Scutellarein Protects Against UVB-Induced Skin Injury in a Mouse Model
Abstract
1. Introduction
2. Results
2.1. UVB Irradiation Leads to Progressive Alterations in Skin Appearance
2.2. UVB Irradiation Induces Increased Transepidermal Water Loss and Release of Inflammatory Factors
2.3. UVB Irradiation-Induced Apoptosis, Neutrophil Infiltration, and Barrier Damage
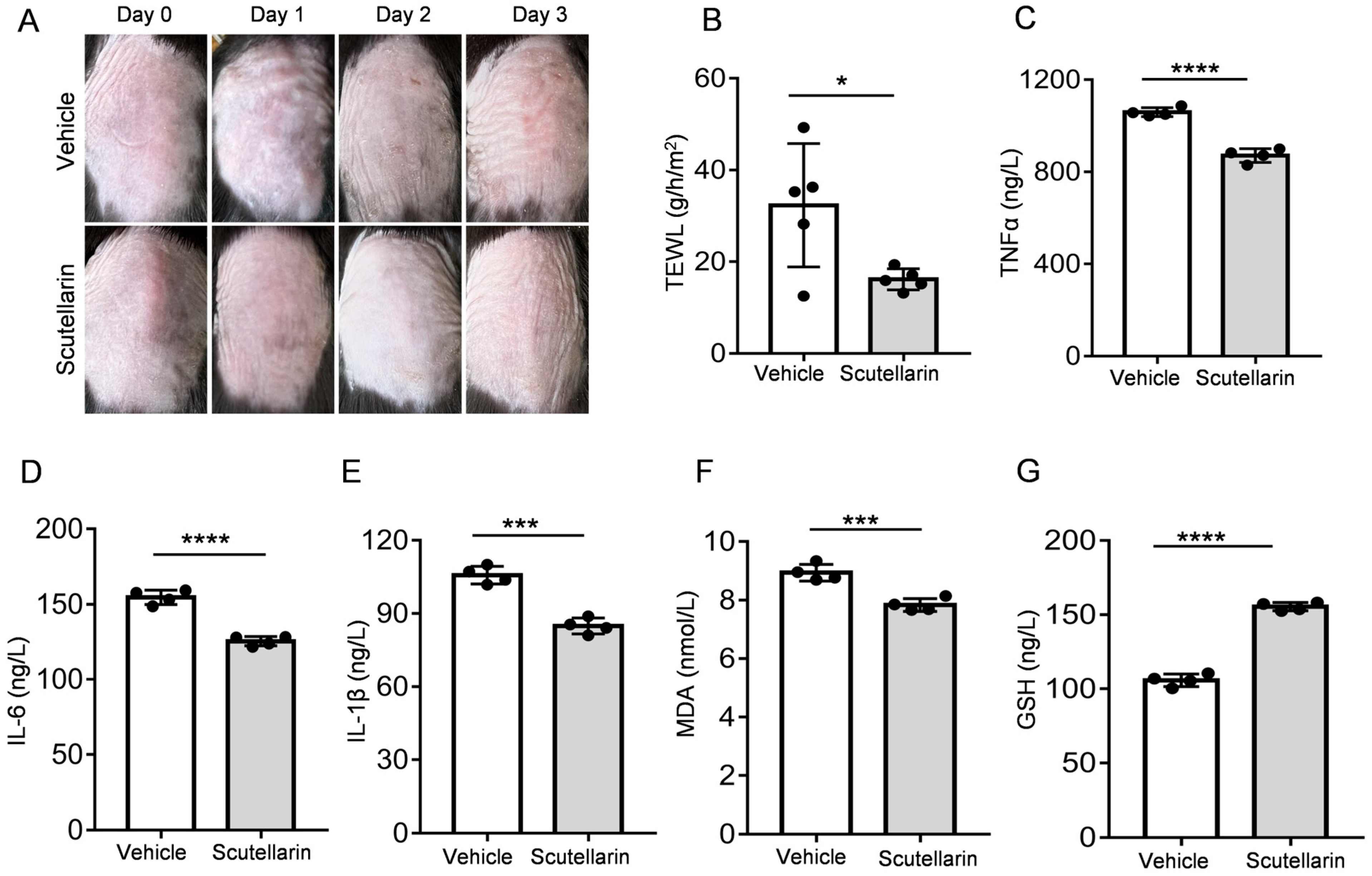
2.4. Scutellarin Ameliorates UVB-Induced Skin Barrier Function
2.5. Scutellarin Attenuates UVB-Induced Inflammatory Cytokines and Oxidative Stress
2.6. Scutellarin Reduces Neutrophil Infiltration and Maintains Skin Barrier Integrity
2.7. Scutellarein Alleviates UVB-Induced Skin Damage by Modulating Inflammatory Responses and Maintaining Extracellular Matrix Homeostasis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Establishment of a Mouse Skin Damage Model Induced by UltravioletB (UVB) Irradiation
4.3. Macroscopic Evaluation of Dorsal Skin
4.4. Measurement of Transepidermal Water Loss (TEWL)
4.5. Detection of Cytokine Levels and Oxidative Stress
4.6. UVB Irradiation-Induced Skin Damage and the Therapeutic Effects of Scutellarein
4.7. Hematoxylin and Eosin (H&E) Staining
4.8. Immunohistochemistry
4.9. RNA-Seq Data Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UVB | Ultraviolet B |
| TEWL | Transepidermal water loss |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| PCA | Principal component analysis |
| CAMs | Cell adhesion molecules |
| NETs | Neutrophil extracellular trap formation |
| ECM | Extracellular matrix |
References
- De Szalay, S.; Wertz, P.W. Protective Barriers Provided by the Epidermis. Int. J. Mol. Sci. 2023, 24, 3145. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, A.; Kendall, A.C. Bioactive lipids in the skin barrier mediate its functionality in health and disease. Pharmacol. Ther. 2024, 260, 108681. [Google Scholar] [CrossRef]
- Dousset, L.; Mahfouf, W.; Younes, H.; Fatrouni, H.; Faucheux, C.; Muzotte, E.; Khalife, F.; Rossignol, R.; Moisan, F.; Cario, M.; et al. Energy metabolism rewiring following acute UVB irradiation is largely dependent on nuclear DNA damage. Free Radic. Biol. Med. 2025, 227, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Pan, J.; Song, C.; Chen, W.; Zhang, Y. Liquiritin Carbomer Gel Cold Paste Promotes Healing of Solar Dermatitis in Mice. Int. J. Mol. Sci. 2024, 25, 3767. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, W.; Liu, T.; Pei, H.; Ma, Y.; Zhao, Y.; Huang, S.; Chen, M. NDRG2 Deficiency Exacerbates UVB-Induced Skin Inflammation and Oxidative Stress Damage. Inflammation 2025, 48, 1313–1325. [Google Scholar] [CrossRef]
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612. [Google Scholar] [CrossRef]
- Saraswat, A.; Lahiri, K.; Chatterjee, M. Topical corticosteroid abuse on the face: A prospective, multicenter study of dermatology outpatients. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 160–166. [Google Scholar] [CrossRef]
- DiRuggiero, D.; DiRuggiero, M. Beyond Skin Deep: The Systemic Impact of Topical Corticosteroids in Dermatology. J. Clin. Aesthet. Dermatol. 2025, 18, S16–S20. [Google Scholar] [PubMed]
- Sitohang, I.B.S.; Makes, W.I.; Sandora, N.; Suryanegara, J. Topical tretinoin for treating photoaging: A systematic review of randomized controlled trials. Int. J. Women’s Dermatol. 2022, 8, e003. [Google Scholar] [CrossRef]
- Jowkar, F.; Namazi, M.R. Statins in dermatology. Int. J. Dermatol. 2010, 49, 1235–1243. [Google Scholar] [CrossRef]
- Lai, J.; Li, C. Review on the pharmacological effects and pharmacokinetics of scutellarein. Arch. Der Pharm. 2024, 357, e2400053. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, W.; Weng, Y.; Zhao, Y.; Chen, H.; Chen, Y.; Qiu, J.; Jiang, B.; Li, C.; Lai, Y. Scutellarin Alleviates CCl4-Induced Liver Fibrosis by Regulating Intestinal Flora and PI3K/AKT Signaling Axis. Int. J. Mol. Sci. 2025, 26, 2997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, L.; Jiao, K.; Xue, C.; Tang, Q.; Jiang, S.; Ren, Y.; Chen, H.; El-Aziz, T.M.A.; Abdelazeem, K.N.M.; et al. Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol. 2022, 179, 4792–4808. [Google Scholar] [CrossRef]
- Thirusangu, P.; Vigneshwaran, V.; Vijay Avin, B.R.; Rakesh, H.; Vikas, H.M.; Prabhakar, B.T. Scutellarein antagonizes the tumorigenesis by modulating cytokine VEGF mediated neoangiogenesis and DFF-40 actuated nucleosomal degradation. Biochem. Biophys. Res. Commun. 2017, 484, 85–92. [Google Scholar] [CrossRef]
- Martinez, R.M.; Fattori, V.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Melo, C.P.B.; Bussmann, A.J.C.; Staurengo-Ferrari, L.; Bezerra, J.R.; Vignoli, J.A.; et al. The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation. Molecules 2020, 25, 2953. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Dömer, D.; Walther, T.; Möller, S.; Behnen, M.; Laskay, T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front. Immunol. 2021, 12, 636954. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Tong, X.; Bridgeman, B.B.; Plebanek, M.P.; Volpert, O.V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20, 930–942. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300. [Google Scholar] [CrossRef]
- Koseki, K.; Kawasaki, H. Assessment of skin barrier function using skin images with topological data analysis. NPJ Syst. Biol. Appl. 2020, 6, 40. [Google Scholar] [CrossRef]
- Thoa, N.T.; Son, N.T. Scutellarein: A review of chemistry and pharmacology. J. Pharm. Pharmacol. 2025, 77, 352–370. [Google Scholar] [CrossRef]
- Sung, N.Y.; Kim, M.Y.; Cho, J.Y. Scutellarein Reduces Inflammatory Responses by Inhibiting Src Kinase Activity. Korean J. Physiol. Pharmacol. 2015, 19, 441–449. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Morita, T.; Hayakawa, T. BNIP3 upregulation via stimulation of ERK and JNK activity is required for the protection of keratinocytes from UVB-induced apoptosis. Cell Death Dis. 2017, 8, e2576. [Google Scholar] [CrossRef]
- Farrukh, M.R.; Nissar, U.A.; Afnan, Q.; Rafiq, R.A.; Sharma, L.; Amin, S.; Kaiser, P.; Sharma, P.R.; Tasduq, S.A. Oxidative stress mediated Ca2+ release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J. Dermatol. Sci. 2014, 75, 24–35. [Google Scholar] [CrossRef]
- Machoń, N.J.; Zdanowska, N.; Klimek-Trojan, P.; Owczarczyk-Saczonek, A. Vascular Cell Adhesion Molecule 1 and E-Selectin as Potential Cardiovascular Risk Biomarkers in Psoriasis. Int. J. Mol. Sci. 2025, 26, 792. [Google Scholar] [CrossRef] [PubMed]
- Segelcke, D.; Pradier, B.; Reichl, S.; Schäfer, L.C.; Pogatzki-Zahn, E.M. Investigating the Role of Ly6G+ Neutrophils in Incisional and Inflammatory Pain by Multidimensional Pain-Related Behavioral Assessments: Bridging the Translational Gap. Front. Pain Res. 2021, 2, 735838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, Y.; Han, Z.; Huang, X.; Zhou, S.; Wang, S.; Zhou, Y.; Han, X.; Chen, H. Exploring mechanisms of skin aging: Insights for clinical treatment. Front. Immunol. 2024, 15, 1421858. [Google Scholar] [CrossRef]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR- and SP1-dependent manner. JCI Insight 2022, 7, e156344. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Angelina, J.; Putra, A.; Trisnadi, S.; Hermansyah, D.; Setiawan, E.; Sumarawati, T.; Amalina, N.D. Hypoxia-conditioned mesenchymal stem cells (MSC) exosomes attenuate ultraviolet-B (UVB)-mediated malondialdehyde (MDA) and matrix metalloproteinase-1 (MMP)-1 upregulation in collagen loss models. Med. Glas. 2025, 22, 9–14. [Google Scholar] [CrossRef]
- Mo, S.; Yue, X.; Qu, Y.; Zhang, G.; Wang, L.; Sun, X. Echinacoside Ameliorates UVB-Induced Skin Damage Through Selective Inhibition of the Cutaneous TRPV3 Channel. Molecules 2025, 30, 2026. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.P.B.; Saito, P.; Martinez, R.M.; Staurengo-Ferrari, L.; Pinto, I.C.; Rodrigues, C.C.A.; Badaro-Garcia, S.; Vignoli, J.A.; Baracat, M.M.; Bussmann, A.J.C.; et al. Aspirin-Triggered Resolvin D1 (AT-RvD1) Protects Mouse Skin against UVB-Induced Inflammation and Oxidative Stress. Molecules 2023, 28, 2417. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, P.; Yang, F.; Zhang, W.; Li, G.; Zhou, L.; Xia, T.; Gao, Y.; Zhou, W. Scutellarein Protects Against UVB-Induced Skin Injury in a Mouse Model. Molecules 2025, 30, 3867. https://doi.org/10.3390/molecules30193867
Sun Y, Zhang P, Yang F, Zhang W, Li G, Zhou L, Xia T, Gao Y, Zhou W. Scutellarein Protects Against UVB-Induced Skin Injury in a Mouse Model. Molecules. 2025; 30(19):3867. https://doi.org/10.3390/molecules30193867
Chicago/Turabian StyleSun, Yue, Pengfei Zhang, Fang Yang, Wang Zhang, Gaofu Li, Lei Zhou, Tiantian Xia, Yue Gao, and Wei Zhou. 2025. "Scutellarein Protects Against UVB-Induced Skin Injury in a Mouse Model" Molecules 30, no. 19: 3867. https://doi.org/10.3390/molecules30193867
APA StyleSun, Y., Zhang, P., Yang, F., Zhang, W., Li, G., Zhou, L., Xia, T., Gao, Y., & Zhou, W. (2025). Scutellarein Protects Against UVB-Induced Skin Injury in a Mouse Model. Molecules, 30(19), 3867. https://doi.org/10.3390/molecules30193867





