | 7.205 | 4.813 |
| 83 | 1,2,3,4-Tetrahydro-6,7-dimethyl-naphthalene | 41.266 | 1.714 | — | — | — |
| 84 | Tetradecane | 41.735 | — | — | 1.012 | 1.482 |
| 85 | trans-1,10-Dimethyl-trans-9-decalinol | 41.753 | 8.485 | — | — | — |
| 86 | (4α,4α,8β)-Octahydro-4,8-dimethyl-4(2H)-naphthalenol | 41.769 | — | 1.307 | — | — |
| 87 | trans-5-(Hexadecyloxy)-2-pentadecyl-1,3-dioxane | 41.872 | — | — | 0.582 | 0.317 |
| 88 | Dodecanal | 42.21 | — | 0.324 | — | — |
| 89 | 4-(Hexadecyloxy)-2-pentadecyl-1,3-dioxane | 42.302 | — | — | 1.308 | 1.142 |
| 90 | 5α-Cholestan-2-one oxime | 42.868 | — | — | — | 0.106 |
| 91 | 1-(5-Ethyl-tetrahydrofuran-2-yl)-3,3-dimethyl-butan-2-one | 43.389 | — | 0.113 | — | — |
| 92 | 1,2,3,4-Tetrahydro-2,5,8-trimethyl-naphthalene | 43.481 | 1.783 | — | — | — |
| 93 | (E)-6,10-Dimethyl-5,9-undecadien-2-one | 44.07 | — | — | 1.678 | 1.593 |
| 94 | 2,6,10-Trimethyl-tetradecane | 44.407 | 0.947 | — | 0.46 | 0.819 |
| 95 | 1-(1,6-Dioxooctadecyl)-pyrrolidine | 44.928 | — | — | 2.001 | 1.549 |
| 96 | 1,12-Tridecadiene | 44.98 | 2.405 | — | — | — |
| 97 | 2-Methyl-1-hexadecanol | 45.3 | — | — | — | 0.33 |
| 98 | cis-1-Chloro-9-octadecene | 45.724 | 0.303 | — | — | — |
| 99 | 2-Tridecanone | 45.901 | 0.67 | — | 0.77 | 0.252 |
| 100 | Pentadecane | 46.038 | — | 0.421 | — | 1.486 |
| 101 | Tridecanal | 46.519 | 1.079 | — | — | — |
| 102 | [1R-(1α,3α,7α)]-1,2,3,6,7,7-hexahydro-2,2,4,7-tetramethyl-1,3-ethano-3H-indene | 46.908 | — | 0.321 | — | — |
| 103 | [1S-(1α,3β,4α,8β)]-Decahydro-1,5,5,8-tetramethyl-1,4-methanoazulen-3-ol | 46.948 | 0.776 | — | — | — |
| 104 | 2-Hexadecanol | 47.921 | — | — | — | 0.466 |
| 105 | Tetradecanal | 49.134 | 5.888 | 0.16 | — | — |
| 106 | 6-Trimethylsilyl-1H-indole-2,3-dione | 49.345 | — | — | 0.599 | 0.359 |
| 107 | Hexadecane | 50.078 | — | 0.561 | — | — |
| 108 | 7-Methyl-Z-tetradecen-1-ol acetate | 52.441 | — | — | — | 0.378 |
| 109 | 2-Methyl-1-Hexadecanol | 52.967 | — | — | — | 0.782 |
| 110 | Hexadecanal | 53.059 | 10.889 | — | — | — |
| 111 | Hexadecyl-oxirane | 53.334 | 7.203 | — | — | — |
| 112 | N-(Acetoxymethyl)-2-(2,4-pentadienyl)-cyclohexanecarboxamide | 53.637 | — | — | 0.458 | — |
| 113 | 2-Pentadecanone | 53.854 | 1.193 | — | — | — |
| 114 | Nonadecane | 53.871 | — | 0.247 | — | — |
| 115 | 3-Acetamido-2-methallylphenol | 53.906 | — | — | 1.316 | — |
| 116 | Pentadecanal | 54.449 | — | 0.26 | — | — |
| 117 | Naphthalene,decahydro-1,1,4a-trimethyl-6-methylene-5-(3-methylene-4-pentenyl) | 54.684 | — | — | — | 0.254 |
| 118 | Octadecanal | 56.727 | 0.802 | 0.824 | — | — |
| 119 | cis-9-Hexadecenal | 57.648 | — | 0.41 | — | — |
| 120 | Propanoic acid 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl) | 61.39 | — | — | — | 0.124 |
| 121 | Tetradecyl-oxirane | 61.396 | — | 0.042 | — | — |
| 122 | n-Hexadecanoic acid | 62.065 | — | 0.703 | — | — |
| 123 | (Z)-9-Octadecenal | 62.392 | 0.888 | 0.721 | — | — |
| 124 | 1,2-Dedihydro-perhydro-histrionicotoxin methyl ether | 65.716 | — | — | — | 0.444 |
| 125 | Heptacosane | 66.443 | — | — | — | 0.112 |
| 126 | Tricosane | 66.454 | — | 0.093 | — | — |
| 127 | 3-Methyl-2-[(trimethylsilyl)oxy]-benzoic acid, trimethylsilyl ester | 66.569 | — | 0.024 | — | — |
| Total | 37 types | 44 types | 47 types | 53 types | ||
| Compound | Content (mg/g) | |||
|---|---|---|---|---|
| TDL | TDP | TDA (M) | TDA (F) | |
| Flavonoids | 155.7 | 13.9 | 35.9 | 34.5 |
| Polyphenols | 25.8 | 23.4 | 25.5 | 67.6 |
| Triterpenes | 22.8 | 39.5 | 15.0 | 18.0 |
| Polysaccharides | 33.5 | 23.0 | 23.3 | 18.9 |
| No. | Amino Acid | Content (mg/g) | ||
|---|---|---|---|---|
| TDA (M) | TDA (F) | |||
| 440 nm | 1 | Asp | 97.27 | 88.36 |
| 2 | Thr | 32.72 | 28.07 | |
| 3 | Ser | 52.98 | 48.39 | |
| 4 | Glu | 136.71 | 117.11 | |
| 5 | Gly | 128.42 | 129.63 | |
| 6 | Ala | 116.92 | 119.15 | |
| 7 | Cys | 0.72 | 0.65 | |
| 8 | Met | 9.75 | 7.90 | |
| 9 | Ile | 53.08 | 48.90 | |
| 10 | Leu | 85.55 | 81.27 | |
| 11 | Tyr | 34.82 | 30.33 | |
| 12 | Phe | 20.83 | 16.79 | |
| 13 | His | 203.48 | 217.17 | |
| 14 | Arg | 40.39 | 34.70 | |
| 570 nm | 15 | Pro | 88.1 | 87.48 |
| Name | Content (mg/g) × 10−6 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Zn | Ga | Al | Bi | Li | Be | B | Ti | V | Cr | Mn | |
| TDL | 135.43 | 3.61 | 1.19 | 25.15 | 5.41 | 0.08 | - | - | 14.62 | 0.19 | 0.61 | 13.62 |
| TDP | 113.23 | 2.14 | 1.26 | - | 11.14 | - | - | 0.16 | 1 | - | - | 4.18 |
| TDA (M) | 32.59 | 6.18 | 1.21 | 2.63 | 9.14 | - | - | - | 1.39 | - | - | 4.72 |
| TDA (F) | 132.54 | 5.33 | 1.39 | - | 25.96 | 0.02 | - | - | 0.92 | - | - | 5.1 |
| Co | Ni | Cu | As | Sr | Cd | Sn | Sb | Ba | TI | Pb | ||
| TDL | 0.14 | 1.58 | 2.52 | 0.71 | 2.72 | 0.28 | - | - | 3.5 | - | 0.23 | |
| TDP | 0.03 | 0.6 | 2.47 | 0.94 | 0.3 | 0.11 | - | - | 0.33 | - | - | |
| TDA (M) | - | 0.82 | 3.32 | 1.82 | 0.2 | - | - | - | 0.07 | - | - | |
| TDA (F) | - | 0.71 | 3.84 | 2.12 | 0.2 | - | - | - | - | - | 0.28 | |
| Compound | Concentration | Cell Inhibition (%) | |||
|---|---|---|---|---|---|
| MKN-45 | K-562 | 5637 | 239T | ||
| TDA (M, C) | 0.5 mg/mL | 86.22 ± 1.19 | 85.42 ± 0.63 | 88.15 ± 3.27 | 97.23 ± 0.60 |
| TDA (F, C) | 0.5 mg/mL | — | 81.28 ± 1.19 | — | — |
| Dox | 10 μM | 79.97 ± 1.95 | 78.13 ± 0.52 | 96.78 ± 0.37 | 95.84 ± 1.32 |
| Free Radical | IC50 (mg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TDL (C) | TDL (W) | TDP (C) | TDP (W) | TDA (F, C) | TDA (F, W) | TDA (M, C) | TDA (M, W) | Vc | |
| DPPH· | 1.155 ± 0.188 | 2.090 ± 0.307 | 1.142 ± 0.653 | 2.315 ± 0.469 | 0.672 ± 0.306 | 1.220 ± 0.222 | 1.008 ± 0.502 | 1.677 ± 0.320 | 0.025 ± 0.163 |
| ·OH | - | - | - | - | - | 1.696 ± 0.649 | 1.573 ± 0.375 | - | 0.016 ± 0.312 |
| ABTS + · | 1.294 ± 0.114 | 1.062 ± 0.100 | 1.436 ± 0.290 | 2.34 ± 0.524 | 2.19 ± 0.701 | 0.589 ± 0.074 | 0.479 ± 0.047 | 0.736 ± 0.470 | 0.015 ± 0.113 |
| ·O2− | 1.220 ± 0.492 | - | 1.416 ± 0.504 | - | - | 1.504 ± 0.309 | 0.773 ± 0.133 | 1.791 ± 0.552 | 0.031 ± 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, D.; Li, Q.; Liu, X.; Zhou, J.; Yin, S.; Zhang, S.; Wang, J.; Zaman, K.A.U.; Bai, H.; Meng, F. Comprehensive Analysis of Trypoxylus dichotomus: Inorganic Elements, Amino Acids, and Bioactive Compounds and Their Anticancer, Antioxidant, and Neuroprotective Properties. Molecules 2025, 30, 220. https://doi.org/10.3390/molecules30020220
Ye D, Li Q, Liu X, Zhou J, Yin S, Zhang S, Wang J, Zaman KAU, Bai H, Meng F. Comprehensive Analysis of Trypoxylus dichotomus: Inorganic Elements, Amino Acids, and Bioactive Compounds and Their Anticancer, Antioxidant, and Neuroprotective Properties. Molecules. 2025; 30(2):220. https://doi.org/10.3390/molecules30020220
Chicago/Turabian StyleYe, Dongyan, Qianhui Li, Xuwen Liu, Jie Zhou, Shuren Yin, Suyi Zhang, Jing Wang, Kh Ahammad Uz Zaman, Helong Bai, and Fanlei Meng. 2025. "Comprehensive Analysis of Trypoxylus dichotomus: Inorganic Elements, Amino Acids, and Bioactive Compounds and Their Anticancer, Antioxidant, and Neuroprotective Properties" Molecules 30, no. 2: 220. https://doi.org/10.3390/molecules30020220
APA StyleYe, D., Li, Q., Liu, X., Zhou, J., Yin, S., Zhang, S., Wang, J., Zaman, K. A. U., Bai, H., & Meng, F. (2025). Comprehensive Analysis of Trypoxylus dichotomus: Inorganic Elements, Amino Acids, and Bioactive Compounds and Their Anticancer, Antioxidant, and Neuroprotective Properties. Molecules, 30(2), 220. https://doi.org/10.3390/molecules30020220





