Extraction and Identification of the Bioactive Metabolites Produced by Curvularia inaequalis, an Endophytic Fungus Collected in Iran from Echium khuzistanicum Mozaff
Abstract
1. Introduction
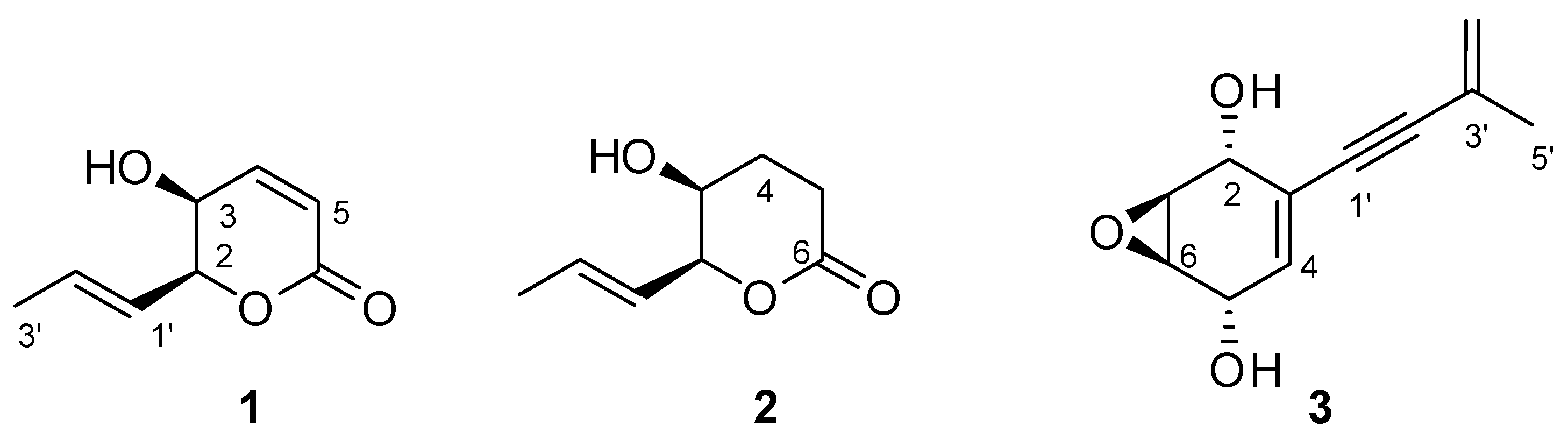
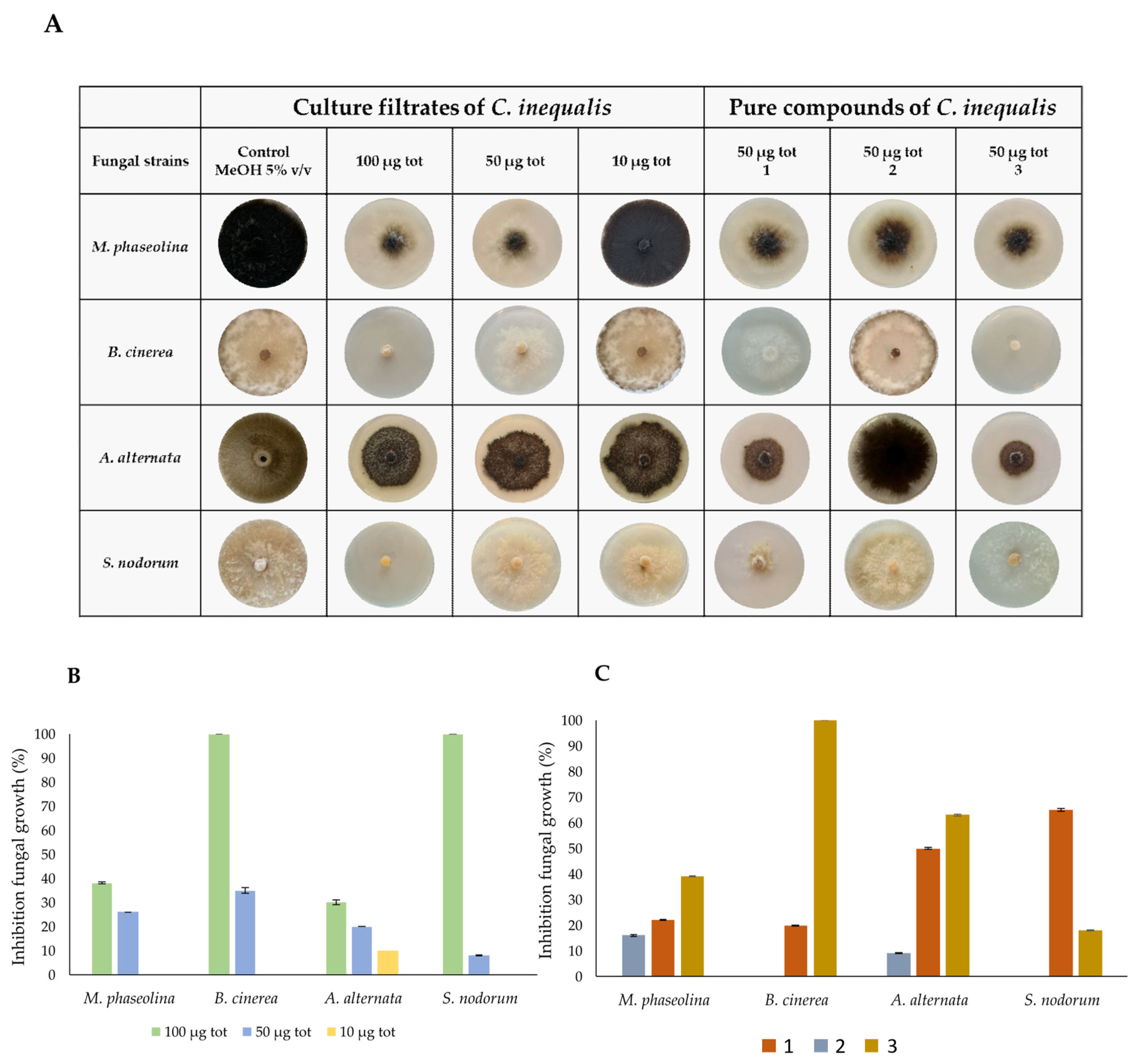
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Fungal Identification
3.3. Fungal Growth
3.4. Extraction and Purification of Bioactive Metabolites
3.5. Phytotoxic Bioassays
3.5.1. Leaf Puncture Assay
3.5.2. Tomato Cutting Assay
3.6. Test Strains and Culture Conditions
3.7. Antimicrobial Assay
3.8. Antifungal Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, nwac206. [Google Scholar] [CrossRef]
- Anke, T.; Thines, E. Fungal metabolites as lead structures for agriculture. In Exploitation of Fungi; Robson, G.D., Ed.; Cambridge University Press: Cambridge, UK, 2007; pp. 45–58. [Google Scholar]
- Demain, A.L.; Adrio, J.L. Contributions of microorganisms to industrial biology. Mol. Biotechnol. 2008, 38, 41–55. [Google Scholar] [CrossRef]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fifty years of drug discovery from fungi. Fungal Divers. 2011, 50, 3–19. [Google Scholar] [CrossRef]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [PubMed]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bustamante, Z.R.; Rivera-Orduña, F.N.; MartínezCárdenas, A.; Flores-Cotera, L.B. Microbial paclitaxel: Advances and perspectives. J. Antibiot. 2010, 63, 460–467. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H.; Liu, L.; Lin, J.; Tang, K. A review: Recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 2010, 86, 1707–1717. [Google Scholar] [CrossRef]
- Liu, W.C.; Gonga, T.; Zhu, P. Advances in exploring alternative Taxol sources. RSC Adv. 2016, 6, 48800–48809. [Google Scholar] [CrossRef]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Evidente, A.; Cimmino, A. Bioactive metabolites from pathogenic and endophytic fungi of forest trees. Curr. Med. Chem. 2018, 25, 208–252. [Google Scholar] [CrossRef]
- El-Metwally, M.M.; Eisa, A.M.; Mekawey, A.A.; Mahmoud, S.F.; El Halmouch, Y. Fungal endophytes of some medicinal plant growing in northwestern coast of Egypt: Isolation, identification and two-way clustering analysis of its antimicrobial activity. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Reveglia, P.; Cinelli, T.; Cimmino, A.; Masi, M.; Evidente, A. The main phytotoxic metabolite produced by a strain of Fusarium oxysporum inducing grapevine plant declining in Italy. Nat. Prod. Res. 2018, 32, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.H., Jr.; Ellestad, G.A.; Kunstmann, M.P. Two new metabolites from an unidentified Nigrospora species. Tetrahedron Lett. 1969, 22, 1791–1794. [Google Scholar] [CrossRef]
- Krasnoff, S.B.; Gupta, S. Identification of the antibiotic phomalactone from the entomopathogenic fungus Hirsutella thompsonii var. synnematosa. J. Chem. Ecol. 1994, 20, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Romero, C.; Ortega-Barría, E.; Arnold, A.E.; Cubilla-Rios, L. Activity against Plasmodium falciparum of lactones isolated from the endophytic fungus Xylaria sp. Pharm. Biol. 2008, 46, 700–703. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Kalinovskii, A.; Khudyakova, Y.V.; Kirichuk, N.N.; Pivkin, M.V.; Afiyatullov, S.S.; Mikhailov, V.V. (–)-Asperpentyn from the facultative marine fungus Curvularia inaequalis. Chem. Nat. Comp. 2014, 50, 1120–1121. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Khudyakova, Y.V.; Kirichuk, N.N.; Yurchenko, E.A.; Afiyatullov, S.S. Metabolitres from the marine isolate of the fungus Curvularia inaequalis. Chem. Nat. Comp. 2013, 49, 163–164. [Google Scholar] [CrossRef]
- Fukushima, T.; Tanaka, M.; Gohbara, M.; Fujomori, T. Phytotoxicity of three lactones from Nigrospora sacchari. Phytochemistry 1998, 48, 625–630. [Google Scholar] [CrossRef]
- Chen, W.; Yu, M.; Chen, S.; Gong, T.; Xie, L.; Liu, J.; Bian, C.; Huang, G.; Zheng, C. Structures and biological activities of secondary metabolites from Xylaria spp. J. Fungi 2024, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Wang, Y.L.; Tan, J.L.; Zhu, C.Y.; Li, D.X.; Huang, R.; Zhang, K.Q.; Niu, X.M. Regulation of the growth of cotton bollworms by metabolites from an entomopathogenic fungus Paecilomyces cateniobliquus. J. Agric. Food Chem. 2012, 60, 5604–5608. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Johnson, R.D.; Techen, N.; Wedge, D.E.; Duke, S.O. Phomalactone from a phytopathogenic fungus infecting Zinnia elegans (Asteraceae) Leaves. J. Chem. Ecol. 2015, 41, 602–612. [Google Scholar] [CrossRef]
- Kim, J.C.; Choi, G.J.; Park, J.H.; Kim, H.T.; Cho, K.Y. Activity against plant pathogenic fungi of phomalactone isolated from Nigrospora sphaerica. Pest Manag. Sci. 2001, 57, 554–559. [Google Scholar] [CrossRef]
- Amaradasa, B.S.; Amundsen, K. First report of Curvularia inaequalis and Bipolaris spicifera causing leaf blight of buffalograss in Nebraska. Plant Dis. 2014, 98, 279. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Xu, J.; Dong, J.; Liu, Y. Nematicidal substances from fungi. Recent Pat. Biotechnol. 2007, 1, 212–233. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.D.; Ribeiro, M.A.D.S.; Garcia, A.; Polonio, J.C.; Santos, C.M.; Silva, A.A.; Orlandelli, R.C.; Castro, J.C.; Abreu-Flho, B.A.; Perira Cbral, M.R.; et al. Secondary metabolites of Curvularia sp. G6-32, an endophyte of Sapindus saponaria, with antioxidant and anticholinesterasic properties. Nat. Prod. Res. 2021, 35, 4148–4153. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Cimmino, A.; Vurro, M.; Fracchiolla, M.; Charudattan, R. Herbicidal potential of ophiobolins produced by Drechslera gigantea. J. Agric. Food Chem. 2006, 54, 1779–1783. [Google Scholar] [CrossRef]
- Ramesha, K.P.; Mohana, N.C.; Nuthan, B.R.; Rakshith, D.; Satish, S. Antimicrobial metabolite profiling of Nigrospora sphaerica from Adiantum philippense L. J. Genet. Eng. Biotechnol. 2020, 18, 66. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; Volume 31, pp. 315–322. [Google Scholar] [CrossRef]
- Berbee, M.L.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Pinkerton, F.; Strobel, G. Serinol as an activator of toxin production in attenuated cultures of Helminthosporium sacchari. Proc. Natl. Acad. Sci. USA 1976, 73, 4007–4011. [Google Scholar] [CrossRef]
- Evidente, A.; Rodeva, R.; Andolfi, A.; Stoyanova, Z.; Perrone, C.; Motta, A. Phytotoxic polyketides produced by Phomopsis foeniculi, a strain isolated from diseased Bulgarian fennel. Eur. J. Plant Pathol. 2011, 130, 173–182. [Google Scholar] [CrossRef]
- Capasso, R.; Iacobellis, N.S.; Bottalico, A.; Randazzo, G. Structure-toxicity relationships of the eremophilane phomenone and PR-toxin. Phytochemistry 1984, 23, 2781–2784. [Google Scholar] [CrossRef]
- Masi, M.; Sautua, F.; Zatout, R.; Castaldi, S.; Arrico, L.; Isticato, R.; Pescitelli, G.; Carmona, M.A.; Evidente, A. Phaseocyclopentenones A and B, phytotoxic penta- and tetrasubstituted cyclopentenones roduced by Macrophomina phaseolina, the causal agent of charcoal rot of soybean in Argentina. J. Nat. Prod. 2021, 84, 459–465. [Google Scholar] [CrossRef]
- Ragucci, S.; Castaldi, S.; Landi, N.; Isticato, R.; Di Maro, A. Antifungal activity of ageritin, a ribotoxin-like protein from Cyclocybe aegerita edible mushroom, against phytopathogenic fungi. Toxins 2023, 15, 578. [Google Scholar] [CrossRef]
- Castaldi, S.; Zorrilla, J.G.; Petrillo, C.; Russo, M.T.; Ambrosino, P.; Masi, M.; Cimmino, A.; Isticato, R. Alternaria alternata isolated from infected pears (Pyrus communis) in Italy produces non-host toxins and hydrolytic enzymes as infection mechanisms and exhibits competitive exclusion against Botrytis cinerea in co-infected host fruits. J. Fungi 2023, 9, 326. [Google Scholar] [CrossRef]

| Tested Strains | Crude Organic Extract | (+)-Phomalactone | Teicoplanin | Colistin | Amphotericin B |
|---|---|---|---|---|---|
| S. aureus (ATCC 6538) | 250 | 62 | 0.5 | a | a |
| MRSA (ATCC 43300) | 250 | 62 | 1 | a | a |
| E. faecalis (ATCC 29212) | 1000 | 250 | 0.5 | a | a |
| P. aeruginosa (ATCC 27853) | 1000 | 500 | a | 1 | a |
| A. baumanii (ATCC BAA747) | 1000 | 125 | a | 0.5 | a |
| C. albicans (ATCC 10231) | 1000 | >500 | a | a | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besharati, M.; Ciavatta, M.L.; Carbone, M.; Cacciapuoti, N.; Aversa, M.; Roscetto, E.; Castaldi, S.; Perrone, G.; Boari, A.; Gialluisi, K.; et al. Extraction and Identification of the Bioactive Metabolites Produced by Curvularia inaequalis, an Endophytic Fungus Collected in Iran from Echium khuzistanicum Mozaff. Molecules 2025, 30, 3870. https://doi.org/10.3390/molecules30193870
Besharati M, Ciavatta ML, Carbone M, Cacciapuoti N, Aversa M, Roscetto E, Castaldi S, Perrone G, Boari A, Gialluisi K, et al. Extraction and Identification of the Bioactive Metabolites Produced by Curvularia inaequalis, an Endophytic Fungus Collected in Iran from Echium khuzistanicum Mozaff. Molecules. 2025; 30(19):3870. https://doi.org/10.3390/molecules30193870
Chicago/Turabian StyleBesharati, Maryam, Maria Letizia Ciavatta, Marianna Carbone, Nadia Cacciapuoti, Martina Aversa, Emanuela Roscetto, Stefany Castaldi, Giancarlo Perrone, Angela Boari, Katia Gialluisi, and et al. 2025. "Extraction and Identification of the Bioactive Metabolites Produced by Curvularia inaequalis, an Endophytic Fungus Collected in Iran from Echium khuzistanicum Mozaff" Molecules 30, no. 19: 3870. https://doi.org/10.3390/molecules30193870
APA StyleBesharati, M., Ciavatta, M. L., Carbone, M., Cacciapuoti, N., Aversa, M., Roscetto, E., Castaldi, S., Perrone, G., Boari, A., Gialluisi, K., Catania, M. R., Moosawi-Jorf, S. A., & Evidente, A. (2025). Extraction and Identification of the Bioactive Metabolites Produced by Curvularia inaequalis, an Endophytic Fungus Collected in Iran from Echium khuzistanicum Mozaff. Molecules, 30(19), 3870. https://doi.org/10.3390/molecules30193870










