Synthesis, Structures and Corrosion Inhibition Properties of 4-Nitrophenylacetato-Rare-Earth(III) 1D Coordination Polymers
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterisation
2.2. X-Ray Crystal Structures
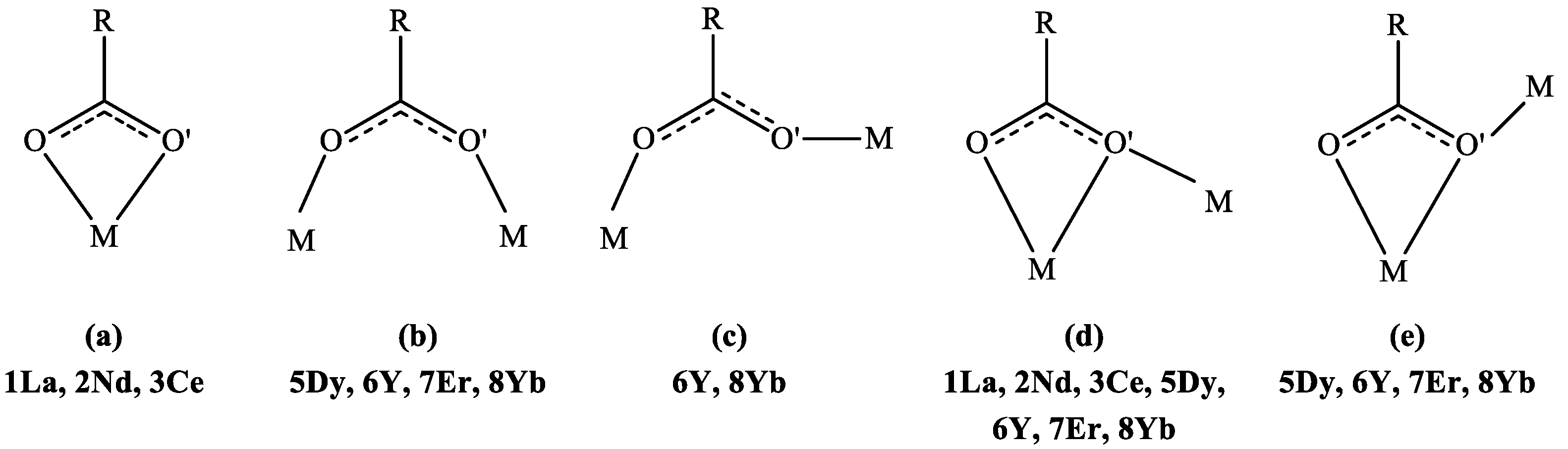
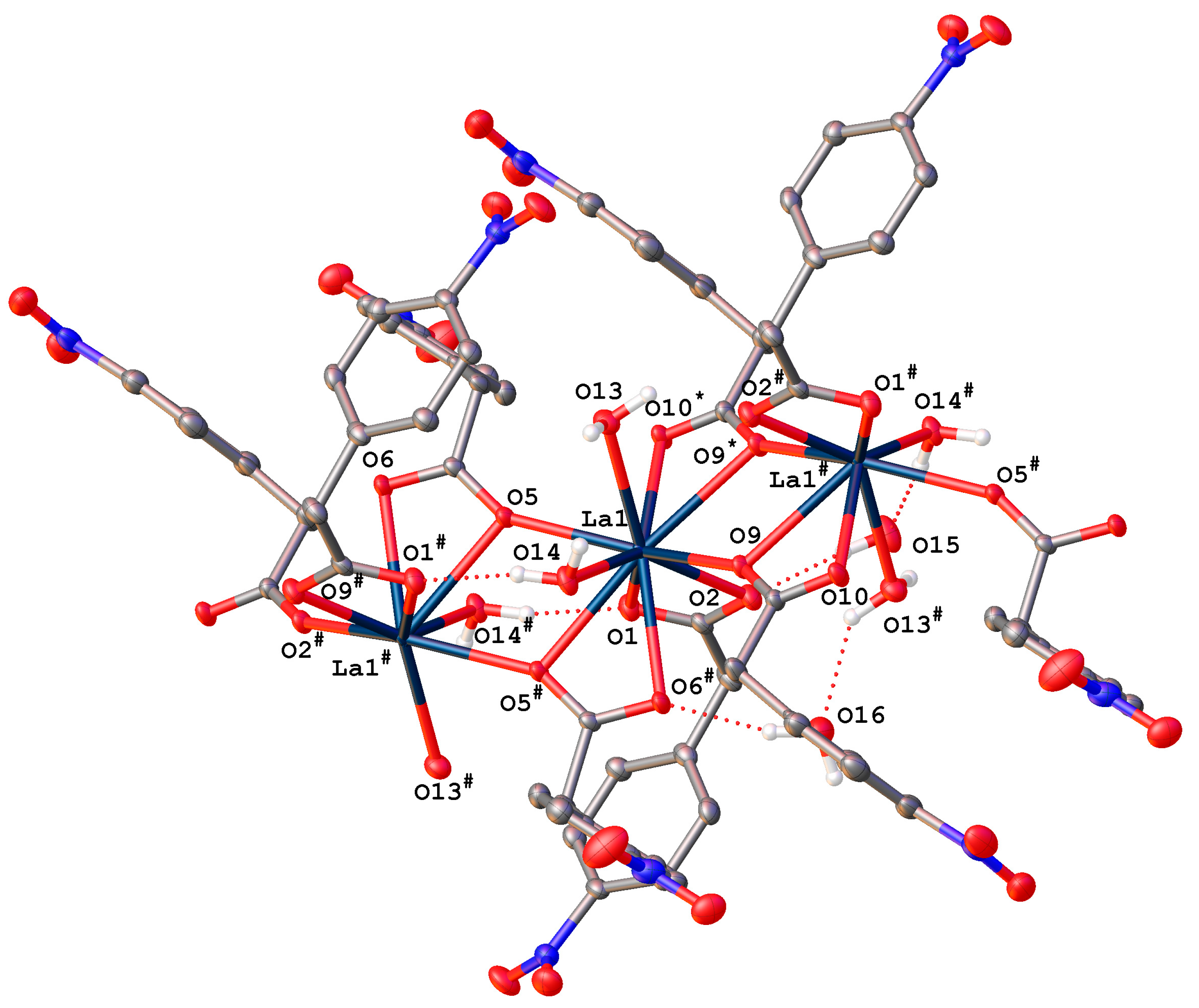
2.2.1. Complexes {[RE(4npa)3(H2O)2]·2H2O}n (RE = La (1La), Nd (2Nd))
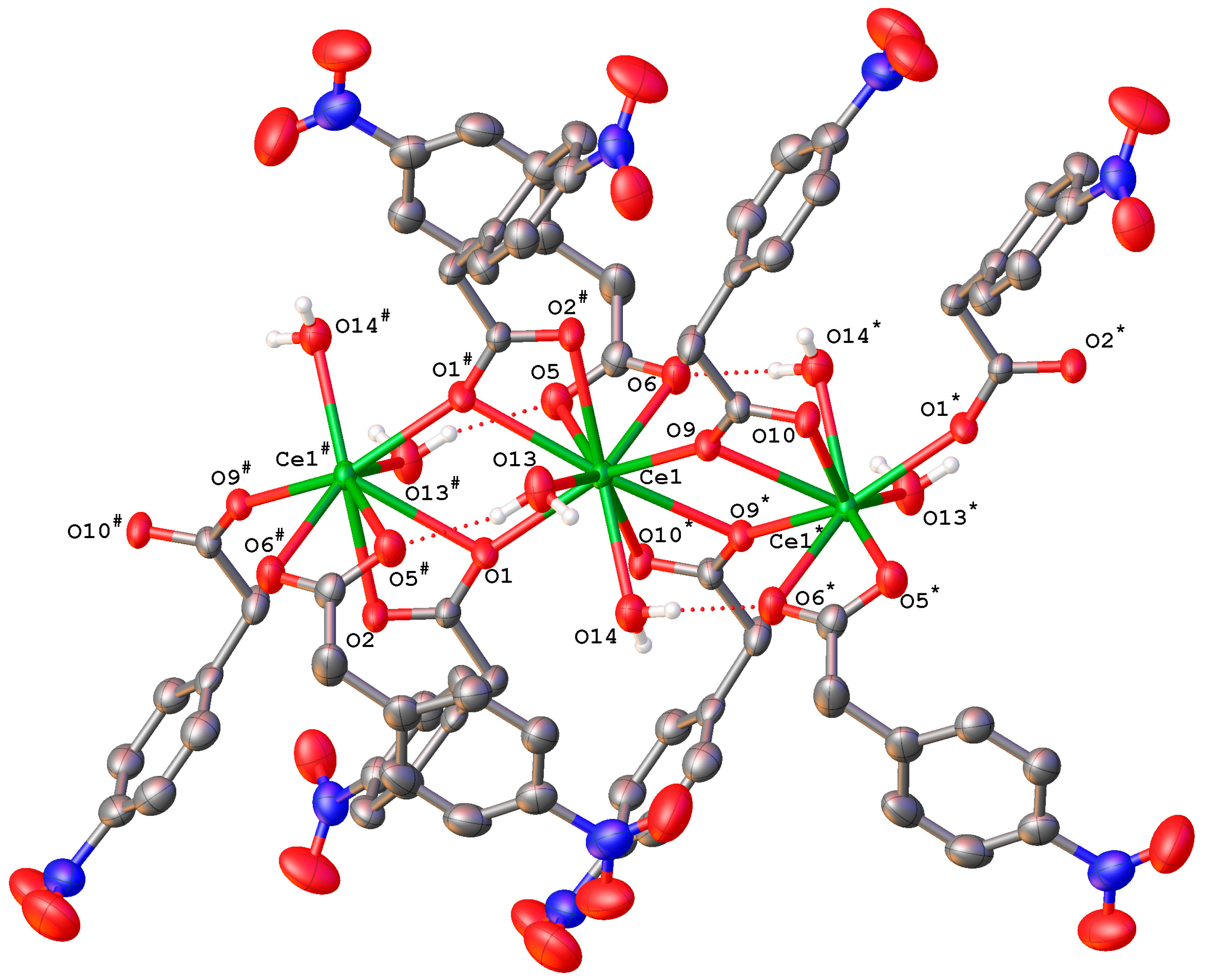
2.2.2. Complex [Ce(4npa)3(H2O)2]n (3Ce)
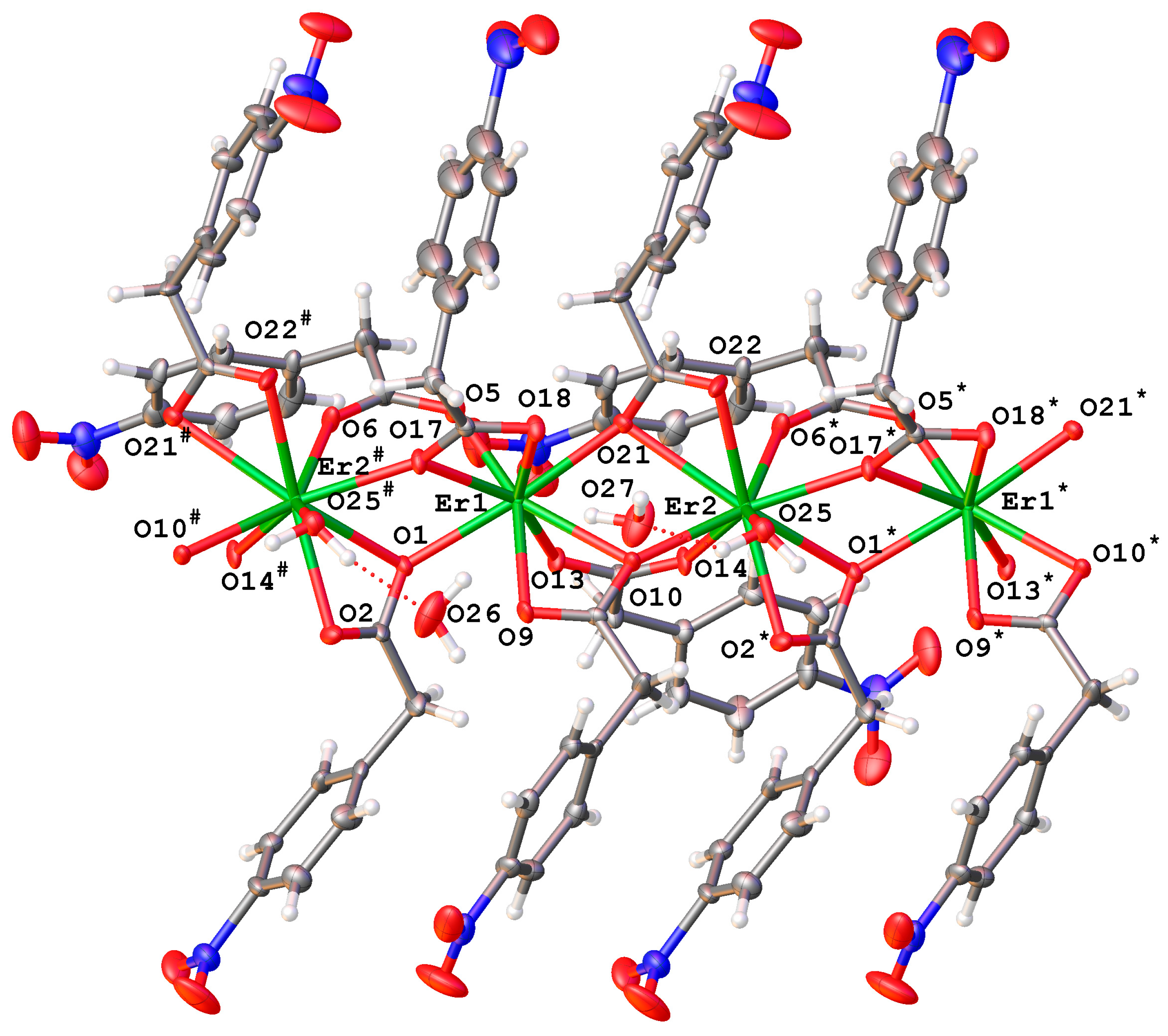
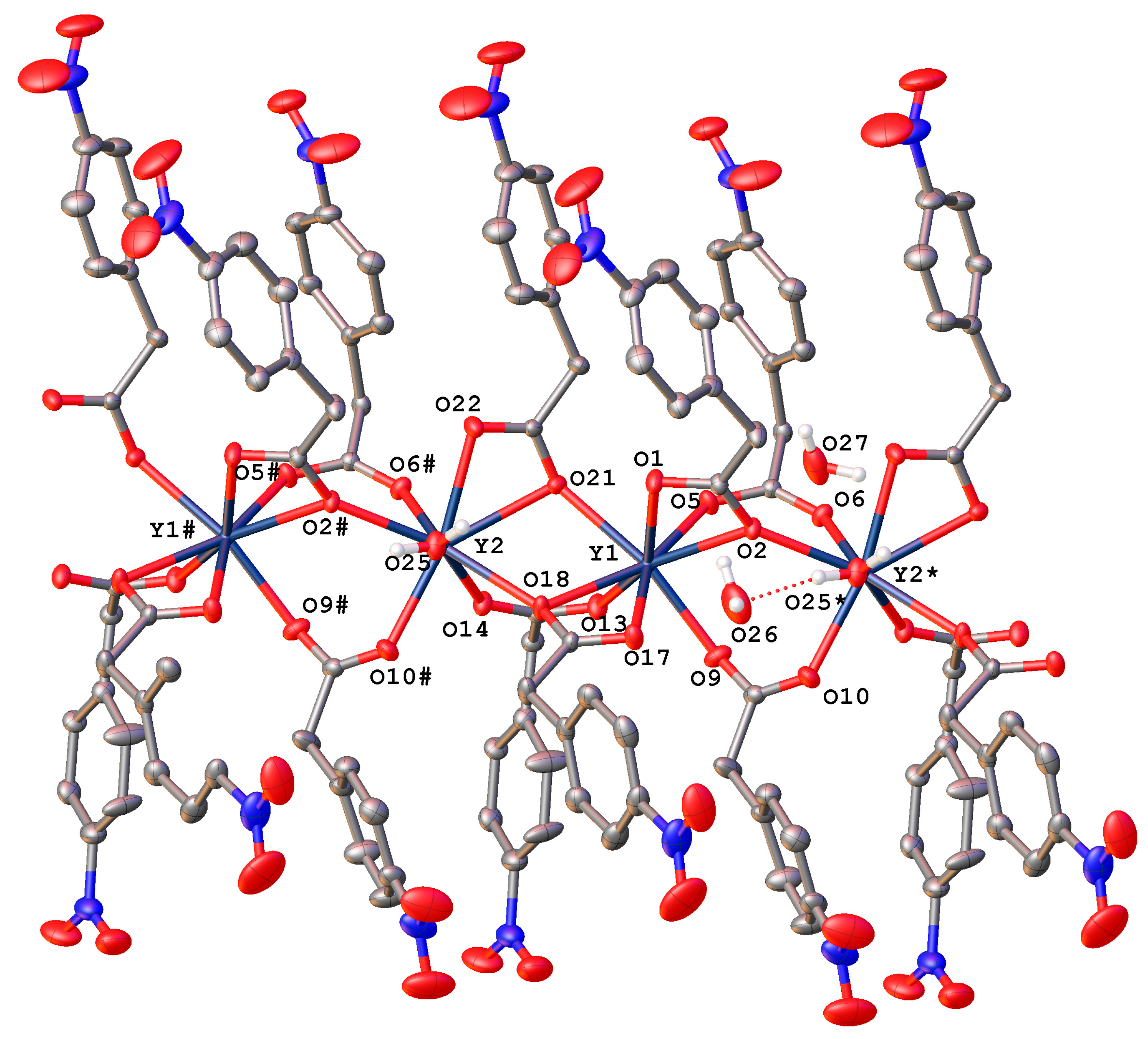
2.2.3. Complexes {[RE2(4npa)6(H2O)]·2H2O}n (RE = Dy (5Dy), Er (7Er))
2.2.4. Complexes {[RE2(4npa)6(H2O)]·2H2O}n (RE = Y (6Y), Yb (8Yb))
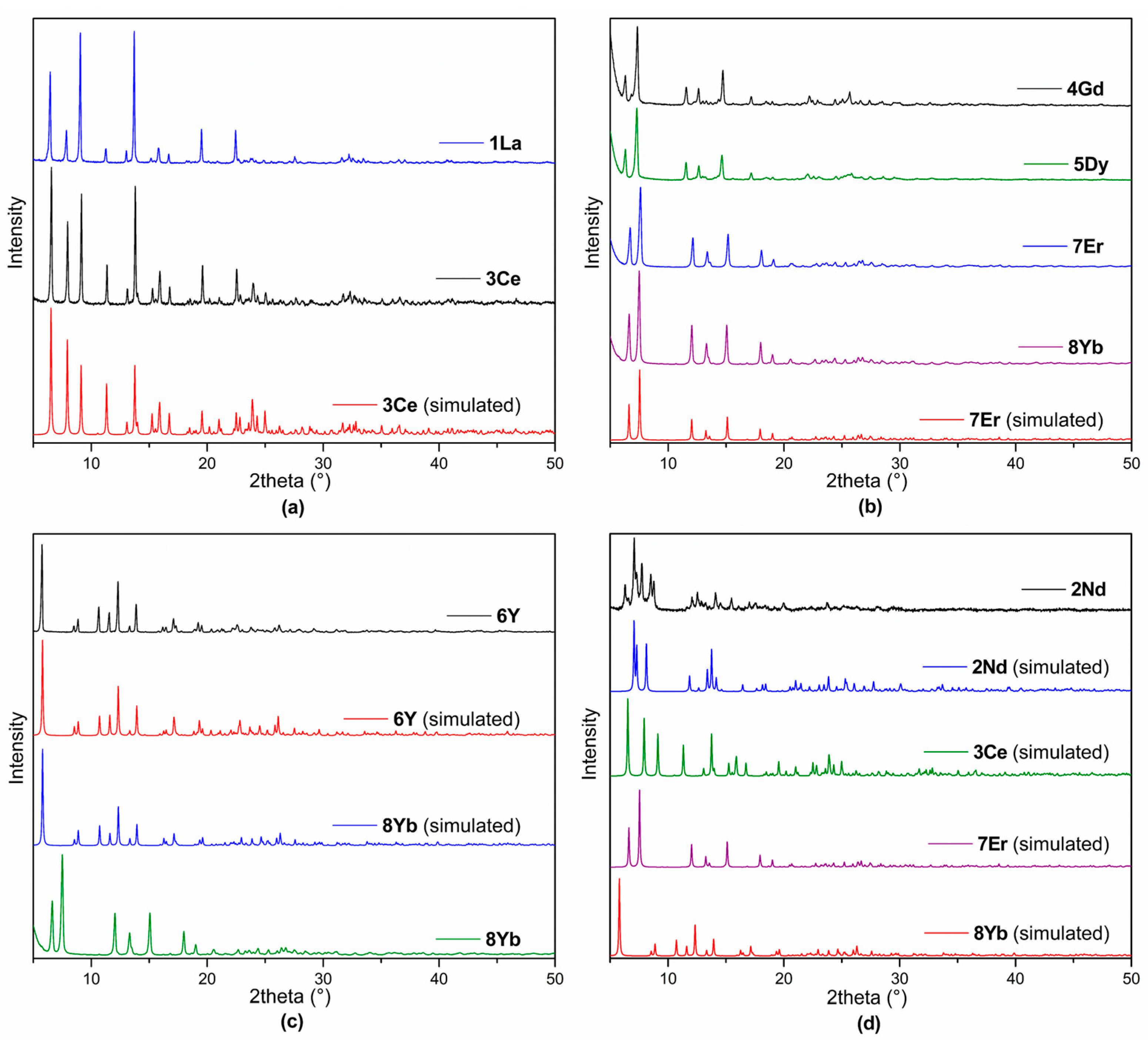
2.3. X-Ray Powder Diffraction
2.4. Corrosion Inhibition
3. Materials and Methods
3.1. General Consideration
3.2. X-Ray Crystallography
3.3. Synthesis of Rare Earth 4-Nitrophenylacetate Complexes
3.4. Immersion Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of rare earth elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- Hu, M.-L.; Morsali, A.; Aboutorabi, L. Lead(II) carboxylate supramolecular compounds: Coordination modes, structures and nano-structures aspects. Coord. Chem. Rev. 2011, 255, 2821–2859. [Google Scholar] [CrossRef]
- Yoshinari, N.; Konno, T. Multitopic metal–organic carboxylates available as supramolecular building units. Coord. Chem. Rev. 2023, 474, 214850. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide coordination chemistry: From old concepts to coordination polymers. J. Coord. Chem. 2014, 67, 3706–3733. [Google Scholar] [CrossRef]
- Liddle, S.T.; Mills, D.P.; Natrajan, L.S. The Lanthanides and Actinides Synthesis, Reactivity, Properties and Applications; World Scientific Publishing Europe Ltd.: Singapore, 2022. [Google Scholar]
- Huang, C.-H. Rare Earth Coordination Chemistry: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Forsyth, M.; Seter, M.; Hinton, B.; Deacon, G.; Junk, P. New ‘Green’ Corrosion Inhibitors Based on Rare Earth Compounds. Aust. J. Chem. 2011, 64, 812–819. [Google Scholar] [CrossRef]
- Somers, A.E.; Peng, Y.; Chong, A.L.; Forsyth, M.; MacFarlane, D.R.; Deacon, G.B.; Hughes, A.E.; Hinton, B.R.W.; Mardel, J.I.; Junk, P.C. Advances in the development of rare earth metal and carboxylate compounds as corrosion inhibitors for steel. Corros. Eng. Sci. Technol. 2020, 55, 311–321. [Google Scholar] [CrossRef]
- Deacon, G.B.; Junk, P.C.; Forsyth, M.; Leeb, W.W. From Chromates to Rare Earth Carboxylates: A Greener Take on Corrosion Inhibition. Chem. Aus. 2008, 75, 18–21. [Google Scholar]
- Gad, S.C. Acute and chronic systemic chromium toxicity. Sci. Total. Environ. 1989, 86, 149–157. [Google Scholar] [CrossRef]
- DesMarais, T.L.; Costa, M. Mechanisms of Chromium-Induced Toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Monticelli, C.; Frignani, A.; Trabanelli, G. Corrosion inhibition of steel in chloride-containing alkaline solutions. J. Appl. Electrochem. 2002, 32, 527–535. [Google Scholar] [CrossRef]
- Vithana, V.P.; Guo, Z.; Deacon, G.B.; Junk, P.C. Syntheses, Structures, and Corrosion Inhibition of Various Alkali Metal Carboxylate Complexes. Molecules 2023, 28, 5515. [Google Scholar] [CrossRef] [PubMed]
- Wormwell, F.; Mercer, A.D. Sodium benzoate and other metal benzoates as corrosion-inhibitors in water and in aqueous solutions. J. Appl. Chem. 1952, 2, 150–160. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Calvino, J.J.; Marcos, M.; RodrÍguez-Chacón, M.A. Lanthanide compounds as environmentally-friendly corrosion inhibitors of aluminium alloys: A review. Corros. Sci. 1998, 40, 1803–1819. [Google Scholar] [CrossRef]
- Gong, J.; Wei, H.; Hao, P.; Li, S.; Zhao, X.; Tang, Y.; Zuo, Y. Study on the Influence of Metal Substrates on Protective Performance of the Coating by EIS. Materials 2024, 17, 378. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, B.; Zhou, T.; Hao, H.; Yang, B.; Sun, H. Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies. Polymers 2019, 11, 635. [Google Scholar] [CrossRef]
- Sathasivam, K.; Wang, M.-Y.; Anbalagan, A.K.; Lee, C.-H.; Yeh, T.-K. Novel photocatalytic coating for corrosion mitigation in 304LSS of dry storage canisters. Front. Mater. 2023, 10, 1129886. [Google Scholar] [CrossRef]
- Monaci, S.; Mezzetta, A.; Guazzelli, L.; Mecerreyes, D.; Forsyth, M.; Somers, A. Lignin-Based Bio Ionic Liquids for Enhanced Corrosion Protection of Metals. ACS Sustain. Chem. Eng. 2025, 13, 2023–2037. [Google Scholar] [CrossRef]
- Catubig, R.A.; Neil, W.C.; McAdam, G.; Yunis, R.; Forsyth, M.; Somers, A.E. Multifunctional Inhibitor Mixtures for Abating Corrosion on HY80 Steel under Marine Environments. J. Electrochem. Soc. 2020, 167, 021503. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Yan, J.; Zhang, J.; Zhang, Q.; Yan, Y. Recent Progress of Polymeric Corrosion Inhibitor: Structure and Application. Materials 2023, 16, 2954. [Google Scholar] [CrossRef]
- Forsyth, M.; Wilson, K.; Behrsing, T.; Forsyth, C.; Deacon, G.B.; Phanasgoankar, A. Effectiveness of Rare-Earth Metal Compounds as Corrosion Inhibitors for Steel. Corrosion 2002, 58, 953–960. [Google Scholar] [CrossRef]
- Blin, F.; Leary, S.G.; Wilson, K.; Deacon, G.B.; Junk, P.C.; Forsyth, M. Corrosion Mitigation of Mild Steel by New Rare Earth Cinnamate Compounds. J. Appl. Electrochem. 2004, 34, 591–599. [Google Scholar] [CrossRef]
- Salpadoru Thuppahige, N.Y.; Guo, Z.; Deacon, G.B.; Somers, A.E.; Junk, P.C. Rare-Earth 4-Hydroxyphenylacetate Complexes: Synthesis, Structural Characterization, and Corrosion Inhibition Properties. Z. Anorg. Allg Chem. 2025, 651, e202400180. [Google Scholar] [CrossRef]
- Mottram, E.; Hamilton, S.; Moon, J.S.; Wang, J.; Bousrez, G.; Somers, A.E.; Deacon, G.B.; Junk, P.C. Synthesis, structure, and corrosion inhibiting properties of phenylacetato-rare earth(III) complexes. J. Coord. Chem. 2020, 73, 2677–2697. [Google Scholar] [CrossRef]
- Bürgstein, M.R.; Roesky, P.W. Nitrophenolate as a building block for lanthanide chains and clusters. Angew. Chem. Int. Ed. 2000, 39, 549–551. [Google Scholar] [CrossRef]
- de Bettencourt-Dias, A.; Bauer, S.; Viswanathan, S.; Maull, B.C.; Ako, A.M. Unusual nitro-coordination of europium (iii) and terbium (iii) with pyridinyl ligands. Dalton Trans. 2012, 41, 11212–11218. [Google Scholar] [CrossRef]
- Dhavskar, K.T.; Butcher, R.J. A comparative structural and property investigation of four new bivalent transition metal complexes based on 4-nitrophenylacetic acid with rigid 4-nitrobenzoate analogues. Inorg. Chim. Acta 2017, 466, 180–187. [Google Scholar] [CrossRef]
- Srinivasan, B.R.; Dhavskar, K.T.; Näther, C. Syntheses, structure and properties of Alkaline-earth metal salts of 4-Nitrophenylacetic acid. J. Chem. Sci. 2016, 128, 1765–1774. [Google Scholar] [CrossRef]
- Yao, H.; Calvez, G.; Daiguebonne, C.; Suffren, Y.; Bernot, K.; Roisnel, T.; Guillou, O. Synthesis, Crystal Structure, and Luminescence Properties of the Iso-Reticular Series of Lanthanide Coordination Polymers Synthesized from Hexa-Lanthanide Molecular Precursors. Inorg. Chem. 2022, 61, 4895–4908. [Google Scholar] [CrossRef]
- Wagner, W.; Hull, C. Treatise on Titrimetry; Dekker: New York, NY, USA, 1971. [Google Scholar]
- Schwarzenbach, G.; Flaschka, H. Complexometric Titrations; Methuen and Co., Ltd.: London, UK, 1969. [Google Scholar]
- West, T.S. Complexometry with EDTA and Related Reagents; BDH Chemicals Ltd.: Poole, UK, 1969. [Google Scholar]
- Wu, J.; Zhou, Y.; Yuan, X.; Wang, J.; Zhao, H. Solubility Modeling, Solvent Effect, and Dissolution Properties of 4-Nitrophenylacetic Acid in Thirteen Solvents Ranging from 283.15 to 328.15 K. J. Chem. Eng. Data 2020, 65, 2894–2902. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra Of Complex Molecules: Advances in Infrared Group Frequencies; Chapman and Hall Ltd.: London, UK, 1975. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rad, N.E.; Junk, P.C.; Deacon, G.B.; Wang, J. New Homoleptic Rare Earth 3,5-Diphenylpyrazolates and 3,5-Di-tert-butylpyrazolates and a Noteworthy Structural Discontinuity. Z. Anorg. Allg Chem. 2019, 645, 877–881. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Deacon, G.B.; Forsyth, C.M.; Junk, P.C.; Hilder, M.; Leary, S.G.; Bromant, C.; Pantenburg, I.; Meyer, G.; Skelton, B.W.; White, A.H. Synthesis and structural properties of anhydrous rare earth cinnamates, [RE(cinn)3]. Z. Anorg. Allg Chem. 2008, 634, 91–97. [Google Scholar] [CrossRef]
- Vithana, V.P.; Guo, Z.; Deacon, G.B.; Somers, A.E.; Junk, P.C. Synthesis, structure, and corrosion inhibiting properties of REIII 3-thiophenecarboxylate complexes. New J. Chem. 2022, 46, 19104–19111. [Google Scholar] [CrossRef]
- Vithana, V.P.; Guo, Z.; Deacon, G.B.; Somers, A.E.; Junk, P.C. RE(III) 3-Furoate Complexes: Synthesis, Structure, and Corrosion Inhibiting Properties. Molecules 2022, 27, 8836. [Google Scholar] [CrossRef]
- Salpadoru Thuppahige, N.Y.; Guo, Z.; Deacon, G.B.; Junk, P.C. Synthesis, structural characterization, and corrosion inhibition properties of rare earth 2-hydroxyphenylacetate coordination polymers. Inorg. Chim. Acta 2025, 587, 122796. [Google Scholar] [CrossRef]
- Behrsing, T.; Deacon, G.B.; Luu, J.; Junk, P.C.; Skelton, B.W.; White, A.H. Structural diversity of lanthanoid salicylate hydrates. Polyhedron 2016, 120, 69–81. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2004. [CrossRef]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Vithana, V.P.; Guo, Z.; Deacon, G.B.; Junk, P.C. Rare Earth 2-Methyl-3-furoate Complexes: Effect of Steric Hindrance on Corrosion Inhibitor Properties. Eur. J. Inorg. Chem. 2024, 27, e202300722. [Google Scholar] [CrossRef]
- Deacon, G.B. Synthesis of Organometallic Compounds by Thermal Decarboxylation. Organomet. Chem. Rev. A 1970, 5, 355–372. [Google Scholar]
- Cowieson, N.P.; Aragao, D.; Clift, M.; Ericsson, D.J.; Gee, C.; Harrop, S.J.; Mudie, N.; Panjikar, S.; Price, J.R.; Riboldi-Tunnicliffe, A. MX1: A bending-magnet crystallography beamline serving both chemical and macromolecular crystallography communities at the Australian Synchrotron. J. Synchrotron Radiat. 2015, 22, 187–190. [Google Scholar] [CrossRef]


| Compound | ν(NO2) | νas(COO−) | νs(COO−) | Δν = (νas − νs) |
|---|---|---|---|---|
| 4npaH | 1508, 1339 | - | - | - |
| {[La(4npa)3(H2O)2]·2H2O}n (1La) | 1510, 1341 | 1556 | 1394 | 162 |
| {[Nd(4npa)3(H2O)2]·2H2O}n (2Nd) | 1508, 1343 | 1560 | 1394 | 166 |
| [Ce(4npa)3(H2O)2]n (3Ce) | 1510, 1340 | 1552 | 1401 | 151 |
| {[Gd2(4npa)6(H2O)]·2H2O}n (4Gd) | 1508, 1341 | 1545 | 1399 | 146 |
| {[Dy2(4npa)6(H2O)]·2H2O}n (5Dy) | 1508, 1339 | 1576 | 1386 | 190 |
| {[Y2(4npa)6(H2O)]·2H2O}n (6Y) | 1504, 1340 | 1541 | 1393 | 148 |
| {[Er2(4npa)6(H2O)]·2H2O}n (7Er) | 1508, 1338 | 1549 | 1400 | 149 |
| {[Yb2(4npa)6(H2O)]·2H2O}n (8Yb) | 1507, 1338 | 1542 | 1401 | 141 |
| Compound | RE1 | RE2 | ||
|---|---|---|---|---|
| CN | RE1-O [Å] | CN | RE2-O [Å] | |
| {[La(4npa)3(H2O)2]·2H2O}n (1La) | 10 | 2.59 | - | - |
| {[Nd(4npa)3(H2O)2]·2H2O}n (2Nd) | 10 | 2.54 | - | - |
| [Ce(4npa)3(H2O)2]n (3Ce) | 10 | 2.58 | - | - |
| {[Dy2(4npa)6(H2O)]·2H2O}n (5Dy) | 8 | 2.36 | 9 | 2.41 |
| {[Y2(4npa)6(H2O)]·2H2O}n (6Y) | 8 | 2.36 | 8 | 2.38 |
| {[Er2(4npa)6(H2O)]·2H2O}n (7Er) | 8 | 2.36 | 9 | 2.41 |
| {[Yb2(4npa)6(H2O)]·2H2O}n (8Yb) | 8 | 2.33 | 8 | 2.35 |
| Solution | Concentration | Solubility | Ave. Weight Loss (mg) | Ave. Corrosion Rate (mm year−1) | IE% | |
|---|---|---|---|---|---|---|
| (ppm) | (mM) | In H2O (ppm) | ||||
| Control (NaCl) | 500 | 0.01 | 14.2 | 0.101 | - | |
| {[La(4npa)3(H2O)2]·2H2O}n (1La) | 500 | 0.666 | 1512 | 4.5 | 0.030 | 71 |
| {[Nd(4npa)3(H2O)2]·2H2O}n (2Nd) | 500 | 0.674 | 1329 | 2.1 | 0.014 | 86 |
| {[Nd2(2hpa)6(H2O)4]⋅3.5H2O}n | 500 | 0.378 | - | - | 0.051 | 48 |
| {[Nd2(4hpa)6(H2O)]·4H2O}n | 500 | 0.389 | - | - | 0.031 | 77 |
| [Nd(PhAc)3(H2O)]n | 500 | - | - | 13.6 | 0.449 | 55 |
| [Ce(4npa)3(H2O)2]n (3Ce) | 500 | 0.708 | 3191 | 10.9 | 0.072 | 29 |
| {[Ce(2hpa)6(H2O)4]⋅3.5H2O}n | 500 | 0.380 | - | - | 0.074 | 24 |
| {[Ce(4hpa)3(H2O)2]·H2O}n | 500 | 0.772 | - | - | 0.043 | 68 |
| {[Gd2(4npa)6(H2O)]·2H2O}n (4Gd) | 500 | 0.347 | 1789 | 3.0 | 0.020 | 80 |
| {[Gd2(2hpa)6(H2O)2]⋅3H2O}n | 500 | 0.381 | - | - | 0.0430 | 57 |
| {[Gd2(4hpa)6(H2O)]·4H2O}n | 500 | 0.381 | - | - | 0.0160 | 88 |
| [Gd(PhAc)3(H2O)]n | 500 | - | - | 16.4 | 0.5430 | 45 |
| {[Dy2(4npa)6(H2O)]·2H2O}n (5Dy) | 500 | 0.351 | 1415 | 1.8 | 0.0116 | 89 |
| {[Dy2(2hpa)6(H2O)2]⋅3H2O}n | 500 | 0.378 | - | - | 0.0590 | 40 |
| {[Dy2(4hpa)6(H2O)]·3H2O}n | 500 | 0.383 | - | - | 0.0360 | 73 |
| [Dy(PhAc)3(H2O)]n | 500 | - | - | 11.0 | 0.3640 | 21 |
| {[Y2(4npa)6(H2O)]·2H2O}n (6Y) | 500 | 0.382 | 3019 | 3.7 | 0.0247 | 76 |
| {[Er2(4npa)6(H2O)]·2H2O}n (7Er) | 500 | 0.345 | 2787 | 3.8 | 0.0253 | 75 |
| {[Yb2(4npa)6(H2O)]·2H2O}n (8Yb) | 500 | 0.340 | 2480 | 4.7 | 0.0313 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neill, J.M.; Salpadoru Thuppahige, N.Y.; Guo, Z.; Deacon, G.B.; Junk, P.C. Synthesis, Structures and Corrosion Inhibition Properties of 4-Nitrophenylacetato-Rare-Earth(III) 1D Coordination Polymers. Molecules 2025, 30, 3940. https://doi.org/10.3390/molecules30193940
Neill JM, Salpadoru Thuppahige NY, Guo Z, Deacon GB, Junk PC. Synthesis, Structures and Corrosion Inhibition Properties of 4-Nitrophenylacetato-Rare-Earth(III) 1D Coordination Polymers. Molecules. 2025; 30(19):3940. https://doi.org/10.3390/molecules30193940
Chicago/Turabian StyleNeill, Jacob M., Naveena Y. Salpadoru Thuppahige, Zhifang Guo, Glen B. Deacon, and Peter C. Junk. 2025. "Synthesis, Structures and Corrosion Inhibition Properties of 4-Nitrophenylacetato-Rare-Earth(III) 1D Coordination Polymers" Molecules 30, no. 19: 3940. https://doi.org/10.3390/molecules30193940
APA StyleNeill, J. M., Salpadoru Thuppahige, N. Y., Guo, Z., Deacon, G. B., & Junk, P. C. (2025). Synthesis, Structures and Corrosion Inhibition Properties of 4-Nitrophenylacetato-Rare-Earth(III) 1D Coordination Polymers. Molecules, 30(19), 3940. https://doi.org/10.3390/molecules30193940






