Three-Dimensional Printable Photocurable Elastomer Composed of Hydroxyethyl Acrylate and Hydroxy Fatty Acid Derived from Waste Cooking Oil: An Innovative Strategy for Sustainable, Highly Flexible Resin Development
Abstract
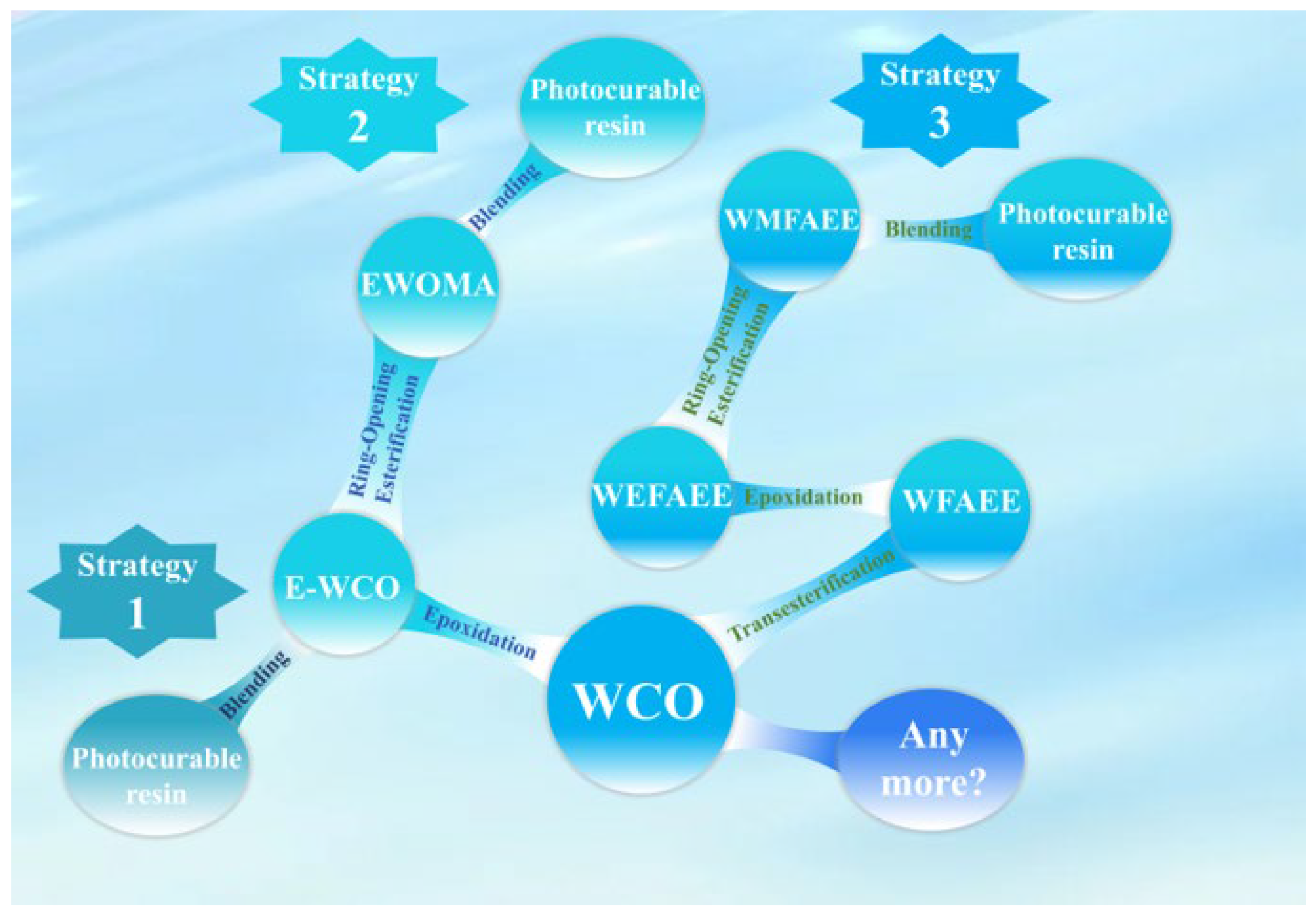
1. Introduction
2. Results and Discussion
2.1. Spectroscopic Analysis of WHFA Based on WCO
2.2. Spectroscopic Analysis of WHFA/HEA Photocurable Elastomer
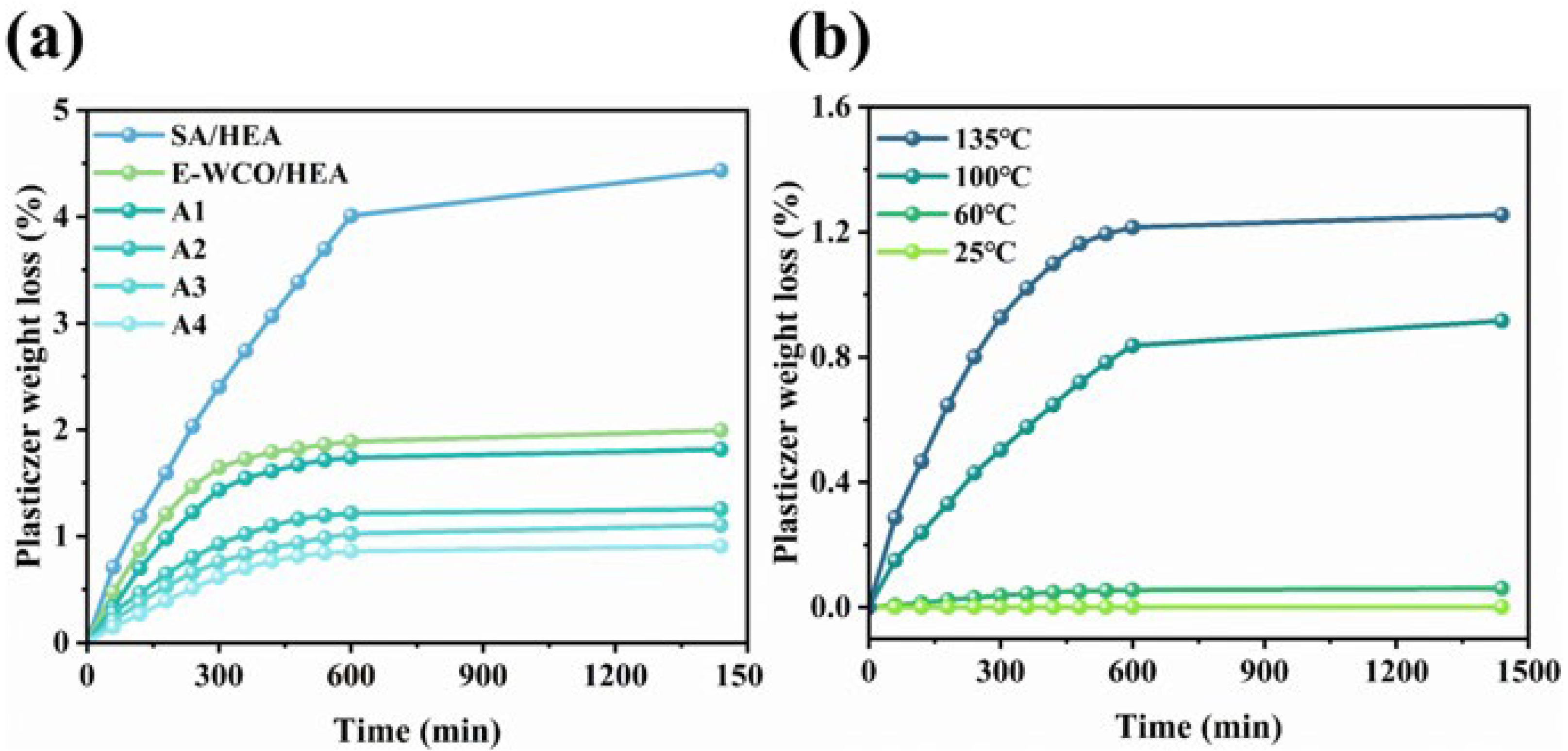
2.3. Migration Rate of WHFA Based on WCO
2.4. Thermal Analysis
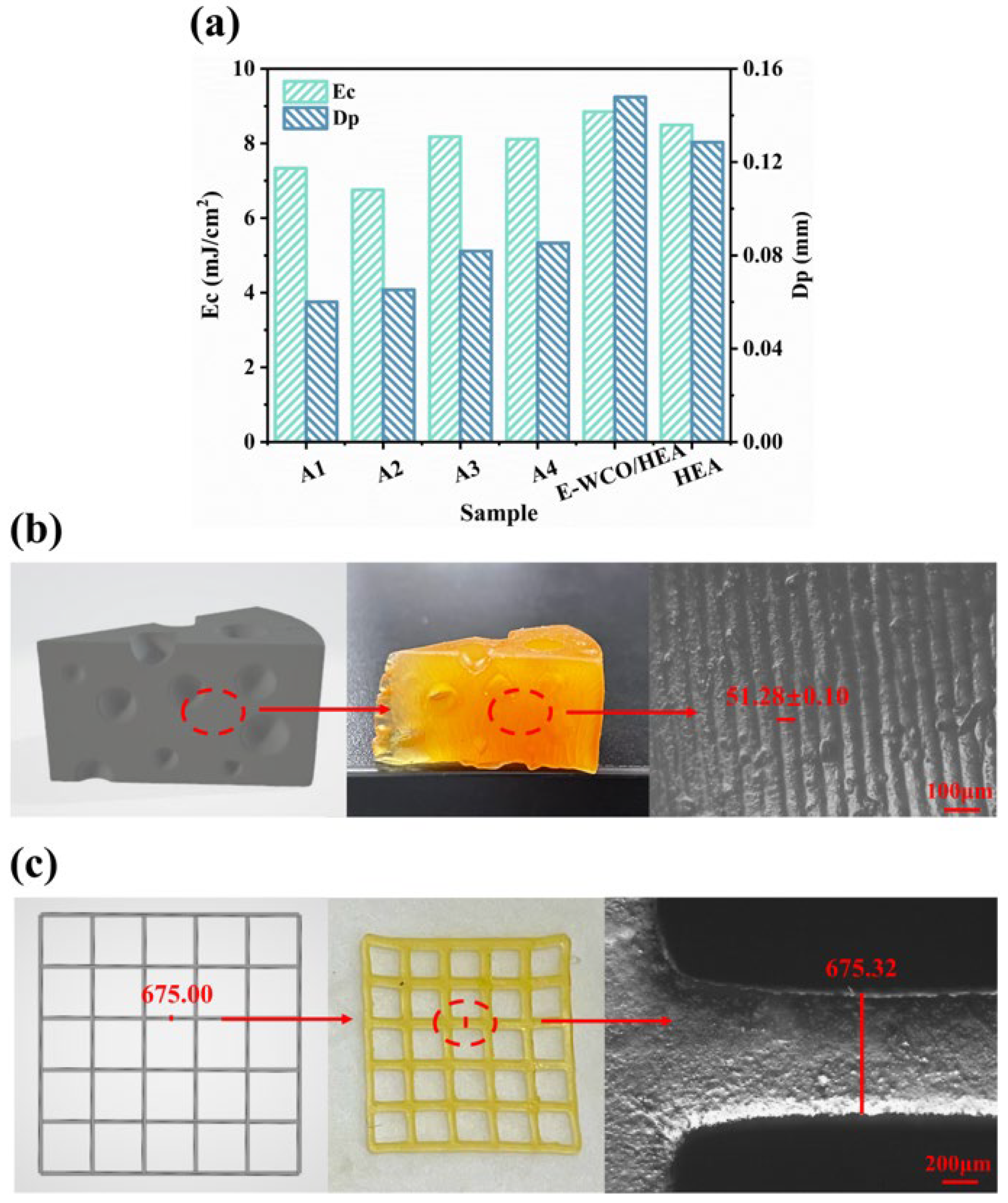
2.5. 3D Printing Behavior
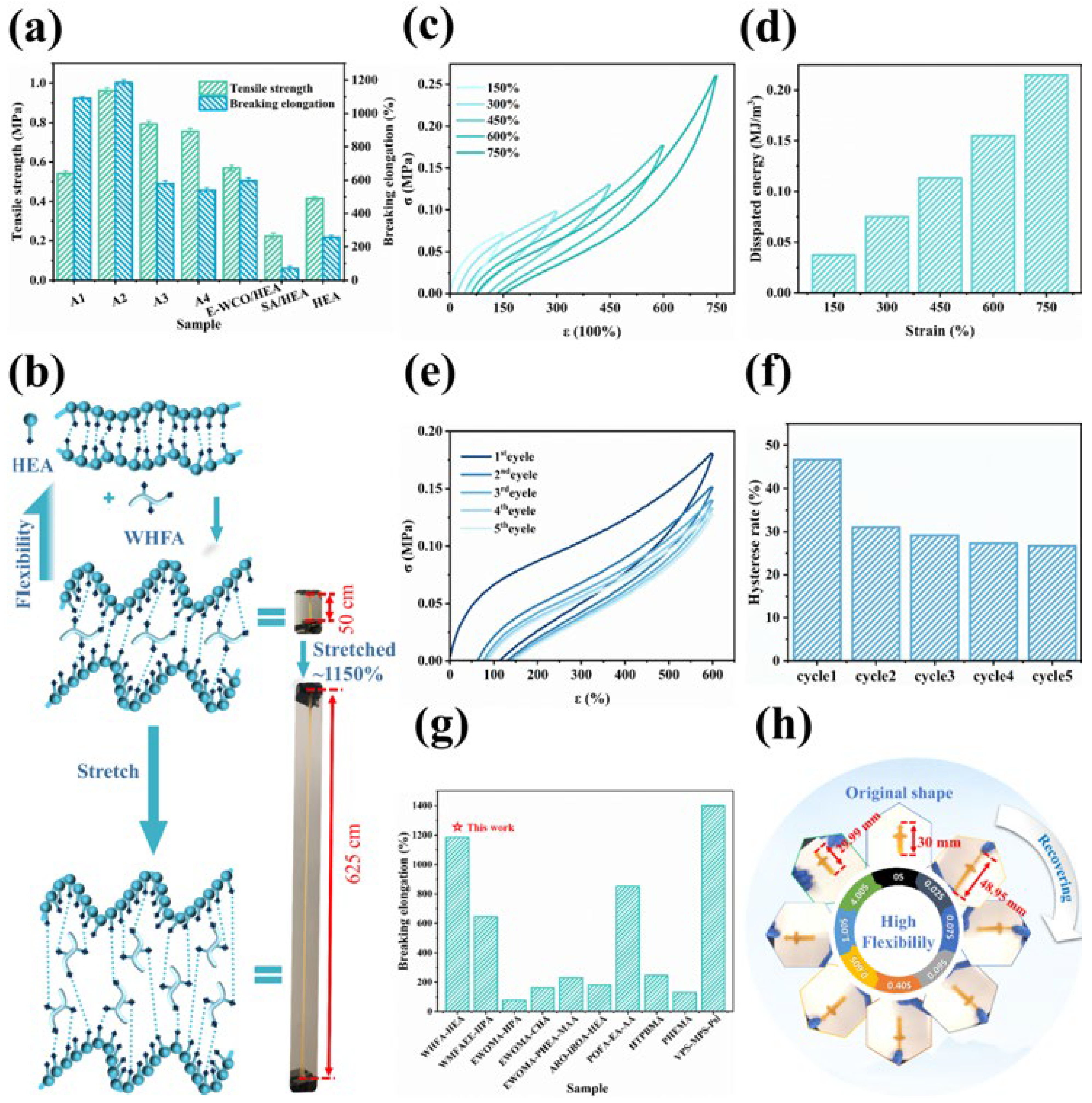
2.6. Mechanical Properties
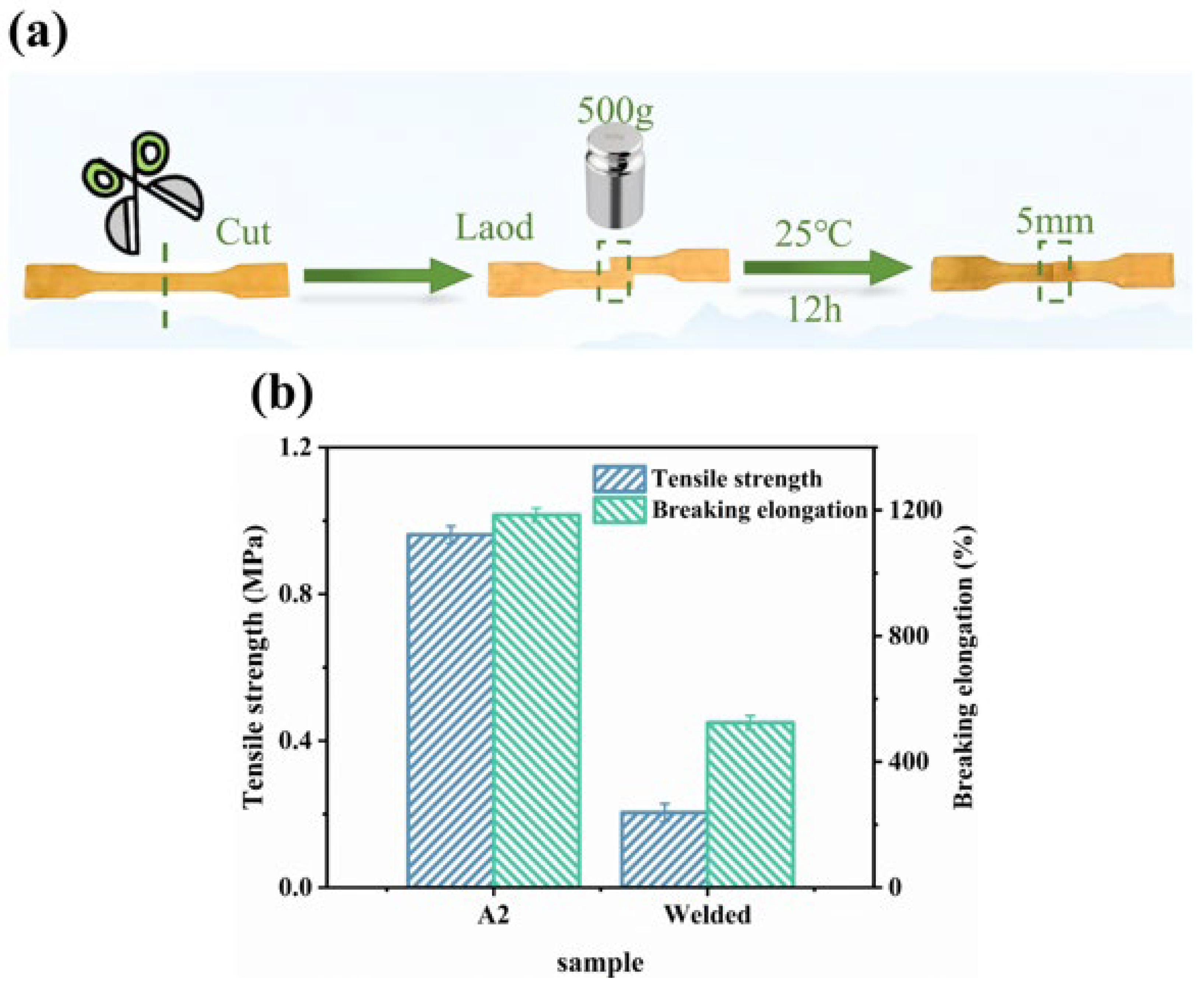
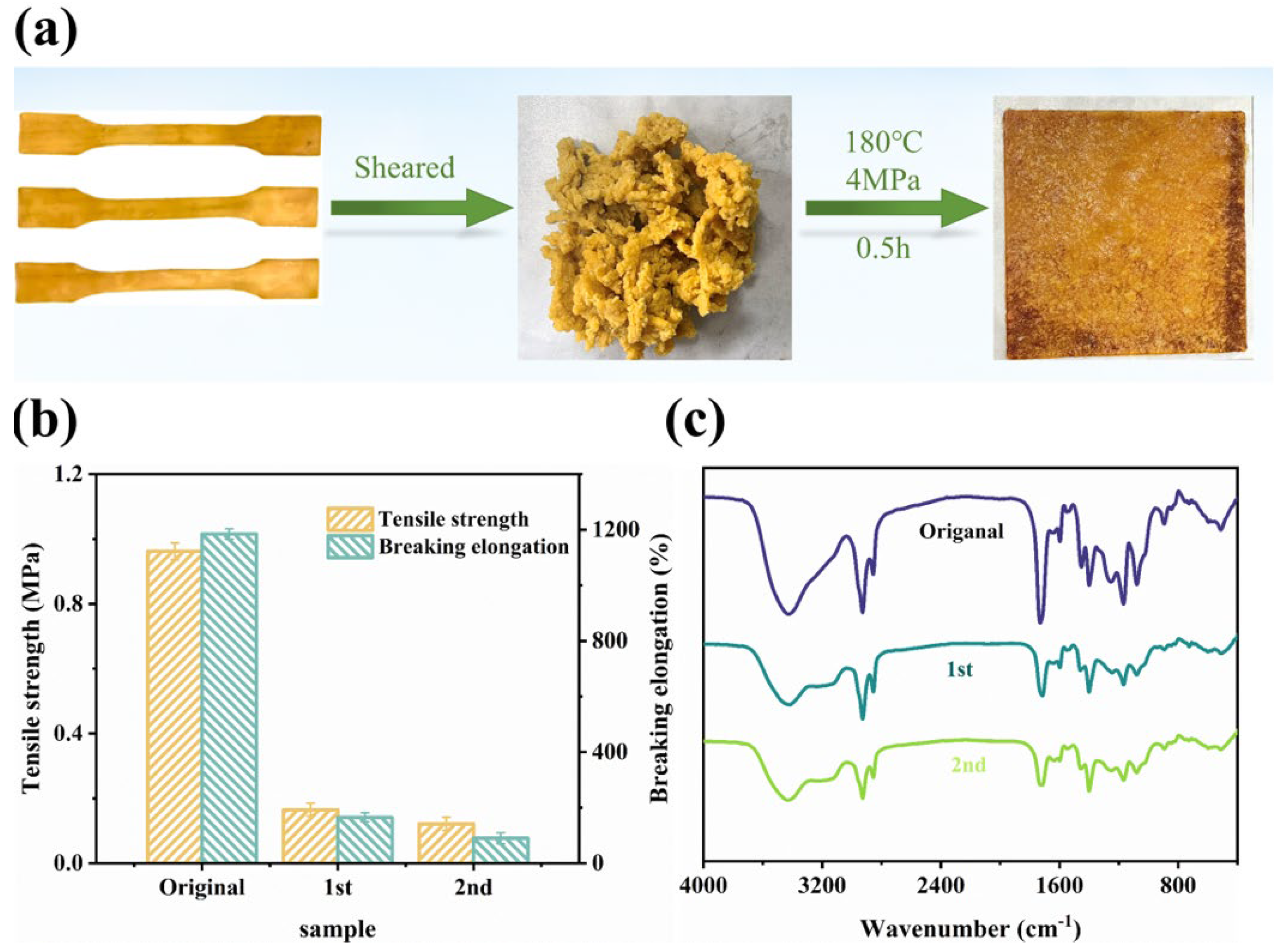
2.7. Welding and Physical Reprocessing Properties
2.8. Pressure Sensitive Adhesive Properties
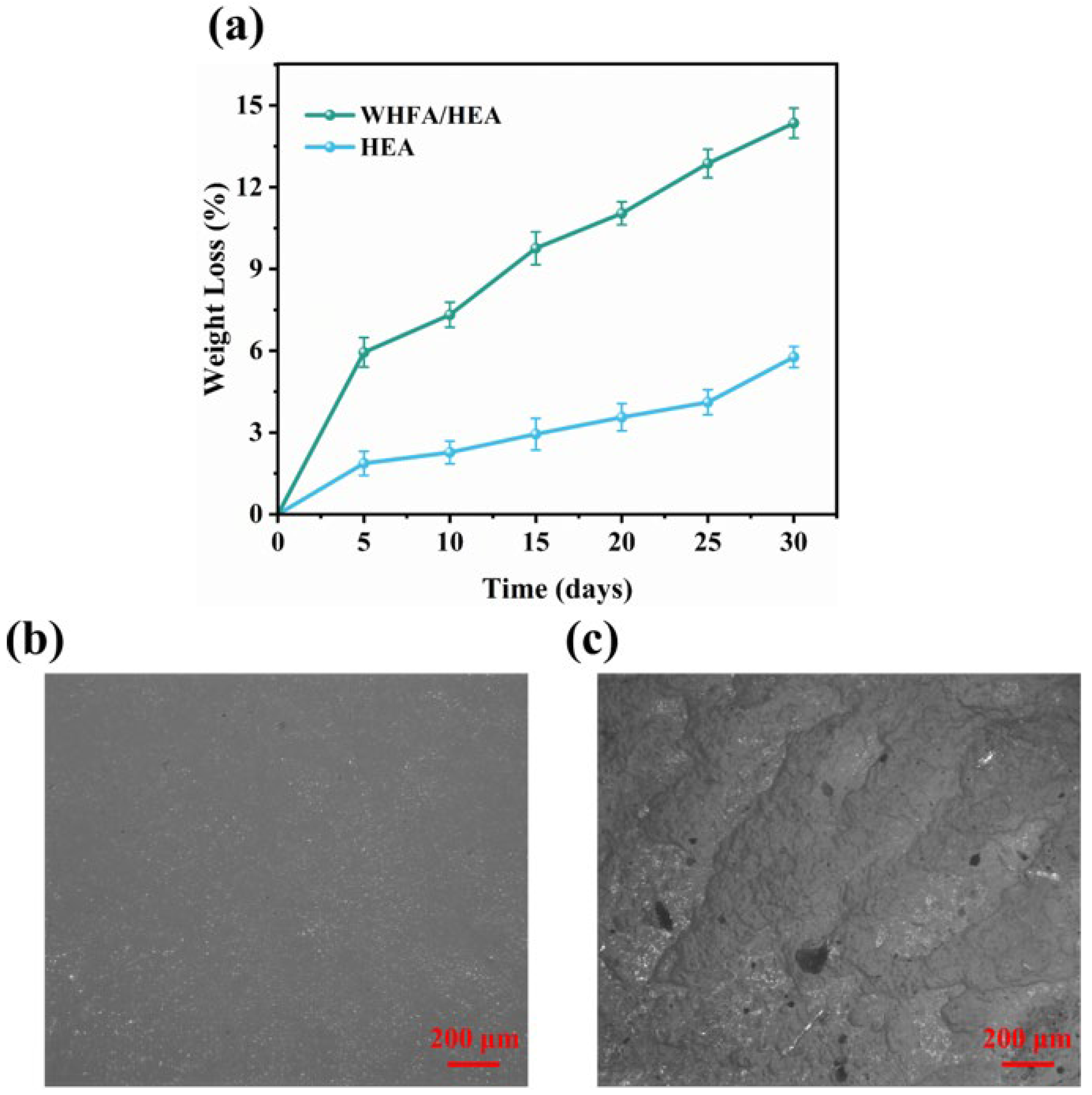
2.9. Biodegradability
3. Materials and Methods
3.1. Materials
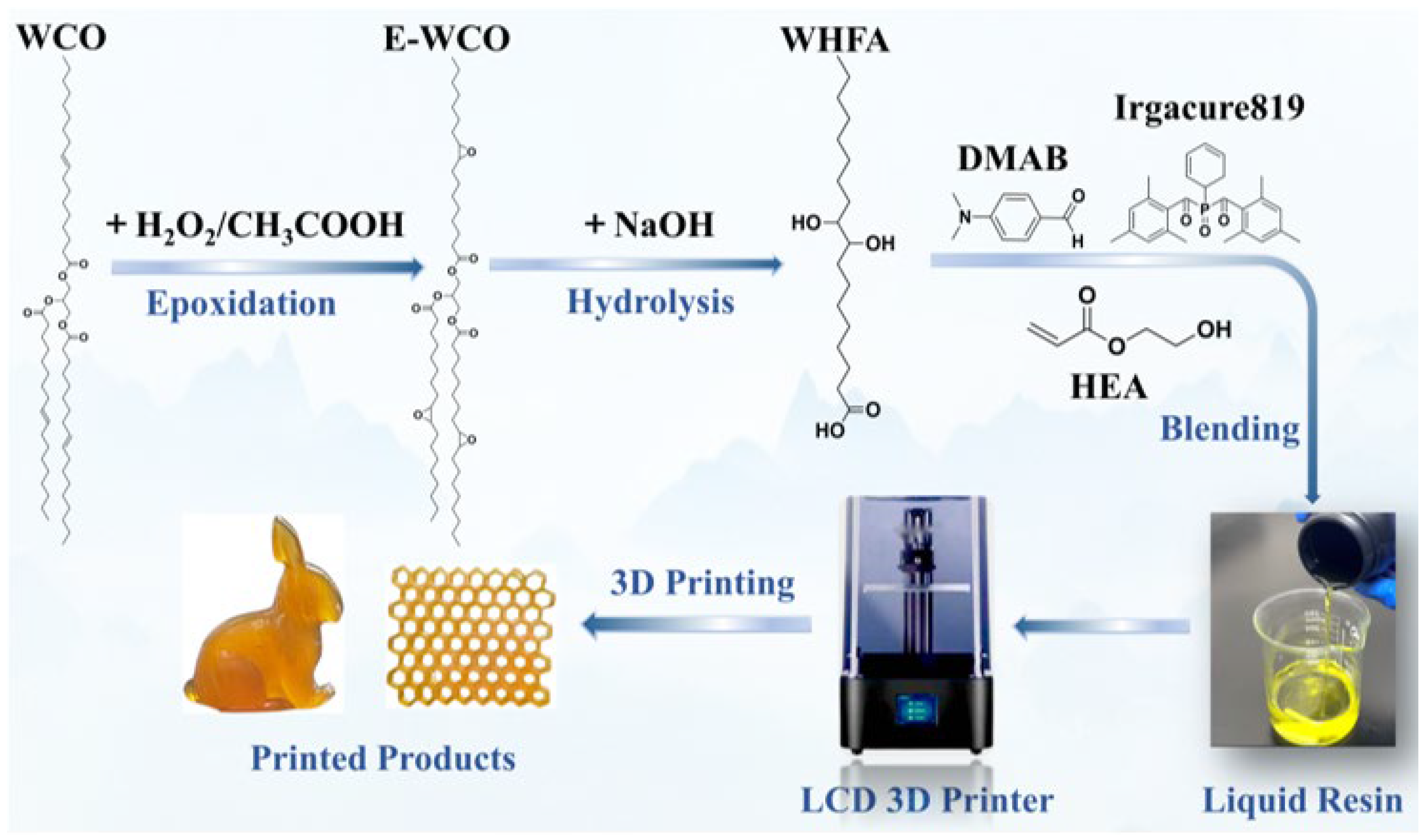
3.2. Preparation of WHFA
3.3. Preparation of WHFA/HEA Photocurable Elastomers
3.4. Molding of WHFA/HEA Photocurable Elastomers
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WCO | Waste cooking oil |
| E-WCO | Epoxidized waste cooking oil |
| WHFA | WCO based hydroxy fatty acid |
| SA | Stearic acid |
| HEA | Hydroxyethyl acrylate |
| Irgacure 819 | Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide |
| DMAB | p-dimethylaminobenzaldehyde |
References
- Caplins, B.W.; Higgins, C.I.; Kolibaba, T.J.; Arp, U.; Miller, C.C.; Poster, D.L.; Zarobila, C.J.; Zong, Y.Q.; Killgore, J.P. Characterizing light engine uniformity and its influence on liquid crystal display based vat photopolymerization printing. Addit. Manuf. 2023, 62, 103381. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, M.Y.; Wu, T.; Yang, X.X.; Qu, Z.W.; Yao, X.L.; He, Y.Z.; Guo, Y.X.; Pu, Q.S.; Wang, X.L. Green Synthesis of Vat Photopolymerization 3D Printing Polyimide. Small 2025, 21, 2502406. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, W.Z.; Liu, F.K.; Cui, J.J.; Feng, S.W.; Liang, C.; Guo, Y.L.; Wang, Z.X.; Mao, Z.J.; Zhang, B. Vat photopolymerization based digital light processing 3D printing hydrogels in biomedical fields: Key parameters and perspective. Addit. Manuf. 2024, 94, 104443. [Google Scholar] [CrossRef]
- Zhou, A.W.; Xu, C.Y.; Kanitthamniyom, P.; Ng, C.S.X.; Lim, G.J.; Lew, W.S.; Vasoo, S.; Zhang, X.S.; Lum, G.Z.; Zhang, Y. Magnetic Soft Millirobots 3D Printed by Circulating Vat Photopolymerization to Manipulate Droplets Containing Hazardous Agents for In Vitro Diagnostics. Adv. Mater. 2022, 34, e2200061. [Google Scholar] [CrossRef]
- Leong, K.M.; Sun, A.Y.; Quach, M.L.; Lin, C.H.; Craig, C.A.; Guo, F.L.; Robinson, T.R.; Chang, M.M.; Olanrewaju, A.O. Democratizing Access to Microfluidics: Rapid Prototyping of Open Microchannels with Low-Cost LCD 3D Printers. Acs Omega 2024, 9, 45537–45544. [Google Scholar] [CrossRef]
- Zuo, X.L.; Zhou, Y.; Hao, K.A.; Liu, C.; Yu, R.H.; Huang, A.R.; Wu, C.; Yang, Y.Y. 3D Printed All-Natural Hydrogels: Flame-Retardant Materials Toward Attaining Green Sustainability. Adv. Sci. 2024, 11, 2306360. [Google Scholar] [CrossRef]
- Maines, E.M.; Porwal, M.K.; Ellison, C.J.; Reineke, T.M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021, 23, 6863–6897. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Hu, J.; Li, J.; Zhou, R.; Lin, S. Green synthesis of soybean oil-derived UV-curable resins for high-resolution 3D printing. Addit. Manuf. 2024, 95, 104543. [Google Scholar] [CrossRef]
- Maturi, M.; Spanu, C.; Locatelli, E.; Sambri, L.; Franchini, M.C. Myrcene-itaconate Diels-Alder cycloadducts in the synthesis of photocurable polyesters for the 3D printing of fully biobased resins. Addit. Manuf. 2024, 92, 104360. [Google Scholar] [CrossRef]
- Lim, W.-B.; Bae, J.-H.; Seo, M.-J.; Min, J.-G.; Lee, J.-H.; Jung, Y.-S.; Huh, P. A novel UV-curable acryl-polyurethane for flexural 3D printing architectures. Addit. Manuf. 2022, 51, 102625. [Google Scholar] [CrossRef]
- Goodarzi Hosseinabadi, H.; Biswas, A.; Bhusal, A.; Yousefinejad, A.; Lall, A.; Zimmermann, W.-H.; Miri, A.K.; Ionov, L. 4D-Printable Photocrosslinkable Polyurethane-Based Inks for Tissue Scaffold and Actuator Applications. Small 2024, 20, 2306387. [Google Scholar] [CrossRef]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 2462. [Google Scholar] [CrossRef]
- Porcarello, M.; Mendes-Felipe, C.; Lanceros-Mendez, S.; Sangermano, M. Design of acrylated epoxidized soybean oil biobased photo-curable formulations for 3D printing. Sustain. Mater. Technol. 2024, 40, e00927. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Talacka, V.; Ostrauskaite, J. High biorenewable content acrylate photocurable resins for DLP 3D printing. J. Appl. Polym. Sci. 2021, 138, 50233. [Google Scholar] [CrossRef]
- Ritere, A.; Jurinovs, M.; Platnieks, O.; Barkane, A.; Gaidukovs, S. A super-tough plant oil based elastomer for UV-light assisted 3D printed soft robotics and shape-memory. J. Mater. Chem. A 2024, 12, 16569–16582. [Google Scholar] [CrossRef]
- Wu, Y.; Fei, M.; Chen, T.; Li, C.; Fu, T.; Qiu, R.; Liu, W. H-bonds and metal-ligand coordination-enabled manufacture of palm oil-based thermoplastic elastomers by photocuring 3D printing. Addit. Manuf. 2021, 47, 102268. [Google Scholar] [CrossRef]
- Nkwonta, C.G.; Auma, C.I.; Gong, Y. Underutilised food crops for improving food security and nutrition health in Nigeria and Uganda-a review. Front. Sustain. Food Syst. 2023, 7, 1126020. [Google Scholar] [CrossRef]
- Tabe-Ojong, M.P.J.; Alamsyah, Z.; Sibhatu, K.T. Oil palm expansion, food security and diets: Comparative evidence from Cameroon and Indonesia. J. Clean. Prod. 2023, 418, 138085. [Google Scholar] [CrossRef]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent advances in the conversion of waste cooking oil into value-added products: A review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Landi, F.F.D.; Fabiani, C.; Castellani, B.; Cotana, F.; Pisello, A.L. Environmental assessment of four waste cooking oil valorization pathways. Waste Manag. 2022, 138, 219–233. [Google Scholar] [CrossRef]
- Qi, Z.F.; Zhang, C.; Wang, Y.; Ping, L.Y.; Gao, B.H.; Sun, T.; Zhang, H.Y. The future of waste cooking oil and its carbon and economic benefits—An automotive energy perspective. Biomass Bioenergy. 2024, 184, 107204. [Google Scholar] [CrossRef]
- Hemanandh, J.; Tureya, H.; Barmavatu, P.; Kumar, G.M.; Bharadwaj, A.S.; Viswanathan, M.R.; Sikarwar, V.S. Experimental investigation on the effect of hydrotreated vegetable oils as a renewable source: Valorization of food waste. Biomass Convers. Biorefin. 2025, 15, 19283–19296. [Google Scholar] [CrossRef]
- Wang, Y.D.; Su, J.Z.; Liu, L.Y.; Liu, Z.M.; Sun, G.Q. Waste cooking oil based capsules for sustainable self-healing asphalt pavement: Encapsulation, characterization and fatigue-healing performance. Constr. Build. Mater. 2024, 425, 136032. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Jeyaseelan, T. Hydrogenation: A novel fuel processing technology for efficient heating with kerosene and waste cooking oil fuel blends. Fuel 2025, 400, 135755. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Farghali, M.; Rashwan, A.K.; Abdelkader, A.; Al-Muhtaseb, A.H.; Ihara, I.; Rooney, D.W. Optimizing biodiesel production from waste with computational chemistry, machine learning and policy insights: A review. Environ. Chem. Lett. 2024, 22, 1005–1071. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Ali, C.H.; Yasin, S.; Jamil, F. Waste quail beaks as renewable source for synthesizing novel catalysts for biodiesel production. Renew. Energy. 2020, 154, 1035–1043. [Google Scholar] [CrossRef]
- Yan, S.; Dong, Q.; Chen, X.Q.; Zhao, X.K.; Wang, X. Performance evaluation of waste cooking oil at different stages and rejuvenation effect of aged asphalt through molecular dynamics simulations and density functional theory calculations. Constr. Build. Mater. 2022, 350, 128853. [Google Scholar] [CrossRef]
- Jeon, K.W.; Gong, J.H.; Kim, M.J.; Shim, J.O.; Jang, W.J.; Roh, H.S. Review on the production of renewable biofuel: Solvent-free deoxygenation. Renew. Sustain. Energy Rev. 2024, 195, 114325. [Google Scholar] [CrossRef]
- Wongsurakul, P.; Rahman, T.; Hongloi, N.; Feyzbar-Khalkhali-Nejad, F.; Aransiola, E.; Bargiela, P.; Zhang, L.H.; Ammar, M.; Baltrusaitis, J.; Kiatkittipong, W. Renewable diesel and bio-aromatics production from waste cooking oil using ethanol as a hydrogen donor in deoxygenation reaction. Chem. Eng. J. 2025, 509, 161170. [Google Scholar] [CrossRef]
- Joshi, J.R.; Bhanderi, K.K.; Karve, M.; Patel, J.V. Chemical modification of waste cooking oil for the bio lubricant production through epoxidation process. Biomass Convers. Biorefin. 2025, 15, 2739–2756. [Google Scholar] [CrossRef]
- Azzena, U.; Montenero, A.; Carraro, M.; Crisafulli, R.; De Luca, L.; Gaspa, S.; Muzzu, A.; Nuvoli, L.; Polese, R.; Pisano, L. Recovery, Purification, Analysis and Chemical Modification of a Waste Cooking Oil. Waste Biomass Valorization 2023, 14, 145–157. [Google Scholar] [CrossRef]
- Teh, J.L.; Walvekar, R.; Ho, K.C.; Khalid, M. Biolubricants from waste cooking oil: A review of extraction technologies, conversion techniques, and performance enhancement using natural antioxidants. J. Environ. Manag. 2025, 375, 124267. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.H.; Hu, X.M.; To, M.H.; Wang, H.M.; Qin, Z.H.; Mou, J.H.; Yan, W.; Kaur, G.; Roelants, S.; Lin, C.S.K. Environmental evaluation of emerging bakery waste oil-derived sophorolipids production by performing a dynamic life cycle assessment. Sustain. Prod. Consum. 2024, 47, 59–70. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhang, T.; Shi, T.Q.; Barceló, D.; Zheng, H.B. Biodegradation and high-value utilization of waste cooking oil: Strategies and mechanisms for producing lipopeptide biosurfactants using Bacillus subtilis YZQ-2. J. Environ. Chem. Eng. 2024, 12, 114549. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.S.; Ng, H.Y.; Wong, J.W.C.; Kim, S.H. Production of biosurfactants from agro-industrial waste and waste cooking oil in a circular bioeconomy: An overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, Z.Y.; Xie, Q.L.; Fang, J.J.; Hu, Y.C.; Lu, M.Z.; Xia, F.; Nie, Y.; Ji, J.B. Structural modification of waste cooking oil methyl esters as cleaner plasticizer to substitute toxic dioctyl phthalate. J. Clean. Prod. 2018, 186, 1021–1030. [Google Scholar] [CrossRef]
- Ma, Y.F.; Fang, Y.; Hu, Y.; Li, Q.G.; Huang, Q.; Shang, Q.Q.; Zhang, M.; Li, S.H.; Jia, P.Y.; Zhou, Y.H. Recent advances in vegetable oil based fine chemicals and polymers. Green Mater. 2024, 1–22, 2400083. [Google Scholar] [CrossRef]
- Li, P.; Niu, B.; Pan, H.L.; Zhang, Y.Y.; Long, D.H. Production of hydrocarbon-rich bio-oil from catalytic pyrolysis of waste cooking oil over nickel monoxide loaded corn cob-derived activated carbon. J. Clean. Prod. 2023, 384, 135653. [Google Scholar] [CrossRef]
- Sharma, P.; Usman, M.; Salama, E.S.; Redina, M.; Thakur, N.; Li, X.K. Evaluation of various waste cooking oils for biodiesel production: A comprehensive analysis of feedstock. Waste Manag. 2021, 136, 219–229. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Li, C.; Chen, X.M.; Peng, W.X.; Aghbashlo, M.; Lam, S.S.; Tabatabaei, M. Managing the hazardous waste cooking oil by conversion into bioenergy through the application of waste-derived green catalysts: A review. J. Hazard. Mater. 2022, 424, 127636. [Google Scholar] [CrossRef]
- Souza, G.P.R.; Correia, T.B.A.; Reis, W.S.M.; Bredda, E.H.; Da Rós, P.C.M.; Pereira, E.B. Enzymatic Hydrolysis of Waste Cooking Oil by Lipase Catalysis: Simplex Mixture Design Optimization. Catal. Lett. 2023, 153, 689–697. [Google Scholar] [CrossRef]
- Beghetto, V. Strategies for the Transformation of Waste Cooking Oils into High-Value Products: A Critical Review. Polymers 2025, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Beghetto, V. Waste Cooking Oils into High-Value Products: Where Is the Industry Going? Polymers 2025, 17, 887. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Miao, W.F.; Wang, N.N.; Chen, H.M.; Wang, X.R.; Wang, J.Y.; Tan, Q.L.; Chen, S.P. Solid alcohol based on waste cooking oil: Synthesis, properties, micromorphology and simultaneous synthesis of biodiesel. Waste Manag. 2019, 85, 295–303. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.Y.; Qi, Y.X.; Jin, X.Y.; Xu, H.R.; Chen, Y.X.; Chen, S.P.; Su, H.P. Synthesis and properties of wax based on waste cooking oil. RSC Adv. 2022, 12, 3365–3371. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.G.; Liu, M.Y.; Wang, L.; Wang, P.Y.; Xu, H.R.; Chen, Y.X.; Chen, S.P. Fatty acid wax from epoxidation and hydrolysis treatments of waste cooking oil: Synthesis and properties. RSC Adv. 2022, 12, 36018–36027. [Google Scholar] [CrossRef]
- Liu, M.Y.; Liu, Y.; Wang, P.Y.; Ying, W.Y.; Liu, Q.; Ding, G.Z.; Chen, S.P. Synthesis and Properties of a Photocurable Coating Based on Waste Cooking Oil. Coatings 2023, 13, 1553. [Google Scholar] [CrossRef]
- Wang, P.Y.; Liu, M.Y.; Sun, J.H.; Li, Y.Y.; Tang, C.Y.; Yang, Y.; Chen, S.P. 4D-printable photocurable pressure sensitive adhesives derived from waste cooking oil. Virtual Phys. Prototyp. 2025, 20, e2499490. [Google Scholar] [CrossRef]
- Liu, M.Y.; Li, G.M.; Wang, P.Y.; Ying, W.Y.; Yang, Y.; Tang, C.Y.; Li, Y.Y.; Chen, S.P. Mechanically enhanced 3D printable photocurable resin composed of epoxy waste cooking oil and triethylene glycol dimethacrylate. J. Polym. Res. 2024, 31, 177. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.Y.; Fan, X.G.; Wang, L.; Liang, J.Y.; Jin, X.Y.; Che, R.J.; Ying, W.Y.; Chen, S.P. Four-Dimensional Printing of Multifunctional Photocurable Resin Based on Waste Cooking Oil. ACS Sustain. Chem. Eng. 2022, 10, 16344–16358. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.Y.; Fan, X.G.; Wang, P.Y.; Chen, S.P. A 4D-Printable Photocurable Resin Derived from Waste Cooking Oil with Enhanced Tensile Strength. Molecules 2024, 29, 2162. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Sun, J.H.; Liu, M.Y.; Tang, C.Y.; Yang, Y.; Ding, G.Z.; Liu, Q.; Chen, S.P. Multifunctional 3D-Printable Photocurable Elastomer with Self-Healing Capability Derived from Waste Cooking Oil. Molecules 2025, 30, 1824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Y.Q.; Di Serio, M.; Zhou, J.J.; Ding, L.R.; Zhang, Y.; Bang, H.B.; Wu, H.P.; Sun, J.Y. Synthesis and Properties of 9,10-Dihydroxystearic Acid Ethoxylate. Tenside Surfactants Deterg. 2019, 56, 237–243. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; De Santis, A.; Di Serio, M.; Esposito, R.; Ruffo, F.; Turco, R. Sustainable Process for Production of Azelaic Acid Through Oxidative Cleavage of Oleic Acid. J. Am. Oil Chem. Soc. 2015, 92, 1701–1707. [Google Scholar] [CrossRef]
- Karakaya, N.O.; Uysal, S. The synthesis and characterization of s-Triazine polymer complexes containing epoxy groups. J. Mol. Struct. 2020, 1203, 127370. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Novel Multifunctional Epoxy (Meth)Acrylate Resins and Coatings Preparation via Cationic and Free-Radical Photopolymerization. Polymers 2021, 13, 1718. [Google Scholar] [CrossRef]
- Herrera-González, A.M.; Caldera-Villalobos, M.; Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; González-López, J.A. Analysis of Double Bond Conversion of Photopolymerizable Monomers by FTIR-ATR Spectroscopy. J. Chem. Educ. 2019, 96, 1786–1789. [Google Scholar] [CrossRef]
- Rudenko, Y.; Prosyankin, E.; Mustafina, A.; Fuki, M.; Borisov, R.; Fedyakova, N.; Bermeshev, M.; Chapala, P. Investigation of the Light Intensity and Temperature Influences on Double Bond Conversion in Resins for Vat Photopolymerization via Fourier Transform Infrared Spectroscopy. Macromol. Chem. Phys. 2025, 226, 2400398. [Google Scholar] [CrossRef]
- Heger, M.; Andersen, J.; Suhm, M.A.; Larsen, R.W. The donor OH stretching-libration dynamics of hydrogen-bonded methanol dimers in cryogenic matrices. Phys. Chem. Chem. Phys. 2016, 18, 3739–3745. [Google Scholar] [CrossRef]
- Xue, R.Q.; Du, H.; Zhang, W.; Sun, C.L.; Li, A.J.; Fang, W.H.; Men, Z. Hydrogen bonding switched symmetric and anti-symmetric vibrations SRS of -CH in aqueous DEG solution. Appl. Phys. Lett. 2023, 123, 221108. [Google Scholar] [CrossRef]
- Olivieri, G.; Cossaro, A.; Capria, E.; Benevoli, L.; Coreno, M.; De Simone, M.; Prince, K.C.; Kladnik, G.; Cvetko, D.; Fraboni, B. Intermolecular Hydrogen Bonding and Molecular Orbital Distortion in 4-Hydroxycyanobenzene Investigated by X-ray Spectroscopy. J. Phys. Chem. C 2015, 119, 121–129. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, R.Q.; Chen, H.Y.; Li, Z.; Liu, E.Z.; Ma, H.X.; Zhou, B.; Hao, H.; Jiao, L.Y. Construction of supramolecular S-scheme heterojunctions assisted by hydrogen bond subtle-tuning actuates highly efficient photocatalytic oxidation. Chem. Eng. J. 2023, 473, 145290. [Google Scholar] [CrossRef]
- Yi, Z.H.; Zhang, X.; Yu, X.Y.; Liu, L.; Xu, X.Z.; Cui, X.B.; Xu, J.Q. Hydrogen-bonded assemblies of a new polyoxometalate-based 3-D supramolecular architecture. J. Coord. Chem. 2013, 66, 1876–1888. [Google Scholar] [CrossRef]
- Kothavade, P.; Yadav, P.; Gopal, A.; Pol, H.; Kafi, A.; Bateman, S.; Shanmuganathan, K. Enhancing the Crystallization Kinetics and Mechanical Properties of Poly(lactic acid) Blends for 3D Printing Application. ACS Appl. Polym. Mater. 2024, 6, 5754–5762. [Google Scholar] [CrossRef]
- Jian, X.Y.; Sun, J.X.; Gu, X.J.; Song, C.T.; Zhang, X.H.; Yang, W.T. Quaternarized cationic poly (vinyl chloride)- co-(vinyl chloroacetate) binary copolymer as non-migration antibacterial additive in PVC matrix. J. Appl. Polym. Sci. 2024, 141, e54979. [Google Scholar] [CrossRef]
- Sharma, P.; Roy, S.; Karimi-Varzaneh, H.A. Impact of Plasticizer Addition on Molecular Properties of Polybutadiene Rubber and its Manifestations to Glass Transition Temperature. Macromol. Theory Simul. 2019, 28, 1900003. [Google Scholar] [CrossRef]
- Choi, I.H.; Kim, J.S. Glass transition temperature of styrene-based ionomer controlled over a very wide temperature range by nonpolar plasticizer content and ion content. J. Appl. Polym. Sci. 2023, 140, e54550. [Google Scholar] [CrossRef]
- Bennett, J. Measuring UV curing parameters of commercial photopolymers used in additive manufacturing. Addit. Manuf. 2017, 18, 203–212. [Google Scholar] [CrossRef]
- Tilve-Martinez, D.; Neri, W.; Horaud, D.; Vukadinovic, N.; Berton, B.; Desmedt, A.; Yuan, J.; Poulin, P. Graphene Oxide Based Transparent Resins For Accurate 3D Printing of Conductive Materials. Adv. Funct. Mater. 2023, 33, 2214954. [Google Scholar] [CrossRef]
- Bodor, M.; Lasagabaster-Latorre, A.; Arias-Ferreiro, G.; Dopico-Garcia, M.S.; Abad, M.J. Improving the 3D Printability and Mechanical Performance of Biorenewable Soybean Oil-Based Photocurable Resins. Polymers 2024, 16, 977. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Shang, Q.; An, R.; Liu, C.; Hu, L.; Zhou, Y. Rubber Seed Oil-Based UV-Curable Polyurethane Acrylate Resins for Digital Light Processing (DLP) 3D Printing. Molecules 2021, 26, 5455. [Google Scholar] [CrossRef]
- Stouten, J.; Schnelting, G.H.M.; Hul, J.; Sijstermans, N.; Janssen, K.; Darikwa, T.; Ye, C.; Loos, K.; Voet, V.S.D.; Bernaerts, K.V. Biobased Photopolymer Resin for 3D Printing Containing Dynamic Imine Bonds for Fast Reprocessability. ACS Appl. Mater. Interfaces 2023, 15, 27110–27119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Cheng, J.; Shang, Q.; Hu, Y.; Liu, C.; Zhang, M.; Hu, L. Self-Healing, Antibacterial, and 3D-Printable Polymerizable Deep Eutectic Solvents Derived from Tannic Acid. Acs Sustain. Chem. Eng. 2022, 10, 7954–7964. [Google Scholar] [CrossRef]
- Ning, H.; Chen, S.Y.; Liu, Y.E.; Qu, B.; Zheng, Y.Y.; Liu, X.Y.; Li, W.J.; Wang, R.; Chen, N.R.; Zhuo, D.X. Polybutadiene polyurethane acrylate photosensitive resin and its application in 3D printing. J. Sci. Adv. Mater. Dev. 2025, 10, 100844. [Google Scholar] [CrossRef]
- Debnath, S.; Kaushal, S.; Ojha, U. Catalyst-Free Partially Bio-Based Polyester Vitrimers. Acs Appl. Polym. Mater. 2020, 2, 1006–1013. [Google Scholar] [CrossRef]
- Zhao, T.T.; Yu, R.; Lo, S.; Li, X.P.; Zhang, Y.; Yang, X.; Zhao, X.J.; Wang, C.; Liu, Z.C.; Dou, R. Superstretchable and Processable Silicone Elastomers by Digital Light Processing 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 14391–14398. [Google Scholar] [CrossRef]
- Huang, L.Y.; Yang, Y.X.; Niu, Z.; Wu, R.Y.; Fan, W.F.; Dai, Q.Q.; He, J.Y.; Bai, C.X. Catalyst-Free Vitrimer Cross-Linked by Biomass-Derived Compounds with Mechanical Robustness, Reprocessability, and Multishape Memory Effects. Macromol. Rapid Commun. 2021, 42, 2100432. [Google Scholar] [CrossRef]
- Jeong, K.; Kwak, M.J.; Kim, Y.; Lee, Y.; Mun, H.; Kim, M.J.; Cho, B.J.; Choi, S.Q.; Im, S.G. Vapor-phase synthesis of a reagent-free self-healing polymer film with rapid recovery of toughness at room temperature and under ambient conditions. Soft Matter. 2022, 18, 6907–6915. [Google Scholar] [CrossRef]
- Das, S.; Kumari, N. A geometrically inspired constitutive framework for damage and intrinsic self-healing of elastomers. Eur. J. Mech. A-Solids 2025, 2503, 18771. [Google Scholar] [CrossRef]
- Wang, X.Z.; Xie, D.M.; Zhao, X.L.; Li, Y.D.; Zeng, J.B. Sustainable, Malleable, and Recyclable Castor Oil-Derived Poly(urethane urea) Networks with Tunable Mechanical Properties and Shape Memory Performance Based on Dynamic Piperazine-Urea Bonds. Macromolecules 2022, 55, 2243–2251. [Google Scholar] [CrossRef]
- Wang, Y.J.; He, Y.; Zheng, S.Y.; Xu, Z.Y.; Li, J.; Zhao, Y.P.; Chen, L.; Liu, W.G. Polymer Pressure-Sensitive Adhesive with A Temperature-Insensitive Loss Factor Operating Under Water and Oil. Adv. Funct. Mater. 2021, 31, 2104296. [Google Scholar] [CrossRef]
- Cui, C.Y.; Liu, B.; Wu, T.L.; Liu, Y.; Fan, C.C.; Xu, Z.Y.; Yao, Y.; Liu, W.G. A hyperbranched polymer elastomer-based pressure sensitive adhesive. J. Mater. Chem. A. 2022, 10, 1257–1269. [Google Scholar] [CrossRef]
- Rosalia, M.; Rubes, D.; Serra, M.; Genta, I.; Dorati, R.; Conti, B. Polyglycerol Sebacate Elastomer: A Critical Overview of Synthetic Methods and Characterisation Techniques. Polymers 2024, 16, 1405. [Google Scholar] [CrossRef]
- Tang, L.S.; He, X.Y.; Huang, R. Advanced elasticity and biodegradability of bio-based copolyester elastomer achieved by the crystallization inhibition of isosorbide and flexibility of 1,6-hexanediol. Polym. Bull. 2025, 82, 649–660. [Google Scholar] [CrossRef]
- Wu, Y.P.; Li, R.; Wang, J.W.; Situ, Y.; Huang, H. A new carbazolyl-basedacylphosphine oxide photoinitiator with high performance and low migration. J. Polym. Sci. 2022, 60, 52–61. [Google Scholar] [CrossRef]
- Fan, L.L.; Zeng, Z.; Zhu, R.X.; Liu, A.P.; Che, H.L.; Huo, M. Polymerization-Induced Self-Assembly Toward Micelle-Crosslinked Tough and Ultrastretchable Hydrogels. Chem. Mater. 2022, 34, 6408–6419. [Google Scholar] [CrossRef]
- Wu, B.; Sufi, A.; Biswas, R.G.; Hisatsune, A.; Moxley-Paquette, V.; Ning, P.; Soong, R.; Dicks, A.P.; Simpson, A.J. Direct Conversion of McDonald’s Waste Cooking Oil into a Biodegradable High-Resolution 3D-Printing Resin. ACS Sustain. Chem. Eng. 2020, 8, 1171–1177. [Google Scholar] [CrossRef]




| Major Compositions | Dp (mm) | Ec (mJ/cm2) | Reference |
|---|---|---|---|
| WHFA/HEA | 0.065 | 6.75 | This work |
| EWOMA -HPA | 0.296 | 41.65 | [48] |
| EWOMA -CHA | 0.195 | 38.03 | [48] |
| WMFAEE-HPA | 0.224 | 55.44 | [52] |
| Commercial resin | 0.314 | 16.32 | [69] |
| IPESO-ETPTA40 | 0.213 | 8.814 | [8] |
| AESO | 1.318 | 77.00 | [70] |
| RSO-PUA/HEA40 % | 0.327 | 15.20 | [71] |
| CROSS−10 | 0.404 | 17.15 | [72] |
| ChCl/HEMA/TA20 | 0.192 | 8.700 | [73] |
| Major Compositions | Elongation at Break (%) | Tensile Strength (MPa) | Reference |
|---|---|---|---|
| Waste cooking oil-derived hydroxy fatty acid (WHFA) Hydroxyethyl acrylate (HEA) | 1184.66 | 0.962 | This work |
| WCO-based methacrylate fatty acid ethyl ester (WMFAEE) Hydroxypropyl acrylate (HPA) | 645.09 | 0.967 | [52] |
| Epoxy waste oil methacrylate (EWOMA) Hydroxypropyl acrylate (HPA) | 78.34 | 0.368 | [48] |
| Epoxy waste oil methacrylate (EWOMA) Cyclohexyl acrylate (CHA) | 162.0 | 0.335 | [48] |
| Epoxy waste oil methacrylate (EWOMA) 2-phenoxyethyl acrylate (PHEA) Methacrylic acid (MAA) | 230.1 | 0.480 | [50] |
| Acrylated rapeseed oil (ARO) Isobornyl acrylate (IBOA) Hydroxyethyl acrylate (HEA) | 180 | - | [15] |
| PO fatty acid-ethyl acrylamide (POFA-EA) Acrylic acid (AA) | 851 | 4.2 | [16] |
| Hydroxyl-terminated polybutadiene (HTPB) Hydroxypropyl methacrylate (HPMA) | 246.1 | 32.9 | [74] |
| Poly(2-hydroxyethyl methacrylate) (PHEMA) | 130 | 25.4 | [75] |
| Vinyl-terminated polydimethylsiloxane (VPS) Branched mercapto-functionalized polysiloxane (MPS) Precipitated silica (PSi) | 1400 | - | [76] |
| Sample | WHFA (g) | HEA (g) | SA (g) | E-WCO (g) | Mass Ratio of HEA to WHFA (or SA, E-WCO) | Irgacure 819 (g) | DMAB (g) |
|---|---|---|---|---|---|---|---|
| A1 | 20 | 40 | - | - | 1:2 | 1.8 | 1.8 |
| A2 | 15 | 45 | - | - | 1:3 | 1.8 | 1.8 |
| A3 | 10 | 50 | - | - | 1:5 | 1.8 | 1.8 |
| A4 | 10 | 100 | - | - | 1:10 | 3.3 | 3.3 |
| SA/HEA (control sample) | - | 45 | 15 | 1:3 | 1.8 | 1.8 | |
| E-WCO/HEA (control sample) | - | 45 | - | 15 | 1:3 | 1.8 | 1.8 |
| HEA (control sample) | - | 100 | - | - | - | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.; Tang, C.; Yang, Y.; Qin, G.; Li, M.; Jiang, H.; Wu, M.; Chen, S. Three-Dimensional Printable Photocurable Elastomer Composed of Hydroxyethyl Acrylate and Hydroxy Fatty Acid Derived from Waste Cooking Oil: An Innovative Strategy for Sustainable, Highly Flexible Resin Development. Molecules 2025, 30, 4000. https://doi.org/10.3390/molecules30194000
Shen F, Tang C, Yang Y, Qin G, Li M, Jiang H, Wu M, Chen S. Three-Dimensional Printable Photocurable Elastomer Composed of Hydroxyethyl Acrylate and Hydroxy Fatty Acid Derived from Waste Cooking Oil: An Innovative Strategy for Sustainable, Highly Flexible Resin Development. Molecules. 2025; 30(19):4000. https://doi.org/10.3390/molecules30194000
Chicago/Turabian StyleShen, Fangping, Chuanyang Tang, Yang Yang, Guangzhi Qin, Minghui Li, Haitian Jiang, Mengyao Wu, and Shuoping Chen. 2025. "Three-Dimensional Printable Photocurable Elastomer Composed of Hydroxyethyl Acrylate and Hydroxy Fatty Acid Derived from Waste Cooking Oil: An Innovative Strategy for Sustainable, Highly Flexible Resin Development" Molecules 30, no. 19: 4000. https://doi.org/10.3390/molecules30194000
APA StyleShen, F., Tang, C., Yang, Y., Qin, G., Li, M., Jiang, H., Wu, M., & Chen, S. (2025). Three-Dimensional Printable Photocurable Elastomer Composed of Hydroxyethyl Acrylate and Hydroxy Fatty Acid Derived from Waste Cooking Oil: An Innovative Strategy for Sustainable, Highly Flexible Resin Development. Molecules, 30(19), 4000. https://doi.org/10.3390/molecules30194000








