1. Introduction
Francisella tularensis is one of the most virulent bacteria on the planet [
1] and the causative agent of tularemia. This organism has an extremely low infectious dose (fewer than 10 colony-forming units [CFU]) and can cause a fatal disease if inhaled. Therefore, the Center for Disease Control and Prevention (CDC) has designated
F. tularensis as a Category A Bioterrorism Agent (which is the category of greatest concern). Weaponization of multidrug-resistant
F. tularensis could be catastrophic. There is no licensed vaccine for tularemia approved for human use, representing a substantial vulnerability to this select agent. Therefore, there is a need to develop novel therapeutics and strategies to reduce the morbidity and mortality caused by this pathogen.
The
Francisella pathogenicity island (FPI) is a 30 kb region of the
F. tularensis genome that is essential for intracellular growth and virulence in mice [
2,
3]. Virulent clades (Type A and Type B) of
F. tularensis contain duplicate copies of the FPI, while the genome of
F. novicida, another member of the
Francisella genus, encodes only a single copy [
4]. Within the FPI, there are 19 genes, including the intracellular growth locus (
iglA-J), the pathogenicity determinant protein family (
pdpA-E),
vsrG,
dotU, and
anmK [
2,
4]. Most of these genes encode a non-canonical Type VI secretion system (T6SS) and its effectors [
2,
5]. The T6SS, which resembles a phage tail, is used to transport virulence factors across the host cell membrane [
6]. The structural components of the T6SS are encoded by
iglA,
iglB,
iglC,
iglD,
pdpA, and
pdpD, with a complex of IglA and IglB forming the sheath, and the inner tube which is composed of IglC [
6]. IglC is the best characterized FPI protein and plays roles in facilitating bacterial escape from the phagosome, NF-κB downregulation, and induction of apoptosis in macrophages [
7,
8,
9]. The spike of the T6SS used to puncture host cell membranes is composed of VgrG and PdpA [
6]. IglE functions to anchor the T6SS to the outer membrane, and this protein interacts with PdpB and DotU to form an inner membrane complex and periplasm-spanning channel [
6]. AnmK is only expressed in
F. novicida and is non-essential for intracellular growth [
10]. The FPI genes
iglH,
iglI, and
iglJ have been shown to be essential for replication in host cells, but their roles are still unknown [
6].
There are multiple transcription factors that regulate the expression of the FPI genes. The main regulators of the FPI are MglA and SspA, global transcriptional regulators of over 100 genes in
F. tularensis [
11]. In order to regulate transcription, MglA forms a heterodimeric complex with SspA to associate with the RNA polymerase [
12]. The orphan response regulator PmrA regulates the expression of genes inside and outside of the FPI, with few genes regulated by both PmrA and MglA [
13,
14]. Regulated by the MglA/SspA complex, FevR operates in tandem with MglA to increase the production of FPI proteins [
14]. MigR is another virulence regulator for
F. tularensis, regulating both FevR and the
iglABCD operon [
15]. Currently, Hfq is the only known negative regulator of the FPI and functions to downregulate the
pdp operon [
16]. A compound that would inhibit MglA/SspA-mediated transcriptional activation could serve as a novel therapeutic for
F. tularensis infections.
Tularemia can be effectively treated with antibiotics if given early in infection [
17,
18]. Antibiotic treatment has been shown to decrease the mortality rate of tularemia from 30–60% to 2% when administered in time [
19]. The three approved tularemia treatments include tetracyclines, and aminoglycosides [
1,
20]. While antibiotic treatments are effective, the symptoms of tularemia are non-specific, which can lead to misdiagnoses, resulting in delayed treatment and reduced survival [
17]. While these treatments are effective in most cases, relapse and treatment failure are possible with tetracycline and gentamicin treatment [
21]. Nephrotoxicity and ototoxicity are also concerns with aminoglycoside antibiotic treatments that need to be accounted for when treating tularemia [
21]. There have been no documented naturally occurring cases of tularemia being resistant to these antibiotics. However, due to
F. tularensis’ bioterrorism potential and past biological weapon research, there is a potential for the release of antibiotic-resistant strains [
19]. Due to these concerns, there is an interest in developing novel therapies for tularemia.
Natural products are a potential source for novel antimicrobial compounds. Natural products are compounds produced by plants, animals, fungi, and microorganisms that have potential medicinal applications [
22,
23]. Based on an analysis by the Food and Drug Administration (FDA), thirty-four percent of new medications approved between the years of 1982 and 2010 were isolated or derived from natural products. Medications isolated from natural products include statins, immunosuppressants, and anti-cancer drugs [
22]. The majority of the antibiotics in use today are natural products, as they were isolated from or based on compounds from bacteria or fungi [
22,
24]. Although the number of synthetic compounds available is approximately 138 times higher than the number of natural products, the “hit rate” for natural products is higher; over 80% have been found to contain pharmacologically active compounds that can expedite the screening process [
22].
Foeniculum vulgare, commonly known as fennel, is a biennial aromatic medicinal plant belonging to the family Apiaceae. A hardy herb with yellow flowers and feathery leaves, fennel is aromatic and flavorful, commonly used in food and traditional medicine [
25]. The bulbs, foliage, and seeds are all edible, being used in many regional cuisines throughout the world. Originally from the Mediterranean coastal regions, fennel has been adopted by cultures across the globe, growing in dry soil close to shorelines and river banks [
25]. In addition to the Mediterranean, fennel is popular in the Middle East and the Indian subcontinent. Fennel is also used medicinally as a carminative, diuretic, and a galactagogue [
25]. With widespread use in traditional medicine, fennel has been the subject of multiple efforts to identify potential drug candidates.
The essential oil of fennel has been shown to contain a wide variety of phenols, flavonoids, and other biologically active compounds [
25]. Some of the main compounds found in
F. vulgare essential oil are anethole, caffeoylquinic acid, acacetin, and oleanolic acid [
25]. There have been multiple studies to evaluate the antibacterial, antifungal, antidiabetic, anti-inflammatory, and antithrombotic properties of the essential oil and its components [
25]. Although fennel essential oil has shown promise in many of these studies, not much progress has been made beyond the initial screen [
25]. Estragole, a main component of fennel essential oil, is a known carcinogen, and it therefore poses a safety concern for the medicinal use of this oil [
25]. However, since numerous screens have shown the therapeutic potential of the essential oil of fennel, additional studies should be conducted to identify bioactive molecules that could be developed into novel therapeutic treatments.
In this paper, we describe the major bioactive secondary metabolite isolated from fennel following a bioassay-guided fractionation methodology. We show that the bioactive compound, dillapiole, limits the intracellular growth of F. tularensis in THP-1 monocyte cells, likely by dampening the expression of major virulence genes within the FPI. Dillapiole and other natural products that diminish bacterial pathogenesis represent a novel paradigm for the treatment of bacterial infections.
3. Discussion
While development of novel antimicrobial compounds has slowed, the threat of antibiotic resistance continues to grow as pathogenic bacteria continually evolve. The aim of this work was to approach this problem from a new perspective, looking for novel compounds that would augment the innate host immune system or dampen the virulence of the pathogenic bacteria. Our screen of a cataloged natural product library identified nine extracts that showed novel antimicrobial action with the inhibition of bacterial growth in an infection model, but no direct killing of these bacteria as is the case with traditional antibiotics. Following the identification of these candidate extracts, we identified dillapiole as the active compound that limited the growth of intracellular F. tularensis from the F. vulgare (fennel) extract.
After dillapiole was identified as the active compound, our investigation shifted to finding the mechanism of action causing the reduction in growth. Our first hypothesis was that dillapiole was acting as an immunomodulator. To test for this possibility, we performed a multiplex cytokine assay, ELISA, and RNA-seq analyses to look at the changes in the immune cells treated with dillapiole. No clear augmentation of host immunity was observed between treatment and control groups that could have been responsible for the observed reduction in intracellular bacteria. However, future investigation into host pathways affected by dillapiole could provide additional insight into the mechanism of this compound. Identification of naturally occurring plant material that augments protective host immunity is not unprecedented. For instance, in a mouse model of tularemia, nasal administration of polysaccharides from Acai enhanced the production of IFN-γ by lung NK cells to levels that were both protective and therapeutic [
32].
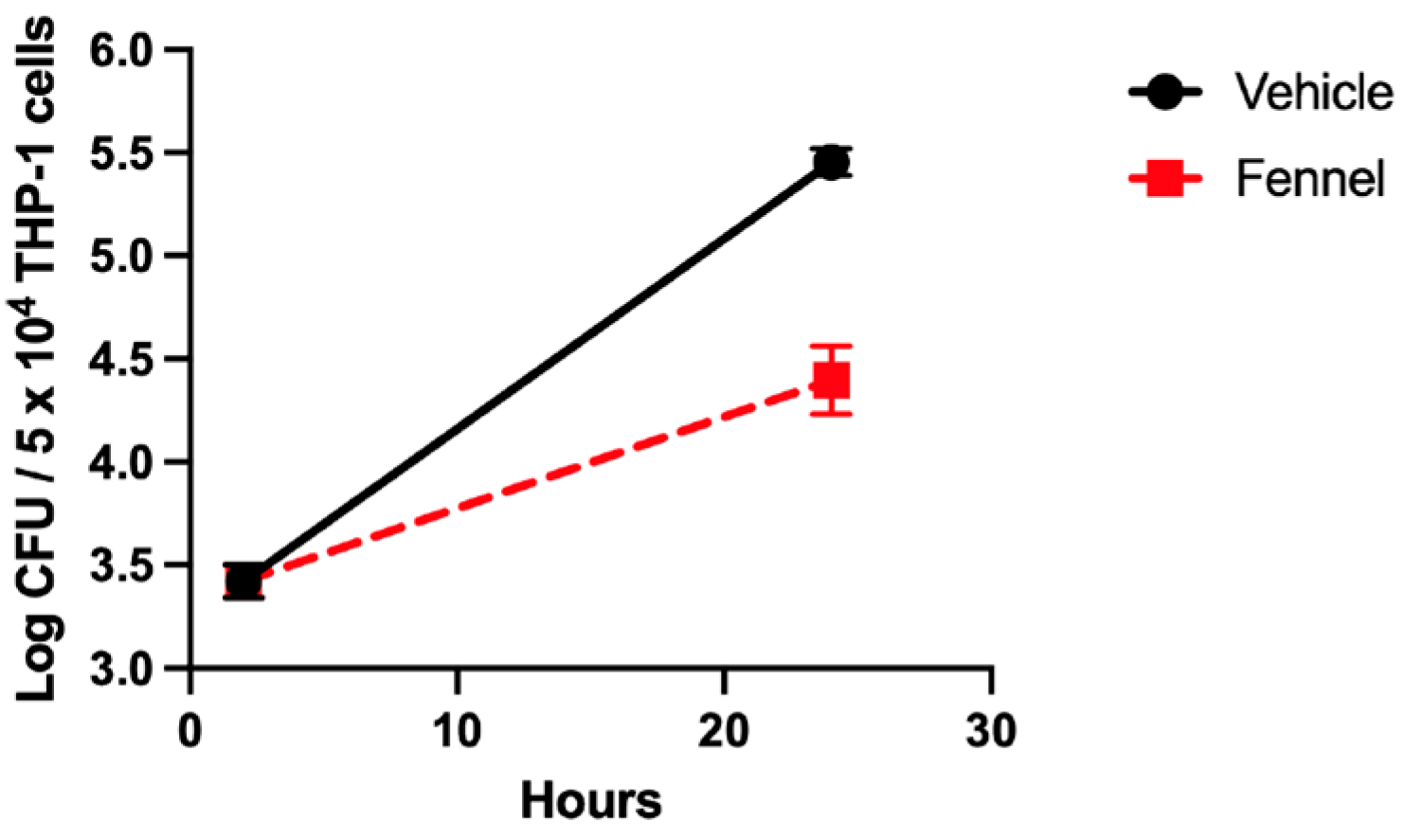
The second possibility was that dillapiole was affecting the expression of bacterial virulence genes. RNA-seq was used to look for global transcriptional changes in F. tularensis when treated with dillapiole. The resulting data showed that dillapiole downregulates the expression of FPI genes and the SspA gene; this suggests that dillapiole attenuates the F. tularensis pathogenesis by impeding the activity or formation of the MglA/SspA regulatory complex by limiting SspA. Western blotting confirmed that FPI-encoded proteins exhibited diminished expression in the presence of dillapiole, which is consistent with the RNA-seq data.
Several small molecules are known to modulate bacterial virulence including SE-1, which inhibits the virulence factor regulator VirF in
Shigella flexneri, leading to a decrease in AraC family proteins needed for pathogenesis [
33]. Another small molecule, M21, reduces the expression of multiple virulence factors in methicillin-resistant
Staphylococcus aureus, returning it to a non-virulent state [
34]. These virulence-regulating molecules could play a key role in the development of novel treatments for the threat of antibiotic resistance to human health. Dillapiole could potentially act by blocking the binding of guanosine tetraphosphate (ppGpp) to the MglA/SspA complex, which is needed to activate full FPI transcription. MglA/SspA trans-activation functionality could be tested utilizing PigR response element reporter assays [
35,
36].
Dillapiole is a phenylpropanoid most commonly found in high concentrations in the essential oil of
Piper aduncum [
23,
30,
31]. With the relative ease of extracting dillapiole from
P. aduncum essential oil, it has been the focus of many studies to identify potential medical uses [
23,
30,
31,
32]. Dillapiole was also found as the major volatile compound in a sample of extract of
F. vulgare collected in the Balkans region in Europe [
33]. The anti-inflammatory effects of dillapiole were evaluated using a rat paw edema model to compare dillapiole and synthesized analogs against indomethacin; however, none of the dillapiole analogs were shown to be as effective as the reference drug [
30]. Dillapiole has also been investigated as a potential treatment for leishmaniasis [
32]. Data from the in vitro studies show that dillapiole is mildly active as an antileishmanial, but falls far short of the effectiveness of the reference drug, amphotericin B [
32]. Another study looked at the potential use of dillapiole to control tick populations. It has been shown to exhibit acaricidal effects against
Amblyomma sculptum larvae [
31]. While dillapiole has been studied for numerous different uses, the novel antimicrobial effects we have demonstrated have not been previously documented.
Future studies should be conducted to explore the potential of dillapiole as a pharmaceutical. These include toxicity studies, pharmacokinetic/pharmacodynamic investigations, and assessment of the therapeutic efficacy of dillapiole in a murine model of tularemia. This work will better assess the effects of plasma protein binding, metabolization, bioavailability, and excretion on the activity of dillapiole. Additional studies should explore whether dillapiole shows efficacy against fully virulent F. tularensis and other bacterial species.
In summary, dillapiole is a novel antibacterial therapeutic that exerts its activity by dampening virulence factor expression in the pathogenic bacterium F. tularensis and potentially others. This treatment paradigm is different from that of existing antibiotics and therefore minimizes the selection for antibiotic resistance. This is because traditional antibiotics target central biological processes that are required for normal viability and growth of the bacteria (even outside the context of infection) while dillapiole only affects the expression of genes required for pathogenesis and does not affect bacterial viability. Therefore, dillapiole does not exert the same degree of selective pressure as traditional antibiotics, which will likely minimize the development of resistance to this novel treatment. Addition of virulence modulators to our expanding repertoire of treatments for infections could lead to a reduction in the morbidity and mortality resulting from antibiotic-resistant infections in the future.
4. Materials and Methods
4.1. Bacterial Culture Conditions for F. tularensis
LVS was provided by Dr. Karen Elkins (U.S. Food and Drug Administration, Silver Spring, MD, USA). Bacteria that were stored as freezer stocks were used to inoculate chocolate II agar plates that were at 37 °C with 5% CO2 for three days. Bacteria from agar plates were used to inoculate sterile filtered TSBc broth [Tryptic Soy Broth (BD, Franklin Lakes, NJ, USA) supplemented with 0.1% L-Cysteine hydrochloride (Fisher, Waltham, MA, USA)]; these bacteria were then grown at 37 °C with shaking for 14–18 h. For F. tularensis LVS/pTC3D, kanamycin (10 μg/mL) was added to the media.
4.2. Natural Product Library Screen
Through a collaboration with the National Center for Natural Products Research, a cataloged library of over 3200 extracts from plants, fungi, and marine life was screened for proinflammatory or anti-pathogenesis activity using a THP-1 infection model [
37]. THP-1 cells (ATCC TIB-202) were cultured in RPMI 1640 medium (no phenol red) supplemented with 10% fetal bovine serum (Gemini Bio-Products, Sacramento, CA, USA), 25 mM HEPES (Corning, Glendale, AZ, USA), 1 mM sodium pyruvate (Gibco, Waltham, MA, USA), and 1% glutamine dipeptide (Gibco). THP-1 cells were plated in flat-bottomed 96-well plates at a density of 5 × 10
4 cells/well.
F. tularensis LVS/pTC3D cultures were adjusted to an OD
600 of 0.3 (~1 × 10
9 CFU/mL) and diluted to a multiplicity of infection (MOI) of 10 in pre-warmed THP-1 media supplemented with kanamycin (10 μg/mL) to ensure the selection of the plasmids harbored by these bacteria. Wells were treated with an extract from the NPX library (8 μg/mL) or tetracycline (20 μg/mL). The plates were incubated at 37 °C, 5% CO
2 for 48 h. Fluorescence readings were taken on a microplate reader (BioTek, Winooski, VT, USA) using an excitation wavelength of 435 nm and an emission wavelength of 495 nm at 0 h, 24 h, and 48 h time points.
4.3. Kirby–Bauer Disk Diffusion Assay
Kirby–Bauer disk diffusion assays were performed on chocolate II agar plates using extracts previously identified to inhibit the replication of F. tularensis in high-throughput testing. F. tularensis cultures were adjusted to an OD600 of 0.1, and 100 μL of this diluted culture was spread and plated onto each chocolate II agar plate. Four sterile Whatman filter disks were placed on each plate, and 5 µL of the extracts, gentamicin (50 mg/mL) or phosphate-buffered saline (PBS), was aliquoted onto each disk. Plates were incubated at 37 °C, 5% CO2 for 48 h. Zones of inhibition were measured in millimeters on perpendicular axes, and these values were averaged.
4.4. Gentamicin Protection Assay
THP-1 cells (ATCC TIB-202) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gemini Bio-Products), 25 mM HEPES (Corning), 1 mM sodium pyruvate (Gibco), and 1% glutamine dipeptide (Gibco). THP-1 cells were plated in flat-bottomed 96-well plates at a density of 5 × 104 cells/well. F. tularensis cultures were adjusted to an OD600 of 0.3 (~1 × 109 CFU/mL) and diluted to a multiplicity of infection (MOI) of 10 in pre-warmed THP-1 medium. The THP-1 cells were then exposed to F. tularensis for 3 h at 37 °C, 5% CO2. Subsequently, cells were incubated with Hank’s Balanced Salt Solution (HBSS) (Corning) containing gentamicin (100 μg/mL) for 60 min to kill extracellular bacteria. The THP-1 cells were then washed twice with HBSS after cells were pelleted at 100× g for 5 min. Following washes, fresh tissue culture medium was then added, and natural products (8 μg/mL) or dillapiole was added. Afterwards, infected THP-1 cells were incubated for an additional 20 h. To detect viable F. tularensis bacteria, at each time point indicated, THP-1 cells were lysed with 0.02% sodium dodecyl sulfate. These lysates were serially diluted and plated onto chocolate II agar plates. Plates were incubated at 37 °C, 5% CO2 for three days to enumerate F. tularensis CFU.
4.5. General Chemistry Procedures
One- and two-dimensional experiments were recorded on Bruker model AMX 400 and 500 NMR spectrometers operating with standard pulse sequences. The instruments were operating at 400 and 500 in 1H and 100 and 125 in 13C, respectively. CDCl3 and C6D6 were used as solvents, and TMS was used as an internal standard. Low-resolution mass spectra (MSs) were obtained on a Micromass Q-Tof Micro mass spectrometer with a lock spray source, as well as by gas chromatography–mass spectrometry (PerkinElmer Clarus SQ 8). Column chromatography was performed on silica gel (70–230 mesh, Merck). Fractions collected from column chromatography were checked by TLC (silica gel 60 F254), while preparative TLC was conducted on silica gel 60 PF254+366 plates (20 × 20 cm, 1 mm thick). Detection of compounds was carried out under UV light.
4.6. Fractionation
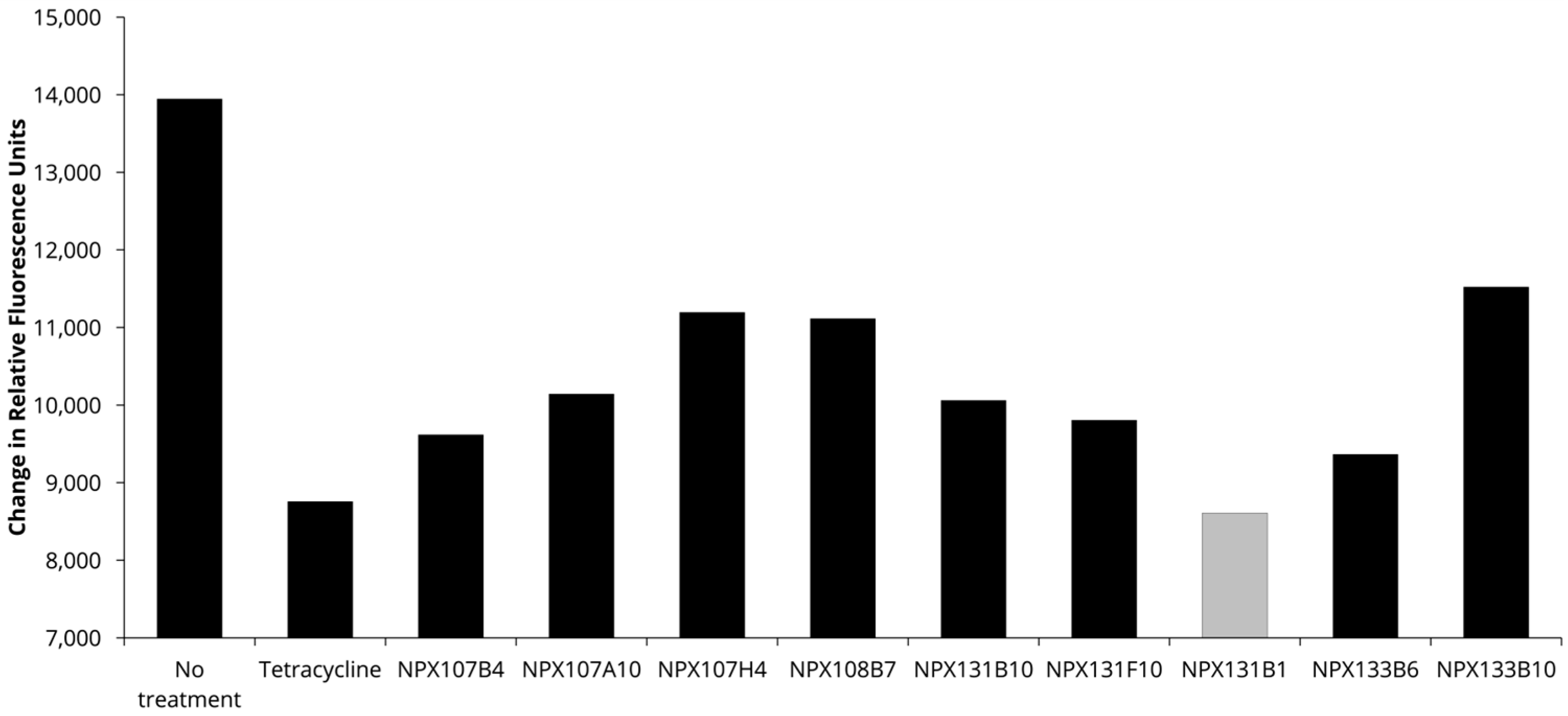
The dried ethanolic extract of F. vulgare was received from the National Center for Natural Product Research at the University of Mississippi. The extract was then dissolved in methanol (MeOH; 250 mL), and silica gel (50 g) was added. The mixture was dried under vacuum. The dried extract supported in silica was then added to a prepared silica gel column, eluted initially with 100% hexanes and then gradient eluted with hexanes: ethyl acetate (EtOAc) at ratios of 80:20, 60:40, 40:60, and 20:80, followed by 100% EtOAc. The 100% EtOAc elution was followed by another gradient solvent system with EtOAc: MeOH at ratios of 90:10 × 2 and 50:50 × 2, followed by 100% MeOH. The fractions were collected in 500 mL increments and monitored by TLC on silica gel using hexanes: EtOAc (80:20). Fractions were concentrated under vacuum to yield fourteen main fractions (Fv-1 to Fv-14). As fraction Fv-5 was determined to contain the active compound, Fv-5 was subjected to an additional round of fractionation using a silica gel column prepared with 100% hexanes. The column was initially eluted with 100% hexanes and then gradient eluted with hexanes: EtOAc at ratios of 99:1, 97:3, 10:90, 80:20, and 60:40, and finally, 100% MeOH. Subfractions were collected in 13 × 100 mm tubes and were changed once each tube was halfway full (~4 mL). Each subfraction was monitored by TLC on silica gel using hexanes (100%), EtOAc: hexanes (5:95), and EtOAc: hexanes (10:90). Similar subfractions were mixed and dried under vacuum to produce thirteen main subfractions (I to XIII).
4.7. Cytokine and Chemokine Assays
TNF-α levels in THP-1 cell supernatants were determined using the Quantikine ELISA Human TNF-α Immunoassay (R&D Systems, Minneapolis, MN, USA) on a microplate reader (BioTek) using the manufacturer’s protocol. To assess the immune response to dillapiole in murine cells, a RAW 264.7 infection model was used. RAW 264.7 cells (ATCC TIB-71) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Gemini Bio-Products), 25 mM HEPES (Corning), 1 mM sodium pyruvate (Gibco), and 1% glutamine dipeptide (Gibco). RAW 264.7 cells were seeded in Primaria 96-well plates 24 h prior to infection at a density of 5 × 104 cells/well. F. tularensis cultures were adjusted to an OD600 of 0.3 (~1 × 109 CFU/mL) and diluted to a multiplicity of infection (MOI) of 10 in pre-warmed RAW 264.7 media. Infected RAW 264.7 cells were treated with dillapiole (8 μg/mL) or the vehicle (100% hexanes). The plates were incubated at 37 °C, 5% CO2 for 24 h followed by the collection of supernatants. Using a Bio-Plex 200 System (Bio-Rad), the Bio-Plex Pro Mouse Cytokine Th1/Th2 Assay (Bio-Rad) was used to determine chemokine and cytokine levels in RAW 264.7 cell supernatants. Bio-Plex Manager 6.0 software (Bio-Rad) was used to calculate cytokine and chemokine concentrations against a standard.
4.8. THP-1 RNA Extraction
THP-1 cells (ATCC TIB-202) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gemini Bio-Products), 25 mM HEPES (Corning), 1 mM sodium pyruvate (Gibco), and 1% glutamine dipeptide (Gibco). THP-1 cells were plated in flat-bottomed 6-well plates at a density of 9 × 106 cells/well. F. tularensis cultures were adjusted to an OD600 of 0.3 (~1 × 109 CFU/mL) and diluted to a multiplicity of infection (MOI) of 10 in pre-warmed THP-1 media. Uninfected and infected THP-1 cells were then treated with dillapiole (8 μg/mL) or hexane 1 h post infection. Plates were incubated for 24 h at 37 °C, 5% CO2.
After incubation, cells were transferred to 15 mL conical tubes and collected by centrifugation. After removing the supernatant, the pellets were resuspended in Trizol and incubated at room temperature for 5 min. Chloroform was then added and vigorously mixed for 15 s until the sample turned milky pink. The mixture was then transferred to an organic matrix tube and centrifuged at max speed for 15 min. After centrifugation, the clear liquid layer was transferred to a new tube, and 70% ethanol was added at a 1:1 ratio. The resulting mixture was then transferred to a spin column (Invitrogen PureLink™ RNA Mini Kit) and spun at max speed for 15 s. The flow through was discarded, and PureLink Dnase (Invitrogen, Waltham, MA, USA) was added directly to the column and incubated for 15 min at room temperature. Following Dnase treatment, Wash Buffer 1 was added to the columns and centrifuged for 15 s. The columns were then washed with Wash Buffer 2 twice, each time being spun for 15 s followed by a final 2 min spin to ensure the column was dry. Spin columns were transferred to recovery tubes, and total RNA was eluted with Rnase-free water. The columns were incubated at room temperature for 2 min before centrifuging for 1 min. RNA concentrations were determined using a NanoDrop spectrophotometer and then stored at −80 °C.
For RNA-Seq analysis of THP-1 cells (±dillapiole), total RNA was extracted from five replicate samples in each experimental group. RNA was quantitated with Qubit RNA fluorimetric assays (ThermoFisher Scientific, Waltham, MA, USA). RNA Integrity Numbers (RINs), assessed using 2100 Bioanalyzer RNA microfluidic chips, were greater than 8.5 for all samples. Illumina-compatible libraries were prepared using a TruSeq Stranded mRNA kit (Illumina, San Diego, CA, USA). RNA libraries were sequenced on an Illumina HiSeq1500 sequencer.
Sequencing reads were trimmed to remove Illumina adapter sequences and low-confidence base calls using Trimmomatic version 0.32 [
38]. The quality of the resulting trimmed reads was verified using FastQC version 0.11.7 [
39]. Reads were aligned to the reference human genome GRCh38 using HISAT2 version 2.1.0. Counts of reads mapping to known genes for each sample were carried out using the R/Bioconductor package GenomicAlignements, version 1.16.10.
Gene expression differences in THP-1 cells were computed using DESeq2 version 1.20.0. Specifically, the expression ratios of treated uninfected cells to untreated uninfected cells and of treated infected cells to untreated infected cells were computed, and then these ratios were compared by using a statistical model of expression as a function of infection, treatment, and their interaction. Genes with a significant interaction effect were identified. A threshold of a Benjamini–Hochberg adjusted p-value less that 0.1 was used to determine significance.
Resulting significant genes were analyzed using the Ingenuity Pathway Analysis tool to identify genetic pathways perturbed by dillapiole treated differently between the infected and uninfected cells.
4.9. F. tularensis RNA Extraction
Broth cultures of F. tularensis LVS treated with vehicle, dillapiole (8 μg/mL), or no treatment were grown to the stationary phase and normalized to the lowest OD600 and pelleted. After removing the supernatant, the pellets were resuspended in Trizol and incubated at room temperature for 5 min. Chloroform was then added and vigorously mixed for 15 s until the sample turned milky pink. The mixture was then transferred to an organic matrix tube and centrifuged at max speed for 15 min. After centrifugation, the aqueous phase was transferred to a new tube, and 70% ethanol was added at a 1:1 ratio. The resulting mixture was then transferred to a spin column (Invitrogen PureLink™ RNA Mini Kit) and spun at max speed for 15 s. After the flow through was discarded, PureLink Dnase (Invitrogen) was added directly to the column and incubated for 15 min at room temperature. Following Dnase treatment, Wash Buffer 1 was added to the columns and centrifuged for 15 s. The columns were then washed with Wash Buffer 2 twice; for each wash, the columns were centrifuged for 15 s followed by a final 2 min spin to ensure the column was dry. Spin columns were transferred to recovery tubes, and Rnase-free water was added to elute RNA. The columns were incubated at room temperature for 2 min before centrifuging for 1 min. The RNA concentrations were determined using a NanoDrop spectrophotometer and then stored at −80 °C.
4.10. F. tularensis RNA-Seq
For RNA-Seq analysis of Francisella cells (hexane vs. dillapiole-treated), total RNA was extracted from eight replicate samples in each experimental group. RNA Integrity Number (RIN) values as determined by Bioanalyzer electrophoresis were all above 9. RNA samples (2 μg) were dissolved in 15 μL TE buffer (10 mM Tris HCL pH 8.0, 1 mM EDTA). 16S and 23S ribosomal RNA (rRNA) were removed using the MICROBExpress Bacterial mRNA Enrichment kit (Life Technologies, Waltham, MA, USA). rRNA-depleted RNA samples were then resuspended in Rnase-free water and quantitated by a fluorescence-based Qubit RNA HS Assay kit (ThermoFisher Scientific) and fragmented for 4 min at 94 °C to be used as inputs for RNA library preparation. Illumina-compatible RNA libraries were prepared using a KAPA RNA HyperPrep Kit (KAPA Biosystems, Inc. Wilmington, MA, USA). The quality of the library and the size of the insert were determined by Agilent DNA High Sensitivity DNA chips; the Qubit dsDNA HS Assay (Thermo Fisher) was used to quantify these libraries. Purified libraries were clustered and sequenced on an Illumina HiSeq1500 in a 2 × 50 paired-end Rapid Run at the Marshall University Genomics Core Facility.
Sequencing reads were trimmed to remove Illumina adapter sequences and low-confidence base calls using Trimmomatic version 0.32 [
38]. The quality of the resulting trimmed reads was verified using FastQC version 0.11.7 [
39]. Counts of reads mapping to known genes for each sample were carried out using Salmon version 0.12 [
40], with transcript sequences ASM924v1 (
Francisella tularensis subsp.
holarctica LVS) from GenBank as the reference transcripts. Resulting quantifications were imported into the R/Bioconductor package DESeq2 [
41], and genes whose expression was altered by dillapiole treatment were identified using a false discovery rate threshold of 10%.
4.11. Western Blotting
Stationary-phase cultures of F. tularensis LVS were normalized to the lowest OD600 and mixed 1:1 with 2X Laemmli Buffer + 2.5% 2-Mercaptoethanol. Samples were boiled at 95 °C for 10 min. Subsequently, samples were loaded onto 4–15% polyacrylamide gels (IglC and IglA) or 12% polyacrylamide gels (LpnA) and run at 150 V for 45 min using Tris-Glycine-SDS running buffer. Proteins were transferred onto 0.45 μm nitrocellulose paper at 250 mA (100 V for 20 min) using Tris-Glycine Transfer Buffer (25 mM Tris, 192 mM Glycine, 20% (v/v) methanol). Blocking was carried out by treating the nitrocellulose membrane with PBS containing 0.02% sodium azide, 0.5% bovine serum albumin, 0.5% casein, and 100 mg/L Phenol Red for 30–60 min. Afterwards, blots were treated with primary antibodies (mouse anti-LpnA, mouse anti-IglC, and rabbit anti-IglA [both from BEI resources], 1:1000 dilution in killer filler) and incubated overnight with shaking at room temperature. Blots were then washed three times using room-temperature PBS. The washed blots were then treated with secondary antibodies (goat anti-rabbit IgG and rabbit anti-mouse, 1:2500 dilution in killer filler) incubated at room temperature for one hour. Following secondary antibody treatment, blots were washed twice with PBS followed by one wash with Tris Buffer. Blots were developed using Naphthol AS-MX phosphate (1 mg/mL) and Fast Red TR salt (2 mg/mL) dissolved in Tris Buffer. Once red bands appeared, nitrocellulose paper was washed thoroughly with tap water to prevent overdevelopment. ImageJ was used to quantify the intensity of each band.
4.12. Statistical Analyses
All statistical calculations were conducted using GraphPad Prism software.