Electronic Structures and Photodetachment of TeO2−, TeO3−, and HTeO4− Anions: A Cryogenic Photoelectron Spectroscopic Study
Abstract
1. Introduction
2. Results and Discussion
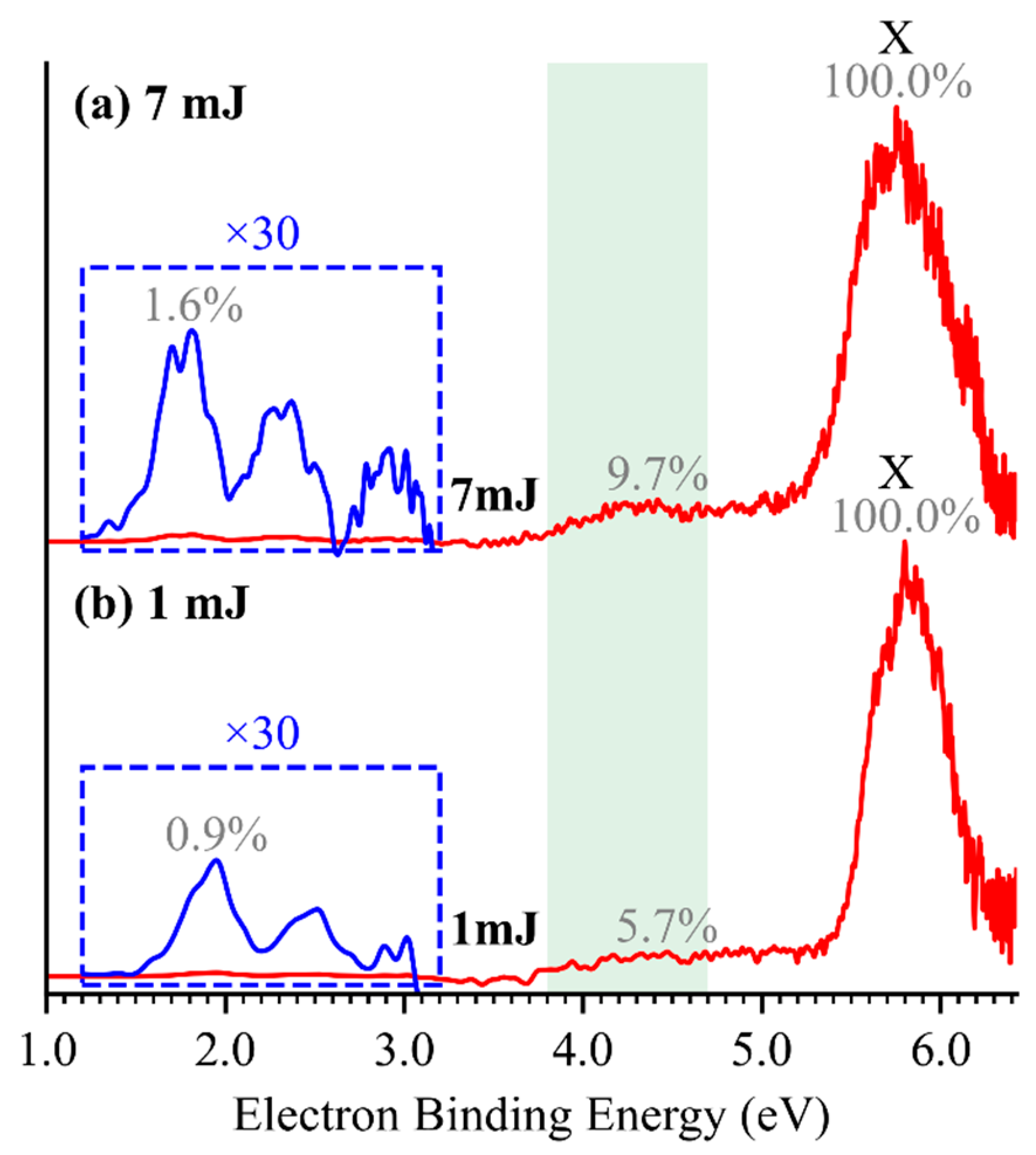
2.1. Photoelectron Spectra of TeO2−, TeO3−, and HTeO4−
2.2. Optimized Structures and Calculated ADEs and VDEs
2.3. Molecular Orbital and Photodetachment Analyses
2.4. Vibrational Excitation and FCF Simulation
2.5. Two-Photon Photodissociation–Photodetachment of HTeO4−
3. Materials and Methods
3.1. Experimental Methods
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADE | adiabatic detachment energy |
| CID | collision-induced dissociation |
| CRACPES | cryogenic anion cluster photoelectron spectroscopy |
| DFT | density functional theory |
| EA | electron affinity |
| EDD | electron density difference |
| eBE | electron binding energy |
| eKE | electron kinetic energy |
| ESI | electrospray ionization |
| FCF | Frank–Condon factor |
| HOMO | highest occupied molecular orbital |
| MO | molecular orbital |
| NIPES | negative-ion photoelectron spectroscopy |
| NPA | natural population analysis |
| TDDFT | time-dependent density functional theory |
| TOF | time-of-flight |
| VDE | vertical detachment energy |
| RF | radio frequency |
| DC | direct current |
References
- Chivers, T.; Laitinen, R.S. Tellurium: A maverick among the chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef]
- Yuan, D.-F.; Trabelsi, T.; Zhang, Y.-R.; Francisco, J.S.; Wang, L.-S. Probing the Electronic Structure and Bond Dissociation of SO3 and SO3– Using High-Resolution Cryogenic Photoelectron Imaging. J. Am. Chem. Soc. 2022, 144, 13740–13747. [Google Scholar] [CrossRef]
- Anstöter, C.S.; Verlet, J.R.R. Photoelectron imaging of the SO3 anion: Vibrational resolution in photoelectron angular distributions*. Mol. Phys. 2021, 119, e1821921. [Google Scholar] [CrossRef]
- Nimlos, M.R.; Ellison, G.B. Photoelectron spectroscopy of sulfur-containing anions (SO2−, S3−, and S2O−). J. Phys. Chem. 1986, 90, 2574–2580. [Google Scholar] [CrossRef]
- Wang, X.-B.; Nicholas, J.B.; Wang, L.-S. Photoelectron Spectroscopy and Theoretical Calculations of SO4− and HSO4−: Confirmation of High Electron Affinities of SO4 and HSO4. J. Phys. Chem. A 2000, 104, 504–508. [Google Scholar] [CrossRef]
- Tran, C.D. Acousto-Optic Devices: Optical Elements for Spectroscopy. Anal. Chem. 1992, 64, 971A–981A. [Google Scholar] [CrossRef] [PubMed]
- Savage, N. Acousto-optic devices. Nat. Photonics 2010, 4, 728–729. [Google Scholar] [CrossRef]
- Maák, P.; Barócsi, A.; Fehér, A.; Veress, M.; Mihajlik, G.; Rózsa, B.; Koppa, P. Acousto-optic deflector configurations optimized for multiphoton scanning microscopy. Opt. Commun. 2023, 530, 129213. [Google Scholar] [CrossRef]
- Mi, Z.; Zhao, H.; Guo, Q. Thermal analysis of TeO2-based acousto-optic tunable filters for spectral imaging. In Proceedings of the Sixth Conference on Frontiers in Optical Imaging and Technology: Novel Imaging Systems, Nanjing, China, 22–24 October 2023; p. 131550X. [Google Scholar] [CrossRef]
- Brofferio, C.; Cremonesi, O.; Dell‘Oro, S. Neutrinoless Double Beta Decay Experiments With TeO2 Low-Temperature Detectors. Front. Phys. 2019, 7, 86. [Google Scholar] [CrossRef]
- Plat, A.; Cornette, J.; Colas, M.; Mirgorodsky, A.P.; Smirnov, M.B.; Noguera, O.; Masson, O.; Thomas, P. Huge susceptibility increase within the (1−x) TeO2 + x TeO3 crystal system: Ab initio calculation study. J. Alloys Compd. 2014, 587, 120–125. [Google Scholar] [CrossRef]
- Roginskii, E.M.; Kuznetsov, V.G.; Smirnov, M.B.; Noguera, O.; Duclère, J.R.; Colas, M.; Masson, O.; Thomas, P. Comparative Analysis of the Electronic Structure and Nonlinear Optical Susceptibility of α-TeO2 and β-TeO3 Crystals. J. Phys. Chem. C 2017, 121, 12365–12374. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, M.; Guo, Q.; Yu, Y.; Lu, S.; Jiang, X.; Zhou, S. A chip-based microcavity derived from multi-component tellurite glass. J. Mater. Chem. C 2015, 3, 5141–5144. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Wang, R.; Li, A.; Zhang, M.; Wang, S.; Wang, P.; Ward, J.M.; Nic Chormaic, S. A tellurite glass optical microbubble resonator. Opt. Express 2020, 28, 32858. [Google Scholar] [CrossRef]
- Jha, A.; Richards, B.D.O.; Jose, G.; Toney Fernandez, T.; Hill, C.J.; Lousteau, J.; Joshi, P. Review on structural, thermal, optical and spectroscopic properties of tellurium oxide based glasses for fibre optic and waveguide applications. Int. Mater. Rev. 2012, 57, 357–382. [Google Scholar] [CrossRef]
- Shen, S.; Jha, A.; Liu, X.; Naftaly, M.; Bindra, K.; Bookey, H.J.; Kar, A.K. Tellurite Glasses for Broadband Amplifiers and Integrated Optics. J. Am. Ceram. Soc. 2002, 85, 1391–1395. [Google Scholar] [CrossRef]
- Castellan, A.; Vaghi, A.; Bart, J.C.J.; Giordano, N. Propylene oxidation on TeO2 · SiO2 catalysts. J. Catal. 1975, 39, 213–224. [Google Scholar] [CrossRef]
- Miki, J.; Osada, Y.; Konoshi, T.; Tachibana, Y.; Shikada, T. Selective oxidation of toluene to benzoic acid catalyzed by modified vanadium oxides. Appl. Catal. A Gen. 1996, 137, 93–104. [Google Scholar] [CrossRef]
- Deng, C.; Ge, B.; Yao, J.; Zhao, T.; Shen, C.; Zhang, Z.; Wang, T.; Guo, X.; Xue, N.; Guo, X.; et al. Surface engineering of TeOx modification on MoVTeNbO creates a high-performance catalyst for oxidation of toluene homologues to aldehydes. Chin. J. Catal. 2024, 66, 268–281. [Google Scholar] [CrossRef]
- Liu, A.; Kim, Y.-S.; Kim, M.G.; Reo, Y.; Zou, T.; Choi, T.; Bai, S.; Zhu, H.; Noh, Y.-Y. Selenium-alloyed tellurium oxide for amorphous p-channel transistors. Nature 2024, 629, 798–802. [Google Scholar] [CrossRef]
- Snodgrass, J.T.; Coe, J.V.; McHugh, K.M.; Freidhoff, C.B.; Bowen, K.H. Photoelectron spectroscopy of selenium- and tellurium-containing negative ions: SeO2−, Se2−, and Te2−. J. Phys. Chem. 1989, 93, 1249–1254. [Google Scholar] [CrossRef]
- Vasiliu, M.; Peterson, K.A.; Christe, K.O.; Dixon, D.A. Electronic Structure Predictions of the Energetic Properties of Tellurium Fluorides. Inorg. Chem. 2019, 58, 8279–8292. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, T.; Hirose, T.; Nagata, T. Electronic structures of (SO2)n− as studied by photoelectron spectroscopy. Int. J. Mass Spectrom. Ion Process. 1997, 171, 273–280. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Hiberty, P.C.; Shaik, S.; Gordon, M.S.; Danovich, D. Orbitals and the Interpretation of Photoelectron Spectroscopy and (e,2e) Ionization Experiments. Angew. Chem. Int. Ed. 2019, 58, 12332–12338. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.; Li, X.; Tang, P.; Yang, F.; Ma, J.; Hu, Z.; Sun, H.; Wang, X.-B.; Sun, Z.; et al. Exploring direct photodetachment and photodissociation–photodetachment dynamics of platinum iodide anions (PtIn−, n = 2–5) using cryogenic photoelectron spectroscopy. J. Chem. Phys. 2024, 161, 214305. [Google Scholar] [CrossRef]
- Chaibi, W.; Peláez, R.J.; Blondel, C.; Drag, C.; Delsart, C. Effect of a magnetic field in photodetachment microscopy. Eur. Phys. J. D 2010, 58, 29–37. [Google Scholar] [CrossRef]
- Quick, C.R.; Donahue, J.B.; Cohen, S.; Bryant, H.C.; Tang, C.Y.; Harris, P.G.; Mohagheghi, A.H.; Reeder, R.A.; Sharifian, H.; Toutounchi, H.; et al. Photodetachment of the H− ion. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1991, 56–57, 205–210. [Google Scholar] [CrossRef]
- Schulz, P.A.; Mead, R.D.; Jones, P.L.; Lineberger, W.C. OH− and OD− threshold photodetachment. J. Chem. Phys. 1982, 77, 1153–1165. [Google Scholar] [CrossRef]
- Tang, P.; Zhang, J.; Li, X.; Yang, F.; Zhao, Q.; Ma, J.; Hu, Z.; Sun, H.; Wang, X.-B.; Sun, Z.; et al. Cryogenic Photoelectron Spectroscopic and Theoretical Study of the Electronic and Geometric Structures of Undercoordinated Osmium Chloride Anions OsCln− (n = 3–5). J. Phys. Chem. A 2024, 128, 5500–5507. [Google Scholar] [CrossRef]
- Peláez, R.J.; Blondel, C.; Delsart, C.; Drag, C. Pulsed photodetachment microscopy and the electron affinity of iodine. J. Phys. B At. Mol. Opt. Phys. 2009, 42, 125001. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.-R.; Wang, X.-B. Examining the Critical Roles of Protons in Facilitating Oxidation of Chloride Ions by Permanganates: A Cluster Model Study. J. Phys. Chem. A 2015, 119, 6244–6251. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post-d group 16-18 elements. J. Chem. Phys. 2003, 119, 11113. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Clark, R.A.; McNamara, B.K.; Barinaga, C.J.; Peterson, J.M.; Govind, N.; Andersen, A.; Abrecht, D.G.; Schwantes, J.M.; Ballou, N.E. Electron Ionization Mass Spectrum of Tellurium Hexafluoride. Inorg. Chem. 2015, 54, 4821–4826. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Musiał, M. Coupled-cluster theory in quantum chemistry. Rev. Mod. Phys. 2007, 79, 291–352. [Google Scholar] [CrossRef]
- Bartlett, R.J. The coupled-cluster revolution. Mol. Phys. 2010, 108, 2905–2920. [Google Scholar] [CrossRef]
- Bartlett, R.J. Coupled-cluster theory and its equation-of-motion extensions. WIREs Comput. Mol. Sci. 2012, 2, 126–138. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Yu, H.S.; He, X.; Truhlar, D.G. MN15-L: A New Local Exchange-Correlation Functional for Kohn–Sham Density Functional Theory with Broad Accuracy for Atoms, Molecules, and Solids. J. Chem. Theory Comput. 2016, 12, 1280–1293. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615. [Google Scholar] [CrossRef]
- Furness, J.W.; Kaplan, A.D.; Ning, J.; Perdew, J.P.; Sun, J. Accurate and Numerically Efficient r2SCAN Meta-Generalized Gradient Approximation. J. Phys. Chem. Lett. 2020, 11, 8208–8215. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta–Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Shao, Y.; Gan, Z.; Epifanovsky, E.; Gilbert, A.T.B.; Wormit, M.; Kussmann, J.; Lange, A.W.; Behn, A.; Deng, J.; Feng, X.; et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 2015, 113, 184–215. [Google Scholar] [CrossRef]
- Gozem, S.; Krylov, A.I. The ezSpectra suite: An easy-to-use toolkit for spectroscopy modeling. WIREs Comput. Mol. Sci. 2022, 12, e1546. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
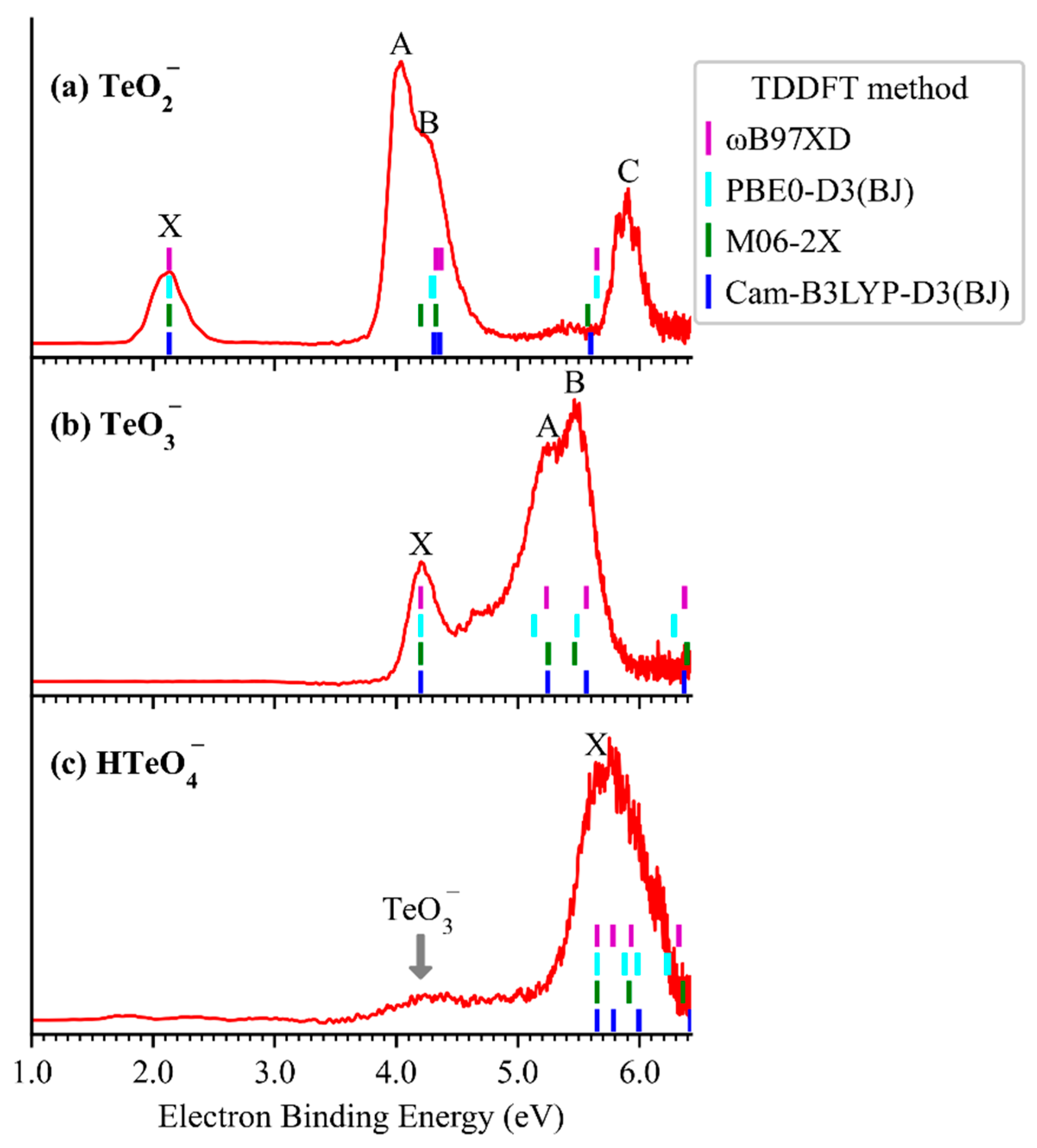
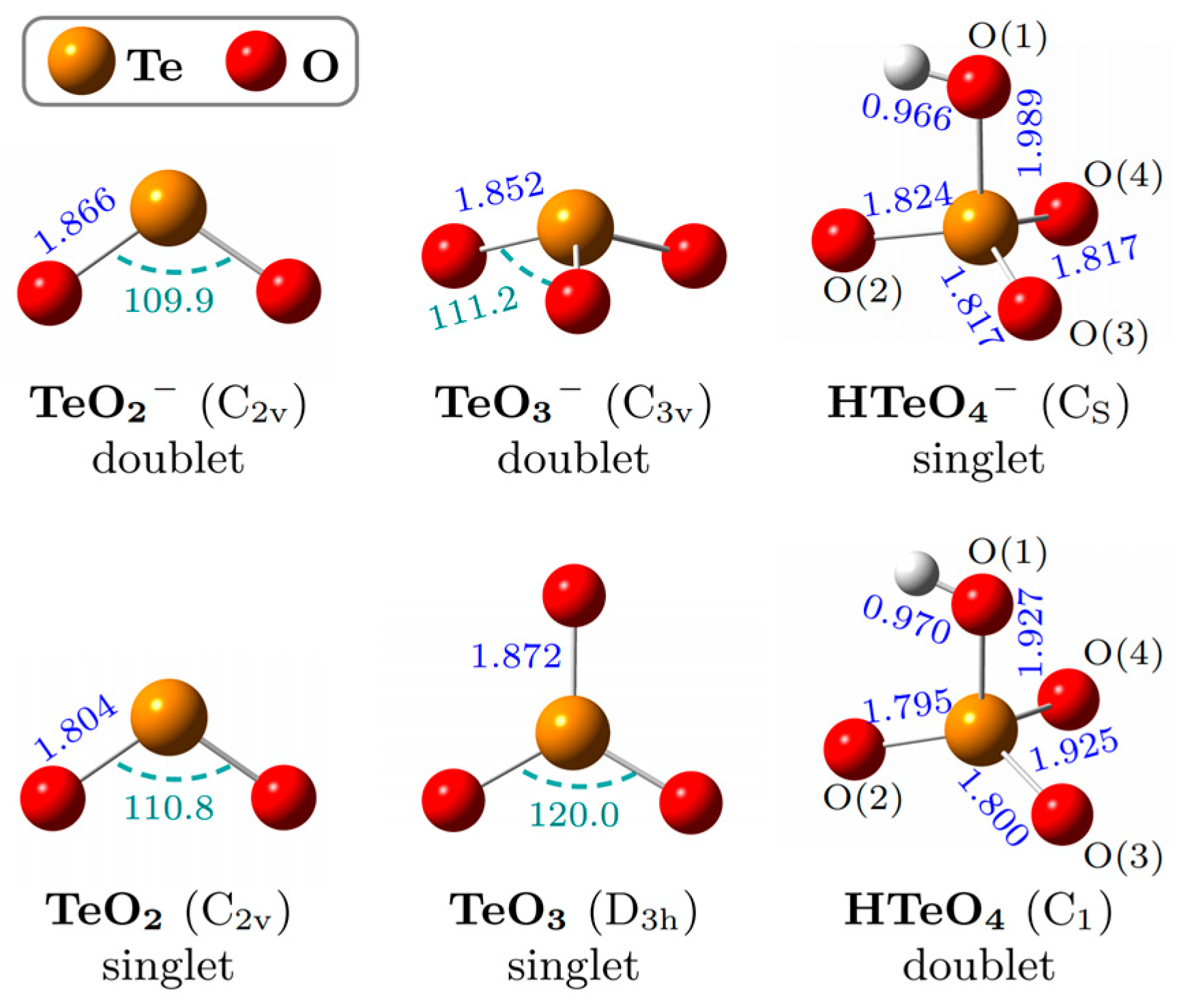
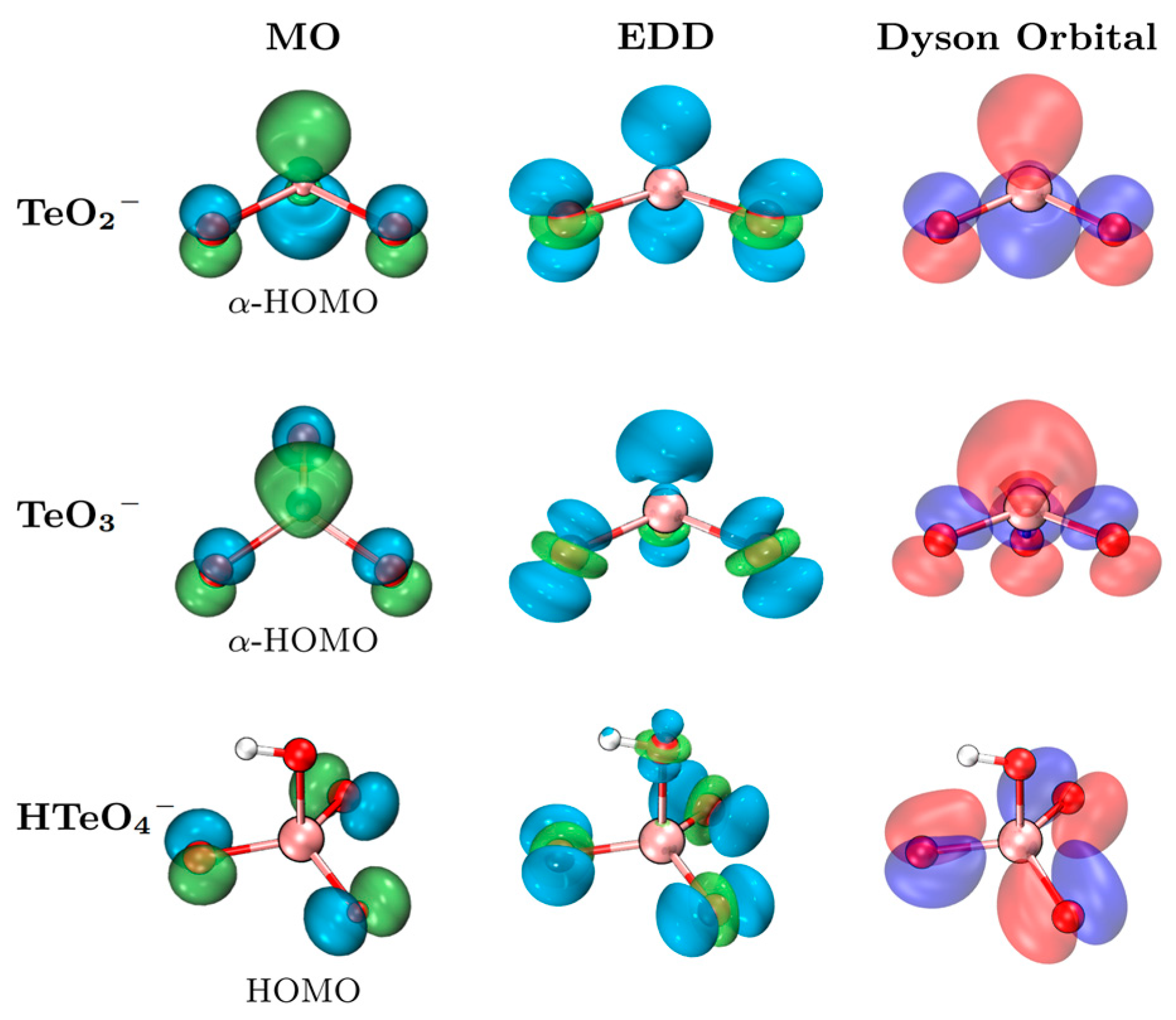
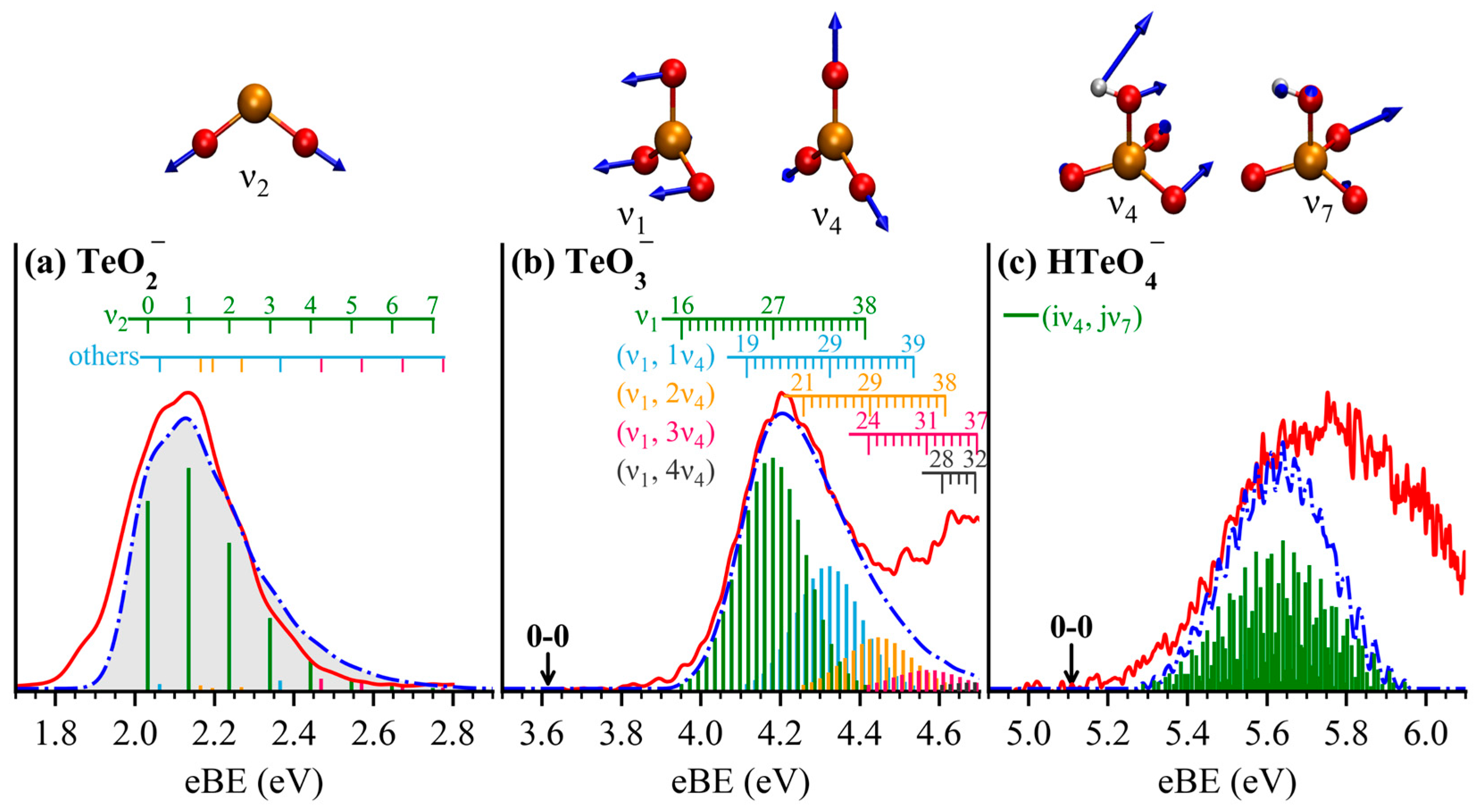
| VDE (eV) | ADE (eV) | |||||
|---|---|---|---|---|---|---|
| TeO2− | TeO3− | HTeO4− | TeO2− | TeO3− | HTeO4− | |
| Expt. | 2.13 | 4.20 | 5.64 | 1.94 | <4.01 a | 5.20 |
| B3LYP-D3(BJ) | 2.50 | 4.66 | 5.48 | 2.38 | 3.99 | 5.11 |
| MN15L-D3(BJ) | 2.23 | 4.29 | 5.34 | 2.06 | 3.52 | 5.00 |
| r2SCAN-3c | 2.14 | 4.32 | 4.96 | 2.09 | 3.68 | 4.77 |
| ωB97XD | 2.53 | 4.74 | 5.75 | 2.31 | 3.91 | 5.12 |
| PEB0-D3(BJ) | 2.53 | 4.68 | 5.34 | 2.33 | 3.87 | 5.00 |
| M06-2X | 2.77 | 4.96 | 6.14 | 2.50 | 3.97 | 5.27 |
| TPSSh-D3(BJ) | 2.34 | 4.43 | 5.17 | 2.23 | 3.75 | 4.88 |
| IP-EOM-CCSD | 2.36 | 4.48 | 5.76 | / | / | / |
| Anion | Neutral | Δ | ||
|---|---|---|---|---|
| TeO2− | Te | 0.00 | 0.76 | 0.76 |
| O | −0.50 × 2 a | −0.38 × 2 | 0.24 | |
| TeO3− | Te | 1.28 | 1.86 | 0.58 |
| O | −1.14 × 3 b | −0.93 × 3 | 0.42 | |
| HTeO4− | H | 0.47 | 0.49 | 0.02 |
| Te | 2.99 | 2.94 | −0.05 | |
| O(1) c | −1.04 | −0.97 | 0.07 | |
| O(2) | −1.16 | −0.86 | 0.30 | |
| O(3) | −1.13 | −0.80 | 0.33 | |
| O(4) | −1.13 | −0.80 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Li, X.; Tang, P.; Zhao, Q.; Zhang, J.; Mei, Y.; Hu, Z.; Sun, Z.; Yang, Y. Electronic Structures and Photodetachment of TeO2−, TeO3−, and HTeO4− Anions: A Cryogenic Photoelectron Spectroscopic Study. Molecules 2025, 30, 3757. https://doi.org/10.3390/molecules30183757
Yang F, Li X, Tang P, Zhao Q, Zhang J, Mei Y, Hu Z, Sun Z, Yang Y. Electronic Structures and Photodetachment of TeO2−, TeO3−, and HTeO4− Anions: A Cryogenic Photoelectron Spectroscopic Study. Molecules. 2025; 30(18):3757. https://doi.org/10.3390/molecules30183757
Chicago/Turabian StyleYang, Fan, Xueying Li, Peng Tang, Qixu Zhao, Jian Zhang, Ye Mei, Zhubin Hu, Zhenrong Sun, and Yan Yang. 2025. "Electronic Structures and Photodetachment of TeO2−, TeO3−, and HTeO4− Anions: A Cryogenic Photoelectron Spectroscopic Study" Molecules 30, no. 18: 3757. https://doi.org/10.3390/molecules30183757
APA StyleYang, F., Li, X., Tang, P., Zhao, Q., Zhang, J., Mei, Y., Hu, Z., Sun, Z., & Yang, Y. (2025). Electronic Structures and Photodetachment of TeO2−, TeO3−, and HTeO4− Anions: A Cryogenic Photoelectron Spectroscopic Study. Molecules, 30(18), 3757. https://doi.org/10.3390/molecules30183757







