Volatile Organic Compounds (VOCs) in Neurodegenerative Diseases (NDDs): Diagnostic Potential and Analytical Approaches
Abstract
1. Introduction
1.1. Neurodegenerative Diseases
1.2. Environmental Risk Factors for NDD Onset
1.3. State of the Art on the Main Environmental Risk Factors
2. VOCs in Biological Matrices as Potential Biomarkers of Neurological Diseases
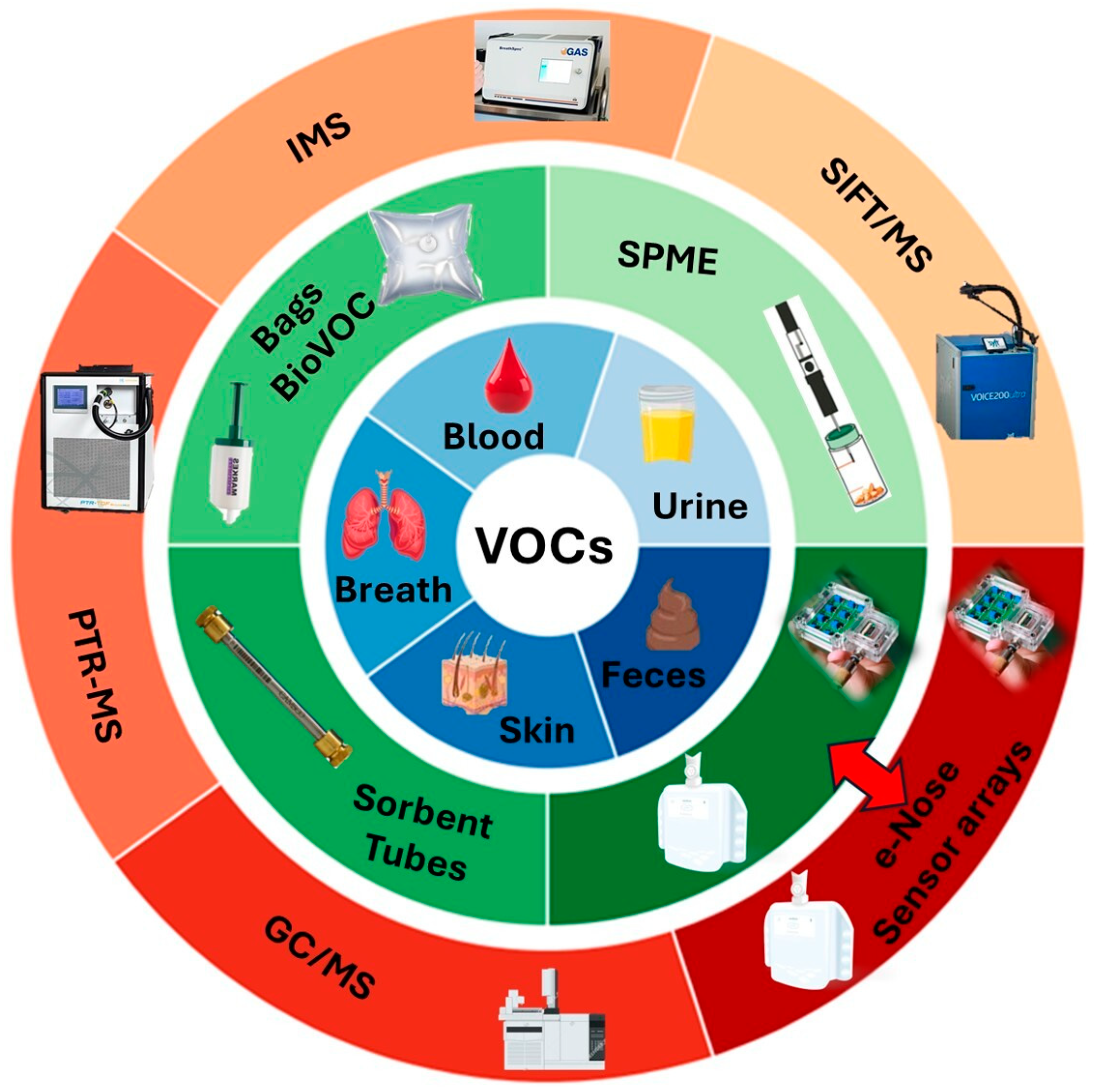
2.1. A Preliminary Overview
2.2. VOCs in Human Breath as Biomarkers of NDDs
2.3. VOCs in Human Feces as Biomarkers of NDDs
2.4. VOCs in Human Sebum as Biomarkers of NDDs
2.5. Challenges and Controversies of VOCs Detection in Biological Matrices for Diagnostic Purposes: A Brief Discussion on Potentialities and Limitations
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| CO | Carbon Monoxide |
| CSM | Cervical Spondylotic Myelopathy |
| CT | Computed Tomography |
| DHS-TD-GC/MS | Dynamic Headspace–Thermal Desorption GC-MS |
| DFA | Discriminant Factor Analysis |
| DL | Deep Learning |
| e-Nose | Electronic Nose |
| FRDA | Friedreich’s Ataxia |
| FTD | Frontotemporal Dementia |
| GC-MS/GC-IMS | Gas Chromatography–Mass Spectrometry/Ion Mobility Spectrometry |
| GDS | Global Deterioration Scale |
| HD | Huntington’s Disease |
| LBD | Lewy Body Dementia |
| LDA | Linear Discriminant Analysis |
| ML | Machine Learning |
| MRI | Magnetic Resonance Imaging |
| NDDs | Neurodegenerative Diseases |
| NOx | Nitrogen Oxides |
| NS | Nitrosative Stress |
| OCPs | Organochlorine Pesticides |
| OR | Odds Ratio |
| OS | Oxidative Stress |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCBs | Polychlorinated Biphenyls |
| PD | Parkinson’s Disease |
| PET | Positron Emission Tomography |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| PM2.5/PM10 | Particulate Matter (2.5/10 μm) |
| POCT | Point-of-Care Testing |
| PTR-MS/SIFT-MS | Proton Transfer Reaction-MS/Selected Ion Flow Tube-MS |
| RF | Random Forest |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| SPME | Solid Phase Microextraction |
| TaClo | 1-trichloromethyl-1,2,3,4-tetrahydro-β-carboline |
| SVM | Support Vector Machine |
| VIP | Variables with an Importance in Prediction |
| VOCs | Volatile Organic Compounds |
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhang, Y.; Zhang, H.; Gao, S.; Wang, L.; Wang, T.; Han, Z.; Sun, B.L.; Liu, G. Mendelian randomization highlights significant difference and genetic heterogeneity in clinically diagnosed Alzheimer’s disease GWAS and self-report proxy phenotype GWAX. Alzheimers Res. Ther. 2022, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Chen-Plotkin, A.S. Genetic modifiers in neurodegeneration. Curr. Genet. Med. Rep. 2018, 6, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Baitharu, I.; Barhwal, K.; Prasad, D.; Singh, S.B.; Ilavazhagan, G.J.C. Enriched environment prevents hypobaric hypoxia induced neurodegeneration and is independent of antioxidant signaling. Cell. Mol. Neurobiol. 2012, 32, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Emard, J.F.; Thouez, J.P.; Gauvreau, D. Neurodegenerative diseases and risk factors: A literature review. Soc. Sci. Med. 1995, 40, 847–858. [Google Scholar] [CrossRef]
- Ogonowski, N.S.; García-Marín, L.M.; Fernando, A.S.; Flores-Ocampo, V.; Rentería, M.E. Impact of genetic predisposition to late-onset neurodegenerative diseases on early life outcomes and brain structure. Transl. Psychiatry 2024, 14, 185. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Bisi, N.; Pinzi, L.; Rastelli, G.; Tonali, N. Early diagnosis of neurodegenerative diseases: What has been undertaken to promote the transition from PET to fluorescence tracers. Molecules 2024, 29, 722. [Google Scholar] [CrossRef]
- Manera, V.; Rovini, E.; Wais, P. Editorial: Early detection of neurodegenerative disorders using behavioral markers and new technologies: New methods and perspectives. Front. Aging Neurosci. 2023, 15, 1149886. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Santos, R.; Bulteau, A.L.; Gomes, C.M. Neurodegeneration, neurogenesis, and oxidative stress. Oxid. Med. Cell Longev. 2016, 2016, 7632025. [Google Scholar] [CrossRef]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef] [PubMed]
- Fusco, M.; Skaper, S.; Coaccioli, S.; Paladini, A.; Varrassi, G. Degenerative joint diseases and neuroinflammation. Pain Pract. 2017, 17, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, G.; Fusco, M.; Skaper, S.D.; Battelli, D.; Zis, P.; Coaccioli, S.; Pace, M.C.; Paladini, A. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: A review. Pain. Ther. 2018, 7, 59–75. [Google Scholar] [CrossRef]
- Boncristiani, C.; Di Gilio, A.; De Castro, F.; Nardini, A.; Palmisani, J.; Martínez Vázquez, R.; de Gennaro, G.; Fanizzi, F.P.; Ciccarella, G.; Vergaro, V. Recent advances in 3D models for multiparametric blood–brain barrier detection in microfluidic systems. J. Mater. Chem. B 2025, 13, 6597–6625. [Google Scholar] [CrossRef]
- GBD 2021. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2024. [Google Scholar]
- Molot, J.; Sears, M.; Marshall, L.M.; Bray, R.I. Neurological susceptibility to environmental exposures: Pathophysiological mechanisms in neurodegeneration and multiple chemical sensitivity. Rev. Environ. Health 2021, 37, 509–530. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and human health impacts of volatile organic compounds: A perspective review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef]
- Mir, R.H.; Sawhney, G.; Pottoo, F.H.; Mohi-Ud-Din, R.; Madishetti, S.; Jachak, S.M.; Ahmed, Z.; Masoodi, M.H. Role of environmental pollutants in Alzheimer’s disease: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 44724–44742. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Fang, Y.; Li, F.L.; Dong, B.; Hua, X.G.; Jiang, W.; Zhang, H.; Lyu, Y.; Zhang, X.J. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environ. Res. 2019, 176, 448–459. [Google Scholar] [CrossRef]
- Delamarre, A.; Meissner, W.G. Epidemiology, environmental risk factors and genetics of Parkinson’s disease. Presse Med. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Air pollution and risk of Parkinson’s disease in a large prospective study of men. Environ. Health Perspect. 2017, 125, 087011. [Google Scholar] [CrossRef] [PubMed]
- Kioumourtzoglou, M.A.; Schwartz, J.D.; Weisskopf, M.G.; Melly, S.J.; Wang, Y.; Dominici, F.; Zanobetti, A. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ. Health Perspect. 2016, 124, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Liu, L.L.; Sun, Y.; Chen, Y.A.; Liu, C.C.; Li, C.Y.; Yu, H.L.; Ritz, B. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environ. Int. 2016, 96, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Young, M.T.; Chen, J.C.; Kaufman, J.D.; Chen, H. Ambient air pollution exposures and risk of Parkinson disease. Environ. Health Perspect. 2016, 124, 1759–1765. [Google Scholar] [CrossRef]
- Ritz, B.; Lee, P.C.; Hansen, J.; Lassen, C.F.; Ketzel, M.; Sorensen, M.; Raaschou-Nielsen, O. Traffic-related air pollution and Parkinson’s disease in Denmark: A case-control study. Environ. Health Perspect. 2016, 124, 351–356. [Google Scholar] [CrossRef]
- Kirrane, E.F.; Bowman, C.; Davis, J.A.; Hoppin, J.A.; Blair, A.; Chen, H.; Patel, M.M.; Sandler, D.P.; Tanner, C.M.; Vinikoor-Imler, L.; et al. Associations of ozone and PM2.5 concentrations with Parkinson’s disease among participants in the agricultural health study. J. Occup. Environ. Med. 2015, 57, 509–517. [Google Scholar] [CrossRef]
- Dutheil, F.; Beaune, P.; Tzourio, C.; Loriot, M.A.; Elbaz, A. Interaction between ABCB1 and professional exposure to organochlorine insecticides in Parkinson disease. Arch. Neurol. 2010, 67, 739–745. [Google Scholar] [CrossRef]
- Bharathi, I.S.S.; Rao, K.S. Copper- and iron-induced differential fibril formation in alpha-synuclein: TEM study. Neurosci. Lett. 2007, 424, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The outdoor air pollution and brain health workshop. Neurotoxicology 2012, 33, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Segalowitz, S.J. Public health, brain health, and the dangers of air pollution for neural development. Brain Cogn. 2008, 68, 115–116. [Google Scholar] [CrossRef]
- Kaplin, A.I.; Williams, M. How common are the “common” neurologic disorders? Neurology 2007, 69, 410–411. [Google Scholar] [CrossRef]
- Kalenik, S.; Zaczek, A.; Rodacka, A. Air pollution-induced neurotoxicity: The relationship between air pollution, epigenetic changes, and neurological disorders. Int. J. Mol. Sci. 2025, 26, 3402. [Google Scholar] [CrossRef]
- Moulton, P.V.; Yang, W. Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public. Health 2012, 2012, 472751. [Google Scholar] [CrossRef]
- Kim, D.-K.; Park, J.-D.; Choi, B.-S. Mercury-induced amyloid-beta (Aβ) accumulation in the brain is mediated by disruption of Aβ transport. J. Toxicol. Sci. 2014, 39, 625–635. [Google Scholar] [CrossRef]
- Ballinger, S.W. Mitochondrial dysfunction as a hallmark of environmental injury. Cells 2021, 11, 110. [Google Scholar] [CrossRef]
- Huang, M.; Bargues-Carot, A.; Riaz, Z.; Wickham, H.; Zenitsky, G.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10808. [Google Scholar] [CrossRef]
- Calabró, V.; Garces, M.; Cáceres, L.; Magnani, N.D.; Marchini, T.; Freire, A.; Vico, T.; Martinefski, M.; Vanasco, V.; Triodi, V.; et al. Urban air pollution induces alterations in redox metabolism and mitochondrial dysfunction in mice brain cortex. Arch. Biochem. Biophys. 2021, 704, 108875–108883. [Google Scholar] [CrossRef]
- Olloquequi, J.; Díaz-Peña, R.; Verdaguer, E.; Ettcheto, M.; Auladell, C.; Camins, A. From Inhalation to Neurodegeneration: Air Pollution as a Modifiable Risk Factor for Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 6928. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Fink, A.L. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: A possible factor in Parkinson’s disease. FEBS Lett. 2001, 500, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein: A possible molecular link between Parkinson’s disease and heavy metal exposure. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Chen, H.; Weisskopf, M.G.; O’Reilly, E.; McCullough, M.L.; Calle, E.E.; Schwarzschild, M.A.; Thun, M.J. Pesticide exposure and risk for Parkinson’s disease. Ann. Neurol. 2006, 60, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, A.; Khuder, S.A.; Schaub, E.A.; Shrivastava, S. A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology 2000, 21, 435–440. [Google Scholar]
- Corrigan, F.M.; Wienburg, C.L.; Shore, R.F.; Daniel, S.; Mann, D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J. Toxicol. Environ. Health A 2000, 59, 229–234. [Google Scholar]
- Le Couteur, D.G.; McLean, A.J.; Taylor, M.C.; Woodham, B.L.; Board, P.G. Pesticides and Parkinson’s disease. Biomed. Pharmacother. 1999, 53, 122–130. [Google Scholar] [CrossRef]
- Gao, H.M.; Hong, J.S.; Zhang, W.; Liu, B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: Relevance to the etiology of Parkinson’s disease. J. Neurosci. 2003, 23, 1228–1236. [Google Scholar] [CrossRef]
- Keane, P.C.; Hanson, P.S.; Patterson, L.; Blain, P.G.; Hepplewhite, P.; Khundakar, A.A.; Judge, S.J.; Kahle, P.J.; LeBeau, F.E.N.; Morris, C.M. Trichloroethylene and its metabolite TaClo lead to degeneration of substantia nigra dopaminergic neurones: Effects in wild type and human A30P mutant α-synuclein mice. Neurosci. Lett. 2019, 711, 134437. [Google Scholar] [CrossRef]
- Zeliger, H.I. Exposure to lipophilic chemicals as a cause of neurological impairments, neurodevelopmental disorders and neurodegenerative diseases. Interdiscip. Toxicol. 2013, 6, 103–110. [Google Scholar] [CrossRef]
- Guehl, D.; Bezard, E.; Dovero, S.; Boraud, T.; Bioulac, B.; Gross, C. Trichloroethylene and parkinsonism: A human and experimental observation. Eur. J. Neurol. 1999, 6, 609–611. [Google Scholar] [CrossRef]
- Liu, M.; Shin, E.-J.; Dang, D.-K.; Jin, C.-H.; Lee, P.H.; Jeong, J.H.; Park, S.-J.; Kim, Y.-S.; Xing, B.; Xin, T.; et al. Trichloroethylene and Parkinson’s disease: Risk assessment. Mol. Neurobiol. 2018, 55, 6201–6214. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.J.; Lewis, L.D.; Gardner, T.B.; Osterling, W.; Eskey, C.J.; Nierenberg, D.W. Two cases of rapid onset Parkinson’s syndrome following toxic ingestion of ethylene glycol and methanol. Clin. Pharmacol. Ther. 2007, 81, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Vardi, J. Progressive parkinsonism in a young experimental physicist following long-term exposure to methanol. Neurotoxicology 2002, 23, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Chavan, H.; Krishnamurthy, P. Polycyclic aromatic hydrocarbons (PAHs) mediate transcriptional activation of the ATP binding cassette transporter ABCB6 gene via the aryl hydrocarbon receptor (AhR). J. Biol. Chem. 2012, 287, 32054–32068. [Google Scholar] [CrossRef]
- Ruder, A.M.; Hein, M.J.; Hopf, N.B.; Waters, M.A. Mortality among 24,865 workers exposed to polychlorinated biphenyls (PCBs) in three electrical capacitor manufacturing plants: A ten-year update. Int. J. Hyg. Environ. Health 2014, 217, 176–187. [Google Scholar] [CrossRef]
- Richardson, J.R.; Roy, A.; Shalat, S.L.; von Stein, R.T.; Hossain, M.M.; Buckley, B.; Gearing, M.; Levey, A.I.; German, D.C. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 2014, 71, 284–290. [Google Scholar] [CrossRef]
- Kilian, J.; Kitazawa, M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease: Evidence from epidemiological and animal studies. Biomed. J. 2018, 41, 141–162. [Google Scholar] [CrossRef]
- Haghani, A.; Morgan, T.E.; Forman, H.J.; Finch, C.E. Air pollution neurotoxicity in the adult brain: Emerging concepts from experimental findings. J. Alzheimers Dis. 2020, 76, 773–797. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garciduenas, L.; Torres-Jardon, R.; Kulesza, R.J.; Mansour, Y.; González-González, L.O.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Mukherjee, P.S. Alzheimer disease starts in childhood in polluted Metropolitan Mexico City: A major health crisis in progress. Environ. Res. 2020, 183, 109137. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Gascon, M.; Gispert, J.D.; Cirach, M.; Sánchez-Benavides, G.; Falcon, C.; Arenaza-Urquijo, E.M.; Gotsens, X.; Fauria, K.; Sunyer, J.; et al. Impact of urban environmental exposures on cognitive performance and brain structure of healthy individuals at risk for Alzheimer’s dementia. Environ. Int. 2020, 138, 105546. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Kulesza, R.J.; González-González, L.O.; Reynoso-Robles, R.; Mukherjee, P.S.; Torres-Jardón, R. Air Pollution, Combustion and Friction Derived Nanoparticles, and Alzheimer’s Disease in Urban Children and Young Adults. J. Alzheimers Dis. 2019, 70, 343–360. [Google Scholar] [CrossRef]
- Tsai, T.-L.; Lin, Y.-T.; Hwang, B.-F.; Nakayama, S.F.; Tsai, C.-H.; Sun, X.-L.; Ma, C.; Jung, C.-R. Fine particulate matter is a potential determinant of Alzheimer’s disease: A systemic review and meta-analysis. Environ. Res. 2019, 177, 108638. [Google Scholar] [CrossRef]
- Younan, D.; Petkus, A.J.; Widaman, K.F.; Wang, X.; Casanova, R.; Espeland, M.A.; Gatz, M.; Henderson, V.W.; Manson, J.E.; Rapp, S.R.; et al. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 2020, 143, 289–302. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, X.; Zhao, H.; Chang, J.Z.; Zhan, G.; Yang, M.; Yao, C.; Huanhuan, Z.; Cunrui, H.; Zengli, Y. Global ambient particulate matter pollution and neurodegenerative disorders: A systematic review of literature and meta-analysis. Environ. Sci. Pollut. Res. 2023, 30, 39418–39430. [Google Scholar] [CrossRef]
- Medehouenou, T.C.M.; Ayotte, P.; Carmichael, P.-H.; Krööger, E.; Verreault, R.; Lindsay, J.; Dewailly, É.; Tyas, S.L.; Bureau, A.; Laurin, D. Exposure to polychlorinated biphenyls and organochlorine pesticides and risk of dementia, Alzheimer’s disease and cognitive decline in an older population: A prospective analysis from the Canadian Study of Health and Aging. Environ. Health 2019, 18, 57. [Google Scholar] [CrossRef]
- Cipriani, G.; Danti, S.; Carlesi, C.; Borin, G. Danger in the air: Air pollution and cognitive dysfunction. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 333–341. [Google Scholar] [CrossRef]
- Babic Leko, M.; Mihelcic, M.; Jurasovic, J.; Perković, M.N.; Španić, E.; Sekovanić, A.; Orct, T.; Zubčić, K.; Horvat, L.L.; Pleić, N.; et al. Heavy metals and essential metals are associated with cerebrospinal fluid biomarkers of Alzheimer’s disease. Int. J. Mol. Sci. 2022, 24, 467. [Google Scholar] [CrossRef]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef]
- Kukull, W.A.; Larson, E.B.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Pfanschmidt, M.L.; Thompson, J.D.; O’Meara, E.S.; Brenner, D.E.; van Belle, G. Solvent exposure as a risk factor for Alzheimer’s disease: A case-control study. Am. J. Epidemiol. 1995, 141, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, G.D.; Still, K.R.; Alexander, W.K.; Nordholm, A.F.; Wilson, C.L.; Rossi, J., III; Mattie, D.R. A review of the neurotoxicity risk of selected hydrocarbon fuels. J. Toxicol. Environ. Health B Crit. Rev. 2001, 4, 223–312. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Schlesinger, I.; Ionescu, R.; Nassar, M.; Axelrod, N.; Robertman, D.; Tessler, Y.; Azar, F.; Marmur, A.; Aharon-Peretz, J.; et al. Detection of Alzheimer’s and Parkinson’s disease from exhaled breath using nanomaterial-based sensors. Nanomedicine 2013, 8, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Mazzatenta, A.; Pokorski, M.; Sartucci, F.; Domenici, L.; Di Giulio, C. Volatile organic compounds (VOCs) fingerprint of Alzheimer’s disease. Respir. Physiol. Neurobiol. 2015, 209, 81–84. [Google Scholar] [CrossRef]
- Tiele, A.; Wicaksono, A.; Daulton, E.; Ifeachor, E.; Eyre, V.; Clarke, S.; Timings, L.; Pearson, S.; Covington, J.A.; Li, X. Breath-based non-invasive diagnosis of Alzheimer’s disease: A pilot study. J. Breath Res. 2020, 14, 026003. [Google Scholar] [CrossRef]
- Xiong, C.; Lu, R.; Bui, Q.; Popp, B.; Schindler, S.; Shriver, L.; Cruchaga, C.; Hassenstab, J.; Benzinger, T.; Agboola, F.; et al. Person-specific digitally measured air pollutant exposure and biomarkers of Alzheimer disease: Findings from a pilot study. J. Alzheimers Dis. 2025, 107, 13872877251362667. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic. Biol. Med. 2010, 49, 1328–1341. [Google Scholar] [CrossRef]
- Al-Mousa, F.; Michelangeli, F. Some commonly used brominated flame retardants cause Ca2+ ATPase inhibition, beta-amyloid peptide release and apoptosis in SH–SY5Y neuronal cells. PLoS ONE 2012, 7, e33059. [Google Scholar] [CrossRef]
- Viberg, H.; Fredriksson, A.; Eriksson, P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 2003, 192, 95–106. [Google Scholar] [CrossRef]
- Murata, H.; Barnhill, L.M.; Bronstein, J.M. Air pollution and the risk of Parkinson’s disease: A review. Mov. Disord. 2022, 37, 894–904. [Google Scholar] [CrossRef]
- Maher, B.A.; Ahmed, I.A.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.; Torres-Jardon, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Subramaniam, N.S.; Bawden, C.S.; Waldvogel, H.; Faull, R.M.L.; Howarth, G.S.; Snell, R.G. Emergence of breath testing as a new non-invasive diagnostic modality for neurodegenerative diseases. Brain Res. 2018, 1691, 75–86. [Google Scholar] [CrossRef]
- Ho, E.; Galougahi, K.K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Capuano, R.; Ciotti, M.; Catini, A.; Bernardini, S.; Di Natale, C. Clinical applications of volatilomic assays. Crit. Rev. Clin. Lab. Sci. 2024, 62, 45–64. [Google Scholar] [CrossRef] [PubMed]
- van Helmond, W.; van Bochove, M.A.; van Herwijnen, A.W.; van Riemsdijk, J.J.H.; de Poot, C.J.; de Puit, M. Chemical profiling of fingerprints using mass spectrometry. Forensic Chem. 2019, 16, 100183. [Google Scholar] [CrossRef]
- Henderson, B.; Slingers, G.; Pedrotti, M.; Pugliese, G.; Malásková, M.; Bryant, L.; Lomonaco, T.; Ghimenti, S.; Moreno, S.; Cordell, R.; et al. The peppermint breath test benchmark for PTR-MS and SIFT-MS. J. Breath Res. 2021, 15, 046005. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.G.; Vidal-de-Miguel, G. Breath Analysis by Secondary Electro-Spray Ionization—Mass Spectrometry to Interrogate Biologically Significant Metabolites Non-Invasively. Crit. Rev. Anal. Chem. 2023, 53, 825–837. [Google Scholar] [CrossRef]
- Lau, H.-C.; Yu, J.-B.; Lee, H.-W.; Huh, J.-S.; Lim, J.-O. Investigation of exhaled breath samples from patients with Alzheimer’s disease using gas chromatography-mass spectrometry and an exhaled breath sensor system. Sensors 2017, 17, 1783. [Google Scholar] [CrossRef]
- Vasilescu, A.; Hrinczenko, B.; Swain, G.M.; Peteu, S.F. Exhaled breath biomarker sensing. Biosens. Bioelectron. 2021, 182, 113193. [Google Scholar] [CrossRef]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart gas sensors: Recent developments and future prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef]
- Wang, C.; Li, M.; Jiang, H.; Tong, H.; Feng, Y.; Wang, Y.; Pi, X.; Guo, L.; Nie, M.; Feng, H.; et al. Comparative analysis of VOCs in exhaled breath of amyotrophic lateral sclerosis and cervical spondylotic myelopathy patients. Sci. Rep. 2016, 6, 26120. [Google Scholar] [CrossRef] [PubMed]
- Feng, T. Applications of artificial intelligence to diagnosis of neurodegenerative diseases. Stud. Health Technol. Inform. 2023, 308, 648–655. [Google Scholar] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, P.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Mansurova, M.; Ebert, B.E.; Blank, L.M.; Ibáñez, A.J. A breath of information: The volatilome. Curr. Genet. 2018, 64, 959–964. [Google Scholar] [CrossRef]
- Mochalski, P.; King, J.; Klieber, M.; Unterkofler, K.; Hinterhuber, H.; Baumann, M.; Amann, A. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst 2013, 138, 2134–2145. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef]
- Drabinska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; De Lacy Costello, B. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath Res. 2021, 15, 034001. [Google Scholar] [CrossRef]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; Di Natale, C.; et al. Volatile signature for the early diagnosis of lung cancer. J. Breath Res. 2016, 10, 016007. [Google Scholar] [CrossRef]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath analysis: Comparison among methodological approaches for breath sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef]
- Palmisani, J.; Di Gilio, A. Sampling Technologies. In Volatile Organic Compounds; Picciariello, A., Altomare, D.F., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Miekisch, W.; Kischkel, S.; Sawacki, A.; Liebau, T.; Mieth, M.; Schubert, J.K. Impact of sampling procedures on the results of breath analysis. J. Breath. Res. 2008, 2, 026007. [Google Scholar] [CrossRef] [PubMed]
- Amorim, L.C.A.; Cardeal, Z.L. Breath air analysis and its use as a biomarker in biological monitoring of occupational and environmental exposure to chemical agents. J. Chromatogr. B 2007, 853, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Herbig, J.; Titzmann, T.; Beauchamp, J.; Kohl, I.; Hansel, A. Buffered end-tidal (BET) sampling—A novel method for real-time breath-gas analysis. J. Breath Res. 2008, 2, 037008. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2643–2651. [Google Scholar] [CrossRef]
- Akman, H.; Bayrakli, I.; Kutluhan, S. Investigation of Correlation between Neurological Diseases and Breath Hexanal. J. Bioanal. Biomed. 2018, 10, 56–59. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Wieczorek, T.; Drabinska, N. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 034001. [Google Scholar] [CrossRef]
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561. [Google Scholar] [CrossRef]
- Aluf, Y.; Vaya, J.; Khatib, S.; Loboda, Y.; Kizhner, S.; Finberg, J.P. Specific oxidative stress profile associated with partial striatal dopaminergic depletion by 6-hydroxydopamine as assessed by a novel multifunctional marker molecule. Free Radic. Res. 2010, 44, 635–644. [Google Scholar] [CrossRef]
- Haripriya, P.; Rangarajan, M.; Pandya, H.J. Breath VOC analysis and machine learning approaches for disease screening: A review. J. Breath Res. 2023, 17, 024001. [Google Scholar] [CrossRef]
- Bach, J.P.; Gold, M.; Mengel, D.; Hattesohl, A.; Lubbe, D.; Schmid, S.; Tackenberg, B.; Rieke, J.; Maddula, S.; Baumbach, J.I.; et al. Measuring compounds in exhaled air to detect Alzheimer’s disease and Parkinson’s disease. PLoS ONE 2015, 10, e0132227. [Google Scholar] [CrossRef]
- Cope, K.A.; Watson, M.T.; Foster, W.M.; Sehnert, S.S.; Risby, T.H. Effects of ventilation on the collection of exhaled breath in humans. J. Appl. Physiol. 2004, 96, 1371–1379. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.C.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of lung cancer with volatile markers in the breath. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Sanchez, J.M. Analytical challenges in breath analysis and its application to exposure monitoring. Trends Anal. Chem. 2013, 44, 79–89. [Google Scholar] [CrossRef]
- Lourenço, C.; Turner, C. Breath Analysis in Disease Diagnosis: Methodological Considerations and Applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hackl, M.; et al. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res. 2012, 6, 036008. [Google Scholar] [CrossRef]
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.W.; Wijmenga, C.; van Schooten, F.J. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef]
- Ubeda, C.; Vázquez-Carretero, M.D.; Luque-Tirado, A.; Ríos-Reina, R.; Rubio-Sánchez, R.; Peral, M.J. Fecal volatile organic compounds and microbiota associated with the progression of cognitive impairment in Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 707. [Google Scholar] [CrossRef]
- De Pablo-Fernandez, E.; Gebeyehu, G.G.; Flain, F.; Slater, R.; Frau, A.; Ijaz, U.Z.; Warner, T.; Probert, C. The faecal metabolome and mycobiome in Parkinson’s disease. Park. Relat. Disord. 2022, 95, 65–69. [Google Scholar] [CrossRef]
- Smiełowska, M.; Ligor, T.; Kupczyk, W.; Szeliga, J.; Jackowski, M.; Buszewski, B. Screening for volatile biomarkers of colorectal cancer by analyzing breath and fecal samples using thermal desorption combined with GC-MS (TD-GC-MS). J. Breath Res. 2023, 17, 047102. [Google Scholar] [CrossRef]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Meng, L.; Shen, L. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef]
- Nazik, H.; Yıldız, B.T. Evaluation of skin disorders, skin sebum and moisture in patients with Parkinson’s disease. Neurol. Asia 2019, 24, 249–254. [Google Scholar]
- Fu, W.; Linxin, X.; Qiwen, Y.; Fang, J.; Zhao, G.; Li, Y.; Pan, C.; Dong, H.; Wang, D.; Ren, H.; et al. Artificial intelligent olfactory system for the diagnosis of Parkinson’s disease. ACS Omega 2022, 7, 5. [Google Scholar] [CrossRef]
- Sinclair, E.; Walton-Doyle, C.; Sarkar, D.; Hollywood, K.A.; Milne, J.; Lim, S.H.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Silverdale, M.; et al. Validating differential volatilome profiles in Parkinson’s disease. ACS Cent. Sci. 2021, 7, 300–306. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Sinclair, E.; Xu, Y.; Sarkar, D.; Walton-Doyle, C.; Liscio, C.; Banks, P.; Milne, J.; Silverdale, M.; Kunath, T.; et al. Discovery of volatile biomarkers of Parkinson’s disease from sebum. ACS Cent. Sci. 2019, 5, 599–606. [Google Scholar] [CrossRef]
- Tsuda, T.; Nonome, T.; Goto, S.; Takeda, J.-I.; Tsunoda, M.; Hirayama, M.; Ohno, K. Application of skin gas GC/MS analysis for prediction of the severity scale of Parkinson’s disease. Chromatography 2019, 40, 149–155. [Google Scholar] [CrossRef]
- Agapiou, A.; Amann, A.; Mochalski, P.; Statheropoulos, M.; Thomas, C. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. Trends Anal. Chem. 2015, 66, 158–175. [Google Scholar] [CrossRef]

| NDDs | VOCs in Main Biological Matrices as Potential Biomarkers of NDDs (Chemical Class—Metabolic Pathway) | ||
|---|---|---|---|
| Breath | Feces | Sebum | |
| AD | Styrene (aromatic hydrocarbon—potentially linked to oxidative stress); 1-methyl-3-(1-methylethyl)benzene (branched aromatic hydrocarbon—unknown); 4-methyloctane (methylated alkane—oxidative stress and lipid peroxidation); 2,6,10-trimethyldodecane (methylated alkane—oxidative stress and lipid peroxidation); 3,7-dimethyldecane (methylated alkane—oxidative stress and lipid peroxidation); butylated hydroxytoluene (branched phenol—unknown) 2,4-dimethyl-1-heptene (methylated alkene/potentially linked lipid peroxidation) [75] | trans-2-decenal (medium-chain fatty aldehyde—potentially associated with gut microbiota defense mechanisms); acetic acid (carboxylic acid—altered gut microbiota); propanoic acid (SCFAs—altered gut microbiota); butanoic acid (SCFAs—altered gut microbiota); pentanoi acid (SCFAs—altered gut microbiota); hexanoic acid (SCFAs—altered gut microbiota); heptanoic acid (SCFAs—altered gut microbiota); 1-butanol (alcohol—unknown) 3-phenyl-propanol (alcohol—unknown); butyl 2-methylbutanoate (ester—unknown); propyl butanoate (ester—unknown) [121] | |
| 2-ethyltoluene (aromatic hydrocarbon—unknown); 3-octanone (ketone—unknown); 2-methylfuran (heteroaromatic compound—unknown) [114] | |||
| 1-phenantherol (polyaromatic hydrocarbon—possibly attributed to endocrine disruption or accumulation of acetylcholinesterase at the synapse); ethyl 3-cyano-2,3-bis(2,5,-dimethyl-3-thienyl)-acrylate (hydrocarbon—unknown) [91] | |||
| acetone (ketone—potentially linked to the decline in brain glucose metabolism); 2-propanol (alcohol); 2-butanone (ketone); hexanal (aldehyde—lipid peroxidation); heptanal (aldehyde—lipid peroxidation); 1-butanol (alcohol—unknown) [77] | |||
| PD | 1-butanol (alcohol—unknown) [114] | 1,3-ditert-butylbenzene (alkylated aromatic hydrocarbon—unknown); γ-terpinene (monoterpene—unknown); α-pinene (bicyclic terpene—unknown) o-cymene (monoterpene—unknown); (R)-(d-limonene) (monoterpene—unknown); 2-methylpropanal (aldehyde—potentially related tolipid perxidation); 3-methylbutanal (aldehyde—potentially related to lipid peroxidation); hexanal (aldehyde—lipid peroxidation); [122] | octanal (aldehyde—oxidative stress, lipid peroxidation) hexyl acetate (ester—unknown) perillic aldehyde (aldehyde—unknown) [129] |
| styrene (aromatic hydrocarbon—potentially increased in PD patients breath due to oxidative stress); 2,3,6,7-tetramethyl-octane (methylated alkane—oxidative stress and lipid perixidation; ethylbenzene (alkylated aromatic hydrocarbon—unknown); 1-methyl-3-(1-methylethyl)benzene (alkylated aromatic hydrocarbon—unknown) [75] | perillic aldehyde (aldehyde—unknown) hippuric acid (carboxylic acid—associated with changes in skin microflora and physiology); eicosane (aliphatic hydrocarbon—associated with changes in skin microflora and physiology); octadecanal (long-chain aldehyde—increased and altered sebum secretion) [130,131] | ||
| 2-butenal (enal—lipid peroxidation); hexanal (aldehyde—lipid peroxidation); heptanal (aldehyde—lipid peroxidation); nonanal (aldehyde—lipid peroxidation); decanal (aldehyde—lipid peroxidation); 2-ethylhexanol (alcohol—unknown); styrene (aromatic hydrocarbon—potentially linked to oxidative stress) ethylbenzene (aromatic hydrocarbon—unknown); p-xylene (aromatic hydrocarbon—unknown); acetone (ketone—potentially indicative of latent insulin resistance); 5-hepten-2-one (ketone—unknown); ethyl acetate (ester—unknown); hexadecane (straight-chain alkane—unknown); tridecane (straight-chain alkane—unknown); dodecane (straight-chain alkane—potentially linked to lipid peroxidation); 6-methyl-o-xylene (methylated aromatic hydrocarbon—unknown); N,N-dimethyl acetamide (amide—unknown) [132] | |||
| MS | hexanal (aldehyde—biomarker of lipid peroxidation) [109] | ||
| ALS | carbamic acid, monoammonium salt (derivative of carbamic acid—decreased levels on ALS patients, involved in synthesis of diethyldithio-carbamic acid, the latter inhibitor of superoxide dismutase elevated in case of oxidative stress); 1-alanine ethylamide (potentially involved in the synthesis of CDDO-ethylamides palying a role in the activation of the endogenous cytoprotective system); guanidine, N,N-dimethyl (decreased levels in ALS patients, inhibitor of NO synthesis); phosphonic acid, p-hydroxyphenyl (organophosphorus compound—decreased levels in ALS patients) [94] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmisani, J.; Aresta, A.M.; Vergaro, V.; Mancini, G.; Mazzola, M.C.; Nisi, M.R.; Pastore, L.; Pizzillo, V.; De Vietro, N.; Boncristiani, C.; et al. Volatile Organic Compounds (VOCs) in Neurodegenerative Diseases (NDDs): Diagnostic Potential and Analytical Approaches. Molecules 2025, 30, 4028. https://doi.org/10.3390/molecules30194028
Palmisani J, Aresta AM, Vergaro V, Mancini G, Mazzola MC, Nisi MR, Pastore L, Pizzillo V, De Vietro N, Boncristiani C, et al. Volatile Organic Compounds (VOCs) in Neurodegenerative Diseases (NDDs): Diagnostic Potential and Analytical Approaches. Molecules. 2025; 30(19):4028. https://doi.org/10.3390/molecules30194028
Chicago/Turabian StylePalmisani, Jolanda, Antonella Maria Aresta, Viviana Vergaro, Giovanna Mancini, Miriana Cosma Mazzola, Marirosa Rosaria Nisi, Lucia Pastore, Valentina Pizzillo, Nicoletta De Vietro, Chiara Boncristiani, and et al. 2025. "Volatile Organic Compounds (VOCs) in Neurodegenerative Diseases (NDDs): Diagnostic Potential and Analytical Approaches" Molecules 30, no. 19: 4028. https://doi.org/10.3390/molecules30194028
APA StylePalmisani, J., Aresta, A. M., Vergaro, V., Mancini, G., Mazzola, M. C., Nisi, M. R., Pastore, L., Pizzillo, V., De Vietro, N., Boncristiani, C., Ciccarella, G., Zambonin, C., de Gennaro, G., & Di Gilio, A. (2025). Volatile Organic Compounds (VOCs) in Neurodegenerative Diseases (NDDs): Diagnostic Potential and Analytical Approaches. Molecules, 30(19), 4028. https://doi.org/10.3390/molecules30194028















