Study of the Thermodynamic Properties in Aqueous Solution of the Cyclocondensation Products of Pyrogallol and Propanaldehyde
Abstract
1. Introduction
2. Results and Discussion
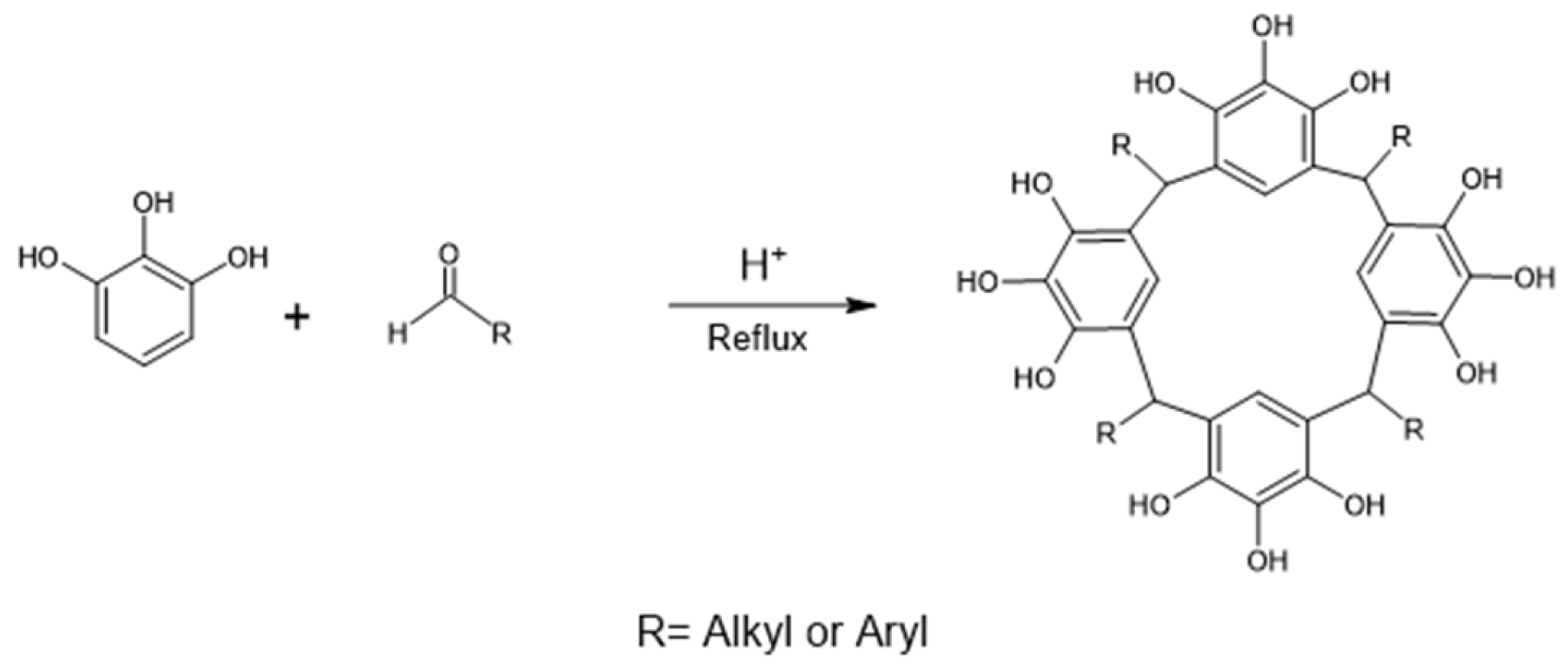
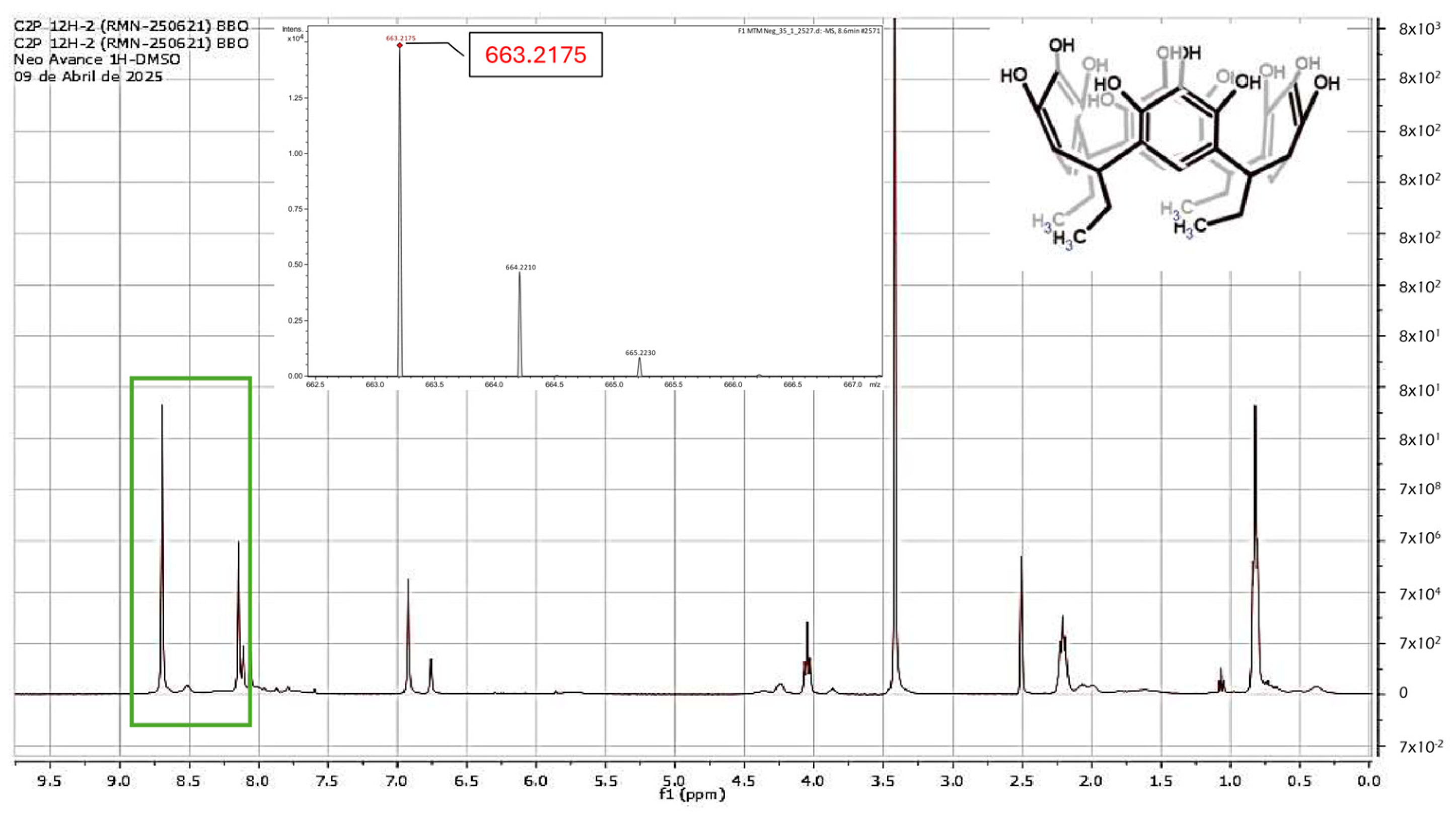
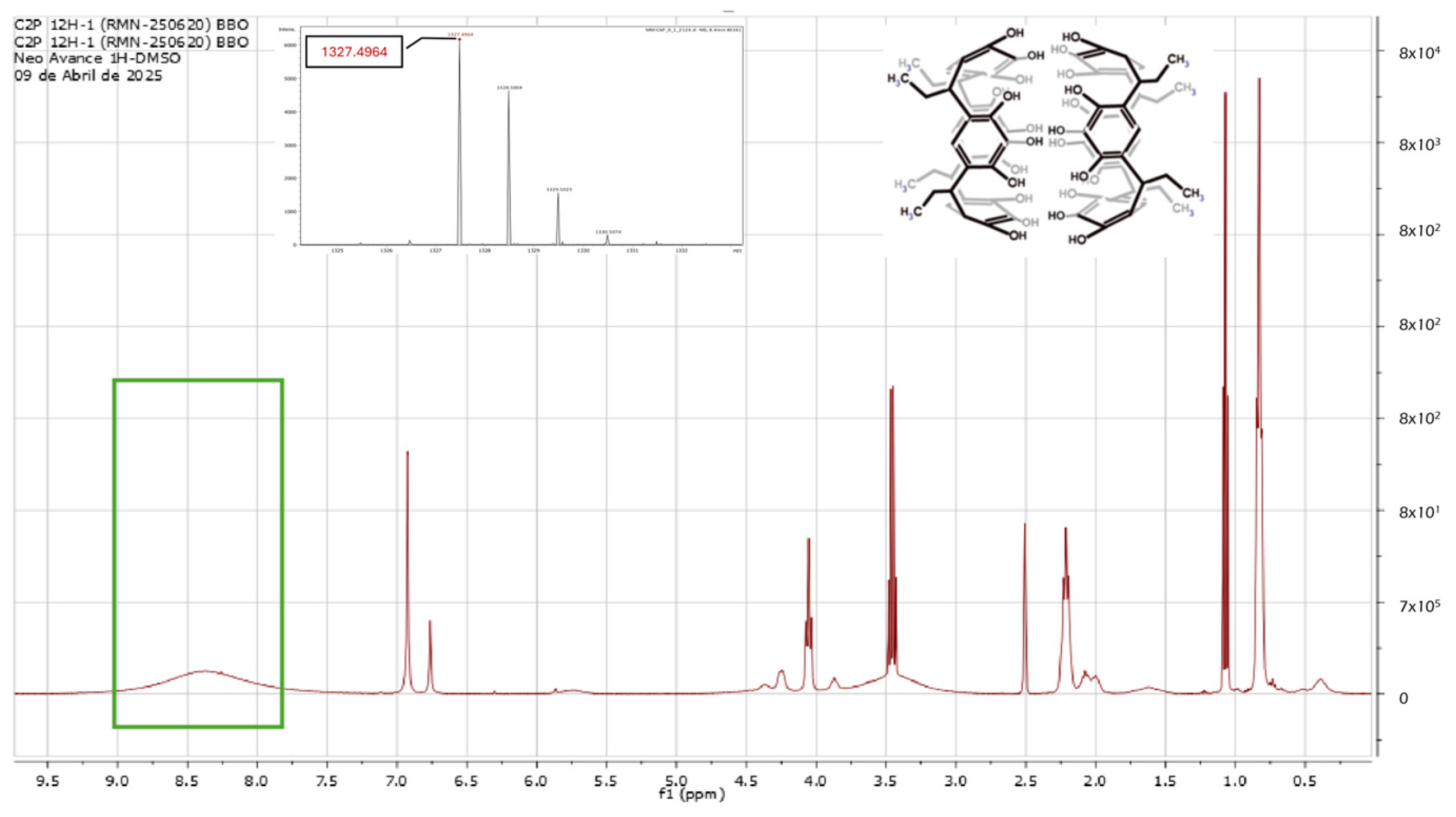
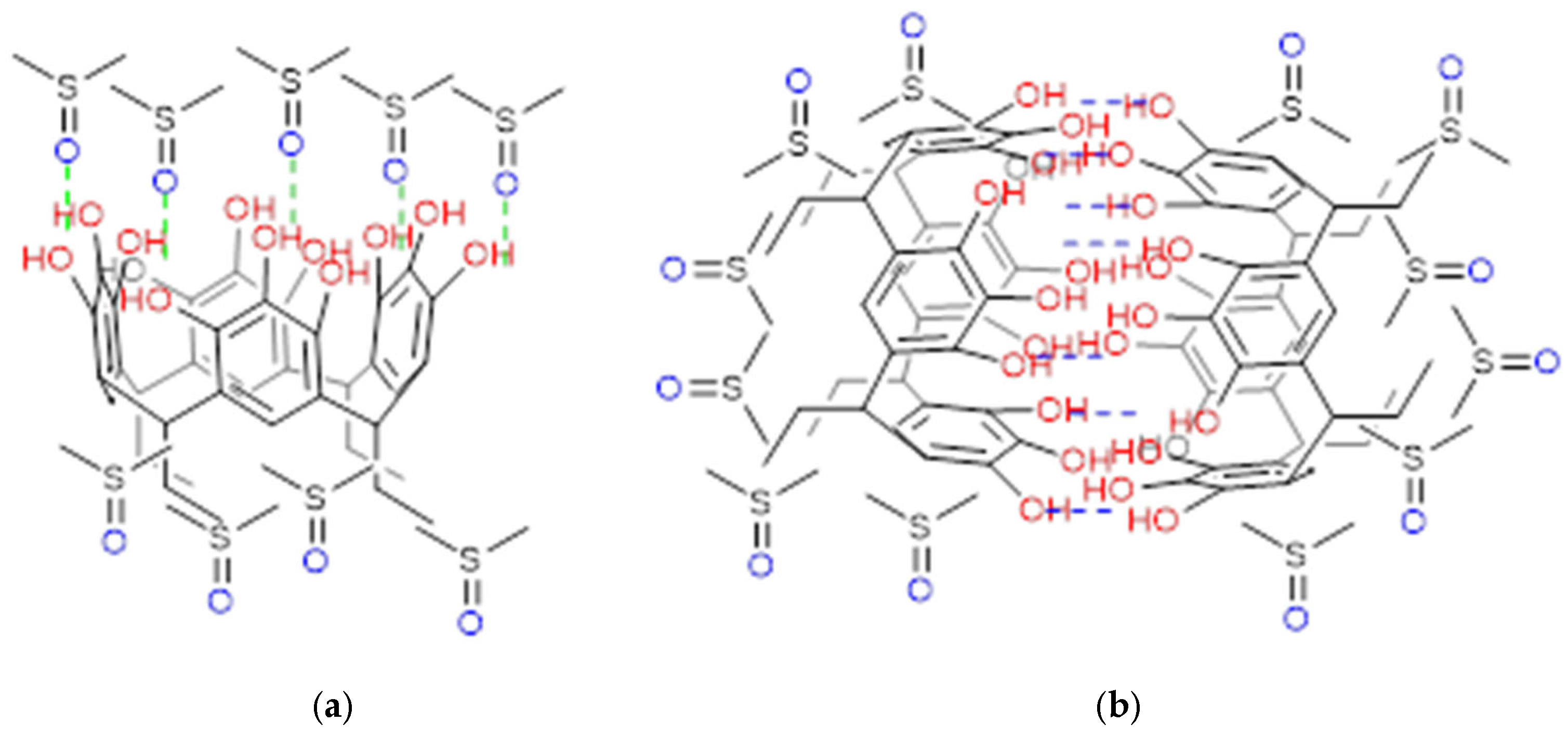
2.1. Reaction of Propanaldehyde with Pyrogallol
2.2. Calculation of Apparent Molar Volume and Standard Molar Expansibility
3. Materials and Methods
3.1. Synthesis of C-tetra(ethyl)pyrogallol[4]arene
3.2. Volumetric Properties of Monomer and Capsule Pyrogallolarene in DMSO Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FT-IR | Fourier Transform Infrared Spectroscopy |
| 1H NMR | 1H-Nuclear Magnetic Resonance |
| 13C NMR | 13C-Nuclear Magnetic Resonance |
| ESI-MS | Electrospray Ionization Mass Spectrometry |
References
- Zambrano, C.H.; Manzano, C.A.; Saltos, A.A.; Dueno, E.E.; Zeller, M. Síntesis de 2,8,14,20-tetra-n-butilpirogalol[4]areno y estudio computacional conformacional. ACI Av. Cienc. Ing. 2010, 2, A22–A29. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S. Macrocycles: Constructions, Chemistry and Nanotechnology Applications; Calixarenes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; Chapter 4; pp. 77–125. [Google Scholar]
- Bowley, N. Synthetic and Structural Studies of Calix[4]Pyrogallol[4]Arenes: Towards Biological Applications. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, 2008. [Google Scholar]
- Griffin, P. Pyrogallo4arenes: A Synthetic Investigation. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2007. [Google Scholar]
- Casas-Hinestroza, J.L.; Cifuentes, A.; Ibáñez, E.; Maldonado, M. Effect of the formation of capsules of tetra(propyl) pyrogallol[4]arene on the host-guest interaction with neurotransmitters. J. Mol. Struct. 2020, 1210, 128063. [Google Scholar] [CrossRef]
- Alshahateet, S.F.; Kooli, F.; Messali, M.; Judeh, Z.M.A.; ElDouhaibi, A.S. Synthesis and Supramolecularity of C-Phenylcalix[4] Pyrogallolarenes: Temperature Effect on the Formation of Different Isomers. J. Mol. Cryst. Liq. Cryst. 2007, 474, 89–110. [Google Scholar] [CrossRef]
- Gibb, B.C.; Chapman, R.G.; Sherman, J.C. Synthesis of Hydroxyl-Footed Cavitands. J. Org. Chem. 1996, 61, 1505–1509. [Google Scholar] [CrossRef]
- Barrett, E.S.; Dale, T.J.; Rebek, J., Jr. Synthesis and Assembly of Monofunctionalized Pyrogallolarene Capsules Monitored by Fluorescence Resonance Energy Transfer. Chem. Commun. 2007, 41, 4224–4226. [Google Scholar] [CrossRef]
- Yang, Y.; Swager, T. Main-chain calixarene polymers: Conformational effects on polymerization. Macromolecules 2006, 39, 2013–2015. [Google Scholar] [CrossRef]
- Grannas, M.; Hoskins, B.; Robson, R. Synthesis and x-ray crystal structures of a calixarene-related, tetraamino, tetraphenolic, polynucleating macrocyclic ligand and its ZnII4 and CoIII3 derivates. Inorg. Chem. 1994, 33, 1071–1079. [Google Scholar] [CrossRef]
- Cave, G.; Dalgarno, S.; Antesberger, J.; Ferrarelli, M.; McKinlay, R.; Atwood, J. Investigations into chain length control over solid-state pyrogallol[4]arene nanocapsule packing. Supramol. Chem. 2008, 20, 157–159. [Google Scholar] [CrossRef]
- Hof, F.; Trembleau, L.; Ullrich, E.C.; Rebek, J., Jr. Acetylcholine Recognition by a Deep, Biomimetic Pocket. Angew. Chem. Int. Ed. 2003, 42, 3150–3153. [Google Scholar] [CrossRef]
- Kim, S.K.; Kang, B.; Koh, H.S.; Yoon, Y.J.; Jung, S.J.; Jeong, B.; Lee, K.; Yoon, J. A New Imidazolium Cavitand for the Recognition of Dicarboxylates. Org. Lett. 2004, 6, 4655–4658. [Google Scholar] [CrossRef]
- Zhang, Q.; Adams, R.D.; Fenske, D. Stable hydrogen-bonded spherical capsules formed from self-assembly of pyrogallol[4]arenes. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 275–279. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Pappalardo, A.; Sfrazzetto, G.T. Applications of supramolecular capsules derived from resorcin[4]arenes, calix[n]arenes and metalloligands: From biology to catalysis. RSC Adv. 2015, 5, 51919–51933. [Google Scholar] [CrossRef]
- Krause, T.; Gruner, M.; Kuckling, D.; Habicher, W.D. Novel starshaped initiators for the controlled radical polymerization based on resorcin[4]- and pyrogallol[4]arenes. Tetrahedron Lett. 2004, 45, 9635–9639. [Google Scholar] [CrossRef]
- Späth, A.; König, B. Molecular recognition of organic ammonium-ions in solution using synthetic receptors. Beilstein J. Org. Chem. 2010, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Negin, S.; Li, R.; Kulikov, O.V.; Daschbach, M.M.; Gokel, G.W. Ion transport through bilayer membranes mediated by pyrogallol[4]arenes. Inorg. Chim. Acta 2014, 417, 177–185. [Google Scholar] [CrossRef]
- Buschmann, H.J.; Schollmeyer, E. Improved adhesion of polyurethane coating to polyester fabrics due to the surface fixation of calixarenes. J. Adhes. Sci. Technol. 2010, 24, 113–121. [Google Scholar] [CrossRef]
- Casas-Hinestroza, J.L.; Pérez-Redondo, A.; Maldonado, M. Inclusion complexation between neurotransmitters with polyacetylated calix[4]pyrogallol arenes: 1H-NMR and crystallographic analysis. Res. Chem. Intermed. 2022, 48, 3091–3107. [Google Scholar] [CrossRef]
- Casas-Hinestroza, J.L.; Maldonado, M. Conformational Aspects of the O-acetylation of C-tetra(phenyl)calixpyrogallol[4]arene. Molecules 2018, 23, 1225. [Google Scholar] [CrossRef]
- Shivanyuk, A.; Rebek, J., Jr. Hydrogen-bonded capsules in polar, protic solvents. Chem. Commun. 2001, 2001, 2374–2375. [Google Scholar] [CrossRef]
- NanJi, S.; Marriot, R. Apparent molar volumes of isoprene and limonene in dense-phase CO2. J. Supercrit. Fluids 2025, 221, 106544. [Google Scholar] [CrossRef]
- Taylor, N.B.; Kuyatt, C.E. Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results; NIST Technical Note 1297; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1994.
- Tiantian, M.; Haiyun, H.; Mengjiao, Z.; Renzhong, L.; Junru, W.; Yanping, D. Effect of temperature on the physicochemical properties and solute–solvent interactions of a dilute solution of [Bmim][OAc] in DMSO. J. Mol. Liq. 2025, 418, 126717. [Google Scholar]
- Parvinder, V.; Kirtanjot, K. Thermodynamic insight of DMSO-DMA and DMSO-DMF binary mixtures across varying temperatures. J. Mol. Liq. 2024, 415, 126286. [Google Scholar]


 ), 298.15 K (
), 298.15 K ( ), 303.15 K (
), 303.15 K ( ), 308.15 K (
), 308.15 K ( ), and 313.15 K (
), and 313.15 K ( ) for: (a) crown pyrogallolarene, and (b) dimer capsule of pyrogallolarene.
) for: (a) crown pyrogallolarene, and (b) dimer capsule of pyrogallolarene.
 ), 298.15 K (
), 298.15 K ( ), 303.15 K (
), 303.15 K ( ), 308.15 K (
), 308.15 K ( ), and 313.15 K (
), and 313.15 K ( ) for: (a) crown pyrogallolarene, and (b) dimer capsule of pyrogallolarene.
) for: (a) crown pyrogallolarene, and (b) dimer capsule of pyrogallolarene.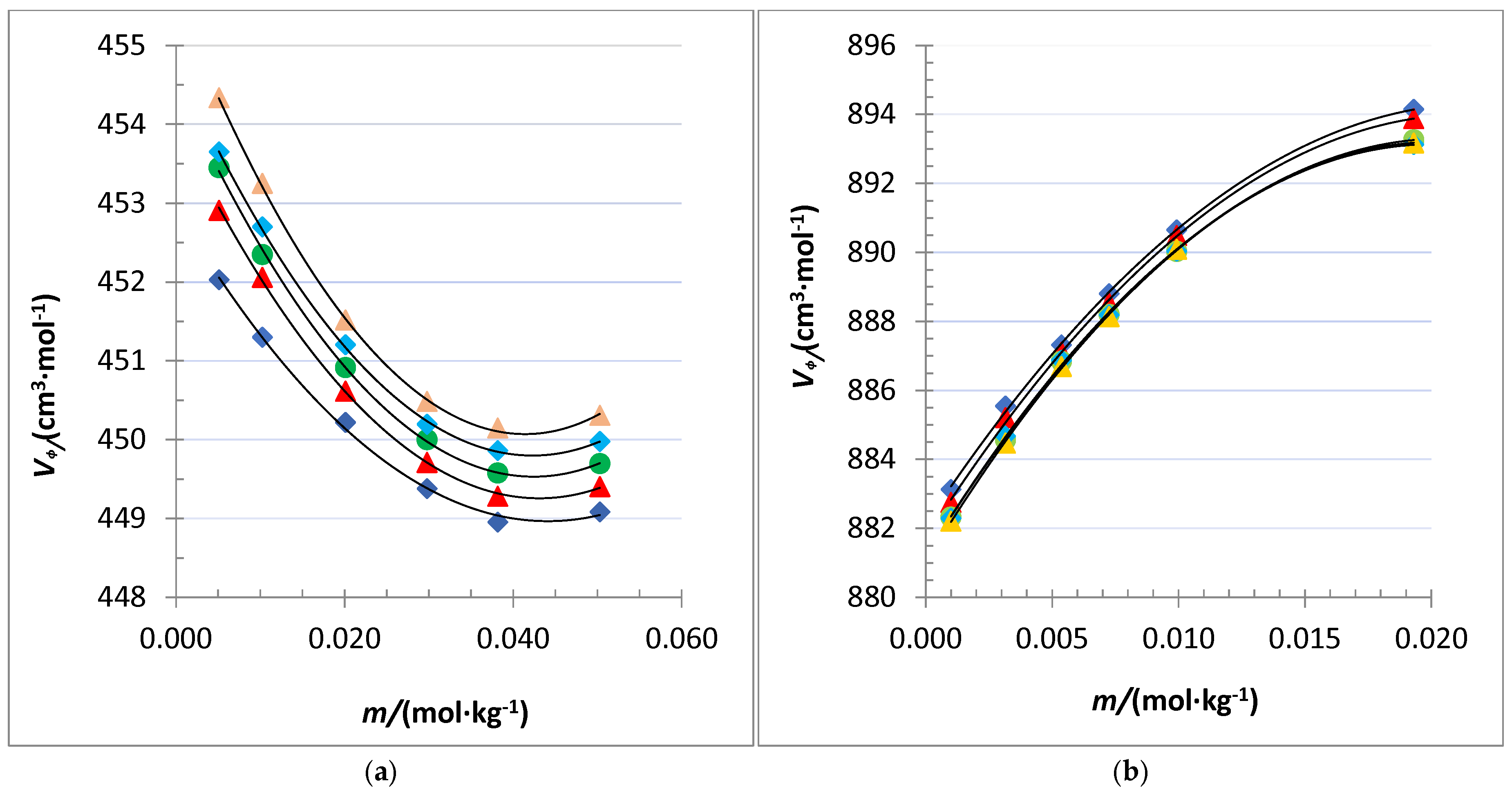
| Conformer/Entry | Retention Time (min) | Yield (%) Solvent EtOH/H2O (1:1) |
|---|---|---|
| Crown (1) | 10.3 | (22%) |
| Dimer capsule (2) | 10.7 | (40%) |
| Chair (3) | 9.9 | (30%) |
| T/K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| m/ (mol∙kg−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) |
| 0.0050758 | 1.101341 | 452.03 | 1.096327 | 452.91 | 1.091316 | 453.45 | 1.086307 | 453.65 | 1.081298 | 454.34 |
| 0.010235 | 1.102292 | 451.34 | 1.097283 | 452.06 | 1.092280 | 452.35 | 1.087276 | 452.71 | 1.082273 | 453.23 |
| 0.020099 | 1.104116 | 450.22 | 1.099122 | 450.62 | 1.094131 | 450.92 | 1.089140 | 451.20 | 1.084151 | 451.52 |
| 0.029782 | 1.105906 | 449.38 | 1.100925 | 449.71 | 1.095946 | 450.00 | 1.090970 | 450.19 | 1.085993 | 450.49 |
| 0.038165 | 1.107444 | 448.95 | 1.102473 | 449.28 | 1.097504 | 449.58 | 1.092535 | 449.86 | 1.087568 | 450.15 |
| 0.050296 | 1.109618 | 449.08 | 1.104662 | 449.40 | 1.099708 | 449.70 | 1.094754 | 449.98 | 1.089799 | 450.31 |
| T/K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| m/ (mol∙kg−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) | ρ/ (g∙cm−3) | Vϕ/ (cm3∙mol−1) |
| 0.00099709 | 1.100766 | 883.13 | 1.095749 | 882.75 | 1.090732 | 882.33 | 1.085719 | 882.29 | 1.080707 | 882.21 |
| 0.0031561 | 1.101603 | 885.55 | 1.096593 | 885.21 | 1.091585 | 884.56 | 1.086578 | 884.68 | 1.081573 | 884.48 |
| 0.0053671 | 1.102448 | 887.32 | 1.097445 | 887.10 | 1.092443 | 886.85 | 1.087443 | 886.87 | 1.082445 | 886.69 |
| 0.0072539 | 1.103159 | 888.80 | 1.098163 | 888.53 | 1.093168 | 888.20 | 1.088174 | 888.19 | 1.083181 | 888.14 |
| 0.0099236 | 1.104152 | 890.65 | 1.099164 | 890.49 | 1.094180 | 890.03 | 1.089194 | 890.04 | 1.084208 | 890.10 |
| 0.019301 | 1.107575 | 894.15 | 1.102621 | 893.88 | 1.097675 | 893.26 | 1.092721 | 893.13 | 1.087763 | 893.18 |
| Pyrogallolarene | T/K | Bv | ||
|---|---|---|---|---|
| Crown (monomer) | 293.15 | 452.92 | −178.96 | 2027.7 |
| 298.15 | 453.99 | −219.79 | 2548.7 | |
| 303.15 | 454.53 | −235.81 | 2778.8 | |
| 308.15 | 454.72 | −233.36 | 2765.4 | |
| 313.15 | 455.60 | −267.27 | 3227.8 | |
| a = 424.02 (cm3∙mol−1) | 0.1004 (cm3∙mol−1∙K−1) | |||
| Dimer capsule | 293.15 | 882.13 | 1108.5 | −25,197 |
| 298.15 | 881.71 | 1147.8 | −26,810 | |
| 303.15 | 881.19 | 1181.9 | −28,845 | |
| 308.15 | 881.19 | 1191.1 | −29,694 | |
| 313.15 | 881.01 | 1209.1 | −29,971 | |
| a = 898.24 (cm3∙mol−1) | −0.0552 (cm3∙mol−1∙K−1) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, M.; Martínez, D.; Crespo, A.; Sanabria, E.; Esteso, M.A. Study of the Thermodynamic Properties in Aqueous Solution of the Cyclocondensation Products of Pyrogallol and Propanaldehyde. Molecules 2025, 30, 3997. https://doi.org/10.3390/molecules30193997
Maldonado M, Martínez D, Crespo A, Sanabria E, Esteso MA. Study of the Thermodynamic Properties in Aqueous Solution of the Cyclocondensation Products of Pyrogallol and Propanaldehyde. Molecules. 2025; 30(19):3997. https://doi.org/10.3390/molecules30193997
Chicago/Turabian StyleMaldonado, Mauricio, Diana Martínez, Almudena Crespo, Edilma Sanabria, and Miguel A. Esteso. 2025. "Study of the Thermodynamic Properties in Aqueous Solution of the Cyclocondensation Products of Pyrogallol and Propanaldehyde" Molecules 30, no. 19: 3997. https://doi.org/10.3390/molecules30193997
APA StyleMaldonado, M., Martínez, D., Crespo, A., Sanabria, E., & Esteso, M. A. (2025). Study of the Thermodynamic Properties in Aqueous Solution of the Cyclocondensation Products of Pyrogallol and Propanaldehyde. Molecules, 30(19), 3997. https://doi.org/10.3390/molecules30193997







