Comparison of the Rate Constants of •OH, SO4•−, CO3•−, Cl2•−, Cl•, ClO• and H• Reactions with Organic Water Contaminants
Abstract
1. Introduction
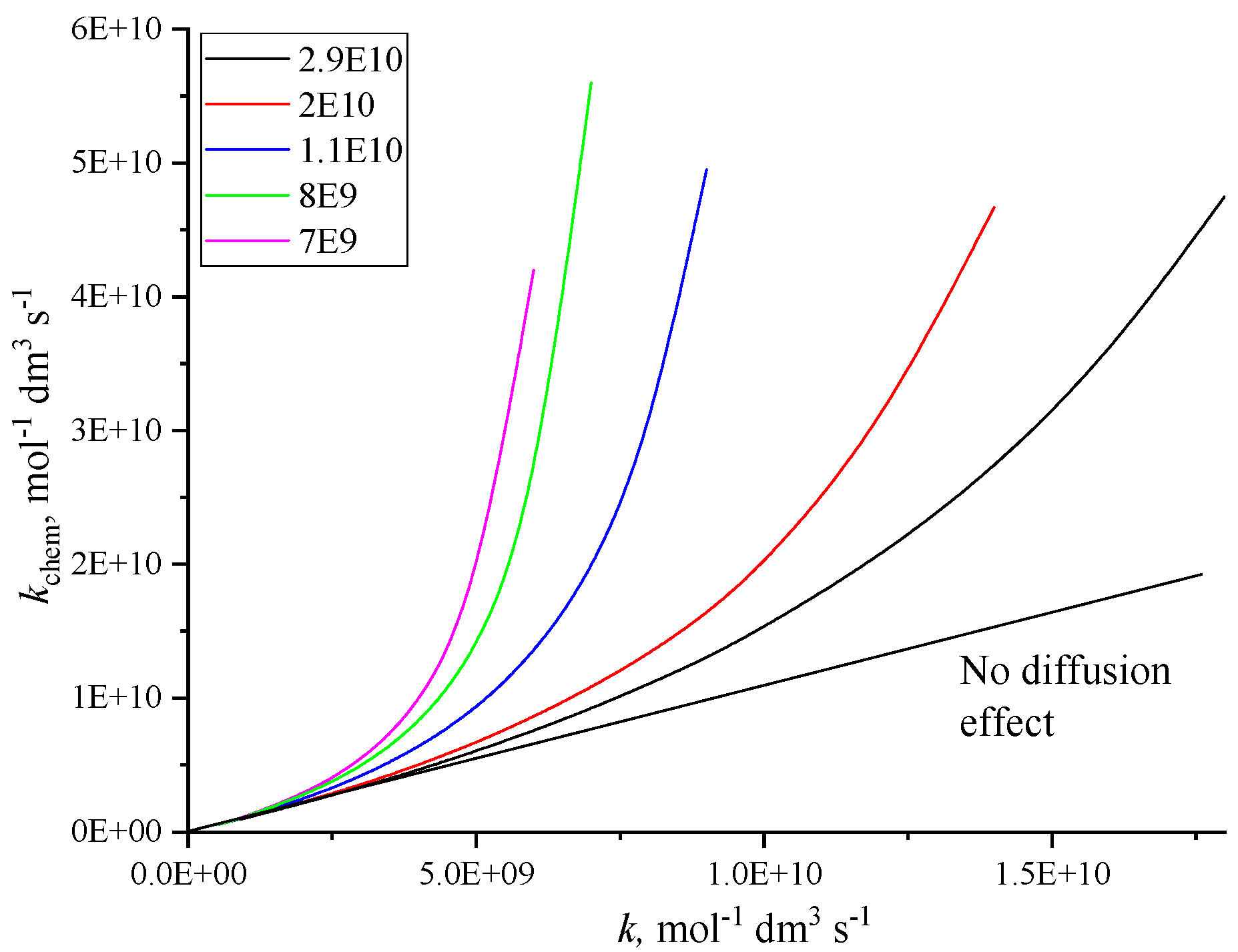
2. The Diffusion Controlled Rate Constant
3. Database
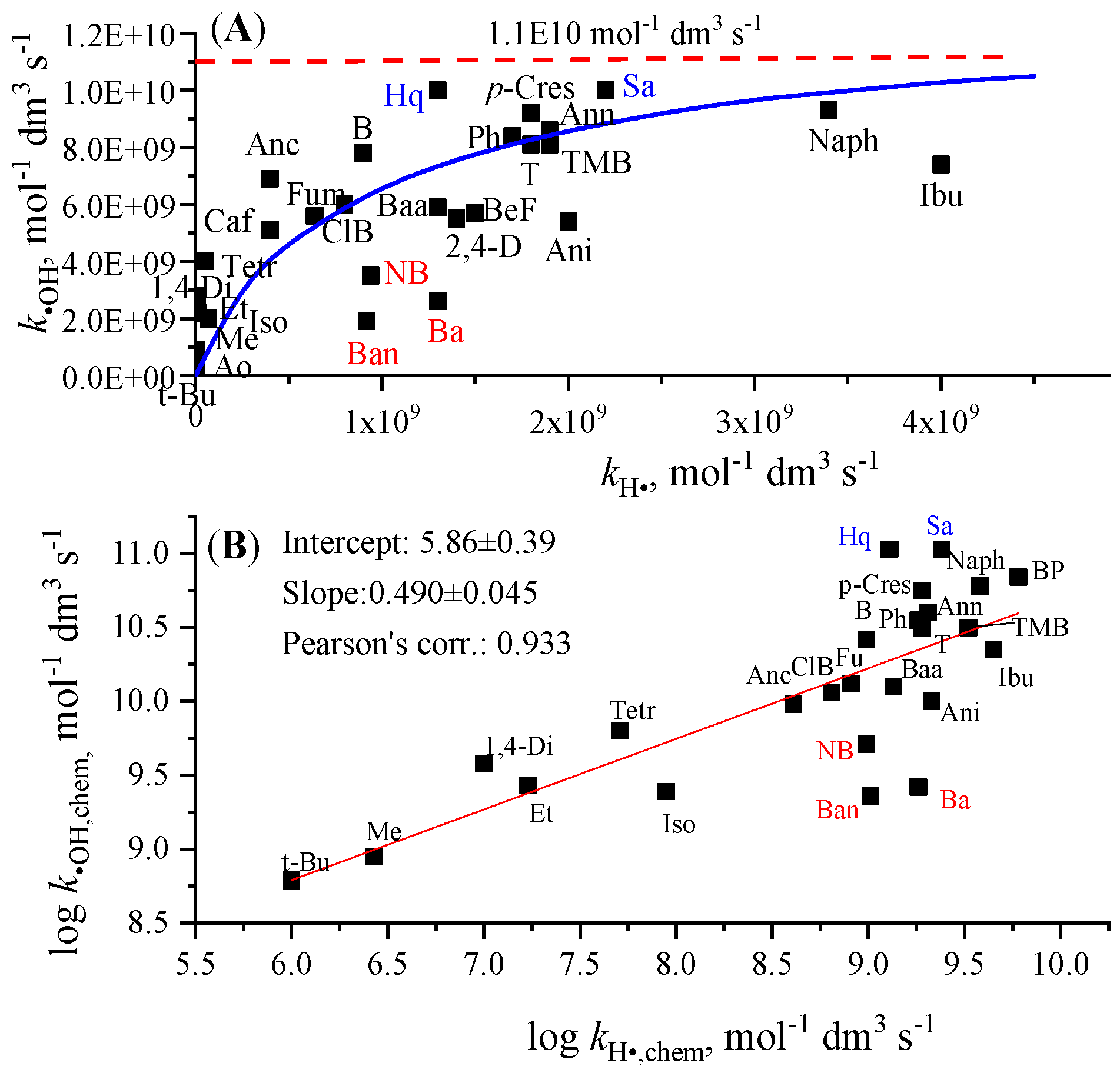
3.1. Connection Between Hydroxyl Radical and Hydrogen Atom Rate Constants
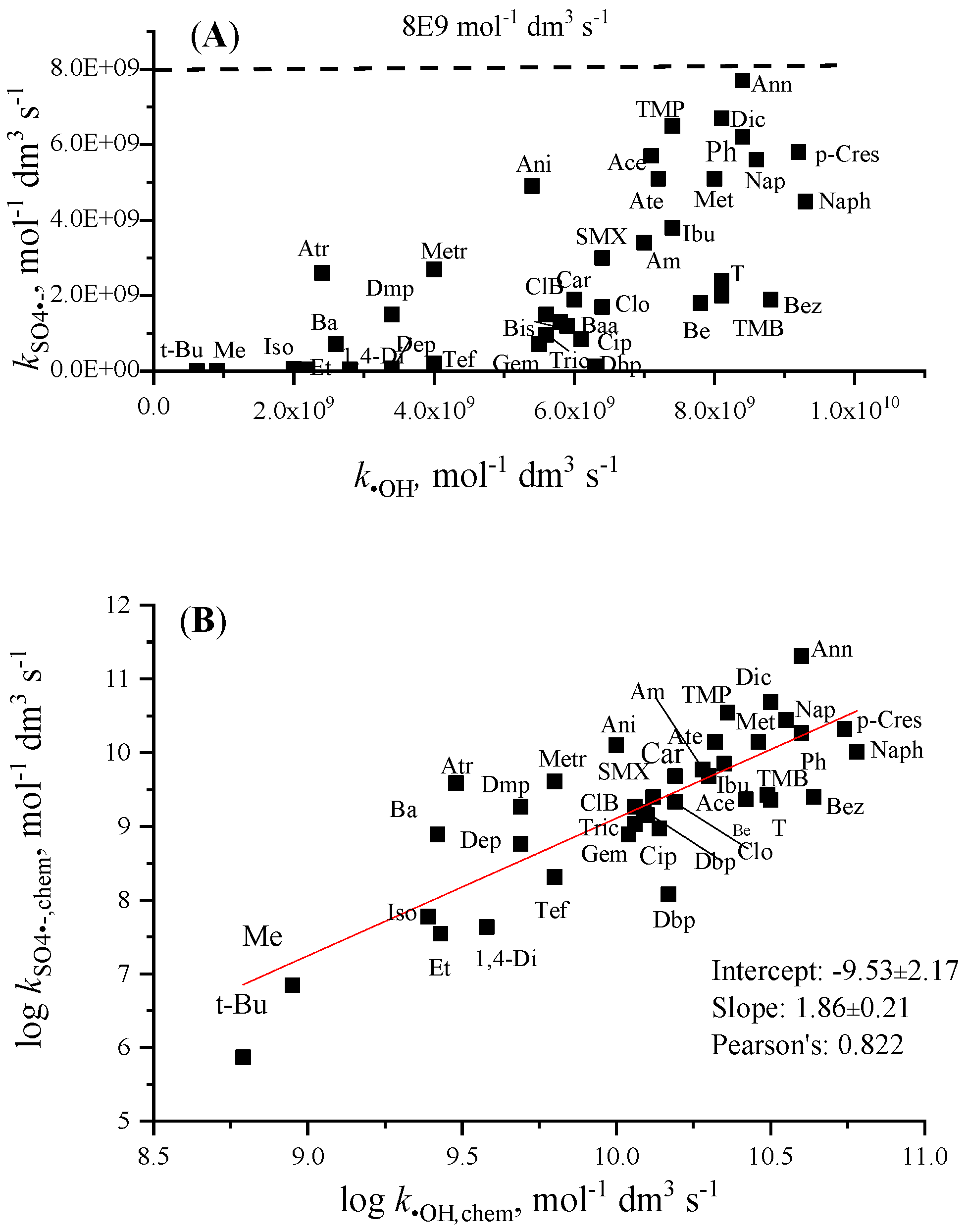
3.2. Rate Constants of Sulfate Radical Anion Reactions with Organic Molecules
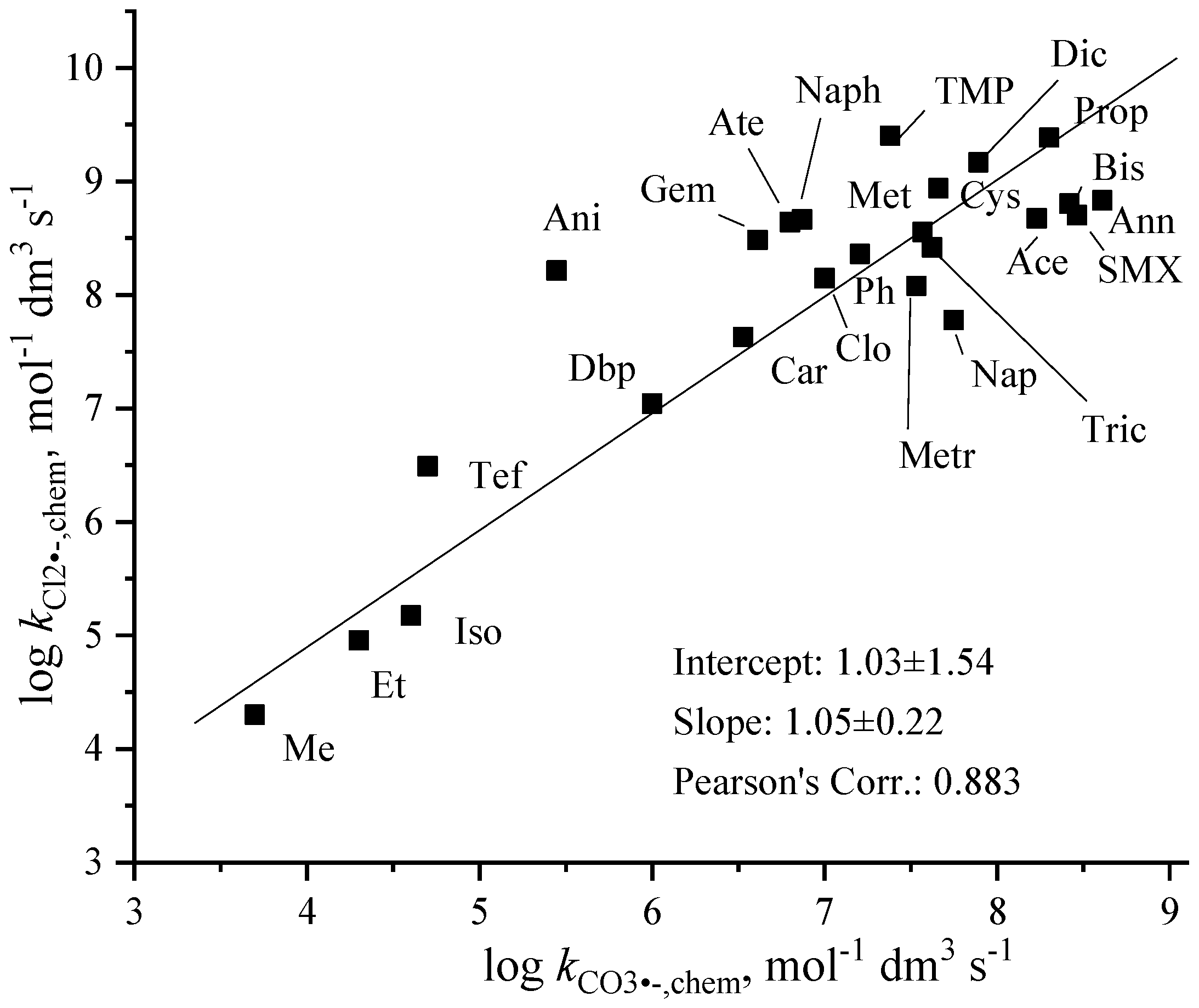
3.3. Correlation Between Carbonate Radical Anion and Dichloride Radical Anion Rate Constants
3.4. Chlorine Atom Reactions
3.5. Chlorine Monoxide Radical Reactions
4. Comparison of the Reactivities of Different Radicals, Connection with the Reduction Potentials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOP | Advanced Oxidation Processes |
| NHE | Normal hydrogen electrode |
| HAT | H-atom transfer |
| SET | Single electron transfer |
| RAF | Radical adduct formation |
References
- Stephan, M.I. (Ed.) Advanced Oxidation Processes for Water Treatment; IWA Publishing: London, UK, 2017; ISBN 97817804071280. [Google Scholar]
- Neta, P.; Huie, R.E.; Ross, B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Bobrowski, K. Radiation Chemistry of Liquid Systems. In Applications of Ionizing Radiation in Materials Processing; Sun, Y., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; Volume 1, pp. 82–117. [Google Scholar]
- Wojnárovits, L.; Tóth, T.; Takács, E. Rate constants of carbonate radical anion reactions with molecules of environmental interest in aqueous solution: A review. Sci. Total Environ. 2020, 137219. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate constants for the reactions of chloride monoxide radical (ClO•) and organic molecules of environmental interest. Water Sci. Technol. 2023, 87, 1925–1944. [Google Scholar] [CrossRef]
- Buxton, G.V.; Bydder, M.; Salmon, G.A.; Williams, J.E. The reactivity of chlorine atoms in aqueous solution. Part III. The reactions of Cl• with solutes. Phys. Chem. Chem. Phys. 2000, 2, 237–245. [Google Scholar] [CrossRef]
- NDRL/NIST, Solution Kinetics Database on the Web. Available online: https://kinetics.nist.gov/solution/ (accessed on 25 June 2025).
- Madden, K.P.; Mezyk, S.P. Critical review of aqueous solution reaction rate constants for hydrogen atoms. J. Phys. Chem. Ref. Data 2011, 40, 023103. [Google Scholar] [CrossRef]
- Neta, P.; Schuler, R.H. Rate constants for reaction of hydrogen atoms with aromatic and heterocyclic compounds. The electrophilic nature of hydrogen atoms. J. Am. Chem. Soc. 1972, 94, 1056–1059. [Google Scholar] [CrossRef]
- Homlok, R.; Mile, V.; Takács, E.; Járvás, G.; Góger, S.; Wojnárovits, L. Comparison of hydrogen atom and hydroxyl radical reactions with simple aromatic molecules in aqueous solution. Chem. Phys. 2020, 534, 110754. [Google Scholar] [CrossRef]
- Ashton, L.; Buxton, G.V.; Stuart, C.R. Temperature dependence of the rate of reaction of OH with some aromatic compounds in aqueous solution. Evidence for the formation of a π-complex intermediate? J. Chem. Soc. Faraday Trans. 1995, 91, 1631–1633. [Google Scholar] [CrossRef]
- Elliot, A.J.; McCracken, D.R.; Buxton, G.V.; Wood, N.D. Estimation of rate constants for near-diffusion-controlled reactions in water at high temperatures. J. Chem. Soc. Faraday Trans. 1990, 86, 1539–1547. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- North, A.M. The Collision Theory of Chemical Reactions in Liquids; Meuthen: London, UK, 1964. [Google Scholar]
- Wojnárovits, L.; Takács, E. Structure dependence of the rate coefficients of hydroxyl radical + aromatic molecule reaction. Radiat. Phys. Chem. 2013, 87, 82–87. [Google Scholar] [CrossRef]
- Rickman, K.A.; Mezyk, S.P. Kinetics and mechanism of sulfate radical oxidation of β-lactam antibiotics in water. Chemosphere 2010, 81, 359–365. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate constants of sulfate radical anion reactions with organic molecules: A review. Chemosphere 2019, 220, 1014–1032. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Radiation induced degradation of organic pollutants in waters and wastewaters. Top. Curr. Chem. 2016, 374, 50. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate constants of dichloride radical anion reactions with molecules of environmental interest in aqueous solution, a review. Environ. Sci. Pollut. Res. 2021, 28, 41552–44157. [Google Scholar] [CrossRef]
- Kazmierczak, L.; Szala-Bilnik, J.; Wolszczak, M.; Swiatla-Wojcik, D. Temperature dependence of the rate constant for hydrogen atom reaction with Cl2−• in water by pulse radiolysis of aqueous HCl solution. Radiat. Phys. Chem. 2015, 117, 7–11. [Google Scholar] [CrossRef]
- Lei, Y.; Cheng, S.; Luo, N.; Yang, X.; An, T. Rate constants and mechanisms of the reactions of Cl• and Cl2•− with trace organic contaminants. Environ. Sci. Technol. 2019, 53, 11170–11182. [Google Scholar] [CrossRef]
- Noyes, R.M. Effects of diffusion rates on chemical kinetics. In Progress in Reaction Kinetics; Porter, G., Ed.; Pergamon: London, UK, 1961; Volume 1, pp. 129–161. [Google Scholar]
- Wojnárovits, L.; Wang, J.; Chu, L.; Takács, E. Rate constants of chlorine atom reactions with organic molecules in aqueous solutions, an overview. Environmen. Sci. Pollut. Res. 2022, 29, 55492–55513. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E.; Dajka, K.; Emmi, S.S.; Russo, M.; D’Angelantonio, M. Re-evaluation of the rate coefficient for the H atom reaction tert-butanol in aqueous solution. Radiat. Phys. Chem. 2004, 69, 217–219. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate coefficients of hydroxyl radical reactions with pesticide molecules and related compounds: A review. Radiat. Phys. Chem. 2014, 96, 120–134. [Google Scholar] [CrossRef]
- Zeng, G.; Yang, R.; Zhou, Z.; Xu, Z.; Lyu, S. Comparative study of naphthalene removal in different radicals-dominated systems: Kinetics, degradation intermediates, and pathways. J. Water Process Eng. 2024, 57, 104659. [Google Scholar] [CrossRef]
- Dong, J.; Yang, P.; Kong, D.; Song, Y.; Lu, J. Formation of nitrated naphthalene in the sulfate radical oxidation process in the presence of nitrite. Water Res. 2024, 255, 121546. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Imren, C. Performance of the persulfate/UV-C process for the treatment of dimethyl phthalate from aquatic environments. Internat. J. Environ. Geoinf. 2018, 5, 189–196. [Google Scholar] [CrossRef]
- Lei, Y.; Luc, J.; Zhu, M.; Xie, J.; Peng, S.; Zhu., C. Radical chemistry of diethyl phthalate oxidation via UV/peroxymonosulfate process: Roles of primary and secondary radicals. Chem. Eng. J. 2020, 379, 122339. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, N.; An, N. Degradation kinetic of dibutyl phthalate (DBP) by sulfate radical- and hydroxyl radical-based advanced oxidation process in UV/persulfate system. Sep. Purif. Technol. 2018, 195, 92–100. [Google Scholar] [CrossRef]
- Mezyk, S.P. Determination of the rate constant for the reaction of hydroxyl and oxide radicals with cysteine in aqueous solution. Radiat. Res. 1996, 145, 102–106. Available online: https://www.jstor.org/stable/3579203 (accessed on 25 June 2025).
- Peller, J.R.; Mezyk, S.P.; Cooper, W.J. Bisphenol A reactions with hydroxyl radicals: Diverse pathways determined between deionized water and tertiary treated wastewater solutions. Res. Chem. Intermed. 2009, 35, 21–34. [Google Scholar] [CrossRef]
- Lin, Z.; Qin, W.; Sun, L.; Xiangjuan, Y.; Dongsheng, X. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of Bisphenol A in VUV/UV/peroxymonosulfate system. J. Water Process Eng. 2020, 38, 101636. [Google Scholar] [CrossRef]
- Illés, E.; Takács, E.; Dombi, A.; Gajda-Schrantz, K.; Rácz, G.; Gonter, K.; Wojnárovits, L. Hydroxyl radical induced degradation of ibuprofen. Sci. Total Environ. 2013, 447, 286–292. [Google Scholar] [CrossRef]
- Kovács, K.; Tóth, T.; Wojnárovits, L. Evaluation of advanced oxidation processes for ß-blockers degradation: A review. Water Sci. Technol. 2022, 85, 685–705. [Google Scholar] [CrossRef]
- Lian, L.S.; Yao, B.; Hou, S.D.; Fang, J.Y.; Yan, S.W.; Song, W.H. Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents. Environ. Sci. Technol. 2017, 51, 2954–2962. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Liu, Y.; Zhang, L.; Feng, L. Modelling study on the effects of chloride on the degradation of bezafibrate and carbamazepine in sulfate radical-based advanced oxidation processes: Conversion of reactive radicals. Chem. Eng. J. 2019, 358, 1332–1341. [Google Scholar] [CrossRef]
- Ayatollahi, S.; Kalnina, D.; Song, W.; Turks, M.; Cooper, W.J. Radiation chemistry of salicylic and methyl substituted salicylic acids: Models for the radiation chemistry of pharmaceutical compounds. Radiat. Phys. Chem. 2013, 92, 93–98. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E.; Tóth, T. Critical evaluation of rate coefficients for hydroxyl radical reactions with antibiotics: A review. Crit. Rev. Environ. Sci. Technol. 2018, 6, 575–613. [Google Scholar] [CrossRef]
- Szabó, L.; Tóth, T.; Takács, E.; Wojnárovits, L. One-electron oxidation of molecules with aromatic and thioether functions: Cl2•−/Br2•− and •OH induced oxidation of penicillins studied by pulse radiolysis. J. Photochem. Photobiol. A Chem. 2016, 326, 50–59. [Google Scholar] [CrossRef]
- Mandal, S. Reaction rate constants of hydroxyl radicals with micropollutants and their significance in advanced oxidation processes. J. Adv. Oxid. Technol. 2018, 21, 178–195. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.K.; Yoon, Y.; Im, J.K.; Kyung-Duk Zoh, K.-D. Kinetics and degradation mechanism of clofibric acid and diclofenac in UV photolysis and UV/H2O2 reaction. Desalination Water Treat. 2014, 52, 6211–6218. [Google Scholar] [CrossRef]
- Karpel Vel Leitner, N. Sulfate radical ion based AOPs. In Advanced Oxidation Processes for Water Treatment; Stefan, M.I., Ed.; IWA Publishing: London, UK, 2018; ISBN 97817804071280. [Google Scholar]
- Buxton, G.V. An overview of the radiation chemistry of liquids. In Radiation Chemistry: From Basics to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 3–16. [Google Scholar]
- Duan, X.; Niu, X.; Gao, J.; Wacławek, S.; Tang, L.; Dionysiou, D.D. Comparison of sulfate radical with other reactive species. Curr. Opin. Chem. Eng. 2022, 38, 100867. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, C.-G.; Park, S.-J.; Moon, J.-K.; Alvarez, P.J.J. pH-dependent contribution of chlorine monoxide radicals and byproducts formation during UV/chlorine treatment on clothianidin. Chem. Eng. J. 2022, 428, 132444. [Google Scholar] [CrossRef]
- Li, Y.; Teng, J.; Wu, J.; Zhang, S.; Zhao, Z.; Li, L. Mechanistic insights into carbonate radical-driven reactions: Selectivity and the hydrogen atom abstraction route. J. Hazard. Mater. 2025, 485, 136930. [Google Scholar] [CrossRef]
- Schuler, R.H.; Albarran, G.; Zajicek, J.; George, M.V.; Fessenden, R.W.; Carmichael, I. On the addition of •OH radicals to the ipso positions of alkyl-substituted aromatics: Production of 4-hydroxy-4-methyl-2,5-cyclohexadien-1-one in the radiolytic oxidation of p-cresol. J. Phys. Chem. A 2002, 106, 12178–12183. [Google Scholar] [CrossRef]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair: A Chemical Perspective; Springer: Heidelberg, Germany, 2006. [Google Scholar]
- Albarran, G.; Schuler, R.H. Hydroxyl radical as a probe of the charge distribution in aromatics: Phenol. J. Phys. Chem. A 2007, 111, 2507–2510. [Google Scholar] [CrossRef]
- Albarran, G.; Mendoza, E.; Schuler, R.H. Concerted effects of substituents in the reaction of •OH radicals with aromatics: The hydroxybenzaldehydes. Radiat. Phys. Chem. 2016, 124, 46–51. [Google Scholar] [CrossRef]
- Anbar, M.; Meyerstein, D.; Neta, P. Reactivity of aromatic compounds towards hydrogen atoms. Nature 1966, 209, 1348. [Google Scholar] [CrossRef]
- Neta, P. Reactions of hydrogen atoms in aqueous solutions. Chem. Rev. 1972, 72, 533–543. [Google Scholar] [CrossRef]
- Sharma, S.B.; Mudaliar, M.; Rao, B.S.M.; Mohan, H.; Mittal, J.P. Radiation chemical oxidation of benzaldehyde, acetophenone, and benzophenone. J. Phys. Chem. A 1997, 101, 8402–8408. [Google Scholar] [CrossRef]
- Geeta, S.; Sharma, S.B.; Rao, B.S.M.; Mohan, H.; Dhanya, S.; Mittal, J.P. Study of kinetics and absorption spectra of OH adducts of hydroxyl derivatives of benzaldehyde and acetophenone. J. Photochem. Photobiol. A Chem. 2001, 140, 99–107. [Google Scholar] [CrossRef]
- Krechkivska, O.; Wilcox, C.M.; Troy, T.P.; Nauta, K.; Chan, B.; Jacob, R.; Reid, S.A.; Radom, L.; Schmidt, T.W.; Kable, S.H. Hydrogen-atom attack on phenol and toluene is ortho-directed. Phys. Chem. Chem. Phys. 2016, 18, 8625. [Google Scholar] [CrossRef]
- Luo, Y.-Q. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Behnami, A.; Aghayani, E.; Benis, K.Z.; Sattari, M.; Pourakbar, M. Comparing the efficacy of various methods for sulfate radical generation for antibiotics degradation in synthetic wastewater: Degradation mechanism, kinetics study, and toxicity assessment. RSC Adv. 2022, 12, 14945. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, F.; Jia, Q.; Wang, Q. Norm index for predicting the rate constants of organic contaminants oxygenated with sulfate radical. Environ. Sci. Pollut. Res. 2020, 27, 974–982. [Google Scholar] [CrossRef]
- Steenken, S.; O’Neill, P.; Schulte-Frohlinde, D. Formation of radical zwitterions from methoxylated benzoic acids. 1. One electron oxidation by Tl2+, Ag2+, and SO4•−. J. Phys. Chem. 1977, 81, 26–30. [Google Scholar] [CrossRef]
- Paul, J.; Naik, D.B.; Bhardwaj, Y.K.; Varshney, L. An insight into the effective advanced oxidation process for treatment of simulated textile dye waste water. RSC Adv. 2014, 4, 39941–39947. [Google Scholar] [CrossRef]
- Buxton, G.V.; Salmon, G.A.; Williams, J.E. The reactivity of biogenic monoterpenes towards •OH and SO4•− radicals in de-oxygenated acidic solution. J. Atmos. Chem. 2000, 36, 111–134. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Shah, N.S.; Khan, H.M.; Hapeshi, E.; Fatta-Kassinos, D.; Dionysiou, D.D. Kinetic and mechanism investigation on the photochemical degradation of atrazine with activated H2O2, S2O82− and HSO5−. Chem. Eng. J. 2014, 252, 393–403. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Shah, N.S.; Sayed, M.; Khan, H.M.; Dionysiou, D.D. Degradation kinetics and mechanism of desethyl-atrazine and desisopropyl-atrazine in water with radical SO4•− based-AOPs. Chem. Eng. J. 2017, 325, 485–494. [Google Scholar] [CrossRef]
- Manoj, P.; Prasanthkumar, K.P.; Manoj, V.M.; Aravind, U.K.; Manojkumar, T.K.; Aravindakumar, C.T. Oxidation of substituted triazines by sulfate radical anion (SO4•−) in aqueous medium: A laser flash photolysis and steady state radiolysis study. J. Phys. Org. Chem. 2007, 20, 122–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Guo, K.; Wu, Z.; Wang, L.; Hua, Z.; Fang, J. Kinetics and pathways of the degradation of PPCPs by carbonate radicals in advanced oxidation processes. Water Res. 2020, 185, 116231. [Google Scholar] [CrossRef]
- Umschlag, T.; Herrmann, H. The carbonate radical (HCO3•/CO3•−) as a reactive intermediate in water chemistry: Kinetics and modelling. Acta Hydrochim. Hydrobio. 1999, 27, 214–222. [Google Scholar] [CrossRef]
- Dell’Arciprete, M.L.; Soler, J.M.; Santos-Juanes, L.; Arques, A.; Mártire, D.O.; Furlong, J.P.; Gonzalez, M.C. Reactivity of neonicotinoid insecticides with carbonate radicals. Water Res. 2012, 46, 3479–3489. [Google Scholar] [CrossRef]
- Buxton, G.V.; Bydder, M.; Salmon, G.A. Reactivity of chlorine atoms in aqueous solution Part 1. The equilibrium Cl−+Cl•− ↔ Cl2•−. J. Chem. Soc. Faraday Trans. 1998, 94, 653–657. [Google Scholar] [CrossRef]
- Wang, J.; Fan, S.; Xu, Z.; Gao, J.; Huang, Y.; Yu, X.; Gan, H. Kinetic and mechanistic insights into the degradation of clofibric acid in saline wastewater by Co2+/PMS process: A modeling and theoretical study. RSC Adv. 2022, 12, 16174–16183. [Google Scholar] [CrossRef]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; von Gunten, U. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. [Google Scholar] [CrossRef]
- Jasper, J.T.; Shafaat, O.S.; Hoffmann, M.R. Electrochemical transformation of trace organic contaminants in latrine wastewater. Environ. Sci. Technol. 2016, 50, 10198–10208. [Google Scholar] [CrossRef]
- Wols, B.A.; Harmsen, D.J.H.; Beerendonk, E.F.; Hofman-Caris, C.H.M. Predicting pharmaceutical degradation by UV (LP)/H2O2 processes: A kinetic model. Chem. Eng. J. 2014, 255, 334–343. [Google Scholar] [CrossRef]
- Hasegawa, K.; Neta, P. Rate constants and mechanisms of reaction of Cl2•− radicals. J. Phys. Chem. 1978, 82, 854–857. [Google Scholar] [CrossRef]
- Cornelius, K. Radiolytic Degradation of Organic Pesticides: A Pulse Radiolysis Study. Ph.D. Thesis, University of Adelaide, Adelaide, SA, Australia, July 1998. Available online: https://digital.library.adelaide.edu.au/dspace/handle/2440/19313 (accessed on 8 September 2025).
- Jacobi, H.-W.; Wicktor, F.; Herrmann, H.; Zellner, R. A laser flash photolysis kinetic study of reactions of the Cl2− radical anion with oxygenated hydrocarbons in aqueous solution. Internat. J. Chem. Kinet. 1999, 31, 169–181. [Google Scholar] [CrossRef]
- Jasper, J.T.; Sedlak, D.L. Phototransformation of wastewater-derived trace organic contaminants in open-water unit process treatment wetlands. Environ. Sci. Technol. 2013, 47, 10781–10790. [Google Scholar] [CrossRef]
- Elango, T.P.; Ramakrishnan, V.; Vancheesan, S.; Kuriacose, J.C. Reactions of the carbonate radical with aliphatic amines. Tetrahedron 1985, 41, 3837–3843. [Google Scholar] [CrossRef]
- Bonifacic, M.; Asmus, K.-D. Stabilization of oxidized sulphur centres by halide ions. Formation and properties of R2S∴X radicals in aqueous solutions. J. Chem. Soc. Perkin Trans. 1980, 2, 758–762. [Google Scholar] [CrossRef]
- Gawandi, V.B.; Mohan, H.; Mitttal, J.P. Investigations on the nature and redox properties of the transients formed on pulse radiolysis of aqueous solutions of 2-(phenylthio)ethanol. Phys. Chem. Chem. Phys. 1999, 1, 1919–1926. [Google Scholar] [CrossRef]
- Naik, D.B.; Mukherjee, T. Nature of the transient species formed in pulse radiolysis of n-allylthiourea in aqueous solutions. Res. Chem. Intermed. 2006, 32, 83–94. [Google Scholar] [CrossRef]
- Larson, R.A.; Zepp, R.G. Reactivity of the carbonate radical with aniline derivatives. Environ. Toxicol. Chem. 1988, 7, 265–274. [Google Scholar] [CrossRef]
- Moore, J.S.; Phillips, G.O.; Sosnowski, A. Reaction of the carbonate radical anion with substituted phenols. Internat. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 31, 603–605. [Google Scholar] [CrossRef]
- Alfassi, Z.B.; Huie, R.E.; Mosseri, S.; Neta, P. Kinetics of one-electron oxidation by the ClO radical. Radiat. Phys. Chem. 1988, 32, 85–88. [Google Scholar] [CrossRef]
- Alegre, M.L.; Gerones, M.; Rosso, J.A.; Bertolotti, S.G.; Braun, A.M.; Mártire, D.O.; Gonzalez, M.C. Kinetic study of the reactions of chlorine atoms and Cl2•− radical anions in aqueous solutions. 1. Reaction with benzene. J. Phys. Chem. A 2000, 104, 3117–3125. [Google Scholar] [CrossRef]
- Mártire, D.O.; Rosso, J.A.; Bertolotti, S.; Le Roux, G.C.; Braun, A.M.; Gonzalez, M.C. Kinetic study of the reactions of chlorine atoms and Cl2•− radical anions in aqueous solutions. II. Toluene, benzoic acid, and chlorobenzene. J. Phys. Chem. A 2001, 105, 5385–5392. [Google Scholar] [CrossRef]
- Wicktor, F.; Donati, A.; Herrmann, H.; Zellner, R. Laser based spectroscopic and kinetic investigations of reactions of the Cl atom with oxygenated hydrocarbons in aqueous solution. Phys. Chem. Chem. Phys. 2003, 5, 2562–2572. [Google Scholar] [CrossRef]
- Gilbert, B.C.; Stell, J.K.; Peet, W.J.; Radford, K.J. Generation and reactions of the chlorine atom in aqueous solution. J. Chem. Soc. Faraday Trans. 1988, 84, 3319–3340. [Google Scholar] [CrossRef]
- Qin, W.; Guo, K.; Chen, C.; Fang, J. Differences in the reaction mechanisms of chlorine atom and hydroxyl radical with organic compounds: From thermodynamics to kinetics. Environ. Sci. Technol. 2024, 58, 17886–17897. [Google Scholar] [CrossRef]
- Minakata, D.; Kamath, D.; Maetzold, S. Mechanistic insight into the reactivity of chlorine-derived radicals in the aqueous-phase UV–Chlorine advanced oxidation process: Quantum mechanical calculations. Environ. Sci. Technol. 2017, 51, 6918–6926. [Google Scholar] [CrossRef]
- Kong, Q.; Lei, X.; Zhang, X.; Cheng, S.; Xu, C.; Yang, B.; Yang, X. The role of chlorine oxide radical (ClO•) in the degradation of polychoro-1,3-butadienes in UV/chlorine treatment: Kinetics and mechanisms. Water Res. 2020, 183, 116056. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, W.; Sun, J.; Zhu, S.; Li, K.; Meng, X.; Luo, J.; Shi, Z.; Zhou, D.; Crittenden, J.C. Oxidation mechanisms of the UV/free chlorine process: Kinetic modeling and quantitative structure activity relationships. Environ. Sci. Technol. 2019, 53, 4335–4345. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, Z.; Wang, P.; Zhang, W.; Bu, L.; Wu, Y.; Dong, B.; Zhou, S. The role of carbonate radicals on the kinetics, radical chemistry, and energy requirement of UV/chlorine and UV/H2O2 processes. Chemosphere 2021, 278, 130499. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Fu, Y.; Ran, Z.; Crittenden, J.C.; Zhang, W.; Wang, H. Degradation of trimethoprim using the UV/Free chlorine process: Influencing factors and optimal operating conditions. Water 2021, 13, 1656. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Yan, S.; Yao, B.; Song, W.; Hua, Z.; Zhang, X.; Kong, X.; Li, X.; Fang, J. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements. Water Res. 2018, 147, 184–194. [Google Scholar] [CrossRef]
- Liu, H.; Hou, Z.; Li, Y.; Lei, Y.; Xu, Z.; Gu, J.; Tian, S. Modeling degradation kinetics of gemfibrozil and naproxen in the UV/chlorine system: Roles of reactive species and effects of water matrix. Water Res. 2021, 202, 117445. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Li, M.; Huo, Y.; Jiang, J.; Zhou, Y.; Jin, Z.; Xie, J.; Zhan, J.; He, M. The pH-dependent contributions of radical species during the removal of aromatic acids and bases in light/chlorine systems. Chem. Eng. J. 2022, 433 Pt 2, 133493. [Google Scholar] [CrossRef]
- Li, M.; Mei, Q.; Wei, B.; An, Z.; Sun, J.; Xie, J.; He, M. Mechanism and kinetics of ClO•-mediated degradation of aromatic compounds in aqueous solution: DFT and QSAR studies. Chem. Eng. J. 2021, 412, 128728. [Google Scholar] [CrossRef]
- Cai, W.-W.; Peng, T.; Yang, B.; Xu, C.; Liu, Y.-S.; Zhao, J.-L.; Gu, F.-L.; Ying, G.-G. Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: Experimental and theoretical studies. Chem. Eng. J. 2020, 402, 126224. [Google Scholar] [CrossRef]
- Li, M.; An, Z.; Huo, Y.; Jiang, J.; Zhou, Y.; Cao, H.; He, M. Simulation degradation of bromophenolic compounds in chlorine-based advanced oxidation processes: Mechanism, microscopic and apparent kinetics, and toxicity assessment. Chemosphere 2022, 291, 133034. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Xu, C.; Yang, L.; Yang, B.; Cai, W.-W.; Gu, F.; Ying, G.-G. Kinetics and mechanism of degradation of reactive radical-mediated probe compounds by the UV/Chlorine process: Theoretical calculation and experimental verification. ACS Omega 2022, 7, 5053–5063. [Google Scholar] [CrossRef] [PubMed]
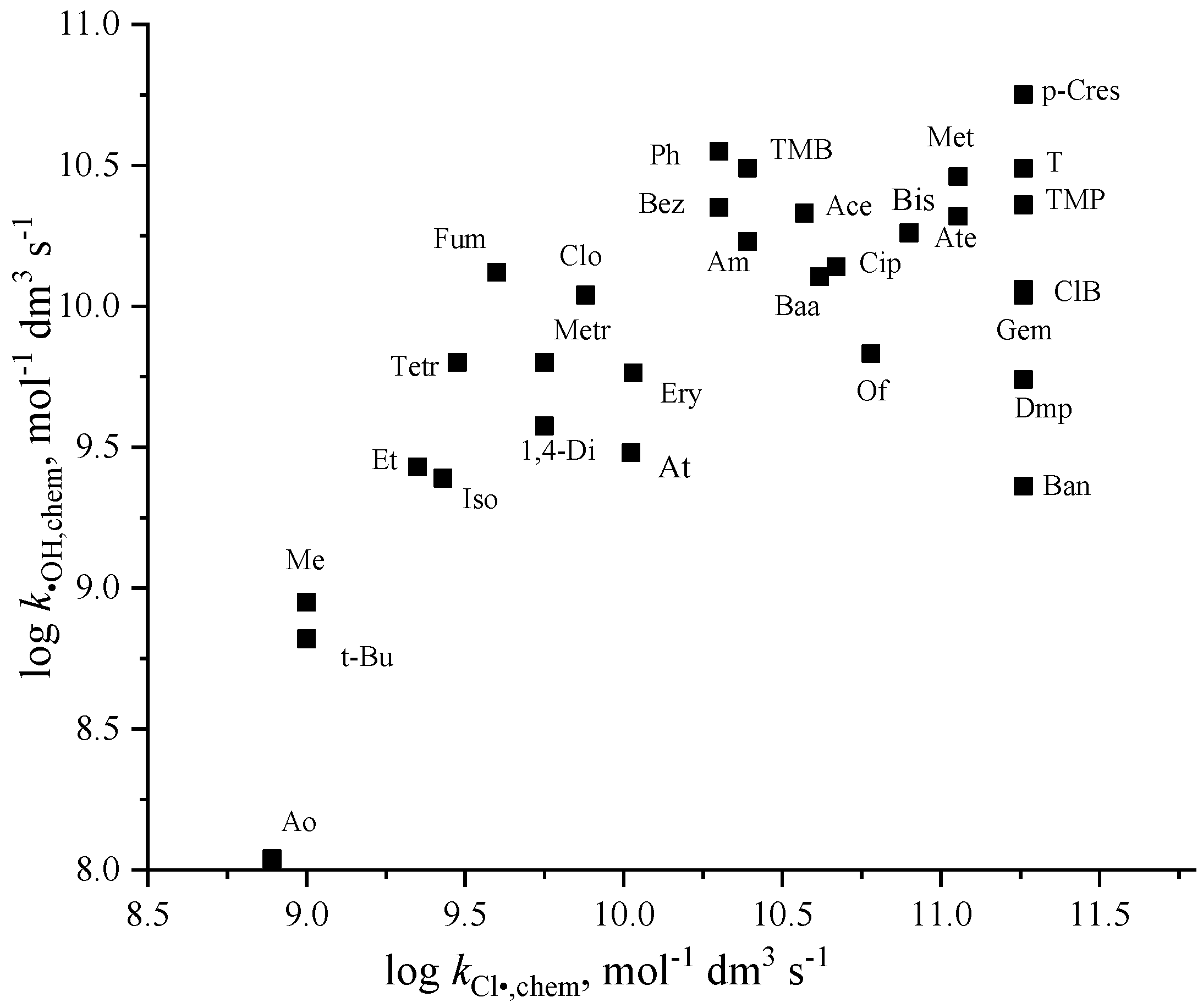
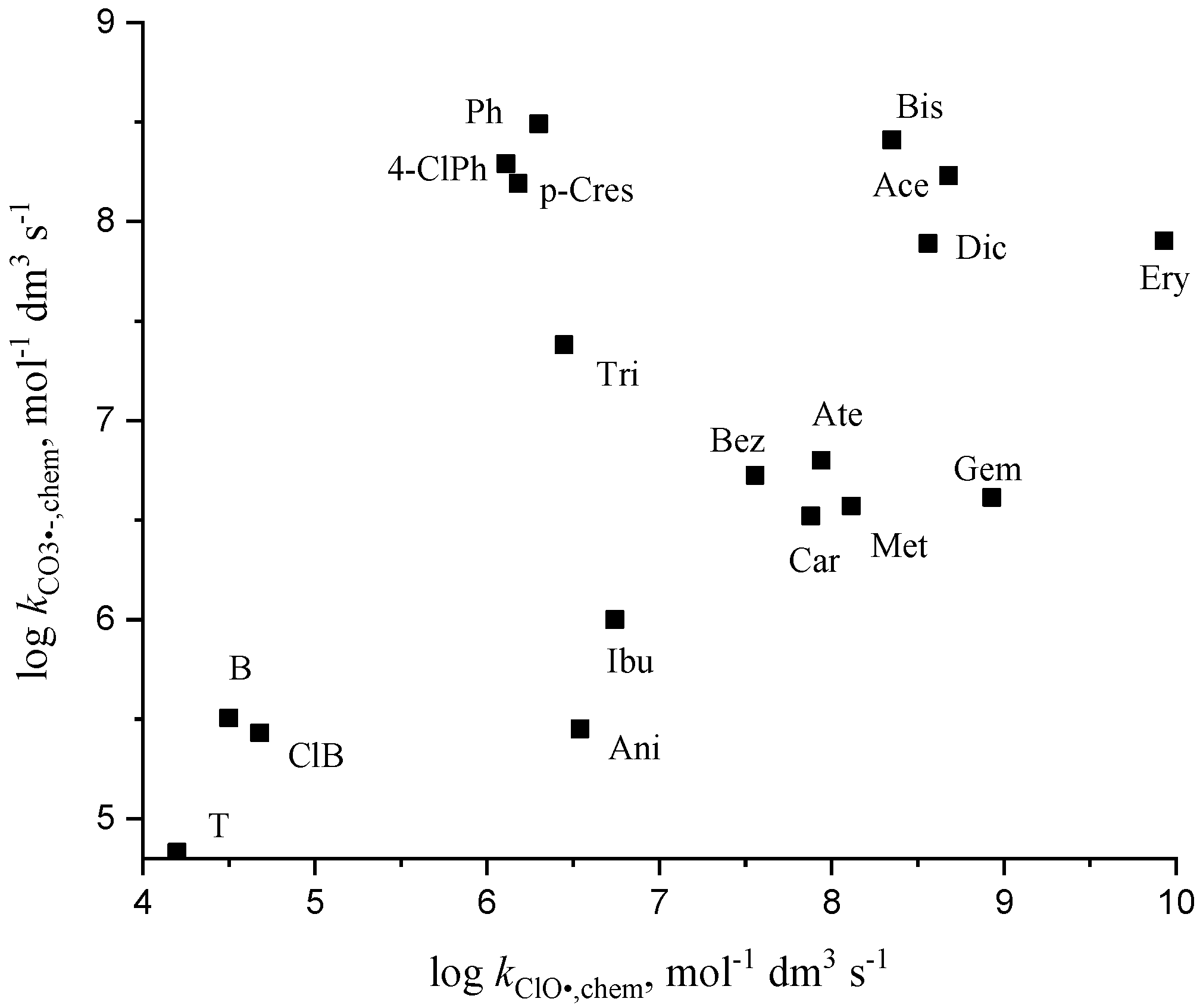
| Radical | Reduction potentials vs. NHE, V |
|---|---|
| •OH/OH− | 2.6 |
| SO4•−/SO42− | 2.43 |
| CO3•−, H+/HCO3−, pH 7 | 1.67 |
| Cl2•−/2Cl− | 2.1 |
| Cl•/Cl− | 2.6 |
| ClO•/ClO− | 1.39 |
| Haq+/H• | −2.9 |
| Radical | kdiff, mol−1 dm3 s−1 |
|---|---|
| •OH | 1.1 × 1010 |
| SO4•− | 8.0 × 109 |
| CO3•− | 7.3 × 109 |
| Cl2•− | 7.3 × 109 |
| Cl• | ~2 × 1010 |
| ClO• | ~1 × 1010 |
| H• | 2.9 × 1010 |
| Compound | •OH | SO4•− | CO3•− | Cl2•− | Cl• | ClO• | H• |
|---|---|---|---|---|---|---|---|
| Methanol (Me) | 9.0 × 108 a/ 8.95 | 7.0 × 106 a/ 6.84 | 5.0 × 103 a/ 3.70 | 2.0 × 104 b/ 4.30 | 1.0 × 109 c/ 9.00 | 2.7 × 106 a/ 6.43 | |
| Ethanol (Et) | 2.2 × 109 a/ 9.43 | 3.5 × 107 a/ 7.57 | 2.2 × 104 a/ 4.34 | 9.0 × 104 b/ 4.95 | 2.0 × 109 c/ 9.35 | 1.7 × 107 a/ 7.23 | |
| Isopropanol (Iso) | 2.0 × 109 a/ 9.39 | 6.0 × 107 a/ 7.78 | 4.0 × 104 a/ 4.60 | 1.6 × 105 b/ 5.20 | 2.4 × 109 c/ 9.43 | 7.0 × 107 a/ 7.85 | |
| tert-BuOH (t-Bu) | 6.2 × 108 a/ 8.82 | 7.4 × 105 a/ 5.87 | <1.6 × 102 a/ <2.20 | 2.6 × 104 b/ 4.42 | 1.0 × 109 c/ 9.01 | negl. d/ | 1.0 × 106 e/ 6.00 |
| Acetone (Ao) | 1.1 × 108 a/ 8.04 | 1.6 × 103 a/ 3.20 | 1.6 × 103 b/ 3.20 | 7.8 × 108 c/ 8.90 | 2.0 × 106 f/ 6.30 | ||
| 1,4-Dioxane (1,4-Di) | 2.8 × 109 a/ 9.58 | 4.3 × 107 a/ 7.64 | 3.3 × 106 b/ 6.52 | 4.4 × 109 c/ 9.75 | negl. d/ | 1.0 × 107 a/ 7.00 | |
| Tetrahydrofuran (Tetr) | 4.0 × 109 a/ 9.80 | 2.0 × 108 g/ 8.31 | 4.9 × 104 d/ 4.69 | 3.0 × 106 b/ 6.48 | 2.6 × 109 f/ 9.48 | 5.2 × 107 a/ 7.71 | |
| Benzene (Be) | 7.8 × 109 h/ 10.42 | 1.8 × 109 i/ 9.37 | 3.2 × 105 j/ 5.50 | <105 b/ <5.00 | 6.0 × 109 c/ 1.2 × 1010 | 3.2 × 104 d/ 4.50 | 9.0 × 108 k/ 8.97 |
| Naphthalene (Naph) | 9.3 × 109 l/ 10.78 | 4.5 × 109 m/ 10.01 | 7.4 × 106 l/ 6.87 | 4.6 × 108 l/ 8.69 | 3.4 × 109 a/ 9.58 | ||
| Toluene (T) | 8.1 × 109 h/ 10.49 | 2.4 × 109 i/ 9.53 | 6.8 × 104 j/ 4.83 | <106 i/ <6.00 | 1.8 × 1010 c/ 11.26 | 1.6 × 104 d/ 4.20 | 1.8 × 109 k/ 9.30 |
| Fluorobenzene (Bef) | 5.7 × 109 h/ 10.07 | 9.8 × 108 i/ 9.05 | 4.8 × 104 d/ 4.68 | 1.5 × 109 k/ 9.19 | |||
| Chlorobenzene (ClB) | 5.6 × 109 h/ 10.06 | 1.5 × 109 i/ 9.27 | 2.7 × 10 5 j/ 5.43 | <106 b/ <6.00 | 1.8 × 1010 c/ 11.26 | 4.8 × 104 d/ 4.68/ | 6.4 × 108 k/ 8.81 |
| Nitrobenzene (NB) | 3.5 × 109 h/ 9.71 | ≤106 i/ <6.00 | 1.4 × 104 j/ 4.14 | negl. b/ | 5.6 × 109 c/ 9.89 | 2.6 × 103 d/ 3.42 | 9.4 × 108 k/ 8.98 |
| Benzoic acid, neutral (Ban) | 1.9 × 109 h/ 9.361 | ≤106 e/ <6.00 | 1.8 × 1010 b/ 11.26 | 5.2 × 103 d/ 3.72 | 9.2 × 108 k/ 8.964 | ||
| Benzoic acid, anion (Baa) | 5.9 × 109 h/ 10.10 | 1.2 × 109 i/ 9.15 | 2 × 106 e/ 6.30 | 1.4 × 1010 c/ 10.62 | <3 × 106 d/ <6.47 | 1.3 × 109 a/ 9.13 | |
| Phenol (Ph) | 8.4 × 109 h/ 10.55 | 6.2 × 109 i/ 10.44 | 3.0 × 108 j/ 8.49 | 2.8 × 108 b/ 8.36 | 1.1 × 1010 c/ 10.301 | 8.0 × 106 d/ 6.30 | 1.7 × 109 k/ 9.26 |
| Aniline, cation (Anc) | 5.1 × 109 h/ 9.98 | 1.2 × 107 e/ 7.08 | 3.3 × 105 d/ 5.52 | 4.0 × 108 a/ 8.60 | |||
| Aniline, neutral (Ann) | 8.6 × 109 h/ 10.60 | 7.7 × 109 i/ 11.31 | 5.0 × 108 a/ 8.73 | 6.8 × 108 b/ 8.88 | 2.7 × 1010 c/ | 1.1 × 1010 d/ 10.39 | 1.9 × 109 k/ 9.31 |
| 1,3,5-Trimethoxybenzene (TMB) | 8.1 × 109 a/ 10.49 | 2.0 × 109 i/ 9.43 | 2.7 × 109 b/ 9.63 | 1.1 × 1010 c/ 10.39 | 1.4 × 109 d/ 9.21 | ~3.0 × 109 a/ 9.52 | |
| Fumaric acid, neutral (Fum) | 6.0 × 109 a/ 10.12 | 1.2 × 105 b/ 5.08 | ~3.3 × 109 c/ 9.60 | 8.0 × 108 a/ 8.91 | |||
| Anisole (Ani) | 5.4 × 109 a/ 10.00 | 4.9 × 109 i/ 10.10 | 2.8 × 105 j/ 5.45 | 1.6 × 108 b/ 8.21 | 3.3 × 106 d/ 6.54 | 2.0 × 109 a/ 9.33 | |
| p-Cresol (p-Cres) | 9.2 × 109 h/ 10.75 | 5.8 × 109 i/ 10.32 | 1.5 × 108 j/ 8.19 | 1.8 × 1010 c/ 11.26 | 1.5 × 106 d/ 6.18 | 1.8 × 109 a/ 9.28 | |
| Benzaldehyde (Ba) | 2.6 × 109 h/ 9.53 | 7.1 × 108 i/ 8.89 | 3.2 × 105 d/ 5.50 | 1.4 × 109 a/ 9.14 | |||
| Catechol, neutral (Catn) | 1.1 × 1010 a/ | 5.7 × 108 b/ 8.79 | 2.8 × 1010 c/ | 1.0 × 107 d/ 7.00 | |||
| Resorcinol, neutral (Rn) | 1.2 × 1010 a/ | 1.4 × 1010 c/ 10.67 | 1.0 × 107 d/ 7.00 | ||||
| Hydroquinone, neutral (Hq) | 1.0 × 1010 h/ 11.04 | 2.3 × 109 j/ 9.53 | 1.2 × 109 b/ 9.16 | 1.0 × 107 d/ 7.00 | 1.3 × 109 a/ 9.14 | ||
| 4-Chlorophenol, neutral (4-ClPh) | 7.6 × 109 a/ 10.39 | 1.9 × 108 j/ 8.29 | 1.3 × 106 d/ 6.11 | ||||
| p-Aminophenol, cation (p-Am) | 4.0 × 109 b/ 9.95 | 8.0 × 105 d/ 5.90 | |||||
| Dimethyl phthalate (Dmp) | 3.7 × 109 n,o/ 9.74 | 4.9 × 108 o/ 9.27 | <1 × 106 o/ <6.00 | 1.4 × 107 o/ 7.15 | 1.8 × 1010 o/ 11.26 | ||
| Diethyl phthalate (Dep) | 3.4 × 109 o/ 9.83 | 5.4 × 108 o/ 8.71 | <1 × 106 o/ <6.00 | 1.1 × 107 o/ 7.04 | 2.0 × 1010 o/ | ||
| Dibutyl phthalate (Dbp) | 6.3 × 10 o,p/ 10.17 | 5.5 × 108 o/ 8.77 | 1.0 × 106 o/ 6.00 | 1.1 × 107 p/ 7.04 | 2.0 × 1010 o/ | ||
| Cysteine (Cys) | 5.4 × 109 r/ 10.01 | 2.1 × 108 a/ 8.33 | 8.5 × 108 a/ 8.98 | ||||
| Bisphenol (Bis) | 6.9 × 109 s/ 10.26 | 4.5 × 109 t/ 9.88 | 2.5 × 108 j/ 8.41 | 5.8 × 108 b/ 8.80 | 1.6 × 1010 i/ 10.90 | 2.2 × 108 d/ 8.35 |
| Compound | •OH | SO4•− | CO3•− | Cl2•− | Cl• | ClO• | H• |
|---|---|---|---|---|---|---|---|
| Ibuprofen (Ibu) | 7.4 × 109 a/ 10.36 | 3.8 × 109 b/ 9.85 | 1.2 × 106 c/ 6.08 | <5 × 106 d/ <6.70 | 2.0 × 1010 e/ | 5.5 × 106 f/ 6.74 | 4.0 × 109 a/ 9.66 |
| Ketoprofen (Ket) | 4.6 × 109 g/ 9.90 | 3.9 × 108 c/ 8.60 | |||||
| Diclofenac (Dic) | 8.1 × 109 g/ 10.49 | 6.7 × 109 b/ 10.61 | 7.8 × 107 c/ 7.89 | 1.2 × 109 d/ 9.16 | 3.8 × 1010 e/ | 3.5 × 108 f/ 8.56 | |
| Acetaminofen (Ace) | 7.1 × 109 g/ 10.30 | 3.0 × 109 b/ 9.68 | 1.7 × 108 c/ 8.23 | 4.4 × 108 d/ 8.67 | 1.3 × 1010 e/ 10.57 | 4.6 × 108 f/ 8.69 | |
| Trimethoprim (TMP) | 7.4 × 109 g/ 10.36 | 6.5 × 109 b/ 10.54 | 2.4 × 107 c/ 7.38 | 1.9 × 109 d/ 9.41 | 1.8 × 1010 e/ 11.26 | 2.8 × 106 f/ 6.45 | |
| Atenolol (Ate) | 7.2 × 109 h/ 10.32 | 5.1 × 109 h/ 10.15 | 6.3 × 106 h/ 6.80 | 4.1 × 108 h/ 8.64 | 1.7 × 1010 h/ 11.05 | 8.7 × 107 h/ 7.94 | |
| Propranolol (Pro) | 1.1 × 1010 h/ | 4.8 × 109 h/ 10.08 | 2.0 × 108 h/ 8.31 | 1.8 × 109 h/ 9.38 | |||
| Metoprolol (Met) | 8.0 × 109 h/ 10.46 | 5.1 × 109 h/ 10.15 | 3.7 × 106 h/ 6.57 | 3.6 × 108 h/ 8.57 | 1.7 × 1010 h/ 11.05 | 1.3 × 108 h/ 8.12 | |
| Gemfibrozil (Gem) | 5.5 × 109 i/ 10.04 | 7.1 × 108 b/ 8.89 | 4.1 × 106 c/ 6.61 | 2.9 × 108 d/ 8.48 | 1.8 × 1010 e/ 11.26 | 7.7 × 108 f/ 8.93 | |
| Carbamazepine (Car) | 6.0 × 109 j/ 10.12 | 1.9 × 109 b/ 9.40 | 3.3 × 106 c/ 6.52 | 4.3 × 107 d/ 7.63 | 3.3 × 1010 e/ | 7.6 × 107 f/ 7.88 | |
| Salicyclic acid (Sa) | 1.0 × 1010 l/ 11.03 | 1.6 × 109 b/ 9.30 | 2.1 × 108 d/ 8.33 | 2.2 × 109 l/ 9.38 | |||
| Amoxicillin (Am) | 6.7 × 109 m/ 10.23 | 3.4 × 109 b/ 9.77 | 1.6 × 109 n/ 9.31 | 1.1 × 1010 e/ 10.39 | |||
| Ciprofloxacin (Cip) | 6.1 × 109 m/ 10.14 | 8.4 × 108 b/ 8.97 | 2.2 × 108 d/ 8.35 | 1.4 × 1010 e/ 10.67 | |||
| Ofloxacin (Of) | 4.2 × 109 m/ 9.83 | 3.5 × 108 d/ 8.57 | 1.5 × 1010 e/ 10.78 | ||||
| Tetracycline (Tet) | 6.5 × 109 m/ 10.20 | 1.2 × 109 d/ 9.16 | 2.0 × 1010 e/ | 8.9 × 108 k/ 8.96 | |||
| Sulfamethoxazole (SMX) | 6.4 × 109 m/ 10.19 | 3.0 × 109 b/ 9.68 | 2.8 × 108 c/ 8.46 | 4.7 × 108 d/ 8.70 | 3.5 × 1010 e/ | <2 × 109 f/ <9.44 | |
| Naproxen (Nap) | 8.6 × 109 o/ 10.60 | 5.6 × 109 b/ 10.27 | 5.6 × 107 c/ 7.75 | 6.6 × 108 d/ 8.86 | 2 × 1010 e/ | <5.7 × 109 f/ <10.41 | |
| Metronidazole (Metr) | 4.0 × 109 m/ 9.80 | 2.7 × 109 b/ 9.61 | 3.4 × 107 c/ 7.53 | 1.2 × 108 d/ 8.08 | 4.4 × 109 e/ 9.75 | <1 × 106 f/ <6.00 | |
| Erythromicin (Ery) | 3.9 × 109 m/ 9.78 | 8 × 107 c/ 7.90 | 7.0 × 109 e/ 10.03 | 4.6 × 109 f/ 10.09 | |||
| Bezafibrate (Bez) | 7.4 × 109 j/ 10.35 | 1.9 × 109 j/ 9.39 | 5.3 × 106 c/ 6.72 | 1.0 × 1010 e/ 10.30 | 3.6 × 107 f/ 7.56 | ||
| Clofibric acid (Clo) | 5.5 × 109 r/ 10.04 | 1.7 × 109 b/ 9.33 | 1.0 × 107 c/ 7.00 | 1.4 × 108 d/ 8.15 | 5.5 × 109 e/ 9.88 | ||
| 2,4-Dichloro- phenoxyacetic acid, (2,4-D) | 5.5 × 109 g/ 10.04 | 1.4 × 109 k/ 9.17 | |||||
| Triclosan (Tric) | 5.6 × 109 m/ 10.06 | 9.6 × 108 b/ 9.03 | 4.2 × 107 c/ 7.62 | 2.5 × 108 d/ 8.41 | 2.8 × 1010 e/ | ||
| Atrazine (At) | 2.4 × 109 p/ 9.487 | 2.6 × 109 b/ 9.58 | 4.0 × 106 d/ 6.60 | 5.0 × 104 r/ 4.70 | 6.9 × 109 e/ 10.02 | <106 f/ <6.00 | |
| Caffeine (Caf) | 4.0 × 109 o/ 9.80 | 9.3 × 108 d/ 9.03 | 1.5 × 1010 e/ 10.78 | 1.4 × 109 f/ 9.24 | 4.0 × 108 k/ 8.61 |
| Compound | •OH | SO4•− | CO3•− | Cl2•− | Cl• | ClO• | H• |
|---|---|---|---|---|---|---|---|
| k, small alcohols | 6 × 108–2 × 109 | 106–108 | 103–104 | 104–105 | 109–2 × 109 | negl. | 106–107 |
| k, simple aromatics | 2 × 109–8 × 109 | 108–8 × 109 | 104–4 × 108 | ≤106–2 × 109 | 1010–2 × 1010 | <104–109 | 4 × 108–2 × 109 |
| Selectivity | aromatics | sulfites, amines | sulfites | amines, sulfites, phenols, methoxyben-zenes | aromatics | ||
| Mechanism | RAF/HAT | RAF/HAT | RAF/HAT/SET | SET/HAT | SET/RAF | RAF | RAF/HAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnárovits, L.; Takács, E. Comparison of the Rate Constants of •OH, SO4•−, CO3•−, Cl2•−, Cl•, ClO• and H• Reactions with Organic Water Contaminants. Molecules 2025, 30, 3741. https://doi.org/10.3390/molecules30183741
Wojnárovits L, Takács E. Comparison of the Rate Constants of •OH, SO4•−, CO3•−, Cl2•−, Cl•, ClO• and H• Reactions with Organic Water Contaminants. Molecules. 2025; 30(18):3741. https://doi.org/10.3390/molecules30183741
Chicago/Turabian StyleWojnárovits, László, and Erzsébet Takács. 2025. "Comparison of the Rate Constants of •OH, SO4•−, CO3•−, Cl2•−, Cl•, ClO• and H• Reactions with Organic Water Contaminants" Molecules 30, no. 18: 3741. https://doi.org/10.3390/molecules30183741
APA StyleWojnárovits, L., & Takács, E. (2025). Comparison of the Rate Constants of •OH, SO4•−, CO3•−, Cl2•−, Cl•, ClO• and H• Reactions with Organic Water Contaminants. Molecules, 30(18), 3741. https://doi.org/10.3390/molecules30183741






