Pharmacokinetic Profile of Extracts from the Chayote (Sechium edule) H387 07 Hybrid and Phytochemical Characterization of Its Segregant H387 M16 for Potential Therapeutic Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization of Sechium H387 07 Hybrid and Segregants
2.1.1. Flavonoids Identified
2.1.2. Cucurbitacins Identified
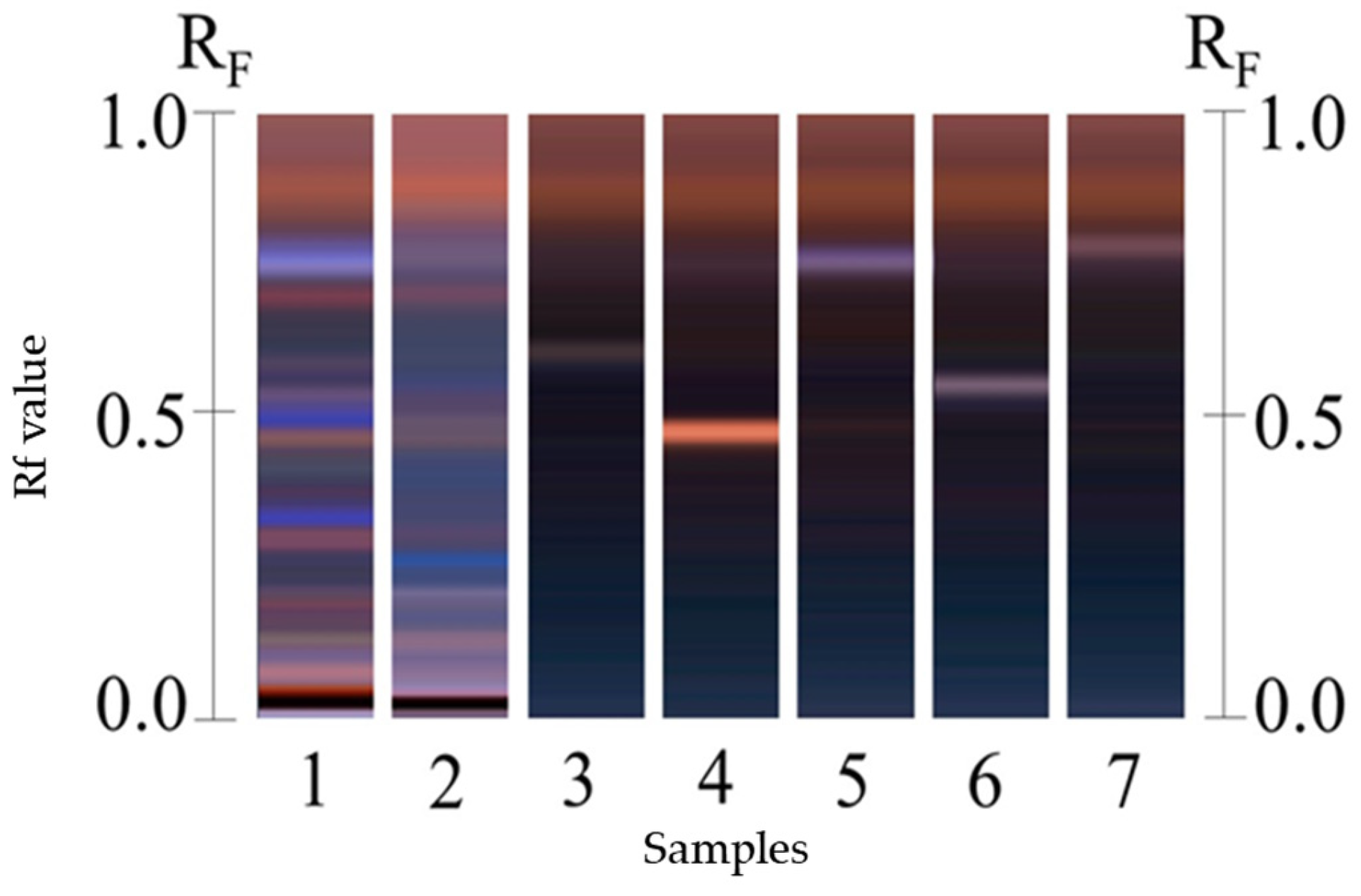
2.1.3. Identification of Cucurbitacins by High-Performance Thin-Layer Chromatography
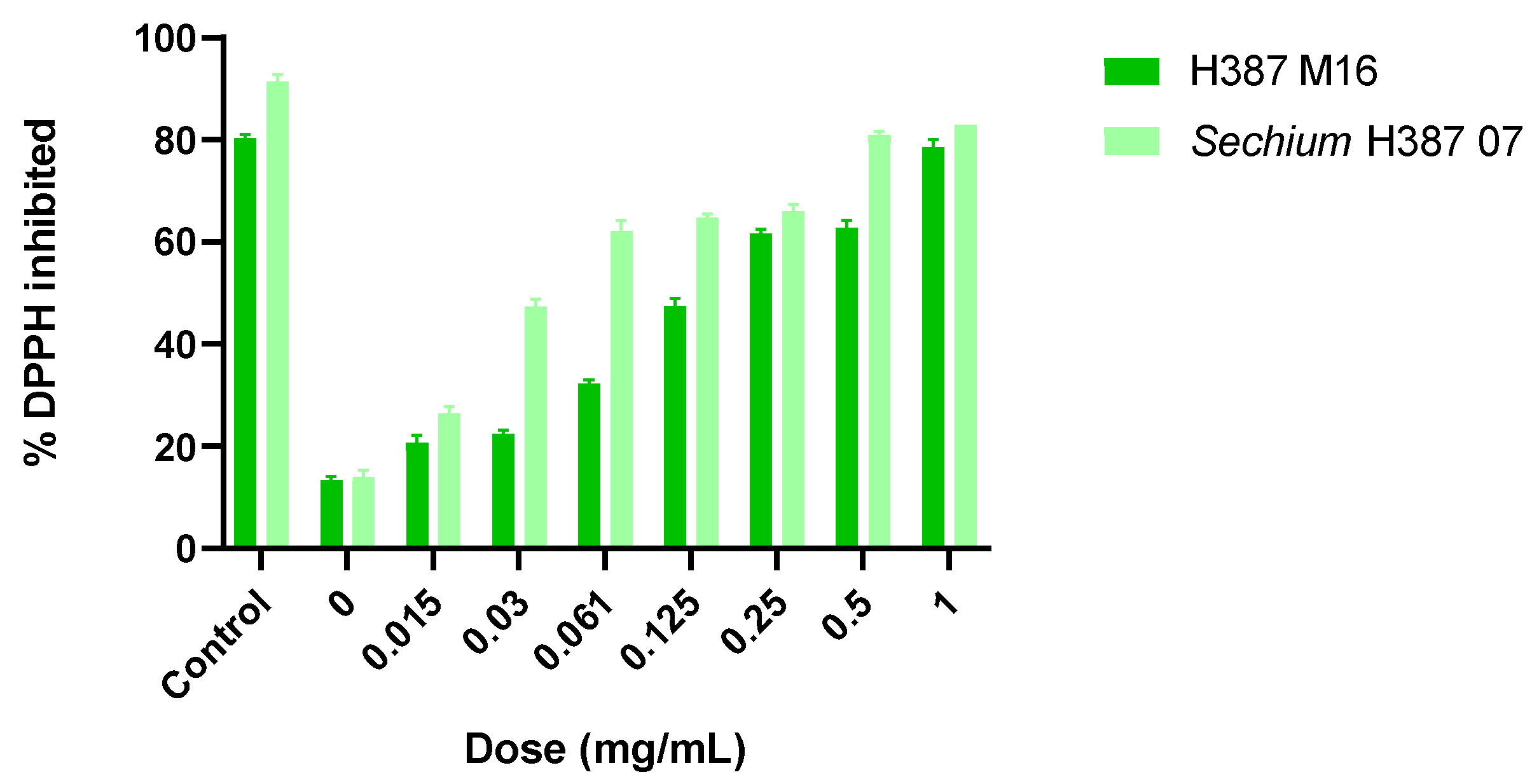
2.2. Antioxidant Capacity of the Extracts by 2,2-Diphenyl-1-Picrylhydrazyl
2.3. Determination of Cardiac, Splenic, Hepatic, Renal, and Cerebral Indices in Mice Administered with the Sechium H387 07 Extract
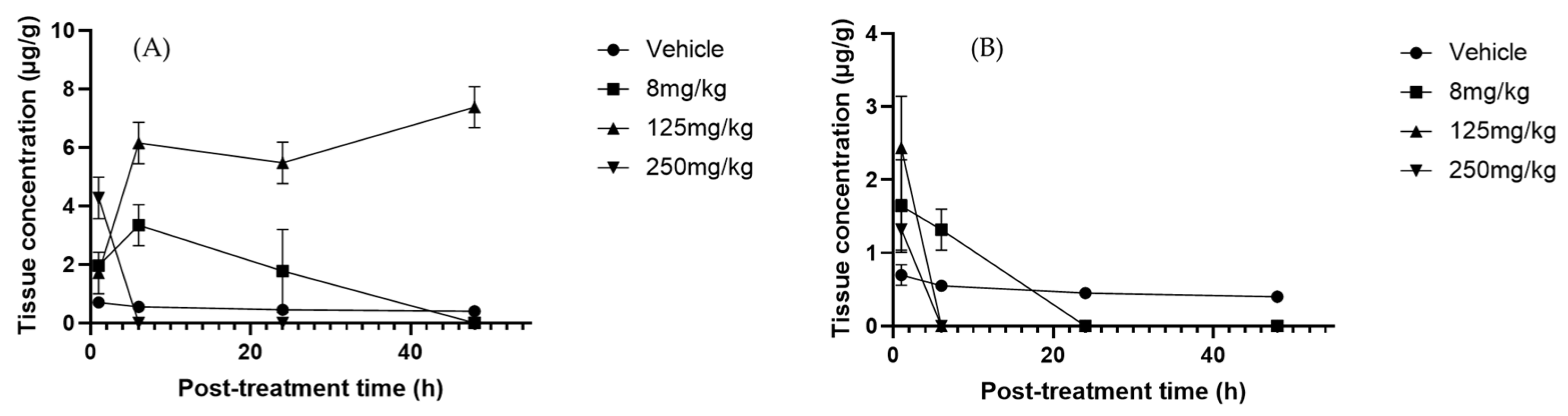
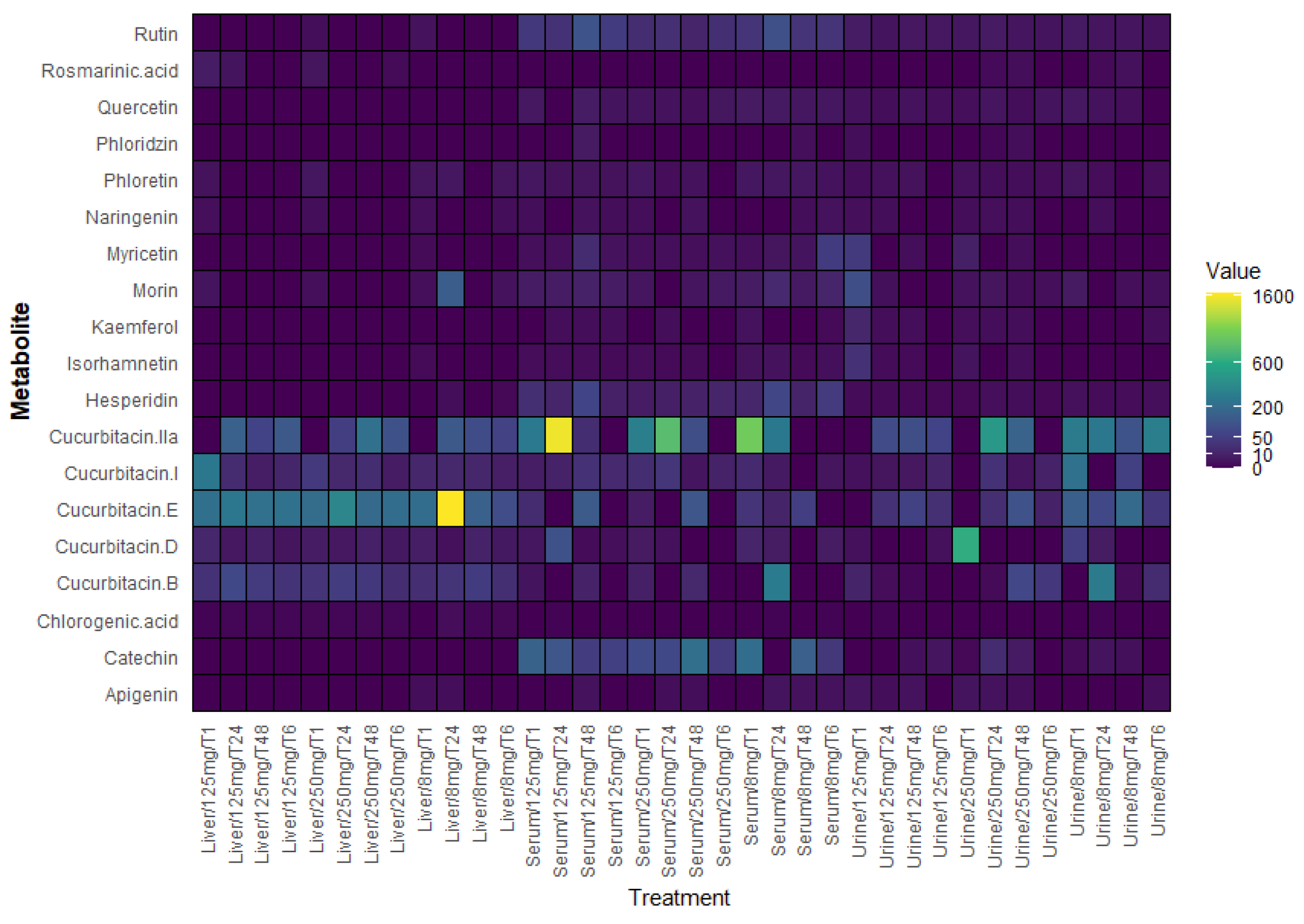
2.4. Polyphenols in Serum, Liver, and Urine
2.5. Identification of Cucurbitacins in the Serum, Liver, and Urine of Mice Treated with Sechium H387 07 Extract
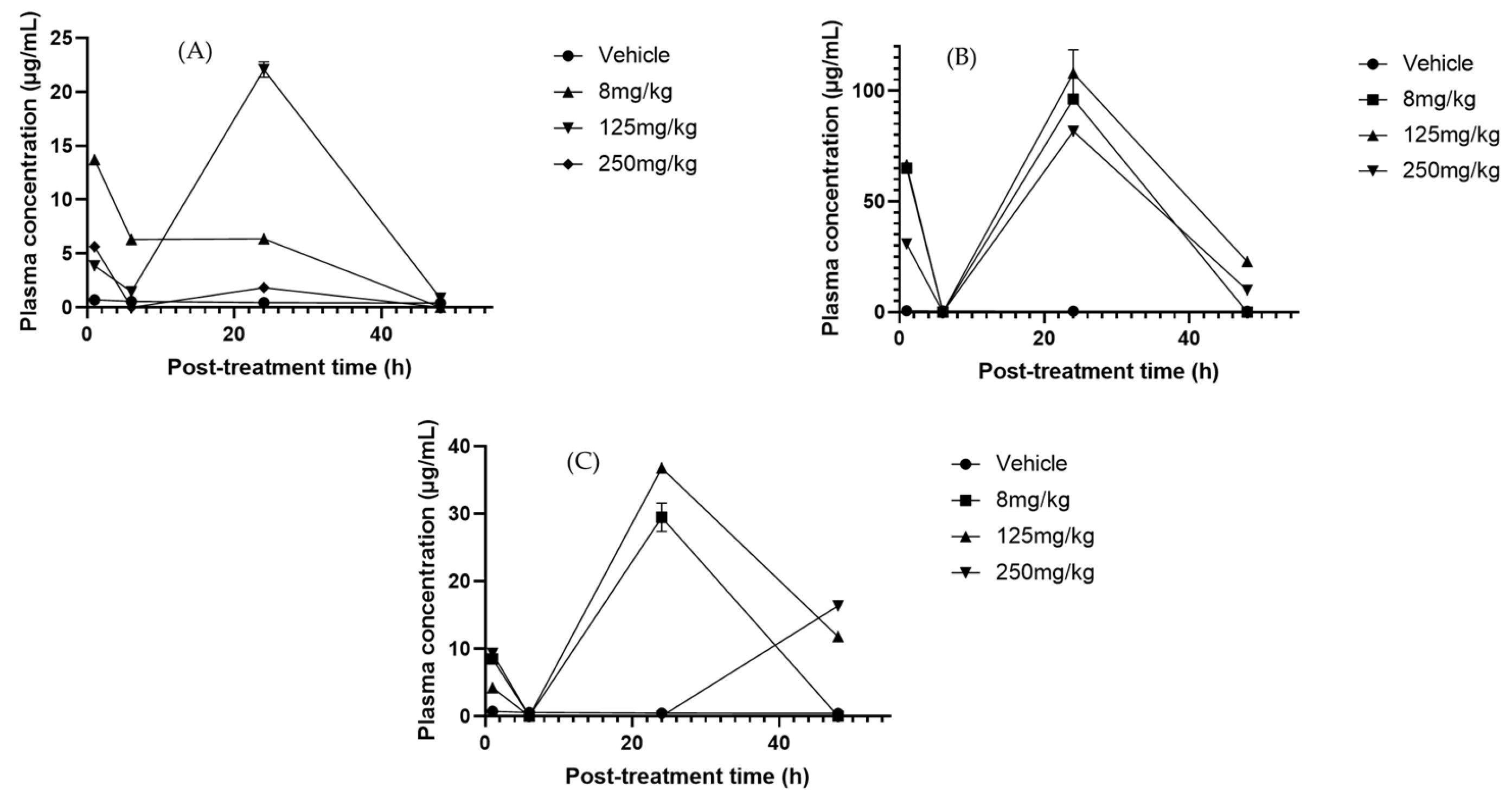
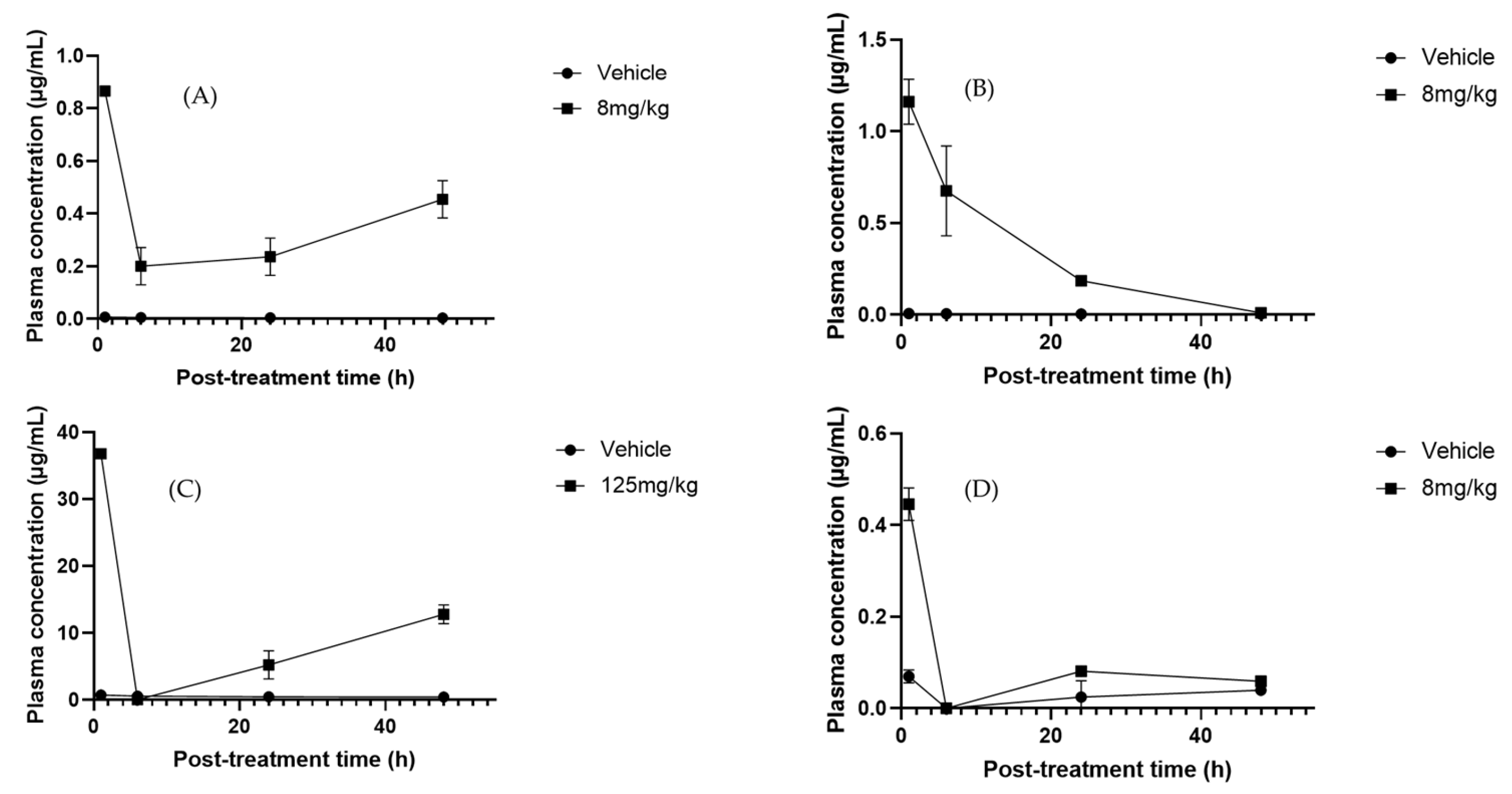
2.6. Pharmacokinetic Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Extract Preparation
3.3. Phytochemical Analysis by Preliminary Tests
3.3.1. Thin-Layer Chromatography Detection
3.3.2. Identification of Secondary Metabolites by High-Performance Liquid Chromatography
3.3.3. Procedure for Identification of Cucurbitacins by High-Performance Thin-Layer Chromatography
4. Assessment of Antioxidant Capacity via 2,2-Diphenyl-1-Picrylhydrazyl Assay
5. Animal Maintenance
5.1. Mouse Treatment
5.2. Pharmacokinetic Evaluation of Secondary Metabolites in Sechium H387 07
5.3. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrera-Guzmán, L.Á.; Cadena-Iñiguez, J.; Legaria-Solano, J.P.; Sahagún-Castellanos, J.; Ramirez-Ojeda, G. Potential distribution of domesticated Sechium edule (Cucurbitaceae) in Mexico. Acta Biol. Colomb. 2022, 27, 326–335. [Google Scholar] [CrossRef]
- Sulistiyani, N.I.; Ainurrofiq, A.; Suryanti, V. Antibacterial Activity of Chayote (Sechium edule Swartz) Squash Extracts and Their Phytochemical Constituents. J. Biodivers. Biotechnol. 2022, 2, 26–32. [Google Scholar] [CrossRef]
- Becerra Ramírez, E. Efecto Antiulceroso in Vivo del Extracto Etanólico del Fruto de Sechium edule (Jacq.) Sw. en Ratas Albinas. Master’s Thesis, Universidad de Lima, Lima, Peru, 2025. [Google Scholar]
- Loizzo, M.R.; Bonesi, M.; Menichini, F.; Tenuta, M.C.; Leporini, M.; Tundis, R. Antioxidant and Carbohydrate-Hydrolysing Enzymes Potential of Sechium edule (Jacq.) Swartz (Cucurbitaceae) Peel, Leaves and Pulp Fresh and Processed. Plant Foods Hum. Nutr. 2016, 71, 381–387. [Google Scholar] [CrossRef]
- Siahaan, J.M. Effect of Antihipoglycemic Sechium edule Jacq. Swartz. Etanol Extract on Histopathologic Changes in Hyperglycemic Mus musculus L. Indones. J. Med. 2017, 2, 86–93. [Google Scholar] [CrossRef]
- Islami, A.I.; Sumarni, S.; Ramlan, D. Siamese Pumpkin Juice (Sechium edule (Jacq.) Sw) to Decreased Blood Pressure Of Postpartum Mother’s Hypertension. J. Kebidanan Dan Kesehat. Tradis. 2022, 7, 72–84. [Google Scholar] [CrossRef]
- Mumtaz, S.M.F.; Paul, S.; Bag, A.K. Effect of Sechium edule on Chemical Induced Kidney Damage in Experimental Animals. Bangladesh J. Pharmacol. 2013, 8, 28–35. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Soto-hernandez, M.; Torres-Salas, A.; Aguiñiga, I.; Ruiz-Posadas, L.; Rivera-Martínez, A.; Avendaño-Arrazate, C.; Santiago-Osorio, E. The Antiproliferative Effect of Chayote Varieties (Sechium edule (Jacq.) Sw.) on Tumour Cell Lines. J. Med. Plant Res. 2013, 7, 455–460. Available online: https://www.researchgate.net/publication/338801500_The_antiproliferative_effect_of_chayote_varieties_Sechium_edule_Jacq_Sw_on_tumour_cell_lines (accessed on 28 September 2022).
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, Y.; Deng, X.; Liu, M.; Luo, W. Cucurbitacin B Supplementation Reduces Inflammatory Responses and Alveolar Bone Loss via Regulating MPO, COX-2 and RANK/RANKL/OPG Signals in a Rodent Model of Ligature-Induced Periodontitis. J. King Saud Univ.-Sci. 2020, 32, 1889–1895. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, F.; Wu, C. Hepatoprotective Effects of Apple Polyphenols on CCl4-Induced Acute Liver Damage in Mice. J. Agric. Food Chem. 2010, 58, 6525–6531. [Google Scholar] [CrossRef]
- Arjaibi, H.M.; Ahmed, M.S.; Halaweish, F.T. Mechanistic Investigation of Hepato-Protective Potential for Cucurbitacins. Med. Chem. Res. 2017, 26, 1567–1573. [Google Scholar] [CrossRef]
- Shawkey, A.M.; Rabeh, M.A.; Abdellatif, A.O. Biofunctional Molecules from Citrullus Colocynthis: An HPLC/MS Analysis in Correlation to Antimicrobial and Anticancer Activities. Adv. Life Sci. Technol. 2014, 17, 51–61. [Google Scholar]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Silvestre, G.F.G.; de Lucena, R.P.; Alves, H.d.S. Cucurbitacins and the Immune System: Update in Research on Anti- Inflammatory, Antioxidant, and Immunomodulatory Mechanisms. Available online: https://www.ingentaconnect.com/content/ben/cmc/pre-prints/content-34994307 (accessed on 11 May 2022).
- Patilaya, P.; Husori, D. Preliminary Study on the Anthelmintic Activity of the Leaf Ethanolic Extract of Indonesian Curanga fel-terrae (Lour.) Merr. Int. J. Pharm. Tech. Res. 2015, 8, 347–351. [Google Scholar]
- Ramdani, D.; Yuniarti, E.; Jayanegara, A.; Chaudhry, A.S. Roles of Essential Oils, Polyphenols, and Saponins of Medicinal Plants as Natural Additives and Anthelmintics in Ruminant Diets: A Systematic Review. Animals 2023, 13, 767. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral Activities of Flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Kapoor, N.; Ghorai, S.M.; Kushwaha, P.K.; Shukla, R.; Aggarwal, C.; Bandichhor, R. Plausible Mechanisms Explaining the Role of Cucurbitacins as Potential Therapeutic Drugs against Coronavirus 2019. Inform. Med. Unlocked 2020, 21, 100484. [Google Scholar] [CrossRef]
- Peter, E.L.; Nagendrappa, P.B.; Ajayi, C.O.; Sesaazi, C.D. Total Polyphenols and Antihyperglycemic Activity of Aqueous Fruits Extract of Abelmoschus esculentus: Modeling and Optimization of Extraction Conditions. PLoS ONE 2021, 16, e0250405. [Google Scholar] [CrossRef]
- Hernández Navia, S.E.; Figueroa-Hernández, J.L.; Rodríguez-Zavala, J.S.; Rodriguez-Sosa, M.; Martínez-Vázquez, M. Anti-Diabetic Effects of Cucurbitacins from Ibervillea lindheimeri on Induced Mouse Diabetes. J. Chem. 2022, 2022, 3379557. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, F.; Cai, Q.; Wu, H.; Jiang, T.; Wang, L.; Chen, X.; Gao, P.; Yang, X.; Chen, Y.; et al. Cardioprotective Polyphenols from Geum japonicum Var. Chinense. Phytochemistry 2024, 218, 113935. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Z.; Wu, Q.-Q.; Jiang, X.-H.; Yuan, Y.; Chang, W.; Bian, Z.Y.; Zhu, J.X.; Tang, Q.-Z. Cucurbitacin B Protects Against Pressure Overload Induced Cardiac Hypertrophy. J. Cell. Biochem. 2017, 118, 3899–3910. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuang, S.; Guan, H.; Li, F.; Zou, H.; Li, D. New Insights into the Anti-Apoptotic Mechanism of Natural Polyphenols in Complex with Bax Protein. J. Biomol. Struct. Dyn. 2024, 42, 3081–3093. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Park, K.A.; Byun, H.S.; Won, M.; Jeon, J.; Lee, Y.; Seok, J.H.; Choi, S.-W.; Lee, S.-H.; et al. Cucurbitacin Induces Autophagy through Mitochondrial ROS Production Which Counteracts to Limit Caspase-Dependent Apoptosis. Autophagy 2012, 8, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-L.; Tao, W.-H.; Yang, T.-X.; Qiao, J.-G. Anticancer Effect of Cucurbitacin B on MKN-45 Cells via Inhibition of the JAK2/STAT3 Signaling Pathway. Exp. Ther. Med. 2016, 12, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Arévalo Galarza, M.L. Desarrollo y transferencia del híbrido amargo de chayote [Sechium edule (Jacq) Sw.] “H 387 07”. Agro Divulg. 2023, 3, 63–78. [Google Scholar] [CrossRef]
- Castellaneta, A.; Losito, I.; Palmitessa, O.D.; Somma, A.; Didonna, A.; Renna, M.; Santamaria, P.; Calvano, C.D.; Cataldi, T.R.I. Confirmation of the Unexpected Occurrence of Bitterness-Inducing Cucurbitacins in Edible Unripe Melons by a Two-Step Workflow Based on Liquid Chromatography with Combined UV and High-Resolution MS Detection. Food Chem. 2025, 469, 142586. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Ruíz-Posadas, L.d.M.; Cadena-Zamudio, J.D.; González-Ugarte, A.K.; Weiss Steider, B.; Santiago-Osorio, E. Fruit Extract from A Sechium edule Hybrid Induce Apoptosis in Leukaemic Cell Lines but Not in Normal Cells. Nutr. Cancer 2015, 67, 250–257. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Aguiñiga-Sánchez, I.; Uriostegui-Arias, M.T.; Santiago-Osorio, E.; Ruiz-Posadas, L.d.M.; Soto-Hernández, M. Antiproliferative Effect of Sechium edule (Jacq.) Sw., Cv. Madre Negra Extracts on Breast Cancer In Vitro. Separations 2022, 9, 230. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Suwalsky, M.; Colina, J.R.; Castillo, I.; Rosado-Pérez, J.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Phytochemical Analysis and Antioxidant and Anti-Inflammatory Capacity of the Extracts of Fruits of the Sechium Hybrid. Molecules 2020, 25, 4637. [Google Scholar] [CrossRef]
- Salazar-Aguilar, S.; Ruiz-Posadas, L.D.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Rivera-Martínez, A.R.; Aguirre-Medina, J.F. Sechium edule (Jacq.) Swartz, a New Cultivar with Antiproliferative Potential in a Human Cervical Cancer HeLa Cell Line. Nutrients 2017, 9, 798. [Google Scholar] [CrossRef]
- Gitonga, H.W.; Kyamanywa, S.; Arusei, P.; Lukanda, M.M.; Edema, R.; Dramadri, I.O. Genotype × Environment Interaction Influence Secondary Metabolite in Cowpea Infested by Flower Bud Thrips. Agronomy 2022, 12, 3210. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I. Potencial Antileucémico in Vitro de Extractos de Cuatro Genotipos de Sechium spp. (Cucurbitaceae). Ph.D. Thesis, Colegio de Postgraduados, Estado de México, Mexico, 2013. [Google Scholar]
- Glassmire, A.E.; Zehr, L.N.; Wetzel, W.C. Disentangling Dimensions of Phytochemical Diversity: Alpha and Beta Have Contrasting Effects on an Insect Herbivore. Ecology 2020, 101, e03158. [Google Scholar] [CrossRef] [PubMed]
- Uckele, K.A.; Jahner, J.P.; Tepe, E.J.; Richards, L.A.; Dyer, L.A.; Ochsenrider, K.M.; Philbin, C.S.; Kato, M.J.; Yamaguchi, L.F.; Forister, M.L.; et al. Phytochemistry Reflects Different Evolutionary History in Traditional Classes versus Specialized Structural Motifs. Sci. Rep. 2021, 11, 17247. [Google Scholar] [CrossRef] [PubMed]
- Napier, J.D.; Heckman, R.W.; Juenger, T.E. Gene-by-Environment Interactions in Plants: Molecular Mechanisms, Environmental Drivers, and Adaptive Plasticity. Plant Cell 2023, 35, 109–124. [Google Scholar] [CrossRef]
- Dandawate, P.; Subramaniam, D.; Panovich, P.; Standing, D.; Krishnamachary, B.; Kaushik, G.; Thomas, S.M.; Dhar, A.; Weir, S.J.; Jensen, R.A.; et al. Cucurbitacin B and I Inhibits Colon Cancer Growth by Targeting the Notch Signaling Pathway. Sci. Rep. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Dittharot, K.; Dakeng, S.; Suebsakwong, P.; Suksamrarn, A.; Patmasiriwat, P.; Promkan, M. Cucurbitacin B Induces Hypermethylation of Oncogenes in Breast Cancer Cells. Planta Med. 2019, 85, 370–378. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B Interacts Synergistically with Antibiotics against Staphylococcus aureus Clinical Isolates and Exhibits Antiviral Activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Xue, Y.; Li, R.; Fang, P.; Ye, Z.-Q.; Zhao, Y.; Zhou, Y.; Zhang, K.-Q.; Li, L. NLRP3 Inflammasome Inhibitor Cucurbitacin B Suppresses Gout Arthritis in Mice. J. Mol. Endocrinol. 2021, 67, 27–40. [Google Scholar] [CrossRef]
- Lin, Y.; Kotakeyama, Y.; Li, J.; Pan, Y.; Matsuura, A.; Ohya, Y.; Yoshida, M.; Xiang, L.; Qi, J. Cucurbitacin B Exerts Antiaging Effects in Yeast by Regulating Autophagy and Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 4517091. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lee, I.-S.; Park, J.Y.; Kim, Y.; An, E.-J.; Jang, H.-J. Cucurbitacin B Induces Hypoglycemic Effect in Diabetic Mice by Regulation of AMP-Activated Protein Kinase Alpha and Glucagon-Like Peptide-1 via Bitter Taste Receptor Signaling. Front. Pharmacol. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Li, J.; Sun, K.; Muroi, M.; Gao, L.; Chang, Y.-T.; Osada, H.; Xiang, L.; Qi, J. Cucurbitacin B Induces Neurogenesis in PC12 Cells and Protects Memory in APP/PS1 Mice. J. Cell. Mol. Med. 2019, 23, 6283–6294. [Google Scholar] [CrossRef]
- Caseys, C.; Stritt, C.; Glauser, G.; Blanchard, T.; Lexer, C. Effects of Hybridization and Evolutionary Constraints on Secondary Metabolites: The Genetic Architecture of Phenylpropanoids in European Populus Species. PLoS ONE 2015, 10, e0128200. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H.; Arévalo-Galarza, M.d.L.; Cisneros-Solano, V.M.; Ruiz-Posadas, L.d.M.; Aguirre-Medina, J.F.; Watanabe, K.; Machida-Hirano, R.; Barrera-Guzmán, L.A. Varietal Descriptors for the Distinction of Underutilized Varieties of Sechium Edule (Jacq) Swartz. Plants 2022, 11, 3309. [Google Scholar] [CrossRef]
- Rivera-Ponce, E.A.; Arévalo-Galarza, M.d.L.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Ramírez-Rodas, Y.; García-Osorio, C. Characteristics and Potential Use of Fruits from Different Varietal Groups of Sechium edule (Jacq.) Sw. Horticulturae 2024, 10, 844. [Google Scholar] [CrossRef]
- Uriostegui-Arias, M.T. Análisis Fitoquímico y Efecto Antiproliferativo de Genotipos de Sechium Edule (Jacq.) Sw. Sobre Cáncer de Mama. Master’s Thesis, Colegio de Postgraduados, Estado de México, Mexico, 2014. [Google Scholar]
- Nakamura, Y.; Ishimitsu, S.; Tonogai, Y. Effects of Quercetin and Rutin on Serum and Hepatic Lipid Concentrations, Fecal Steroid Excretion and Serum Antioxidant Properties. J. Health Sci. 2000, 46, 229–240. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Dong, L.; Li, J.; He, W.; Chen, X.; Hu, Z. Investigation of the Interaction between Naringin and Human Serum Albumin. J. Mol. Struct. 2008, 875, 1–8. [Google Scholar] [CrossRef]
- Kaci, H.; Bodnárová, S.; Fliszár-Nyúl, E.; Lemli, B.; Pelantová, H.; Valentová, K.; Bakos, É.; Özvegy-Laczka, C.; Poór, M. Interaction of Luteolin, Naringenin, and Their Sulfate and Glucuronide Conjugates with Human Serum Albumin, Cytochrome P450 (CYP2C9, CYP2C19, and CYP3A4) Enzymes and Organic Anion Transporting Polypeptide (OATP1B1 and OATP2B1) Transporters. Biomed. Pharmacother. 2023, 157, 114078. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. Bioavailability of Phloretin and Phloridzin in Rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Gao, M.; Wu, C.; Yang, C.; Yang, J.; Wu, G.; Yang, B.; Kuang, H. Simultaneous Determination of Cucurbitacin B and Cucurbitacin E in Rat Plasma by UHPLC-MS/MS: A Pharmacokinetics Study after Oral Administration of Cucurbitacin Tablets. J. Chromatogr. B 2017, 1065–1066, 63–69. [Google Scholar] [CrossRef]
- Thomas, S.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, Y.; Liao, F.; Ou-Yang, Y.; Liu, Y.; Yang, X. Probing the Binding of Morin to Human Serum Albumin by Optical Spectroscopy. J. Pharm. Biomed. Anal. 2008, 46, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-W.; Choi, S.H.; Hong, J.-K.; Kim, K.M.; Kang, S.C.; Yoon, I.-S. Pharmacokinetics and Extensive Intestinal First-Pass Effects of Apigenin and Its Active Metabolite, Apigenin-7-O-Glucuronide, in Rats. J. Pharm. Investig. 2024, 54, 467–481. [Google Scholar] [CrossRef]
- Gradolatto, A.; Basly, J.-P.; Berges, R.; Teyssier, C.; Chagnon, M.-C.; Siess, M.-H.; Canivenc-Lavier, M.-C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab. Dispos. 2005, 33, 49–54. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and Polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar]
- Lněničková, K.; Procházková, E.; Skálová, L.; Matoušková, P.; Bártíková, H.; Souček, P.; Szotáková, B. Catechins Variously Affect Activities of Conjugation Enzymes in Proliferating and Differentiated Caco-2 Cells. Molecules 2016, 21, 1186. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Zarco, N.; Casas-Grajales, S.; Ramos-Tovar, E.; Flores-Beltrán, R.E.; Arauz, J.; Shibayama, M.; Favari, L.; Tsutsumi, V.; Segovia, J.; et al. Naringenin Prevents Experimental Liver Fibrosis by Blocking TGFβ-Smad3 and JNK-Smad3 Pathways. World J. Gastroenterol. 2017, 23, 4354–4368. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, M. Mutual Interactions between Flavonoids and Enzymatic and Transporter Elements Responsible for Flavonoid Disposition via Phase II Metabolic Pathways. RSC Adv. 2012, 2, 7948–7963. [Google Scholar] [CrossRef]
- Lü, J.; Zhang, D.; Zhang, X.; Sa, R.; Wang, X.; Wu, H.; Lin, Z.; Zhang, B. Network Analysis of the Herb–Drug Interactions of Citrus Herbs Inspired by the “Grapefruit Juice Effect”. ACS Omega 2022, 7, 35911–35923. [Google Scholar] [CrossRef]
- Alsanea, S.; Gao, M.; Liu, D. Phloretin Prevents High-Fat Diet-Induced Obesity and Improves Metabolic Homeostasis. AAPS J. 2017, 19, 797–805. [Google Scholar] [CrossRef]
- Li, Y.; Paxton, J.W. The Effects of Flavonoids on the ABC Transporters: Consequences for the Pharmacokinetics of Substrate Drugs. Expert Opin. Drug Metab. Toxicol. 2013, 9, 267–285. [Google Scholar] [CrossRef]
- Liu, Z.; Kumar, M.; Devi, S.; Kabra, A. The Mechanisms of Cucurbitacin E as a Neuroprotective and Memory-Enhancing Agent in a Cerebral Hypoperfusion Rat Model: Attenuation of Oxidative Stress, Inflammation, and Excitotoxicity. Front. Pharmacol. 2021, 12, 794933. [Google Scholar] [CrossRef]
- Wang, S.; Guan, X.; Zhong, X.; Yang, Z.; Huang, W.; Jia, B.; Cui, T. Simultaneous Determination of Cucurbitacin IIa and Cucurbitacin IIb of Hemsleya amabilis by HPLC–MS/MS and Their Pharmacokinetic Study in Normal and Indomethacin-Induced Rats. Biomed. Chromatogr. 2016, 30, 1632–1640. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, J.; Huang, Q.; Ren, Y.; Li, T.; Zhang, X.; Yao, R.; Sun, J. Cucurbitacin IIa: A Review of Phytochemistry and Pharmacology. Phytother. Res. 2021, 35, 4155–4170. [Google Scholar] [CrossRef]
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and Cancer Intervention: Chemistry, Biology and Mechanisms (Review). Int. J. Oncol. 2018, 52, 19–37. [Google Scholar] [CrossRef]
- Hunsakunachai, N.; Nuengchamnong, N.; Jiratchariyakul, W.; Kummalue, T.; Khemawoot, P. Pharmacokinetics of Cucurbitacin B from Trichosanthes cucumerina L. in Rats. BMC Complement. Altern. Med. 2019, 19, 157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhao, Q.; Wu, Q.; Chang, J.; Xue, H.; Liu, C.; Liu, X. A New Sensitive UPLC-MS/MS Method for the Determination of Cucurbitacin B in Rat Plasma: Application to an Absolute Bioavailability Study. RSC Adv. 2018, 8, 30978–30985. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Ranjan, A.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Kumar, M.; Dhama, K.; Lee, E.H.C.; et al. Cucurbitacins as Potent Chemo-Preventive Agents: Mechanistic Insight and Recent Trends. Biomolecules 2023, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic Properties and Drug Interactions of Apigenin, a Natural Flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- Chen, Z.; Ying, X.; Meng, S.; Zhu, X.; Jiang, H.; Cao, Q.; Li, X.; Meng, F. High-Performance Liquid Chromatographic Determination and Pharmacokinetic Study of Apigenin-7-O-β-D-Glucoside in Rat Plasma after Intravenous Administration. Arch. Pharm. Res. 2011, 34, 741–746. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and Metabolism of the Flavonoid Chrysin in Normal Volunteers. Br. J. Clin. Pharmacol. 2001, 51, 143–146. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Mi, L.; Shen, X.; Feng, T.; Liu, X.; Wang, Q. Study on pharmacokinetics, tissue distribution, and excretion of phloretin and its prodrug 2′,4′,6′,4-Tetra-O-acetylphloretin in rats using LC–MS/MS. Acta Chromatogr. 2019, 31, 63–70. [Google Scholar] [CrossRef]
- Lee, D.H.; Iwanski, G.B.; Thoennissen, N.H. Cucurbitacin: Ancient Compound Shedding New Light on Cancer Treatment. Sci. World J. 2010, 10, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Magnusson, B.M.; Burczynski, F.J.; Weiss, M. Enterohepatic Circulation: Physiological, Pharmacokinetic and Clinical Implications. Clin. Pharmacokinet. 2002, 41, 751–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-R.; Gao, M.-X.; Yang, K. Cucurbitacin B Inhibits Cell Proliferation and Induces Apoptosis in Human Osteosarcoma Cells via Modulation of the JAK2/STAT3 and MAPK Pathways. Exp. Ther. Med. 2017, 14, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Soto-Hernandez, M.; Avendaño-Arrazate, C.; Ruiz-Posadas, L.; Santiago-Osorio, E.; Ramos, M.; Cisneros, V.; Aguirre Medina, J. Production, Genetics, Postharvest Management and Pharmacological Characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. [Google Scholar]
- Harborne, A.J. Phytochemical Methods A Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; ISBN 978-0-412-57260-9. [Google Scholar]

| Group of Metabolites | Sechium H387 07 | H387 M16 |
|---|---|---|
| Tannins | + | − |
| Phenols | +++ | + |
| Flavonoids | + | + |
| Alkaloids | − | − |
| Saponins | + | ++ |
| Terpenoids | +++ | +++ |
| Segregant 387 M16 | Sechium H387 07 | |
|---|---|---|
| Flavonoids | (mg of Metabolite/g of Extract) | |
| Myricetin | 0.00134 | 0.00594 |
| Kaempferol | 0.00209 | 0.00217 |
| Isorhamnetin | 0.00247 | 0.00124 |
| Apigenin | 0.0035 | 0.00249 |
| Rutin | 0.0668 | 0.00993 |
| Morin | 0.01840 | 0.01049 |
| Quercetin | 0.01365 | 0.00628 |
| Catechin | 0.14132 | 0.09205 |
| Hesperidin | 0.05008 | 0.03749 |
| Phloridzin | 0.03711 | 0.02945 |
| Naringenin | 0.00510 | 0.00526 |
| Phloretin | 0.00465 | 0.01233 |
| Total | 0.34669 | 0.21513 |
| Segregant H387 M16 | Sechium H387 07 | |
|---|---|---|
| Cucurbitacins | (mg of Metabolite/g of Extract) | |
| Cucurbitacin D | N/I | N/I |
| Cucurbitacin I | 0.039 | 3.061 |
| Cucurbitacin B | 2.63 | 4.595 |
| Cucurbitacin IIA | N/I | N/I |
| Cucurbitacin E | 0.0779 | 0.594 |
| Total | 2.747 | 8.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Tiburcio, E.E.; Soto-Hernández, R.M.; Aguiñiga-Sánchez, I.; Cadena-Iñiguez, J.; Ruiz-Posadas, L.d.M.; Peña-Valdivia, C.B.; Gómez-Yáñez, H. Pharmacokinetic Profile of Extracts from the Chayote (Sechium edule) H387 07 Hybrid and Phytochemical Characterization of Its Segregant H387 M16 for Potential Therapeutic Applications. Molecules 2025, 30, 3948. https://doi.org/10.3390/molecules30193948
Delgado-Tiburcio EE, Soto-Hernández RM, Aguiñiga-Sánchez I, Cadena-Iñiguez J, Ruiz-Posadas LdM, Peña-Valdivia CB, Gómez-Yáñez H. Pharmacokinetic Profile of Extracts from the Chayote (Sechium edule) H387 07 Hybrid and Phytochemical Characterization of Its Segregant H387 M16 for Potential Therapeutic Applications. Molecules. 2025; 30(19):3948. https://doi.org/10.3390/molecules30193948
Chicago/Turabian StyleDelgado-Tiburcio, Eugenia Elisa, Ramón Marcos Soto-Hernández, Itzen Aguiñiga-Sánchez, Jorge Cadena-Iñiguez, Lucero del Mar Ruiz-Posadas, Cecilia B. Peña-Valdivia, and Héctor Gómez-Yáñez. 2025. "Pharmacokinetic Profile of Extracts from the Chayote (Sechium edule) H387 07 Hybrid and Phytochemical Characterization of Its Segregant H387 M16 for Potential Therapeutic Applications" Molecules 30, no. 19: 3948. https://doi.org/10.3390/molecules30193948
APA StyleDelgado-Tiburcio, E. E., Soto-Hernández, R. M., Aguiñiga-Sánchez, I., Cadena-Iñiguez, J., Ruiz-Posadas, L. d. M., Peña-Valdivia, C. B., & Gómez-Yáñez, H. (2025). Pharmacokinetic Profile of Extracts from the Chayote (Sechium edule) H387 07 Hybrid and Phytochemical Characterization of Its Segregant H387 M16 for Potential Therapeutic Applications. Molecules, 30(19), 3948. https://doi.org/10.3390/molecules30193948







