A Comprehensive Review of Substitutional Silicon-Doped C60 Fullerenes and Their Endohedral/Exohedral Complexes: Synthetic Strategies and Molecular Modeling Approaches
Abstract
1. Introduction
2. Synthesis of Si-Doped C60 Fullerenes
3. Theoretical Investigations of Si-Doped C60 Fullerenes
3.1. Substitutional Si-Doped C60 Fullerenes
3.2. Exohedral Complexes of Substitutional Si-Doped C60 Fullerenes
| Exohedral Complexes of Substitutional Si-Doped C60 Fullerenes | |||||||
|---|---|---|---|---|---|---|---|
| Si-Doped Fullerene Derivative (Host) | Adsorbed Guest | Type of Calculation | Calculation Method | Computational Software | Aim of Study | (Ref.) | |
| Semiempirical | DFT (Functional and Basis Set) | ||||||
| C60−nSin (n = 1, 2, 4, 6) | mH2 (m = 1–6, 12, 24, 36) | GO, ESPA, Eads | SCC-DFTB | LDA/DPZ | SIESTA code DFTB+ code | Evaluation of H2 adsorption capacity and binding energy on Si-doped C60 via exohedral interaction (also S-doped and P-doped C60) | [58] |
| C59Si | Li+ | GO, ESPA, CINEB, Eads | - | PBE-D3 | VASP | Investigation of Si-doping effects on structural stability, electronic properties, and lithium-ion adsorption/migration in C60 (also C59B, C59P, C59S, C59N, and pristine C60) | [91] |
| C59Si | SO2, H2S, thiophene | GO, ESPA, PCM | - | ωB97XD/6-31+G(d) | Gaussian 09 | Evaluation of adsorption capacity of C59Si toward SO2, H2S and thiophene in gas and aqueous phase (also C59B, C59P, C59S, C59N, and C59Al) | [93] |
| C59Si | CO, O2 | GO ESPA Eads (with BSSE), PCM | - | B3LYP-gCP-D3/6-31G(d) | Gaussian 09 | Assessment of O2 and CO co-adsorption and catalytic oxidation activity of C59Si (also C59B, C59P, C59S, and pristine C60) | [94] |
| C59Si | O2, HOO·, HO·O·, O·, HO·HO·, HO· | GO, ESPA, Eads | B3LYP/6-311+G(d), B3LYP/6-311G(d,p), wB97XD/6-311G(d,p) | Gaussian09 | Evaluation of adsorption and activation of oxygen reduction reaction intermediates on Si-doped fullerene catalyst C59Si (in comparison to C60) | [95] | |
| C59Si | amphetamine | GO, ESPA, Eads (with BSSE), NICS | - | B3LYP-D/3-21G(d), B3LYP-D/6-31G, B3LYP-D/6-311++G(d,p), GIAO | GAMESS | Investigation of the adsorption process and aromaticity response of Si-doped C60 as a molecular sensor for amphetamine (in comparison to C59B, C59Ga, C59Ge, C59Al, and pristine C60) | [74] |
| C59Si | 5-fluorouracil | GO, ESPA, Eads (with BSSE), PCM | - | B3LYP/6-31G(d), ωB97x-D/6-31G(d), M06-2X/6-31G(d) | GAMESS | Evaluation of the adsorption affinity and electronic effects of 5-fluorouracil on Si-doped C60 (in comparison to C59B, C59Al, and pristine C60) | [75] |
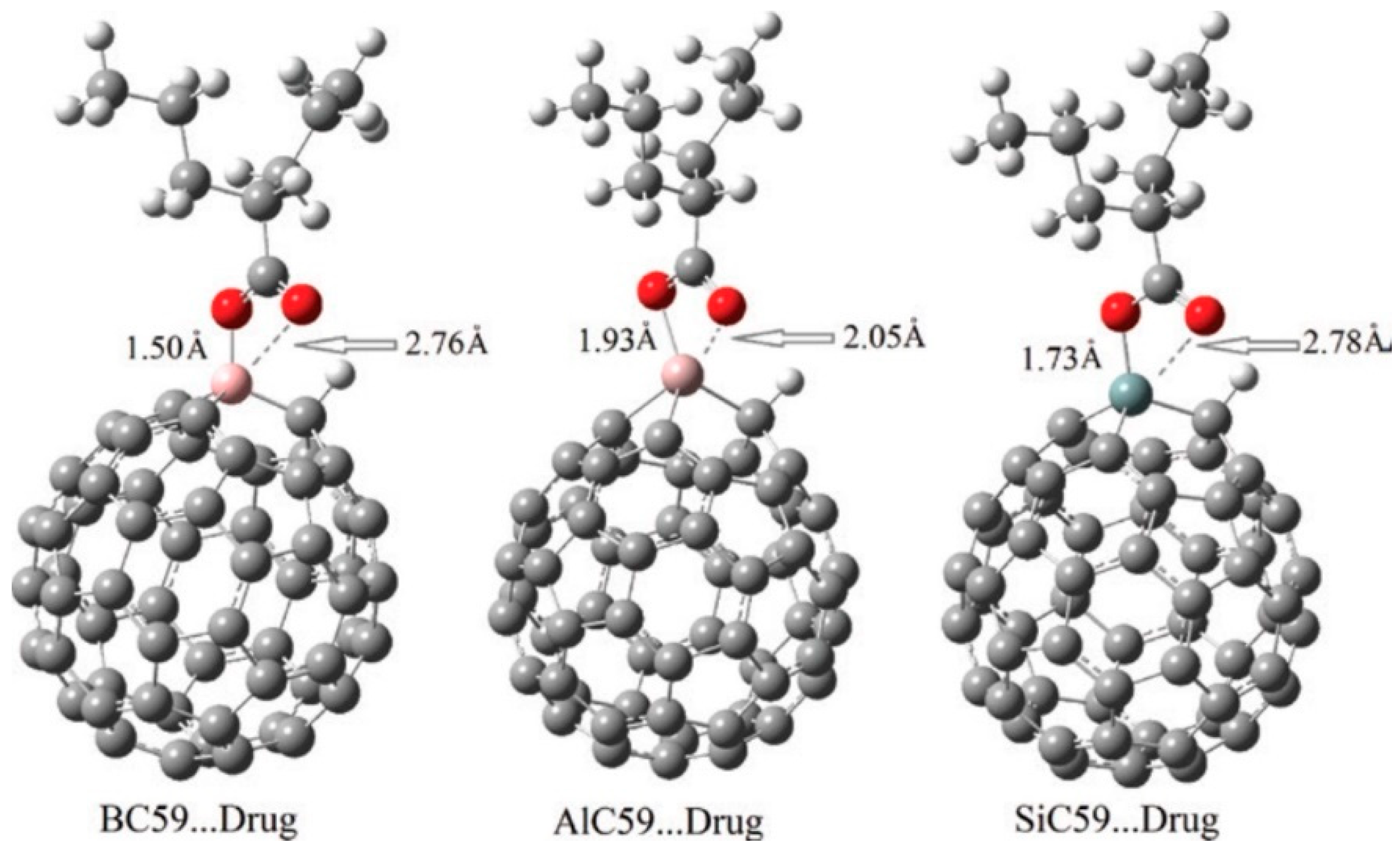
| C59Si | 6-chloro-3-hydroxy-2-pyrazinecarboxamide | GO, ESPA, Eads, PCM | - | B3LYP/6-31G(d), M06-2X/6-31G(d) | Gaussian 09 | Evaluation of adsorption energy and electronic properties of Si-doped C60 interacting with pyrazinamide derivative (in comparison to C59Al, and pristine C60) | [121] |
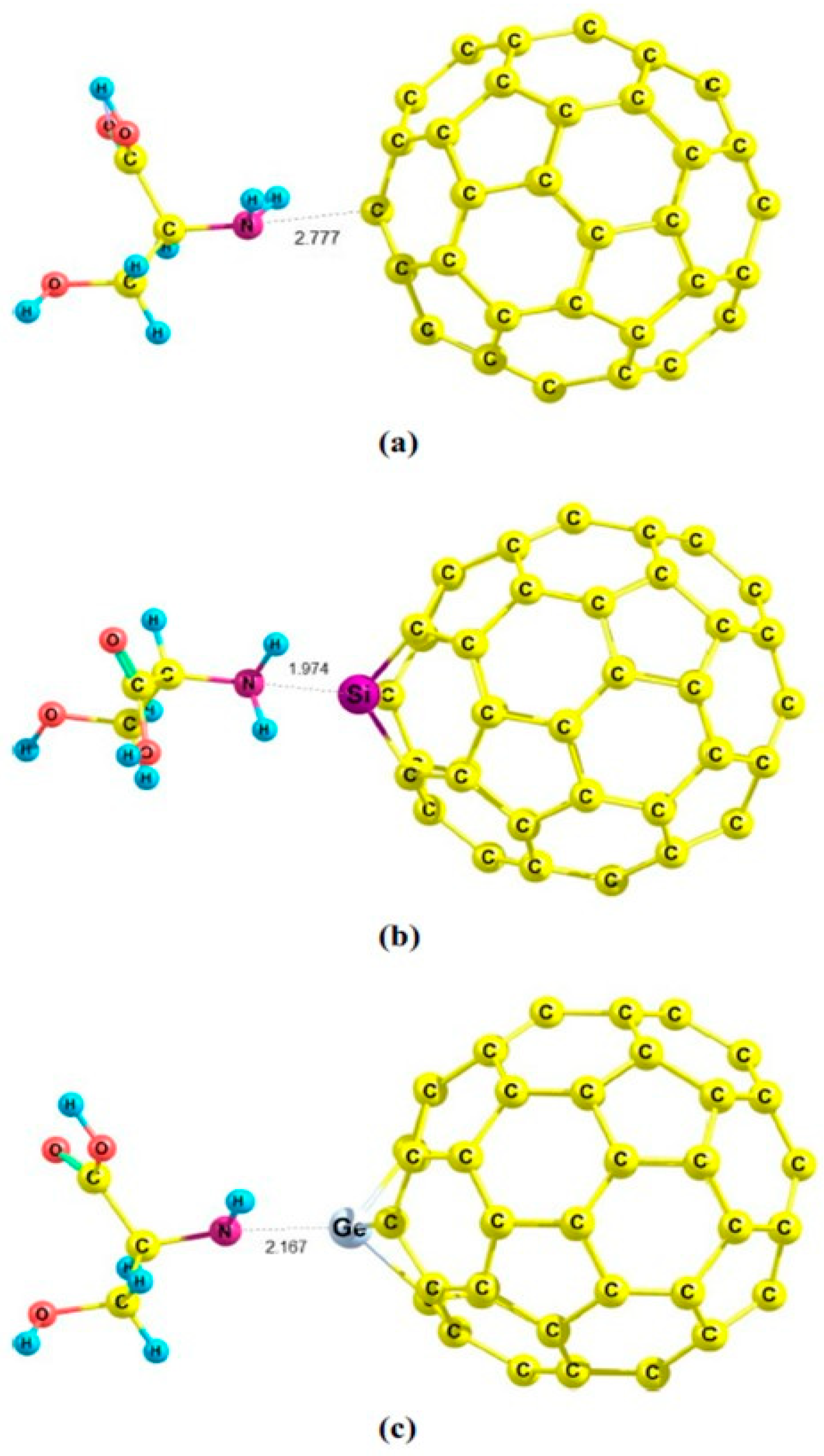
| C59Si | amantadine | GO, ESPA, Eads (with BSSE), IR, NMR, PCM | - | B3LYP/6-31G(d), B3LYP/cc-pVDZ | Gaussian 09 | Investigation of noncovalent interactions between Si-doped C60 and amantadine in gas and aqueous phases (in comparison to C59B, C59Ga, C59Ge, C59Al, and pristine C60) | [62] |
| C59Si | favipiravir | GO, ESPA, BSSE, Eads, PCM | - | M06-2X/6-31G(d) | Gaussian 09 | Investigation of the adsorption mechanism, and the structural and electronic properties of favipiravir on Si-doped C60 and pristine C60 in gas and aqueous phases for potential drug delivery applications | [63] |
| C59Si, C58Si2 | 2,4,6-trinitrotoluene (TNT) | GO, ESPA, Eads | - | B3LYP/6-31G(d) | Gaussian 09 | Assessment of the adsorption mechanism, stability, reactivity and sensing performance of single- and double-Si-doped C60 toward explosive substance TNT in gas phase | [96] |
| C59Si | β-propiolactone | GO, ESPA, Eads (with BSSE), PCM | - | B3LYP/6-31G(d) | Gaussian 09 | Investigation of the adsorption properties, electronic structure, and solvent effects on β-propiolactone binding to Si-doped C60 (also C59Al) for potential sensing applications | [99] |
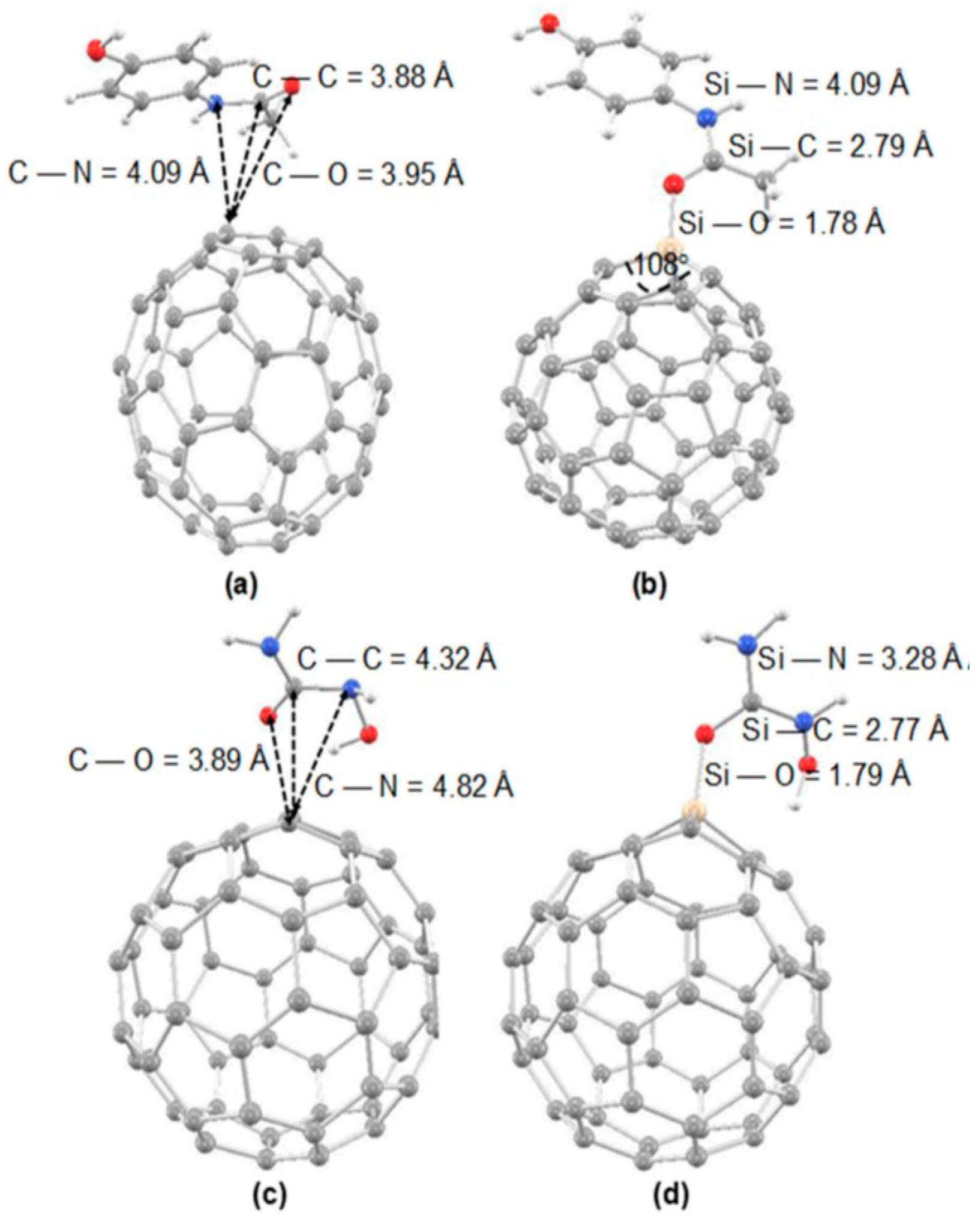
| C59Si | paracetamol | GO, ESPA, BSSE, Eads (with BSSE), PCM | - | B3LYP/6-31G(d) | Gaussian 16 | Assessment of the adsorption strength, structural and electronic properties of paracetamol binding to Si-doped C60 (also other C60 derivatives) for potential drug delivery and sensing applications | [112] |
| C59Si | amantadine | GO, ESPA, Eads | - | HSEH1PBE/6-311G(d), B3LYP-D3/6-311G(d), wB97XD/6-311G(d), M062X/6-311G(d) | Gaussian 16 | Assessment of the adsorption strength and electronic reactivity descriptors for amantadine on Si-doped C60 (in comparison to C59B, C59Ga, C59Ge, C59Al, C59P, C59As, C59N, and pristine C60) | [110] |
| C59Si | 4-phenylpyridine (4-PHPY) | GO, ESPA, Eads | - | M062X/6-31G(d), B3LYP/6-31G(d) | Gaussian 09 | Evaluation of the adsorption strength and electronic properties of 4-PHPY on Si-doped C60 (in comparison to C59B) | [98] |
| C59Si | piperazine-2,3,5,6-tetraone (PPTO) | GO, ESPA, Eads (with BSSE), PCM, NMR | - | B3LYP/6-31G(d) | Gaussian 09 | Evaluation of the adsorption strength and electronic reactivity of Si-doped C60 as drug carrier or sensor for PPTO (in comparison to C59Al) | [100] |
| C59Si | 1-(3-trifluoromethylphenyl)piperazine (TFMPP) | GO, ESPA, Eads (with BSSE), | - | B3LYP/cc-pVDZ | Gaussian 09 | Assessment of binding affinity and sensing ability of Si-doped C60 (in comparison to C59Al) for an ecstasy analog (TFMPP) with CNS activity. | [101] |
| C59Si | phenylalanine | GO, ESPA, Eads, IR | - | B3LYP/6-31G(d), M062X/6-311G(d) | Gaussian 09 | Investigation of the structural stability, charge distribution and adsorption behavior of phenylalanine on Si-doped C60 in gas and aqueous phase (in comparison to C59Al and pristine C60) | [104] |
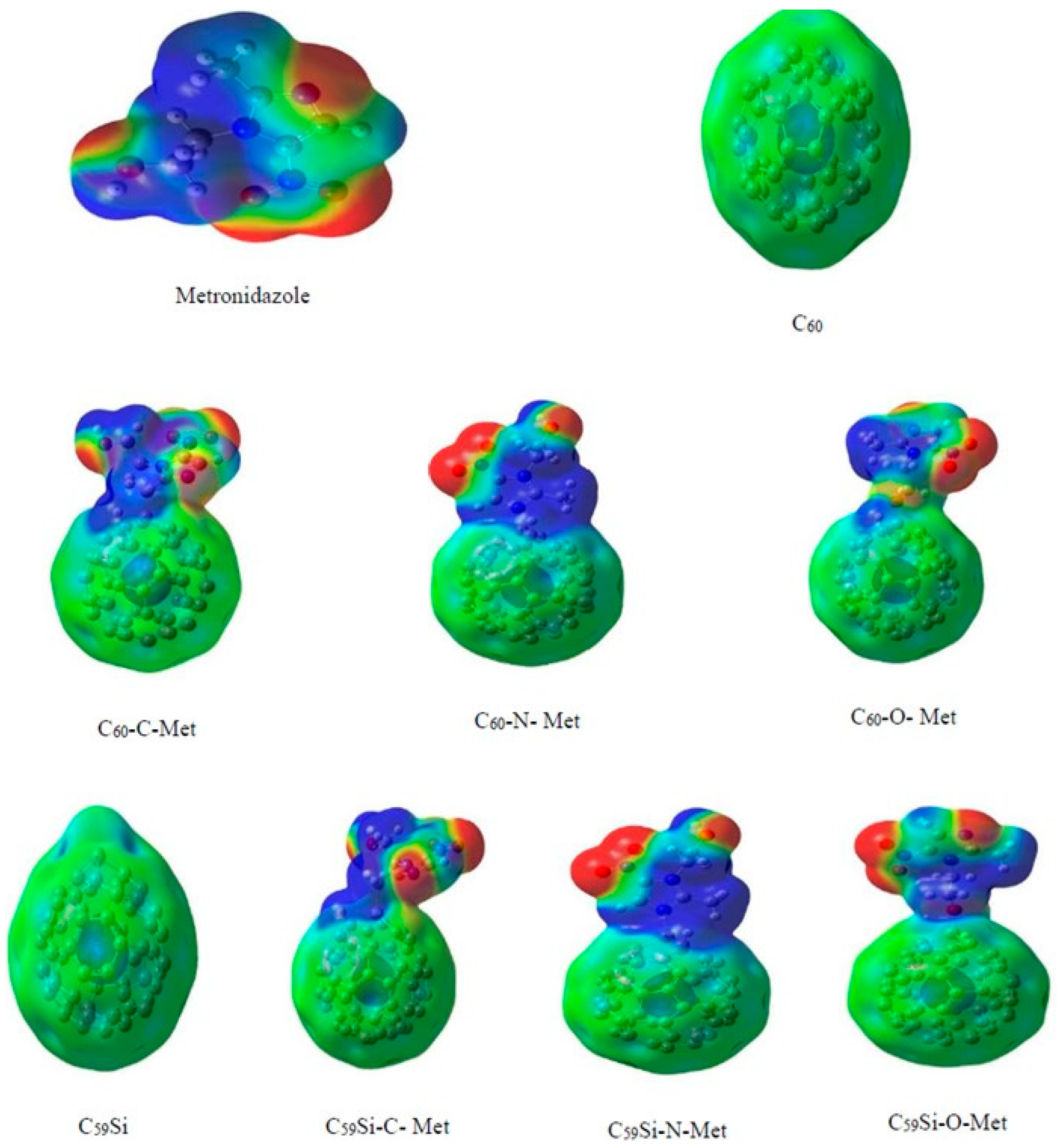
| C59Si | metronidazole | GO, ESPA, Eads | - | B3LYP/6-31G(d,p) | Gaussian 09 | Investigation of the electronic structure, adsorption energy and quantum reactivity descriptors of metronidazole adsorbed on Si-doped C60 (in comparison to C59B, C59Al, and pristine C60) | [114] |
| C59Si | ornidazole | GO, ESPA, Eads | - | B3LYP/6-31G(d) | Gaussian 09 | Investigation of the adsorption energy, molecular electrostatic potential and frontier orbital properties of ornidazole on Si-doped C60 (in comparison to C59B, C59Al and pristine C60) | [25] |
| C59Si | molnupiravir | GO, ESPA, Eads, PCM, IR | - | B3LYP/cc-pVDZ | Gaussian 09 | Investigation of the quantum reactivity descriptors, adsorption mechanism and intermolecular interactions between Si-doped C60 and molnupiravir | [111] |
| C59Si | carbamazepine | GO, ESPA, Eads (with BSSE), PCM | - | ωB97XD/6-31G(d), M06L/6-31G(d) | Gaussian 09 | Investigation of the interaction energy, solvent effects, and electronic properties of carbamazepine adsorbed on Si-doped C60 (in comparison to C59B, C59Ga, C59Ge, C59Al, C59P, C59N and pristine C60) | [24] |
| C59Si | nimesulide, diclofenac, mefenamic acid | GO, ESPA, Eads (with BSSE), PCM, SMD, IR | - | B3LYP/6-31G(d) | Gaussian 09 | Investigation of the solvent effects, electronic structure, and nonlinear optical properties of Si-doped C60 interacting with NSAID drugs (in comparison to pristine C60) | [116] |
| C59Si | histamine | GO, ESPA, Eads (with BSSE), PCM | - | B3LYP-D3/6-31G(d,p) | Gaussian 16 | Investigation of the adsorption behavior, electronic reactivity descriptors and solvent effects in histamine detection by Si-doped C60 (in comparison to C59B and pristine C60) | [107] |
| C59Si | tyramine | GO, ESPA, Eads (with BSSE), MD | - | B3LYP/6-31G(d,p) | Gaussian 16 | Investigation of the non-covalent interactions, adsorption stability and electronic reactivity of Si-doped C60 toward tyramine (in comparison to C59B and pristine C60) | [108] |
| C59Si | hydroxyurea, paracetamol | GO, ESPA, Eads | - | PBE | PHASE/0 code | Investigation of the adsorption energy, charge transfer and electronic properties of pharmacologically active compounds paracetamol and hydroxyurea adsorbed on Si-substituted C60 | [113] |
| C59Si | methadone | GO, ESPA, Eads (with BSSE), TDDFT | - | M062X/6-31G(d), B3LYP/6-31G(d) | GAMESS | Investigation of the structural, spectroscopic and electronic response of Si-doped C60 to methadone adsorption using TDDFT and quantum reactivity descriptors (in comparison to C59B, C59Ge, and pristine C60) | [14] |
| C59Si | epigallocatechin-3-gallate (EGCG) | GO, ESPA, Eads (with BSSE) | B3LYP-D3/6-31G(d) | Gaussian 16, | Analysis of the non-covalent interactions and electronic reactivity in EGCG adsorption on Si-substituted C60 (in comparison to C59B, C59Ga, C59Ge, C59Al, C59P and pristine C60) | [118] | |
| C60−nSin (n = 2, 4, 6, 8) | 1,3-butadiene | GO, ESPA, Eads (with BSSE), NICS | - | M06-2X/6–311+G(d) | GAMESS | Investigation of the aromaticity and Diels–Alder reactivity of Si-substituted C60 with increasing silicon content | [88] |
| C60−nSin (n = 1–4, 6), C60Sim (m = 1–6) | Sim (m = 1–6) | GO, ESPA, MD | DFTB-based TBMD | - | DFTB code | Investigation of the fragmentation dynamics of substitutional and adsorbed Si-doped C60 fullerenes | [60] |
| C60−nSin (n = 1), C60Sim (m = 1–2) | Sim (m = 1, 2) | GO, ESPA, MD | - | PW91 | “Dacapo” code | Investigation of the thermal stability, structural and electronic properties of substitutional vs. exohedral doping behavior of Si-substituted C60 | [45] |
| C60−nSin (n = 1–12) C60Sim (m = 1–15) | Sim (m = 1–15) | GO, ESPA, MD | DFTB-based TBMD | - | DFTB code | Investigation of the thermal behavior and fragmentation mechanisms of Si-doped C60 fullerenes, including substitutional derivatives (C60−nSin) and exohedral adducts (C60Sim) | [61] |
| C59Si | Sim (m = 10, 15, 20) | GO, ESPA, Eads | - | PW91, PAW | VASP | Investigation of the structural and electronic properties of nanostructures C59Si–Sim (m = 10, 15, 20) | [89] |
| C59Si | phenylpropanolamine | GO, ESPA, Eads (with BSSE), NICS | - | B3LYP-D/3-21G(d), GIAO | GAMESS | Investigation of the structural, electronic, adsorption and aromatic properties of C59Si with a pharmacologically active compound (in comparison to C59Al and pristine C60) | [120] |
| C59Si | H2S | GO, ESPA, Eads | - | B3LYP/3-21G(d) | Gaussian 09 | Investigation of the structural, electronic and adsorption properties of C59Si for H2S gas sensing (in comparison to C59B, C59N, C59S, C59P and pristine C60) | [92] |
| C59Si | valproic acid | GO, ESPA, Eads (with BSSE), PCM, IR, NMR | - | B3LYP/cc-pVDZ | Gaussian 09 | Investigation of the interaction mechanism and stability of valproic acid adsorbed on Si-doped C60 (in comparison to C59B, and C59Al) | [115] |
| C58Si2 | salicylic acid, flurbiprofen | GO, ESPA, Eads (with BSSE), PCM, IR | - | B3LYP/6-31G(d) | Gaussian 09 | Assessment of the structural, electronic and adsorption properties of C58Si2 as a nanocarrier for two NSAIDs | [119] |
| C59Si | hydroquinone | GO, ESPA, Eads (with BSSE), PCM | - | B3LYP/6-31G(d), M06-2X/cc-pVDZ | Gaussian 09 | Investigation of the adsorption mechanism and electronic properties of hydroquinone on Si-doped C60 (in comparison to C59B) | [97] |
| C59Si | Li+, Na+, K+, Be2+, Mg2+, Ca2+ | GO, ESPA, Eads (with BSSE) | - | B3LYP/6-31G(d) | GAMESS | Investigation of the structural, electronic, and adsorption properties of alkali and alkaline earth cations on Si-doped C60 nanocage for potential sensor applications (compared with pristine C60) | [90] |
| C57SiAlB | ifosfamide | GO, ESPA, Eads (with BSSE), PCM, IR | - | B3LYP/6-31G(d), wB97XD/6-31G(d) | Gaussian 09 | Investigation of the structural, electronic, adsorption, and vibrational properties of tri-doped C60 interacting with ifosfamide for potential drug delivery applications | [117] |
| C59Si | epinephrine | GO, ESPA, Eads, PCM | - | B3LYP/6-31G(d,p) | Gaussian 16 | Evaluation of the adsorption behavior, structural, electronic, and energetic properties of Si-doped C60 interacting with epinephrine (compared with C59B and pristine C60) | [109] |
| C59Si | serine | GO, ESPA, Eads | PM6 | PBE0/6-311G(d), B3LYP-D3/6-311G(d), ωB97XD/6-311G(d), M06-2X/6-311G(d) | Gaussian 16 | Assessment of the intermolecular interactions, structural, electronic, and adsorption properties of Si-doped C60 with serine (compared with C59Ge and pristine C60) | [105] |
| C59Si | cysteine | GO, ESPA, Eads | PM6 | PBE0/6-311G(d), B3LYP-D3/6-311G(d), ωB97XD/6-311G(d), M06-2X/6-311G(d) | Gaussian 16 | Investigation of the intermolecular interactions, structural, electronic, and adsorption properties of Si-doped C60 with cysteine (compared with C59Ge and pristine C60) | [106] |
| C59Si | acrolein | GO, ESPA, Eads | PM6 | PBE0/6-311G(d), B3LYP-D3/6-311G(d), ωB97XD/6-311G(d), M06-2X/6-311G(d) | Gaussian 16 | Investigation of the intermolecular interactions, structural, electronic, and adsorption properties of Si-doped C60 with acrolein (compared with C59Ge and pristine C60) | [102] |
| C59Si | Hexachlorobenzene (HCB) | GO, ESPA, Eads | PM6 | PBE0/6-311G(d), B3LYP-D3/6-311G(d), ωB97XD/6-311G(d), M06-2X/6-311G(d) | Gaussian 16 | Investigation of the adsorption mechanism, structural, electronic, and reactivity properties of Si-doped C60 with HCB (compared with C59Ge and pristine C60) | [103] |
| C60−nSin (n = 30), C60Sim (m = 1–30) | Sim (m = 1–30) | GO, ESPA, FTMD | SCC-DFTB | LSDA/6-31G(d), PBE/6-31G(d), B3LYP/6-31G(d) | Gaussian 03, “Dylax” DFTB code | Investigation of the thermal stability and formation mechanisms of substitutional and exohedral Si-doped C60 | [59] |
3.3. Endohedral Complexes of Substitutional Si-Doped C60 Fullerenes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A New Form of Carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Krätschmer, W.; Fostiropoulos, K.; Huffman, D.R. The Infrared and Ultraviolet Absorption Spectra of Laboratory-Produced Carbon Dust: Evidence for the Presence of the C60 Molecule. Chem. Phys. Lett. 1990, 170, 167–170. [Google Scholar] [CrossRef]
- Lin, Y.L.; Nori, F. Electronic Structure of Single- and Multiple-Shell Carbon Fullerenes. Phys. Rev. B 1994, 49, 5020–5023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bethune, D.S.; Johnson, R.D.; Salem, J.R.; de Vries, M.S.; Yannoni, C.S. Atoms in Carbon Cages: The Structure and Properties of Endohedral Fullerenes. Nature 1993, 366, 123–128. [Google Scholar] [CrossRef]
- Honeychuck, R.V. Science of Fullerenes and Carbon Nanotubes; Dresselhaus, M.S., Dresselhaus, G., Eklund, P.C., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. xviii, 965. [Google Scholar]
- Kroto, H.W. The Stability of the Fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature 1987, 329, 529–531. [Google Scholar] [CrossRef]
- Clancy, A.J.; Bayazit, M.K.; Hodge, S.A.; Skipper, N.T.; Howard, C.A.; Shaffer, M.S.P. Charged Carbon Nanomaterials: Redox Chemistries of Fullerenes, Carbon Nanotubes, and Graphenes. Chem. Rev. 2018, 118, 7363–7408. [Google Scholar] [CrossRef]
- Vassallo, A.M.; Pang, L.S.K.; Cole-Clarke, P.A.; Wilson, M.A. Emission FTIR Study of C60 Thermal Stability and Oxidation. J. Am. Chem. Soc. 1991, 113, 7820–7821. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Altalhi, T.; Liu, D.J.; Yakobson, B.I. A Quantum Mechanical MP2 Study of the Electronic Effect of Nonplanarity on the Carbon Pyramidalization of Fullerene C60. Nanomaterials 2024, 14, 1576. [Google Scholar] [CrossRef]
- Daniel, G.B.; Nachimuthu, K.; Nallasivam, J.L. Functionalization of [C60]-Fullerene: A Recent Update. Chem. Asian J. 2025, 20, e202401800. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic Solar Cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Smalley, R.E. Discovering the Fullerenes. Rev. Mod. Phys. 1997, 69, 723–730. [Google Scholar] [CrossRef]
- Hoseininezhad-Namin, M.S.; Javanshir, Z.; Jouyban, A.; Pargolghasemi, P.; Rahimpour, E. Effects of Ge, Si, and B Doping on the Adsorption and Detection Properties of C60 Fullerene towards Methadone in Gas and Aqua Phases: A DFT Study. J. Mol. Model. 2023, 29, 71. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.V.; Benzigar, M.R.; Talapaneni, S.N.; Singh, G.; Karakoti, A.S.; Yi, J.; Al-Muhtaseb, A.A.; Ariga, K.; Ajayan, P.M.; Vinu, A. Self-Assembled Fullerene Nanostructures: Synthesis and Applications. Adv. Funct. Mater. 2022, 32, 2106924. [Google Scholar] [CrossRef]
- Vostrowsky, O.; Hirsch, A. Heterofullerenes. Chem. Rev. 2006, 106, 5191–5207. [Google Scholar] [CrossRef] [PubMed]
- Erbahar, D.; Susi, T.; Rocquefelte, X.; Bittencourt, C.; Scardamaglia, M.; Blaha, P.; Guttmann, P.; Rotas, G.; Tagmatarchis, N.; Zhu, X.; et al. Spectromicroscopy of C60 and Azafullerene C59N: Identifying Surface Adsorbed Water. Sci. Rep. 2016, 6, 35605. [Google Scholar] [CrossRef]
- Chen, K.Y.; Wu, S.Y.; Chen, H.T. Unraveling Catalytic Mechanisms for CO Oxidation on Boron-Doped Fullerene: A Computational Study. ACS Omega 2020, 5, 28870–28876. [Google Scholar] [CrossRef]
- Moreno-Vicente, A.; Alías-Rodríguez, M.; Dunk, P.W.; de Graaf, C.; Poblet, J.M.; Rodríguez-Fortea, A. Highly Oxidized U(VI) within the Smallest Fullerene: Gas-Phase Synthesis and Computational Study of Boron-Doped U@C27B. Inorg. Chem. Front. 2023, 10, 908–914. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, S.; Zhang, X.; Zhao, X. Structural and Electronic Properties of Oxygen-Doped Heterofullerene: Is the O–C Single Bond Shorter than the C–C Single Bond? Solid State Commun. 2001, 118, 247–250. [Google Scholar] [CrossRef]
- Muhr, H.J.; Nesper, R.; Schnyder, B.; Kötz, R. The Boron Heterofullerenes C59B and C69B: Generation, Extraction, Mass Spectrometric and XPS Characterization. Chem. Phys. Lett. 1996, 249, 399–405. [Google Scholar] [CrossRef]
- Hummelen, J.C.; Knight, B.; Pavlovich, J.; González, R.; Wudl, F. Isolation of the Heterofullerene C59N as Its Dimer (C59N)2. Science 1995, 269, 1554–1556. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Pellarin, M.; Lermé, J.L.; Vialle, J.L.; Broyer, M.; Blase, X.; Mélinon, P.; Kéghélian, P.; Perez, A. Synthesis and Structure of Silicon-Doped Heterofullerenes. Phys. Rev. Lett. 1998, 80, 5365–5368. [Google Scholar] [CrossRef]
- Lemos Silva, R.A.; Scalabrini Machado, D.F.; de Oliveira, H.C.B.; Ribeiro, L.; da Silva Filho, D.A. Theoretical Study of the Interaction of Fullerenes with the Emerging Contaminant Carbamazepine for Detection in Aqueous Environments. Sci. Rep. 2022, 12, 15848. [Google Scholar] [CrossRef]
- Fekri, M.H.; Bazvand, R.; Solymani, M.; Razavi Mehr, M. Adsorption Behavior, Electronical and Thermodynamic Properties of Ornidazole Drug on C60 Fullerene Doped with Si, B and Al: A Quantum Mechanical Simulation. Phys. Chem. Res. 2021, 9, 151–164. [Google Scholar]
- Rezaee, P.; Asl, S.A.; Javadi, M.H.; Rezaee, S.; Morad, R.; Akbari, M.; Arab, S.S.; Maaza, M. DFT Study on CO2 Capture Using Boron, Nitrogen, and Phosphorus-Doped C20 in the Presence of an Electric Field. Sci. Rep. 2024, 14, 12388. [Google Scholar] [CrossRef]
- de Silva, S.W.; Du, A.; Senadeera, W.; Gu, Y. Neutral and Charged Boron-Doped Fullerenes for CO2 Adsorption. Beilstein J. Nanotechnol. 2014, 5, 413–418. [Google Scholar] [CrossRef]
- Kuganathan, N. Activation of CO2 on the Surfaces of Bare, Ti-Adsorbed and Ti-Doped C60. Fuels 2022, 3, 176–183. [Google Scholar] [CrossRef]
- Hebard, A.F.; Rosseinsky, M.J.; Haddon, R.C.; Murphy, D.W.; Glarum, S.H.; Palstra, T.T.; Ramirez, A.P.; Kortan, A.R. Superconductivity at 18 K in Potassium-Doped C60. Nature 1991, 350, 600–601. [Google Scholar] [CrossRef]
- Chang, X.; Xu, Y.; von Delius, M. Recent Advances in Supramolecular Fullerene Chemistry. Chem. Soc. Rev. 2024, 53, 47–83. [Google Scholar] [CrossRef]
- Garg, I.; Sharma, H.; Dharamvir, K.; Jindal, V.K. Substitutional Patterns in Boron Doped Heterofullerenes C60−nBn (n = 1–12). J. Comput. Theor. Nanosci. 2011, 8, 642–655. [Google Scholar] [CrossRef]
- Pattanayak, J.; Kar, T.; Scheiner, S. Boron−Nitrogen (BN) Substitution of Fullerenes: C60 to C12B24N24 CBN Ball. J. Phys. Chem. A 2002, 106, 2970–2978. [Google Scholar] [CrossRef]
- Soltani, A.; Tazikeh-Lemeski, E.; Javan, M.B. A Comparative Theoretical Study on the Interaction of Pure and Carbon Atom Substituted Boron Nitride Fullerenes with Ifosfamide Drug. J. Mol. Liq. 2020, 297, 111894. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Abolghasemzadeh, S. Sc Functionalized Boron-Rich C60 Fullerene for Efficient Storage and Separation of CO2 Molecules: A DFT Investigation. Comput. Theor. Chem. 2022, 1208, 113557. [Google Scholar] [CrossRef]
- Chen, Z.; Reuther, U.; Hirsch, A.; Thiel, W. Theoretical Studies on the Substitution Patterns in Heterofullerenes C70−xNx and C70−xBx (x = 2−10). J. Phys. Chem. A 2001, 105, 8105–8110. [Google Scholar] [CrossRef]
- Yadav, V.K. Exploring the Effect of BN Doping in Two-Dimensional Fullerene Networks through First Principle Simulations. FlatChem 2024, 45, 100655. [Google Scholar] [CrossRef]
- Miri Jahromi, A.; Zandi, P.; Khedri, M.; Ghasemy, E.; Maleki, R.; Tayebi, L. Molecular Insight into Optimizing the N- and P-Doped Fullerenes for Urea Removal in Wearable Artificial Kidneys. J. Mater. Sci. Mater. Med. 2021, 32, 49. [Google Scholar] [CrossRef]
- Khan, A.A.; Ahmad, I.; Ahmad, R. Influence of Electric Field on CO2 Removal by P-Doped C60-Fullerene: A DFT Study. Chem. Phys. Lett. 2020, 742, 137155. [Google Scholar] [CrossRef]
- Ewels, C.P.; El Cheikh, H.; Suarez-Martinez, I.; Van Lier, G. Oxidation and Reactivity of Nitrogen- and Phosphorus-Doped Heterofullerenes. Phys. Chem. Chem. Phys. 2008, 10, 2145–2148. [Google Scholar] [CrossRef]
- Chuang, S.C.; Santhosh, K.C.; Lin, C.H.; Wang, S.L.; Cheng, C.H. Synthesis and Chemistry of Fullerene Derivatives Bearing Phosphorus Substituents. Unusual Reaction of Phosphines with Electron-Deficient Acetylenes and C60. J. Org. Chem. 1999, 64, 6664–6669. [Google Scholar] [CrossRef]
- Solà, M.; Cabrera-Trujillo, J.M.; Tenorio, F.; Mayorga, O.; Cases, M.; Duran, M.; Robles, J. Pristine and Silicon-Substituted Small-Carbon Clusters and Fullerenes: Electronic Structure and Reactivity. In Reviews of Modern Quantum Chemistry; World Scientific: Singapore, 2002; pp. 1367–1420. [Google Scholar]
- Guirado-López, R. Stability and Electronic Properties of Si-Doped Carbon Fullerenes. Phys. Rev. B 2002, 65, 165421. [Google Scholar] [CrossRef]
- Matsubara, M.; Massobrio, C. First Principles Study of Extensive Doping of C60 with Silicon. Mater. Sci. Eng. C 2006, 26, 1224–1227. [Google Scholar] [CrossRef]
- Umran, N.M.; Kumar, R. Theoretical Investigation of Endohedral Complexes of Si and Ge with C60 Molecule. Physica B 2014, 437, 47–52. [Google Scholar] [CrossRef]
- Marcos, P.A.; Alonso, J.A.; López, M.J.; Hernández, E. Tight Binding Studies of Exohedral Silicon Doped C60. Compos. Sci. Technol. 2003, 63, 1499–1505. [Google Scholar] [CrossRef]
- Kimura, T.; Sugai, T.; Shinohara, H. Production and Characterization of Boron- and Silicon-Doped Carbon Clusters. Chem. Phys. Lett. 1996, 256, 269–273. [Google Scholar] [CrossRef]
- Cao, B.; Zhou, X.; Shi, Z.; Gu, Z.; Xiao, H.; Wang, J. Synthesis of Silicon-Containing Fullerenes. Chem. Lett. 1998, 27, 735–736. [Google Scholar] [CrossRef]
- Fye, J.L.; Jarrold, M.F. Structures of Silicon-Doped Carbon Clusters. J. Phys. Chem. A 1997, 101, 1836–1840. [Google Scholar] [CrossRef]
- Pellarin, M.; Ray, C.; Lermé, J.; Vialle, J.L.; Broyer, M.; Blase, X.; Kéghélian, P.; Mélinon, P.; Perez, A. Photolysis Experiments on SiC Mixed Clusters: From Silicon Carbide Clusters to Silicon-Doped Fullerenes. J. Chem. Phys. 1999, 110, 6927–6935. [Google Scholar] [CrossRef]
- Iwaszczuk, J.; Baj, A.; Wałejko, P. Sialic Acids—Structure and Properties. Prospect. Pharm. Sci. 2024, 22, 31–38. [Google Scholar] [CrossRef]
- Blase, X.; Broyer, M.; Kéghélian, P.; Lermé, J.; Mélinon, P.; Pellarin, M.; Perez, A.; Ray, C.; Vialle, J.L. Production and Stability of Silicon-Doped Heterofullerenes. Eur. Phys. J. D 1999, 9, 49–54. [Google Scholar] [CrossRef]
- Billas, I.M.; Tast, F.; Branz, W.; Malinowski, N.; Heinebrodt, M.; Martin, T.P.; Boero, M.; Massobrio, C.; Parrinello, M. Experimental and Computational Studies of Si-Doped Fullerenes. Eur. Phys. J. D 1999, 9, 337–340. [Google Scholar] [CrossRef]
- Ohara, M.; Nakamura, Y.; Negishi, Y.; Miyajima, K.; Nakajima, A.; Kaya, K. Behavior of Silicon and Germanium Clusters on a C60 Fullerene. J. Phys. Chem. A 2002, 106, 4498–4501. [Google Scholar] [CrossRef]
- Bulina, N.V.; Lopatin, V.A.; Vnukova, N.G.; Osipova, I.V.; Churilov, G.N.; Krätschmer, W. Arc Synthesis of Silicon-Doped Heterofullerenes in Plasma at Atmospheric Pressure. Fuller. Nanotub. Carbon Nanostruct. 2007, 15, 395–400. [Google Scholar] [CrossRef]
- Lu, J.; Luo, Y.; Huang, Y.; Zhang, X.; Zhao, X. Semiempirical Calculations on Heterofullerene C59Si: Structural and Electronic Localization. Solid State Commun. 2001, 118, 309–312. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, Z.; Liu, L.; Shen, Z.; Kuo, J.L. Theoretical Study on Structural Stability of Alloy Cages: A Case of Silicon-Doped Heterofullerenes. Commun. Comput. Phys. 2010, 8, 289–303. [Google Scholar] [CrossRef]
- Ibrahim, M.; El-Haes, H.; Jalbout, A.F. Semiempirical Molecular Modelling Study of C60 Doped with Silicon, Germanium, and Aluminium. Chin. J. Phys. 2006, 44, 432–439. [Google Scholar]
- Ganji, M.D.; Ahmadian, N.; Goodarzi, M.; Khorrami, H.A. Molecular Hydrogen Interacting with Si-, S- and P-Doped C60 Fullerenes and Carbon Nanotube. J. Comput. Theor. Nanosci. 2011, 8, 1392–1399. [Google Scholar] [CrossRef]
- Wongchoosuk, C.; Wang, Y.; Kerdcharoen, T.; Irle, S. Nonequilibrium Quantum Chemical Molecular Dynamics Simulations of C60 to SiC Heterofullerene Conversion. Carbon 2014, 68, 285–295. [Google Scholar] [CrossRef]
- Fu, C.C.; Fava, J.; Weht, R.; Weissmann, M. Molecular Dynamics Study of the Fragmentation of Silicon-Doped Fullerenes. Phys. Rev. B 2002, 66, 045405. [Google Scholar] [CrossRef]
- Marcos, P.A.; Alonso, J.A.; López, M.J. Simulating the Thermal Behavior and Fragmentation Mechanisms of Exohedral and Substitutional Silicon-Doped C60. J. Chem. Phys. 2005, 123, 204323. [Google Scholar] [CrossRef] [PubMed]
- Parlak, C.; Alver, Ö. A Density Functional Theory Investigation on Amantadine Drug Interaction with Pristine and B, Al, Si, Ga, Ge Doped C60 Fullerenes. Chem. Phys. Lett. 2017, 678, 85–90. [Google Scholar] [CrossRef]
- Parlak, C.; Alver, Ö.; Şenyel, M. Computational Study on Favipiravir Adsorption onto Undoped- and Silicon-Decorated C60 Fullerenes. J. Theor. Comput. Chem. 2017, 16, 1750011. [Google Scholar] [CrossRef]
- Lan, Y.Z.; Kang, H.L.; Niu, T. One- and Two-Photon Absorptions of the Cn and Cn−1Si Fullerenes in Gas Phase and Solution. Eur. Phys. J. D 2015, 69, 69. [Google Scholar] [CrossRef]
- Bai, H.; Ji, W.; Liu, X.; Wang, L.; Yuan, N.; Ji, Y. Doping the Buckminsterfullerene by Substitution: Density Functional Theory Studies of C59X (X = B, N, Al, Si, P, Ga, Ge, and As). J. Chem. 2013, 2013, 571709. [Google Scholar] [CrossRef]
- Koponen, L.; Puska, M.J.; Nieminen, R.M. Photoabsorption Spectra of Small Fullerenes and Si-Heterofullerenes. J. Chem. Phys. 2008, 128, 154307. [Google Scholar] [CrossRef]
- Ghafouri, R.; Anafcheh, M. A Computational NICS and 13C NMR Characterization of C60−nSin Heterofullerenes (n = 1, 2, 6, 12, 20, 24, 30). J. Clust. Sci. 2012, 23, 469–480. [Google Scholar] [CrossRef]
- Beigi, H.S.I. DFT Study on a Fullerene Doped with Si and N. Russ. J. Phys. Chem. A 2013, 87, 1537–1541. [Google Scholar] [CrossRef]
- Fu, C.C.; Weissmann, M.; Machado, M.; Ordejón, P. Ab Initio Study of Silicon-Multisubstituted Neutral and Charged Fullerenes. Phys. Rev. B 2001, 63, 085411. [Google Scholar] [CrossRef]
- Scipioni, R.; Matsubara, M.; Ruiz, E.; Massobrio, C.; Boero, M. Thermal Behavior of Si-Doped Fullerenes vs. Their Structural Stability at T = 0 K: A Density Functional Study. Chem. Phys. Lett. 2011, 510, 14–17. [Google Scholar] [CrossRef]
- Li, J.; Xia, Y.; Zhao, M.; Liu, X.; Song, C.; Li, L.; Li, F. From Pure C60 to Silicon Carbon Fullerene-Based Nanotube: An Ab Initio Study. J. Chem. Phys. 2008, 128, 154719. [Google Scholar] [CrossRef]
- Jiao, H.; Chen, Z.; Hirsch, A.; Thiel, W. Structures and Magnetic Properties of Mono-Doped Fullerenes C59Xn and C59X(6−n)− (X = B−, N+, P+, As+, Si): Isoelectronic Analogues of C60 and C606−. J. Mol. Model. 2003, 9, 34–38. [Google Scholar] [CrossRef]
- Fan, B.B.; Shi, C.Y.; Zhang, R.; Jia, Y. Ground States of Silicon-Multisubstituted Fullerene: First-Principles Calculations and Monte Carlo Simulations. Chin. Phys. Lett. 2013, 30, 106101. [Google Scholar] [CrossRef]
- Bashiri, S.; Vessally, E.; Bekhradnia, A.; Hosseinian, A.; Edjlali, L. Utility of Extrinsic [60] Fullerenes as Work Function Type Sensors for Amphetamine Drug Detection: DFT Studies. Vacuum 2017, 136, 156–162. [Google Scholar] [CrossRef]
- Hazrati, M.K.; Hadipour, N.L. Adsorption Behavior of 5-Fluorouracil on Pristine, B-, Si-, and Al-Doped C60 Fullerenes: A First-Principles Study. Phys. Lett. A 2016, 380, 937–941. [Google Scholar] [CrossRef]
- Matsubara, M.; Kortus, J.; Parlebas, J.C.; Massobrio, C. Dynamical Identification of a Threshold Instability in Si-Doped Heterofullerenes. Phys. Rev. Lett. 2006, 96, 155502. [Google Scholar] [CrossRef]
- Matsubara, M.; Massobrio, C. Stable Highly Doped C60−mSim Heterofullerenes: A First Principles Study of C40Si20, C36Si24, and C30Si30. J. Phys. Chem. A 2005, 109, 4415–4418. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Massobrio, C. Charge Effects in Silicon-Doped Heterofullerenes. Appl. Phys. A 2007, 86, 289–292. [Google Scholar] [CrossRef]
- Matsubara, M.; Massobrio, C. An Extensive Study of Charge Effects in Silicon Doped Heterofullerenes. Solid State Phenom. 2007, 129, 95–103. [Google Scholar] [CrossRef]
- An, W.; Shao, N.; Bulusu, S.; Zeng, X.C. Ab Initio Calculation of Carbon Clusters. II. Relative Stabilities of Fullerene and Nonfullerene C24. J. Chem. Phys. 2008, 128, 084301. [Google Scholar] [CrossRef]
- Yanov, I.; Leszczynski, J. The Molecular Structure and Electronic Spectrum of the C@C60 Endohedral Complex: An Ab Initio Study. J. Mol. Graph. Model. 2001, 19, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Paulus, B. Electronic and Structural Properties of the Cage-Like Molecules C20 to C36. Phys. Chem. Chem. Phys. 2003, 5, 3364–3367. [Google Scholar] [CrossRef]
- Jaworski, A.; Hedin, N. Local Energy Decomposition Analysis and Molecular Properties of Encapsulated Methane in Fullerene (CH4@C60). Phys. Chem. Chem. Phys. 2021, 23, 21554–21567. [Google Scholar] [CrossRef]
- Taylor, P.R.; Bylaska, E.; Weare, J.H.; Kawai, R. C20: Fullerene, Bowl or Ring? New Results from Coupled-Cluster Calculations. Chem. Phys. Lett. 1995, 235, 558–563. [Google Scholar] [CrossRef]
- Fukuda, R.; Ehara, M. Electronic Excitations of C60 Fullerene Calculated Using the Ab Initio Cluster Expansion Method. J. Chem. Phys. 2012, 137, 134304. [Google Scholar] [CrossRef]
- Simeon, T.M.; Yanov, I.; Leszczynski, J. Ab Initio Quantum Chemical Studies of Fullerene Molecules with Substitutes C59X [X = Si, Ge, Sn], C59X− [X = B, Al, Ga, In], and C59X [X = N, P, As, Sb]. Int. J. Quantum Chem. 2005, 105, 429–436. [Google Scholar] [CrossRef]
- Matsubara, M.; Massobrio, C.; Parlebas, J.C. First Principles Calculation Study of Multi-Silicon Doped Fullerenes. Comput. Mater. Sci. 2005, 33, 237–243. [Google Scholar] [CrossRef]
- Anafcheh, M.; Khanmohammadi, H.; Zahedi, M. Diels–Alder Cycloaddition of the Silicon–Silicon Bonds at Pentagon Junctions of Si-Doped Non-IPR and SW Defective Fullerenes. Monatsh. Chem. 2021, 152, 241–250. [Google Scholar] [CrossRef]
- Wu, M.M.; Zhou, X.; Zhou, J.; Sun, Q.; Wang, Q.; Jena, P. Interaction of C59Si with Si Based Clusters: A Study of Janus Nanostructures. J. Phys. Condens. Matter 2010, 22, 275303. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, A.; Poor Heravi, M.R.; Khanmohammadi, A. Electronic Sensors for Alkali and Alkaline Earth Cations Based on Fullerene-C60 and Silicon Doped on C60 Nanocages: A Computational Study. J. Mol. Model. 2022, 28, 148. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Li, J.; Bai, Z.; Wang, C.; Lv, X. Atomic Insights of Structural, Electronic Properties of B, N, P, S, Si-Doped Fullerenes and Lithium Ion Migration with DFT-D Method. J. Mol. Model. 2024, 30, 422. [Google Scholar] [CrossRef]
- Yousefian, Z.; Ghasemy, E.; Askarieh, M.; Rashidi, A. Theoretical Studies on B, N, P, S, and Si Doped Fullerenes toward H2S Sensing and Adsorption. Physica E 2019, 114, 113626. [Google Scholar] [CrossRef]
- Amiraslanzadeh, S. The Effect of Doping Different Heteroatoms on the Interaction and Adsorption Abilities of Fullerene. Heteroat. Chem. 2016, 27, 23–31. [Google Scholar] [CrossRef]
- Deddouche, N.; Chemouri, H. Air Purification of the Carbon Monoxide Molecule CO Using the Nanocatalytic Oxidation. Computational Study. Theor. Found. Chem. Eng. 2022, 56, 760–767. [Google Scholar] [CrossRef]
- Vashchenko, A.V.; Kuzmin, A.V.; Shainyan, B.A. Single Si-Doped Fullerene as a Catalyst in the Oxygen Reduction Reaction: A Quantum Chemical Insight. Int. J. Quantum Chem. 2021, 121, e26565. [Google Scholar] [CrossRef]
- Parlak, C.; Alver, Ö. Single and Double Silicon Decoration of Fullerene C60 and Single Walled Carbon Nanotubes for Adsorption and Detection of TNT. J. Mol. Struct. 2019, 1198, 126881. [Google Scholar] [CrossRef]
- Ergürhan, O.; Parlak, C.; Alver, Ö.; Şenyel, M. Conformational and Electronic Properties of Hydroquinone Adsorption on C60 Fullerenes: Doping Atom, Solvent and Basis Set Effects. J. Mol. Struct. 2018, 1167, 227–231. [Google Scholar] [CrossRef]
- Gökpek, Y.; Bilge, M.; Bilge, D.; Alver, Ö.; Parlak, C. Adsorption Mechanism, Structural and Electronic Properties: 4-Phenylpyridine & Undoped or Doped (B or Si) C60. J. Mol. Liq. 2017, 238, 225–228. [Google Scholar] [CrossRef]
- Parlak, C.; Tepe, M.; Bağlayan, Ö.; Alver, Ö. Investigation of Detection and Adsorption Properties of β-Propiolactone with Silicon and Aluminum Doped Fullerene C60 Using Density Functional Theory. J. Mol. Struct. 2020, 1217, 128346. [Google Scholar] [CrossRef]
- Alver, Ö.; Parlak, C.; Ramasami, P.; Şenyel, M. Interaction between Doped C60 Fullerenes and Piperazine-2,3,5,6-Tetraone: DFT Simulation. Main Group Met. Chem. 2018, 41, 63–66. [Google Scholar] [CrossRef]
- Özkan, E.; Bilge, M.; Bilge, D.; Alver, Ö.; Parlak, C.; Şenyel, M.; Ramasami, P. Sensor Application of Doped C60 Fullerenes in Detection of 1-(3-Trifluoromethylphenyl)piperazine as an Alternative to Ecstasy. Main Group Met. Chem. 2019, 42, 23–27. [Google Scholar] [CrossRef]
- Doust Mohammadi, M.; Abdullah, H.Y. Ab Initio Investigation for the Adsorption of Acrolein onto the Surface of C60, C59Si, and C59Ge: NBO, QTAIM, and NCI Analyses. Struct. Chem. 2022, 33, 363–378. [Google Scholar] [CrossRef]
- Mohammadi, M.D.; Abdullah, H.Y.; Louis, H.; Etim, E.E.; Edet, H.O.; Godfrey, O.C. Hexachlorobenzene (HCB) Adsorption onto the Surfaces of C60, C59Si, and C59Ge: Insight from DFT, QTAIM, and NCI. Chem. Phys. Impact 2023, 6, 100234. [Google Scholar] [CrossRef]
- Kaya, M.F.; Alver, Ö.; Parlak, C.; Ramasami, P. Theoretical Insight of Alpha Amino Acid Phenylalanine Adsorption on Pristine and Decorated Fullerenes. Main Group Met. Chem. 2019, 42, 135–142. [Google Scholar] [CrossRef]
- Doust Mohammadi, M.; Abdullah, H.Y. Intermolecular Interactions between Serine and C60, C59Si, and C59Ge: A DFT Study. Silicon 2022, 14, 6075–6088. [Google Scholar] [CrossRef]
- Doust Mohammadi, M.; Abdullah, H.Y. Non-Covalent Interactions of Cysteine onto C60, C59Si, and C59Ge: A DFT Study. J. Mol. Model. 2021, 27, 330. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Shakerzadeh, E.; Vishwkarma, A.K.; Singh, P.K.; Pathak, A.; Chakroborty, S.; Pandey, F.P.; Moharana, S.; Kumar, R. Histamine Sensing by Boron and Silicon Doped C60 Fullerenes: A First Principles Investigation. Diam. Relat. Mater. 2023, 140, 110471. [Google Scholar] [CrossRef]
- Pattanaik, S.; Vishwkarma, A.K.; Yadav, T.; Shakerzadeh, E.; Sahu, D.; Chakroborty, S.; Tripathi, P.K.; Zereffa, E.A.; Malviya, J.; Barik, A.; et al. In Silico Investigation on Sensing of Tyramine by Boron and Silicon Doped C60 Fullerenes. Sci. Rep. 2023, 13, 22264. [Google Scholar] [CrossRef]
- Yadav, T.; Vishwkarma, A.K.; Shakerzadeh, E.; Adam, J.; Kumar, P.; Vaithiyanathan, V.; Pathak, A.; Nguyen, M.T. Theoretical Investigation on Sensing of the Epinephrine Neurotransmitter by Doped C60 Fullerenes. Colloids Surf. A Physicochem. Eng. Asp. 2025, 716, 136649. [Google Scholar] [CrossRef]
- Mohammadi, M.D.; Salih, I.H.; Abdullah, H.Y. An Ultimate Investigation on the Adsorption of Amantadine on Pristine and Decorated Fullerenes C59X (X = Si, Ge, B, Al, Ga, N, P, and As): A DFT, NBO, and QTAIM Study. J. Comput. Biophys. Chem. 2021, 20, 23–39. [Google Scholar] [CrossRef]
- Parlak, C.; Alver, Ö.; Bağlayan, Ö. Quantum Mechanical Simulation of Molnupiravir Drug Interaction with Si-Doped C60 Fullerene. Comput. Theor. Chem. 2021, 1202, 113336. [Google Scholar] [CrossRef]
- Parlak, C.; Alver, Ö. Paracetamol Adsorption on C60 Fullerene and Its Derivatives: In Silico Insights. J. Indian Chem. Soc. 2022, 99, 100769. [Google Scholar] [CrossRef]
- Apriati, Y.N.; Kristiawan, B.; Jannah, N.; Nugraheni, A.D.; Sholihun, S. Drug-Molecule Adsorption onto Silicon-Doped Fullerene: A Density Functional Theory Study. Indones. J. Chem. 2023, 23, 1742–1752. [Google Scholar] [CrossRef]
- Fekri, M.H.; Bazvand, R.; Soleymani, M.; Razavi Mehr, M. Adsorption of Metronidazole Drug on the Surface of Nano Fullerene C60 Doped with Si, B and Al: A DFT Study. Int. J. Nanosci. Nanotechnol. 2024, 11, 346–354. [Google Scholar]
- Alver, Ö.; Parlak, C.; Şenyel, M.; Ramasami, P. Density Functional Theory Study on the Adsorption of Valproic Acid to Doped Fullerenes. Main Group Met. Chem. 2018, 41, 67–71. [Google Scholar] [CrossRef]
- Njeumen, C.A.; Ejuh, G.W.; Tadjouteu Assatse, Y.; Yossa Kamsi, R.A.; Ndjaka, J.M.B. DFT Studies of Physico-Chemical, Electronic and Nonlinear Optical Properties of Interaction between Doped-Fullerenes with Non-Steroidal Anti-Inflammatory Drugs. Physica B 2023, 665, 415041. [Google Scholar] [CrossRef]
- Kolsuz, D.; Bağlayan, Ö.; Parlak, C.; Alver, Ö. B, Al and Si Doping of Fullerene-C60 and the Limits of Selective Drug Delivery Assessments of Ifosfamide: A DFT Approach. J. Indian Chem. Soc. 2024, 101, 101128. [Google Scholar] [CrossRef]
- Singh, P.P.; Dey, C.; Dhal, B.; Muduli, S.B.; Chakraborty, S. In Silico Scrutinization of (Al, Ga, B, Si, Ge, and P)-Doped C60 in Sensing EGCG: An Application Based DFT Study. Comput. Theor. Chem. 2025, 1244, 115001. [Google Scholar] [CrossRef]
- Çatal, E.; Bağlayan, Ö.; Köroğlu, A.; Parlak, C.; Alver, Ö. Assessing a Double Silicon Decorated Fullerene for the Delivery of Interacting Flurbiprofen and Salicylic Acid Drugs: A DFT Approach. J. Indian Chem. Soc. 2023, 100, 101046. [Google Scholar] [CrossRef]
- Moradi, M.; Nouraliei, M.; Moradi, R. Theoretical Study on the Phenylpropanolamine Drug Interaction with the Pristine, Si and Al Doped [60] Fullerenes. Physica E 2017, 87, 186–191. [Google Scholar] [CrossRef]
- Parlak, C.; Alver, Ö.; Ramasami, P. Adsorption Mechanisms of 6-Chloro-3-Hydroxy-2-Pyrazinecarboxamide on Pristine, Si- and Al-Doped C60 Fullerenes: A DFT Study. J. Clust. Sci. 2017, 28, 2645–2652. [Google Scholar] [CrossRef]
- Javan, M.B.; Ganji, M.D. Theoretical Investigation on the Encapsulation of Atomic Hydrogen into Heterofullerene Nanocages. Curr. Appl. Phys. 2013, 13, 1525–1531. [Google Scholar] [CrossRef]
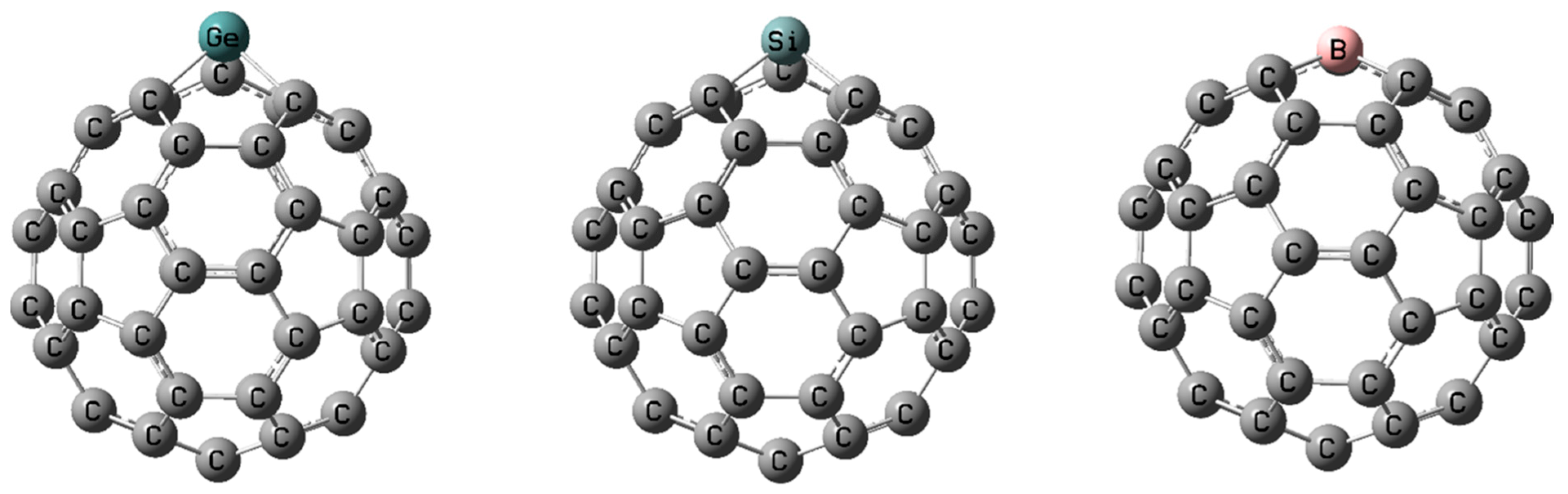
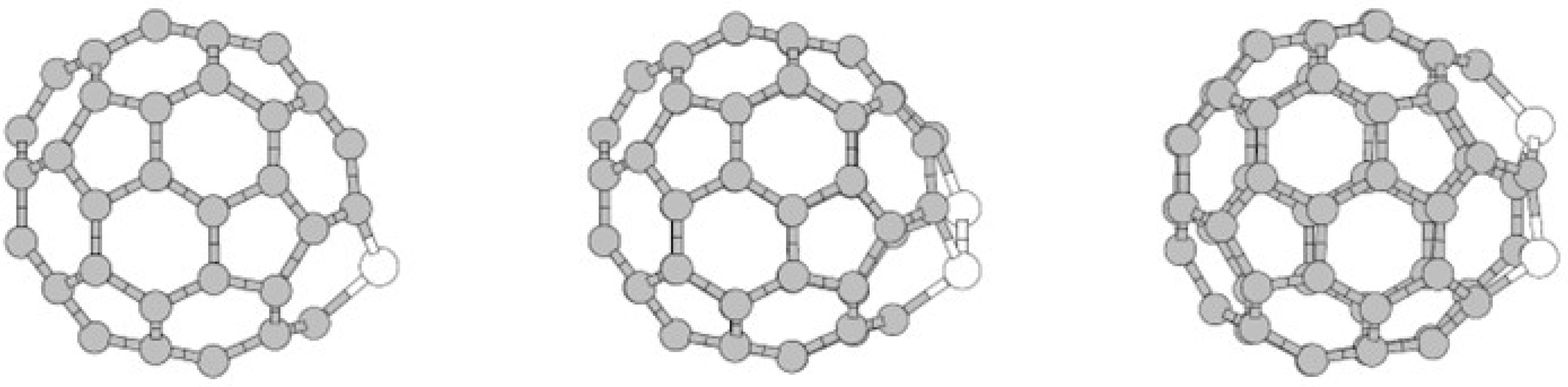
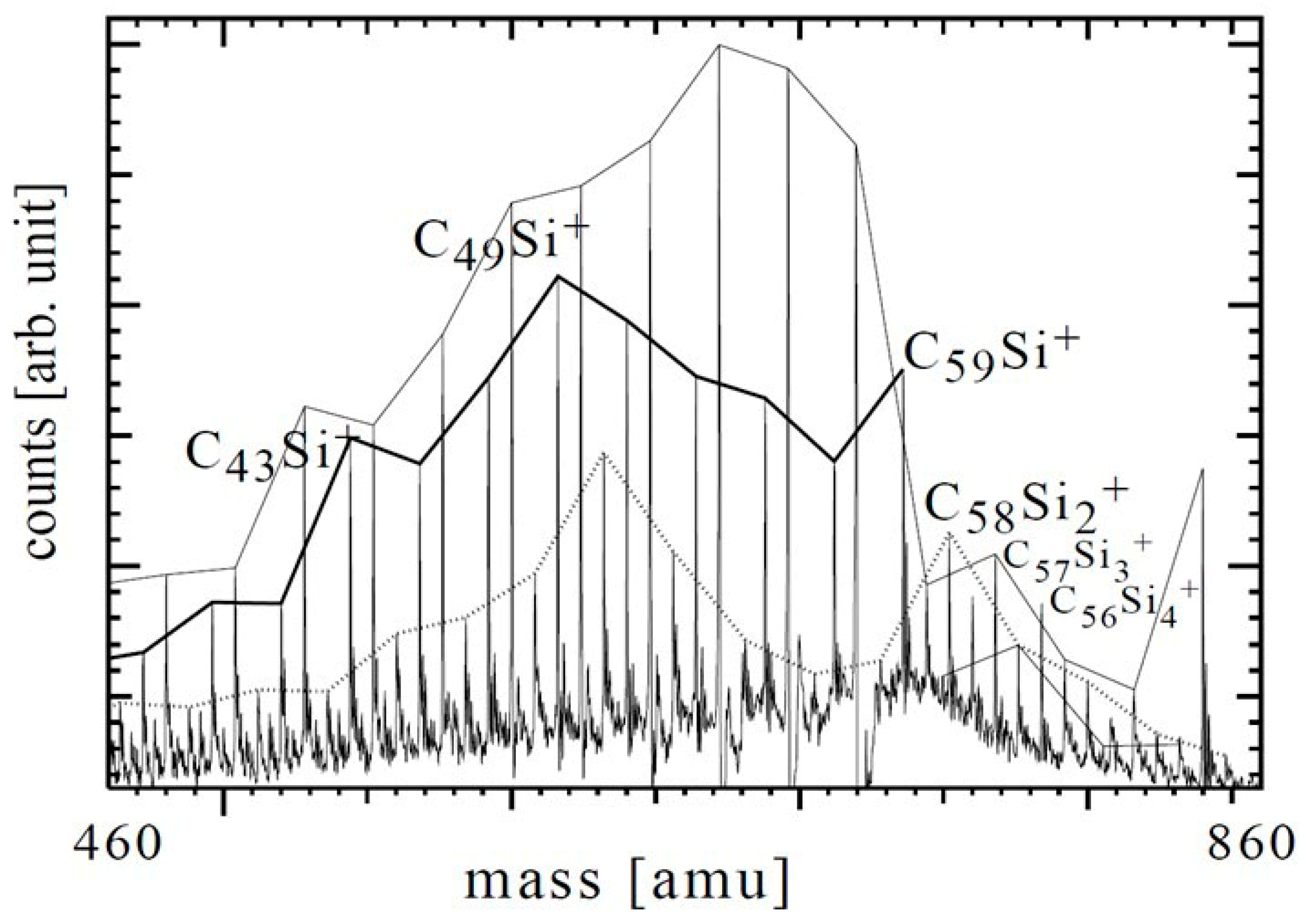
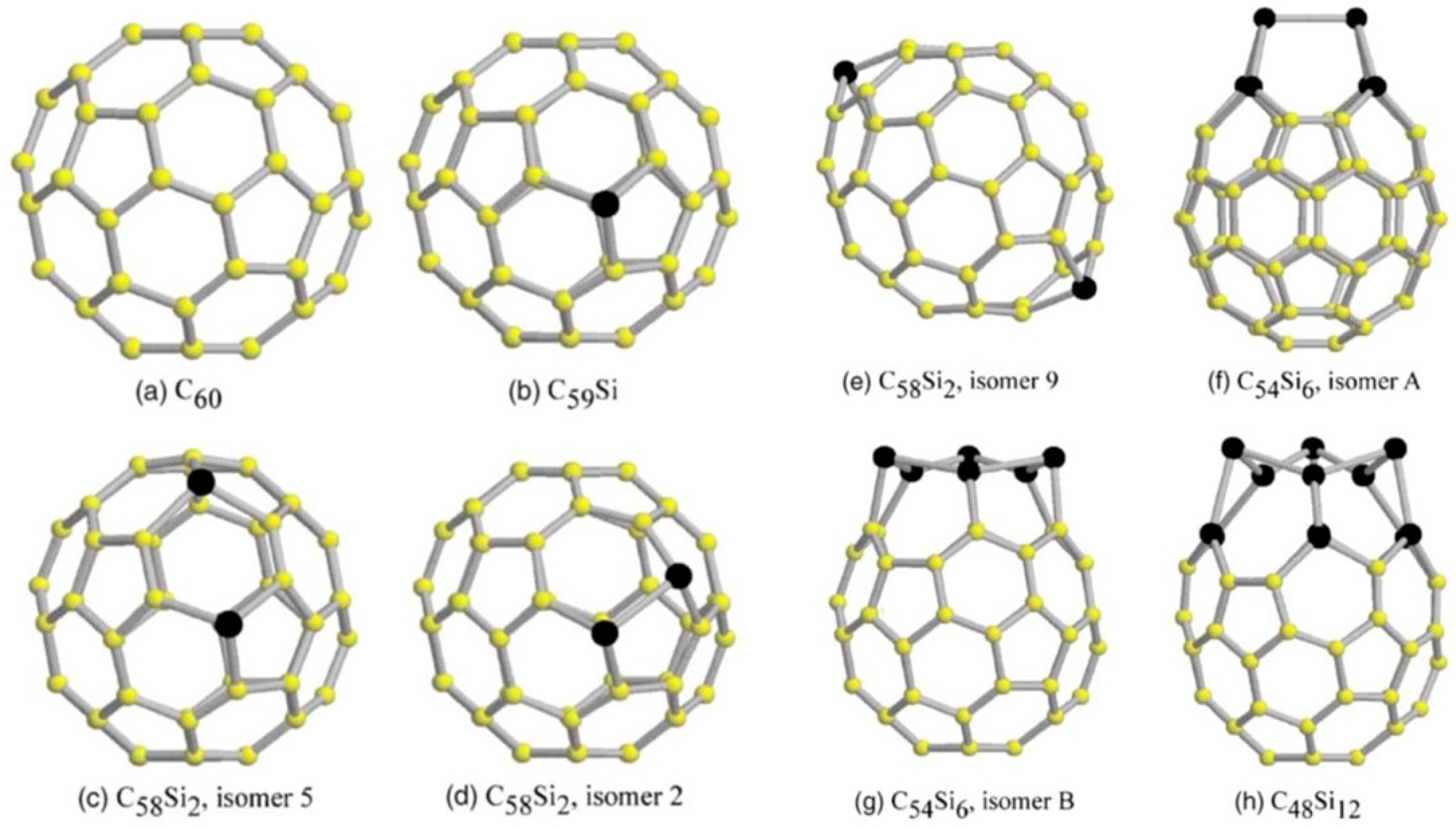
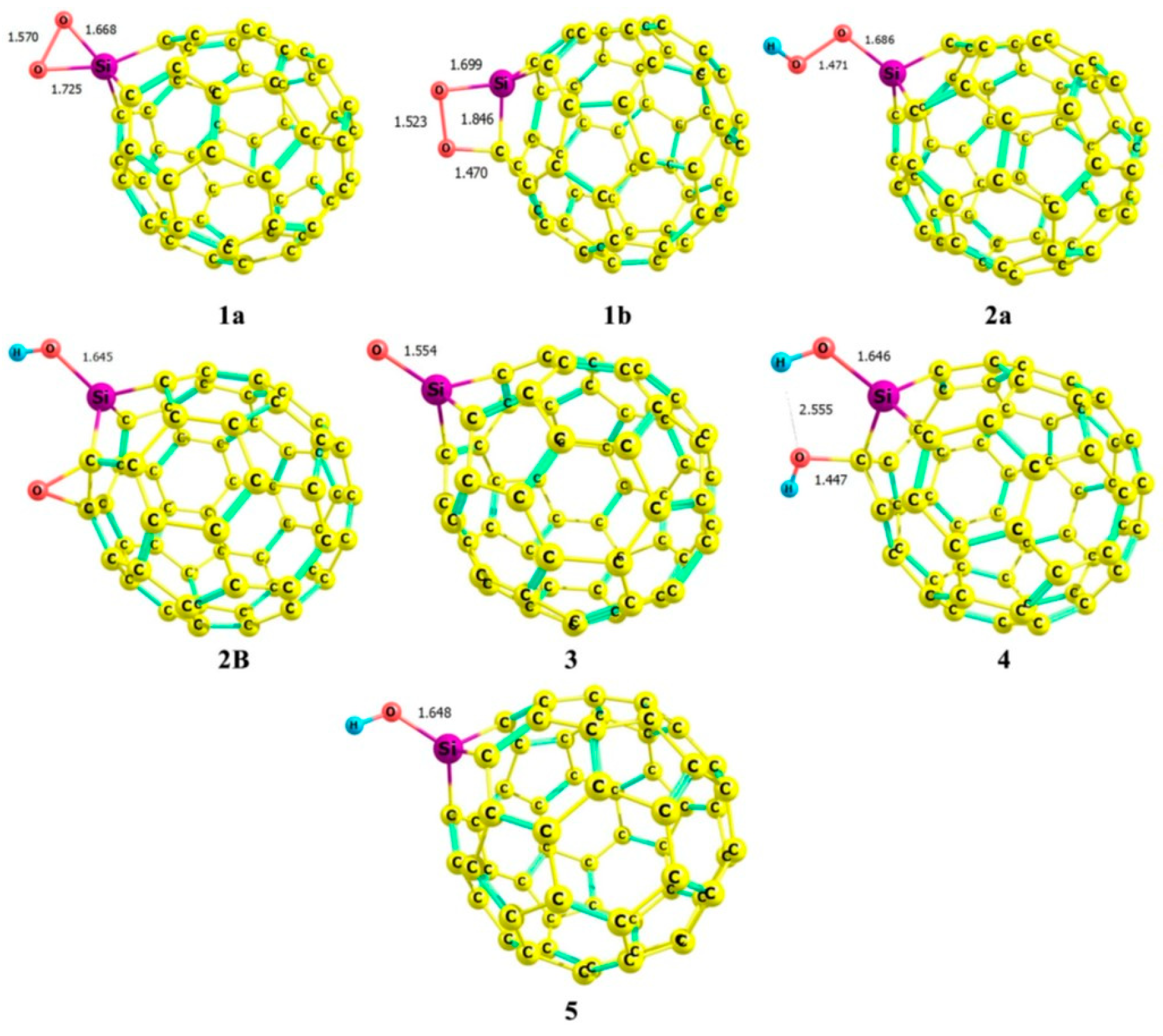
| Study | Synthesis Method | Analytical Techniques | Proposed Products/Findings | Limitations |
|---|---|---|---|---|
| Kimura et al. (1996) [46] | Laser vaporization of Si-doped graphite | TOF-MS | SiCn (n ≈ 56–61) clusters in fullerene-size window; heterofullerene-like distributions | Cannot distinguish substitutional vs. exohedral MS fragmentation (C2 loss); complicates interpretation |
| Fye and Jarrold (1997) [48] | Laser vaporization; drift-tube ion mobility with MS | Ion mobility + MS, fragmentation, water-adduct reactivity | C2n−1Si+ consistent with substitutional doping; C2nSi+ consistent with exohedral Si | Gas-phase only; no bulk isolation |
| Cao et al. (1997) [47] | Arc evaporation of SiC-loaded electrodes; extraction in CS2 | MS, HPLC | Bulk Si-containing fullerene fractions obtained | No unambiguous assignment between substitutional and exohedral structures |
| Ray et al. (1998) [23] | Laser vaporization of Si:C mixed targets (carbon-rich; clusters formed as cations) | Reflectron TOF-MS (abundance patterns) and excimer-laser photofragmentation of size-selected clusters | C2n−qSiq (q = 1, 2) mirror pure fullerene stability pattern; first neutral losses are SiC and Si2 from Si-doped parents, then “fullerene-like” C2 losses; spectra support cagelike geometry with neighboring Si atoms | Gas-phase only; mass coincidences prevent unique assignment for >2 Si; no bulk isolation/crystallography. |
| Pellarin et al. (1999) [49] | Laser vaporization from SixC1−x mixed targets (x = 0–50%); both stoichiometric SiC and carbon-rich; photoionization/photofragmentation sequence | High-fluence photoionization MS of neutrals; photofragmentation MS of size-selected cations | Laser annealing yields stable Si-doped fullerenes; early losses of Si2C/Si3C; patterns indicate cagelike substitution with up to ~12 Si atoms tolerated | Inferences based on MS patterns; isomass overlaps complicate assignments at higher number of Si atoms; no structural (solid-state) confirmation. |
| Billas et al. (1999) [52] | Inert-gas condensation: mixing preformed C60 vapor with laser-vaporized Si in He; subsequent photoionization/photofragmentation | TOF-MS (photoionization), photofragmentation of C60Six parents; plus ab initio DFT support | Transformation from exohedral C60Six clusters to in-cage (substitutional) C59Si/C58Si2 upon photofragmentation; evidence for ≥3 Si substitution deduced from fragment patterns despite mass coincidence | Assignment beyond two Si limited by mass coincidence; conclusions rely on fragmentation diagnostics; no bulk isolation/crystal structures. |
| Ohara et al. (2002) [53] | Two-rod, two-laser vaporization (C60 and Si/Ge) in He carrier gas | TOF-MS of anions/cations; anion photoelectron spectroscopy (PES) with magnetic-bottle detector | Exohedral C60Sin/C60Gem formed; size distributions limited to n ≤ 4 (m ≤ 3); PES shows that added Si/Ge largely cluster on the surface; electronic signatures for n = 3–4 resemble C60− (charge remains on cage) | Gas-phase clusters only; no evidence for substitutional incorporation; hot-source conditions limit survival of larger n; no extractable bulk material. |
| Bulina et al. (2007) [54] | Atmospheric-pressure arc in carbon–He plasma with injected Si powder (AC 44 kHz); soot collected | Workup: benzene extraction (pure fullerenes), then pyridine extraction (derivatives); MS (laser desorption TOF); XRD; emission spectral analysis | MS of pyridine extract shows peaks assigned to C52Si8+ and C62Si8+ (content ≤ 1%); C52Si8/C62Si8 ≈ 7/3 (analogous to C60/C70 ratio); discussion consistent with closed cages and adjacent Si atoms | Very low abundance; assignment based solely on MS in extract; no high-resolution structural proof or IMS; alternative interpretations (fragments/adducts) cannot be fully excluded. |
| Species and Isomer | C–C (Å) | C–Si (Å) | Si–Si (Å) |
|---|---|---|---|
| C59Si | 1.40–1.43 hh; 1.46–1.51 hp | 1.848 hh; 1.900 hp | … |
| C58Si2, 5 | 1.40–1.45 hh; 1.47–1.50 hp | 1.862 hh; 1.88–1.92 hp | … |
| C58Si2, 2 | 1.40–1.44 hh; 1.46–1.50 hp | 1.847 hh; 1.916 hp | 2.303 hp |
| C58Si2, 9 | 1.40–1.43 hh; 1.46–1.51 hp | 1.848 hh; 1.900 hp | … |
| C54Si6, A | 1.40–1.43 hh; 1.45–1.50 hp | 1.872 hh; 1.928 hp | 2.661 hh; 2.411 hp |
| C54Si6, B | 1.41–1.42 hh; 1.46–1.53 hp | 1.90–1.98 hp | 2.360 hh; 2.372 hp |
| C48Si12 | 1.41–1.44 hh; 1.46–1.48 hp | 1.83–1.85 hh; 1.82–1.84 hp | 2.425 hh; 2.32–2.48 hp |
| Substitutional Si-Doped C60 Fullerenes | ||||||
|---|---|---|---|---|---|---|
| Si-Doped C60 Derivative | Type of Calculation | Calculation Method | Computational Software | Aim of Study | First Author/Publication Year DOI (Ref.) | |
| Semiempirical | DFT (Functional and Basis Set) | |||||
| C59Si | GO, ESPA, LR-TDDFT, QR-TDDFT, PCM | - | BLYP/3-21+G(d), B3LYP/3-21+G(d), B3LYP/6-31G(d), B3LYP/cc-pVDZ, B3LYP/aug-cc-pVDZ, CAMB3LYP/6-31+G(d), LDA/3-21+G(d), LDA/6-31+G(d), BP86/3-21+G(d), BP86/6-31+G(d), PBE/6-31+G(d) | Gaussian 03, DALTON2013 | Investigation of OPA/TPA spectra of Si-monosubstituted fullerenes (Cn−1Si for n = 20, 30, 40, 50, and 60) in gas/solution to assess substitution and solvent influences | [64] |
| C59Si | GO, ESPA | PM3 | - | Gaussian 98 | Investigation of structural deformation and electronic localization in Si-substituted C60 | [55] |
| C59Si | GO, ESPA, TDDFT, IR, NICS | - | B3LYP/6-31G(d), GIAO | Gaussian 09 | Investigation of structural, electronic, vibrational, dielectric and aromatic property changes in C59Si relative to pristine C60 and C60 with other dopants (B, N, Al, As, P, Ga, Ge) | [65] |
| C59Si | GO, ESPA IR, SCRF, | - | B3LYP/aug-cc-pVDZ | Gaussian 98 | Investigation of structural, energetic and vibrational differences in Si-substituted C59Si compared to C60 and C59N | [68] |
| C59Si, C59Si6− | GO, ESPA, NICS | - | B3LYP/6-31G(d), SCF/3-21G, GIAO | Gaussian 98 | Investigation of the geometry, electronic structure, and magnetic properties of Si-doped fullerene C60 and C606− as isoelectronic analogs of C60 and C606− | [72] |
| C60−nSin (n = 1, 2) | CPMD | - | BLYP | CPMD code | Investigation of structural deformation, charge localization and bonding patterns in C59Si and C58Si2 relative to C60 | [52] |
| C60−nSin (n = 1, 2, 3, 6, 12) | GO, ESPA | - | LDA(PZ)/TZP (TM) | SIESTA code | Investigation of stability, structural preference and thermal fragmentation behavior of Si-substituted C60 isomers | [69] |
| C60−nSin (n = 1, 2, 6, 12) | GO, ESPA, TDDFT, CPMD | - | BLYP, PBE, LDA | SIESTA code, OCTOPUS 2.0.1., CPMD code | Investigation of photoabsorption spectra of Si-substituted C60 (up to C48Si12) to assess spectral changes upon increasing silicon content | [66] |
| C60−nSin (n = 1, 2, 6, 12, 20, 24, 30) | GO, NICS, NMR | - | B3LYP/6-311G(d), GIAO | Gaussian 98 | Investigation of electronic structure and 13C NMR shielding parameters (NICS) in Si-substituted C60 to assess the impact of silicon doping on aromaticity | [67] |
| C60−nSin (n = 5, 6, 10, 12, 14, 18, 22, 24, 26, 30) | GO, ESPA, CMCS, GA (CBG model) | MNDO | B3LYP/3-21G, B3LYP/6-31G(d), PBE/DNP | Gaussian 03, DMol3 | Investigation of structural stability and Si-substitution patterns in C60 | [56] |
| C60−nSin (n = 20, 24, 30) | GO, ESPA, CPMD | - | BLYP, TM-NCPP | CPMD code | Investigation of structural stability and charge transfer in highly Si-substituted C60 | [77] |
| C60−nSin (n = 20, 24, 30) | GO, ESPA, FPMD | - | BLYP, TM-NCPP | CPMD code | Investigation of thermal instability threshold and fragmentation mechanisms in highly Si-doped C60 | [76] |
| C30Si30 | GO, ESPA, CPMD | - | BLYP | CPMD code | Investigation of effects of ±1e charge on structure and electronic properties of Si-substituted C60 | [78] |
| C30Si30 | GO, ESPA, FPMD | - | PBE-PP, BLYP-PP, BLYP/6-311G, BLYP/6-311G(d), B3LYP/6-311G, B3LYP/6-311G(d), BLYP/TZV, BLYP/TZVP | CPMD code, Gaussian 09 | Investigation of topological conditions underlying the thermal stability of C30Si30 | [70] |
| C60−nSin (n = 1–4) | GO, ESPA | MNDO | BLYP/STO-3G, BLYP/6-311G | Gaussian 98 | Assessment of structural stability and electronic properties of substitutional Si-doped C60 | [42] |
| C59Si | GO, ESPA, IR | PM3 | - | MOPAC 2002 via CAChe 1.33 | Evaluation of structural, electronic and vibrational properties of Si-doped C60 in comparison to pristine C60 C59Al and C59Ge using semiempirical method | [57] |
| C48Si12 | GO, ESPA, CPMD | - | BLYP | CPMD code | Investigation of structural stability and electronic properties of C48Si12 heterofullerene isomers with compact Si patterns | [87] |
| C30Si30 | GO, ESPA, CPMD | - | BLYP | CPMD code | Investigation of structural and electronic effects of positive and negative charging on highly Si-doped fullerene | [79] |
| C60−nSin (n = 1–12) | MCS, GO, ESPA | - | PW91 | VASP code, DFTB+ code | Investigation of ground-state structures and stability of silicon-multisubstituted C60 fullerenes | [73] |
| C48Si12, C36Si24 | GO, ESPA | - | PBE/DZP (TM) | SIESTA code | Investigation of energetic, geometrical, and electronic properties of silicon–carbon fullerene derivatives C48Si12 and C36Si24 | [71] |
| Endohedral Complexes of Substitutional Si-Doped C60 Fullerenes | |||||||
|---|---|---|---|---|---|---|---|
| Si-Doped Fullerene Derivative (Host) | Guest | Type of Calculation | Calculation Method | Computational Software | Aim of Study | (Ref) | |
| Semiempirical | DFT (Functional and Basis Set) | ||||||
| C59Si | mH (m = 1) | GO, ESPA, Eads (with BSSE) | - | PBE/LCPAO (TM) | OpenMX code | Investigation of the structural and electronic effects of endohedral atomic H encapsulation in Si-substituted C60 (in comparison to C59P, C59B, C59S, C59O and pristine C60) | [122] |
| C59Si | mH2 (m = 1, 2, 3, 4) | GO, ESPA, Eads | - | PBE/DZP (TM-NCPP) | SIESTA code | Estimation of the stability and storage capacity of molecular H2 in Si-substituted C60 via encapsulation of 1–4 H2 molecules (in comparison to C59N, C59B and pristine C60) | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska-Pisklak, M.; Siekacz, P.; Stokłosa, Z.; Szeleszczuk, Ł. A Comprehensive Review of Substitutional Silicon-Doped C60 Fullerenes and Their Endohedral/Exohedral Complexes: Synthetic Strategies and Molecular Modeling Approaches. Molecules 2025, 30, 3912. https://doi.org/10.3390/molecules30193912
Zielińska-Pisklak M, Siekacz P, Stokłosa Z, Szeleszczuk Ł. A Comprehensive Review of Substitutional Silicon-Doped C60 Fullerenes and Their Endohedral/Exohedral Complexes: Synthetic Strategies and Molecular Modeling Approaches. Molecules. 2025; 30(19):3912. https://doi.org/10.3390/molecules30193912
Chicago/Turabian StyleZielińska-Pisklak, Monika, Patrycja Siekacz, Zuzanna Stokłosa, and Łukasz Szeleszczuk. 2025. "A Comprehensive Review of Substitutional Silicon-Doped C60 Fullerenes and Their Endohedral/Exohedral Complexes: Synthetic Strategies and Molecular Modeling Approaches" Molecules 30, no. 19: 3912. https://doi.org/10.3390/molecules30193912
APA StyleZielińska-Pisklak, M., Siekacz, P., Stokłosa, Z., & Szeleszczuk, Ł. (2025). A Comprehensive Review of Substitutional Silicon-Doped C60 Fullerenes and Their Endohedral/Exohedral Complexes: Synthetic Strategies and Molecular Modeling Approaches. Molecules, 30(19), 3912. https://doi.org/10.3390/molecules30193912








