Synthesis of New Brassinosteroid Analogs with Androstane Skeleton and Heterocyclic Acyl Side Chains: Preliminary Molecular Docking Studies
Abstract
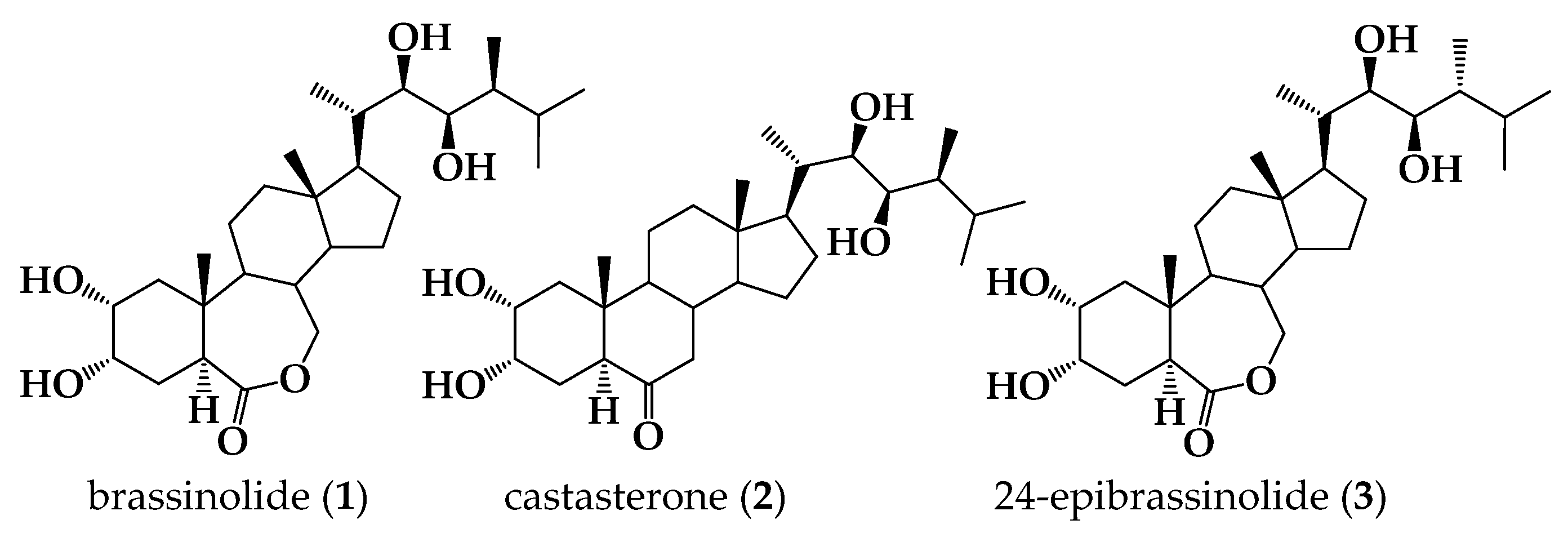
1. Introduction
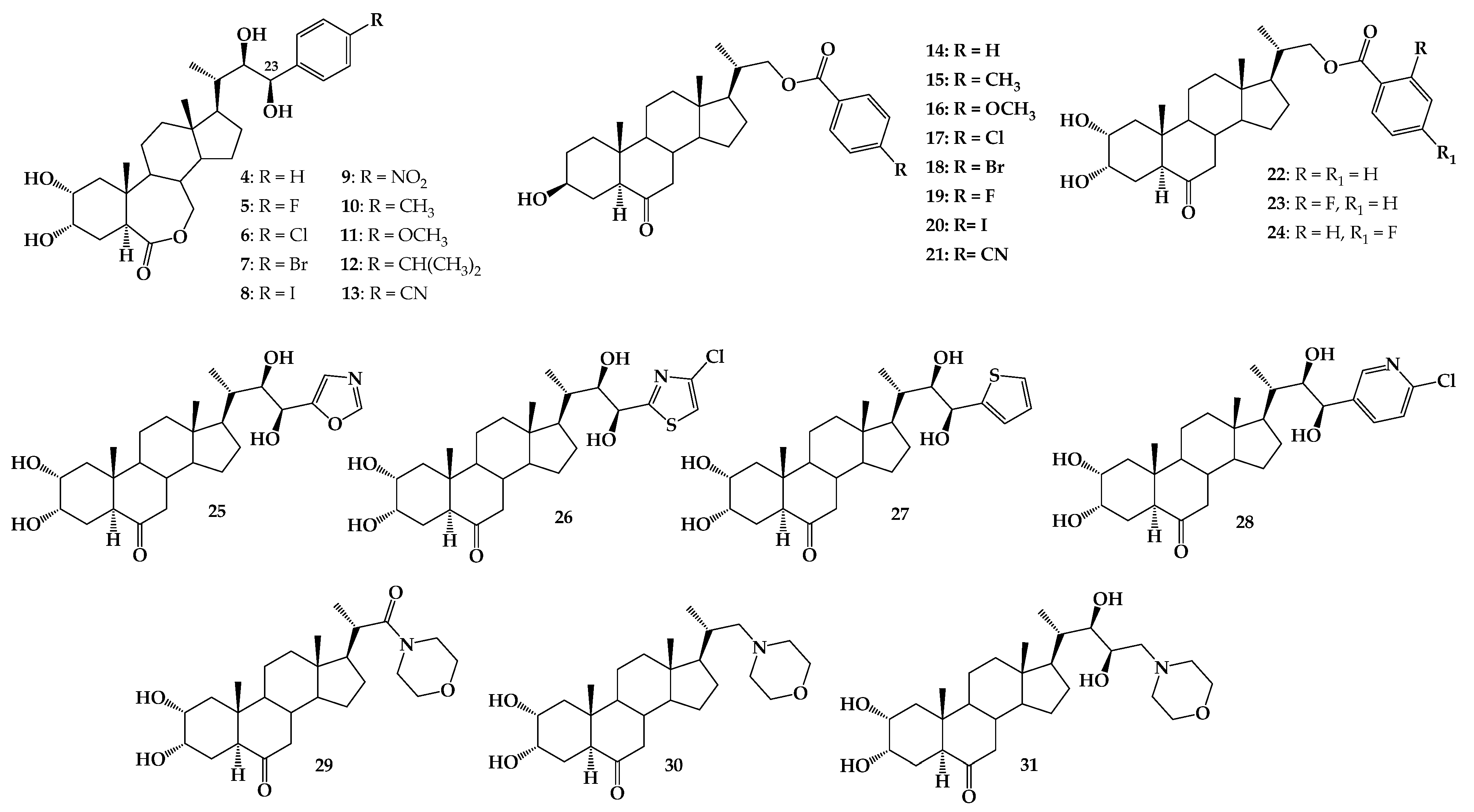
2. Results and Discussion
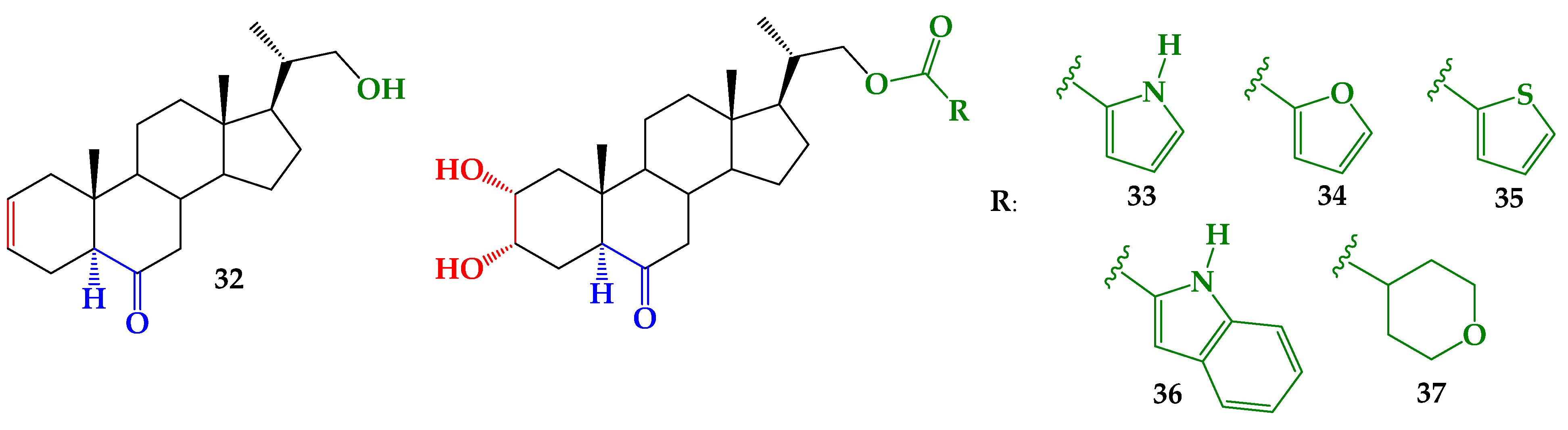
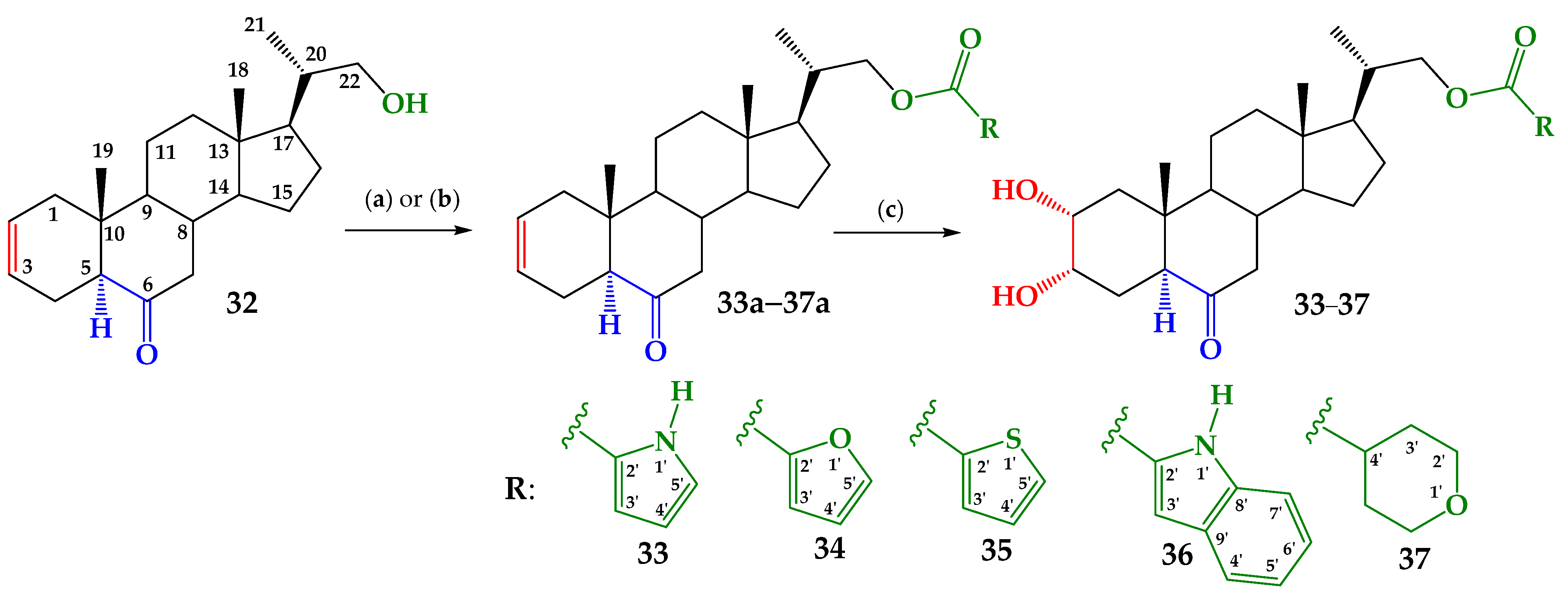
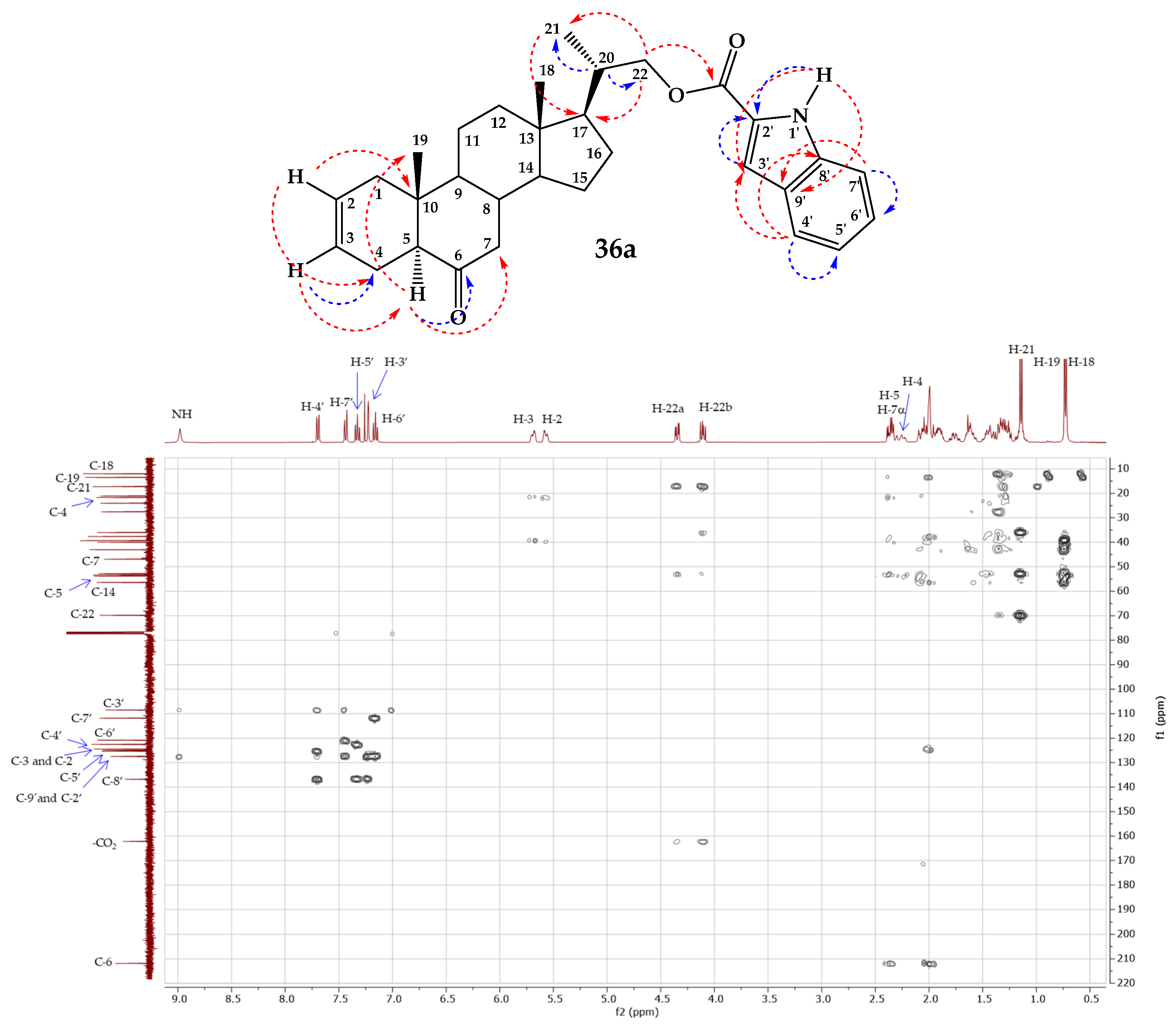
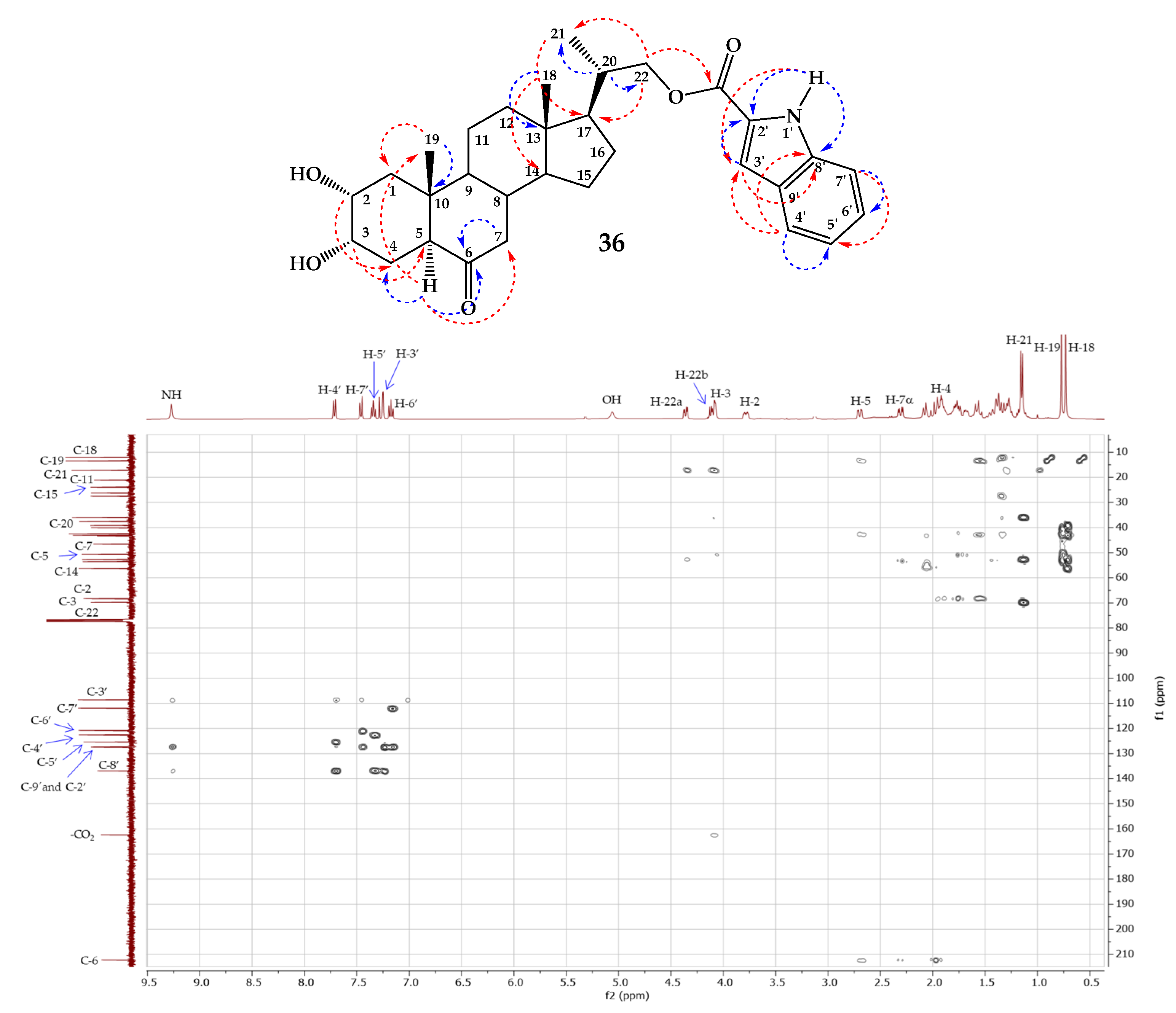
2.1. Chemical Synthesis
2.2. Molecular Docking Study
3. Materials and Methods
3.1. General Chemicals and Methods
3.2. Synthesis
3.2.1. General Procedure of Steglich Esterification
- 6-Oxo-23,24-dinor-5α-Cholan-2-en-22-yl-1H-pyrrole-2-carboxylate (33a).
- 6-Oxo-23,24-dinor-5α-Cholan-2-en-22-yl-1H-indole-2-carboxylate (36a).
3.2.2. Esterification Reaction with Heterocyclic Acid Chlorides of Compounds 34a, 35a, and 37a
- 6-Oxo-23,24-dinor-5α-Cholan-2-en-22-yl-furan-2-carboxylate (34a).
- 6-Oxo-23,24-dinor-5α-Cholan-2-en-22-yl-thiophene-2-carboxylate (35a).
- 6-Oxo-23,24-dinor-5α-Cholan-2-en-22-yl-tetrahydro-2H-pyran-4-carbocarboxylate (37a).
3.2.3. Sharpless Dihydroxylation of Olefins 33–37
- 2α,3α-Dihydroxy-6-oxo-23,24-dinor-5α-cholan-22-yl-1H-pyrrole-2-carboxylate (33).
- 2α,3α-Dihydroxy-6-oxo-23,24-dinor-5α-cholan-22-yl-furan-2-carboxylate (34).
- 2α,3α-Dihydroxy-6-oxo-23,24-dinor-5α-cholan-22-yl-thiophene-2-carboxylate (35).
- 2α,3α-Dihydroxy-6-oxo-23,24-dinor-5α-cholan-22-yl-1H-indole-2-carboxylate (36).
- 2α,3α-Dihydroxy-6-oxo-23,24-dinor-5α-cholan-22-yl-2H-pyran-4-carbocarboxylate (37).
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the Sixth Class of Phytohormones: A Molecular View from the Discovery to Hormonal Interactions in Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive Overview of the Brassinosteroid Biosynthesis Pathways: Substrates, Products, Inhibitors, and Connections. Front. Plant Sci. 2020, 11, 1034. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A. Metabolism of brassinosteroids in plants. Plant Physiol. Biochem. 2007, 45, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Auñón, F.; Puente-Moreno, J.; García-Pastor, M.E.; Serrano, M.; Valero, D. Brassinosteroids: An Innovative Compound Family That Could Affect the Growth, Ripening, Quality, and Postharvest Storage of Fleshy Fruits. Plants 2024, 13, 3082. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, J. Brassinosteroids Regulate Root Growth, Development, and Symbiosis. Mol. Plant 2016, 9, 86–100. [Google Scholar] [CrossRef]
- Wendeborn, S.; Lachia, M.; Jung, P.M.J.; Leipner, J.; Brocklehurst, D.; De Mesmaeker, A.; Gaus, K.; Mondière, R. Biological Activity of Brassinosteroids—Direct Comparison of Known and New Analogs in planta. Helv. Chim. Acta 2017, 100. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ashraf, M.; Bajguz, A.; Ahmad, P. Brassinosteroids Regulate Growth in Plants Under Stressful Environments and Crosstalk with Other Potential Phytohormones. J. Plant Growth Regul. 2018, 37, 1007–1024. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Brassinosteroids Implicated in Growth Stress Responses. In Phytohormones: A Window to Metabolism Signaling Biotechnological Applications; Tran, L.-S.P., Pal, S., Eds.; Springer: New York, NY, USA, 2014; pp. 163–190. [Google Scholar]
- Siddiqui, H.; Hayat, S.; Bajguz, A. Regulation of photosynthesis by brassinosteroids in plants. Acta Physiol. Plant 2018, 40, 59. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Fedina, E.; Yarin, A.; Mukhitova, F.; Blufard, A.; Chechetkin, I. Brassinosteroid-induced changes of lipid composition in leaves of Pisum sativum L. during senescence. Steroids 2017, 117, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Tretyn, A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 2003, 62, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Ohnishi, T.; Shibata, K.; Asahina, M.; Nomura, T.; Fujita, T.; Ishizaki, K.; Kohchi, T. Occurrence of brassinosteroids in non-flowering land plants, liverwort, moss, lycophyte and fern. Phytochemistry 2017, 136, 46–55. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroids in Microalgae: Application for Growth Improvement and Protection Against Abiotic Stresses. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer: Singapore, 2019; pp. 45–58. [Google Scholar]
- Zullo, M.A.T.; Bajguz, A. The Brassinosteroids Family—Structural Diversity of Natural Compounds and Their Precursors. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer: Singapore, 2019; pp. 1–44. [Google Scholar]
- Hayat, S.; Ahmad, A. Brassinosteroids: Bioactivity and Crop Productivity; Springer Science+Business Media: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Temmem, O.; Uguen, D.; De Cian, A.; Gruber, N. Toward a total synthesis of brassinosteroids; structure assessment of the Ireland–Claisen products of geranyl and neryl esters. Tetrahedron Lett. 2002, 43, 3169–3173. [Google Scholar] [CrossRef]
- Temmem, O.; Zoller, T.; Uguen, D. Toward a total synthesis of brassinosteroids; stereoselective generation of the hydrindane ring system. Tetrahedron Lett. 2002, 43, 3181–3184. [Google Scholar] [CrossRef]
- Back, T.G. Stereoselective synthesis of brassinosteroids. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 16, pp. 321–364. [Google Scholar]
- Kovganko, N.V.; Ananich, S.K. Advances in the chemical synthesis of brassinosteroids. Chem. Nat. Compd. 1997, 33, 389–416. [Google Scholar] [CrossRef]
- Khripach, V.A.; Zhabinskii, V.N.; de Groot, A.E. Chapter VI—Basic synthetic methods and formal syntheses. In Brassinosteroids. A New Class of Plant Hormones; Khripach, V.A., Zhabinskii, V.N., de Groot, A.E., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 73–135. [Google Scholar]
- Khripach, V.; Zhabinskii, V.; Ermolovich, Y. Synthetic Aspects of Brassinosteroids. Stud. Nat. Prod. Chem. 2015, 44, 309–352. [Google Scholar]
- Back, T.G.; Janzen, L.; Nakajima, S.K.; Pharis, R.P. Effect of Chain Length and Ring Size of Alkyl and Cycloalkyl Side-Chain Substituents upon the Biological Activity of Brassinosteroids. Preparation of Novel Analogues with Activity Exceeding that of Brassinolide. J. Org. Chem. 2000, 65, 3047–3052. [Google Scholar] [CrossRef]
- Huang, L.F.; Zhou, W.S. Studies on Steroidal Plant-Growth Regulators. Part 33. Novel Method for Construction of the Side-Chain of 23-Arylbrassinosteroids Via Heck Arylation and Asymmetric Dihydroxylation As Key Steps. J. Chem. Soc. Perkin Trans. 1 1994, 3579–3585. [Google Scholar] [CrossRef]
- Kvasnica, M.; Oklestkova, J.; Bazgier, V.; Rárová, L.; Korinkova, P.; Mikulík, J.; Budesinsky, M.; Béres, T.; Berka, K.; Lu, Q.; et al. Design, synthesis and biological activities of new brassinosteroid analogues with a phenyl group in the side chain. Org. Biomol. Chem. 2016, 14, 8691–8701. [Google Scholar] [CrossRef]
- Korinkova, P.; Bazgier, V.; Oklestkova, J.; Rarova, L.; Strnad, M.; Kvasnica, M. Synthesis of novel aryl brassinosteroids through alkene cross-metathesis and preliminary biological study. Steroids 2017, 127, 46–55. [Google Scholar] [CrossRef]
- Aitken, V.; Diaz, K.; Soto, M.; Olea, A.F.; Cuellar, M.A.; Nuñez, M.; Espinoza-Catalán, L. New Brassinosteroid Analogs with 23,24-Dinorcholan Side Chain, and Benzoate Function at C-22: Synthesis, Assessment of Bioactivity on Plant Growth, and Molecular Docking Study. Int. J. Mol. Sci. 2024, 25, 419. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.; Wang, Y.; Russinova, E.; Estévez-Braun, A.; Amesty, A.; Olea, A.F.; Mellado, M.; Díaz, K.; Espinoza Catalán, L. Synthesis, Biological Activity, and Molecular-Docking Studies of New Brassinosteroid Analogs. Int. J. Mol. Sci. 2024, 25, 10158. [Google Scholar] [CrossRef] [PubMed]
- Diachkov, M.V.; Ferrer, K.; Oklestkova, J.; Rarova, L.; Bazgier, V.; Kvasnica, M. Synthesis and Biological Activity of Brassinosteroid Analogues with a Nitrogen-Containing Side Chain. Int. J. Mol. Sci. 2021, 22, 155. [Google Scholar] [CrossRef] [PubMed]
- Rárová, L.; Sedlák, D.; Oklestkova, J.; Steigerová, J.; Liebl, J.; Zahler, S.; Bartůněk, P.; Kolář, Z.; Kohout, L.; Kvasnica, M.; et al. The novel brassinosteroid analog BR4848 inhibits angiogenesis in human endothelial cells and induces apoptosis in human cancer cells in vitro. J. Steroid Biochem. Mol. Biol. 2018, 178, 263–271. [Google Scholar] [CrossRef]
- Munawar, S.; Zahoor, A.F.; Hussain, S.M.; Ahmad, S.; Mansha, A.; Parveen, B.; Ali, K.G.; Irfan, A. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon 2024, 10, e23416. [Google Scholar] [CrossRef]
- Vert, G.; Nemhauser, J.L.; Geldner, N.; Hong, F.; Chory, J. Molecular mechanisms of steroid hormone signaling in plants. Annu. Rev. Cell Dev. Biol. 2005, 21, 177–201. [Google Scholar] [CrossRef]
- Diaz, K.; Espinoza, L.; Carvajal, R.; Conde-Gonzalez, M.; Niebla, V.; Olea, A.F.; Coll, Y. Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs. Int. J. Mol. Sci. 2020, 21, 1832. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya, O.; Núñez, M.; Mellado, M.; Olea, A.F.; Espinoza-Catalán, L. Synthesis of New Brassinosteroid Analogs with Androstane Skeleton and Heterocyclic Acyl Side Chains: Preliminary Molecular Docking Studies. Molecules 2025, 30, 4011. https://doi.org/10.3390/molecules30194011
Araya O, Núñez M, Mellado M, Olea AF, Espinoza-Catalán L. Synthesis of New Brassinosteroid Analogs with Androstane Skeleton and Heterocyclic Acyl Side Chains: Preliminary Molecular Docking Studies. Molecules. 2025; 30(19):4011. https://doi.org/10.3390/molecules30194011
Chicago/Turabian StyleAraya, Omara, María Núñez, Marco Mellado, Andrés F. Olea, and Luis Espinoza-Catalán. 2025. "Synthesis of New Brassinosteroid Analogs with Androstane Skeleton and Heterocyclic Acyl Side Chains: Preliminary Molecular Docking Studies" Molecules 30, no. 19: 4011. https://doi.org/10.3390/molecules30194011
APA StyleAraya, O., Núñez, M., Mellado, M., Olea, A. F., & Espinoza-Catalán, L. (2025). Synthesis of New Brassinosteroid Analogs with Androstane Skeleton and Heterocyclic Acyl Side Chains: Preliminary Molecular Docking Studies. Molecules, 30(19), 4011. https://doi.org/10.3390/molecules30194011






