From Plants to Protection: Cardiorenal Benefits in Non-Diabetic Chronic Kidney Disease and Heart Failure
Abstract
1. Introduction
2. Search Strategy and Compound Selection
3. Non-Diabetic Chronic Kidney Disease and Heart Failure-Biomarkers of Diagnostic and Prognostic
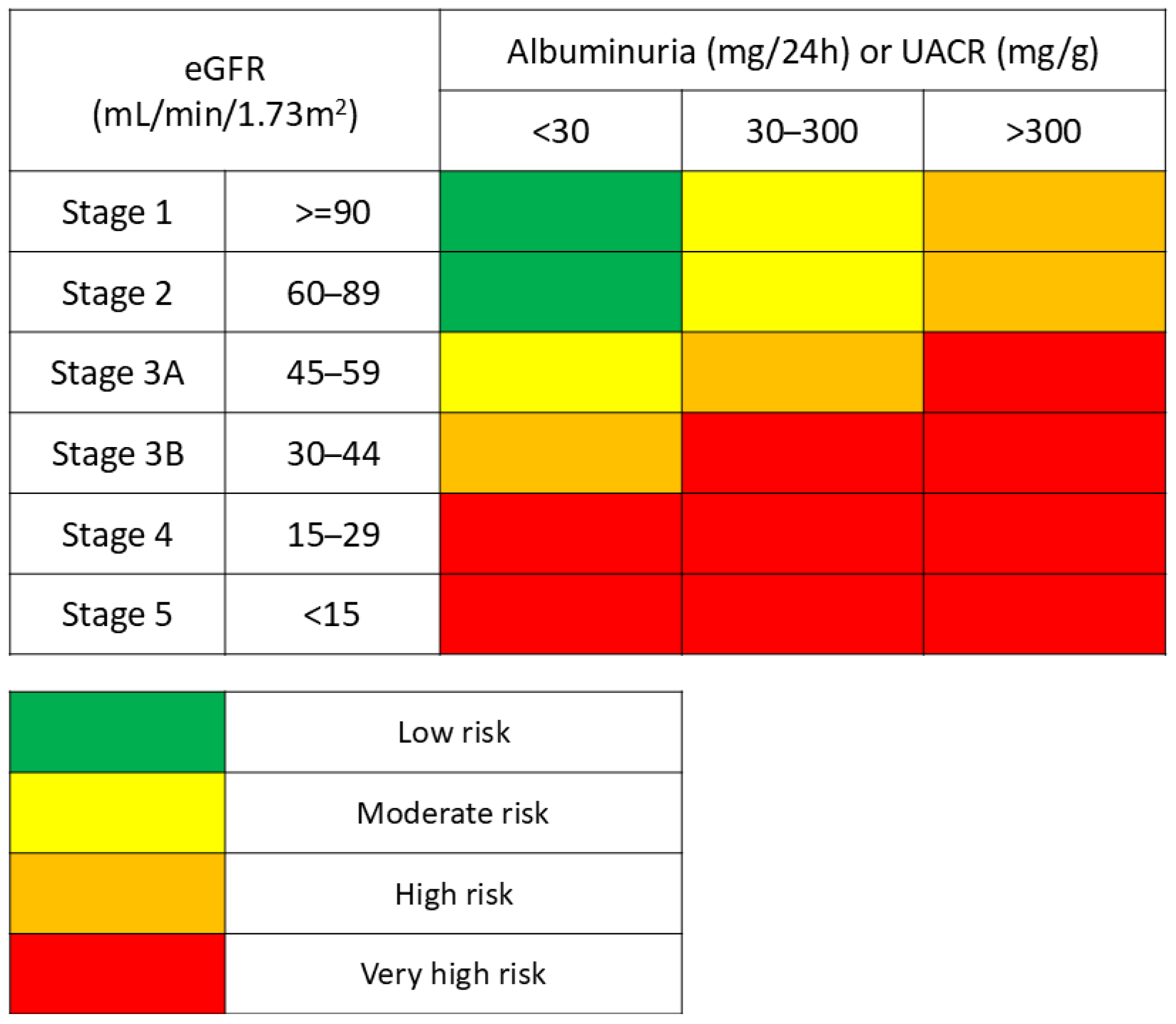
3.1. Non-Diabetic Chronic Kidney Disease
3.2. Heart Failure
3.3. Biomarkers in Non-Diabetic Chronic Kidney Disease and Heart Failure: Interplay and Interpretation
4. Therapeutic Strategies and Limitations
5. Natural Compounds
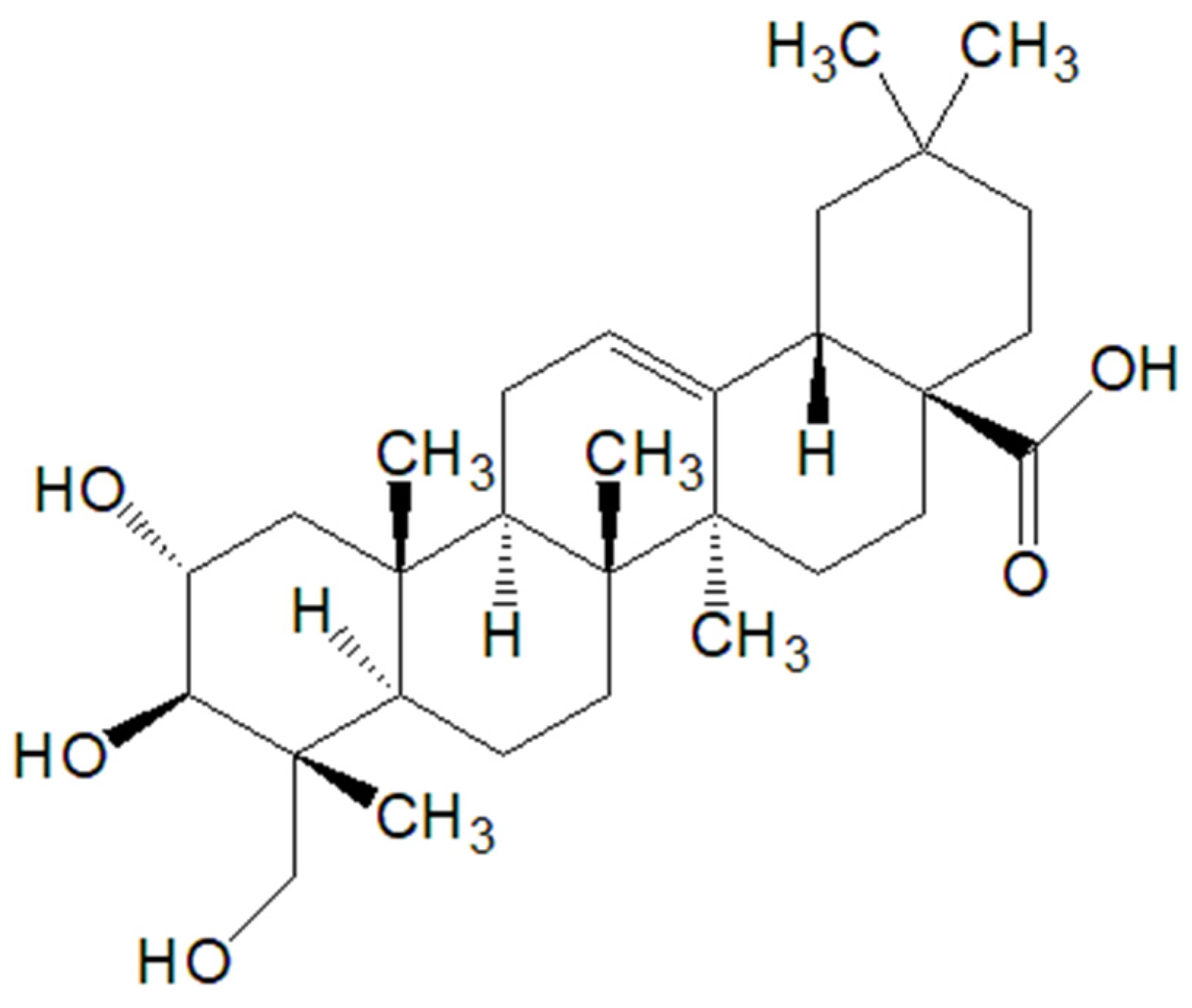
5.1. Arjunolic Acid
5.2. Kaempferol
5.3. Luteolin
5.4. Resveratrol
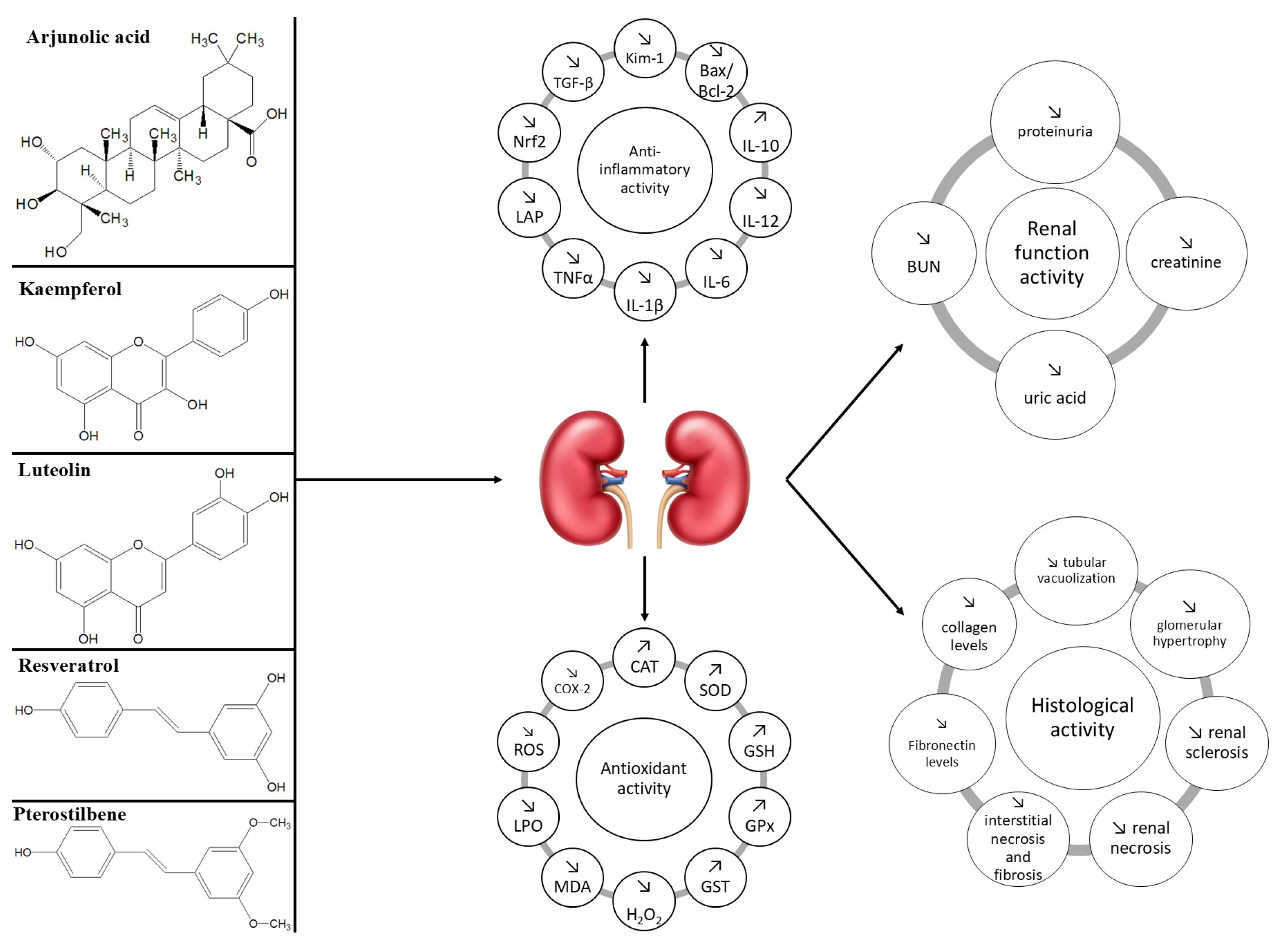
5.5. Common and Distinct Mechanisms of Action and Effects of Natural Compounds in Non-Diabetic Chronic Kidney Disease
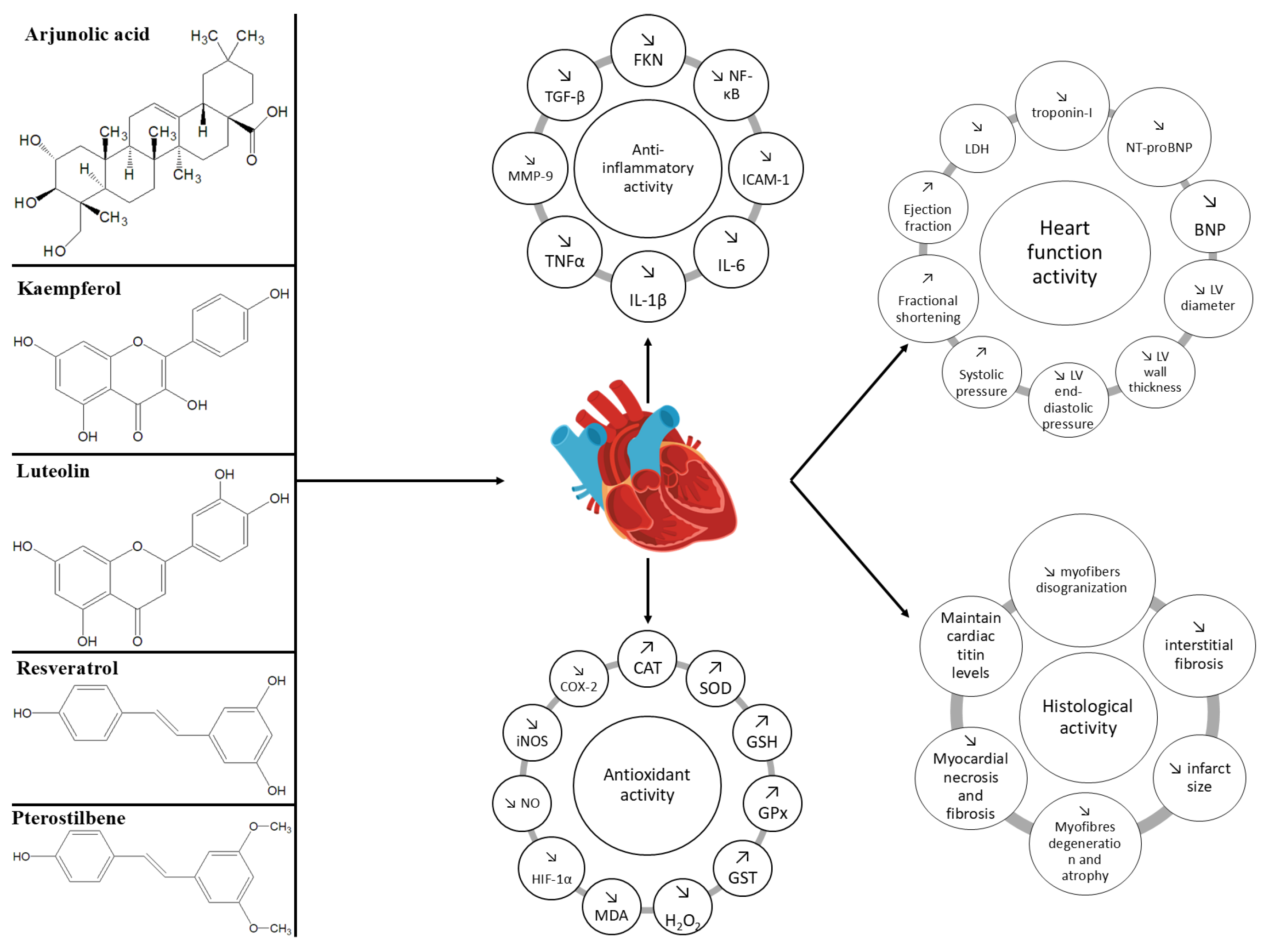
5.6. Common and Distinct Mechanisms of Action and Effects of Natural Compounds in Heart Failure
6. Knowledge Gaps and Future Directions
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.M.; Yang, C.W. Chronic Kidney Disease: Global Dimension and Perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Ikizler, T.A. Hemodialysis. N. Engl. J. Med. 2010, 363, 1833–1845. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide Access to Treatment for End-Stage Kidney Disease: A Systematic Review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Narayanan, M.; Setia, S. Chronic Kidney Disease. In The Perioperative Medicine Consult Handbook, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 66, pp. 301–305. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Shin, J.Y. Trends in the Prevalence and Management of Diabetes in Korea: 2007-2017. Epidemiol. Health 2019, 41, e2019029. [Google Scholar] [CrossRef]
- Krastev, E.; Abanos, S.; Kovachev, P.; Tcharaktchiev, D. Diabetes Prevalence and Duration Data Extracted from Outpatient Records Representative for the Bulgarian Population. Stud. Health Technol. Inform. 2023, 305, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223. [Google Scholar] [CrossRef]
- Dorans, K.S.; Mills, K.T.; Liu, Y.; He, J. Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The Global Burden of Disease Attributable to High Body Mass Index in 195 Countries and Territories, 1990–2017: An Analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17. [Google Scholar] [CrossRef]
- Li, Q.; Blume, S.W.; Huang, J.C.; Hammer, M.; Ganz, M.L. Prevalence and Healthcare Costs of Obesity-Related Comorbidities: Evidence from an Electronic Medical Records System in the United States. J. Med. Econ. 2015, 18, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.T.; Chao, C.T.; Lin, S.H. Chronic Kidney Disease: Strategies to Retard Progression. Int. J. Mol. Sci. 2021, 22, 10084. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Astor, B.C.; Lewis, J.; Hu, B.; Appel, L.J.; Lipkowitz, M.S.; Toto, R.D.; Wang, X.; Wright, J.T.; Greene, T.H. Longitudinal Progression Trajectory of GFR Among Patients With CKD. Am. J. Kidney Dis. 2012, 59, 504. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure with Reduced Ejection Fraction: A Review. JAMA—J. Am. Med. Assoc. 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; Von Haehling, S. Heart Failure and Kidney Dysfunction: Epidemiology, Mechanisms and Management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Li, J.W.H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Barnes, E.C.; Kumar, R.; Davis, R.A. The Use of Isolated Natural Products as Scaffolds for the Generation of Chemically Diverse Screening Libraries for Drug Discovery. Nat. Prod. Rep. 2016, 33, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Off J Int Soc Nephrol. 2013. Ann. Intern. Med. 2013, 158, 825–831. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic Kidney Disease and Mortality Risk: A Systematic Review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef]
- Blum, M.F.; Surapaneni, A.; Stewart, J.D.; Liao, D.; Yanosky, J.D.; Whitsel, E.A.; Power, M.C.; Grams, M.E. Particulate Matter and Albuminuria, Glomerular Filtration Rate, and Incident CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 311–319. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease–A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Sunderraj, A.; Wong, M.; Gutiérrez, O.M.; Wolf, M.; Akhabue, E.; Carnethon, M.R.; Yancy, C.W.; Isakova, T. Associations of FGF23 with 10-Year Change in EGFR and UACR and with Incident CKD in the CARDIA Cohort. Kidney360 2023, 4, E1236–E1244. [Google Scholar] [CrossRef]
- Chu, C.D.; Xia, F.; Du, Y.; Singh, R.; Tuot, D.S.; Lamprea-Montealegre, J.A.; Gualtieri, R.; Liao, N.; Kong, S.X.; Williamson, T.; et al. Estimated Prevalence and Testing for Albuminuria in US Adults at Risk for Chronic Kidney Disease. JAMA Netw. Open 2023, 6, E2326230. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Flagg, A.J. Chronic Renal Therapy. Nurs. Clin. N. Am. 2018, 53, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Rees, O.L.; Wheen, P.; Anderson, L.J. Updates in Heart Failure. Clin. Med. 2023, 23, 432. [Google Scholar] [CrossRef]
- Boorsma, E.M.; ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in Heart Failure: A Contemporary Look at Physiology, Diagnosis and Treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Bennett, J.A.; Riegel, B.; Bittner, V.; Nichols, J. Validity and Reliability of the NYHA Classes for Measuring Research Outcomes in Patients with Cardiac Disease. Hear. Lung J. Acute Crit. Care 2002, 31, 262–270. [Google Scholar] [CrossRef]
- Ghali, J.K. Subjective versus Objective Classification of NYHA Class IV. J. Card. Fail. 2010, 16, 707. [Google Scholar] [CrossRef]
- Caraballo, C.; Desai, N.R.; Mulder, H.; Alhanti, B.; Wilson, F.P.; Fiuzat, M.; Felker, G.M.; Piña, I.L.; O’Connor, C.M.; Lindenfeld, J.; et al. Clinical Implications of the New York Heart Association Classification. J. Am. Hear. Assoc. Cardiovasc. Cerebrovasc. Dis. 2019, 8. [Google Scholar] [CrossRef]
- Gilbert, E.M.; Xu, W.D. Rationales and Choices for the Treatment of Patients with NYHA Class II Heart Failure. Postgrad. Med. 2017, 129, 619–631. [Google Scholar] [CrossRef]
- Almenar, L.; Díaz, B.; Quesada, A.; Crespo, C.; Martí, B.; Mealing, S.; Linde, C.; Daubert, C. Cost-Effectiveness Analysis of Cardiac Resynchronization Therapy in Patients with NYHA I and NYHA II Heart Failure in Spain. Int. J. Technol. Assess. Health Care 2013, 29, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Zhang, R.; Wu, X.; Zhou, X. Optimal Pharmacologic Treatment of Heart Failure With Preserved and Mildly Reduced Ejection Fraction: A Meta-Analysis. JAMA Netw. Open 2022, 5, E2231963. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the Diagnosis and Management of Heart Failure. Heart Fail. Rev. 2022, 27, 625. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of SST2 in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative Stress and Inflammation in the Evolution of Heart Failure: From Pathophysiology to Therapeutic Strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Guaricci, A.I.; Sturdà, F.; Russo, R.; Basile, P.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Fazzari, F.; Bertandino, F.; Monitillo, F.; et al. Assessment and Management of Heart Failure in Patients with Chronic Kidney Disease. Heart Fail. Rev. 2024, 29, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Halimi, J.M.; Rossignol, P.; Sarafidis, P.; De Caterina, R.; Giugliano, R.; Zannad, F. From Cardiorenal Syndrome to Chronic Cardiovascular and Kidney Disorder: A Conceptual Transition. Clin. J. Am. Soc. Nephrol. 2024, 19, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Axelsson, J.; Machowska, A.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Lindström, K.; Stenvinkel, P.; Qureshi, A.R. Biomarkers of Cardiovascular Disease and Mortality Risk in Patients with Advanced CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1163–1172. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Selvin, E.; Liang, M.; Ballantyne, C.M.; Hoogeveen, R.C.; Aguilar, D.; McEvoy, J.W.; Grams, M.E.; Coresh, J. Plasma Galectin-3 Levels Are Associated with the Risk of Incident Chronic Kidney Disease. Kidney Int. 2018, 93, 252–259. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, J.; Zhang, M.; Shen, C.; Jiang, Z.; Zhang, T.; Gao, F. The Diagnostic Accuracy of N-Terminal Pro-B-Type Natriuretic Peptide and Soluble ST2 for Heart Failure in Chronic Kidney Disease Patients: A Comparative Analysis. Med. Sci. Monit. 2023, 29, e940641. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Barasch, J. Creatinine and Cystatin C: Not the Troponin of the Kidney. Circulation 2018, 137, 2029. [Google Scholar] [CrossRef]
- Banerjee, D.; Rosano, G.; Herzog, C.A. Management of Heart Failure Patient with CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 1131. [Google Scholar] [CrossRef]
- Savira, F.; Magaye, R.; Liew, D.; Reid, C.; Kelly, D.J.; Kompa, A.R.; Sangaralingham, S.J.; Burnett, J.C.; Kaye, D.; Wang, B.H. Cardiorenal Syndrome: Multi-organ Dysfunction Involving the Heart, Kidney and Vasculature. Br. J. Pharmacol. 2020, 177, 2906. [Google Scholar] [CrossRef]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Masarone, D.; Martucci, M.L.; Errigo, V.; Pacileo, G. The Use of β-Blockers in Heart Failure with Reduced Ejection Fraction. J. Cardiovasc. Dev. Dis. 2021, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.V.; Roberts, M.A.; Hawley, C.M.; Cass, A.; Garg, A.X.; Krum, H.; Tonkin, A.; Perkovic, V. Effects of Beta-Adrenergic Antagonists in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2011, 58, 1152–1161. [Google Scholar] [CrossRef]
- The Consensus Trial Study Group. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N. Engl. J. Med. 1987, 316, 1429–1435. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Zhang, W.; Xing, Y.; Guo, Y.; Wang, F.; Jia, J.; Yan, T.; Liu, Y.; Lin, S. ACE Inhibitor Benefit to Kidney and Cardiovascular Outcomes for Patients with Non-Dialysis Chronic Kidney Disease Stages 3–5: A Network Meta-Analysis of Randomised Clinical Trials. Drugs 2020, 80, 797. [Google Scholar] [CrossRef]
- Heran, B.S.; Musini, V.M.; Bassett, K.; Taylor, R.S.; Wright, J.M. Angiotensin Receptor Blockers for Heart Failure. Cochrane Database Syst. Rev. 2012, 2012. [Google Scholar] [CrossRef]
- Kobori, H.; Mori, H.; Masaki, T.; Nishiyama, A. Angiotensin II Blockade and Renal Protection. Curr. Pharm. Des. 2013, 19, 3033. [Google Scholar] [CrossRef]
- Tsai, Y.N.; Cheng, W.H.; Chang, Y.T.; Hsiao, Y.W.; Chang, T.Y.; Hsieh, Y.C.; Lin, Y.J.; Lo, L.W.; Chao, T.F.; Kuo, M.J.; et al. Mechanism of Angiotensin Receptor-Neprilysin Inhibitor in Suppression of Ventricular Arrhythmia. J. Cardiol. 2021, 78, 275–284. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 132–133. [Google Scholar] [CrossRef]
- Vizzardi, E.; Regazzoni, V.; Caretta, G.; Gavazzoni, M.; Sciatti, E.; Bonadei, I.; Trichaki, E.; Raddino, R.; Metra, M. Mineralocorticoid Receptor Antagonist in Heart Failure: Past, Present and Future Perspectives. Int. J. Cardiol. Hear. Vessel. 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and Kidney Outcomes with Finerenone in Patients with Type 2 Diabetes and Chronic Kidney Disease: The FIDELITY Pooled Analysis. Eur. Heart J. 2022, 43, 474–484A. [Google Scholar] [CrossRef]
- Magdy, J.S.; McVeigh, J.; Indraratna, P. Diuretics in the Management of Chronic Heart Failure: When and How. Aust. Prescr. 2022, 45, 200. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Navaneethan, S.D.; Virani, S.S.; Gregg, L.P. Revisiting Diuretic Choice in Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2022, 31, 406–413. [Google Scholar] [CrossRef]
- Talha, K.M.; Anker, S.D.; Butler, J. SGLT-2 Inhibitors in Heart Failure: A Review of Current Evidence. Int. J. Hear. Fail. 2023, 5, 82. [Google Scholar] [CrossRef]
- Thomas, M.C.; Neuen, B.L.; Twigg, S.M.; Cooper, M.E.; Badve, S.V. SGLT2 Inhibitors for Patients with Type 2 Diabetes and CKD: A Narrative Review. Endocr. Connect. 2023, 12, e230005. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; Hanssen, H.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) Wi. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, H.; Yang, X.; Zheng, S.; Li, Y.; Liu, S.; Xu, X. Traditional Chinese Medicine and Renal Regeneration: Experimental Evidence and Future Perspectives. Chin. Med. 2024, 19, S13020–S13024. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Prathiviraj, R.; Sivakumar, T.R.; Ma, Y.; Sekar, S. GC-MS and Network Pharmacology Analysis of the Ayurvedic Fermented Medicine, Chandanasava, Against Chronic Kidney and Cardiovascular Diseases. Appl. Biochem. Biotechnol. 2023, 195, 2803–2828. [Google Scholar] [CrossRef] [PubMed]
- Tekiner, H. Aretaeus of Cappadocia and His Treatises on Diseases. Turk. Neurosurg. 2015, 25, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Sumitra, M.; Manikandan, P.; Kumar, D.A.; Arutselvan, N.; Balakrishna, K.; Manohar, B.M.; Puvanakrishnan, R. Experimental Myocardial Necrosis in Rats: Role of Arjunolic Acid on Platelet Aggregation, Coagulation and Antioxidant Status. Mol. Cell. Biochem. 2001, 224, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Al-Gayyar, M.M.H.; Al Youssef, A.; Sherif, I.O.; Shams, M.E.E.; Abbas, A. Protective Effects of Arjunolic Acid against Cardiac Toxicity Induced by Oral Sodium Nitrite: Effects on Cytokine Balance and Apoptosis. Life Sci. 2014, 111, 18–26. [Google Scholar] [CrossRef]
- Bansal, T.; Chatterjee, E.; Singh, J.; Ray, A.; Kundu, B.; Thankamani, V.; Sengupta, S.; Sarkar, S. Arjunolic Acid, a Peroxisome Proliferator-Activated Receptor Agonist, Regresses Cardiac Fibrosis by Inhibiting Non-Canonical TGF- Signaling. J. Biol. Chem. 2017, 292, 16440–16462. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Eladl, M.A.; Al-Gayyar, M.M.H. Renal Protective Effects of Arjunolic Acid in a Cisplatin-Induced Nephrotoxicity Model. Cytokine 2016, 77, 26–34. [Google Scholar] [CrossRef]
- Sherif, I.O. Amelioration of Cisplatin-Induced Nephrotoxicity in Rats by Triterpenoid Saponin of Terminalia Arjuna. Clin. Exp. Nephrol. 2015, 19, 591–597. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Effect of Kaempferol and Its Glycoside Derivatives on Antioxidant Status of HL-60 Cells Treated with Etoposide. Molecules 2022, 27, 333. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Khafaji, S.S. Antioxidant, Anti-Inflammatory, and Anti-Reprotoxic Effects of Kaempferol and Vitamin E on Lead Acetate-Induced Testicular Toxicity in Male Rats. Open Vet. J. 2023, 13, 1683–1695. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Wang, Y.; Geng, J.; Han, S. The Protective Effect of Kaempferol on Heart via the Regulation of Nrf2, NF-Κβ, and PI3K/Akt/GSK-3β Signaling Pathways in Isoproterenol-Induced Heart Failure in Diabetic Rats. Drug Dev. Res. 2019, 80, 294–309. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, W.; Sun, X.; Wang, Y.; Zhou, M. Kaempferol Ameliorates Cisplatin Induced Nephrotoxicity by Modulating Oxidative Stress, Inflammation and Apoptosis via ERK and NF-ΚB Pathways. AMB Express 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Quan, D.; Chen, K.; Kang, L.; Yang, D.; Wu, H.; Yan, M.; Wu, S.; Lv, L.; Zhang, G. Kaempferol Inhibits Renal Fibrosis by Suppression of the Sonic Hedgehog Signaling Pathway. Phytomedicine 2023, 108, 154246. [Google Scholar] [CrossRef]
- Du, Y.; Han, J.; Zhang, H.; Xu, J.; Jiang, L.; Ge, W. Kaempferol Prevents against Ang II-Induced Cardiac Remodeling through Attenuating Ang II-Induced Inflammation and Oxidative Stress. J. Cardiovasc. Pharmacol. 2019, 74, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Sun, X.; Liu, X.; Hutterer, G.; Pummer, K.; Hager, B.; Ye, Z.; Chen, Z. Kaempferol Alleviates Calcium Oxalate Crystal-Induced Renal Injury and Crystal Deposition via Regulation of the AR/NOX2 Signaling Pathway. Phytomedicine 2021, 86, 153555. [Google Scholar] [CrossRef]
- Chen, D.; Shen, F.; Liu, J.; Tang, H.; Teng, X.; Yang, F.; Liu, H. Luteolin Enhanced Antioxidant Capability and Induced Pyroptosis through NF-ΚB/NLRP3/Caspase-1 in Splenic Lymphocytes Exposure to Ammonia. Sci. Total Environ. 2024, 919, 70699. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.C.; Lin, J.H.; Lee, W.S.; Liu, C.H.; Lin, T.Y.; Yang, K.T. Baicalein and Luteolin Inhibit Ischemia/Reperfusion-Induced Ferroptosis in Rat Cardiomyocytes. Int. J. Cardiol. 2023, 375, 74–86. [Google Scholar] [CrossRef]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a Modulator of Skin Aging and Inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an Anti-Inflammatory and Neuroprotective Agent: A Brief Review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective Effects of Flavone Luteolin in Neuroinflammation and Neurotrauma. Biofactors. 2021, 47, 190–197. [Google Scholar] [CrossRef]
- Hu, W.; Xu, T.; Wu, P.; Pan, D.; Chen, J.; Chen, J.; Zhang, B.; Zhu, H.; Li, D. Luteolin Improves Cardiac Dysfunction in Heart Failure Rats by Regulating Sarcoplasmic Reticulum Ca2+-ATPase 2a. Sci. Rep. 2017, 7, 41017. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Pugel, E.P.; Zagorac, G.B.; Mahmutefendić, H.; Škoda, M. Luteolin Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Platinum Accumulation, Inflammation and Apoptosis in the Kidney. Toxicology 2013, 310, 115–123. [Google Scholar] [CrossRef]
- Arslan, B.Y.; Arslan, F.; Erkalp, K.; Alagöl, A.; Sevdi, M.S.; Yıldız, G.; Küçük, S.H.; Altınay, S. Luteolin Ameliorates Colistin-Induced Nephrotoxicity in the Rat Models. Ren. Fail. 2016, 38, 1735–1740. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Lu, X.; Bao, P.; Zhao, X. Luteolin Ameliorates Cardiac Failure in Type I Diabetic Cardiomyopathy. J. Diabetes Complicat. 2012, 26, 259–265. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Akinrinde, A.S.; Adebiyi, O.E.; Jarikre, T.A.; Omobowale, T.O.; Ola-Davies, O.E.; Saba, A.B.; Emikpe, B.O.; Adedapo, A.A. Luteolin Supplementation Ameliorates Cobalt-Induced Oxidative Stress and Inflammation by Suppressing NF-KB/Kim-1 Signaling in the Heart and Kidney of Rats. Environ. Toxicol. Pharmacol. 2020, 80, 103488. [Google Scholar] [CrossRef]
- Kalbolandi, S.M.; Gorji, A.V.; Babaahmadi-Rezaei, H.; Mansouri, E. Luteolin Confers Renoprotection against Ischemia–Reperfusion Injury via Involving Nrf2 Pathway and Regulating MiR320. Mol. Biol. Rep. 2019, 46, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Alekhya Sita, G.J.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Rama Sireesha, K.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A.; et al. Protective Role of Luteolin against Bisphenol A-Induced Renal Toxicity through Suppressing Oxidative Stress, Inflammation, and Upregulating Nrf2/ARE/HO-1 Pathway. IUBMB Life 2019, 71, 1041–1047. [Google Scholar] [CrossRef]
- Owumi, S.E.; Lewu, D.O.; Arunsi, U.O.; Oyelere, A.K. Luteolin Attenuates Doxorubicin-Induced Derangements of Liver and Kidney by Reducing Oxidative and Inflammatory Stress to Suppress Apoptosis. Hum. Exp. Toxicol. 2021, 40, 1656–1672. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of Grape Pomace Antioxidant Extract on Oxidative Stress and Inflammation in Diet Induced Obese Mice. J. Agric. Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols From Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 1522. [Google Scholar] [CrossRef]
- Shen, M.; Wu, R.X.; Zhao, L.; Li, J.; Guo, H.T.; Fan, R.; Cui, Y.; Wang, Y.M.; Yue, S.Q.; Pei, J.M. Resveratrol Attenuates Ischemia/Reperfusion Injury in Neonatal Cardiomyocytes and Its Underlying Mechanism. PLoS ONE 2012, 7, e51223. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.O.; Rizzetto, F.; Grimmer, G.H.; Ribeiro-Alves, M.; Daleprane, J.B.; Carraro-Eduardo, J.C.; Mafra, D. Effects of Resveratrol Supplementation in Nrf2 and NF-ΚB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J. Ren. Nutr. 2016, 26, 401–406. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Justo, M.L.; Claro, C.M.; Vila, E.; Parrado, J.; Herrera, M.D.; Alvarez De Sotomayor, M. Endothelium-Dependent Vasodilator and Antioxidant Properties of a Novel Enzymatic Extract of Grape Pomace from Wine Industrial Waste. Food Chem. 2012, 135, 1044–1051. [Google Scholar] [CrossRef]
- Xuan, W.; Wu, B.; Chen, C.; Chen, B.; Zhang, W.; Xu, D.; Bin, J.; Liao, Y. Resveratrol Improves Myocardial Ischemia and Ischemic Heart Failure in Mice by Antagonizing the Detrimental Effects of Fractalkine. Crit. Care Med. 2012, 40, 3026–3033. [Google Scholar] [CrossRef]
- Raj, P.; Sayfee, K.; Parikh, M.; Yu, L.; Wigle, J.; Netticadan, T.; Zieroth, S. Comparative and Combinatorial Effects of Resveratrol and Sacubitril/Valsartan alongside Valsartan on Cardiac Remodeling and Dysfunction in Mi-Induced Rats. Molecules 2021, 26, 5006. [Google Scholar] [CrossRef]
- Gupta, P.K.; DiPette, D.J.; Supowit, S.C. Protective Effect of Resveratrol against Pressure Overload-Induced Heart Failure. Food Sci. Nutr. 2014, 2, 218–229. [Google Scholar] [CrossRef]
- Ma, E.; Wu, C.; Chen, J.; Wo, D.; Ren, D.N.; Yan, H.; Peng, L.; Zhu, W. Resveratrol Prevents Ang II-Induced Cardiac Hypertrophy by Inhibition of NF-ΚB Signaling. Biomed. Pharmacother. 2023, 165, 115275. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Tang, B.; Kang, P.; Zhang, H.; Shi, C. Resveratrol Inhibits Ferroptosis and Decelerates Heart Failure Progression via Sirt1/P53 Pathway Activation. J. Cell. Mol. Med. 2023, 27, 3075–3089. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Chopra, K. Possible Role of Nitric Oxide in the Protective Effect of Resveratrol in 5/6th Nephrectomized Rats. J. Surg. Res. 2006, 133, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Chen, Y.Y.; Hsiao, C.M.; Pan, M.H.; Wang, B.J.; Chen, Y.C.; Ho, C.T.; Huang, K.C.; Chen, R.J. Induction of Autophagy by Pterostilbene Contributes to the Prevention of Renal Fibrosis via Attenuating NLRP3 Inflammasome Activation and Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2020, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Song, Z.; Chen, Y.; Li, S.; Zhang, Y.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Resveratrol Protects Against Renal Damage via Attenuation of Inflammation and Oxidative Stress in High-Fat-Diet-Induced Obese Mice. Inflammation 2019, 42, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tian, S.; Han, J.; Xiong, P. Resveratrol as a Therapeutic Agent for Renal Fibrosis Induced by Unilateral Ureteral Obstruction. Ren. Fail. 2014, 36, 285–291. [Google Scholar] [CrossRef]
- Li, P.; Song, X.; Zhang, D.; Guo, N.; Wu, C.; Chen, K.; Liu, Y.; Yuan, L.; Chen, X.; Huang, X. Resveratrol Improves Left Ventricular Remodeling in Chronic Kidney Disease via Sirt1-Mediated Regulation of FoxO1 Activity and MnSOD Expression. BioFactors 2020, 46, 168–179. [Google Scholar] [CrossRef]
- Sung, M.M.; Byrne, N.J.; Robertson, I.M.; Kim, T.T.; Samokhvalov, V.; Levasseur, J.; Soltys, C.L.; Fung, D.; Tyreman, N.; Denou, E.; et al. Resveratrol Improves Exercise Performance and Skeletal Muscle Oxidative Capacity in Heart Failure. Am. J. Physiol.–Hear. Circ. Physiol. 2017, 312, H842–H856. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, Z.; Zhang, J.; Li, X.; Yang, W.; Wei, Y.; Guo, X. Pterostilbene Attenuates Heart Failure by Inhibiting Myocardial Ferroptosis through SIRT1/GSK-3β/GPX4 Signaling Pathway. Heliyon 2024, 10, e24562. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Takahara, S.; Maayah, Z.H.; Parajuli, N.; Byrne, N.J.; Shoieb, S.M.; Soltys, C.L.M.; Beker, D.L.; Masson, G.; El-Kadi, A.O.S.; et al. Resveratrol Improves Cardiac Function and Exercise Performance in MI-Induced Heart Failure through the Inhibition of Cardiotoxic HETE Metabolites. J. Mol. Cell. Cardiol. 2018, 125, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Das, S.K.; Levasseur, J.; Byrne, N.J.; Fung, D.; Kim, T.T.; Masson, G.; Boisvenue, J.; Soltys, C.L.; Oudit, G.Y.; et al. Resveratrol Treatment of Mice with Pressure-Overloadinduced Heart Failure Improves Diastolic Function and Cardiac Energy Metabolism. Circ. Hear. Fail. 2015, 8, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ahmet, I.; Tae, H.J.; Lakatta, E.G.; Talan, M. Long-Term Low Dose Dietary Resveratrol Supplement Reduces Cardiovascular Structural and Functional Deterioration in Chronic Heart Failure in Rats. Can. J. Physiol. Pharmacol. 2016, 95, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, M.; Chen, J.; Yang, Z.; Sun, J.; Wang, Z.; Huang, X.; Yuan, T.; Shen, X.; Xian, S. Resveratrol Ameliorates Pressure Overload-Induced Cardiac Dysfunction and Attenuates Autophagy in Rats. J. Cardiovasc. Pharmacol. 2015, 66, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.S.; Wang, Z.B.; Ye, Z.; Lei, J.P.; Li, L.; Su, D.F.; Zheng, X. Resveratrol, an Activator of SIRT1, Upregulates AMPK and Improves Cardiac Function in Heart Failure. Genet. Mol. Res. 2014, 13, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Lu, M.; Han, Y.; Zhou, H.; Liu, W.; Li, L.; Jin, R. Resveratrol Improves Mitochondrial Function in the Remnant Kidney from 5/6 Nephrectomized Rats. Acta Histochem. 2017, 119, 392–399. [Google Scholar] [CrossRef]
- Basta, M.; Saleh, S.R.; Aly, R.G.; Dief, A.E. Resveratrol Ameliorates the Behavioural and Molecular Changes in Rats Exposed to Uninephrectomy: Role of Hippocampal SIRT1, BDNF and AChE. J. Physiol. Biochem. 2023, 79, 273–285. [Google Scholar] [CrossRef] [PubMed]
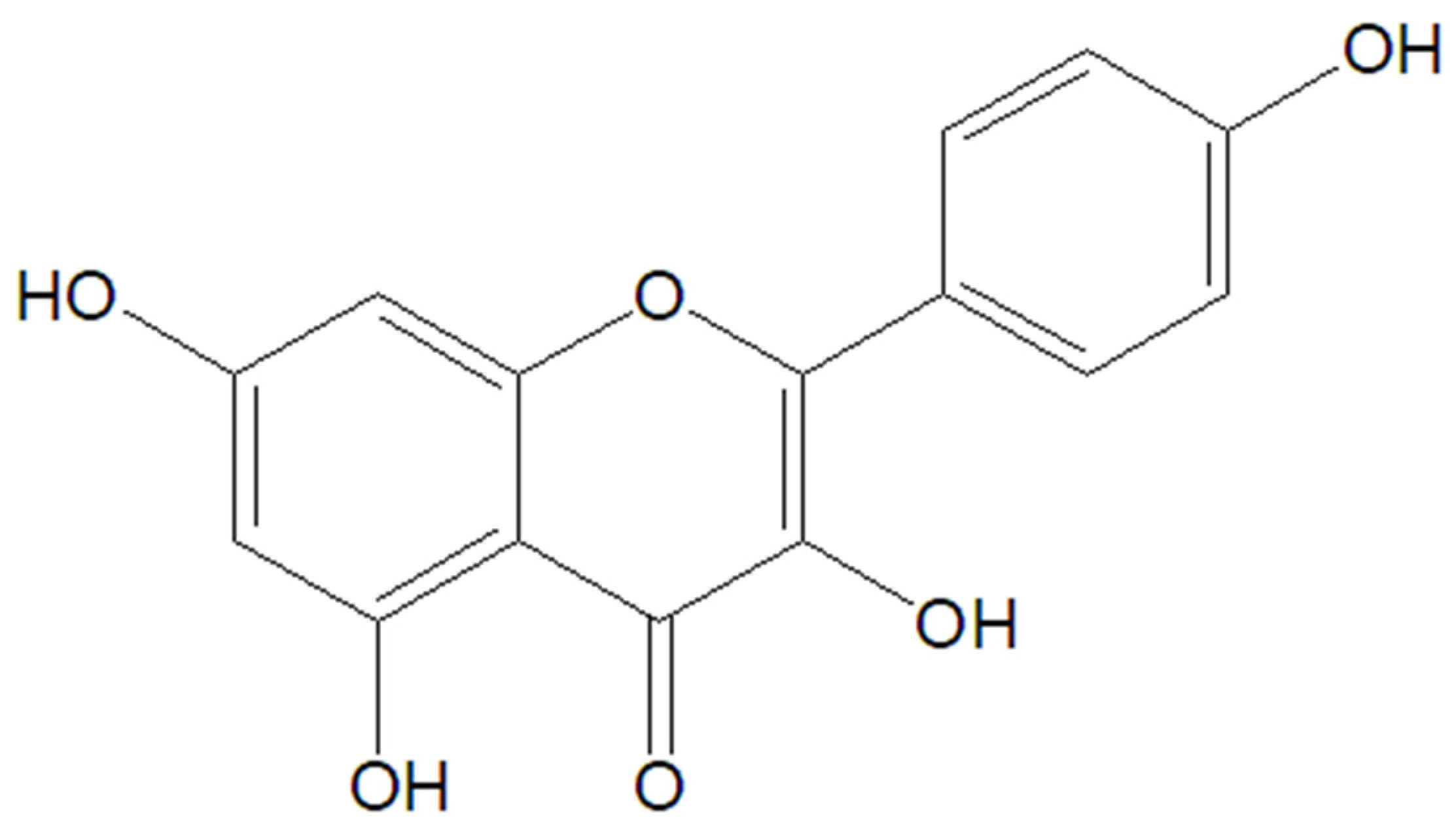
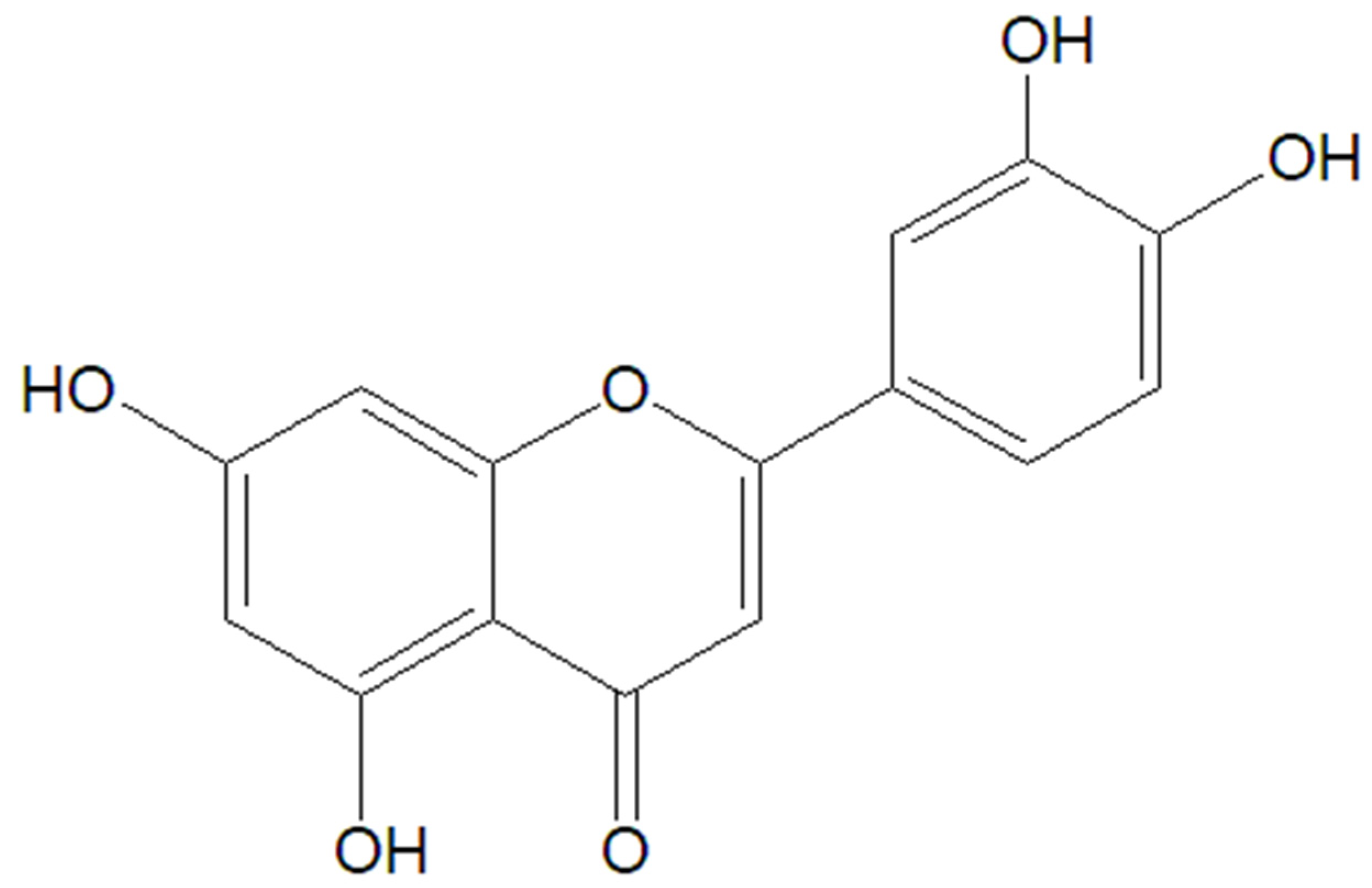
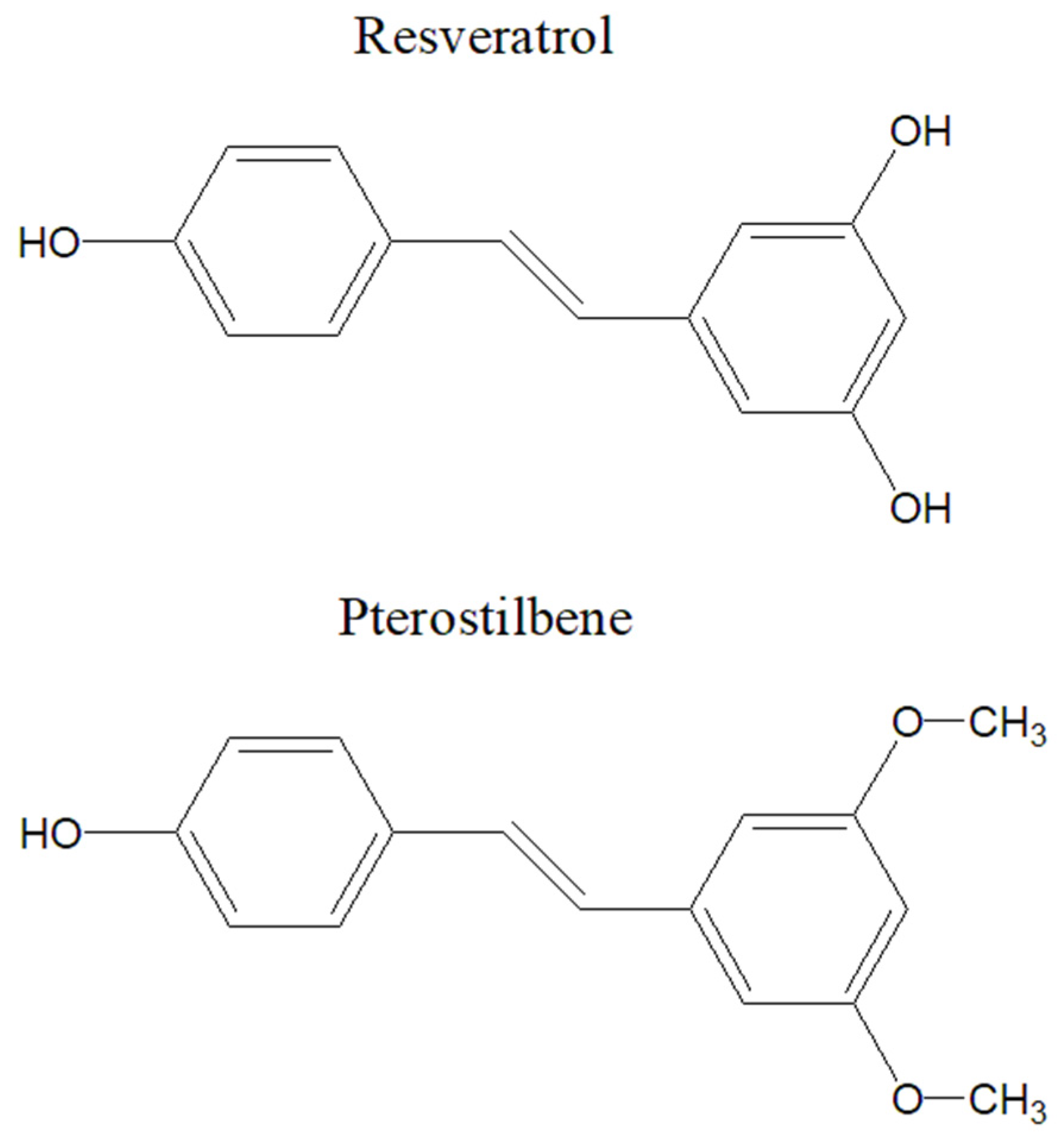
| Therapeutic Class | Representants | Mechanism of Action | Therapeutic Outcomes | References |
|---|---|---|---|---|
| Beta Blockers | Bisoprolol Carvedilol Metoprolol | Beta-adrenergic blocking agents | CV *: Improve morbidity and mortality in chronic HFrEF by decreasing the heart rate and the contractility | [63] |
| R **: Ameliorate renal function by reducing systemic vascular resistance–only in patients with heart failure | [64] | |||
| Inhibitors of Angiotensin Converting Enzyme (ACEI) | Lisinopril Captopril Perindopril Ramipril | Decreases the formation of angiotensin II | CV: Improve morbidity and mortality in chronic HFrEF by decreasing left ventricular afterload, leading to an increase in cardiac output and a decrease in heart-filling pressure | [65] |
| R: Ameliorate renal function by reducing systemic vascular resistance | [66] | |||
| Angiotensin receptor blocker (ARB) | Candesartan Losartan Olmesartan Valsartan Irbesartan | Displaces angiotensin II from angiotensin I receptor | CV: Improve morbidity and mortality in chronic HFrEF by decreasing left ventricular afterload, leading to an increase in cardiac output and a decrease in heart-filling pressure | [67] |
| R: Ameliorate renal function by reducing systemic vascular resistance | [68] | |||
| Angiotensin receptor blocker + Neprilysin inhibitor (ARNI) | Valsartan + Sacubitril | Displaces angiotensin II from angiotensin I receptor + prevents the breakdown of natriuretic peptides | CV: Improve morbidity and mortality in chronic HFrEF by preventing and reversing electrical and structural remodeling that appears in heart failure | [69] |
| R: Prevent the progression of chronic kidney disease and lead to a need for lower doses of diuretic drugs | [70] | |||
| Mineralocorticoid receptor antagonist (MRA) | Spironolactone Eplerenone | Blocks mineralocorticoid receptors from the activation by aldosterone and 11-deoxycorticosterone | CV: Improve morbidity and mortality in chronic HFrEF by modulating the RAA system, leading to a reduction in ventricular remodeling | [71] |
| Non-steroidal mineralocorticoid receptor antagonist (nsMRA) | Finerenone | Selectively blocks mineralocorticoid receptors * differs from steroidal MRAs by a distinct cofactor-binding profile and lower risk of hyperkalaemia | CV: Improve morbidity in patients with HFrEF and diabetes or CKD by reducing hospitalization for heart failure through modulation of fibrosis and inflammation | [72] |
| R: Ameliorate renal function by slowing the decline in eGFR | ||||
| Diuretics | Furosemide Bumetanide Indapamide | Blocks sodium reabsorption, which leads to an increase in urinary water and sodium losses | CV: Improve the morbidity in chronic HFrEF, HFmrEF, and HFpEF by improving the volume overload, especially in a decompensated state | [73] |
| R: Ameliorate renal function by maintaining an effective diuresis | [74] | |||
| SGLT2 inhibitors | Dapagliflozin Empagliflozin | Blocks the activity of the sodium-glucose cotransporter 2 in the proximal renal tubules, thereby lowering the renal threshold for glucose | CV: Improve morbidity and mortality in chronic HFrEF, HFmrEF, and HFpEF by increasing natriuresis, promoting ketone production (alternative source of energy for cardiomyocytes), and blocking RAA system | [75] |
| R: Prevent the progression of diabetic chronic kidney disease by better glycemic control | [76] |
| Different Models of Heart Failure | ||||
|---|---|---|---|---|
| Dosage of Arjunolic Acid | Model | Anti-inflammatory and Antioxidant Effects | Beneficial Heart Effects | Reference |
| Arjunolic acid (10 mg/kg/ alternate day–intraperitoneal from 6th to 14th day) | 21 male Wistar rats with ligated right renal artery-induced cardiac hypertrophy | - down-regulated TGF-β activity | - mitigated collagen-1 and collagen-3 expression - improved cardiac function - up-regulated PPARα expression | [84] |
| Different Models of Non-Diabetic Chronic Kidney Disease | ||||
| Dosage of arajunolic acid | Model | Anti-inflammatory and antioxidant effects | Beneficial kidney effects | Reference |
| Arajunolic acid (100 or 250 mg/kg–oral on 1st, 4th, 7th day) | 50 male Sprague Dawley rats with cisplatin-induced nephropathy | - reduced TGF-β, MDA, and LAP levels in renal homogenate - reduced NADPH oxidase activity in renal homogenate - reduced NF-κB, TNFα, IL-1β, caspase-8 and caspase-9 levels in renal homogenate | - increased survival rate - significantly reduced plasma creatinine and urea levels - histological: mitigated dilatation of Bowman’s space, shrinkage of glomerular tufts | [85] |
| Arajunolic acid (20 mg/kg/day–oral for 10 days) | 30 male Sprague-Dawley rats with cisplatin-induced nephropathy | - significantly reduced MDA and NO levels in renal homogenate - significantly increased GSH activity in renal homogenate - down-regulated TGF-β, Kim-1, and NF-κB expression in kidney tissue - up-regulated Bcl-2 expression in kidney tissue | - significantly reduced serum creatinine and BUN levels - histological: reduced tubular cell necrosis and degeneration | [86] |
| Different Models of Heart Failure | ||||
|---|---|---|---|---|
| Dosage of Kaempferol | Model | Anti-inflammatory and Antioxidant Effects | Beneficial Heart Effects | Reference |
| Kaempferol (10/20 mg/kg/day–oral for 42 days) | 60 male Wistar rats with isoproterenol-induced heart failure | - decreased MDA levels - increased CAT, GPx, SOD, GR, and GST levels - significantly decreased IL-6, IL-1β, TNF-α, and NF-κB levels | - significantly reduced LDH, BNP, and troponin-I levels - maintained systolic blood pressure - significantly increased Nrf-2, γ-GCS and HO-1 mRNA expression - significantly decreased Keap1 expression | [91] |
| Kaempferol (10 mg/kg/alternative day–oral for 28 days) | Male C57BL/6 rats with Angiotensin-II-induced heart failure | - | - mitigated decreases in ejection fraction and fractional shortening induced by Angiotensin-II - mitigated increases in diastolic dysfunction - reduced NT-proBNP levels - histological: reduced numbers of deranged cellular structures, disorganized myofibers; maintained cardiac titin levels | [94] |
| Different Models of Non-Diabetic Chronic Kidney Disease | ||||
| Dosage of Kaempferol | Model | Anti-inflammatory and antioxidant effects | Beneficial kidney effects | Reference |
| Kaempferol (100 or 200 mg/kg/day–oral for 14 days) | 30 male Balb/C rats with cisplatin-induced nephropathy | - histological: decreased accumulation of inflammatory cells - both doses increased GR, SOD, and GST levels; the 200mg dose increased CAT, GSH, and NQO1 levels - decreased TBARS and iNOS levels - the 200 mg dose increased Nrf2 and HO-1 expression in renal tissue - down-regulated Bax/Bcl-2 and TP53 expression in renal homogenate - significantly reduced TNF-α and IL-12 levels in renal tissue - down-regulated JNK, p38, and ERK1/2 expression | - reduced serum creatinine and BUN levels - histological: decreased renal injuries | [92] |
| Kaempferol (25 or 50 mg/kg/day–oral for 10 days) | 40 male C57BL/6 rats with calcium oxalate crystal-induced nephropathy | - down-regulated iNOS, NOX2, and NF-κB expression - decreased IL-6, IL-1β, and TNF-α levels - increased IL-4, IL-10, and Arg1 levels - reduced ROS, MDA, and H2O2 levels - increased SOD and GSH levels | - significantly reduced serum creatinine and BUN levels - histological: reduced formation of calcium oxalate crystal deposits in kidneys; reduced tubular damage | [95] |
| Kaempferol (10, 20, or 40 mg/kg/day–oral for 84 days) | 25 male rats with spontaneously hypertensive-induced chronic kidney disease + 5 Wistar-Kyoto rats | - reduced MCP-1, TNF-α, and IL-1β levels - histological: reduced accumulation of inflammatory cells - reduced α-SMA levels in renal homogenate | - maintained blood pressure - reduced serum BUN and creatinine levels - reduced mALB level - down-regulation of TGF-β1, collagen I, and collagen III expression - histological: reduced tubular dilatation and atrophy | [93] |
| Different Models of Heart Failure | ||||
|---|---|---|---|---|
| Dosage of Luteolin | Model | Anti-Inflammatory and Antioxidant Effects | Beneficial Heart Effects | Reference |
| Luteolin (200 mg/kg/day–oral for 56 days) | 40 male Sprague-Dawley rats with streptozotocin-induced diabetic cardiomyopathy | - | - increased left ventricular systolic pressure and left ventricular developed pressure - decreased left ventricular end-diastolic pressure - increased heart rate | [105] |
| Luteolin (10 μg/kg/day–intraperitoneal for 14 days) | 91 male Sprague-Dawley rats with pressure overload via abdominal aortic constriction-induced heart failure | - | - up-regulated SERCA2a and Akt expression - decreased left ventricular internal diameters of end-systole and end-diastole - increased ejection fraction and fractional shortening - histological: reduced myocardium fibrosis and apoptosis | [102] |
| Luteolin (100 mg/kg/day–oral for 8 days) | 32 male Wistar rats with cobalt chloride-induced cardiopathy | - reduced MDA, NO, and H2O2 levels - reduced MPO activity - increased GSH, GPx, and GST levels - down-regulated NF-κB expression in heart tissue | - histological: mitigated degeneration and atrophy of the myofibres | [106] |
| Different Models of Non-Diabetic Chronic Kidney Disease | ||||
| Dosage of Luteolin | Model | Anti-inflammatory and antioxidant effects | Beneficial kidney effects | Reference |
| Luteolin (50 mg/kg/day–oral for 3 days) | 30 rats with ischemia–reperfusion-induced nephropathy | - significantly increased CAT, SOT, and GPx - reduced MDA levels in renal tissue - down-regulated miR320 and Nrf2 expression in renal tissue | - significantly reduced serum creatinine and BUN levels - histological: significantly mitigated renal injury | [107] |
| Luteolin (100 mg/kg/day–oral for 8 days) | 32 male Wistar rats with cobalt chloride-induced nephropathy | - reduced MDA and H2O2 levels - increased GSH, GPx, and GST levels - down-regulated NF-κB expression in kidney tissue | - histological: mitigated tubular atrophy and nephron necrosis | [106] |
| Luteolin (10 mg/kg/day–intraperitoneal for 3 days) | 30 male BALB/cN rats with cisplatin-induced nephropathy | - significantly reduced 3-NT and 4HNE levels in renal tissue - increased GSH level - down-regulated NF-κB, COX-2, and TNF-α expression in kidney tissue | - reduced serum creatinine and BUN levels - histological: mitigated tubular dilatation and tubular necrosis; down-regulated caspase-3 and p53 expression | [103] |
| Luteolin (10 mg/kg/day–intraperitoneal for 7 days) | 28 male Wistar rats with cisplatin-induced nephropathy | - | - reduced serum creatinine level - histological: mitigated renal injury | [104] |
| Luteolin (100 or 200 mg/kg/day–oral for 28 days) | 28 male Wistar rats with bisphenol A-induced nephropathy | - reduced serum IL-6, IL-1β, and TNF-α levels - reduced MDA level in renal tissue - increased SOD, GPx, and GSH levels in renal tissue | - reduced serum creatinine, BUN, and uric acid levels - histological: mitigated renal injury such as glomerular hypertrophy, epithelial cell edema, and accumulation of inflammatory cells | [108] |
| Luteolin (50 or 100 mg/kg/day–oral for 14 days) | 50 male Wistar rats with doxorubicin-induced nephropathy | - increased SOD, CAT, GPx, GST, GSH, and TSH levels in kidney tissue - reduced RONS, LPO, and MDA levels in kidney tissue - reduced NO levels and MPO activity in kidney tissue - reduced IL-1β and TNF-α levels in kidney tissue - increased IL-10 level in kidney tissue | - increased survival rate - decreased serum creatinine and urea levels - decreased caspase-3 and caspase-9 activity - histological: decreased glomerular hypercellularity and preserved kidney architecture | [109] |
| Different Models of Heart Failure | ||||
|---|---|---|---|---|
| Dosage of Resveratrol | Model | Anti-Inflammatory and Antioxidant Effects | Beneficial Heart Effects | Reference |
| Resveratrol (45.51 mg/kg/day–oral for 28 days) | 24 rats with angiotensin II-induced cardiopathy | - down-regulated NF-kB expression | - increased left ventricular ejection fraction and fractional shortening - reduced left ventricle wall thickness - mitigated angiotensin II-induced cardiac hypertrophy - reduced interstitial fibrosis - down-regulated ACTA1, ANP, BNP, collagen-I, and collagen-III expression - down-regulated Ang-II/AT1R signal transduction | [118] |
| Resveratrol (20mg/kg/day–intraperitoneal for 42 days) | 95 C57BL/6 rats with left coronary artery ligation-induced cardiopathy | - down-regulated ANP, ICAM-1, MMP-9, FKN, procollagen-I, and procollagen-III expression | - increased survival rate - echocardiography: reduced left ventricular end-diastolic and end-systolic diameters; increased left ventricular fractional shortening - reduced left ventricle infarct size | [115] |
| Resveratrol (450 mg/kg/day–oral for 14 days) | 44 C57B1/6N male rats with transverse aortic constriction pressure-overload-induced heart failure | - | - increased total basal physical activity level - increased metabolic rate - up-regulated insulin signaling in the skeletal muscle | [126] |
| Pterostilbene (50 mg/kg/day–oral for 56 days) | 30 C57BL/6 male rats with transverse aortic constriction-induced heart failure | - up-regulated Sirt1 and GPX4 expression - down-regulated p-GSK-3β expression | - increased left ventricular ejection fraction and fractional shortening - increased cardiac index - decreased left ventricular internal dimensions at systole and diastole - ameliorated cardiac remodeling by reducing cardiac collagen volume fraction | [127] |
| Resveratrol (15 mg/kg/day–oral for 56 days) | 30 Wistar male rats with isoproterenol-induced heart failure | - reduced nitrotyrosine levels - increased Akt-1and GSK-3β levels - reduced phosphorylation of ERK1/2 and p38-MAPK - down-regulated iNOS and COX-2 expression | - reduced serum BNP level - echocardiography: reduced left ventricular wall thickness, end-systolic volume, and systolic-left ventricular inner diameter - histological: decreased interstitial fibrosis | [128] |
| Resveratrol (22.5 mg/kg/day–oral for 14 days) | 48 Sprague-Dawley male rats with left anterior descending artery ligated-induced heart failure | - did not change Sirt1, AMPK and Akt levels - did not change Mn-SOD and GPx1 levels - down-regulated CYP1B1 expression | - echocardiography: increased left ventricular ejection fraction and reduced cardiac remodeling by decreasing left atrial mass and left ventricular end-diastolic dimension - increased exercise capacity - improved cardiac energy metabolism | [129] |
| Resveratrol (320 mg/kg/day–oral for 14 days) | 67 C57B1/6 male rats with transverse aortic constriction pressure-overload-induced heart failure | - restored AMPK activation | - increased survival rate - histological: reduced cardiac fibrosis - down-regulated cardiac hypertrophy gene expression: anf, ska, bnp, β-mhc - improved diastolic function and reduced left atrial volume - improved myocardial insulin sensitivity | [130] |
| Resveratrol (10 mg/kg/day–oral for 28 days) | 18 C57BL6 male rats with transverse aortic constriction pressure-overload-induced heart failure | - significantly reduced left ventricular macrophage and mast cell infiltration - down-regulated 4-HNE and 8-OHdG expression - reduced HIF-1α levels - increased SOD and glutathione activity in left ventricle homogenate | - reduced diastolic-left ventricular internal dimension and diastolic-left ventricular posterior wall thickness - increased left ventricular ejection fraction and fractional shortening - histological: reduced perivascular and interstitial fibrosis | [117] |
| Resveratrol (50 mg/kg/day–oral for 10 months) | 52 C57BL/6J rats with aortic coarctation-induced heart failure | - up-regulated SOD, GSH, GPX4, and SLC7A11 expression | - down-regulated BNP and sST2 expression - increased left ventricular ejection fraction - reduced myocardial edema, hypertrophy, and fibrosis | [119] |
| Resveratrol (5mg/kg/day–oral for 10 months) | 110 Wistar male rats with left coronary artery ligation-induced heart failure | - | Echocardiography - reduced aortic stiffness - improved left ventricular systolic function | [131] |
| Resveratrol (8mg/kg/day–intraperitoneal for 28 days) | 30 Sprague Dawley male rats with abdominal aorta ligated, pressure-overload-induced heart failure | - | - increased left ventricular ejection fraction and fractional shortening - down-regulated BNP mRNA expression - reduced autophagy by down-regulating beclin-1 and lamp-1 expression - increased myocardial ATP level | [132] |
| Resveratrol (2.5 mg/kg/day–oral for 56 days) | 30 Sprague Dawley male rats with left anterior descending artery ligated-induced heart failure | - reduced MDA and TNF-α levels in left ventricular homogenate | - reduced systolic and diastolic left ventricular internal diameter - reduced end-diastolic and end-systolic volumes - increased left ventricular ejection fraction and fractional shortening - histological: reduced collagen levels - reduced BNP levels | [116] |
| Resveratrol (2.5 mg/kg/day–oral for 112 days) | 50 male rats with the left anterior descending artery ligated-induced heart failure | - increased phosphorylation of AMPK - up-regulated Sirt1 expression | - increased survival rate - reduced BNP levels - increased left ventricular ejection fraction | [133] |
| Resveratrol (100 mg/kg/day–oral for 3 months) | 60 patients with heart failure with reduced ejection fraction in a single-center, randomized, double-blind, placebo-controlled study | - significantly reduced IL-6 and IL-1 levels | - significantly reduced galectin-3 and NT-proBNP levels - echocardiography: increased left ventricular ejection fraction, stroke volume; decreased left ventricular end-systolic volume; improved global longitudinal strain - increased exercise capacity - improved respiratory parameters - improved quality of life | [120] |
| Different Models of Non-Diabetic Chronic Kidney Disease | ||||
| Dosage of Resveratrol | Model | Anti-inflammatory and Antioxidant Effects | Beneficial Kidney Effects | Reference |
| Resveratrol (20 mg/kg/day–oral for 28 days) | 116 Sprague Dawley male rats with 5/6 nephrectomy-induced chronic kidney disease | Renal mitochondria - increased MMP and ATP levels - decreased ROS levels - increased complex-I and complex-III activity - increased Sirt1 activity | - | [134] |
| Resveratrol (100 mg/kg/day–oral for 28 days) | 30 C57BL/6 male rats with high-fat diet-induced nephropathy | - decreased TNF-α and IL-6 levels in renal homogenate - increased IL-10 levels in renal homogenate - decreased MDA levels and increased T-SOD and GPx levels in renal homogenate - down-regulated TLR4, MCP-1, CD11c and F4/80 expression | - decreased serum creatinine and BUN levels - histological: reduced kidney hypertrophy, enlargement of the mesangial area, and tubular vacuolization | [123] |
| Pterostilbene (200 mg/kg/day–oral for 7 days) | 20 ICR male rats with a high-adenine diet-induced chronic kidney disease | - histological: reduced accumulation of inflammatory cells such as macrophages and CD68+ - down-regulated renal TGF-β and fibronectin expression - up-regulated E-cadherin expression | - reduced serum creatinine, uric acid, and BUN levels, increasing these levels in urine - histological: reduced tubular dilatation and interstitial fibrosis | [122] |
| Resveratrol (20 mg/kg/day–oral for 14 days) | 24 C57BL/6J male rats with unilateral ureteral obstruction-induced nephropathy | - increased SOD levels and decreased MDA and 8-OHdG levels in renal homogenate - down-regulated fibronectin, TGF-β, TNF-α, and ICAM-1 expression - up-regulated Sirt1 expression | -histological: mitigated renal fibrosis by reducing glomerular injury and collagen accumulation | [124] |
| Resveratrol (20 mg/kg/day–oral for 7 months) | 26 male Wistar rats with uninephrectomy-induced chronic kidney disease | - reduced TNF-α, IDO, and IL-1β levels | - reduced serum creatinine and urea levels - reduced proteinuria - insignificantly reduced urine protein-to-creatinine ratio | [135] |
| Resveratrol (5 mg/kg/day–oral for 84 days) | 50 male Wistar rats with 5/6 nephrectomy-induced chronic kidney disease | - | - significantly increased survival rate - reduced proteinuria - reduced serum creatinine, urea, and BUN levels - histological: reduced glomeruli sclerosis and interstitial fibrosis | [121] |
| Resveratrol (500 mg/day–oral for 28 days) | 20 non-dialyzed CKD patients in a randomized, crossover, double-blind trial | - no significant activity on Nrf2 and NF-κB expression - no significant activity on CRP, IL-6, TNF-α, CAT, SOD, GPx activity | - no significant activity on serum creatinine and urea levels | [113] |
| Different Models of both Heart Failure and Non-Diabetic Chronic Kidney Disease | ||||
| Dosage of Resveratrol | Model | Anti-inflammatory and Antioxidant Effects | Beneficial Kidney Effects | Reference |
| Resveratrol (20 mg/kg/day–oral for 84 days) | 12 Sprague–Dawley and wild-type C57BL/6J rats with 5/6-nephrectomy-induced chronic kidney disease | - increased Sirt1 activity in the heart - decreased 8-OHdG level in the heart - up-regulated MnSOD expression in the heart | - reduced serum creatinine and BUN levels - renal histology: mitigated renal injury, such as glomerular hypertrophy and sclerosis, necrosis of tubulointerstitial tissue - reduced fibronectin and collagen-1 levels - ameliorated dilatation and wall thickening of the heart on echocardiography - heart histology: reduced myocyte hypertrophy, perivascular and interstitial fibrosis | [125] |
| Mechanisms/Effects | Arjunolic Acid | Kaempferol | Luteolin | Resveratrol | Shared Pathways in ND-CKD |
|---|---|---|---|---|---|
| Antioxidant activity | ↗ GSH ↘ MDA, NO, LAP | ↗ SOD, CAT, GPx, GST ↘ MDA, iNOS, TBARS, ROS | ↗ SOD, CAT, GPx ↘ MDA, H2O2 | ↗ SOD, GPx, T-SOD ↘ MDA, ROS | Reduction in oxidative stress and ROS |
| Anti-inflammatory activity | ↘ NF-κB, TNF-α, IL-1β, Kim-1 | ↘ TNF-α, IL-1β, IL-6, JNK, ERK1/2 | ↘ TNF-α, IL-1β, COX-2, NF-κB | ↗ IL-10 ↘ TNF-α, IL-6 | Downregulation of pro-inflammatory cytokines |
| Antifibrotic effects | ↘ TGF-β | ↘ TGF-β, α-SMA, collagen I/III | ↘ TGF-β, fibronectin, collagen I | ↘ TGF-β, fibronectin, collagen I | Suppression of fibrosis and ECM deposition |
| Apoptosis regulation | ↗ Bcl-2 ↘ Caspase-8/9 | ↘ Bax/Bcl-2, TP53 | ↗ Bcl-2 ↘ p53, Bax, caspase-3 | ↗ Sirt1 ↘ Bax | Protection from cell death via apoptotic modulation |
| Mitochondrial effects | Not prominently described | Not prominently described | Not prominently described | ↗ ATP, Sirt1, complex-I/III activity | Enhancement of mitochondrial function (resveratrol-specific) |
| Renal function improvement | ↗ survival rate ↘ creatinine, BUN, urea | ↘ creatinine, BUN, tubular necrosis | ↘ creatinine, BUN, structural damage | ↗ renal histology ↘ creatinine, BUN, proteinuria | Functional and structural nephroprotection |
| Mechanisms/Effects | Arjunolic Acid | Kaempferol | Luteolin | Resveratrol | Shared Pathways in HF |
|---|---|---|---|---|---|
| Antioxidant activity | Not prominently described | ↗ SOD, CAT, GPx, GST ↘ MDA | ↗ GPx, GST, GSH ↘ MDA, NO | ↗ SOD, GPx, GSH ↘ MDA, 4-HNE, 8-OHdG | Reduction in oxidative burden in myocardium |
| Anti-inflammatory activity | ↘ TGF-β | ↘ TNF-α, IL-1β, IL-6, NF-κB | ↘ TNF-α, IL-1β, NF-κB | ↘ TNF-α, IL-6, IL-1, NF-κB | Attenuation of cardiac inflammation |
| Improvement in cardiac function | ↗ PPARα ↗ Ejection fraction ↘ Collagen I/III | ↘ Nrf2 ↗ Ejection fraction Maintained SBP ↘ BNP | ↗ Ejection fraction and Fractional shortening ↘ LV diameters | ↗ Ejection fraction, fractional shortening, and hemodynamics ↘ BNP | Enhancement of systolic and diastolic function |
| Remodeling and fibrosis inhibition | ↘ Collagen I/III | ↗ Titin preservation ↘ Myocardial fibrosis | ↘ Myocardial apoptosis, fibrosis | ↘ Collagen, fibronectin, hypertrophy genes | Structural cardiac protection via antifibrotic actions |
| Apoptosis regulation | Not prominently described | ↗ Nrf2 ↘ NF-κB | ↘ Caspase-3, p53, Bax | ↗ Akt, Sirt1 ↘ apoptosis, ICAM-1 | Cardiomyocyte survival via anti-apoptotic mechanisms |
| Energy metabolism/Mitochondrial effects | ↗ PPARα | Mild or indirect involvement | ↗ SERCA2a, Akt | ↗ AMPK, Sirt1, ATP, insulin sensitivity | Improved cardiac bioenergetics and metabolic efficiency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Măgureanu, D.C.; Pop, R.M.; Bocsan, I.C.; Neag, M.A.; Cozma, A.; Levai, A.M.; Chedea, V.S.; Buzoianu, A.D. From Plants to Protection: Cardiorenal Benefits in Non-Diabetic Chronic Kidney Disease and Heart Failure. Molecules 2025, 30, 3982. https://doi.org/10.3390/molecules30193982
Măgureanu DC, Pop RM, Bocsan IC, Neag MA, Cozma A, Levai AM, Chedea VS, Buzoianu AD. From Plants to Protection: Cardiorenal Benefits in Non-Diabetic Chronic Kidney Disease and Heart Failure. Molecules. 2025; 30(19):3982. https://doi.org/10.3390/molecules30193982
Chicago/Turabian StyleMăgureanu, Dan Claudiu, Raluca Maria Pop, Ioana Corina Bocsan, Maria Adriana Neag, Angela Cozma, Antonia Mihaela Levai, Veronica Sanda Chedea, and Anca Dana Buzoianu. 2025. "From Plants to Protection: Cardiorenal Benefits in Non-Diabetic Chronic Kidney Disease and Heart Failure" Molecules 30, no. 19: 3982. https://doi.org/10.3390/molecules30193982
APA StyleMăgureanu, D. C., Pop, R. M., Bocsan, I. C., Neag, M. A., Cozma, A., Levai, A. M., Chedea, V. S., & Buzoianu, A. D. (2025). From Plants to Protection: Cardiorenal Benefits in Non-Diabetic Chronic Kidney Disease and Heart Failure. Molecules, 30(19), 3982. https://doi.org/10.3390/molecules30193982








