Lepidiline-Derived Imidazole-2(3H)-Thiones: (3+2)-Cycloadditions vs. Nucleophilic Additions in Reactions with Fluorinated Nitrile Imines
Abstract
1. Introduction
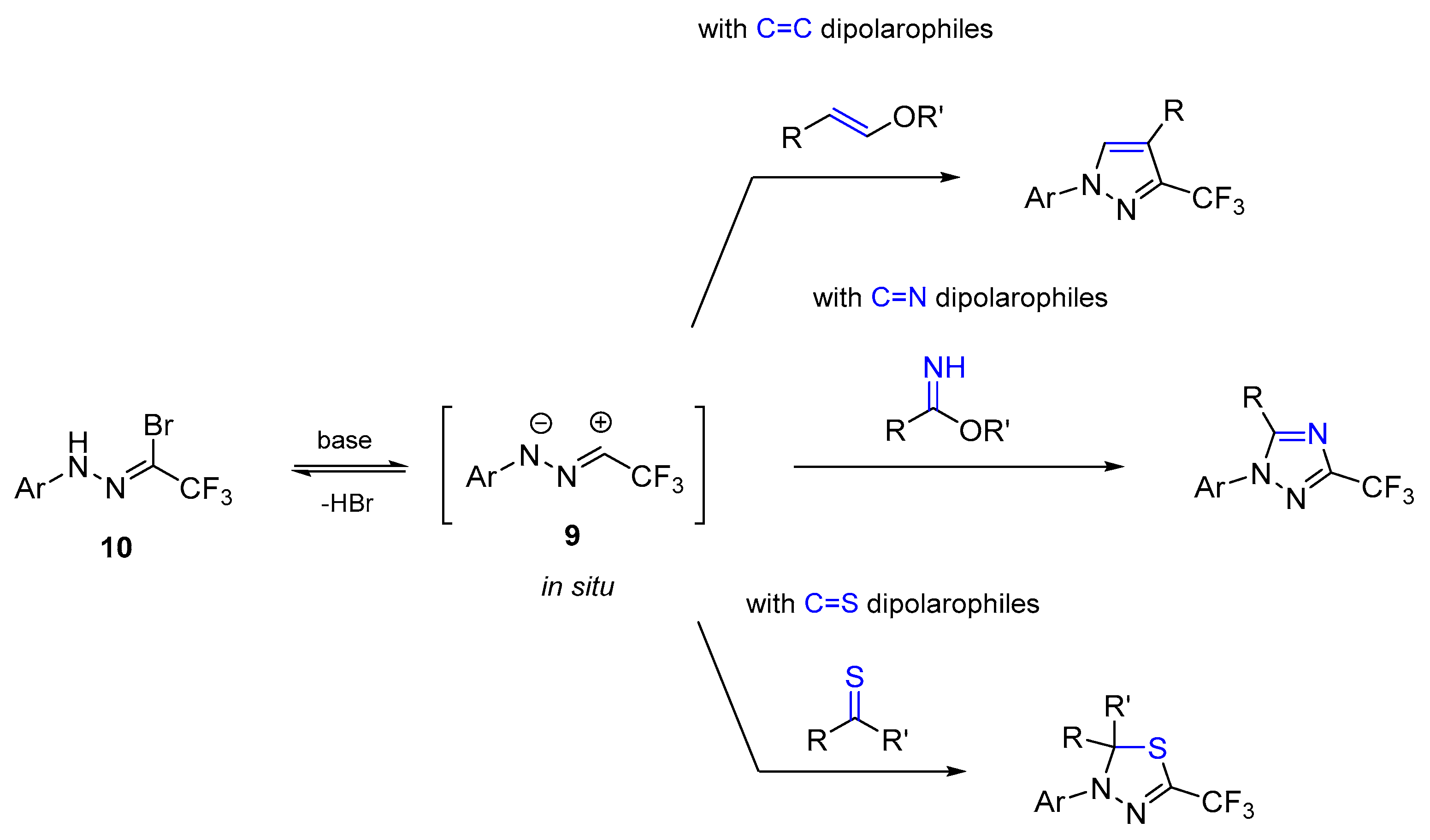
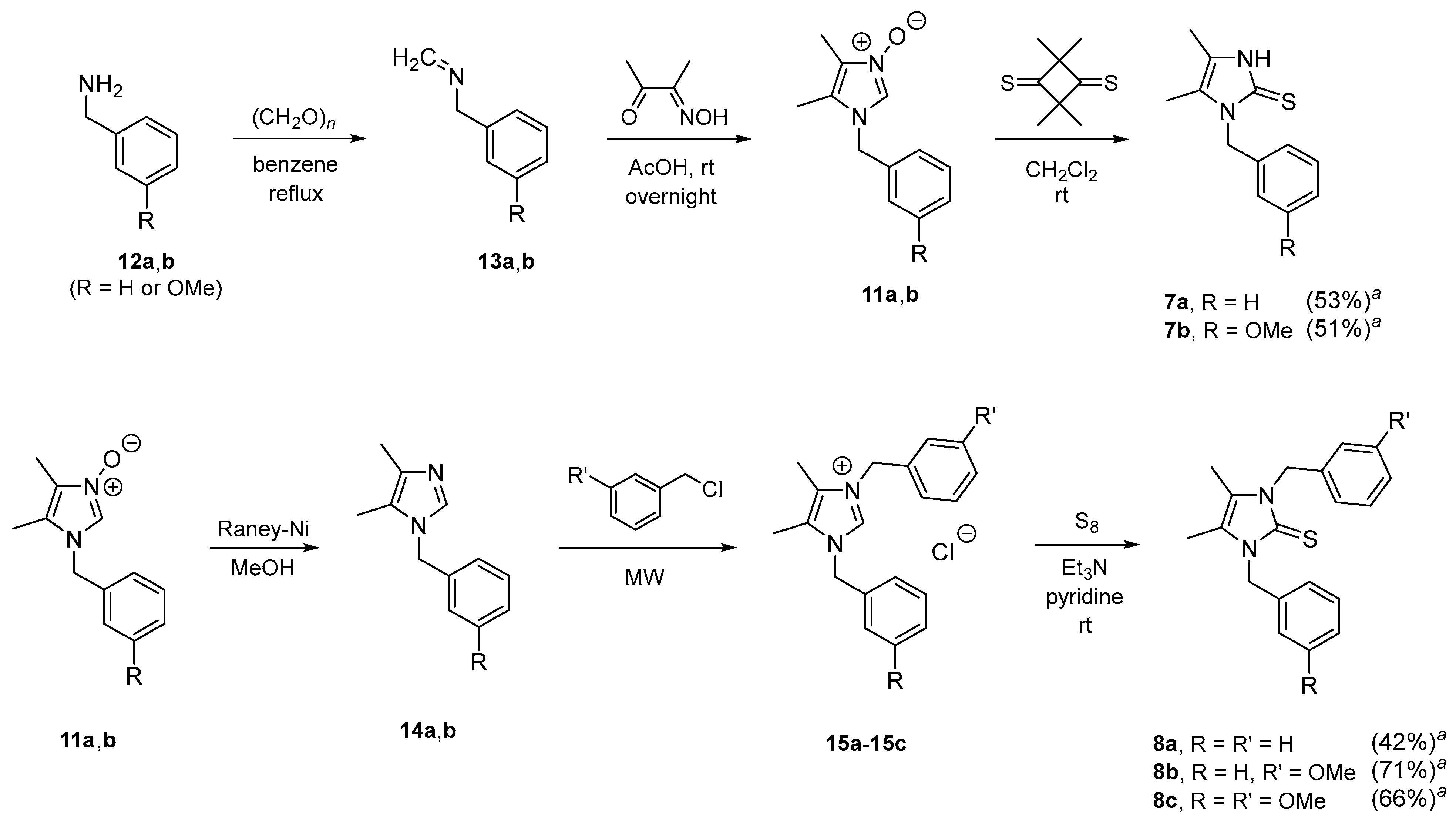
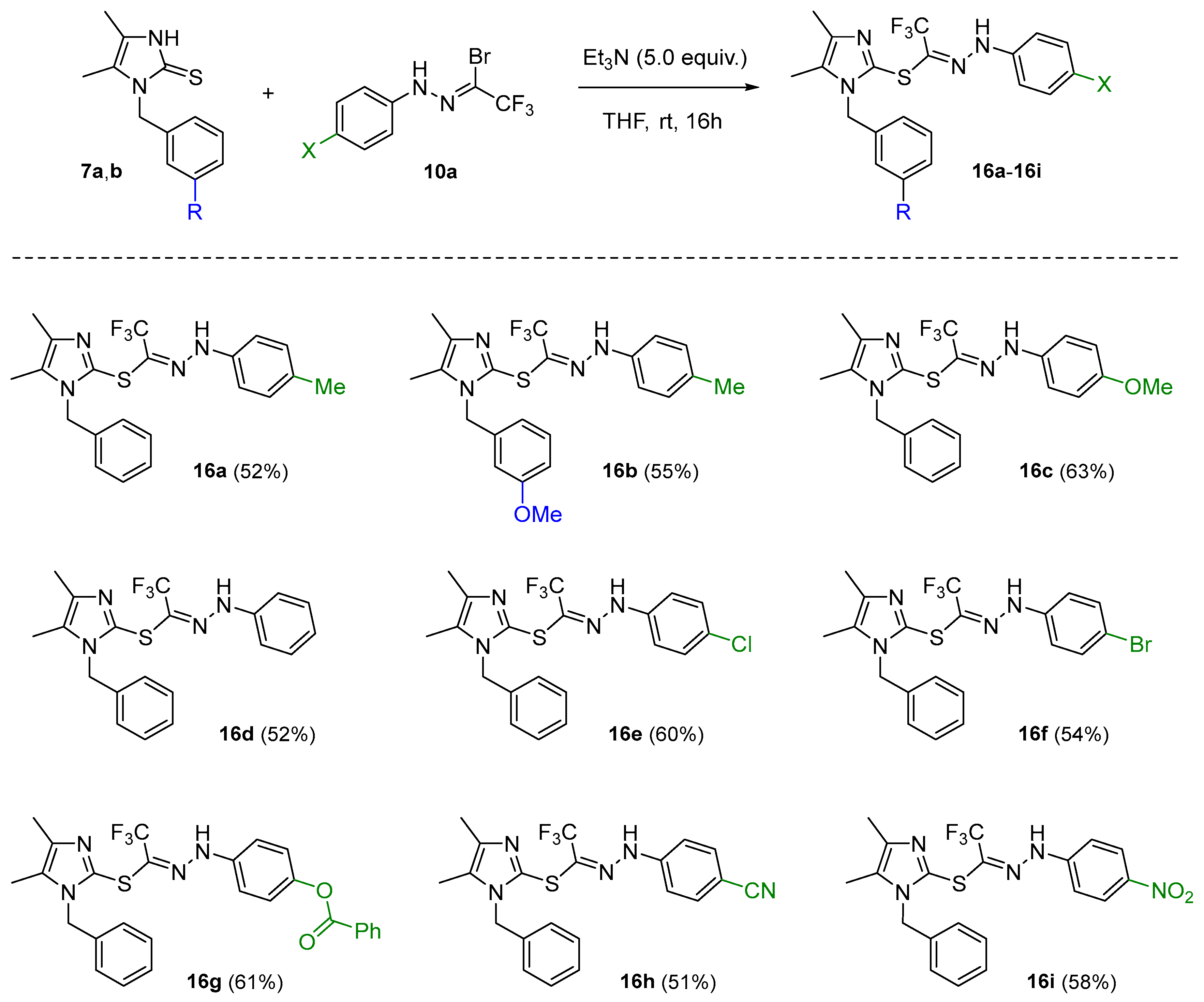
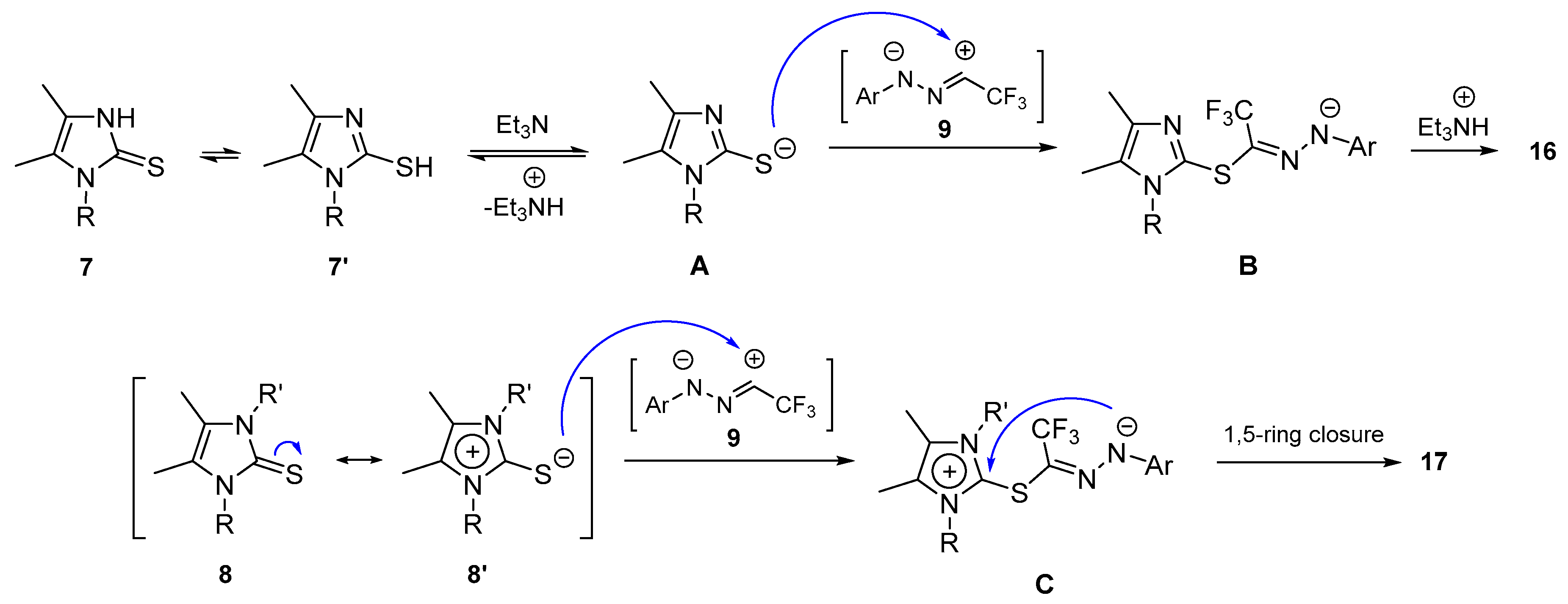
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Synthesis General Methods
3.2. General Procedure for Synthesis of Cycloadducts 16 and 17
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maat, L.; Beyerman, H.C. The Imidazole Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; Academic Press: Cambridge, MA, USA, 1984; Chapter 5; pp. 281–333. [Google Scholar] [CrossRef]
- Jin, Z. Imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2006, 23, 464–496. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.-M.; Damu, G.L.V.; Geng, R.-X.; Zhou, C.-H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef] [PubMed]
- Teli, P.; Sahiba, N.; Sethiya, A.; Soni, J.; Agarwal, S. Imidazole derivatives: Impact and prospects in antiviral drug discovery. In Imidazole-Based Drug Discovery; Elsevier Inc.: Amsterdam, Netherlands, 2022; Chapter 4; pp. 167–193. [Google Scholar] [CrossRef]
- Kapourani, A.; Kontogiannopoulos, K.N.; Barmpalexis, P. A review on the role of pilocarpine on the management of xerostomia and the importance of the topical administration systems development. Pharmaceuticals 2022, 15, 762. [Google Scholar] [CrossRef]
- Maier, U.H.; Gundlach, H.; Zenk, M.H. Seven imidazole alkaloids from Lepidium sativum. Phytochem. 1998, 49, 1791–1795. [Google Scholar] [CrossRef]
- Gacemi, S.; Benarous, K.; Imperial, S.; Yousfi, M. Lepidine B & E as new target inhibitors from Lepidium sativum seeds against four enzymes of the pathogen Candida albicans: In vitro and in sillico studies. Endocr. Metab. Immune. Disord. Drug Targets 2020, 20, 127–138. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole alkaloids from Lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef]
- Jin, W.; Chen, X.; Dai, P.; Yu, L. Lepidiline C and D: Two new imidazole alkaloids from Lepidium meyenii Walpers (Brassicaceae) roots. Phytochem. Lett. 2016, 17, 158–161. [Google Scholar] [CrossRef]
- Le, H.T.N.; Van Roy, E.; Dendooven, E.; Peeters, L.; Theunis, M.; Foubert, K.; Pieters, L.; Tuenter, E. Alkaloids from Lepidium meyenii (Maca), structural revision of macardine and UPLC-MS/MS feature-based molecular networking. Phytochemistry 2021, 190, 112863. [Google Scholar] [CrossRef] [PubMed]
- Ulloa del Carpio, N.; Alvarado-Corella, D.; Quiñones-Laveriano, D.M.; Araya-Sibaja, A.; Vega-Baudrit, J.; Monagas-Juan, M.; Navarro-Hoyos, M.; Villar-López, M. Exploring the chemical and pharmacological variability of Lepidium meyenii: A comprehensive review of the effects of maca. Front. Pharmacol. 2024, 15, 1360422. [Google Scholar] [CrossRef]
- Minich, D.M.; Ross, K.; Frame, J.; Fahoum, M.; Warner, W.; Meissner, H.O. Not all Maca is created equal: A review of colors, nutrition, phytochemicals, and clinical uses. Nutrients 2024, 16, 530. [Google Scholar] [CrossRef]
- Curran, D.; Müller-Bunz, H.; Bär, S.I.; Schobert, R.; Zhu, X.; Tacke, M. Novel anticancer NHC*-gold(I) complexes inspired by lepidiline A. Molecules 2020, 25, 3474. [Google Scholar] [CrossRef]
- Cochrane, A.R.; Kennedy, A.R.; Kerr, W.J.; Lindsay, D.M.; Reid, M.; Tuttle, T. The natural product lepidiline A as an N-heterocyclic carbene ligand precursor in complexes of the type [Ir(cod)(NHC)PPh3]X: Synthesis, Characterisation, and application in hydrogen isotope exchange catalysis. Catalysts 2020, 10, 161. [Google Scholar] [CrossRef]
- Tóth, S.; Szlávik, M.F.; Mendel, R.; Fekecs, F.; Tusnády, G.; Vajda, F.; Varga, N.; Apáti, A.; Bényei, A.; Paczal, A.; et al. Synthesis and systematic investigation of lepidiline A and its gold(I), silver(I), and copper(I) complexes using in vitro cancer models and multipotent stem cells. ACS Omega 2024, 9, 32226–32234. [Google Scholar] [CrossRef] [PubMed]
- Mlostoń, G.; Kowalczyk, M.; Celeda, M.; Jasiński, M.; Denel-Bobrowska, M.; Olejniczak, A.B. Fluorinated analogues of lepidilines A and C: Synthesis and screening of their anticancer and antiviral activity. Molecules 2022, 27, 3524. [Google Scholar] [CrossRef]
- Poper, W.K.; Denel-Bobrowska, M.; Olejniczak, A.B.; Jasiński, M. First lepidiline-inspired 1,3-dibenzyl 2-CF3S-imidazoliums: Design, synthesis and cytotoxic activity study. Biomed. Pharmacother. 2025, 192, 118606. [Google Scholar] [CrossRef]
- Jamieson, C.; Livingstone, K. The Nitrile Imine 1,3-dipole; Properties, Reactivity and Applicationse; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Dongxu, Z. Trifluoromethylated hydrazones and acylhydrazones as potent nitrogen-containing fluorinated building blocks. Beilstein J. Org. Chem. 2023, 19, 1741–1754. [Google Scholar] [CrossRef]
- Yamaletdinova, N.R.; Gataullin, R.R. advances in the synthesis of heterocycles with two and three heteroatoms using hydrazonoyl halides. Helv. Chim. Acta 2024, 107, e202400058. [Google Scholar] [CrossRef]
- Świątek, K.; Utecht-Jarzyńska, G.; Palusiak, M.; Ma, J.-A.; Jasiński, M. One-pot synthesis of 1-aryl-3-trifluoromethylpyrazoles sing nitrile imines and mercaptoacetaldehyde as a surrogate of acetylene. Org. Lett. 2023, 25, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Świątek, K.; Utecht-Jarzyńska, G.; Jasiński, M. Exercise in 1-aryl-3-CF3-1H-pyrazoles: Regioselective synthesis of 4-/5-iodides and cross-coupling reactions. RSC. Adv. 2025, 15, 9225–9229. [Google Scholar] [CrossRef]
- Tanaka, K.; Maeno, S.; Mitsuhashi, K. Preparation of trifluoroacetonitrile phenylimine and its reactions with some dipolarophiles. Chem. Lett. 1982, 11, 543–546. [Google Scholar] [CrossRef]
- Utecht, G.; Fruziński, A.; Jasiński, M. Polysubstituted 3-trifluoromethylpyrazoles: Regioselective (3+2)-cycloaddition of trifluoroacetonitrile imines with enol ethers and functional group transformations. Org. Biomol. Chem. 2018, 16, 1252–1257. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Utecht-Jarzyńska, G.; Mlostoń, G.; Jasiński, M. A straightforward access to 3-trifluoromethyl-1H-indazoles via (3+2)-cycloaddition of arynes with nitrile imines derived from trifluoroacetonitrile. J. Fluorine Chem. 2021, 241, 109691. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Utecht-Jarzyńska, G.; Mlostoń, G.; Jasiński, M. Trifluoromethylated pyrazoles via sequential (3+2)-cycloaddition of fluorinated nitrile imines with chalcones and solvent-dependent deacylative oxidation reactions. Org. Lett. 2022, 24, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, H.; Cheung, C.W.; Zhang, F.-G.; Jasiński, M.; Ma, J.-A.; Nie, J. Regioselective [3+2]-cycloaddition of di/trifluoromethylated hydrazonoyl chlorides with fluorinated nitroalkenes: A facile access to 3-di/trifluoroalkyl-5-fluoropyrazoles. Org. Biomol. Chem. 2023, 21, 5040–5045. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, J.-L.; Chen, Z.; Wang, R. Base-promoted (3+2)-cycloaddition of trifluoroacetohydrazonoyl chlorides with imidates en route to trifluoromethyl-1,2,4-triazoles. J. Org. Chem. 2022, 87, 14514−14522. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wan, X.; Zhou, Y.; Liu, J.; Cai, J.; Deng, G.-J. Facile synthesis of fully substituted 1,2,4-triazoles vis [3+2] cycloaddition of nitrile imines with amidine under transition metal-free conditions. Asian. J. Org. Chem. 2023, e202200674. [Google Scholar] [CrossRef]
- Cen, K.; Wei, J.; Feng, Y.; Liu, Y.; Wang, X.; Liu, Y.; Yin, Y.; Yu, J.; Wang, D.; Cai, J. Synthesis of fused 3-trifluoromethyl-1,2,4-triazoles via base-promoted [3+2] cycloaddition of nitrile imines and 1H-benzo[d]imidazole-2-thiols. Org. Biomol. Chem. 2023, 21, 7095–7099. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Utecht, G.; Lentz, D.; Jasiński, M. Trifluoromethylated 2,3-dihydro-1,3,4-thiadiazoles via the regioselective [3+2]-cycloadditions of fluorinated nitrile imines with aryl, hetaryl, and ferrocenyl thioketones. J. Fluorine Chem. 2016, 192, 147–154. [Google Scholar] [CrossRef]
- Utecht, G.; Sioma, J.; Jasiński, M.; Mlostoń, G. Expected and unexpected results in reactions of fluorinated nitrile imines with (cyclo)aliphatic thioketones. J. Fluorine Chem. 2017, 201, 68–75. [Google Scholar] [CrossRef]
- Grzelak, P.; Utecht, G.; Jasiński, M.; Mlostoń, G. First (3+2)-cycloadditions of thiochalcones as C=S dipolarophiles: Efficient synthesis of 1,3,4-thiadiazoles via reactions with fluorinated nitrile imines. Synthesis 2017, 49, 2129–2137. [Google Scholar] [CrossRef]
- Huisgen, R.; Langhals, E. Thiones as superdipolarophiles. Tetrahedron Lett. 1989, 30, 5369–5372. [Google Scholar] [CrossRef]
- Huisgen, R.; Fišera, L.; Giera, H.; Sustmann, R. Thiones as superdipolarophiles. Rates and equilibria of nitrone cycloadditions to thioketones. J. Am. Chem. Soc. 1995, 117, 9671–9678. [Google Scholar] [CrossRef]
- Mlostoń, G.; Kowalczyk, M.; Celeda, M.; Gach-Janczak, K.; Janecka, A.; Jasiński, M. Synthesis and cytotoxic activity of lepidilines A-D: Comparison with some 4,5-diphenyl analogues and related imidazole-2-thiones. J. Nat. Prod. 2021, 84, 3071–3079. [Google Scholar] [CrossRef]
- Mlostoń, G.; Celeda, M.; Heimgartner, H.; Duda, D.; Obijalska, E.; Jasiński, M. Synthesis and selected transformations of 2-unsubstituted imidazole N-oxides using a ball-milling mechanochemical approach. Catalysts 2022, 12, 589. [Google Scholar] [CrossRef]
- Poper, W.K.; Ma, J.-A.; Jasiński, M. One-pot telescoping S-transfer and trifluoromethylation for the synthesis of 2-CF3S-imidazoles with N-oxides as convenient precursors. J. Org. Chem. 2024, 89, 15331–15335. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Habib, I.; Hossain, M. Functionalization of imidazole N-oxide: A recent discovery in organic transformations. Beilstein J. Org. Chem. 2022, 18, 1575–1588. [Google Scholar] [CrossRef]
- Kutasevich, A.V.; Perevalov, V.P.; Mityanov, V.S. Recent progress in non-catalytic C-H functionalization of heterocyclic N-oxides. Eur. J. Org. Chem. 2021, 2021, 357–373. [Google Scholar] [CrossRef]
- Mlostoń, G.; Jasiński, M.; Wróblewska, A.; Heimgartner, H. Recent progress in the chemistry of 2-unsubstituted 1H-imidazole 3-oxides. Curr. Org. Chem. 2016, 20, 1359–1369. [Google Scholar] [CrossRef]
- Mlostoń, G.; Gendek, T.; Heimgartner, H. First examples of reactions of azole N-oxides with thioketones: A novel type of sulfur-transfer reaction. Helv. Chim. Acta 1998, 81, 1585–1595. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Jasiński, M.; Kaszyński, P. Tautomeric equilibrium in trifluoroacetaldehyde arylhydrazones. Tetrahedron 2015, 71, 2349–2356. [Google Scholar] [CrossRef]
- Utecht, G.; Mlostoń, G.; Jasiński, M. A straightforward access to trifluoromethylated spirobipyrazolines through a double (3+2)-cycloaddition of fluorinated nitrile imines with alkoxyallenes. Synlett 2018, 29, 1753–1758. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Utecht-Jarzyńska, G.; Jasiński, M. w-(3-Trifluoromethylpyrazol-4-yl)alkanoic acids via (3+2)-cycloaddition of nitrile imines with cyclic enones ad deacylative aromatization. J. Fluorine Chem. 2023, 272, 110206. [Google Scholar] [CrossRef]
- Utecht-Jarzyńska, G.; Michalak, A.; Banaś, J.; Mlostoń, G.; Jasiński, M. Trapping of trifluoroacetonitrile imines with mercaptoacetaldehyde and mercaptocarboxylic acids: An access to fluorinated 1,3,4-thiadiazine derivatives via (3+3)-annulation. J. Fluorine Chem. 2019, 222-223, 8–14. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Świątek, K.; Celeda, M.; Utecht-Jarzyńska, G.; Jaskulska, A.; Gach-Janczak, K.; Jasiński, M. Trifluoromethylated 4,5-dihydro-1,2,4-triazin-6(1H)-ones via (3+3)-annulation of nitrile imines with α-amino esters. Materials 2023, 16, 856. [Google Scholar] [CrossRef] [PubMed]
- Utecht-Jarzyńska, G.; Kowalczyk, A.; Jasiński, M. Fluorinated and non-fluorinated 1,4-diarylpyrazoles via MnO2-mediated mechanochemical deacylative oxidation of 5-acylpyrazolines. Molecules 2022, 27, 8446. [Google Scholar] [CrossRef] [PubMed]
- Utecht-Jarzyńska, G.; Jarzyński, S.; Jasiński, M. Trapping in situ generated CF3-nitrile imines with maleimides under solvent-free mechanochemical conditions. RSC Mechanochem. 2025, 2, 79–82. [Google Scholar] [CrossRef]
- Utecht-Jarzyńska, G.; Mykhaylychenko, S.S.; Rusanov, E.B.; Shermolovich, Y.G.; Jasiński, M.; Mlostoń, G. Highly fluorinated 2,3-dihydro-1,3,4-thiadiazole derivatives via (3+2)-cycloadditions of tertiary thioamides with nitrile imines derived from trifluoroacetonitrile. J. Fluorine Chem. 2021, 242, 109702. [Google Scholar] [CrossRef]
- Abramov, N.D.; Trzhtsinskaya, B.V. Structure and properties of imidazole-2-thiones. Chem. Heterocycl. Compd. 1988, 24, 1309–1321. [Google Scholar] [CrossRef]
- Lingareddy, E.; Nadagouda, A.; Sai, L.; Saradhi, T.P.; Bansod, S.; Tangutur, A.D.; Nanubolu, J.B.; Kumbhare, R.M. Lewis acid-catalyzed substituted spiro 4-thia-1,2,6,9-tetraazaspiro [4.4]non-2-ene-7,8-diones via 1,3-dipolar [3+2]cycloaddition. ChemistrySelect 2024, 9, e202402070. [Google Scholar] [CrossRef]
- Maqbool, M.; Solangi, M.; Khan, K.M.; Özil, M.; Baltaş, N.; Salar, U.; Tariq, S.S.; Ul Haq, Z.; Taha, M. Imidazole-thiadiazole hybrids: A multitarget de novo drug design approach, in vitro evaluation, ADME/T, and in silico studies. Arch. Pharm. 2024, 357, 2400325. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, T.; Aslam, S.; Yaseen, M.; Saif, M.J.; Ahmad, M.; Al-Hussain, S.A.; Zaki, M.E.A. Recent synthetic strategies of medicinally important imidazothiadiazoles. J. Saudi Chem. Soc. 2023, 27, 101679. [Google Scholar] [CrossRef]
- Mijoba, A.; Parra-Giménez, N.; Fernandez-Moreira, E.; Ramírez, H.; Serrano, X.; Blanco, Z.; Espinosa, S.; Charris, A.E. Synthesis of hybrid molecules with imidazole-1,3,4-thiadiazole core and evaluation of biological activity on Trypanosoma cruzi and Leishmania donovani. Molecules 2024, 29, 4125. [Google Scholar] [CrossRef] [PubMed]
- Mlostoń, G.; Celeda, M.; Kowalczyk, M.; Oliver, G.A.; Werz, D.B. Ring-opening reactions of donor-acceptor cyclopropanes with enolizable 5-mercapto-1H-tetrazoles. Eur. J. Org. Chem. 2024, 27, e202400831. [Google Scholar] [CrossRef]
- Mlostoń, G.; Kowalczyk, M.; Celeda, M.; Oliver, G.A.; Werz, D.B. Ring-opening reactions of donor-acceptor cyclopropanes with some enolizable azaheterocyclic thiones: S- versus N-attack. Synlett 2025, 36, 1548–1552. [Google Scholar] [CrossRef]


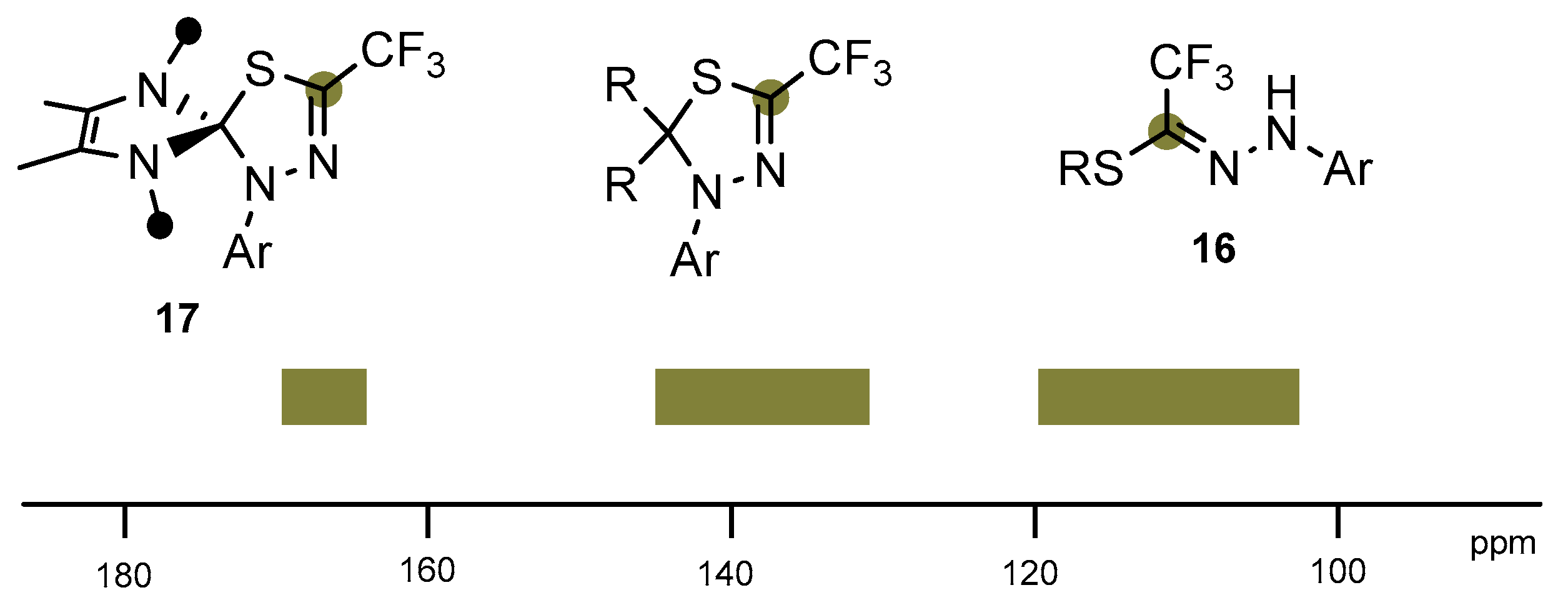
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poper, W.K.; Świątek, K.; Urbaniak, K.; Olszewska, B.; Jasiński, M. Lepidiline-Derived Imidazole-2(3H)-Thiones: (3+2)-Cycloadditions vs. Nucleophilic Additions in Reactions with Fluorinated Nitrile Imines. Molecules 2025, 30, 3851. https://doi.org/10.3390/molecules30193851
Poper WK, Świątek K, Urbaniak K, Olszewska B, Jasiński M. Lepidiline-Derived Imidazole-2(3H)-Thiones: (3+2)-Cycloadditions vs. Nucleophilic Additions in Reactions with Fluorinated Nitrile Imines. Molecules. 2025; 30(19):3851. https://doi.org/10.3390/molecules30193851
Chicago/Turabian StylePoper, Wiktor K., Kamil Świątek, Katarzyna Urbaniak, Barbara Olszewska, and Marcin Jasiński. 2025. "Lepidiline-Derived Imidazole-2(3H)-Thiones: (3+2)-Cycloadditions vs. Nucleophilic Additions in Reactions with Fluorinated Nitrile Imines" Molecules 30, no. 19: 3851. https://doi.org/10.3390/molecules30193851
APA StylePoper, W. K., Świątek, K., Urbaniak, K., Olszewska, B., & Jasiński, M. (2025). Lepidiline-Derived Imidazole-2(3H)-Thiones: (3+2)-Cycloadditions vs. Nucleophilic Additions in Reactions with Fluorinated Nitrile Imines. Molecules, 30(19), 3851. https://doi.org/10.3390/molecules30193851






