1. Introduction
Prostate cancer (PC) is the second most prominent type of cancer in men, which accounts for >35,000 annual deaths [
1]. PC survival and growth are driven by androgen receptor (AR) signaling. Thus, PC treatment initially revolves around the depletion of circulating androgens, usually through castration. However, PC cells can adapt to androgen deprivation therapy (ADT), which may result in progression to the more aggressive castration-resistant prostate cancer (CRPC). Evidence for the role of cholesterol in PC pathogenesis has been progressively growing, with several studies identifying hypercholesterolemia as a potentially modifiable risk factor in PC development [
2]. Cholesterol is the biosynthetic precursor to androgens and a key substrate for de novo steroidogenesis in prostate cells. The mCRPC cells rely on de novo cholesterol biosynthesis rather than transcellular uptake, and recent studies have shown that androgen-independent (AI) PC cells’ growth can be promoted by the low-density lipoprotein cholesterol (LDL-C) availability [
3].
The proprotein convertase subtilisin/kexin type 9 (PCSK9) regulates LDL-C levels by binding to the low-density lipoprotein receptor (LDLR), resulting in its degradation by the lysosome. The LDLR in the liver represents the primary pathway for the removal of plasma LDL-C [
4]. Thus, an overexpression of PCSK9 in this organ can lead to higher levels of circulating LDL-C. PCSK9 inhibitors not only reduce systemic levels of cholesterol but also reduce the supply of cholesterol to cancer cells and are therefore indicated for familial hypercholesterolemia in which statins are not effective [
4].
Alirocumab and evolocumab are currently FDA-approved, marketed humanized monoclonal antibodies (mAbs) that interfere with the PCSK9-LDLR PPI, considerably lowering LDL-C levels [
4]. However, mAbs are large molecules that are unable to cross the plasma membrane and fail to act intracellularly. Furthermore, manufacturing of mAbs is expensive and should be formulated in parenteral forms. Therefore, there are several non-mAb PCSK9 inhibitors being developed as potential alternative treatments for reducing circulating LDL-C.
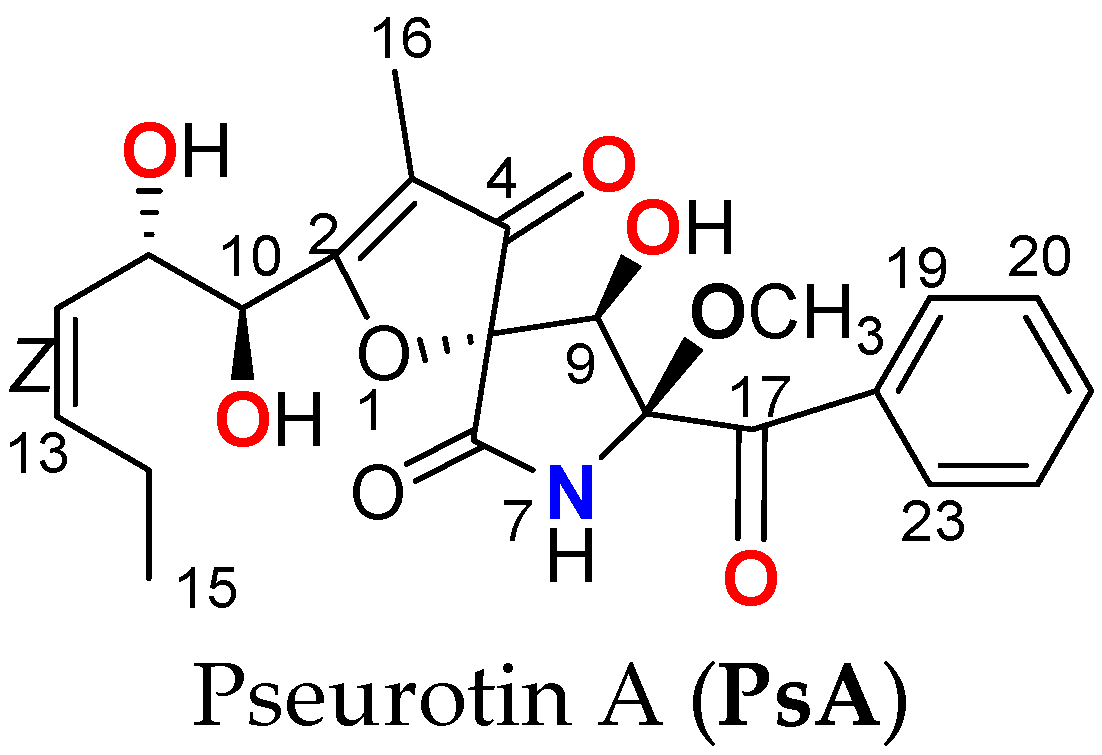
Pseurotins are spiroheterocyclic γ-lactam alkaloids isolated from
Aspergillus fumigatus and other fungi fermentation. Among the topmost bioactive pseurotins are pseurotin A (PsA,
Scheme 1) and the related pseurotin D. Both PsA and pseurotin D showed relevant antitumor and immunomodulatory effects [
5]. PsA dually inhibited PSCK9 expression and interaction with LDLR in mouse livers and various tumor models with a promising safety profile [
6,
7,
8,
9]. PsA showed in vitro dose-dependent reduction of PCSK9 expression in the hormone-dependent BT-474 luminal B breast cancer (BC) cells [
6]. PsA suppressed the in vivo progression and recurrences of the BT-474 BC cells in a nude mouse xenograft model by reducing the liver and plasma PCSK9 levels and normalizing the liver LDLR level [
6]. This established PsA as a novel first-in-class small molecule lead dually reducing the PCSK9 expression and suppressing its PPI with LDLR in hormone-dependent BC and possibly other hormone-dependent malignancies [
6]. Subsequent studies revealed PsA’s suppression of PCSK9 expression, migration, and colony formation in CRPC cell lines PC-3 and 22Rv1 [
7,
8]. PsA lowered the progression, locoregional and distant recurrences of the CRPC cell lines PC-3 and CWR-R1ca in vivo in xenografted nude mouse models, validating the novel concept of targeting the PCSK9-LDLR axis to control CRPC pathogenesis [
7,
8]. Studies on the PsA in vitro toxicity against RWPE-1 and CCD 841 CoN human non-tumorigenic prostate and colon cells proved cellular death reached at the 10-fold level of literature-reported therapeutic activity [
9]. PsA-mediated induction of non-malignant cells’ apoptosis was at very high concentrations [
9]. The Up-and-Down acute toxicity procedure showed a PsA LD
50 value of >550 mg/kg in male and female
Swiss albino mice [
9]. A 14-day acute toxicity assay of PsA 10, 250, and 500 mg/kg by oral gavage versus vehicle control in
Swiss albino mice reported neither major organ toxicity in the liver, lung, heart, brain, or kidneys nor any alarming changes in the hematological and biochemical parameters [
9]. The 500 mg/kg female dosing group showed a 45% decrease in body weight after 14 days but displayed no other signs of toxicity [
9]. Single oral doses of PsA, up to 50-fold the therapeutic dose, did not pose acute organ toxicity in
Swiss albino mice [
9]. While this study displayed this wide PsA acute safety index, there is still a dire need to assess its chronic safety since the effect of PCSK9 inhibitors on neurodegenerative diseases is still controversial [
10].
The hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitory-LDLR expression promoter statins are standard interventions for hypercholesterolemia [
11]. Literature linked HMG-CoA reductase inhibition with lowered cognitive performance and potential long-term harmful neurocognitive effects [
11]. Many patients using statins encounter muscle-related rhabdomyolysis, leading to intolerance and treatment discontinuation [
11,
12]. PC-specific mortality risk was lower among statin users; however, statin use did not reduce the risk of PC recurrence [
12]. Statins also proved to stimulate PCSK9 transcription, which adversely affects their long-term efficacy [
12].
PCSK9 binds to the LDLR, resulting in its degradation by the lysosomes, yet there are conflicting views on whether the ubiquitin-proteasome system (UPS) could also be involved in PCSK9-induced LDLR degradation. The sterol-responsive nuclear liver X receptor (LXR) inhibits the LDLR pathway through induction of the E3 ubiquitin ligase, known as the inducible degrader of the LDLR (Idol), which triggers the ubiquitination of the LDLR and ensures its degradation [
13]. LDLR ubiquitination was detected following PCSK9-targeting treatments, suggesting that a different E3 ligase other than Idol is involved in PCSK9-induced LDLR ubiquitination [
14]. LDLR degradation mediated by PCSK9 does not require proteasome function or ubiquitination of the LDLR cytoplasmic tail [
15]. These inconsistent findings suggest that the clear mechanism of PCSK9-mediated LDLR degradation is yet to be delineated.
The UPS serves as the most prominent driver for the regulation of proteolysis in all eukaryotic cells. The UPS plays a key role in regulating a wide range of cellular pathways that include apoptosis, neuronal signaling, growth, and proliferation through the cell cycle and immune response [
16]. Dysregulation of UPS-linked pathways correlated with neurodegenerative disorders like Alzheimer’s disease, Parkinson’s, and certain cancers [
17]. Clinical trials on the proteasome inhibitor bortezomib have demonstrated significant improvement in survival rates in patients suffering from multiple myeloma [
18]. This established proteasome inhibition as a key molecular target in cancer therapy, which was further validated by subsequent studies in a variety of cancers [
19,
20,
21,
22]. A dysfunctional UPS and associated genes are prominent molecular markers in mCRPC cells [
21,
23,
24].
The uniqueness of PsA as the first small-molecule dual suppressor of PCSK9 expression and inhibitor of its PPI with LDLR highlights its future potential as a novel cancer recurrence suppressive lead entity. This study presents the PsA comprehensive chronic toxicity, bioavailability, biodistribution, recurrence suppressive efficacy in a clinical-mimicking mCRPC model, and RNA-sequencing-based mechanistic molecular insights.
3. Discussion
Despite the progress in early PC diagnosis, many diagnosed patients have locally advanced disease and distant metastasis at initial diagnosis. ADT and APIs are initially effective in CSPC suppression, but inevitably, they develop resistance. The mitogenic role of PCSK9 in several malignancies has been recently documented. PsA was validated as a novel orally active dual inhibitor of PCSK9 secretion and PCSK9-LDLR PPI [
6,
7,
8]. PsA has also shown promising in vivo results in nude mouse xenograft models by suppressing BC and mCRPC progression and recurrence in daily oral doses as low as 10 mg/kg [
6,
7,
8]. Initial appraisal of pharmacologically validated hits and leads is a critical endpoint at the early stages of the drug development pipeline. PsA has a definite promising therapeutic potential against mCRPC, coupled with acute single-dose safety results in a murine model, displaying no major organ toxicity at doses up to 500 mg/kg, presenting an initial safety profile for this molecule going forward [
9].
Following a 90-day daily oral dosing safety study of up to 80 mg/kg PsA, male and female
Swiss albino mice fed on HFD showed no significant change in body weight nor abnormalities in their neurological or autonomic responses. Sub-chronic doses of 10 mg/kg, 40 mg/kg, and 80 mg/kg were chosen based on the findings of the Up-and-Down toxicity procedure previously performed by this study team that resulted in an LD
50 of >550 mg/kg for PsA oral dosing in
Swiss albino mice [
9]. The male 10 and 40 mg/kg dosing groups saw a significant decrease in the heart weights as compared to VC; however, this was not mirrored in the female nor in the male mice 80 mg/kg dosing groups. Knocking out PCSK9 in experimental animals can lead to heart failure with preserved ejection fraction [
38]. However, this cardiotoxic effect resulted in an increase in overall heart weight with a significant increase in the diastolic relative wall thickness versus wild-type animal control. Thus, the sex-specific decrease in average heart weight is not cardiotoxicity due to the PCSK9 inhibitory effects of PsA. Chronic drug exposure can potentially lower heart weight through mechanisms like cardiac atrophy, drug-induced oxidative stress, or mitochondrial dysfunction. PsA in vivo testing for effects on oxidative stress and mitochondrial dysfunction might be required to further assess the potential sex-specific long-term cardiac safety.
Elevation of the liver transaminases AST and ALT is a reliable marker of liver injury/necrosis. However, in this study, systemic AST level for both males and females and ALT level for the female mice were significantly lower than those of the VC group. A study screened >12,000 mice and determined the average male AST levels to be ~50 U/L, female AST levels to be ~48 U/L, male ALT levels to be ~26 U/L, and female ALT levels to be ~30 U/L [
39]. The slight increase above the baseline levels for AST and ALT in the control mice is likely caused by the HFD use over the study course, inducing a non-alcoholic fatty liver disease (NAFLD), characterized by excessive lipid accumulation in hepatocytes [
40]. The most severe form of NAFLD is nonalcoholic steatohepatitis (NASH), which can lead to liver fibrosis/cirrhosis. The increase in liver AST level due to the HFD was more pronounced in the female VC group. Observing the significant decrease in the AST and ALT levels in treatment groups, nearly to the healthy levels, ~48 U/L and ~30 U/L, respectively, demonstrated the PsA potential hepatoprotective effects against the long-term HFD use. The most significant decrease in AST levels was more pronounced in the female treatment groups. PsA possibly protected mouse livers against the buildup of triglycerides by long-term intake of HFD and subsequently reduced the AST and ALT levels back to the baseline levels. While PCSK9 inhibitors are effective for hyperlipidemia, there is inconclusive evidence correlating circulating PCSK9 levels with the development or reversal of NAFLD [
41]. The absence of NAFLD features in treated groups clearly indicates the safety and anti-hyperlipidemic uniqueness of PsA.
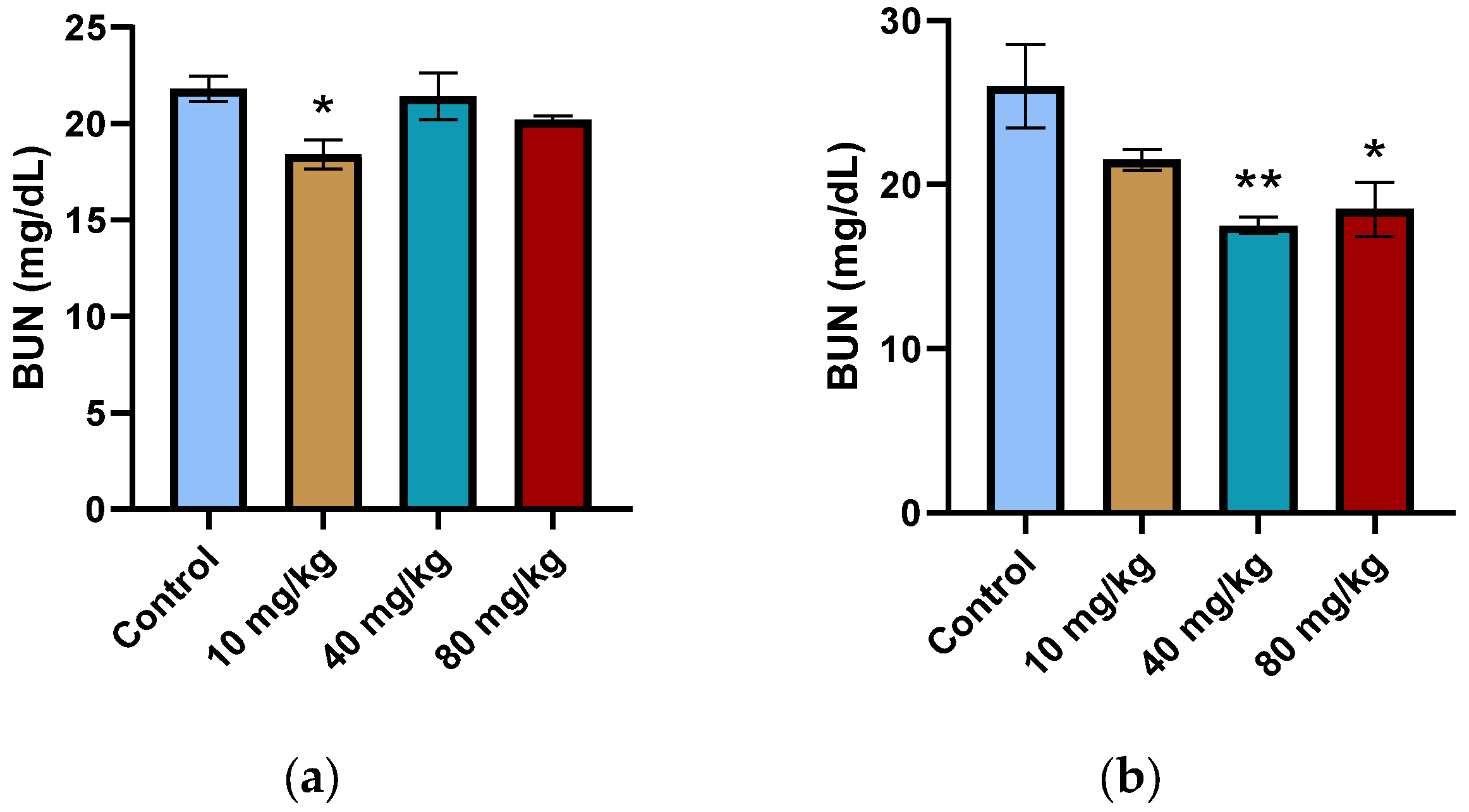
Elevated levels of BUN or CREAT are common nephrotoxicity indicators. A decrease in BUN levels is usually linked to malnutrition, overhydration, or a low-protein diet. The CREAT levels of both male and female treatment groups showed no significant change over the 90-day dosing regimen (
Supplementary Tables S3 and S4). This, paired with the lack of necrosis, inflammation, or other signs of nephrotoxicity in the histopathological examination of the kidney tissue sections, justifies that the decrease in BUN is not due to an overt toxicity on the nephrons (
Supplementary Figure S5b).
Hematological parameters remain important markers for the prediction of injury due to xenobiotic exposure in humans and animals. Negative hematological results in animals have a high correlation with similar negative results in humans [
42]. The average WBC-H, RBC-H, HGB, HCT, MCHC, and PLT count in PsA-treated mice indicates no signs of infection, inflammation, aplastic or hemolytic anemia, or thrombocytopenia, as the levels were in accordance with literature parameters observed in healthy mice [
43]. Only the MCV of the male 40 mg/kg treatment group value of 55.4 ± 4.8 fL was slightly lower, but still well within the reference interval of 57.81 ± 7.79 fL seen in healthy male
Swiss albino mice [
43]. The cause of the missing hematological data for the female 40 mg/kg and 80 mg/kg treatment groups was due to an error in proper blood collection during animal sacrifice, leading to blood samples clotting before analysis. While this lack of data does weaken the safety study, the absence of deleterious effects in the hematological parameters of the male treatment groups up to 80 mg/kg as well as the lack of significant change in any of the hematological parameters between the female control and the 10 mg/kg-treated group suggest the lack of drastic changes in the hematological parameters for the 40 mg/kg and 80 mg/kg female dosing groups. This hypothesis is strengthened by the hematological data collected earlier in the 14-day acute PsA toxicity assessment, which observed healthy levels of WBC-H, RBC-H, HGB, HCT, MCV, MCHC, and PLT count with doses up to 500 mg/kg level [
9].
Motivated by the HFD use over the 90-day sub-chronic safety study course, PsA’s ability to reduce plasma cholesterol in treated
Swiss albino mice was assessed. Daily PsA oral dosing greatly reduced the total plasma cholesterol levels in both female and male treatment groups, relative to the VC. The male mice displayed a greater relative plasma cholesterol reduction response. PsA anti-hypercholesterolemic effect was not only comparable with the FDA-approved marketed PCSK9-LDLR PPI inhibitory humanized mAb evolocumab whose clinical trials reported total cholesterol reduction by −26.5% after 8 dosing weeks, but also was in accordance with a propensity score matching analysis that showed greater relative % reduction for LDL-C in males over females (−54.4% versus −42.7%,
p < 0.001) [
44]. This clearly favors PsA anti-hyperlipidemic validity as an orally active PCSK9-targeting lead and rationalizes its use for the control of PCSK9-sensitive mCRPC.
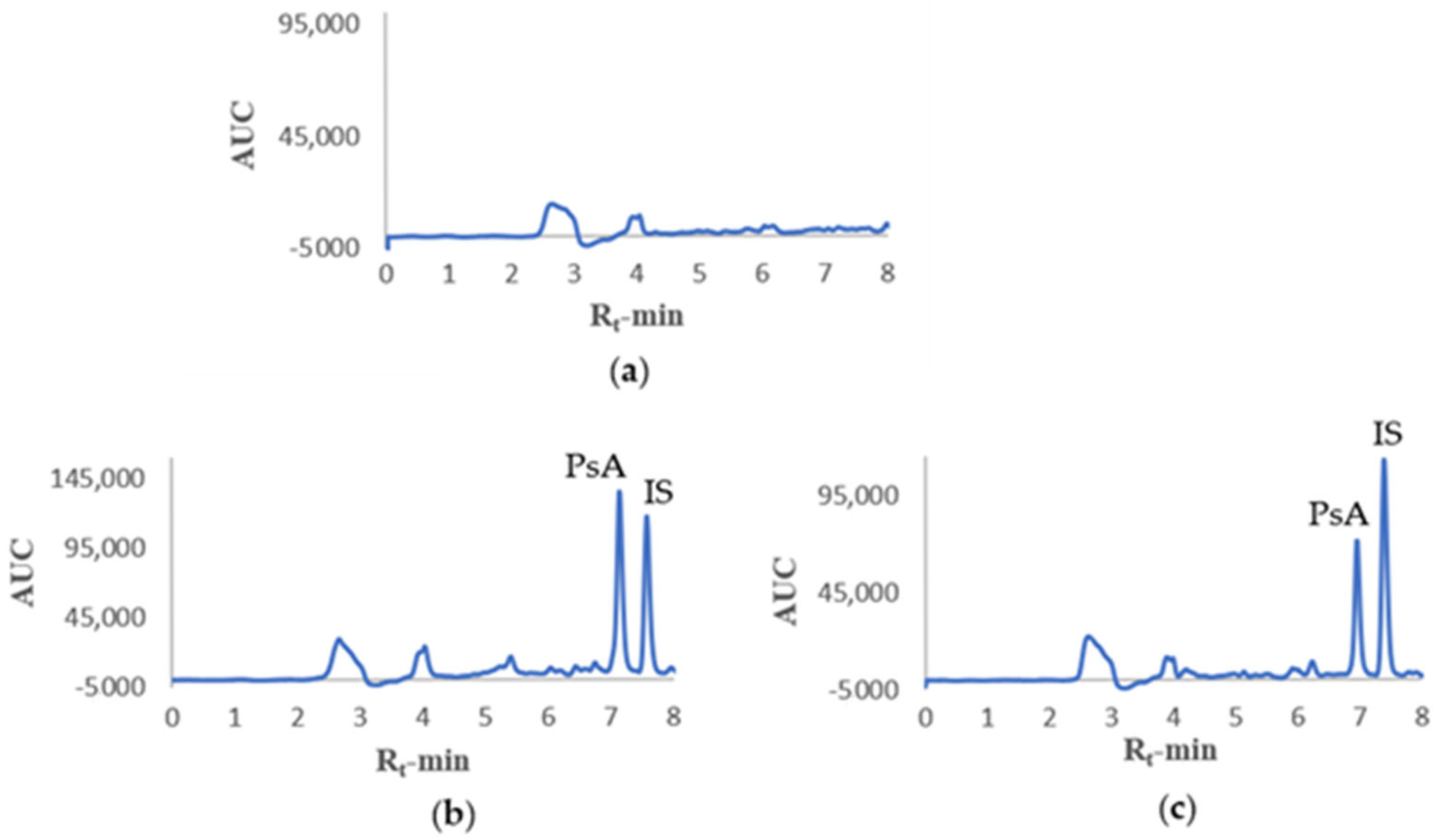
Development of a sensitive, specific, reproducible, and reliable analytical assay is paramount to assess PKs. An HPLC method was first developed for the detection and separation of PsA from mouse plasma constituents and later optimized for linearity, selectivity, sensitivity, recovery, accuracy, stability, and precision. The developed HPLC analytical method in mouse plasma was in accordance with the guidelines for bioanalytical method validation of the Food and Drug Administration [
29]. It is imperative to understand PsA PKs properties before subsequent preclinical testing as a valid anti-PC recurrence lead. Deciding on the IV dosing concentration of 50 mg/kg for the PK study was due to limitations in PsA aqueous solubility and limited maximum IV volume administration in mice. An IP dosing concentration of 125 mg/kg afforded prominent HPLC peaks utilized for the PsA qualitative tissue distribution assessment. Yet, the quantitative distribution of PsA in mouse organs was still unknown. IP dosing allowed higher dosing volumes of PsA without causing harm to the animals. The developed HPLC method was sensitive enough to easily detect much lower PsA concentrations.
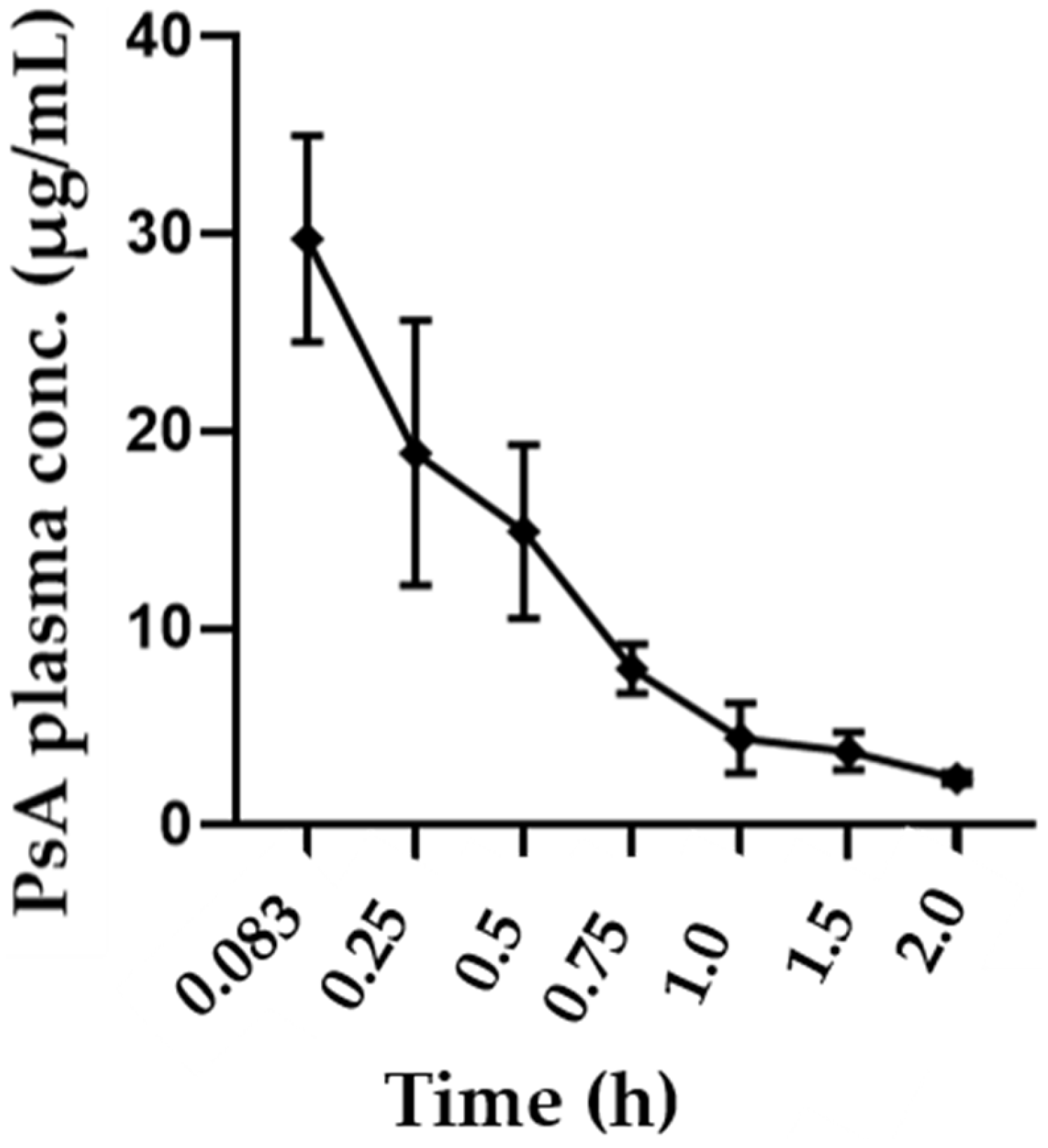
Following IV administration, PsA demonstrated rapid distribution and elimination in the mouse body. This was determined using the calculated parameters, including an initial concentration range of 24.6–34.2 µg/mL, an average T
1/2 of 0.53 h, an average V
d of 1.83 L/kg, and an average clearance rate of 2.39 L/h. These parameters fit into a non-compartment model. The notable pharmacodynamics effect of PsA in PC and BC further evidenced its wide distribution into the mouse tissues. The volume of distribution of 1.83 L/kg was significantly higher than the average total body water volume in mice (0.6 L/kg), indicating potentially elevated binding to peripheral tissues [
45]. The short half-life of 0.53 h does pose challenges for future druggability and would require frequent doses to be administered. However, knowledge surrounding drug half-life is considered insufficient whenever the tested parent lead has active metabolites that might prolong its activity duration. Although PsA was not detectable in the plasma through the validated HPLC method following oral dosing, it was detectable following IP dosing. This was parallel to notable anticancer pharmacodynamic effects observed in cancer models [
6,
7,
8]. It is thus assumed that PsA is being extensively metabolized by the gut microbiota in the oral route. Future research is needed to uncover PsA active metabolites to further elucidate its pharmaceutical potential.
PsA showed effective mCRPC recurrence suppression in a clinical-mimicking model of nude mice xenografted with the mCRPC CWR-R1ca-Luc exposed to neoadjuvant DTX (3 weeks), ENZ (4 weeks), followed by primary tumor surgical excision. PsA daily oral dosing over 60 days effectively suppressed the mCRPC locoregional and distant recurrences at both 10 mg and 20 mg/kg. Specifically, PsA showed superior bone, lung, liver, and spleen mCRPC distant recurrences suppression versus VC. PsA did not prevent mCRPC brain distant recurrences in both treatment doses versus the VC group, which was in-line with its poor distribution into the brain due to potential failure to cross the BBB.
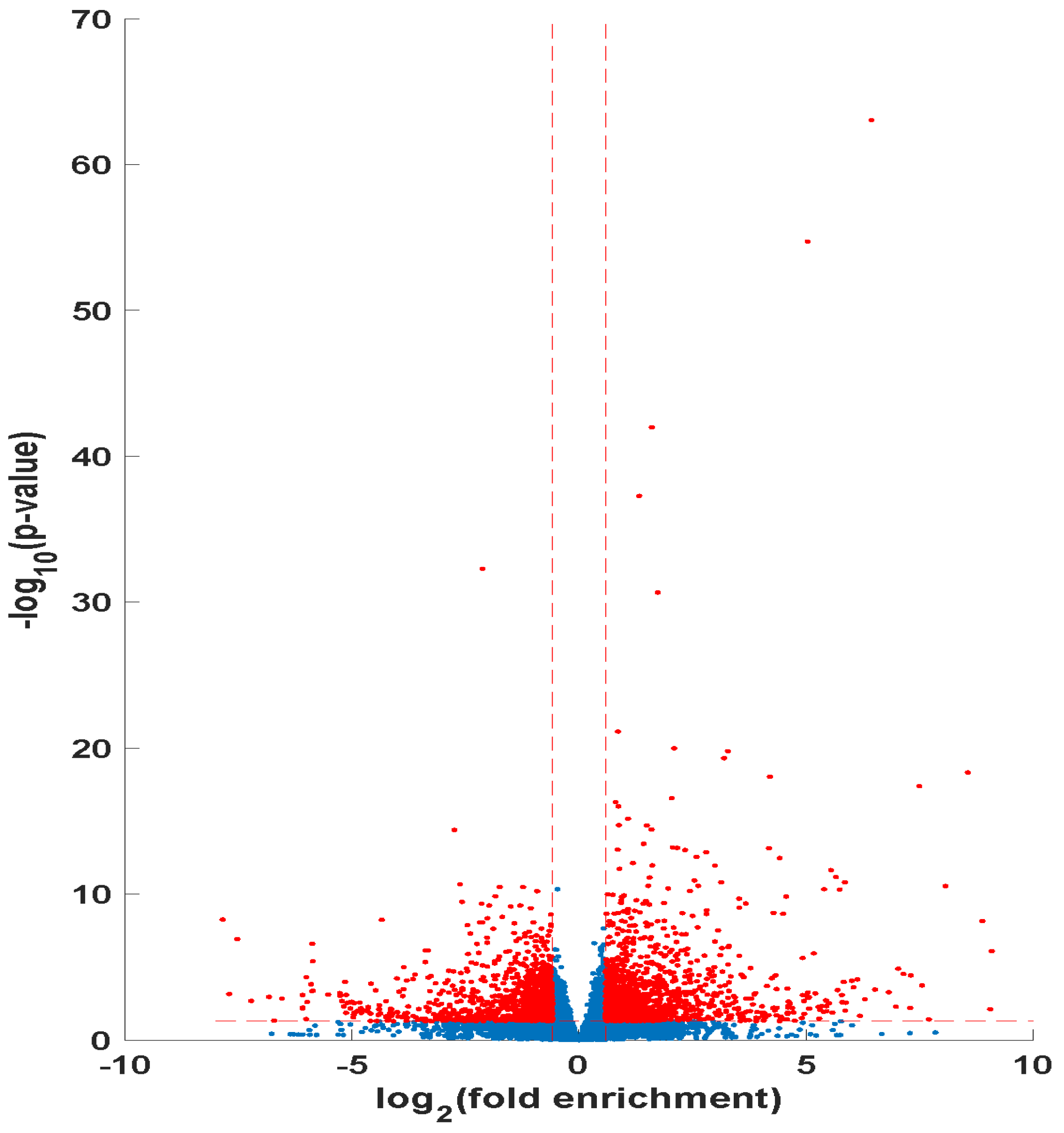
RNA-sequencing was used to develop a genomic profile for PsA 20 mg/kg treatment effects on the only locoregionally-recurred mCRPC tumor in the aforementioned nude mice model (
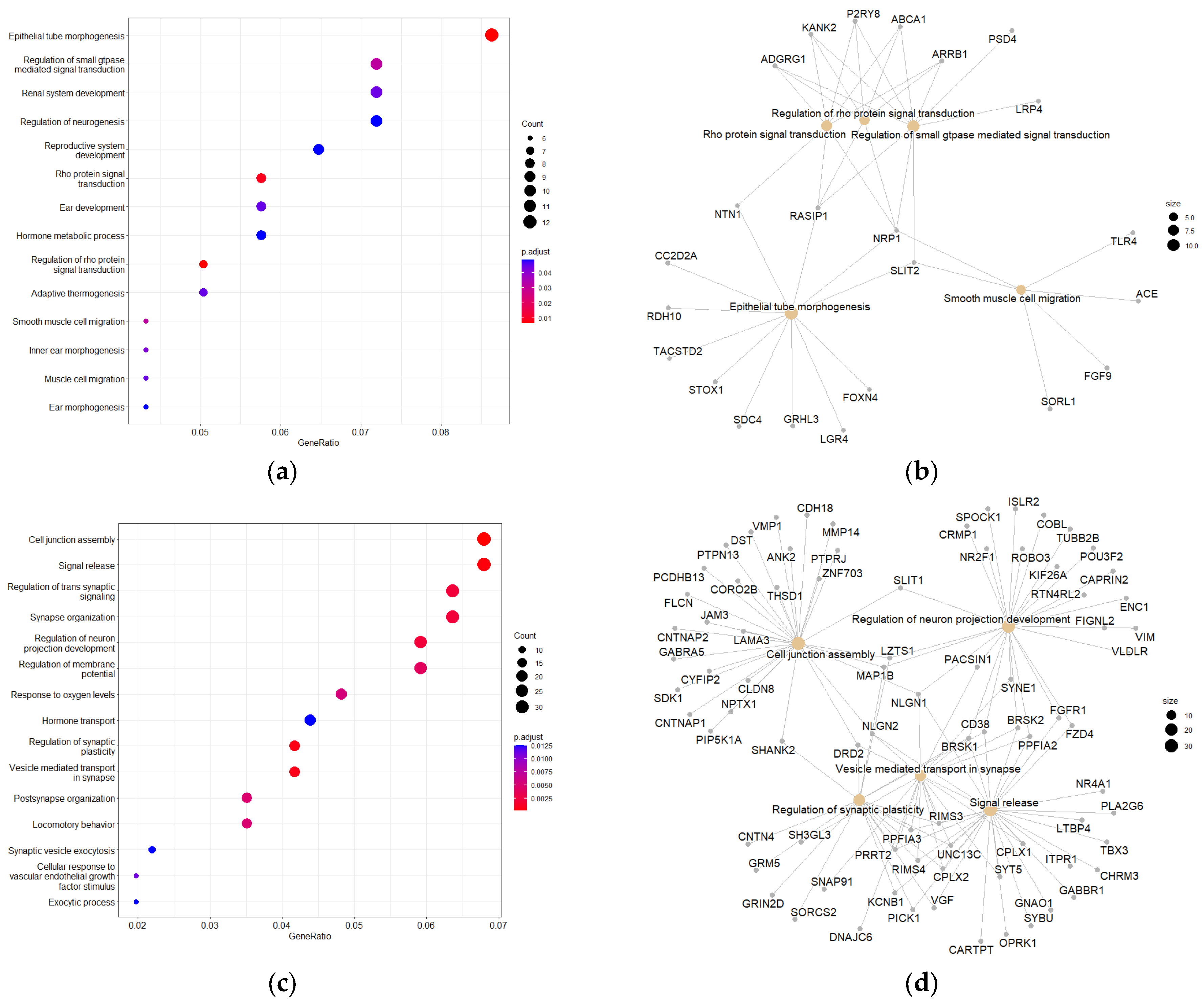
Table 5). PEA and PPI were subsequently utilized to help identify potential molecular targets, predict disease pathways, and elucidate the underlying therapeutic mechanism of PsA. The KEGG and GO pathway enrichment analysis of the biological processes of the most significantly enriched upregulated and downregulated DEGs displayed strong evidence for neurological disorders, tumor suppression, and anti-migratory effects. Cell junction assembly was identified as the most significantly enriched GO pathway associated with upregulated DEGs (
Supplementary Figure S6a). Intercellular adhesion can resist the action of EMT and reduce the invasion potential of cancer cells. Thus, upregulation of genes associated with this pathway following PsA treatment provided strong correlation and mechanistic insights into its PC recurrences suppressive effects observed in previous studies [
7,
8]. The most significantly enriched GO pathways associated with the downregulated DEGs were epithelial tube morphogenesis, Rho protein signal transduction, and regulation of Rho protein signal transduction (
Figure 8a). During EMT, epithelial cells are converted into more active and invasive mesenchymal cells and lose their characteristics that promote differentiation [
46]. This includes cell-cell adhesion, which correlates with the most significantly enriched GO pathway associated with the upregulated genes. The Rho GTPases facilitate a wide range of malignant cellular processes, including cell migration, survival, and proliferation [
47]. Downregulation of Rho GTPases provides further mechanistic justification for the PsA anti-PC effects.
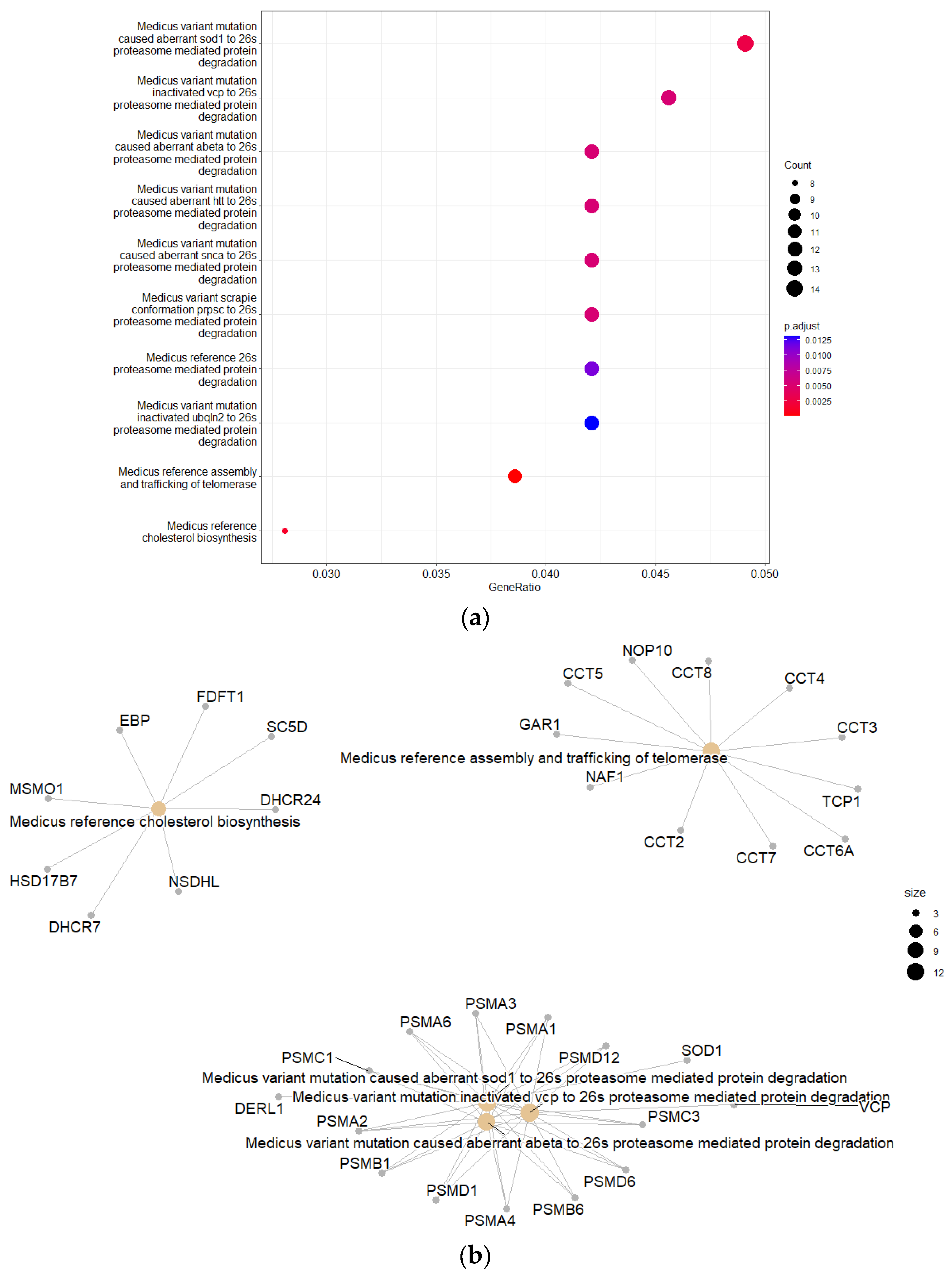
KEGG PEA of the downregulated genes resulted in eight enriched canonical pathways associated with 26S proteasome-mediated protein degradation. The 26S proteasome is widely known to be the major degradative machinery of the UPS, which is the cell’s foremost protein degradation system [
13,
14,
15,
16,
17]. The UPS is responsible for the bulk of protein degradation at the endoplasmic reticulum (ER).
A substantial number of cell, animal, and human studies have correlated the disruption of the UPS as a primary or secondary source in the pathogenesis of numerous neurodegenerative conditions [
13,
14,
15,
16,
17]. The PEA also mapped multiple downregulated genes to the assembly and trafficking of telomerase. Downregulated telomerase can cause telomere attrition, which may be linked to the development of neurodegenerative diseases [
13,
14,
15,
16,
17]. However, data analyses of PsA acute and chronic safety studies did not show any prominent neurodegenerative markers in the brain histopathology, such as Lewy bodies. There were also no changes in behavioral, autonomic, or neurological responses in either study that would hint at neurotoxicity. PsA failed to cross the BBB and was not detectable in the brain, though it was detected in four other organs following a single 125 mg/kg IP dosing (
Figure 6). These observations provided compelling evidence for PsA’s lack of potential neurotoxicity. Since the RNA-Seq data were acquired on recurrent mCRPC tumors and not normal nervous or neuronal tissues, the anticancer effects associated with the downregulation of UPS-related genes hold more potential than the neurotoxic effects. Multiple UPS inhibitors have gained FDA approval for the control of multiple myeloma and non-solid malignancies.
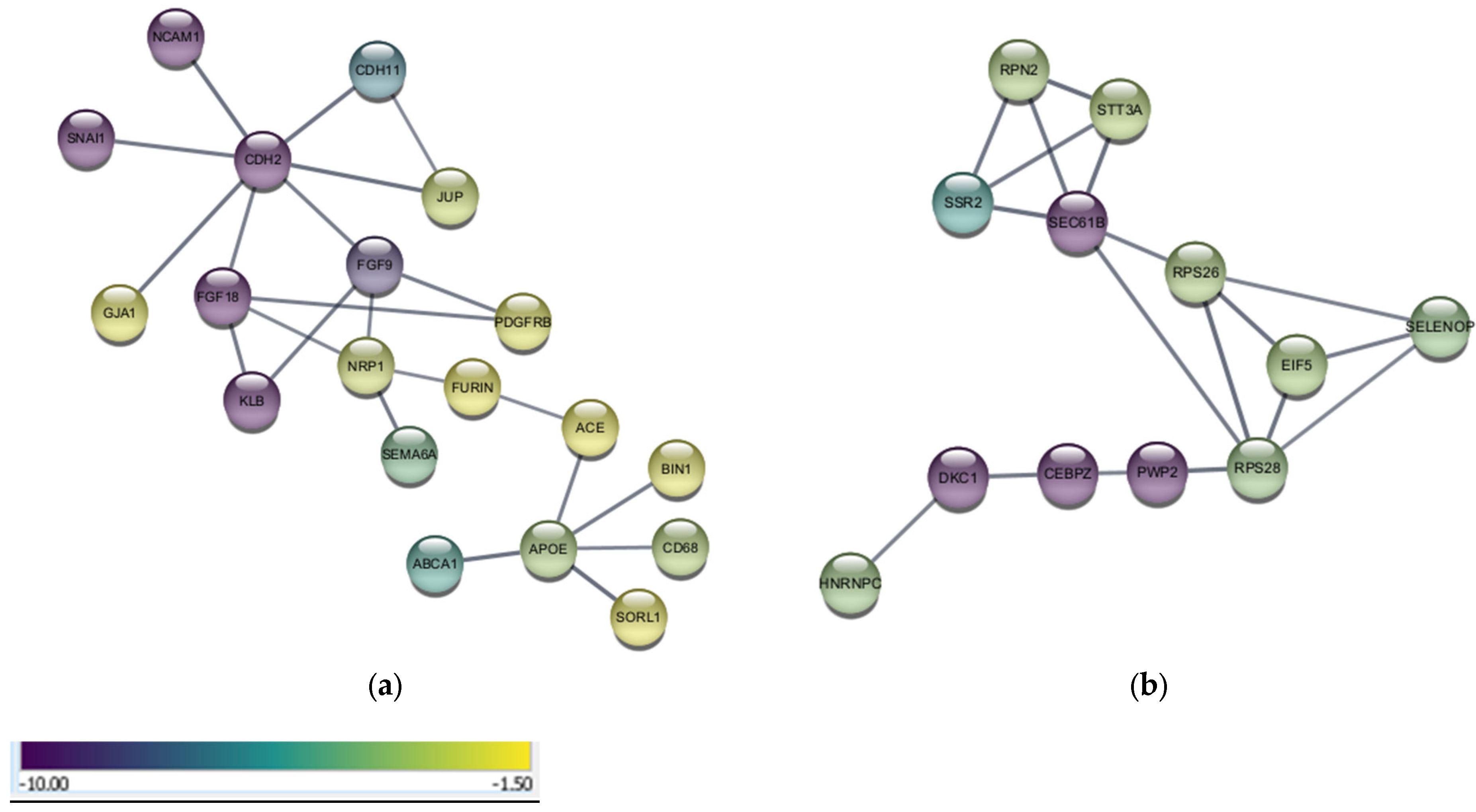
Analyses of functional networks from the PPIs of upregulated and downregulated DEGs was in line with the results from the PEA and exhibited gene/protein networks with researched associations to potential neurological disorders and anticancer effects. Analysis of downregulated DEGs resulted in networks and pathways that showed direct and indirect associations with the UPS. Cancer cells are more dependent on the upregulated UPS than normal cells to degrade the tumor suppressor proteins and prevent apoptosis [
48]. Downregulation of UPS can diminish cancer cells’ ability to respond to stress by inhibiting ubiquitination at sites related to oncogenic pathways [
49]. Downregulation of the UPS by PsA treatments cannot reduce efficiency in degrading damaged and misfolded proteins in the brain, nor can it raise DEGs neurodegenerative markers, since it will not cross BBB. Furthermore, the UPS was proven to be implemented in the progression of the CSPC-to-CRPC phenotypes with predominance in the mCRPC [
21,
24]. The UPS and specifically the 26S proteasome complex is an emerging molecular target for cancer therapy. This study shows that PsA might uniquely inhibit the UPS in recurrent mCRPC, in addition to targeting the PCSK9-LDLR axis. Dual targeting of the PCSK9-LDLR axis and UPS by PsA is a novel mCRPC-controlling strategy.
This is the first study to report the sub-chronic safety, PKs, biodistribution, and transcriptomics of PsA using murine models. This initial preclinical assessment data highlights the PsA potential as a novel mCRPC recurrence suppressor lead.
4. Materials and Methods
4.1. Experimental Animals
Twenty male athymic nude mice (Strain Foxnnu-Foxn1+), 7–8 weeks old, were used for the therapeutic recurrence model (Protocol # 21DEC-KES-01). Sixteen male and fourteen female Swiss albino mice, 19–20 weeks old, were used for PKs and distribution studies (Protocol # 21DEC-KES-02). Twenty male and twenty female Swiss albino mice, 7–8 weeks old, were used for a chronic toxicity study (Protocol # 21DEC-KES-03). Mice were purchased from Envigo (Indianapolis, IN, USA). The animals were given a 2-week acclimatization period and maintained under clean room conditions with a relative humidity of 55–65%, a temperature of 22 ± 2 °C, a 12:12 h light/dark cycle, Alpha-Dri bedding, and free access to drinking water and pelleted rodent chow. Animals were housed in group cages (male, n = 5; and female, n = 5) and kept in the same environmental conditions. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), University of Louisiana at Monroe, and were conducted in strict accordance with good animal practice as defined by the National Institutes of Health (NIH) guidelines.
4.2. Systemic Cholesterol Monitoring Assay
Plasmatic cholesterol concentrations in mice were determined using a total cholesterol kit (EnzyChromTM Cholesterol Assay Kit ECCH-100 BioAssay Systems, Hayward, CA, USA), adhering to the manufacturer’s instructions. Colorimetric quantitation was measured at 340 nm using a Synergy 2 microplate reader (BioTek Instruments Inc., Winooski, VT, USA). Blood was collected through tail vein extraction on the day before treatment, day 35, and day 90. Blood was quickly collected into green-topped Lithium Heparin 400 µL blood collection tubes (BD Microtainer REF# 365965 Becton, Dickinson and Company, Franklin Lakes, NJ, USA), centrifuged at 13,000× g for 10 min, and the upper layer of plasma was transferred to 1.5 mL Eppendorf tubes (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −20 °C until analysis.
4.3. Chemicals and Reagents
HPLC grade CH
3CN (Millipore–Sigma, Darmstadt, Germany) and 18 Ω water (PHARMCO-AAPER, Brookfield, CT, USA) containing 0.1% formic acid (Millipore–Sigma, Darmstadt, Germany) were used for the mobile phase. 2-Benzofuran carboxylic acid purchased from Aldrich Chemical Company Inc. (Milwaukee, WI, USA) was used as IS. PsA was extracted from a 14-day
Aspergillus fumigatus fermentation broth [
6,
7,
8]. It was then further isolated on Sephadex LH20 using CH
2Cl
2-EtOAc (VWR Chemicals BDH, Radnor, PA, USA) gradient elution and finally purified on LiChroprep RP-18, 25–40 µm, C-18 RP (Millipore–Sigma, Darmstadt, Germany) gravity column using isocratic 30% CH
3CN in H
2O (PHARMCO-AAPER, Brookfield, CT, USA) to a purity of >99% guided by HPLC and q
1H NMR. EtOAc and isopropanol purchased from Avantor Science Central (Allentown, PA, USA) were used in the extraction of PsA from mouse plasma. Dried remains were reconstituted in HPLC-grade methanol (PHARMCO-AAPER, Brookfield, CT, USA).
4.4. The 90-Day Sub-Chronic Toxicity Study Design
For the 90-day daily oral dosing sub-chronic toxicity study, twenty male and twenty female healthy 8-week-old
Swiss albino mice were randomly selected and randomized to 8 groups (
n = 5/sex/group). Mice received free access to purified drinking water and high-fat diet (HFD) pelleted rodent chow (11.4% total fat, no. 7004, Envigo/Teklad, Madison, WI, USA) until the day before sacrifice, at which time they were fasted for 8 h and allowed only free access to drinking water. Daily doses of PsA (10, 40, 80 mg/kg body weight) were administered to the mice formulated in <5% DMSO (Mediatech Inc., Manassas, VA, USA) and <0.2% Tween 80 (Croda Inc., Princeton, NJ, USA) at a volume not exceeding 1% of body weight by metal oral gavage (2 mm diameter with stainless steel bite protector, 18-gauge, 3.81 cm long). The mice were observed for mortality, morbidity, and behavioral signs of pain/toxicity at 1, 2, 4, 6, 12, and 24 h and then once every subsequent day for a total of 14 days. The mice were observed weekly until the completion of the study. The body weights of the mice were measured in grams using a Scout
TM Pro (Ohaus Corp., Pine Brook, NJ, USA) on day 0, 4, 8, 11, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, 83, and 90 after initial treatment. On day 90, all mice were anesthetized using isoflurane (USP-vaporizer method, Matrix VIP-3000, Covetrus, Dublin, OH, USA, exposure of animals in an anesthesia chamber at 3% isoflurane vaporization rate. Exposure time 2 min). Mice were then euthanized by cervical dislocation according to the 2020 AVMA Guidelines on Euthanasia and dissected [
50]. Mice organs (brain, liver, kidney, heart, lungs, spleen) were excised and weighed for histopathological examination. The organs were stored in 10% neutral buffered formalin (Avantor Science Central, Allentown, PA, USA) for 24 h and then transferred to 70% ethanol (AAPER ALCOHOL, Shelbyville, KY, USA). This study was performed following the OECD guideline 408 for the testing of chemicals [
51].
4.4.1. Hematological and Biochemical Evaluation
On the day of sacrifice, mice were decapitated, and the blood was quickly collected into a green-topped Lithium Heparin 400 µL blood collection tube (BD Microtainer REF# 365965) as well as a purple-topped K2 EDTA (K2E) 500 µL blood collection tube (BD Microtainer, Cat# 365974) to ensure all blood samples were collected free of clots. The Li Heparin samples were immediately centrifuged at 13,000× g for 10 min, and the plasma was transferred to 1.5 mL Eppendorf tubes free of Li heparin for biochemical analysis. Plasma samples were analyzed for AST, ALT, ALP, glucose, BUN, and creatinine levels using the Beckman AU680 clinical chemistry analyzer system (Beckman Coulter, Atlanta, GA, USA). The ETDA samples were analyzed for hematological parameters, and WBC, RBC, Hgb, Hct, MCV, MCHC, and PLT were determined using the Siemens Advia 120 hematology analyzer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). All blood samples were analyzed at the LSU School of Veterinary Medicine Clinical Pathology Laboratory at Baton Rouge, Louisiana.
4.4.2. Paraffin Embedding and Staining
The experimental animals’ organs were carefully excised and quickly fixed in 10% neutral buffered formalin for 24 h before being transferred to 70% ethanol. The organs were embedded in paraffin before sectioning and staining with hematoxylin and eosin (H&E). All sectioning and staining were conducted at the AML Laboratories (Jacksonville, FL, USA). The paraffin-embedded tissues were sliced into 5 µm-thick sections before being mounted on a positively charged slide. The 5 µm-thick sections were then dewaxed using xylene, rinsed using 80–95% ethanol, and rehydrated using H2O. They were subsequently stained with H&E before being dehydrated again using 80–95% ethanol to xylene.
4.4.3. Statistical Analysis
Results for the hematological and biochemical parameters were analyzed separately by One-way Analysis of Variance (ANOVA), followed by multiple comparisons with Dunnett’s test. All statistical analyses were performed using GraphPad Prism version 8 software (San Diego, CA, USA). Data in this study were expressed as a mean ± SD (standard deviation) and mean ± SEM (standard error of mean). A probability value of <0.05 was considered statistically significant (* p < 0.05; ** p < 0.01; and *** p < 0.001).
4.5. HPLC and Chromatographic Conditions
Chromatographic analysis was conducted on a Shimadzu HPLC system (Shimadzu USA Manufacturing Inc., Canby, OR, USA) equipped with an SPD-20A UV detector, 20 µL manual sampler, LC-20AD pump, a degasser, and an automated temperature-controlled column compartment. The UV detector, column oven, and LC-20AD pump were coupled to the Shimadzu system and controlled through Lab Solutions software, v5.111. Stationary phase separation was carried out using a Shimadzu Shim-Pack GWS C18 column (4.6 × 250 mm, 5 µm) equipped with a Zorbax ODS guard column (4.6 × 12.5 mm, 5 µm) by gradient elution with the column temperature maintained at 35 °C. The UV detector wavelength was set and measured at 254 nm. A constant flow rate of 1.0 mL/min and manual injection of 20 µL were employed throughout the HPLC analysis. A mobile phase consisting of aqueous HCOOH (0.1%, v/v, mobile system A) and HPLC grade CH3CN (mobile system B) utilized with a gradient elution of 30–60% B at 0–5 min, 60–85% B at 5–8 min, 85–95% B at 8–11.5 min, and 95% B at 11.5–18 min. The post-run re-equilibration time was 5 min.
4.5.1. Calibration Standard and Sample Preparation
For preparation of the stock solution, 1.7 mg of >99% pure PsA was weighed and dissolved in 1 mL HPLC-grade methanol. Subsequent dilutions were carried out using 18 Ω water to reach standard solutions of 50, 40, 30, 20, 10, 8, and 4 µg/mL PsA. They were stored at −20 °C and used within 4 h for analysis. 2.0 mg of 2-benzofuran carboxylic acid internal standard (IS) was weighed and dissolved in 1 mL HPLC-grade methanol before being diluted using 18 Ω water to a working concentration of 50 µg/mL. It was stored at −20 °C until use throughout HPLC analyses. For the intravenous (IV) and intraperitoneal (IP) injections, ~5 mg, respectively, of >99% pure PsA was dissolved in 15 µL DMSO before being combined with 230 µL sterilized phosphate buffer saline (PBS) and 5 µL Tween 80 to make a stock volume of 250 µL. The PsA concentration injected was 50 mg/kg for IV and 125 mg/kg for IP dosing based on each mouse’s individual weight.
4.5.2. PsA Extraction Method
Five µL of the IS (50 µL/mL) and 20 µL of 50, 40, 30, 20, 10, 8, and 4 µg/mL PsA standard solutions were combined with 15 µL freshly extracted blank mouse plasma. 300 µL of a 70% EtOAc/30% isopropanol mixture was added and vortexed for 30 s. Following centrifugation at 15,000× g for 10 min, the supernatant was transferred to a 2 mL Eppendorf tube for nitrogen evaporation. The residues were reconstituted in 25 µL HPLC-grade MeOH before being subjected to 30 s sonication followed by centrifugation at 15,000× g for 10 min. Afterwards, the entire sample was immediately injected for RP-HPLC analysis. The mouse plasma samples used in the PK study were subjected to the same extraction method, minus the addition of the PsA standard solutions. After weighing, each mouse organ was reconstituted in 500 µL PBS, then homogenized using the MISONIX Sonicator (Division of QSonica LLC, Newtown, CT, USA). Samples were centrifuged at 15,000× g for 10 min, and the supernatant was collected and transferred to a 2 mL Eppendorf. About 5 µL of IS (25 µL/mL) and 300 µL of a 70% EtOAc/30% isopropanol mixture were added to 100 µL of supernatant and vortexed for 30 s. Following centrifugation at 15,000× g for 10 min, the supernatant was transferred to a 2 mL Eppendorf tube and evaporated under N2. The remains were reconstituted in 40 µL HPLC-grade methanol before being subjected to 30 s sonication followed by centrifugation at 15,000× g for 10 min.
4.5.3. Method Validation
The HPLC method validation was conducted for linearity, selectivity, sensitivity, recovery, accuracy, stability, and precision of PsA in mouse plasma in accordance with the guidelines for Bioanalytical Method Validation of the Food and Drug Administration [
29]. Calibration curves were constructed using the peak-area ratios of the PsA analyte to the internal standard vs. plasma concentrations. Quality control (QC) samples at four concentrations (4, 32, 50, and 250 µg/mL) were used to assess method validation.
4.5.4. Linearity
A calibration curve prepared from seven calibration points by linear regression with a weighting factor of 1/x was used to quantify the concentration of PsA in mouse plasma samples. A ratio for analyte concentration was determined by plotting analyte/IS peak area under the curve, and the linear calibration equation with its correlation coefficient (r) was determined.
4.5.5. Accuracy and Precision
The intra-day precision and accuracy were evaluated by analyzing fresh mouse plasma spiked with respective concentrations at three replicates within the same day. Inter-day precision and accuracy were evaluated in the same way for four consecutive days (
n = 3). The precision was calculated using the coefficient of variation (CV) for the analysis of QC samples. The CV for each QC did not deviate by more than ±15%, in accordance with the guidelines for Bioanalytical Method Validation of the FDA [
29].
4.5.6. Stability
The stability of PsA at room temperature was determined by preparing six separate mouse plasma samples for each concentration. Three samples for each concentration were immediately extracted and injected into the HPLC. The three remaining samples were left on a lab bench protected from light for 24 h before being extracted and injected into the HPLC. The concentrations measured at the 0 and 24 h time points were compared. The stability of PsA after three freeze-thaw cycles was assessed by preparing six separate mouse plasma samples for each concentration. Three samples for each concentration were immediately extracted and injected into the HPLC. The three remaining samples for each concentration were frozen and thawed in a −80 °C freezer three separate times before being extracted and injected into the HPLC. The stability of PsA at −20 °C for 2 weeks was determined by preparing six separate mouse plasma samples for each concentration. Three samples for each concentration were immediately extracted and injected into the HPLC. The three remaining samples were left in a −20 °C freezer for two weeks before being thawed, extracted, and analyzed on the HPLC.
4.5.7. Recovery
Recovery of PsA was determined by comparing the mean peak area under the curve (AUC) obtained from the extracted mouse plasma with the AUC obtained by the direct injection of the corresponding spiked standard solutions.
4.5.8. Pharmacokinetics Analysis
The mean plasma concentration-time curve was plotted using GraphPad Prism software version 8.0.2 (La Jolla, CA, USA). PK parameters were obtained through non-compartmental analysis following intravenous bolus input using PK Solver 2.0 [
52].
4.6. PsA PKs Study in Swiss Albino Mice
For the PKs study, twelve male and twelve female healthy Swiss albino mice were randomly selected and placed in 3 groups (n = 4/sex/group). The mice received free access to drinking water and pelleted regular rodent chow (5% fat content, Cat# 7012, Envigo/Teklad, Madison, WI, USA) until the day before experimentation, after which they were fasted for 8 h and allowed only free access to drinking water. Mice were administered PsA formulated in sterile PBS-DMSO (925:75) + 0.2% Tween 80 at a volume not exceeding 100 µL intravenously through recto-orbital injection. Each animal was anaesthetized using Isoflurane (Piramal Critical Care Inc., Bethlehem, PA, USA) for 1–2 min before injection. Disposable sterilized syringes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) were used for blood collection as well as administration of PsA at a dose of 50 mg/kg and volume, adjusted to the mouse body weight, no greater than 100 µL for animal safety considerations. Blood samples of around 50–70 µL were collected through the tail vein in 600 µL Eppendorf tubes at time points 0.25, 0.75, and 1.5 h for groups 1 and 4, 0.083, 0.5, and 1 h for groups 2 and 5, in addition to 2 and 4 h for groups 3 and 6 following intravenous injection and subjected to immediate centrifugation. From the plasma, 15 µL was transferred into clean 600 µL Eppendorf tubes and immediately subjected to the extraction method listed above and analyzed using HPLC.
4.7. PsA Biodistribution Study in Swiss Albino Mice
For the biodistribution study, four healthy male and two healthy female Swiss albino mice were randomly selected and placed in 2 groups (n = 3/group). The mice received free access to drinking water and pelleted regular rodent chow (Cat# 7012, Envigo/Teklad, Madison, WI, USA) until the day before experimentation, after which they were fasted for 8 h and allowed only free access to drinking water. One group was IP administered 125 mg/kg PsA formulated in PBS-DMSO (925:75) + 0.2% Tween 80 at a body weight-adjusted volume not exceeding 300 µL. Mice were then sacrificed through cervical dislocation 7 min after injection. Disposable sterilized syringes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) were used for the injection. The blood was collected in 600 µL Eppendorf tubes. The brain, liver, kidney, spleen, and heart were excised, weighed, and homogenized using the MISONIX Sonicator (Division of QSonica LLC, Newtown, CT, USA). Collected samples were immediately extracted using the described method above and analyzed using HPLC.
4.8. The mCRPC Recurrence Prevention Study in Athymic Nude Mice
For the recurrence prevention study, nineteen 7–8-week-old athymic male nude mice (Strain Foxn
nu-Foxn
1+) were xenografted with 2 × 10
6 the mCRPC CWR-R1ca-Luc cells at the suprascapular region in 100 µL 1:1 serum-free Dulbecco’s Modified Eagle Medium (DMEM) medium-Matrigel. Mice were fed on an HFD (11.4% total fat, Cat# 7004, Envigo/Teklad, Madison, WI, USA) for 7 days before the tumor cells xenografting. When the tumors reached 100 mm
3, mice were subjected to weekly IV injections of 10 mg/kg docetaxel (DTX) (Combi-Blocks, San Diego, CA, USA) dissolved in Tween 80 (20 mg/mL) for four consecutive weeks, followed by daily IP injections of 5 mg/kg ENZ (Combi-Blocks, San Diego, CA, USA) in sterile PBS for three weeks. Following these neoadjuvant regimens, mice were randomly selected and placed into three groups. Groups 1 (
n = 7) received daily oral doses of 20 mg/kg PsA (prepared in sterile PBS-DMSO 925:75). Group 2 (
n = 5) received daily oral PsA 10 mg/kg. Group 3
(n = 7) received daily oral doses of PsA-free vehicle control. Treatments continued for 60 days. Isoflurane-anesthetized mice were subjected to weekly tumor monitoring using PerkinElmer IVIS Lumina Series III (Waltham, MA, USA) 30 min after an IP injection of (+)-luciferin (75 mg/kg, IP, PerkinElmer, Waltham, MA, USA) for efficiency assessment of excision surgery, tumor progression, and tumor metastasis. Following treatment regimen completion, mice were anesthetized using isoflurane (USP-vaporizer method, Matrix VIP-3000), exposure of animals in an anesthesia chamber at 3% isoflurane vaporization rate. Exposure time 2 min. Mice were then euthanized by cervical dislocation according to the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2020 Edition, and tumors were collected [
50]. Tumors were snap frozen using Isopentane (J.T. Baker, Radnor, PA, USA) in liquid nitrogen before storage at −80 °C.
4.9. Tumor RNA Extraction
Following spraying hands with RNaseZapTM (Invitrogen Thermo Fisher Scientific, Waltham, MA, USA), excised tumor cells from the mCRPC recurrence prevention study were defrosted from −80 °C and 85–95 mg were weighed and transferred to a 1.7 mL RNase/DNase-free Eppendorf (Genesee Scientific, Morrisville, NC, USA). About 1 mL of TRizol (Invitrogen-Thermo Scientific, Waltham, MA, USA) was added to each Eppendorf before being homogenized using a MISONIX Sonicator (Division of QSonica LLC, Newtown, CT, USA). Afterwards, samples were stored on ice for 2 h. About 200 µL of molecular-grade CHCl3 (ThermoFisher Scientific, Waltham, MA, USA) was added to each Eppendorf and incubated for 3 min at room temperature. Samples were vortexed for 30 s, then put on ice for 15 min to allow for phase separation. The upper layer was then collected and centrifuged at 4 °C and 12,000× g for 15 min. The supernatant was collected, and 500 µL of molecular-grade isopropyl alcohol (ThermoFisher Scientific, Waltham, MA, USA) was added before incubation on ice for 30 min. Samples were centrifuged at 4 °C and 12,000× g for 10 min, isopropyl alcohol was decanted, and the RNA pellet was reconstituted with 1 mL 70% molecular grade ethanol (Decon Labs, King of Prussia, PA, USA). Samples were then centrifuged at 4 °C and 12,000× g for 10 min, the ethanol supernatant was removed, and an ethanol wash was repeated twice more. RNA pellet was allowed to air dry for 5 min to remove excess traces of ethanol before being suspended in 20–50 µL RNase-free water (VWR International, LLC, Radnor, PA, USA). Samples were stored in −80 °C freezer until the next day, when they were measured using a Reichert (Reichert Technologies, Depew, NY, USA) nanodrop. Nearly 1000 ng were prepared and submitted for sequencing analysis.
4.9.1. RNA-Seq Data Collection and DEG Identification
RNA-Sequencing was performed at a strand-specific 100-cycle paired-end resolution, in an Illumina NovaSeq 6000 sequencing machine (Illumina, San Diego, CA, USA). The samples were multiplexed in two lanes of a flow-cell, resulting in between 48.71 and 40.04 million reads per sample. The read quality was assessed using the FastQC software, v0.12.0 [
53]. On average, the per-sequence quality score measured in the Phred quality scale was above 30 for all the samples. As expected for RNA-sequencing data, QC did not reveal the presence of adapters that required trimming in the sequenced reads. The reads were mapped to the joint human (GRCh38) and mouse (GRCm39) genomes using the STAR software, version 2.7.11b [
54]. On average, 91% of the sequenced reads mapped to the combined genome, resulting in between 42.9 and 36.0 million mapped reads per sample, of which, on average, 80.4% were uniquely mapped reads. Transcript abundance estimates were calculated using the FeatureCounts software, v2.07 [
55]. Expression normalization and differential gene expression calculations were performed using the DESeq2 software, v1.48.2, to identify statistically significant differentially expressed genes [
56]. The significance
p-values were adjusted for multiple hypothesis testing by the Benjamini and Hochberg method, establishing a false discovery rate (FDR) for each gene [
57]. The cut-off criteria for assessing the DEGs were log2FC ≥ 1.5 or ≤−1.5, adjusted
p-value ≤ 0.05, and an FDR ≤ 0.1.
4.9.2. RNA-Seq Data Analysis
Statistical software R (version 4.2.3,
https://www.r-project.org) was implemented in the statistical calculation and interpretation of the DEGs. Pathway enrichment analysis (PEA) was performed using the gene set enrichment analysis (GSEA) database [
58]. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Medicus gene sets were downloaded from the GSEA database and utilized for the PEA [
59,
60,
61]. The Bioconductor packages, v3.21, clusterProfiler, v4.12.6, enrichR, v1.24.4 and DOSE were used to perform the hierarchical clustering and enrichment analysis of the DEGs [
62,
63,
64,
65,
66,
67]. Biological processes, cellular components, and molecular functions of the DEGs were explored using GO term enrichment analysis. Pathway enrichment analysis of the DEGs was performed using KEGG Medicus. Data visualizations were created using packages in tidyverse [
68].
4.9.3. Protein-Protein Interaction and Gene Regulatory Network Analysis
Both lists of upregulated and downregulated DEGs were separately uploaded and analyzed using the STRINGapp in Cytoscape Software, v3.10.4 [
69,
70]. A confidence cutoff of >0.8 was implemented. This confidence indicator ranges from 0 to 1, with 1 being the highest, and indicates how likely STRING judges an interaction to be true, based on evidence from genomic context, gene co-expression, interaction experiments, molecular pathways, automatic text mining, and curated knowledge.