Impacts of Tween-20, Glycerol, and Trehalose on Hyaluronidase Activity: Insights from Microscale Thermophoresis and Capillary Electrophoresis
Abstract
1. Introduction
2. Results and Discussion
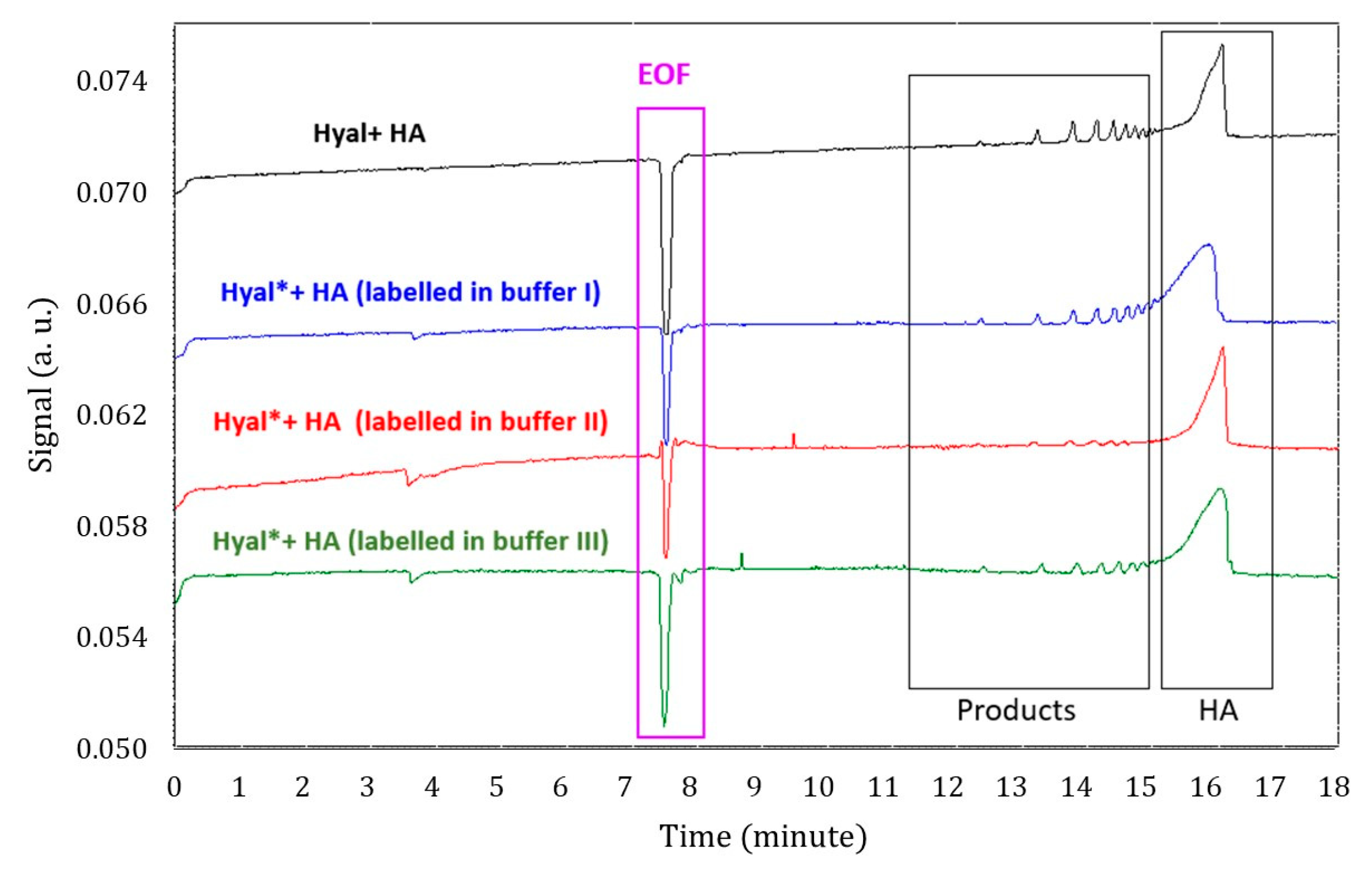
2.1. Optimization of MST Conditions and Catalytic Activity Assay for Hyal*
2.2. Additives’ Effects on MST Signals and Catalytic Activity of Hyaluronidase
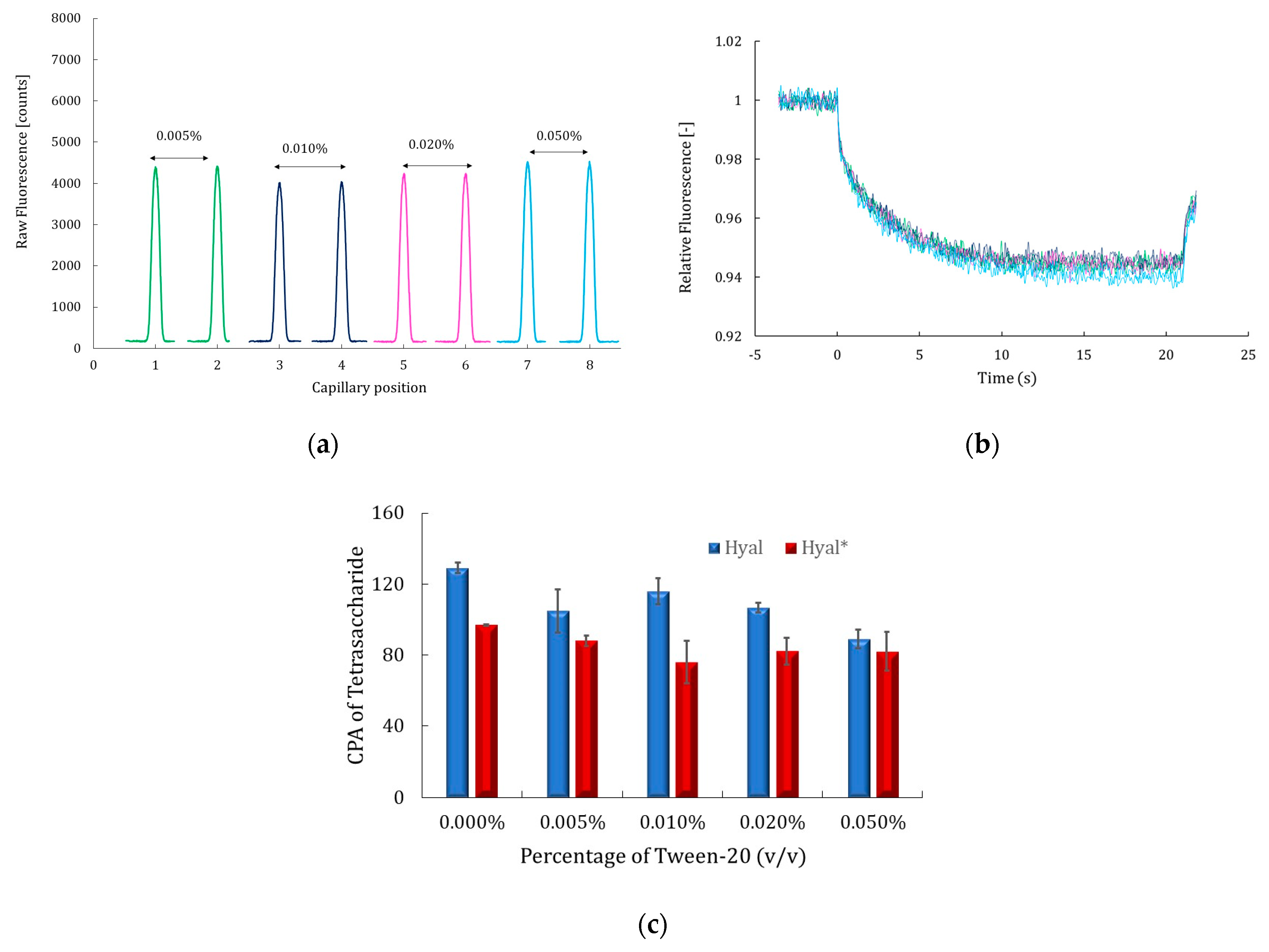
2.2.1. Effect of Tween-20
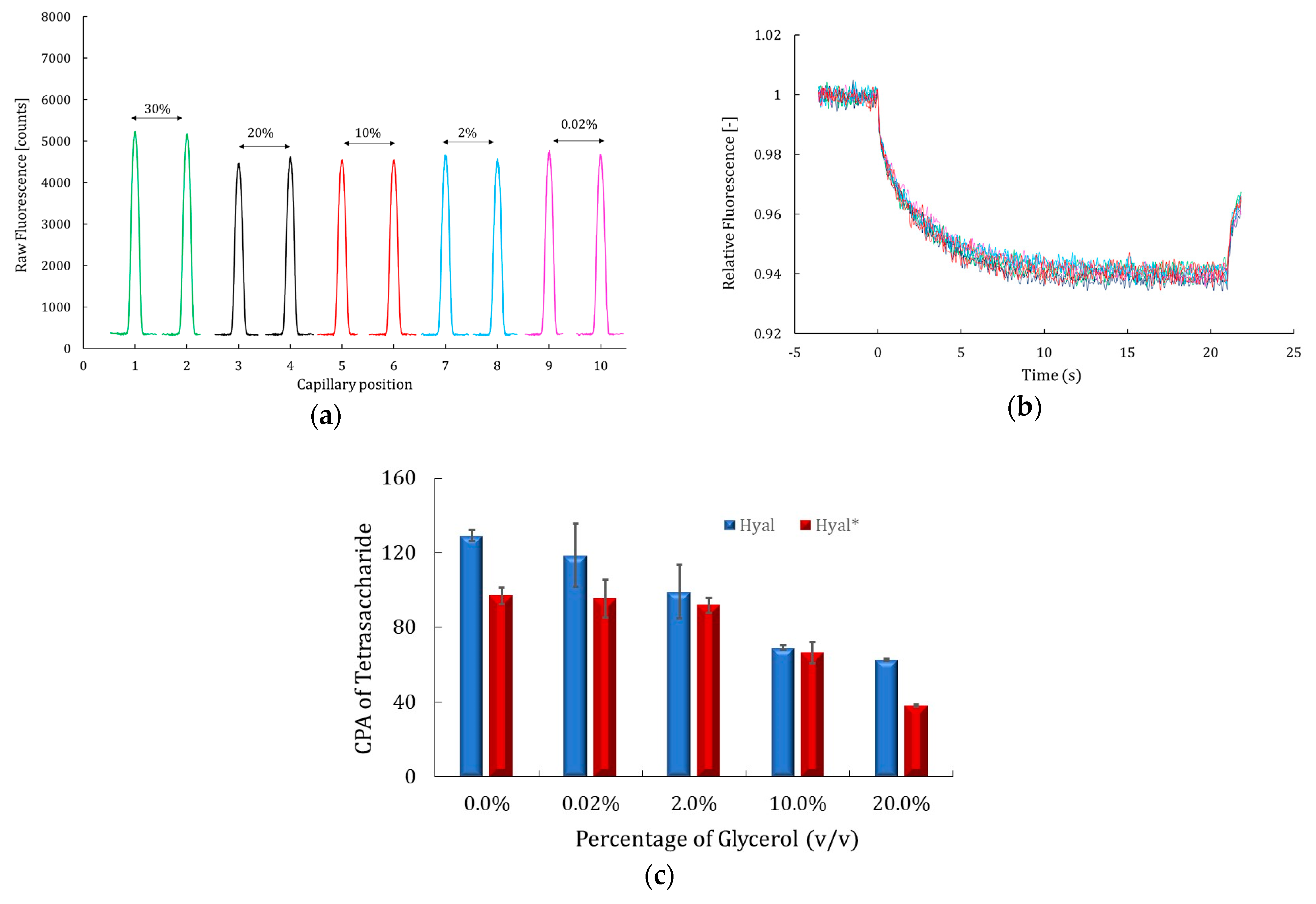
2.2.2. Effect of Glycerol
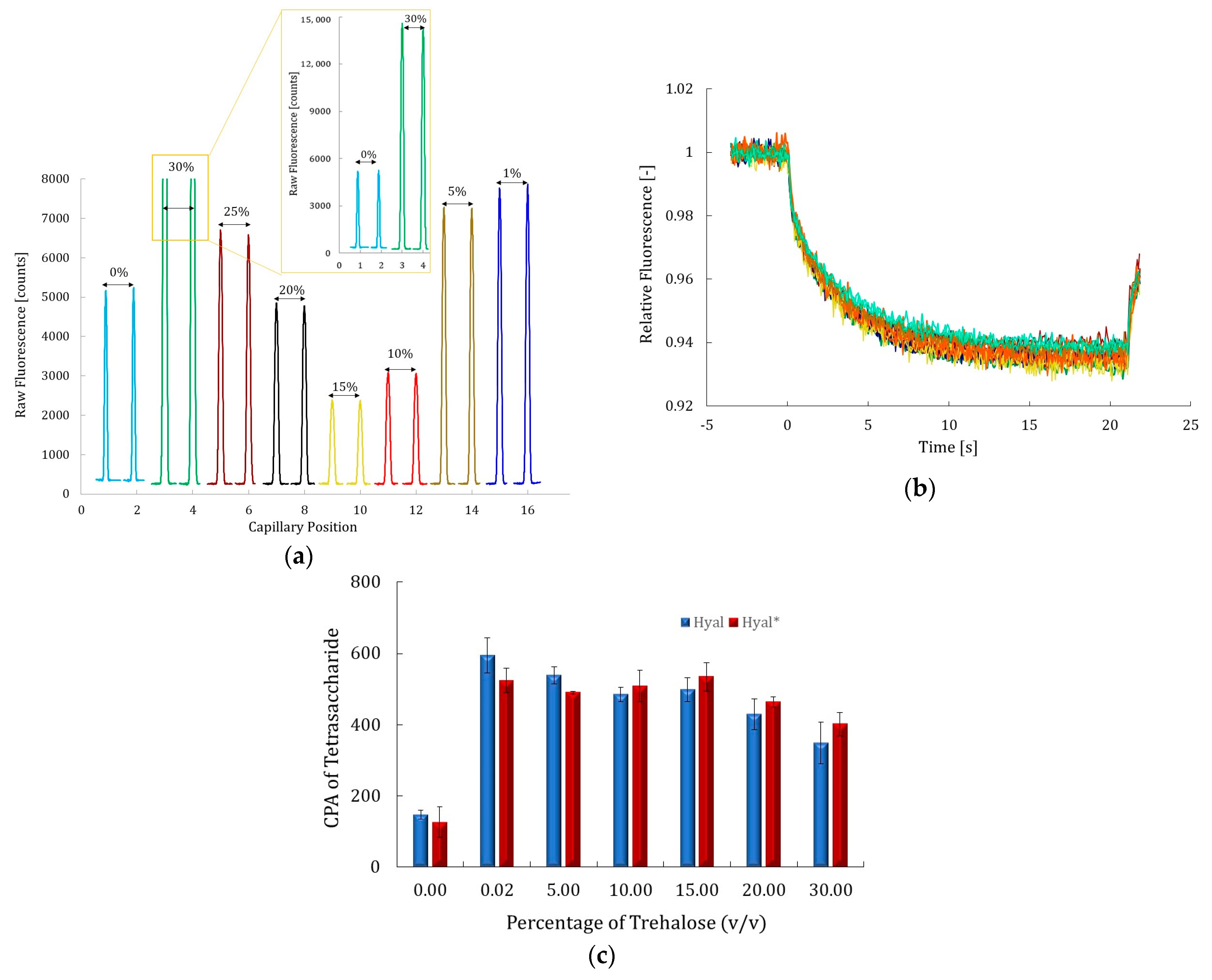
2.2.3. Effect of Trehalose
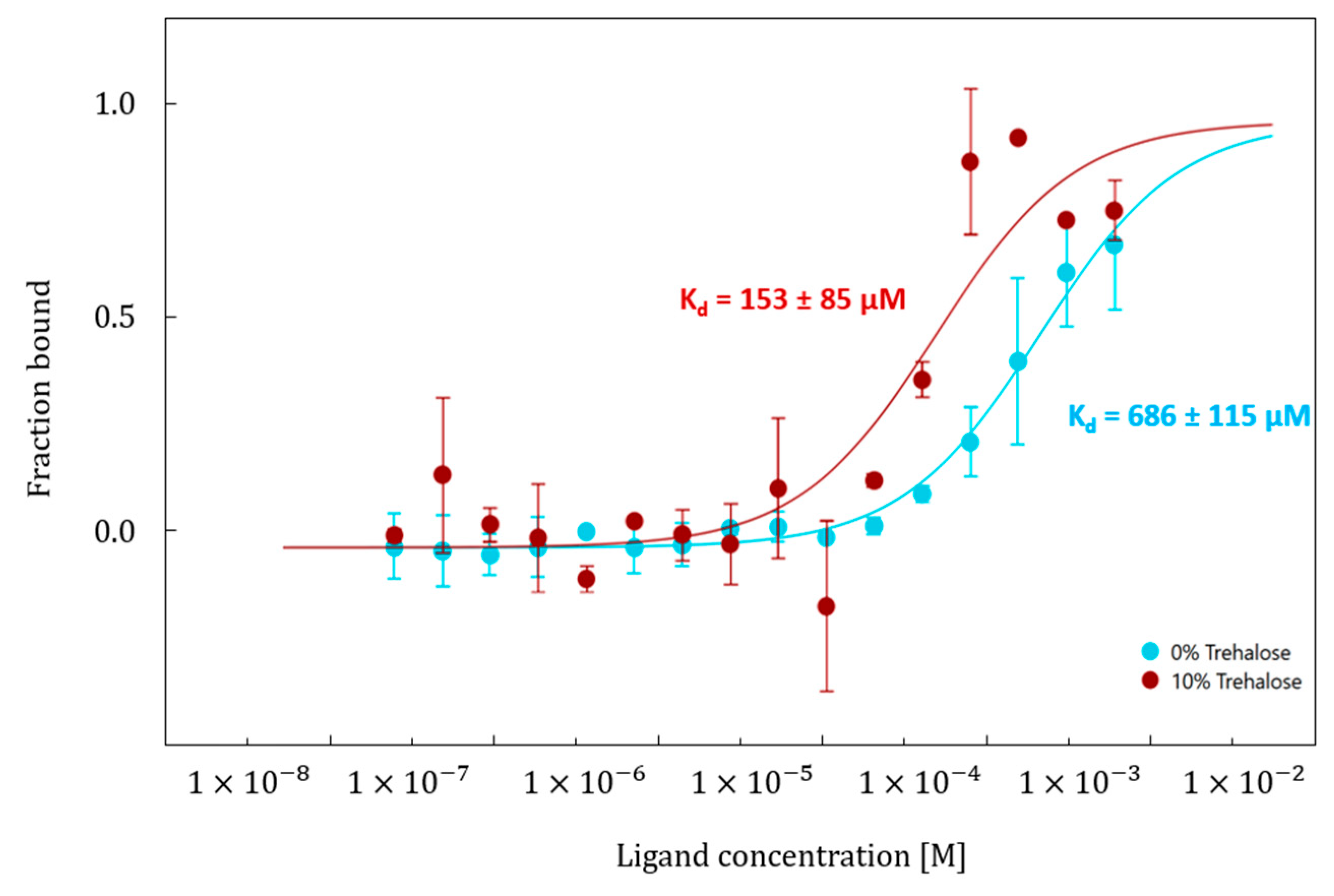
2.3. Binding Affinity Between EGCG and Hyal* in Presence of Trehalose at 10% (v/v)
2.4. Evaluation of Inhibition Effect in the Presence and Absence of 10% Trehalose by CE
3. Materials and Methods
3.1. Chemicals
3.2. Buffer Solutions
3.2.1. Labeling Buffers
3.2.2. MST Assay Buffer
3.2.3. Buffers for CE Analysis
3.3. Reagent Stock Solutions
3.4. Additive Stock Solutions
3.4.1. Tween-20
3.4.2. Glycerol
3.4.3. Trehalose
4. Instrumentation and Operating Conditions
4.1. Capillary Electrophoresis Analysis Conditions
4.2. Microscale Thermophoresis for Hyal* Binding Assays
4.2.1. Hyaluronidase Labeling
4.2.2. Quantification of Labeled Hyaluronidase by UV Spectrophotometry
4.2.3. Binding Affinity Between EGCG and Hyal*
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, E.L.; Poddar, S.; Iftekhar, S.; Suh, K.; Woolfork, A.G.; Ovbude, S.; Pekarek, A.; Walters, M.; Lott, S.; Hage, D.S. Affinity Chromatography: A Review of Trends and Developments over the Past 50 Years. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1157, 122332. [Google Scholar] [CrossRef] [PubMed]
- Asmari, M.; Michalcová, L.; Ibrahim, A.E.; Glatz, Z.; Wätzig, H.; El Deeb, S. Studying Molecular Interactions via Capillary Electrophoresis and Microscale Thermophoresis: A Review. Electrophoresis 2023, 44, 1114–1142. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.A.; Koropatkin, N.M. Isothermal Titration Calorimetry for Quantification of Protein-Carbohydrate Interactions. Methods Mol. Biol. 2023, 2657, 129–140. [Google Scholar] [CrossRef]
- Becker, W.; Bhattiprolu, K.C.; Gubensäk, N.; Zangger, K. Investigating Protein–Ligand Interactions by Solution Nuclear Magnetic Resonance Spectroscopy. ChemPhysChem 2018, 19, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Burris, D.M.; Gillespie, S.W.; Campbell, E.J.; Ice, S.N.; Yadav, V.; Picking, W.D.; Lorson, C.L.; Singh, K. Applications of Surface Plasmon Resonance (SPR) to the Study of Diverse Protein—Ligand Interactions. Curr. Protoc. 2024, 4, e1030. [Google Scholar] [CrossRef]
- Müller-Esparza, H.; Osorio-Valeriano, M.; Steube, N.; Thanbichler, M.; Randau, L. Bio-Layer Interferometry Analysis of the Target Binding Activity of CRISPR-Cas Effector Complexes. Front. Mol. Biosci. 2020, 7, 98. [Google Scholar] [CrossRef]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular Interaction Studies Using Microscale Thermophoresis. ASSAY Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef]
- Bartoschik, T.; Galinec, S.; Kleusch, C.; Walkiewicz, K.; Breitsprecher, D.; Weigert, S.; Muller, Y.A.; You, C.; Piehler, J.; Vercruysse, T.; et al. Near-Native, Site-Specific and Purification-Free Protein Labeling for Quantitative Protein Interaction Analysis by MicroScale Thermophoresis. Sci. Rep. 2018, 8, 4977. [Google Scholar] [CrossRef]
- Entzian, C.; Schubert, T. Studying Small Molecule–Aptamer Interactions Using MicroScale Thermophoresis (MST). Methods 2016, 97, 27–34. [Google Scholar] [CrossRef]
- Nasreddine, R.; Nehmé, R. Microscale Thermophoresis for Studying Protein-Small Molecule Affinity: Application to Hyaluronidase. Microchem. J. 2021, 170, 106763. [Google Scholar] [CrossRef]
- Mueller, A.M.; Breitsprecher, D.; Duhr, S.; Baaske, P.; Schubert, T.; Längst, G. MicroScale Thermophoresis: A Rapid and Precise Method to Quantify Protein–Nucleic Acid Interactions in Solution. In Functional Genomics; Humana Press: New York, NY, USA, 2017; pp. 151–164. ISBN 978-1-4939-7231-9. [Google Scholar]
- Magnez, R.; Bailly, C.; Thuru, X. Microscale Thermophoresis as a Tool to Study Protein Interactions and Their Implication in Human Diseases. Int. J. Mol. Sci. 2022, 23, 7672. [Google Scholar] [CrossRef]
- Rainsford, P.; Rylandsholm, F.G.; Jakubec, M.; Silk, M.; Juskewitz, E.; Ericson, J.U.; Svendsen, J.-S.; Engh, R.A.; Isaksson, J. Label-Free Measurement of Antimicrobial Peptide Interactions with Lipid Vesicles and Nanodiscs Using Microscale Thermophoresis. Sci. Rep. 2023, 13, 12619. [Google Scholar] [CrossRef] [PubMed]
- Chartier, S.; Claude, B.; Nasreddine, R.; Launay, A.; Martins, M.; Elodie, V.-R.; Vallée, B.; Sebban, M.; Coadou, G.; Nehmé, R. Using CE to Confirm the Activity of Fluorescent miRFP670-LIMK1 Protein Produced for MST Assays Directly in Cell Lysate. Anal. Chim. Acta 2025, 1360, 344145. [Google Scholar] [CrossRef]
- Chou, D.K.; Krishnamurthy, R.; Randolph, T.W.; Carpenter, J.F.; Manning, M.C. Effects of Tween 20 and Tween 80 on the Stability of Albutropin during Agitation. J. Pharm. Sci. 2005, 94, 1368–1381. [Google Scholar] [CrossRef]
- Al Hamoui Dit Banni, G.; Nasreddine, R.; Fayad, S.; Colas, C.; Marchal, A.; Nehmé, R. Investigation of Lipase-Ligand Interactions in Porcine Pancreatic Extracts by Microscale Thermophoresis. Anal. Bioanal. Chem. 2021, 413, 3667–3681. [Google Scholar] [CrossRef]
- Shen, L.; Guo, A.; Zhu, X. Tween Surfactants: Adsorption, Self-Organization, and Protein Resistance. Surf. Sci. 2011, 605, 494–499. [Google Scholar] [CrossRef]
- Liang, H.; Fan, X.; Gao, X.; Li, A.; Zhou, C. Effects of Glycerol on the Freezing Behaviors and Physicochemical Properties of Pork Patties under Freeze-Thaw Cycles. Food Meas. 2024, 18, 7172–7184. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of Trehalose on Protein Structure. Protein Sci. Publ. Protein Soc. 2009, 18, 24. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Uribe, S. Trehalose-Enzyme Interactions Result in Structure Stabilization and Activity Inhibition. The Role of Viscosity. Mol. Cell. Biochem. 2004, 256–257, 319–327. [Google Scholar] [CrossRef]
- Murray, A.; Kilbride, P.; Gibson, M.I. Trehalose in Cryopreservation. Applications, Mechanisms and Intracellular Delivery Opportunities. RSC Med. Chem. 2024, 15, 2980–2995. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Mittag, J.J.; Herling, T.W.; Genst, E.D.; Dobson, C.M.; Knowles, T.P.J.; Braun, D.; Buell, A.K. Quantitative Thermophoretic Study of Disease-Related Protein Aggregates. Sci. Rep. 2016, 6, 22829. [Google Scholar] [CrossRef]
- El Deeb, S.; Al-Harrasi, A.; Khan, A.; Al-Broumi, M.; Al-Thani, G.; Alomairi, M.; Elumalai, P.; Sayed, R.A.; Ibrahim, A.E. Microscale Thermophoresis as a Powerful Growing Analytical Technique for the Investigation of Biomolecular Interaction and the Determination of Binding Parameters. Methods Appl. Fluoresc. 2022, 10, 042001. [Google Scholar] [CrossRef]
- Wang, W.-F.; Yang, J.-L. Advances in Screening Enzyme Inhibitors by Capillary Electrophoresis. Electrophoresis 2019, 40, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Gattu, S.; Crihfield, C.L.; Lu, G.; Bwanali, L.; Veltri, L.M.; Holland, L.A. Advances in Enzyme Substrate Analysis with Capillary Electrophoresis. Methods 2018, 146, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Fayad, S.; Nehmé, R.; Langmajerová, M.; Ayela, B.; Colas, C.; Maunit, B.; Jacquinet, J.-C.; Vibert, A.; Lopin-Bon, C.; Zdeněk, G.; et al. Hyaluronidase Reaction Kinetics Evaluated by Capillary Electrophoresis with UV and High-Resolution Mass Spectrometry (HRMS) Detection. Anal. Chim. Acta 2017, 951, 140–150. [Google Scholar] [CrossRef]
- Cao-Ngoc, P.; Leclercq, L.; Rossi, J.-C.; Hertzog, J.; Tixier, A.-S.; Chemat, F.; Nasreddine, R.; Al Hamoui Dit Banni, G.; Nehmé, R.; Schmitt-Kopplin, P.; et al. Water-Based Extraction of Bioactive Principles from Blackcurrant Leaves and Chrysanthellum Americanum: A Comparative Study. Foods 2020, 9, 1478. [Google Scholar] [CrossRef]
- Nasreddine, R.; Orlic, L.; Al Hamoui Dit Banni, G.; Fayad, S.; Marchal, A.; Piazza, F.; Lopin-Bon, C.; Hamacek, J.; Nehmé, R. Polyethylene Glycol Crowding Effect on Hyaluronidase Activity Monitored by Capillary Electrophoresis. Anal. Bioanal. Chem. 2020, 412, 4195–4207. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase Inhibitors: A Biological and Therapeutic Perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Mio, K.; Stern, R. Inhibitors of the Hyaluronidases. Matrix Biol. 2002, 21, 31–37. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An Overview of Its Properties, Applications, and Side Effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef]
- Fayad, S.; Ayela, B.; Chat, C.; Morin, P.; Lopin-Bon, C.; Nehmé, R. Effect of Modified Di- and Trisaccharides on Hyaluronidase Activity Assessed by Capillary Electrophoresis-Based Enzymatic Assay. Carbohydr. Res. 2019, 475, 56–64. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and Evaluation of Dermo-Cosmetic Activities of the Invasive Plant Species Polygonum Cuspidatum. Plants 2023, 12, 83. [Google Scholar] [CrossRef]
- Hermanson, G.T. Homobifunctional Crosslinkers. In Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 275–298. ISBN 978-0-12-382239-0. [Google Scholar]
- Streit, M.; Budiarta, M.; Jungblut, M.; Beliu, G. Fluorescent Labeling Strategies for Molecular Bioimaging. Biophys. Rep. 2025, 5, 100200. [Google Scholar] [CrossRef]
- Kharrat, O.; Yamaryo-Botté, Y.; Nasreddine, R.; Voisin, S.; Aumer, T.; Cammue, B.P.A.; Madinier, J.-B.; Knobloch, T.; Thevissen, K.; Nehmé, R.; et al. The Antimicrobial Activity of ETD151 Defensin Is Dictated by the Presence of Glycosphingolipids in the Targeted Organisms. Proc. Natl. Acad. Sci. USA 2025, 122, e2415524122. [Google Scholar] [CrossRef]
- Kharrat, O.; Paquet, F.; Nasreddine, R.; Madinier, J.-B.; Nehmé, R.; Aucagne, V.; Bulet, P.; Warschawski, D.; Landon, C. Molecular Recognition of Fungal Methylated Glucosylceramides by ETD151 Defensin. J. Biol. Chem. 2025, 301, 110587. [Google Scholar] [CrossRef]
- Katoch, R. Buffers and Their Preparation. In Analytical Techniques in Biochemistry and Molecular Biology; Katoch, R., Ed.; Springer: New York, NY, USA, 2011; pp. 29–37. ISBN 978-1-4419-9785-2. [Google Scholar]
- Salameh, M.A.; Wiegel, J. Effects of Detergents on Activity, Thermostability and Aggregation of Two Alkalithermophilic Lipases from Thermosyntropha Lipolytica. Open Biochem. J. 2010, 4, 22–28. [Google Scholar] [CrossRef]
- Hirai, M.; Ajito, S.; Sugiyama, M.; Iwase, H.; Takata, S.; Shimizu, N.; Igarashi, N.; Martel, A.; Porcar, L. Direct Evidence for the Effect of Glycerol on Protein Hydration and Thermal Structural Transition. Biophys. J. 2018, 115, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Umezurike, G.M. The Effect of Glycerol on the Activity of β-Glucosidase from Botryodiplodia Theobromae Pat. Biochem. J. 1988, 254, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Mejri, M.; Mathlouthi, M. Effect of Small Carbohydrates on the Catalytic Activity of a Protease and Two Glycohydrolases. Carbohydr. Polym. 2001, 45, 161–167. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, N.; Yu, X.; Wang, Y.; Sun, Q.; Dong, X. Cryoprotective Effect of Trehalose on Myofibrillar Protein of Snakehead Fish (Channa argus) during Freeze–Thaw Cycles. Food Chem. 2025, 474, 143213. [Google Scholar] [CrossRef]
- Sarin, D.; Chakraborty, D.; Sreenivasan, S.; Mishra, A.; Rathore, A.S. Higher Concentration of Trehalose Dihydrate Stabilizes Recombinant IgG1 under Forced Stress Conditions. J. Pharm. Sci. 2025, 114, 1398–1409. [Google Scholar] [CrossRef]
- Sola-Penna, M.; Meyer-Fernandes, J.R. Stabilization against Thermal Inactivation Promoted by Sugars on Enzyme Structure and Function: Why Is Trehalose More Effective than Other Sugars? Arch. Biochem. Biophys. 1998, 360, 10–14. [Google Scholar] [CrossRef] [PubMed]
- D’Alfonso, L.; Collini, M.; Cannone, F.; Chirico, G.; Campanini, B.; Cottone, G.; Cordone, L. GFP-Mut2 Proteins in Trehalose-Water Matrixes: Spatially Heterogeneous Protein-Water-Sugar Structures. Biophys. J. 2007, 93, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zaroog, M.S.; Abdul Kadir, H.; Tayyab, S. Stabilizing Effect of Various Polyols on the Native and the Denatured States of Glucoamylase. Sci. World J. 2013, 2013, 570859. [Google Scholar] [CrossRef] [PubMed]
- Stefan, A.; Hochkoeppler, A. Not Only an Inhibitor: Trehalose Enhances the Catalytic Action Exerted on Oxaloacetate by Rabbit Lactate Dehydrogenase. Protein Sci. 2025, 34, e70304. [Google Scholar] [CrossRef]
- Chmielewski, R.; Lebiedowska, A.; Barańska-Rybak, W. Evaluation of the Anti-Glycation Protective Effect of an Injectable Product Based on a Combination of Two Different Ranges of Molecular Weights of Hyaluronic Acid and Trehalose on Human Skin Explants. Int. J. Mol. Sci. 2025, 26, 3217. [Google Scholar] [CrossRef]
- Aït-Mohand, K.; Lopin-Bon, C.; Jacquinet, J.-C. Synthesis of Variously Sulfated Biotinylated Oligosaccharides from the Linkage Region of Proteoglycans. Carbohydr. Res. 2012, 353, 33–48. [Google Scholar] [CrossRef]
- ATTO 647N. Available online: https://www.atto-tec.com/ATTO-647N.html?language=en (accessed on 25 July 2025).
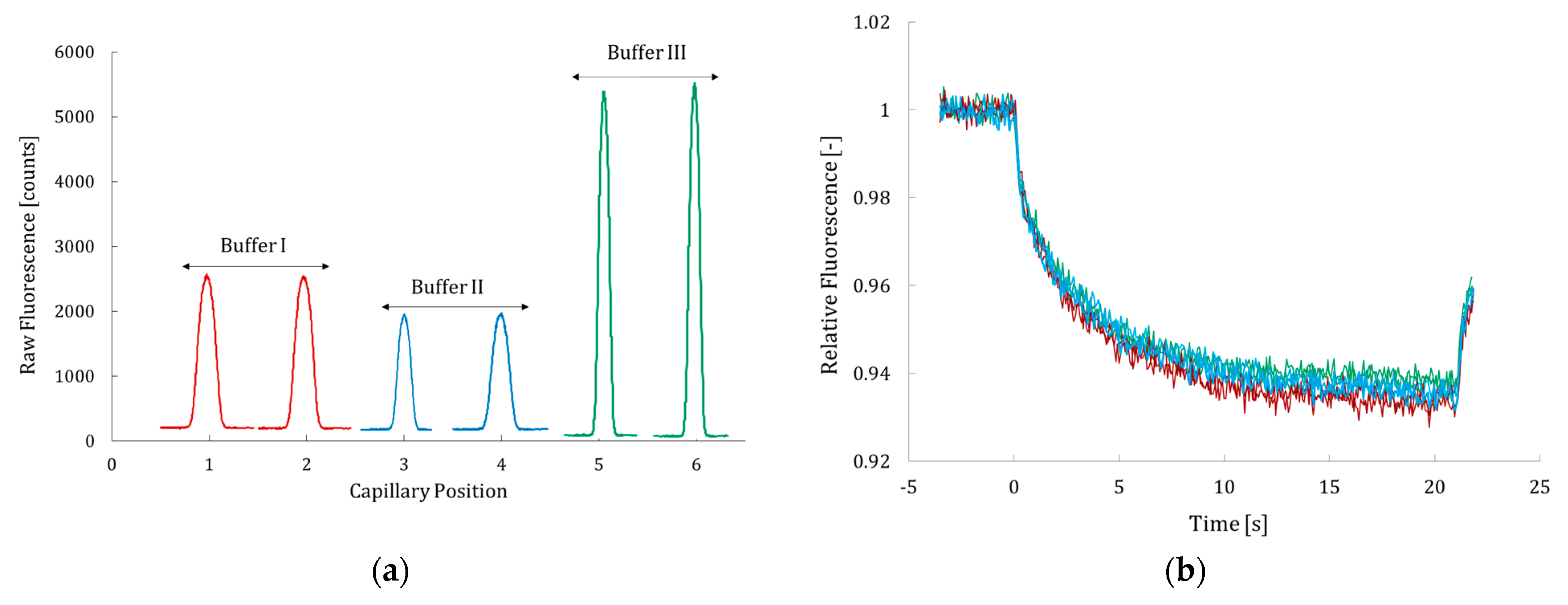
| Concentration of Hyal*—Equation (2) | DOL— Equation (3) | |
|---|---|---|
| Phosphate–ammonium (pH 6.6) (buffer I) | 6.1 µM (~0.3 g/L) | 1.1 |
| Carbonate buffer (pH 8.2) (buffer II) | 8.1 µM (~0.4 g/L) | 1.2 |
| PBS buffer (pH 7.5) (buffer III) | 8.5 µM (~0.5 g/L) | 1.0 |
| Compound | Inhibition at 1 mg/mL—Equation (1) | |
|---|---|---|
| 0% Trehalose | +10% (v/v) Trehalose | |
Apigenin-7-glucoside (432 g/mol)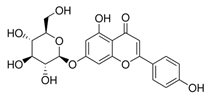 | 25 ± 5% | 55 ± 7% (↑ × 2) |
CS-4 (1406 g/mol) | 32 ± 3% | 91 ± 5% (↑ × 3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasreddine, R.; Cecić Vidoš, J.; Launay, A.; Nehmé, R. Impacts of Tween-20, Glycerol, and Trehalose on Hyaluronidase Activity: Insights from Microscale Thermophoresis and Capillary Electrophoresis. Molecules 2025, 30, 4008. https://doi.org/10.3390/molecules30194008
Nasreddine R, Cecić Vidoš J, Launay A, Nehmé R. Impacts of Tween-20, Glycerol, and Trehalose on Hyaluronidase Activity: Insights from Microscale Thermophoresis and Capillary Electrophoresis. Molecules. 2025; 30(19):4008. https://doi.org/10.3390/molecules30194008
Chicago/Turabian StyleNasreddine, Rouba, Josipa Cecić Vidoš, Alexandra Launay, and Reine Nehmé. 2025. "Impacts of Tween-20, Glycerol, and Trehalose on Hyaluronidase Activity: Insights from Microscale Thermophoresis and Capillary Electrophoresis" Molecules 30, no. 19: 4008. https://doi.org/10.3390/molecules30194008
APA StyleNasreddine, R., Cecić Vidoš, J., Launay, A., & Nehmé, R. (2025). Impacts of Tween-20, Glycerol, and Trehalose on Hyaluronidase Activity: Insights from Microscale Thermophoresis and Capillary Electrophoresis. Molecules, 30(19), 4008. https://doi.org/10.3390/molecules30194008






