A Novel Polyacrylamide Film-Forming Agent for Maintaining Wellbore Stability
Abstract
1. Introduction
2. Results and Discussion
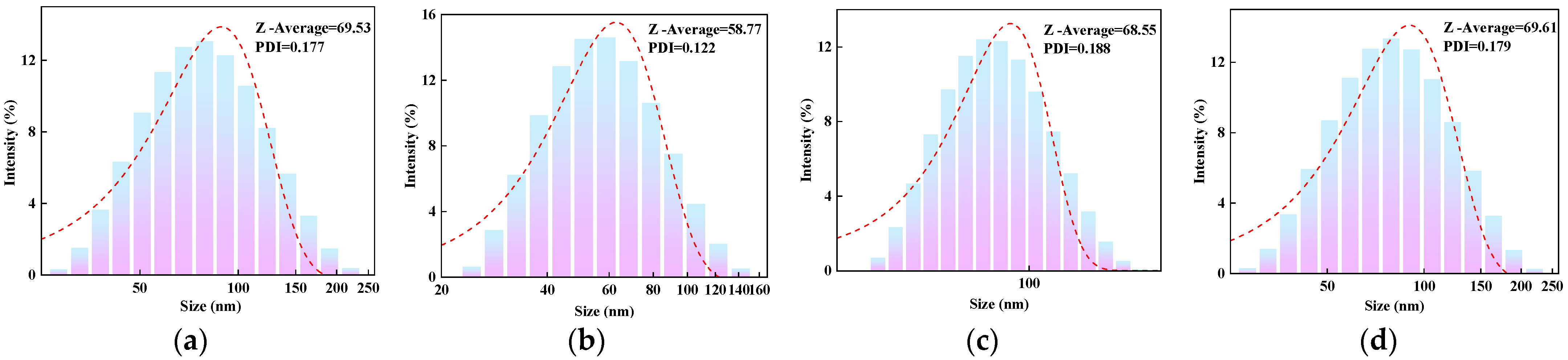
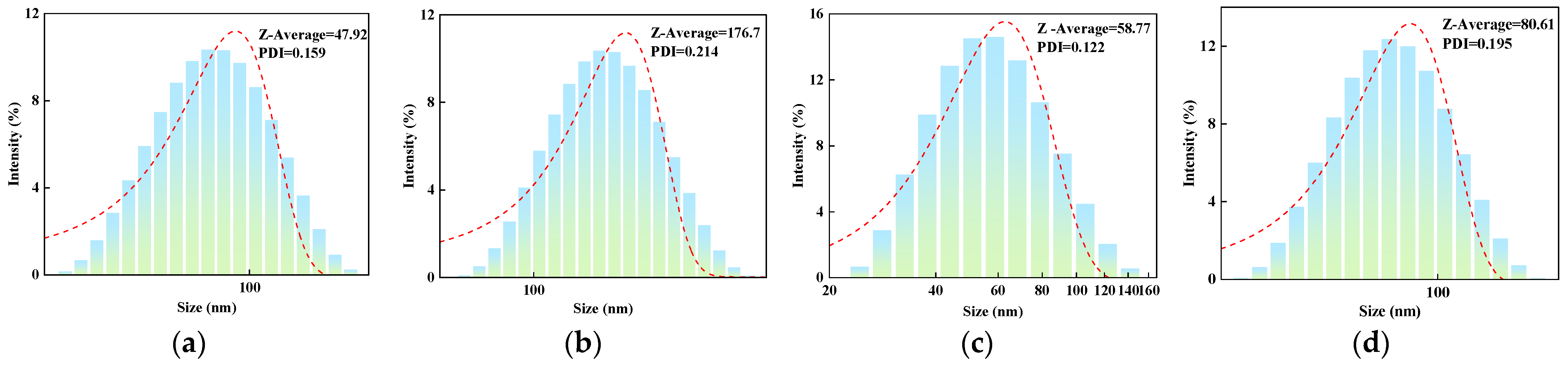
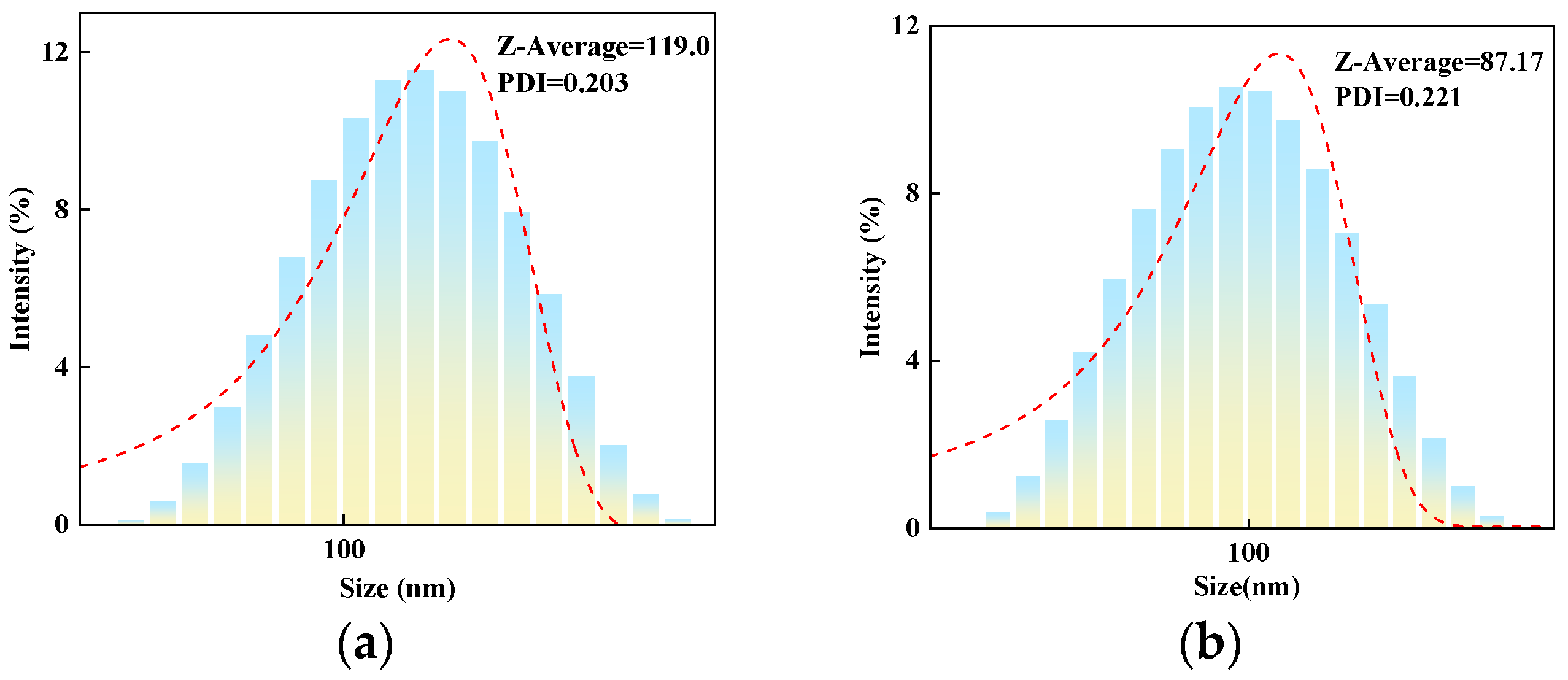
2.1. Particle Size Analysis
2.1.1. Effect of AM Content on the Particle Size of AMVA
2.1.2. Effect of Molar Ratio of VAc to AM on the Particle Size of AMVA
2.1.3. Effect of Cellulose Content on the Particle Size of AMVA
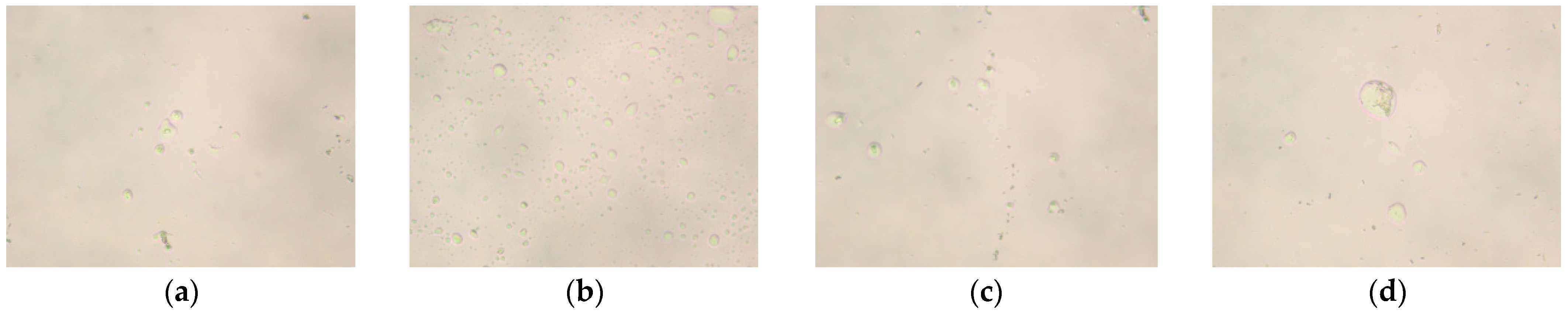
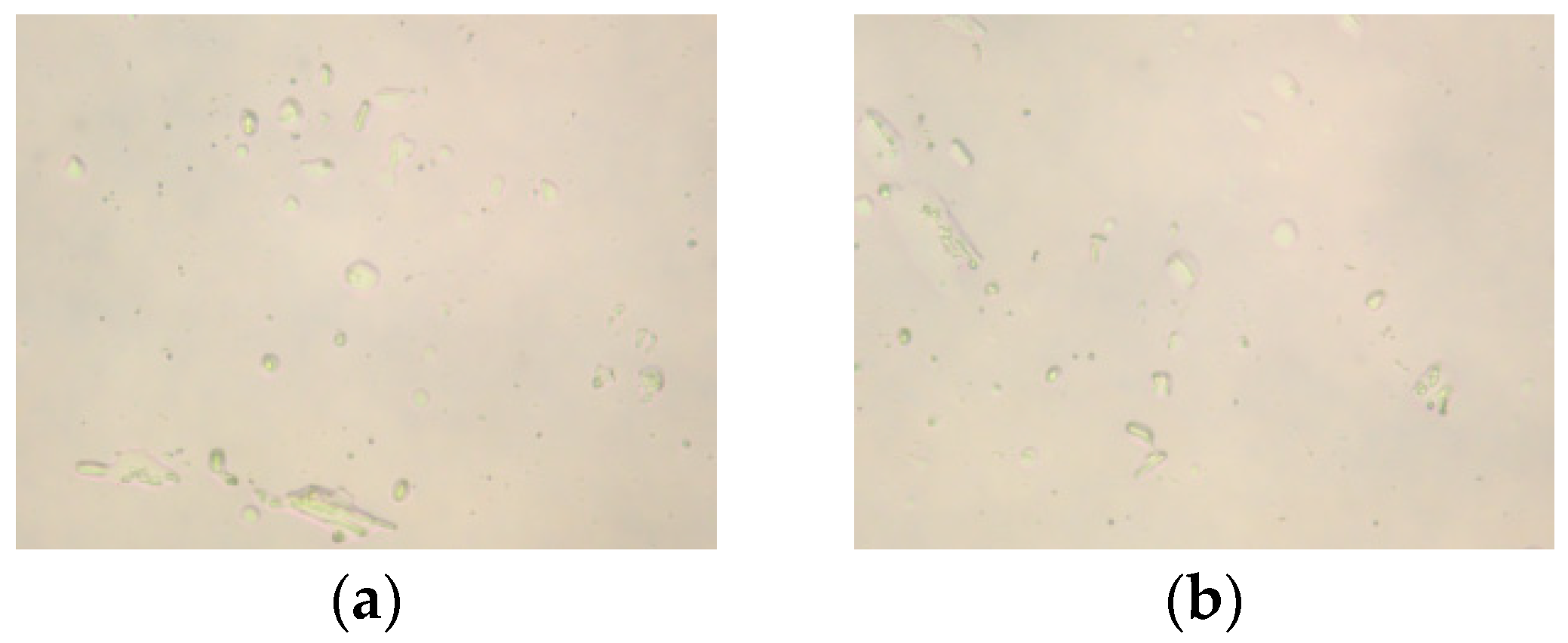
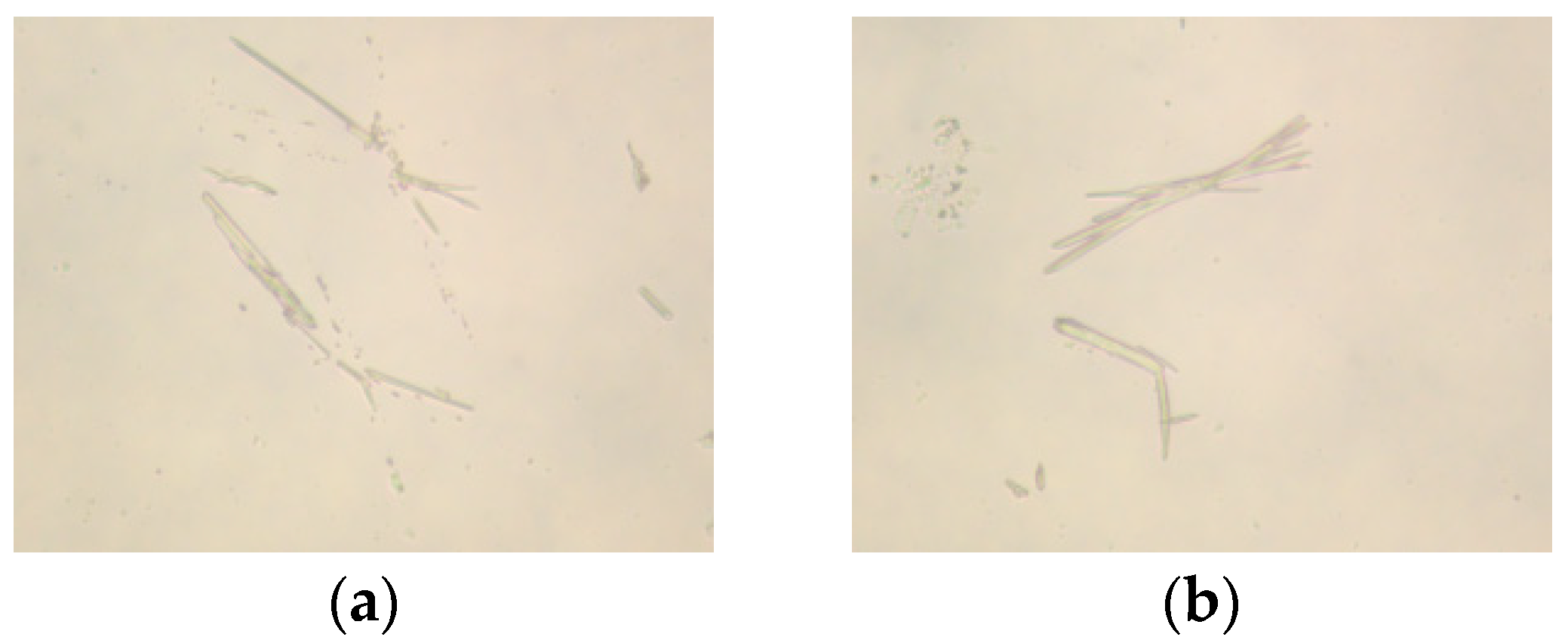
2.2. Polarizing Microscope Analysis
2.2.1. Effect of Molar Ratio of VAc to AM on the Polarized Optical Microscopy Analysis of AMVA
2.2.2. Effect of Cellulose Content on the Polarized Optical Microscopy Analysis of AMVA
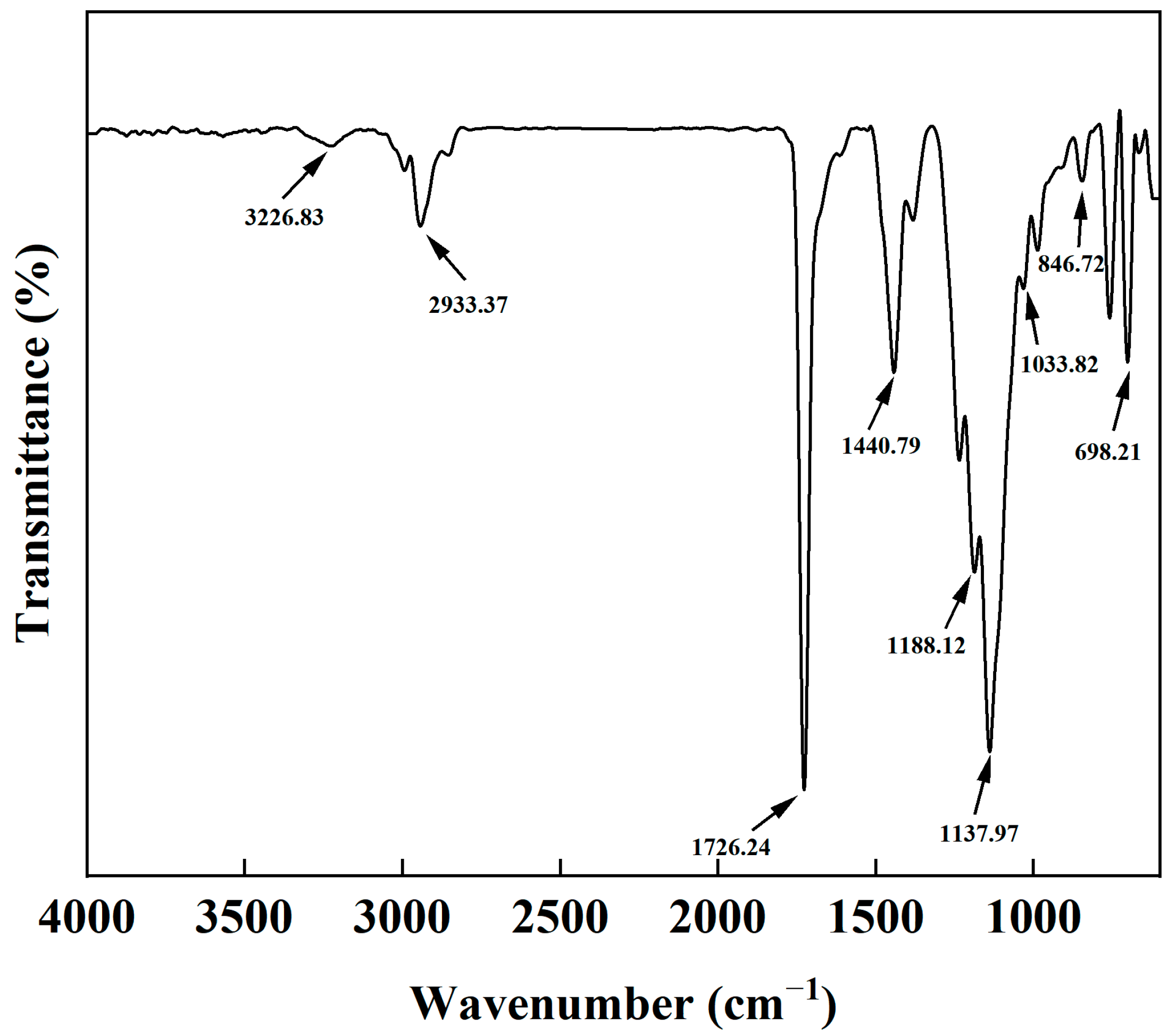
2.3. FTIR of AMVA
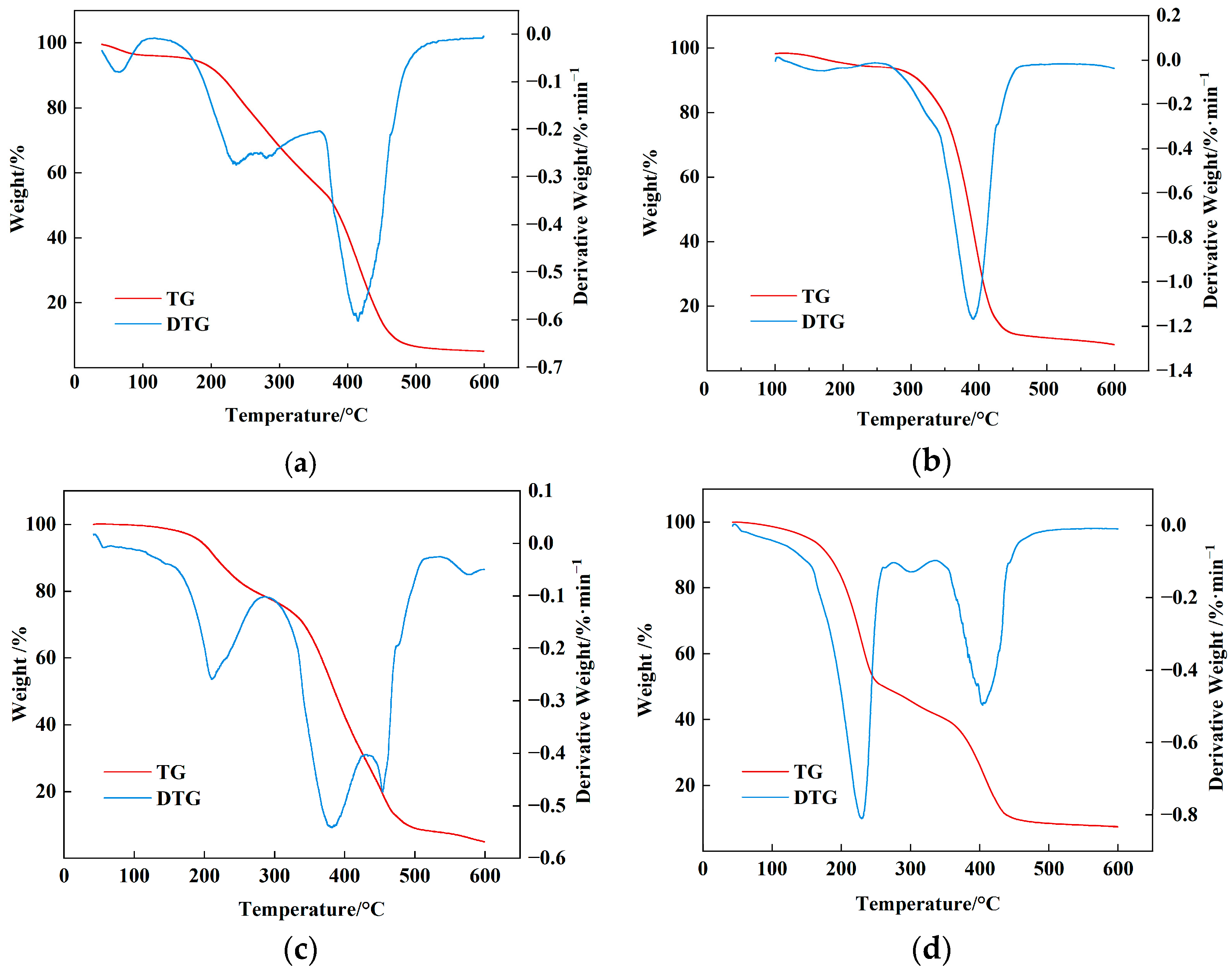
2.4. Thermogravimetric Analysis of AMVA
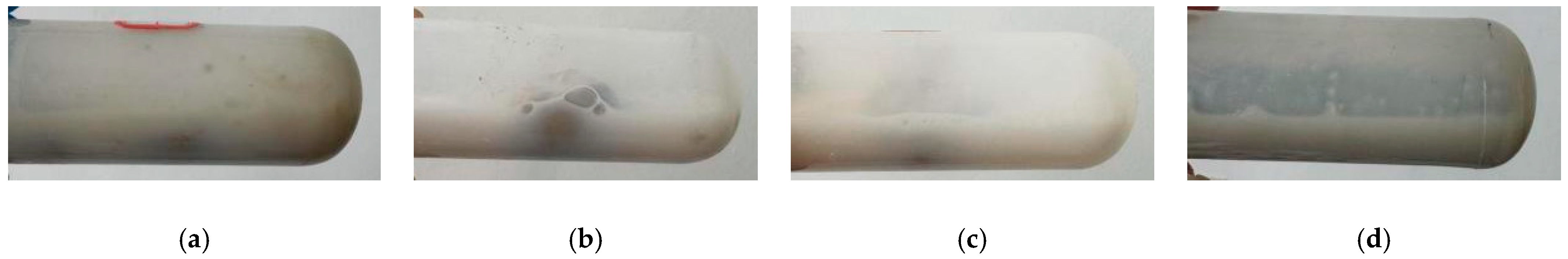
2.5. Moldability Test
2.5.1. Effect of Acrylamide Content on Film-Forming Properties of AMVA Emulsions
2.5.2. Effect of Molar Ratio of VAc to AM on Film-Forming Properties of AMVA Emulsions
2.5.3. Effect of Cellulose Content on Emulsion Film-Forming Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
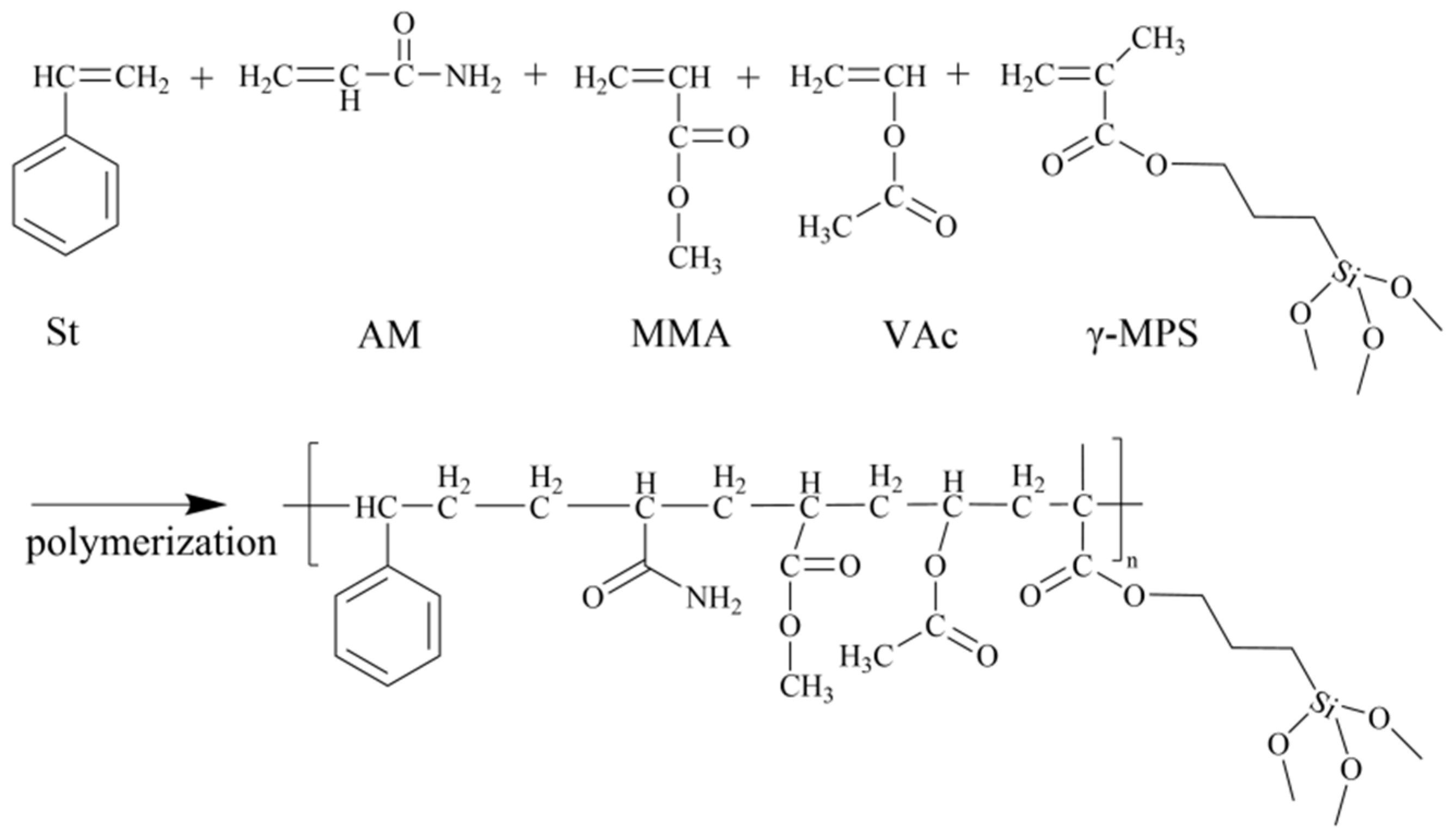
3.3. Mechanism Analysis
3.3.1. Particle Size Testing
3.3.2. Microscopic Analysis
3.3.3. FTIR
3.3.4. TG Analysis
3.3.5. Film-Forming Ability Test
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alhusseini, A.K.; Hamzah, S.H. Geomechanical Characteristics and Wellbore Instability for Nahr Umr Oil Field. Petrol. Chem. 2024, 64, 771–780. [Google Scholar]
- Luo, Z.; Jiang, D.; Ma, C.; Liu, K.; Yu, X. Simulation Analysis of Wellbore Instability Considering the Influence of Hydration Effect on the Physical Properties of Brittle Shale. Chem. Technol. Fuels Oils 2024, 60, 652–661. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, R.; Gao, S.; Huang, H.; Zhang, Z.; Ren, H.; Du, W. Study on the Shale Hydration Inhibition Performance of Triethylammonium Acetate. Minerals 2022, 12, 620. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, D.Y.; Zhuang, G.Z.; Li, X.L. Advanced development of chemical inhibitors in water-based drilling fluids to improve shale stability: A review. Pet. Sci. 2025, 22, 1977–1996. [Google Scholar] [CrossRef]
- Abbas, M.A.; Zamir, A.; Elraies, K.A.; Mahmood, S.M.; Rasool, M.H. A critical parametric review of polymers as shale inhibitors in water-based drilling fluids. J. Petrol. Sci. Eng. 2021, 204, 108745. [Google Scholar]
- Parneta, O.; Stasiuk, I.; Dilay, I.; Demkiv, I. Measures to Improve Metrological and Technical Characteristics of the Film Gas Flowmeter. Energy Eng. Control Syst. 2024, 10, 110–119. [Google Scholar] [CrossRef]
- Nzenguet, A.M.; Essamlali, Y.; Zahouily, M.; Amadine, O. Development and characterization of eco-friendly starch/polyacrylamide/graphene oxide-based slow-release fertilizers for sustainable agriculture. J. Polym. Res. 2025, 32, 1–16. [Google Scholar]
- Yang, W.; Lai, X.; Wang, L.; Shi, H.; Li, H.; Chen, J.; Wang, W. Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking. Polymers 2024, 16, 2884. [Google Scholar] [CrossRef]
- Jakhongir, K.; Zokirjon, T. Electrochemical formation of polyacrylamide-sulfur composite coatings on titanium surfaces. Results Opt. 2025, 19, 100804. [Google Scholar] [CrossRef]
- Zaman, S.U.; Mehdi, M.S. Dialysis treatment, in vitro, and anticoagulation activity of polysulfone-polyacrylamide based-blend membranes: An experimental study. J. Biomat. Sci.-Polym. E. 2025, 36, 169–190. [Google Scholar]
- Prouvé, E.; Rémy, M.; Feuillie, C.; Molinari, M.; Chevallier, P.; Drouin, B.; Laroche, G.; Durrieu, M.C. Interplay of matrix stiffness and stress relaxation in directing osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2022, 10, 4978–4996. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Poly (acrylic acid) nanocomposites: Design of advanced materials. J. Plast. Film Sheet. 2021, 37, 409–428. [Google Scholar] [CrossRef]
- Ma, J.; Xia, B.; Yu, P.; An, Y. Comparison of an emulsion-and solution-prepared acrylamide/AMPS copolymer for a fluid loss agent in drilling fluid. ACS Omega 2020, 5, 12892–12904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Lv, K.; Lu, H.; Geng, Y.; Huang, X. Thermosensitive core-shell polymer microspheres for enhanced wellbore stability in deep-water water-based drilling fluids. J. Mol. Liq. 2025, 418, 126690. [Google Scholar] [CrossRef]
- Tatarchuk, V.; Gromilov, S.; Plyusnin, P. One-step synthesis and characterization of gold nanoparticles doped polyacrylamide hydrogels. J. Sol-Gel. Sci. Technol. 2024, 110, 377–390. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Shi, C.; Wang, G.; Zhao, Y.; Liu, Y.; Zhang, D. Preparation and Characterization of Phosphoric Acid Doped Polyacrylamide/β-Cyclodextrin High-Temperature Proton Exchange Membrane. Macromol. Chem. Phys. 2022, 223, 2200006. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Xiang, X. Enhancing Conductivity of Polyacrylamide Hydrogel With Surface-Coated Hydroxylated Carbon Nanotubes for Wearable Device Applications. J. Appl. Polym. Sci. 2025, 142, e56645. [Google Scholar] [CrossRef]
- Ali, B.; Ezzo, H.A.; Karam, S.; Said, M.; Atassi, Y. Synergistic effect of calcium chloride as coagulant and (chitosan-graft-polyacrylamide) as flocculant for anionic dyes removal from wastewater. Polym. Bull. 2025, 82, 3805–3822. [Google Scholar] [CrossRef]
- Abou El-Reash, Y.G.; Abdelghany, A.M.; Leopold, K. Solid-phase extraction of Cu2+ and Pb2+ from waters using new thermally treated chitosan/polyacrylamide thin films; adsorption kinetics and thermodynamics. Int. J. Environ. Anal. Chem. 2017, 97, 965–982. [Google Scholar] [CrossRef]
- Padhan, A.; Singh, V. Reusable Underwater Superoleophobic Polyacrylamide Membranes for Effective Oil-Water Separation and Dye Removal. ChemistrySelect 2024, 9, e202304653. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhang, N.; Yu, L.; Liu, Z.; Liu, H.; Song, G. Non-ionic polymeric polyacrylamide (PAM) modified SnO2 electron transport layer for high-efficiency perovskite solar cells. Sol. Energy Mater. Sol. Cells 2024, 272, 112907. [Google Scholar] [CrossRef]
- Lopes, R.G.; Vieira, A.S.; Soares, A.T.; Menezes, R.S.; Antoniosi Filho, N.R.; Owatari, M.S.; Arana, L.A.V.; Derner, R.B. Impacts of cationic polyacrylamide flocculant and elevated pH on the harvesting of the diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2025, 37, 1815–1822. [Google Scholar] [CrossRef]
- Yin, X.; Li, Z.; Chai, F.; Tian, Y.; Tan, Y.; Wang, M. Synthesis of a micro-crosslinked polyacrylamide flocculant and its application in treatment of oily produced water. Energy Fuels 2021, 35, 18396–18405. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, F.; Liu, B.; Wang, Z.; Lin, X.; Zhao, H.; Wang, Y. A biomimetic closed-loop recyclable, long-term durable, extreme-condition resistant, flame-retardant nanocoating synthesized by reversible flocculation assembly. Mater. Horiz. 2023, 10, 4551–4561. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Di, H.; Liu, D.; Wang, X.; Zhao, Y. Influence of anionic polyacrylamide on the freeze-thaw resistance of silty clay. Cold Reg. Sci. Technol. 2024, 219, 104111. [Google Scholar] [CrossRef]
- Habibi, M.; Mobarakeh, M.N.; Chamani, A.; Abdullah, L.C.; Zamani-Ahmadmahmoodi, R. The efficiency of polyaluminum chloride and anionic polyacrylamide in removing the hot rolling steel factory effluent turbidity. Int. J. Environ. Sci. Technol. 2024, 21, 2765–2772. [Google Scholar] [CrossRef]
- Morsi, M.A.; Abdelrazek, E.M.; Ramadan, R.M.; Elashmawi, I.S.; Rajeh, A. Structural, optical, mechanical, and dielectric properties studies of carboxymethyl cellulose/polyacrylamide/lithium titanate nanocomposites films as an application in energy storage devices. Polym. Test. 2022, 114, 107705. [Google Scholar] [CrossRef]
- El-Hoshoudy, A.N.; Desouky, S.E.M.; Al-Sabagh, A.M.; Betiha, M.A.; El-kady, M.Y.; Mahmoud, S. Evaluation of solution and rheological properties for hydrophobically associated polyacrylamide copolymer as a promised enhanced oil recovery candidate. Egypt. J. Pet. 2017, 26, 779–785. [Google Scholar] [CrossRef]
- Xie, G.; Xia, L.; Bai, Y.; Huang, D.; Zhang, L. Investigation on the plugging mechanism of nanocomposite polyacrylate copolymers in water-based drilling fluids: Experiments and applications. Geoenergy Sci. Eng. 2024, 242, 213223. [Google Scholar] [CrossRef]
- Abd-Elaal, A.A.; Tawfik, S.M.; Abd-Elhamid, A.; Salem, K.G.; El-Hoshoudy, A.N. Experimental and theoretical investigation of cationic-based fluorescent-tagged polyacrylate copolymers for improving oil recovery. Sci. Rep. 2024, 14, 27689. [Google Scholar]
- Huang, X.; Sun, J.; Jin, J.; Lv, K.; Li, H.; Rong, K.; Meng, X. Use of silicone quaternary ammonium salt for hydrophobic surface modification to inhibit shale hydration and mechanism study. J. Mol. Liq. 2021, 341, 117369. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, F.; Lv, K.; Chang, X. A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids. e-Polymers 2019, 19, 574–578. [Google Scholar] [CrossRef]



| Sample Name | AM/wt% | MMA/wt% | VAc/wt% | Cellulose/wt% | γ-MPS/wt% | St/wt% |
|---|---|---|---|---|---|---|
| AMVA-8 | 3.7 | 55.6 | 14.8 | 1 | 7.4 | 18.5 |
| AMVA-9 | 3.7 | 55.6 | 14.8 | 1.6 | 7.4 | 18.5 |
| Sample Name | TInitial/°C | T1/2/°C | Tmax/°C |
|---|---|---|---|
| AMVA-1 | 117 | 379 | 415 |
| AMVA-2 | 246 | 386 | 392 |
| AMVA-3 | 160 | 385 | 380 |
| AMVA-4 | 120 | 261 | 229 |
| Sample Name | w(AM)/% | Product Stability | Bentonite Agglomeration Test |
|---|---|---|---|
| AMVA-1 | 1 wt% | Milky white colloidal | Poor clumping after centrifugation |
| AMVA-2 | 3.7 wt% | Milky white liquid with bluish color | Clumping after centrifugation |
| AMVA-3 | 7 wt% | Milky white consistency, large amount of precipitation | Clumping after centrifugation |
| AMVA-4 | 10 wt% | Milky white consistency, large amount of precipitation | Poor clumping after centrifugation |
| Sample Name | n(VAc):n(AM) | Product Stability | Bentonite Agglomeration Test |
|---|---|---|---|
| AMVA-5 | 1:2 | Milky white color is lighter, blue light is not obvious | Clusters after centrifugation, dispersed after shaking |
| AMVA-6 | 1:3 | bluish milky white emulsion | Clumps after centrifugation, does not disperse when shaken |
| AMVA-2 | 1:4 | Milky white color is lighter, blue light is not obvious | Clumps after centrifugation, does not disperse when shaken |
| AMVA-7 | 1:5 | Milky white color is lighter, blue light is not obvious | Clusters after centrifugation, dispersed after shaking |
| Sample Name | Fiber Content/wt% | Product Stability | Bentonite Agglomeration Test |
|---|---|---|---|
| AMVA-8 | 1.0 | Lighter milky white | Centrifuged and clumped |
| AMVA-9 | 1.6 | Lighter milky white | Centrifuged and clumped |
| Sample Name | AM/(wt%) | MMA/(wt%) | VAc/(wt%) | γ-MPS/(wt%) | St/(wt%) |
|---|---|---|---|---|---|
| AMVA-1 | 1.0 | 55.6 | 14.8 | 7.4 | 18.5 |
| AMVA-2 | 3.7 | 55.6 | 14.8 | 7.4 | 18.5 |
| AMVA-3 | 7.0 | 55.6 | 14.8 | 7.4 | 18.5 |
| AMVA-4 | 10.0 | 55.6 | 14.8 | 7.4 | 18.5 |
| AMVA-5 | 3.7 | 55.6 | 7.4 | 7.4 | 18.5 |
| AMVA-6 | 3.7 | 55.6 | 11.1 | 7.4 | 18.5 |
| AMVA-7 | 3.7 | 55.6 | 18.5 | 7.4 | 18.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Wei, W.; Yang, Y.; Hao, C.; Zhang, Y.; Xu, G. A Novel Polyacrylamide Film-Forming Agent for Maintaining Wellbore Stability. Molecules 2025, 30, 3877. https://doi.org/10.3390/molecules30193877
Ma G, Wei W, Yang Y, Hao C, Zhang Y, Xu G. A Novel Polyacrylamide Film-Forming Agent for Maintaining Wellbore Stability. Molecules. 2025; 30(19):3877. https://doi.org/10.3390/molecules30193877
Chicago/Turabian StyleMa, Guoyan, Wenjing Wei, Yanzhe Yang, Chao Hao, Yaru Zhang, and Guoqiang Xu. 2025. "A Novel Polyacrylamide Film-Forming Agent for Maintaining Wellbore Stability" Molecules 30, no. 19: 3877. https://doi.org/10.3390/molecules30193877
APA StyleMa, G., Wei, W., Yang, Y., Hao, C., Zhang, Y., & Xu, G. (2025). A Novel Polyacrylamide Film-Forming Agent for Maintaining Wellbore Stability. Molecules, 30(19), 3877. https://doi.org/10.3390/molecules30193877







