Polymer Films of 2-(Azulen-1-yldiazenyl)-5-(thiophen-2-yl)-1,3,4-thiadiazole: Surface Characterization and Electrochemical Sensing of Heavy Metals †
Abstract
1. Introduction
2. Results
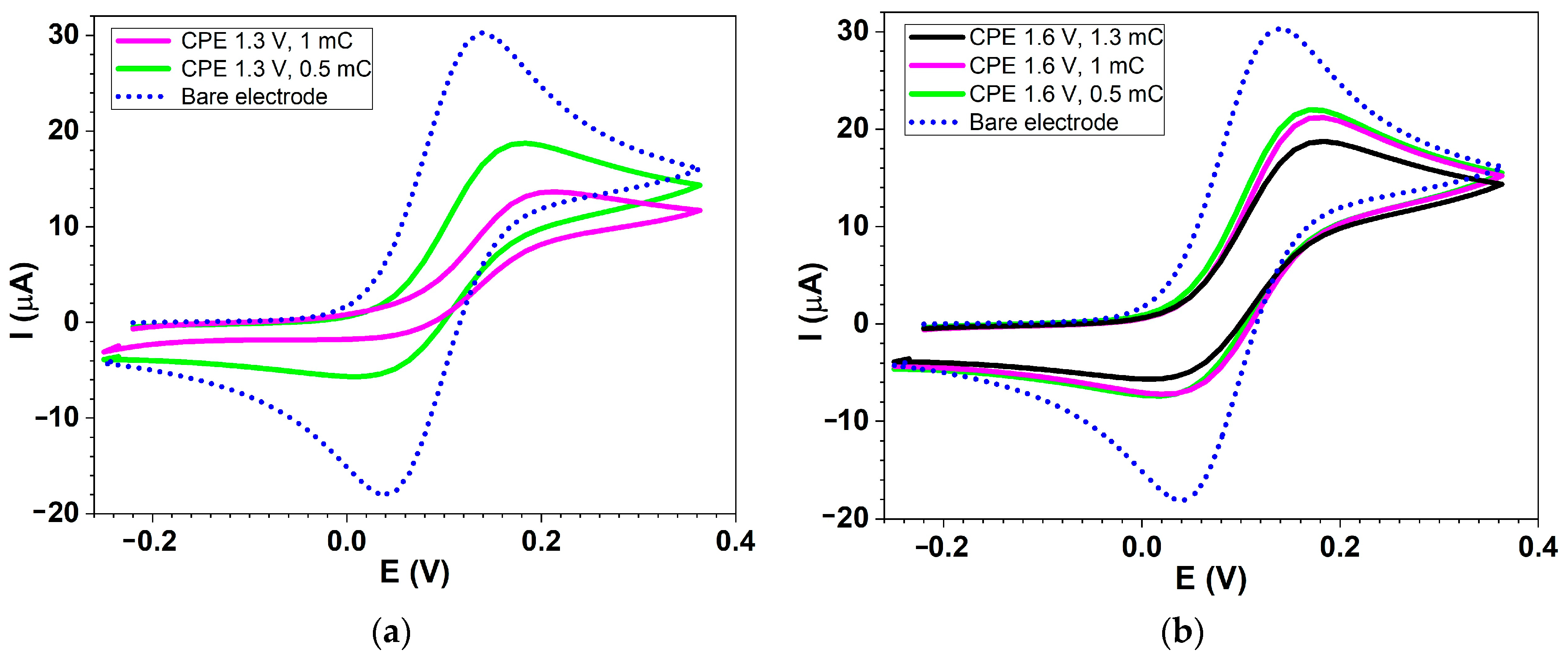
2.1. Preparation of Polymer Films and Electrochemical Characterization of CMEs
2.2. SEM Characterization of CMEs
2.3. AFM Characterization of CMEs
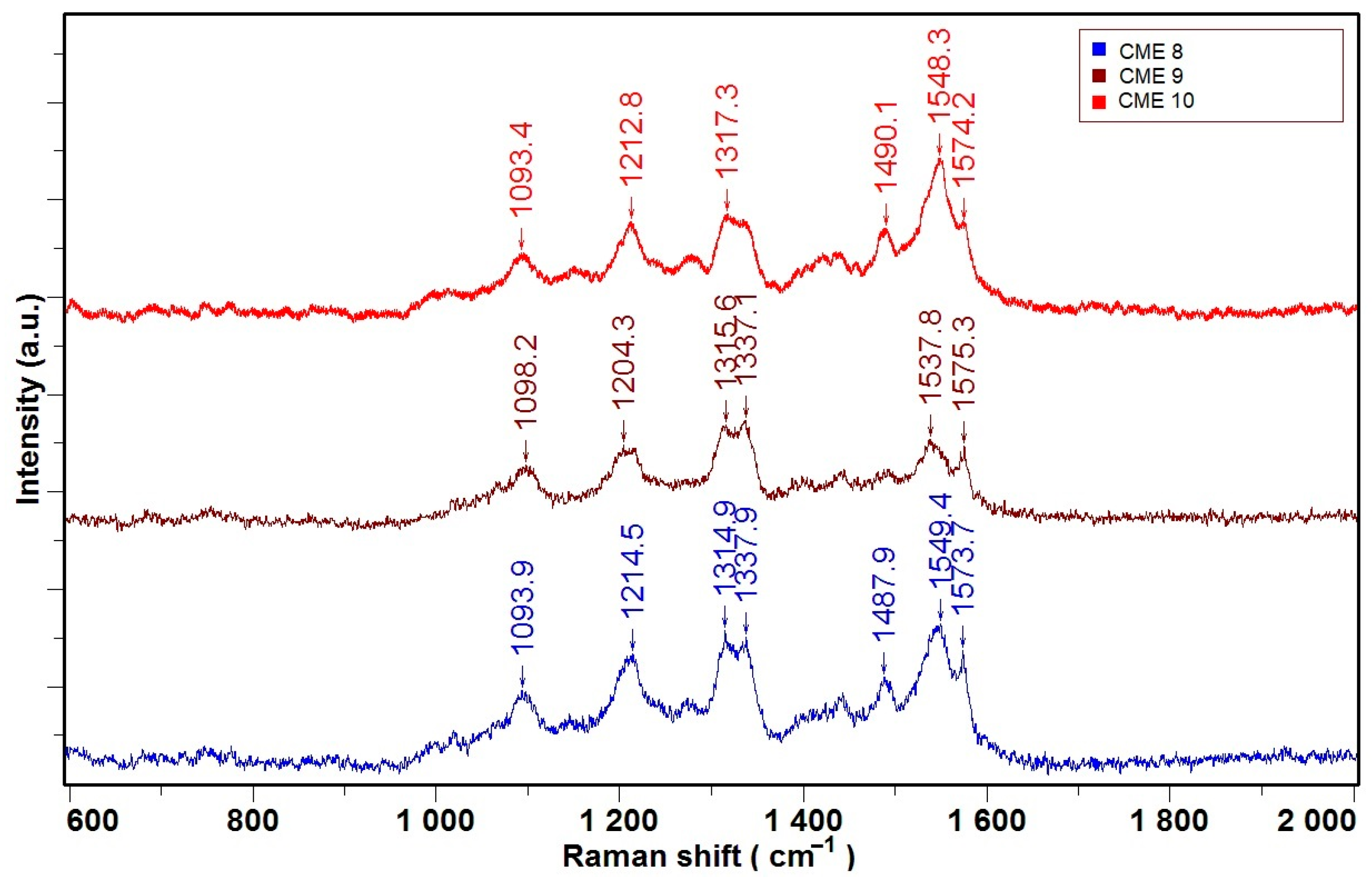
2.4. Raman Characterization of CMEs
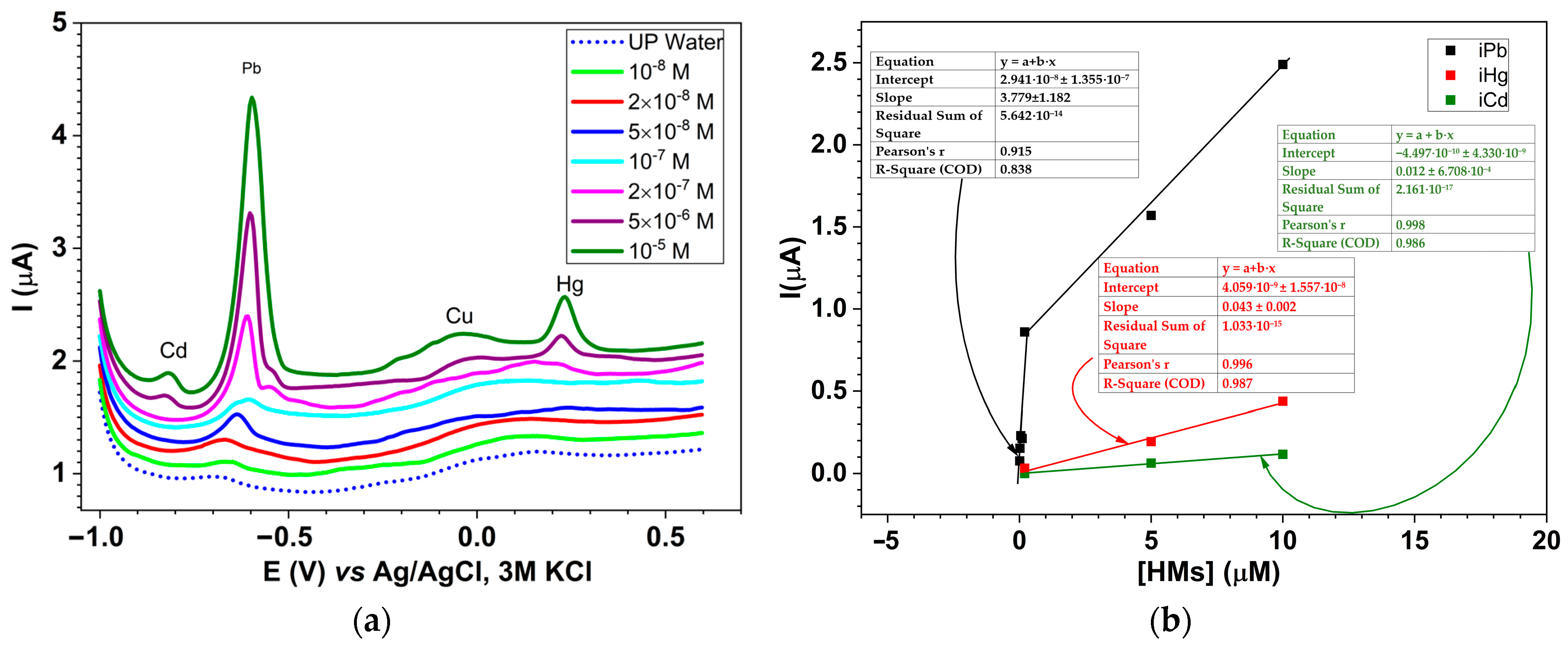
2.5. Electrochemical Sensing of Heavy Metals (HMs) Using CMEs
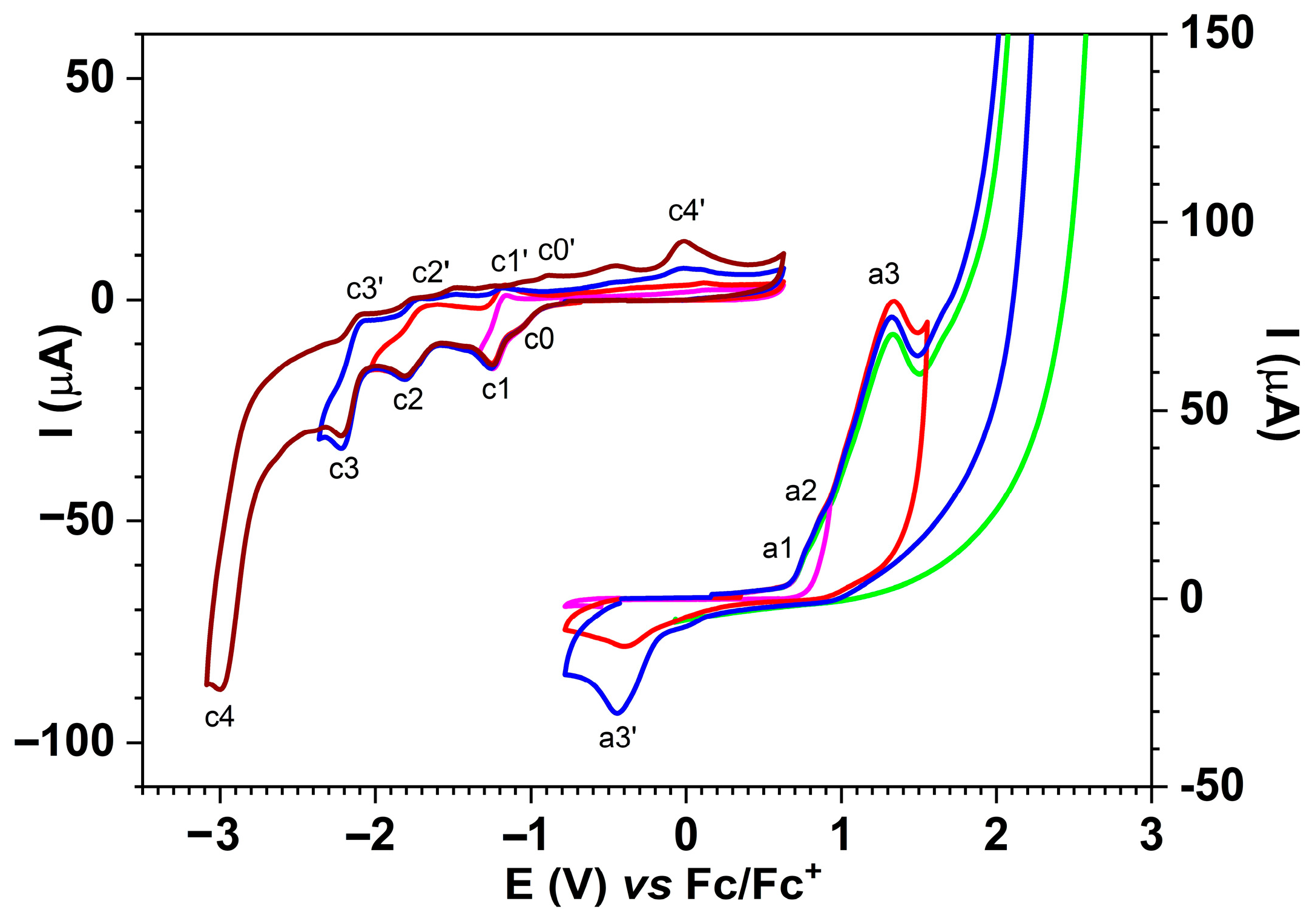
2.6. Investigation of the Ligand’s Electrochemical Properties
2.6.1. Electrochemical Study of the Ligand Through DPV
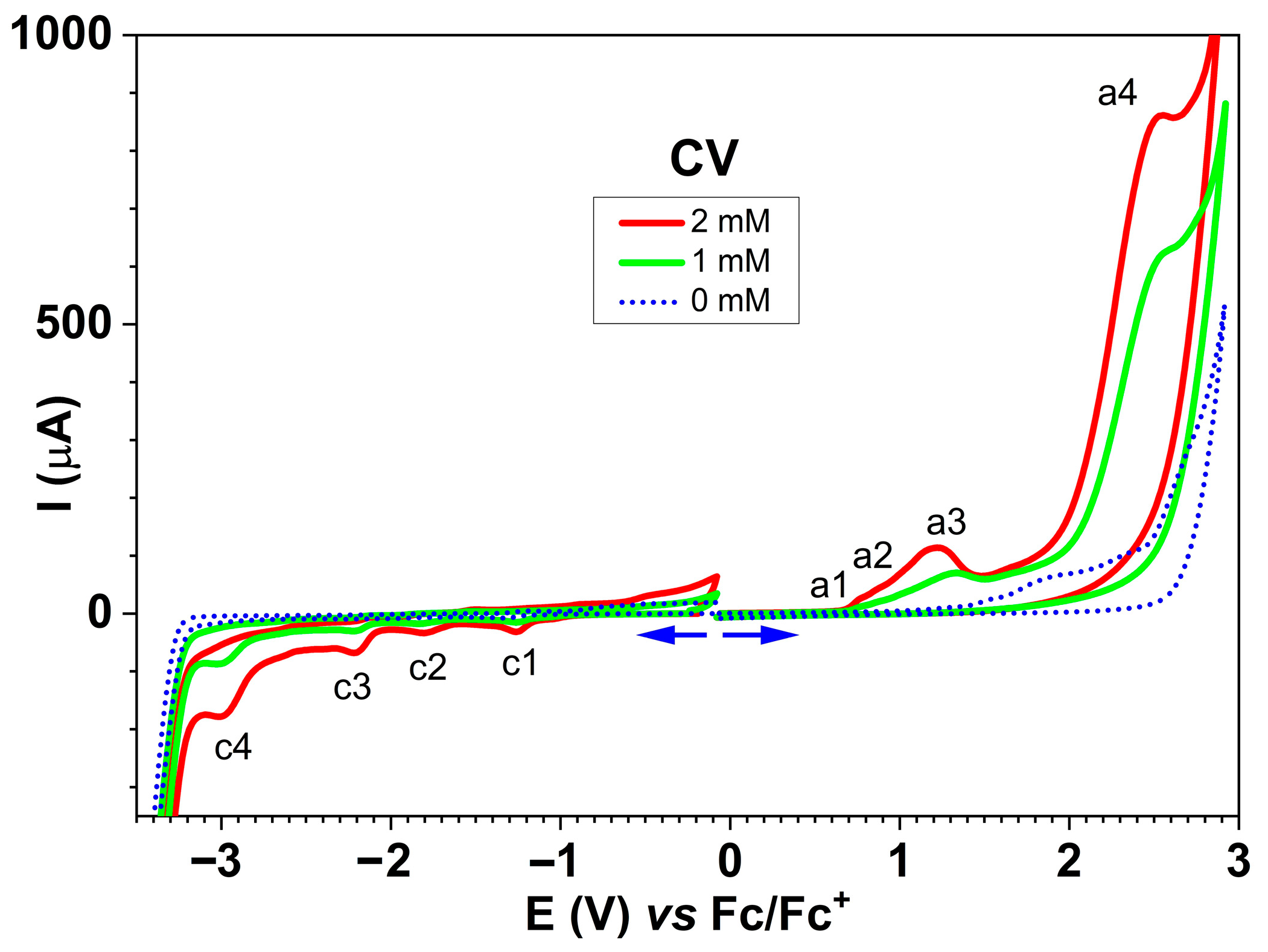
2.6.2. Electrochemical Study of the Ligand Through CV
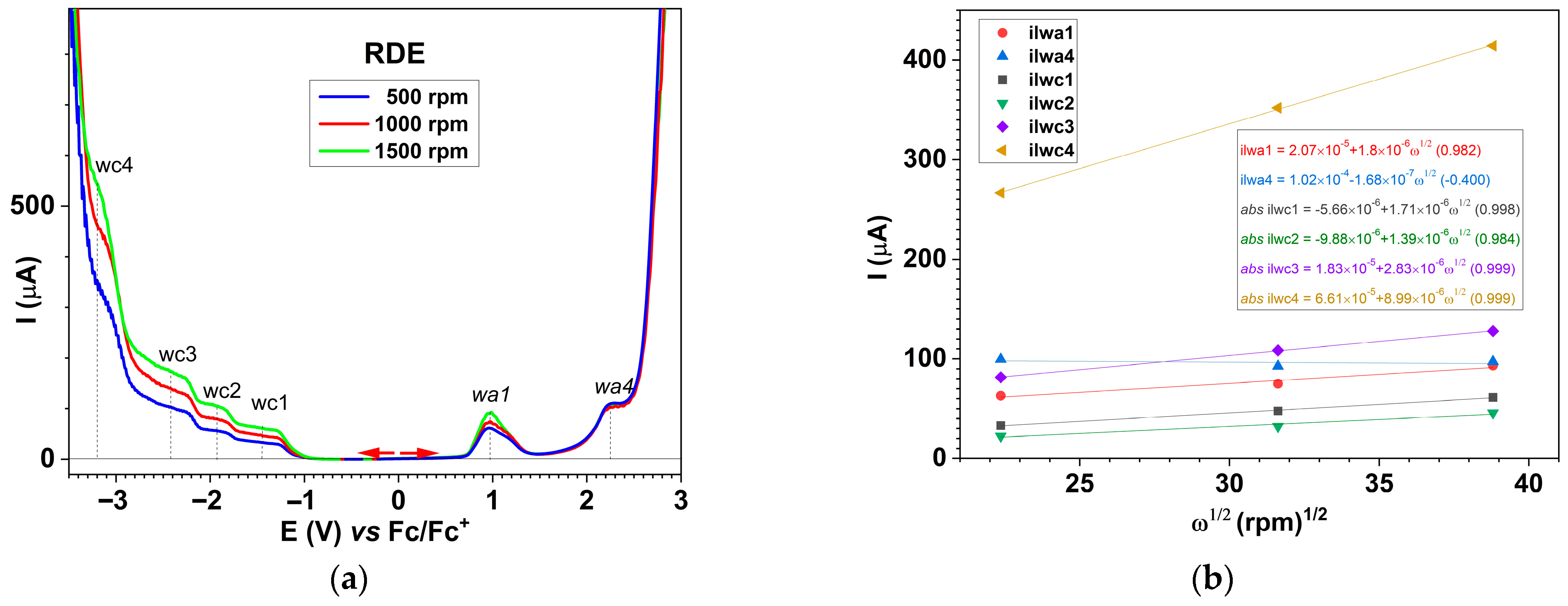
2.6.3. Electrochemical Study of the Ligand Through RDE
3. Discussion
Polymer Film Preparations and Electrochemical Characterization
- CME 8: teff = 161 nm (an extra 6 nm compared with the underlying 155 nm);
- CME 9: teff = 73 nm (an extra 63 nm compared with the underlying 10 nm);
- CME 10: teff = 39 nm (an extra 13 nm compared with the underlying 26 nm).
- -
- 1093 cm−1—C—H bonds;
- -
- 1212 cm−1—C—H bonds (from vinylene group);
- -
- 1314 cm−1, 1337 cm−1—C—C bonds (possible in the range of 1202–1367 cm−1);
- -
- 1482–1509 cm−1—(C–C=C) group;
- -
- 1490 cm−1—C=C—thiophene group;
- -
- 1548 cm−1, 1574 cm−1—C=C bonds from aromatic/olefinic groups.
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Răzuș, A.C. Azulene, Reactivity, and Scientific Interest Inversely Proportional to Ring Size; Part 1: The Five-Membered Ring. Symmetry 2023, 15, 310. [Google Scholar] [CrossRef]
- Răzuș, A.C. A Century of Azulene Chemistry; A Brief Look at Azulenes Building. Symmetry 2025, 17, 335. [Google Scholar] [CrossRef]
- Anchidin-Norocel, L.; Gutt, G.; Tătăranu, E.; Amariei, S. Electrochemical sensors and biosensors: Effective tools for detecting heavy metals in water and food with possible implications for children’s health. Int. J. Environ. Sci. 2024, 40, 100643. [Google Scholar] [CrossRef]
- Sulthana, S.F.; Iqbal, U.M.; Suseela, S.B.; Anbazhagan, R.; Chinthaginjala, R.; Chitathuru, D.; Ahmad, I.; Kim, T.H. Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review. ACS Omega 2024, 9, 25493–25512. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yue, W. Carbon Nanotubes Heavy Metal Detection with Stripping Voltammetry: A Review Paper. Electroanalysis 2017, 29, 1832–1846. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Liu, G. Recent Advances in Chemically Modified Electrodes, Microfabricated Devices and Injection Systems for the Electrochemical Detection of Heavy Metals: A Review. Int. J. Electrochem. Sci. 2017, 12, 8622–8641. [Google Scholar] [CrossRef]
- Răzuș, A.C.; Bîrzan, L.; Cristea, M.; Tecuceanu, V.; Drăghici, C.; Hăngănu, A.; Măgănu, M.; Pintilie, L.; Ungureanu, E.M. New (azulen-1-yldiazenyl) heteroaromatic compounds containing 1,3,4-thiadiazol-3-yl moieties. Rev. Chim. 2019, 70, 1518–1529. [Google Scholar] [CrossRef]
- Helim, R.; Zazoua, A.; Korri-Youssoufi, H. Sustainable Biopolymer-Based Electrochemical Sensors for Trace Heavy Metal Determination in Water: A Comprehensive Review. Chemosensors 2024, 12, 267. [Google Scholar] [CrossRef]
- Manikandan, R.; Rajarathinam, T.; Jayaraman, S.; Jang, H.; Yoon, J.-H.; Lee, J.; Paik, H.-J.; Chang, S.-C. Recent advances in miniaturized electrochemical analyzers for hazardous heavy metal sensing in environmental samples. Coord. Chem. Rev. 2024, 499, 215487. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, J.; Qiao, J.; Ge, F.; Yang, Y.; Zhang, Q. Advancements in Electrochemical Sensing Technology for Heavy Metal Ions Detection. Food Chem. X 2025, 25, 102204. [Google Scholar] [CrossRef]
- Ding, Q.; Li, C.; Wang, H.; Xu, C.; Kuang, H. Electrochemical detection of heavy metal ions in water. Chem. Commun. 2021, 57, 7215–7224. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, H.; Wang, L. Electropolymerized thiadiazole films for simultaneous Pb(II)/Cd(II) detection. Anal. Methods 2012, 4, 3586–3592. [Google Scholar] [CrossRef]
- Anăstăsoaie, V.; Matica, O.T.; Lete, C.; Isopescu, R.; Miskovic-Stankovic, V.; Ungureanu, E.-M. Electrochemical Studies of Azulene Modified Electrodes. Symmetry 2023, 15, 514. [Google Scholar] [CrossRef]
- Otutu, J.O.; Efurhievwe, E.M.; Ameru, S.U. Synthesis and Application of Disazo Dyes Derived from 2-Amino-5-Mercapto-1,3, 4-Thiadiazole and 2-Chloroaniline on Acrylic Fabric and Nylon 66 Fabric. Curr. Res. Chem. 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Arcoria, A.; De Giorgi, M.R.; Fatuzzo, F.; Longo, M.L. Dyeing properties of basic azo-dyes from 2-amino thiadiazole. Dyes Pigment. 1993, 21, 67–74. [Google Scholar] [CrossRef]
- Skripnik, L.I.; Ol’shevskaya, I.A.; Fedorova, L.N.; Rybalka, N.I.; Plaksienko, N.F. Synthesis and Study of the Properties of Tetrazoles, Azides, Triazenes, and Azo Compounds of the Thiadiazole Series. Chem. Heterocycl. Compd. 1980, 16, 721–724. [Google Scholar] [CrossRef]
- Giles, R.R.; Weaver, M.A. Nitrogen and/or Sulfur Containing Heterocyclic Azo Dyes with Aniline, Tetrahydroquinoline and Benzomorpholine Couplers Having Sulfate Group. U.S. Patent 4255326, 10 March 1981. [Google Scholar]
- Giles, R.R.; Weaver, M.A. Azo Dyes from 1,3,4-thiadiazol-2-yl and 1,2,4-thiadiazol-5-yl Moieties Bearing Sulfated Hydroxyalkoxycarbonyl or N-(hydroxyalkyl)carbamoyl Groups on Their Rings. U.S. Patent 4302387, 24 November 1981. [Google Scholar]
- Weaver, M.A.; Coates, C.A. Azo Dyes from 2-amino-5-cyanomethylthio-1,3,4-thiadiazole and Aniline and Tetrahydroquinoline Couplers. U.S. Patent 4528368, 9 July 1985. [Google Scholar]
- Mikoshiba, H.; Kamio, T. Azo Dye Compound Having 1,2,4-triazole as the Azo Component. U.S. Patent 6458194B1, 1 October 2002. [Google Scholar]
- Raghav, M.; Patel, V. Synthesis and Application of 2-Aminothiadiazole Disperse Dyes for Nylon Fabrics. J. Serb. Chem. Soc. 2001, 66, 87–93. [Google Scholar] [CrossRef]
- Maradiya, H. Monoazo Disperse Dyes Based on 2-Amino-1,3,4-Thiadiazole Derivatives. J. Serb. Chem. Soc. 2002, 67, 709–718. [Google Scholar] [CrossRef]
- Austin, P.W.; Fishwick, B.R. Disperse Monoazo Dyes, Their Preparation and Their Application to Synthetic Textile Materials. Euro Patent 0023386A1, 4 February 1981. [Google Scholar]
- Keerthi Kumar, C.T.; Keshavayya, J.; Rajesh, T.N.; Peethambar, S.K.; Shoukat Ali, A.R. Synthesis, Characterization, and Biological Activity of 5-Phenyl-1,3,4-Thiadiazole-2-Amine Incorporated Azo Dye Derivatives. Org. Chem. Int. 2013, 2013, 370626. [Google Scholar] [CrossRef]
- Kamel, M.G.; Sroor, F.M.; Othman, A.M.; Hassaneen, H.M.; Abdallah, T.A.; Saleh, F.M.; Teleb, M.A.M. Synthesis and Biological Evaluation of New 1,3,4-Thiadiazole Derivatives as Potent Antimicrobial Agents. Monatshefte Für Chem. Chem. Mon. 2022, 153, 929–937. [Google Scholar] [CrossRef]
- Keerthi Kumar, C.T.; Keshavayya, J.; Rajesh, T.; Peethambar, S.K.; Shoukat Ali, R.A. Synthesis, Characterization and Antimicrobial Activity of Heterocyclic Azodyes Derived from Thiadiazole. Chem. Sci. Trans. 2013, 2, 1346. [Google Scholar] [CrossRef]
- Sahoo, J.; Paidesetty, S.K. Medicinal Interest of Azo-Based Organic Compounds: A Review. Asian J. Pharm. Clin. Res. 2016, 9, 33–39. [Google Scholar]
- Răzuș, A.C.; Birzan, L.; Nae, S.; Răzuș, S.A.; Cimpeanu, V.; Stanciu, C. Azulene-1-Azopyridines. Synthesis and N-Alkylation at Pyridine Moiety. Synth. Commun. 2002, 32, 825–837. [Google Scholar] [CrossRef]
- Răzuș, A.C.; Birzan, L.; Nae, S.; Cristian, L.; Chiraleu, F.; Cimpeanu, V. Azulene-1-azopyridine 1’-oxides. Dye. Pigm. 2003, 57, 223–233. [Google Scholar] [CrossRef]
- Răzuș, A.C.; Birzan, L.; Nae, S.; Surugiu, M.N.; Cimpeanu, V. Azulene-1-azo-2′-thiazoles. Synthesis and Properties. J. Heterocycl. Chem. 2003, 40, 995–1004. [Google Scholar] [CrossRef]
- Răzuș, A.C.; Birzan, L.; Surugiu, N.M.; Corbu, A.C.; Chiraleu, F. Syntheses of Azulen-1-Yl-Benzothiazol-2-Yl Diazenes. Dye. Pigm. 2007, 74, 26–33. [Google Scholar] [CrossRef]
- Cristian, L.; Sasaki, I.; Lacroix, P.G.; Donnadieu, B.; Asselberghs, I.; Clays, K.; Răzuș, A.C. Donating Strength of Azulene in Various Azulen-1-Yl-Substituted Cationic Dyes: Application in Nonlinear Optics. Chem. Mater. 2004, 16, 3543–3551. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Y.-H.; Han, M.-Y. Stimuli-Responsive Conjugated Copolymers Having Electro-Active Azulene and Bithiophene Units in the Polymer Skeleton: Effect of Protonation and p-Doping on Conducting Properties. Macromolecules 2004, 37, 3222–3230. [Google Scholar] [CrossRef]
- Brotea, A.-G.; Matica, O.-T.; Musina, C.; Pandele, A.M.; Trusca, R.; Ungureanu, E.-M. Chemically Modified Electrodes Based on 4-((5-Isopropyl-3,8-dimethylazulen-1-yl)methylene)-2-phenyloxazol-5(4H)-one. Symmetry 2024, 16, 245. [Google Scholar] [CrossRef]
- Anăstăsoaie, V.; Omocea, C.; Enache, L.-B.; Anicăi, L.; Ungureanu, E.-M.; van Staden, J.F.; Enăchescu, M. Surface Characterization of New Azulene-Based CMEs for Sensing. Symmetry 2021, 13, 2292. [Google Scholar] [CrossRef]
- Enache, L.B.; Anăstăsoaie, V.; Bîrzan, L.; Ungureanu, E.M.; Diao, P.; Enăchescu, M. Polyazulene-Based Materials for Heavy Metal Ion Detection: Tetrazole-Functionalized Azulene Monomers for Pb(II) Sensing (LOD ≈ 5 × 10−8 M). Coatings 2020, 10, 869. [Google Scholar] [CrossRef]
- Irfan, A.; Kalam, A.; Al-Sehemi, A.G.; Dubey, M. Investigation of the Effect of Substituents on Electronic and Charge Transport Properties of Benzothiazole Derivatives. Molecules 2022, 27, 8672. [Google Scholar] [CrossRef]
- Anăstăsoaie, V. Modified Electrodes for Electrocatalytic Oxidation of Water and for Detection of Heavy Metals. Ph.D. Thesis, Faculty of Chemical Engineering and Biotechnology, Politehnica University of Bucharest, Bucharest, Romania, 2022. [Google Scholar]
- Meana-Esteban, B.; Lete, C.; Kvarnström, C.; Ivaska, A. Raman and in Situ FTIR-ATR Characterization of Polyazulene Films and Its Derivate. J. Phys. Chem. B 2006, 110, 23343–23350. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.F.; Trevelyan, J.E.; Smith, T.; Williams, K.P.J. A Multi-Wavelength Raman Study of Some Oligothiophenes and Polythiophene. Physchem 2023, 3, 210–219. [Google Scholar] [CrossRef]
- Al-Yasari, R.K. Analysis of molecular structures and spectroscopic properties of thiophene molecules. J. Chem. Pharm. Sci. 2017, 10, 940–944. [Google Scholar]
- Larkin, P. Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Edwards, H.G.M. Spectra–Structure Correlations in Raman Spectroscopy. Society for Applied Spectroscopy. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1838–1871. [Google Scholar]
- University of California, Irvine. Raman Band Correlation Table. Department of Chemistry. Available online: https://www.chem.uci.edu/~dmitryf/manuals/Raman%20correlations.pdf (accessed on 4 August 2025).
- HORIBA Scientific. Optimizing Carbon Material Analysis with Raman Spectroscopy (Application Note AN-RA93). Available online: https://static.horiba.com/fileadmin/Horiba/Application/Materials/Material_Research/Material_Research/AN-RA93-Optimizing_Carbon_Material_Analysis_with_Raman_Spectroscopy.pdf (accessed on 4 August 2025).




| CME | [L] (mM) | Preparation Potential/ Electrode | Electric Charge (mC) | CME’s Characterization |
|---|---|---|---|---|
| 1 | 1.3 | 1.3 V/GC3 | 0.5 | Fc *a |
| 2 | 1.3 | 1.3 V/GC3 | 1 | Fc *a |
| 3 | 1.3 | 1.6 V/GC3 | 0.5 | Fc *a |
| 4 | 1.3 | 1.6 V/GC3 | 1 | Fc *a |
| 5 | 1.3 | 1.6 V/GC3 | 1.3 | Fc *a |
| 6 | 1 | 1.3 V/GC3 | 1 | Chronoamperometry, Fc *b |
| 7 | 1 | 1.3 V/GC3 | 1.2 | Fc *a |
| 8 | 1 | 0.9 V/GC6 | 4 | SEM, AFM, Raman |
| 9 | 1 | 1.3 V/GC6 | 4 | SEM, AFM, Raman |
| 10 | 1 | 1.3 V/GC6 | 14 | SEM, AFM, Raman |
| AFM Topography | Line Profile Across Indentation | Thickness (nm) | |
|---|---|---|---|
| CME 8 | 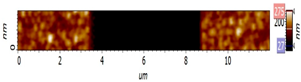 | 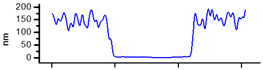 | 155 |
| CME 9 |  | 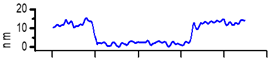 | 10 |
| CME 10 |  | 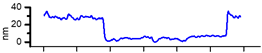 | 25 |
| Peak | Method | Characteristics of the Peak | |
|---|---|---|---|
| DPV | CV | ||
| a1 | 0.74 | 0.86 | Irreversible |
| a2 | 1.04 | 1.14 | Irreversible |
| a3 | 1.23 | 1.32 | Irreversible |
| a4 | 2.27 | 2.55 | Irreversible |
| c1 | −1.22 | −1.25 | Reversible |
| c2 | −1.77 | −1.83 | Reversible |
| c3 | −2.16 | −2.2 | Reversible |
| c4 | −2.94 | −3.01 | Irreversible |
| No. | Conditions\Fc Parameter | Epa (V) | 10−5 · ipa (A) | Epc (V) | 10−6 · ipc(A) | ΔEp *1 | Ef *2 |
|---|---|---|---|---|---|---|---|
| 1 | Bare electrode | 0.138 | 3.025 | 0.037 | −0.181 | 0.101 | 0.087 |
| 2 | CPE at 1.3 V, 0.5 mC | 0.183 | 1.887 | 0.015 | −5.624 | 0.168 | 0.099 |
| 3 | CPE at 1.3 V, 1 mC | 0.205 | 1.356 | −0.007 | −1.882 | 0.212 | 0.099 |
| 4 | CPE at 1.6 V, 0.5 mC | 0.171 | 2.215 | 0.015 | −7.34 | 0.156 | 0.094 |
| 5 | CPE at 1.6 V, 1 mC | 0.175 | 2.124 | 0.027 | −7.138 | 0.148 | 0.104 |
| 6 | CPE at 1.6 V, 1.3 mC | 0.179 | 1.881 | 0.012 | −5.619 | 0.167 | 0.094 |
| L-CME | Condition | Morphology | Aggregation | Porosity |
|---|---|---|---|---|
| CME 8 | +0.9 V, 4 mC | Fine, granular | Low | Low |
| CME 9 | +1.3 V, 4 mC | Clustered, porous | Moderate | Moderate |
| CME 10 | +1.3 V, 14 m | Dense, networked | High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musina, C.; Cristea, M.; Gavrilă, R.; Brincoveanu, O.; Comănescu, F.C.; Anăstăsoaie, V.; Stanciu, G.; Ungureanu, E.-M. Polymer Films of 2-(Azulen-1-yldiazenyl)-5-(thiophen-2-yl)-1,3,4-thiadiazole: Surface Characterization and Electrochemical Sensing of Heavy Metals. Molecules 2025, 30, 3959. https://doi.org/10.3390/molecules30193959
Musina C, Cristea M, Gavrilă R, Brincoveanu O, Comănescu FC, Anăstăsoaie V, Stanciu G, Ungureanu E-M. Polymer Films of 2-(Azulen-1-yldiazenyl)-5-(thiophen-2-yl)-1,3,4-thiadiazole: Surface Characterization and Electrochemical Sensing of Heavy Metals. Molecules. 2025; 30(19):3959. https://doi.org/10.3390/molecules30193959
Chicago/Turabian StyleMusina (Borsaru), Cornelia, Mihaela Cristea, Raluca Gavrilă, Oana Brincoveanu, Florin Constantin Comănescu, Veronica Anăstăsoaie, Gabriela Stanciu, and Eleonora-Mihaela Ungureanu. 2025. "Polymer Films of 2-(Azulen-1-yldiazenyl)-5-(thiophen-2-yl)-1,3,4-thiadiazole: Surface Characterization and Electrochemical Sensing of Heavy Metals" Molecules 30, no. 19: 3959. https://doi.org/10.3390/molecules30193959
APA StyleMusina, C., Cristea, M., Gavrilă, R., Brincoveanu, O., Comănescu, F. C., Anăstăsoaie, V., Stanciu, G., & Ungureanu, E.-M. (2025). Polymer Films of 2-(Azulen-1-yldiazenyl)-5-(thiophen-2-yl)-1,3,4-thiadiazole: Surface Characterization and Electrochemical Sensing of Heavy Metals. Molecules, 30(19), 3959. https://doi.org/10.3390/molecules30193959







