Nitazenes: The Emergence of a Potent Synthetic Opioid Threat
Abstract
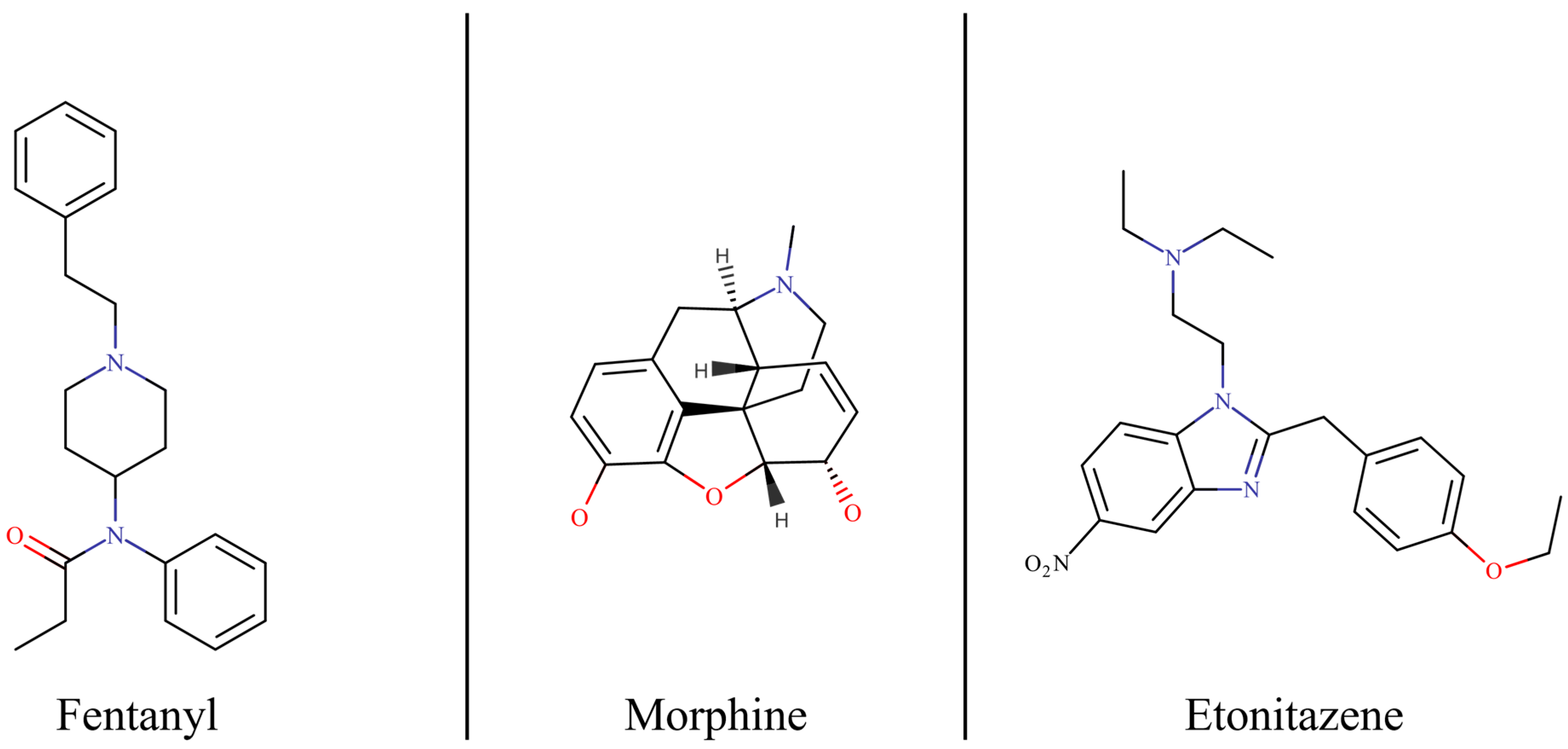
1. Introduction
- Ease of synthesis: Nitazene analogues are relatively straightforward to synthesise, do not require controlled precursors, and can be produced in small-scale clandestine laboratories worldwide.
- Economic appeal: Their high potency means a few grams can yield thousands of doses, making them easier to conceal and transport, which is economically advantageous for traffickers.
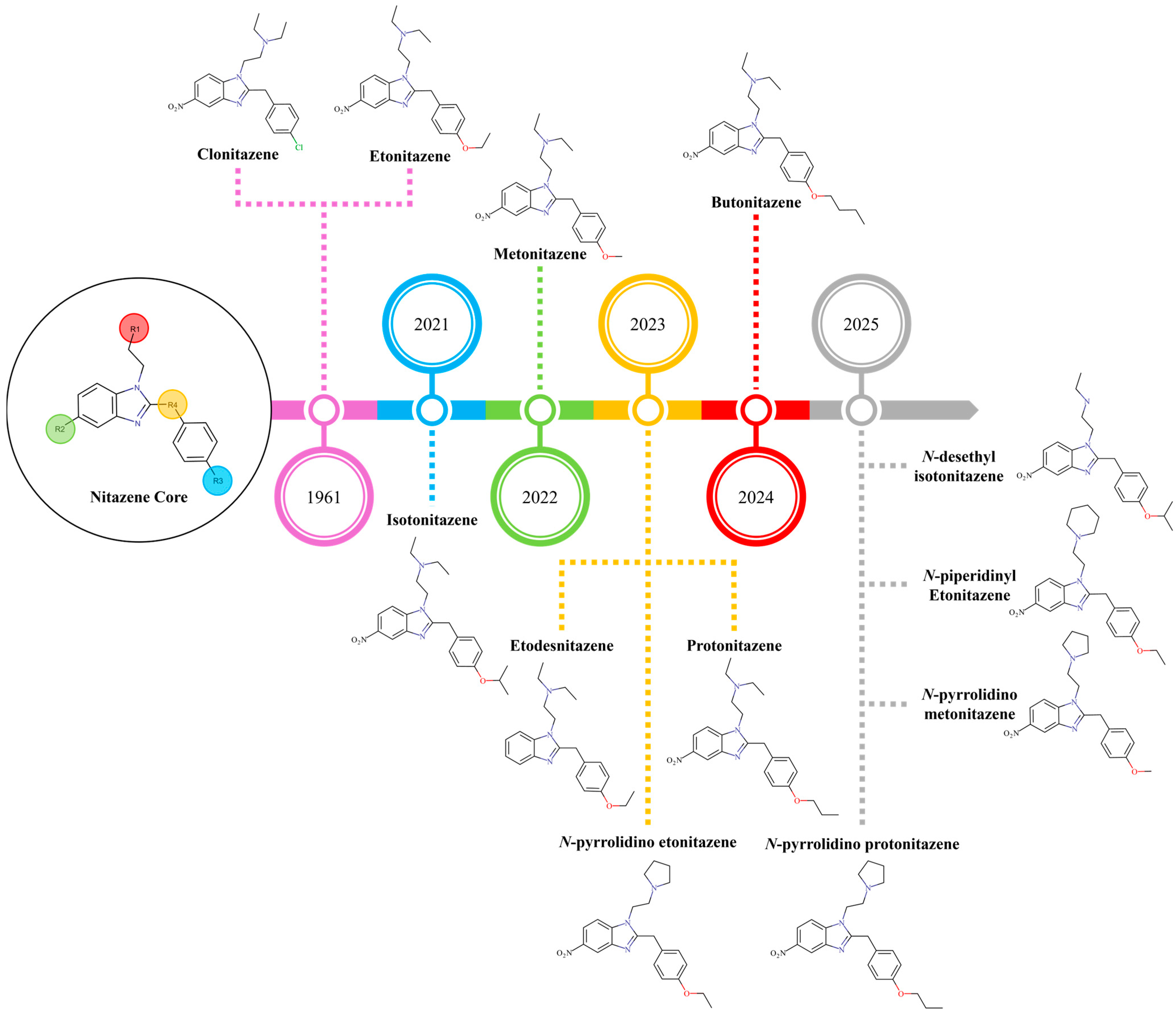
- Regulatory evasion: The rapid and consistent emergence of novel nitazene analogues with slight chemical modifications enables them to circumvent existing drug laws, which are often based on specific substances, creating a regulatory lag. This trend intensified after class-wide bans on fentanyl and its analogues in the late 2010s, prompting illicit chemists to seek alternative NSOs from the historical pharmacological literature.
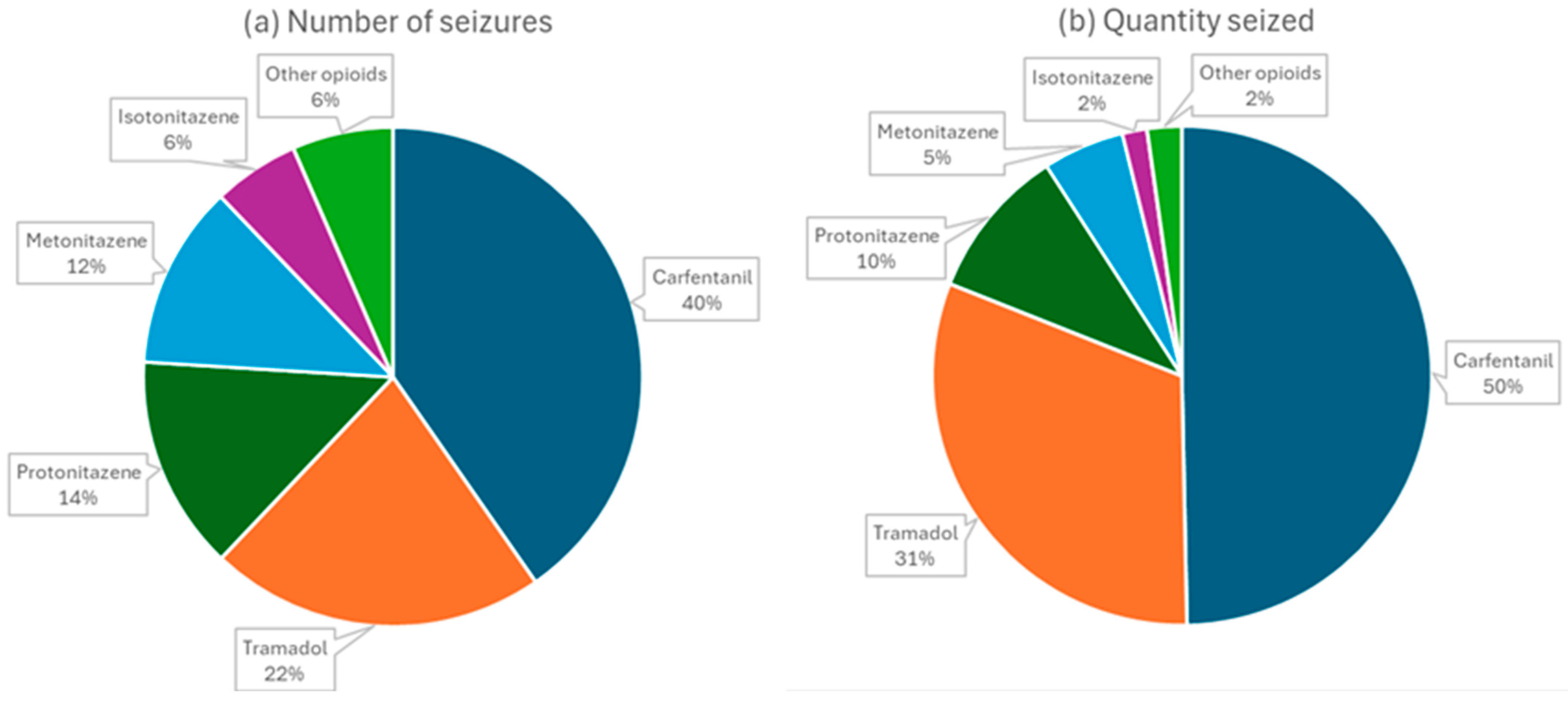
2. The Situation in the World: A Growing Concern
3. Pharmacological Profile and Toxicity of Nitazenes
4. Analytical Challenges in Forensic and Toxicological Investigations
5. Future Directions and Strategic Responses
- Enhanced detection and surveillance:
- Optimised clinical management:
- Proactive public health and harm reduction:
- Focused research and policy development:
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime. World Drug Report 2025. 2025. Available online: https://www.unodc.org/unodc/data-and-analysis/world-drug-report-2025.html (accessed on 22 August 2025).
- European Union Drugs Agency. European Drug Report 2025 Trends and Developments. 2025. Available online: https://www.euda.europa.eu/publications/european-drug-report/2025_en (accessed on 22 August 2025).
- Lassi, N.; Jiang, S. The Future of Deadly Synthetic Opioids: Nitazenes and Their International Control. Glob. Policy 2025. [Google Scholar] [CrossRef]
- Pergolizzi, J., Jr.; Raffa, R.; LeQuang, J.A.K.; Breve, F.; Varrassi, G. Old Drugs and New Challenges: A Narrative Review of Nitazenes. Cureus 2023, 15, e40736. [Google Scholar] [CrossRef]
- Advisory Council on the Misuse of Drugs. ACMD Advice on 2-Benzyl Benzimidazole and Piperidine Benzimidazolone Opioids (Accessible Version). 2025. Available online: https://www.gov.uk/government/publications/acmd-advice-on-2-benzyl-benzimidazole-and-piperidine-benzimidazolone-opioids/acmd-advice-on-2-benzyl-benzimidazole-and-piperidine-benzimidazolone-opioids-accessible-version (accessed on 22 August 2025).
- Isoardi, K.Z.; Alfred, S.; Weber, C.; Harris, K.; Soderstrom, J.; Syrjanen, R.; Thompson, A.; Schumann, J.; Stockham, P.; Sakrajda, P.; et al. Clinical Toxicity of Nitazene Detections in Two Australian Emergency Department Toxicosurveillance Systems. Drug Alcohol. Rev. 2025. [Google Scholar] [CrossRef]
- Dugues, P.; Rabai, A.; Chenorhokian, S.; Pfau, G.; Cherki, S.; Bellouard, M.; Alvarez, J.C.; Larabi, I.A. Emergence of Counterfeit Oxycodone Tablets Containing Nitazenes in France: First National Alert and Analytical Characterization. Toxicol. Anal. Clin. 2025. [Google Scholar] [CrossRef]
- Gonçalves de Araújo, K.R.; Fabris, A.L.; Junior, L.F.N.; Soares, A.L.; Costa, J.L.; Yonamine, M. Synthetic Illicit Opioids in Brazil: Nitazenes Arrival. Forensic Sci. Int. Rep. 2024, 10, 100375. [Google Scholar] [CrossRef]
- Meyer, M.; Westenberg, J.N.; Jang, K.L.; Choi, F.; Schreiter, S.; Mathew, N.; King, C.; Lang, U.E.; Vogel, M.; Krausz, R.M. Shifting Drug Markets in North America—A Global Crisis in the Making? Int. J. Ment. Health Syst. 2023, 17, 36. [Google Scholar] [CrossRef]
- Glatfelter, G.C.; Vandeputte, M.M.; Chen, L.; Walther, D.; Tsai, M.H.M.; Shi, L.; Stove, C.P.; Baumann, M.H. Alkoxy Chain Length Governs the Potency of 2-Benzylbenzimidazole ‘Nitazene’ Opioids Associated with Human Overdose. Psychopharmacology 2023, 240, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.C.; Severe, A.D.; Jalloh, M.S.; Manini, A.F. Naloxone Dosing and Hospitalization for Nitazene Overdose: A Scoping Review. J. Med. Toxicol. 2025, 21, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Stangeland, M.; Dale, O.; Skulberg, A.K. Nitazenes: Review of Comparative Pharmacology and Antagonist Action. Clin. Toxicol. 2025, 63, 393–406. [Google Scholar] [CrossRef]
- Vandeputte, M.M.; Stove, C.P. Navigating Nitazenes: A Pharmacological and Toxicological Overview of New Synthetic Opioids with a 2-Benzylbenzimidazole Core. Neuropharmacology 2025, 275, 110470. [Google Scholar] [CrossRef]
- Monti, M.C.; De Vrieze, L.M.; Vandeputte, M.M.; Persson, M.; Gréen, H.; Stove, C.P.; Schlotterbeck, G. Detection of N-Desethyl Etonitazene in a Drug Checking Sample: Chemical Analysis and Pharmacological Characterization of a Recent Member of the 2-Benzylbenzimidazole “Nitazene” Class. J. Pharm. Biomed. Anal. 2024, 251, 116453. [Google Scholar] [CrossRef]
- Keller, E.L.; Peake, B.; Simpson, B.S.; Longo, M.; Trobbiani, S.; White, J.M.; Gerber, C. Searching for a Needle in a Haystack: Chemical Analysis Reveals Nitazenes Found in Drug Paraphernalia Residues. Drug Alcohol. Rev. 2025. [Google Scholar] [CrossRef]
- Mammoliti, E.; Nielsen, S.; Roxburgh, A. A Scoping Review of the Emergence of Novel Synthetic Opioids in Australian Drug Markets: What Does This Mean for Harm Reduction Responses? Drug Alcohol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime. February 2025-UNODC EWA: Increasing Availability of Nitazenes Calls for Global Response. 2025. Available online: https://www.unodc.org/LSS/Announcement/Details/b47cf39e-f557-4001-98a8-536af5673e9e (accessed on 22 August 2025).
- Schumann, J.L.; Dwyer, J.; Brown, J.A.; Jauncey, M.; Roxburgh, A. Identification of Nitazene-Related Deaths in Australia: How Do We Make It Accurate and Timely? Drug Alcohol. Rev. 2025. [Google Scholar] [CrossRef]
- Holland, A.; Copeland, C.S.; Shorter, G.W.; Connolly, D.J.; Wiseman, A.; Mooney, J.; Fenton, K.; Harris, M. Nitazenes—Heralding a Second Wave for the UK Drug-Related Death Crisis? Lancet Public Health 2024, 9, e71–e72. [Google Scholar] [CrossRef] [PubMed]
- Lo Faro, A.F.; Berardinelli, D.; Cassano, T.; Dendramis, G.; Montanari, E.; Montana, A.; Berretta, P.; Zaami, S.; Busardò, F.P.; Huestis, M.A. New Psychoactive Substances Intoxications and Fatalities during the COVID-19 Epidemic. Biology 2023, 12, 273. [Google Scholar] [CrossRef]
- Verbeek, J.; Brinkman, D.J. A Comprehensive Narrative Review of Protonitazene: Pharmacological Characteristics, Detection Techniques, and Toxicology. Basic. Clin. Pharmacol. Toxicol. 2025, 137, e70078. [Google Scholar] [CrossRef]
- Roberts, A.; Korona-Bailey, J.; Mukhopadhyay, S. Nitazene-Related Deaths—Tennessee, 2019–2021. Morb. Mortal. Wkly. Rep. 2022, 71, 1196–1197. [Google Scholar] [CrossRef]
- Griffiths, P.N.; Seyler, T.; De Morais, J.M.; Mounteney, J.E.; Sedefov, R.S. Opioid Problems Are Changing in Europe with Worrying Signals That Synthetic Opioids May Play a More Significant Role in the Future. Addiction 2024, 119, 1334–1336. [Google Scholar] [CrossRef]
- Darke, S.; Duflou, J.; Farrell, M.; Lappin, J.; Peacock, A. Emergence of Deaths Due to Nitazene Toxicity in Australia. Drug Alcohol Rev. 2024, 43, 2093–2094. [Google Scholar] [CrossRef] [PubMed]
- Oelrichs, R. A National Perspective. Med. J. Aust. 2025, 222, 215. [Google Scholar] [CrossRef]
- European Union Drugs Agency. EU Drug Market: New Psychoactive Substances-Distribution and Supply in Europe: New Opioids. 2025. Available online: https://www.euda.europa.eu/publications/eu-drug-markets/new-psychoactive-substances/distribution-and-supply/new-opioids_en (accessed on 22 August 2025).
- Zawilska, J.B.; Adamowicz, P.; Kurpeta, M.; Wojcieszak, J. Non-Fentanyl New Synthetic Opioids—An Update. Forensic Sci. Int. 2023, 349, 111775. [Google Scholar] [CrossRef]
- Giommoni, L. How to Improve the Surveillance of the Taliban Ban’s Impact on European Drug Markets. Int. J. Drug Policy 2024, 124, 104320. [Google Scholar] [CrossRef]
- Malcolm, N.J.; Palkovic, B.; Sprague, D.J.; Calkins, M.M.; Lanham, J.K.; Halberstadt, A.L.; Stucke, A.G.; McCorvy, J.D. Mu-Opioid Receptor Selective Superagonists Produce Prolonged Respiratory Depression. iScience 2023, 26, 107121. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.M.; Glatfelter, G.C.; Walther, D.; Layle, N.K.; St. Germaine, D.M.; Ujváry, I.; Iula, D.M.; Baumann, M.H.; Stove, C.P. Characterization of Novel Nitazene Recreational Drugs: Insights into Their Risk Potential from in Vitro µ-Opioid Receptor Assays and in Vivo Behavioral Studies in Mice. Pharmacol. Res. 2024, 210, 107503. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.M.; Chen, L.; Baumann, M.H.; Canals, M.; Javitch, J.A.; Lane, J.R.; Shi, L. In Vitro Functional Profiling of Fentanyl and Nitazene Analogs at the μ-Opioid Receptor Reveals High Efficacy for Gi Protein Signaling. ACS Chem. Neurosci. 2024, 15, 854–867. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, L.M.; Walton, S.E.; Pottie, E.; Papsun, D.; Logan, B.K.; Krotulski, A.J.; Stove, C.P.; Vandeputte, M.M. In Vitro Structure–Activity Relationships and Forensic Case Series of Emerging 2-Benzylbenzimidazole ‘Nitazene’ Opioids. Arch. Toxicol. 2024, 98, 2999–3018. [Google Scholar] [CrossRef] [PubMed]
- Magny, R.; Schiestel, T.; M’Rad, A.; Lefrère, B.; Raphalen, J.H.; Ledochowski, S.; Labat, L.; Mégarbane, B.; Houzé, P. Comparison of the Metabolic Profiles Associated with Protonitazene and Protonitazepyne in Two Severe Poisonings. Metabolites 2025, 15, 371. [Google Scholar] [CrossRef]
- Tomiyama, K.I.; Funada, M. The Synthetic Opioid Isotonitazene Induces Locomotor Activity and Reward Effects through Modulation of the Central Dopaminergic System in Mice. Toxicol. Appl. Pharmacol. 2025, 500, 117361. [Google Scholar] [CrossRef]
- Baldo, B.A. Opioid-Induced Respiratory Depression: Clinical Aspects and Pathophysiology of the Respiratory Network Effects. Am. J. Physiol. Lung Cell Mol. Physiol. 2025, 328, L267–L289. [Google Scholar] [CrossRef]
- Jadhav, G.R.; Fasinu, P.S. Metabolic Characterization of the New Benzimidazole Synthetic Opioids—Nitazenes. Front. Pharmacol. 2024, 15, 1434573. [Google Scholar] [CrossRef]
- Ameline, A.; Gheddar, L.; Pichini, S.; Stove, C.; Aknouche, F.; Maruejouls, C.; Raul, J.S.; Kintz, P. In Vitro Characterization of Protonitazene Metabolites, Using Human Liver Microsomes, and First Application to Two Urines Collected from Death Cases. Clin. Chim. Acta 2024, 561, 119764. [Google Scholar] [CrossRef]
- Hancox, J.C.; Wang, Y.; Copeland, C.S.; Zhang, H.; Harmer, S.C.; Henderson, G. Nitazene Opioids and the Heart: Identification of a Cardiac Ion Channel Target for Illicit Nitazene Opioids. J. Mol. Cell. Cardiol. Plus 2024, 10, 100118. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.; Shi, L.; Robertson, M.J.; Skiniotis, G.; Michaelides, M.; Stavitskaya, L.; Shen, J. A Putative Binding Model of Nitazene Derivatives at the μ-Opioid Receptor. Neuropharmacology 2025, 273, 110437. [Google Scholar] [CrossRef] [PubMed]
- Hataoka, K.; Hojo, M.; Nomura, S.; Nakagawa, Y.; Kawai, A.; Nakamura, M.; Ikushima, K.; Alexander, D.B.; Suzuki, J.; Suzuki, T.; et al. Evaluation of Rewarding Effects of Nitazene Analogs: Results from Conditioned Place Preference Tests and in Vivo Microdialysis Experiments in Mice. J. Toxicol. Sci. 2025, 50, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Singh Jutley, G.; Looms, J.; Ford, L. N-Desethyl Isotonitazene Detected in Polydrug Users Admitted to Hospital in Birmingham, United Kingdom. Clin. Toxicol. 2024, 62, 19–25. [Google Scholar] [CrossRef]
- Amaducci, A.; Aldy, K.; Campleman, S.L.; Li, S.; Meyn, A.; Abston, S.; Culbreth, R.E.; Krotulski, A.; Logan, B.; Wax, P.; et al. Naloxone Use in Novel Potent Opioid and Fentanyl Overdoses in Emergency Department Patients. JAMA Netw. Open 2023, 6, e2331264. [Google Scholar] [CrossRef]
- Alhosan, N.; Cavallo, D.; Santiago, M.; Kelly, E.; Henderson, G. Slow Dissociation Kinetics of Fentanyls and Nitazenes Correlates with Reduced Sensitivity to Naloxone Reversal at the μ-Opioid Receptor. Br. J. Pharmacol. 2025, 182, 969–987. [Google Scholar] [CrossRef]
- Bade, R.; Nadarajan, D.; Driver, E.M.; Halden, R.U.; Gerber, C.; Krotulski, A.; Hall, W.; Mueller, J.F. Wastewater-Based Monitoring of the Nitazene Analogues: First Detection of Protonitazene in Wastewater. Sci. Total Environ. 2024, 920, 170781. [Google Scholar] [CrossRef]
- Curtis, B.; Lawes, D.J.; Caldicott, D.; McLeod, M.D. Identification of the Novel Synthetic Opioid N-Pyrrolidino Isotonitazene at an Australian Drug Checking Service. Drug Test. Anal. 2025. [Google Scholar] [CrossRef]
- Palmquist, K.B.; Truver, M.T.; Shoff, E.N.; Krotulski, A.J.; Swortwood, M.J. Review of Analytical Methods for Screening and Quantification of Fentanyl Analogs and Novel Synthetic Opioids in Biological Specimens. J. Forensic Sci. 2023, 68, 1643–1661. [Google Scholar] [CrossRef]
- Sisco, E.; Appley, M.G.; Pyfrom, E.M.; Banta-Green, C.J.; Shover, C.L.; Molina, C.A.; Biamont, B.; Robinson, E.L. Beyond Fentanyl Test Strips: Investigating Other Urine Drug Test Strips for Drug Checking Applications. Forensic Chem. 2024, 40, 100594. [Google Scholar] [CrossRef]
- Pacana, A.L.; Skillman, B.N. Evaluation of Enzyme-Linked Immunosorbent Assay Screening Kits for the Detection of Nitazene Analogs. J. Forensic Sci. 2025, 70, 1609–1614. [Google Scholar] [CrossRef]
- De Vrieze, L.M.; Stove, C.P.; Vandeputte, M.M. Nitazene Test Strips: A Laboratory Evaluation. Harm Reduct. J. 2024, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Keller, E.L.; Peake, B.; Simpson, B.S.; White, J.M.; Gerber, C. Comprehensive Method to Detect Nitazene Analogues and Xylazine in Wastewater. Environ. Sci. Pollut. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.; Hardwick, E.K.; Couch, A.N.; Davidson, J.T. Development and Validation of a Combined Selected Ion Monitoring-Scan GC-EI-MS Method for Nitazene Analogs. J. Forensic Sci. 2025, 70, 1949. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, E.K.; Tyler Davidson, J. Structural Characterization of Nitazene Analogs Using Electron Ionization-Mass Spectrometry (EI-MS). Forensic Chem. 2024, 40, 100605. [Google Scholar] [CrossRef]
- Hardwick, E.K.; Davidson, J.T. Structural Characterization of Nitazene Analogs Using Electrospray Ionization–Tandem Mass Spectrometry (ESI–MS/MS). Drug Test. Anal. 2025. [Google Scholar] [CrossRef]
- Ververi, C.; Galletto, M.; Massano, M.; Alladio, E.; Vincenti, M.; Salomone, A. Method Development for the Quantification of Nine Nitazene Analogs and Brorphine in Dried Blood Spots Utilizing Liquid Chromatography—Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2024, 241, 115975. [Google Scholar] [CrossRef]
- Wachełko, O.; Tusiewicz, K.; Szpot, P.; Zawadzki, M. The UHPLC-MS/MS Method for the Determination of 26 Synthetic Benzimidazole Opioids (Nitazene Analogs) with Isomers Separation. J. Pharm. Biomed. Anal. 2025, 260, 116796. [Google Scholar] [CrossRef]
- Schüller, M.; Lucic, I.; Øiestad, Å.M.L.; Pedersen-Bjergaard, S.; Øiestad, E.L. High-Throughput Quantification of Emerging “Nitazene” Benzimidazole Opioid Analogs by Microextraction and UHPLC-MS-MS. J. Anal. Toxicol. 2023, 47, 787–796. [Google Scholar] [CrossRef]
- Gao, G.; Yang, S.; Wang, X.; Xiang, P.; Ma, L.; Yan, F.; Shi, Y. UHPLC-MS/MS-Based Analysis of 17 Nitazenes in Human Hair for Practical Forensic Casework with Simultaneous Separation of 6 Groups of Isomers. J. Pharm. Biomed. Anal. 2025, 257, 116707. [Google Scholar] [CrossRef]
- Liu, C.M.; Huang, B.Y.; Hua, Z.D.; Jia, W.; Li, Z.-Y. Characterization of Mass Spectrometry Fragmentation Patterns Under Electron-Activated Dissociation (EAD) for Rapid Structure Identification of Nitazene Analogs. Rapid Commun. Mass. Spectrom. 2025, 39, e10030. [Google Scholar] [CrossRef]
- Hollerbach, A.L.; Lin, V.S.; Ibrahim, Y.M.; Ewing, R.G.; Metz, T.O.; Rodda, K.E. Elucidating the Gas-Phase Behavior of Nitazene Analog Protomers Using Structures for Lossless Ion Manipulations Ion Mobility-Orbitrap Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2024, 35, 1609–1621. [Google Scholar] [CrossRef]
- Killoran, S.; McNamara, S.; Kavanagh, P.; O’Brien, J.; Lakes, R. Identification of N-Pyrrolidino Protonitazene in Powders Sold as Heroin and Associated with Overdose Clusters in Dublin and Cork, Ireland. Drug Test. Anal. 2025, 17, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Kriikku, P.; Pelander, A.; Jylhä, A.; Ojanperä, I. Post-Mortem Identification and Toxicological Findings of Fluetonitazepyne and Isotonitazepyne. Drug Test. Anal. 2025. [Google Scholar] [CrossRef]
- Kintz, P.; Ameline, A.; Gheddar, L.; Pichini, S.; Mazoyer, C.; Teston, K.; Aknouche, F.; Maruejouls, C. Testing for Protonitazene in Human Hair Using LC–MS-MS. J. Anal. Toxicol. 2024, 48, 630–635. [Google Scholar] [CrossRef]
- Pardi, J.; Ford, S.; Cooper, G. Validation of an Analytical Method for Quantitation of Metonitazene and Isotonitazene in Plasma, Blood, Urine, Liver and Brain and Application to Authentic Postmortem Casework in New York City. J. Anal. Toxicol. 2023, 47, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Bade, R.; Nadarajan, D.; Hall, W.; Brown, J.A.; Schumann, J. Early Identification of the Use of Potent Benzylbenzimidazoles (Nitazenes) through Wastewater Analysis: Two Years of Data from 22 Countries. Addiction 2025, 120, 1739. [Google Scholar] [CrossRef]
- Nalakath, J.; Thacholil, R.P.; Kadry, A.P.S.; Praseen, O.K. LCMS Detection and Characterization of In Vitro Metabolites of Isotonitazene, a Targeted Strategy for Novel Psychoactive Substance Control in Camel Racing. Rapid Commun. Mass. Spectrom. 2025, 39, e9982. [Google Scholar] [CrossRef] [PubMed]
- Nalakath, J.; Palathinkal, A.B.; Naduvilakkandy, R.; Vazhat, R.A.; Komathu, P.O. In Vitro Metabolism of Metonitazene in Camels: High-Resolution Mass Spectrometric Characterization for Doping Control. Rapid Commun. Mass. Spectrom. 2025, 39, e10111. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime. The Challenge of New Psychoactive Substances—A Technical Update. 2024. Available online: https://www.unodc.org/unodc/en/scientists/the-challenge-of-new-psychoactive-substances.html (accessed on 22 August 2025).
- Bhuiyan, I.; Tobias, S.; Ti, L. Responding to Changes in the Unregulated Drug Supply: The Need for a Dynamic Approach to Drug Checking Technologies. Am. J. Drug Alcohol. Abus. 2023, 49, 685. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Park-Lee, E.; Birdsey, J.; West, A.; Cornelius, M.; Cooper, M.R.; Cowan, H.; Wang, J.; Sawdey, M.D.; Cullen, K.A.; et al. Morbidity and Mortality Weekly Report Tobacco Product Use Among Middle and High School Students-National Youth Tobacco Survey, United States, 2024. Weekly 2024, 73, 917. [Google Scholar]
- Kozell, L.B.; Eshleman, A.J.; Wolfrum, K.M.; Swanson, T.L.; Schutzer, K.A.; Schutzer, W.E.; Abbas, A.I. Pharmacology of Newly Identified Nitazene Variants Reveals Structural Determinants of Affinity, Potency, Selectivity for Mu Opioid Receptors. Neuropharmacology 2025, 276, 110512. [Google Scholar] [CrossRef]
- Gray, A.; Douglas, S.; Tiller, M.; Bleakley, M. Using Forensic Intelligence as a Model for Determining Future Toxicology Methods: TBI Forensic Toxicology and Forensic Drug Chemistry Nitazene Identification. J. Anal. Toxicol. 2024, 48, 463–467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.R.P.; Quintas, A.; Neng, N.R. Nitazenes: The Emergence of a Potent Synthetic Opioid Threat. Molecules 2025, 30, 3890. https://doi.org/10.3390/molecules30193890
Pereira JRP, Quintas A, Neng NR. Nitazenes: The Emergence of a Potent Synthetic Opioid Threat. Molecules. 2025; 30(19):3890. https://doi.org/10.3390/molecules30193890
Chicago/Turabian StylePereira, Joana R. P., Alexandre Quintas, and Nuno R. Neng. 2025. "Nitazenes: The Emergence of a Potent Synthetic Opioid Threat" Molecules 30, no. 19: 3890. https://doi.org/10.3390/molecules30193890
APA StylePereira, J. R. P., Quintas, A., & Neng, N. R. (2025). Nitazenes: The Emergence of a Potent Synthetic Opioid Threat. Molecules, 30(19), 3890. https://doi.org/10.3390/molecules30193890






