Synthesis and Characterization of Cholesterol-Based Liquid Crystals Linked with Perfluorinated Alkyl Chains
Abstract
1. Introduction
2. Results and Discussion
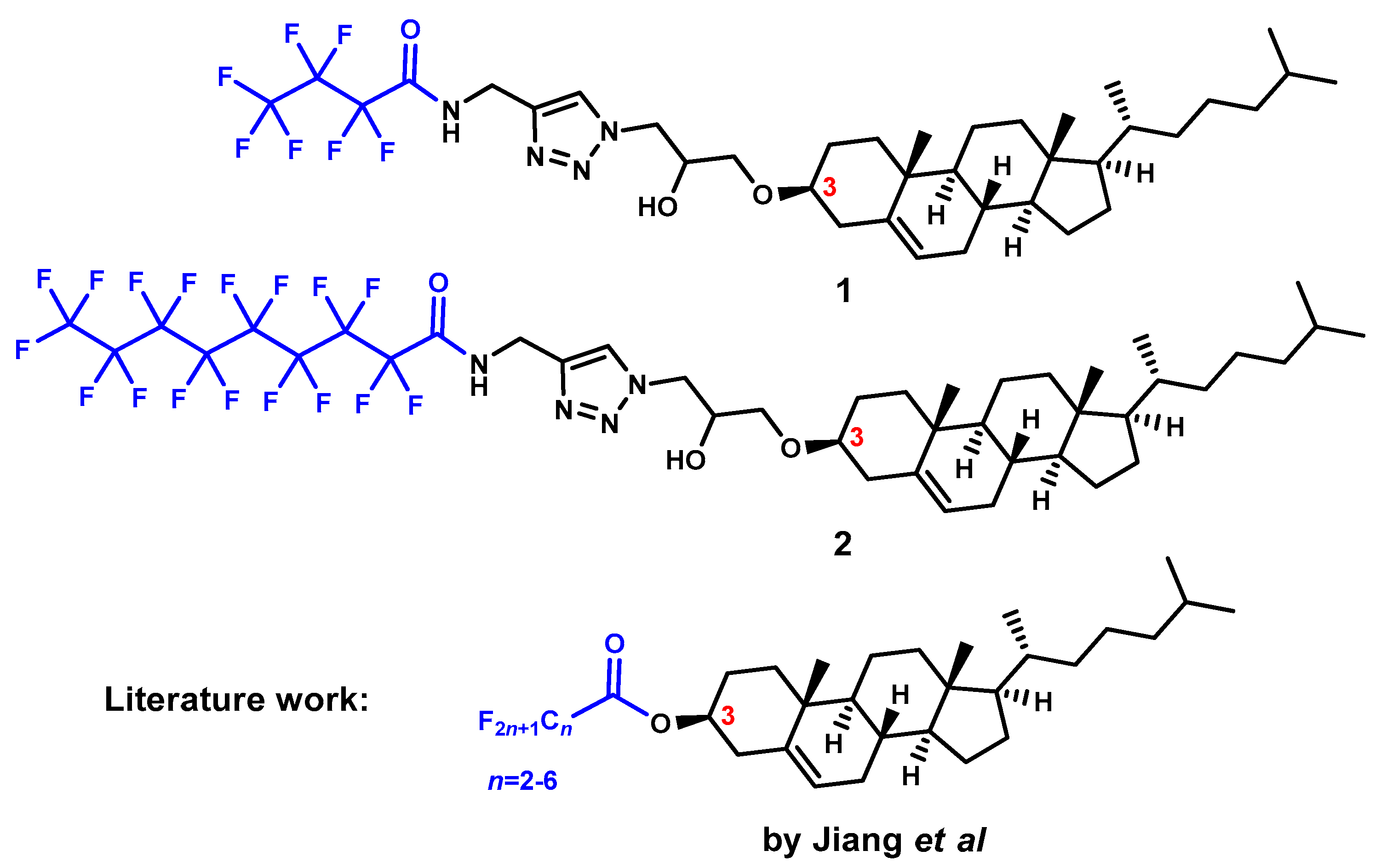
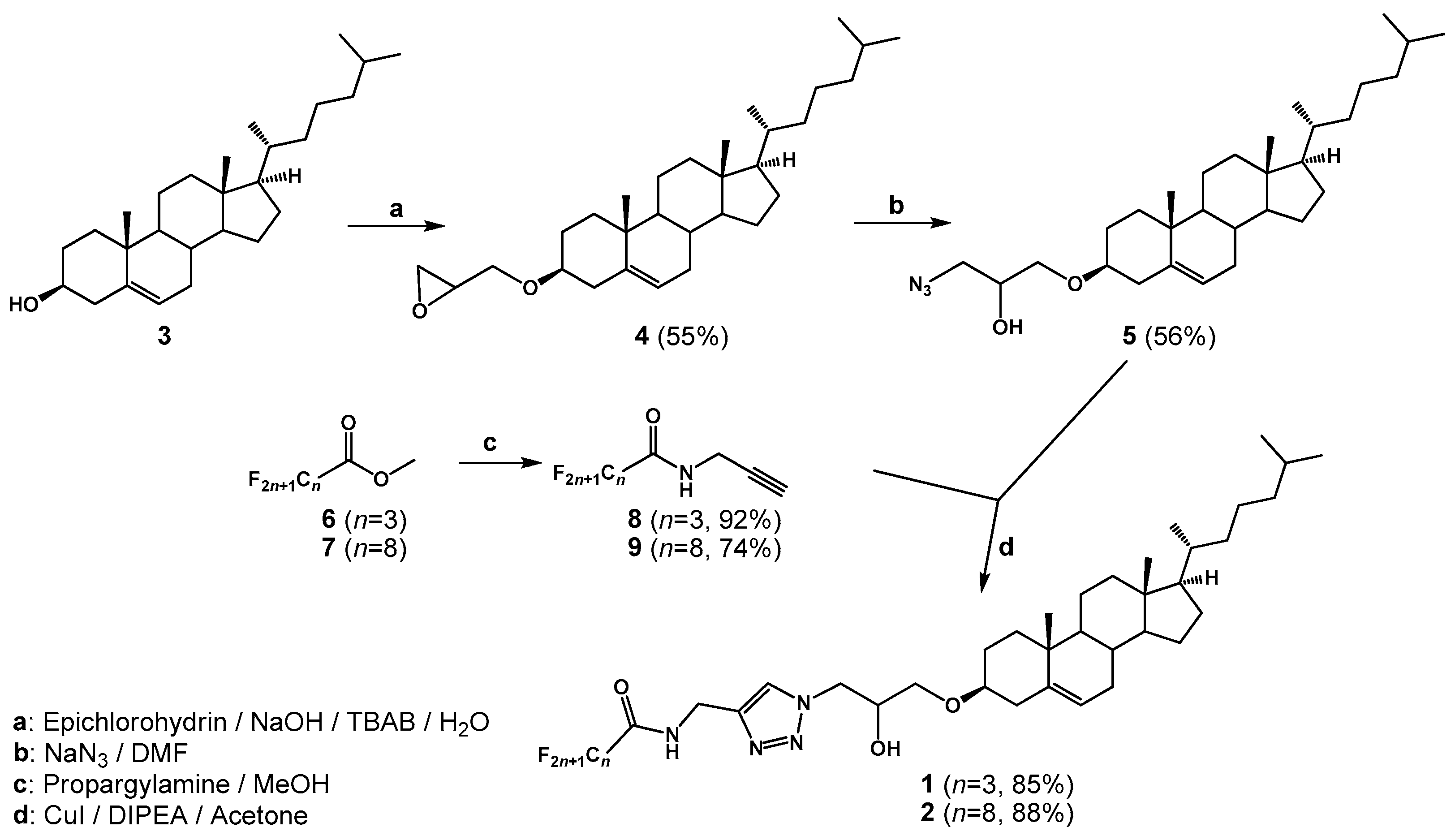
2.1. Chemical Synthesis and Characterization
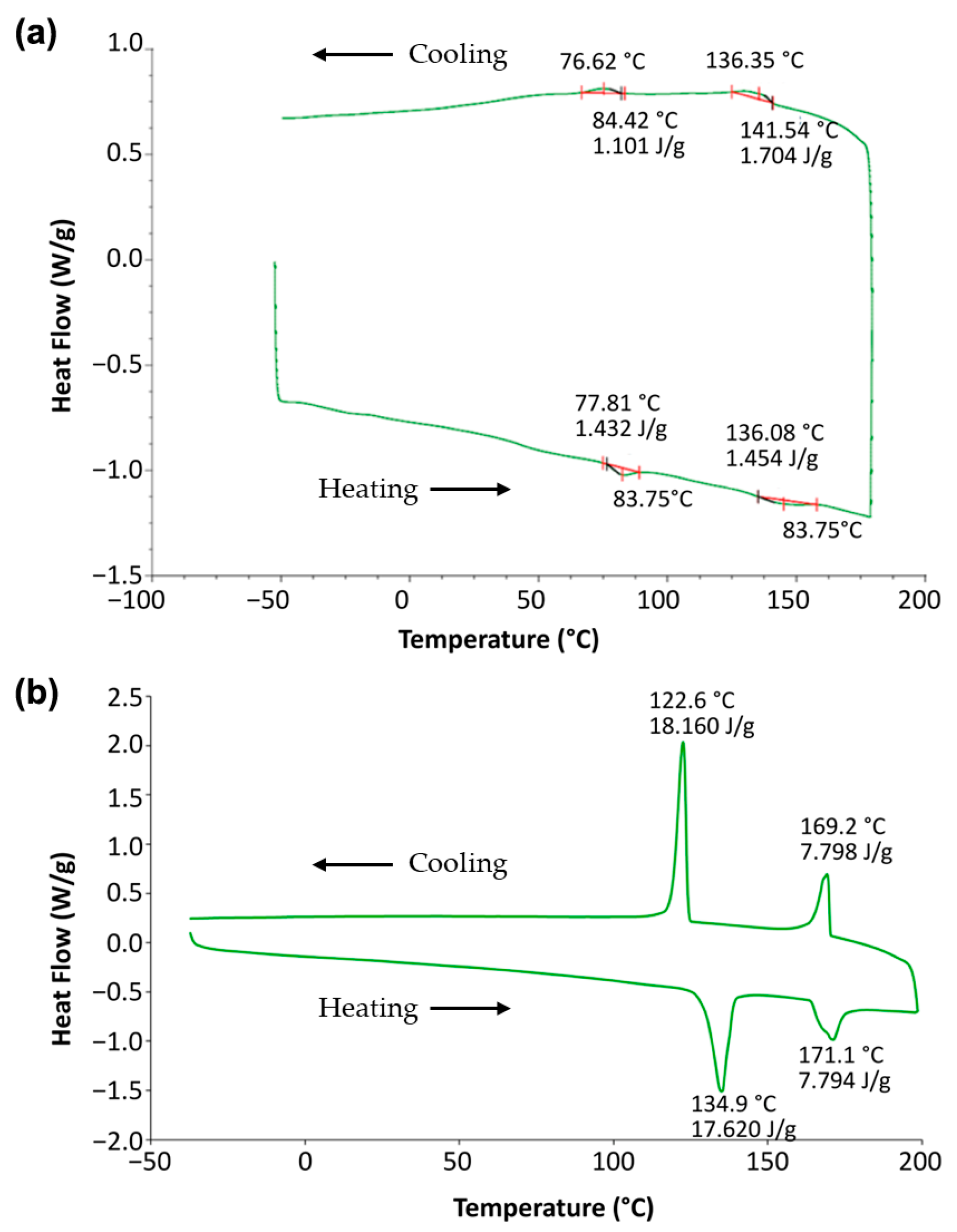
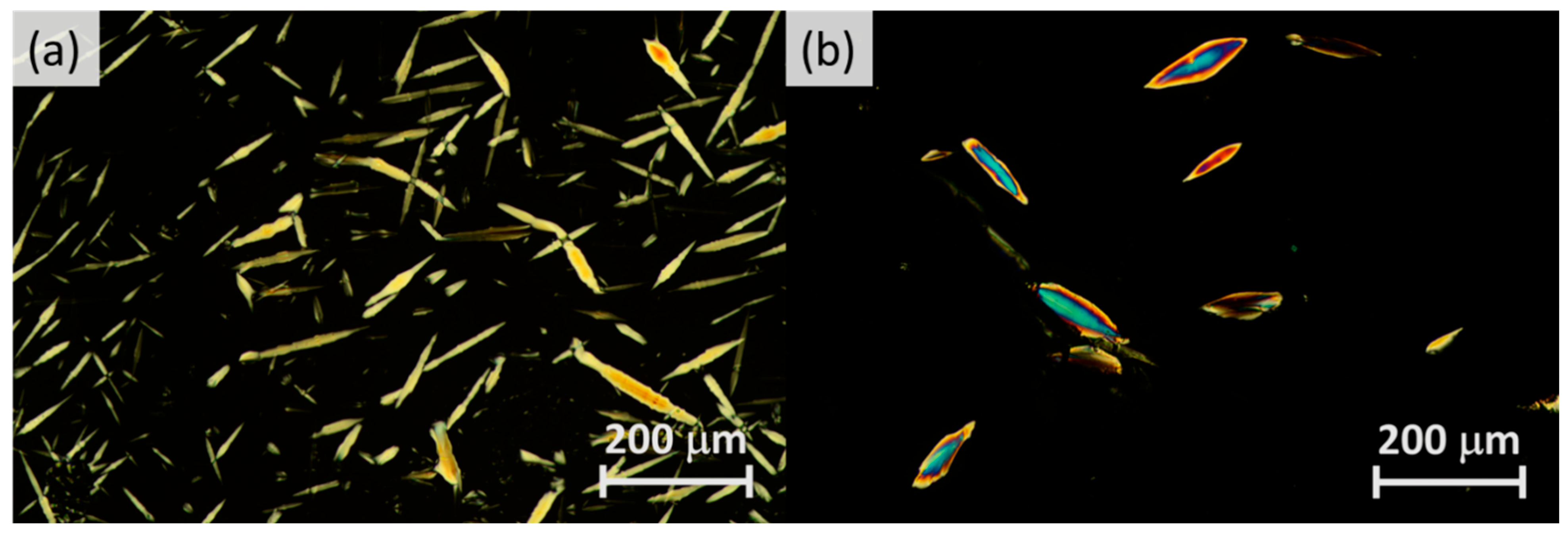
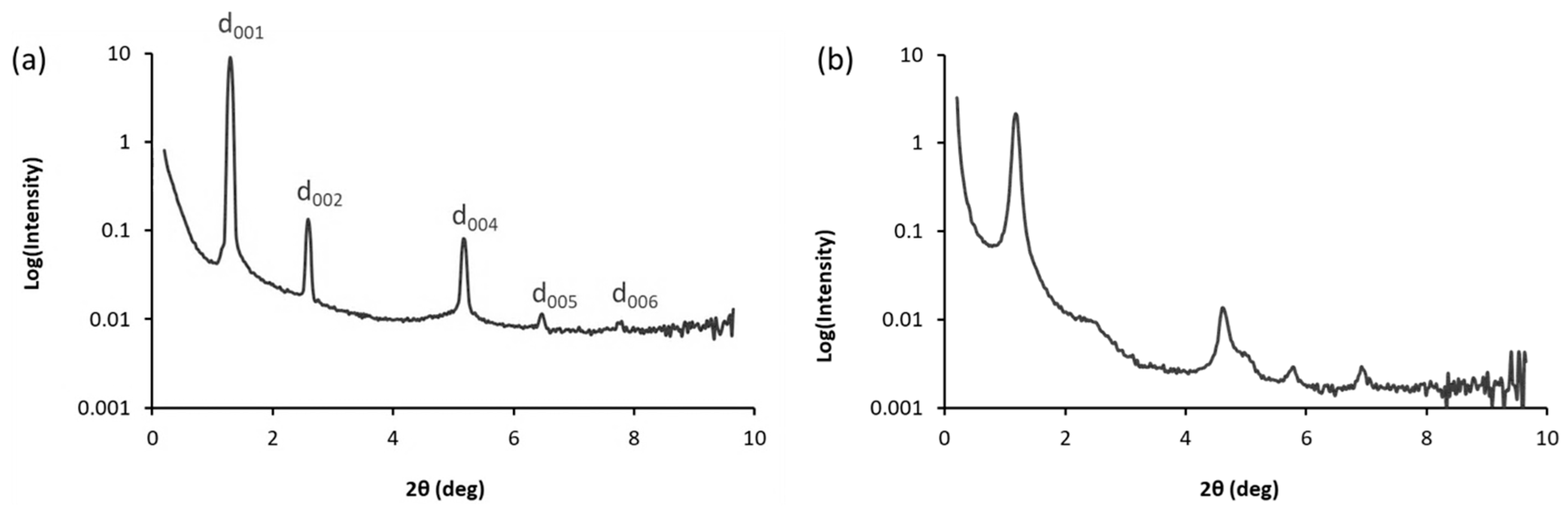
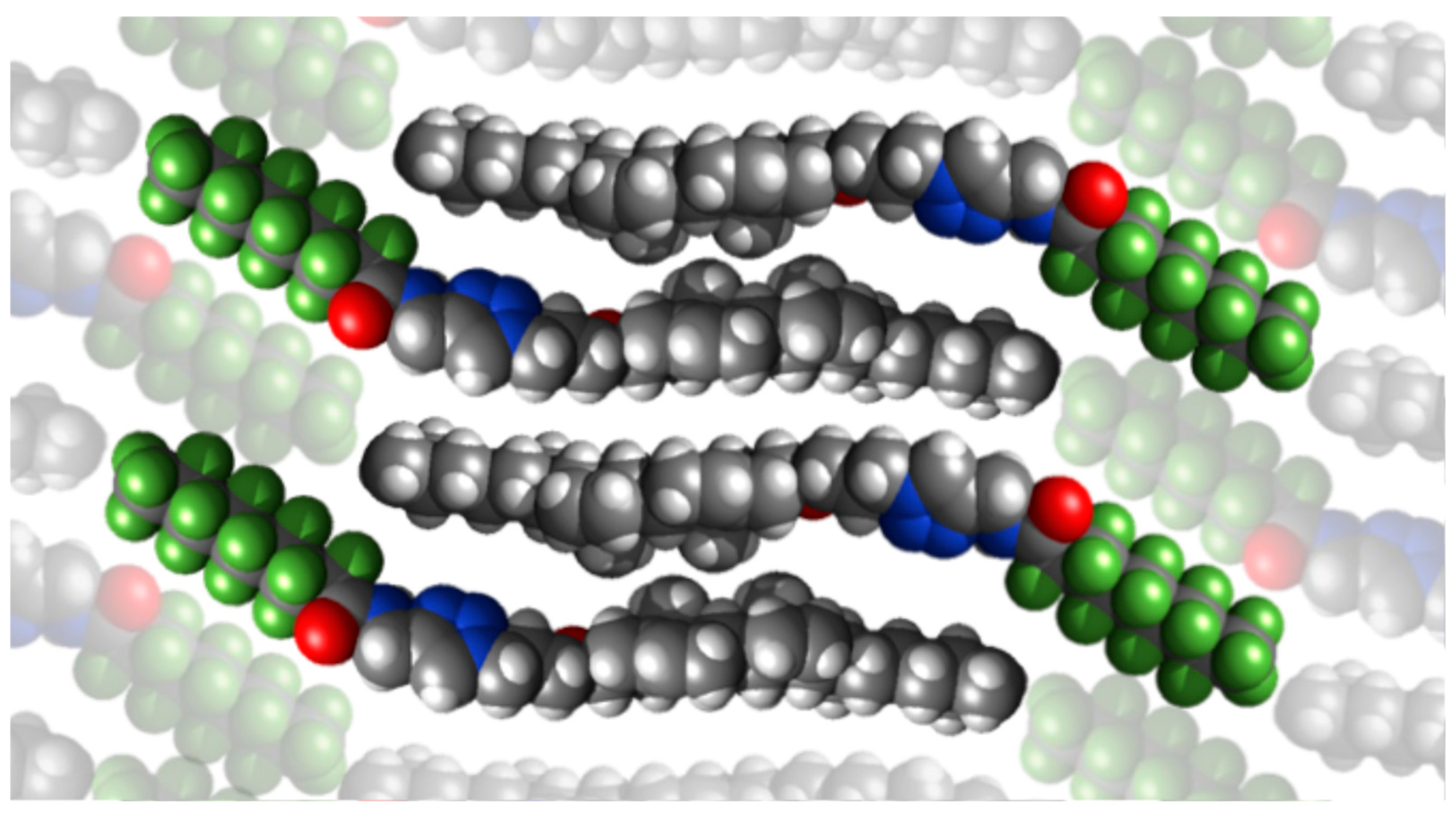
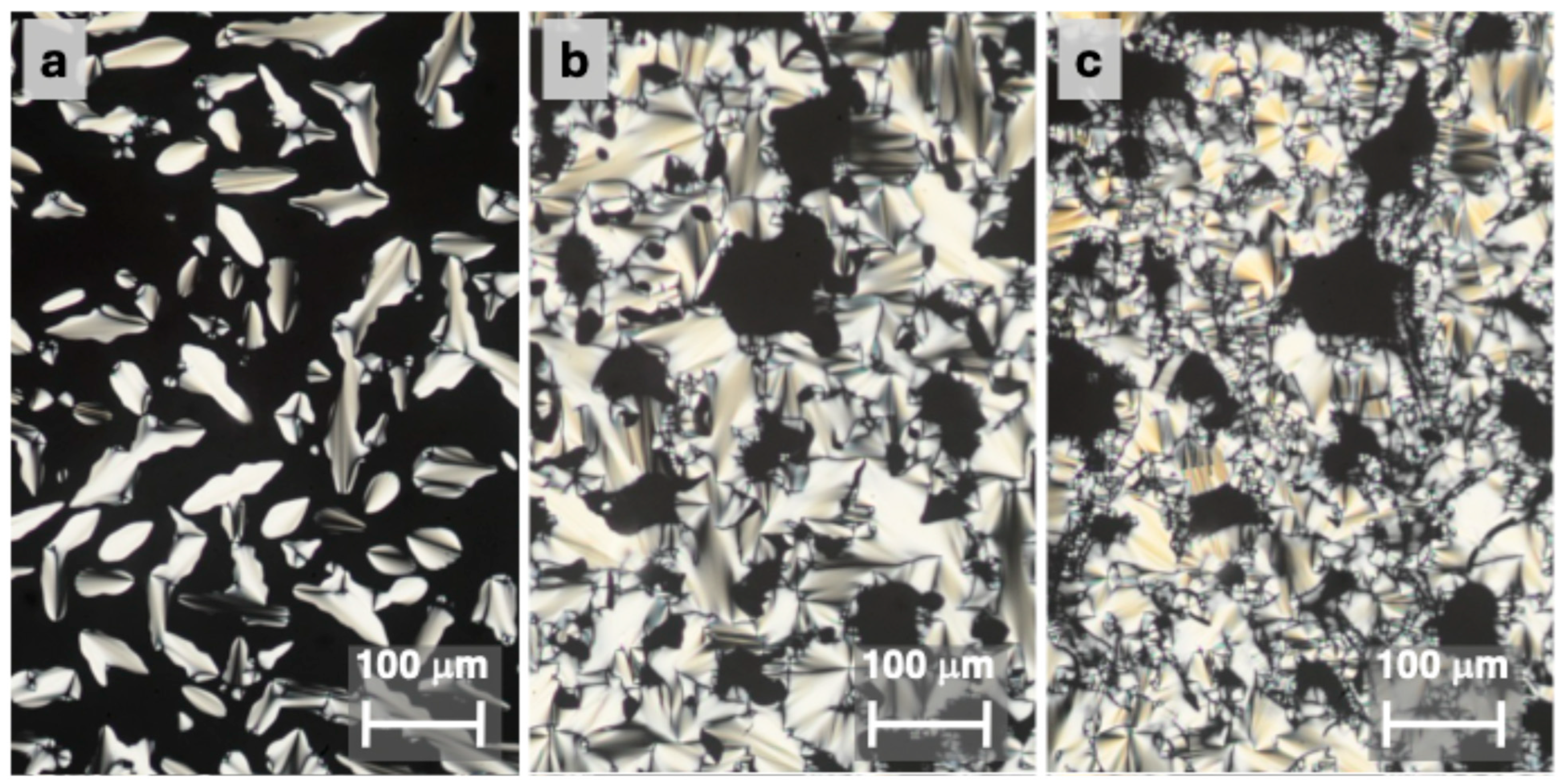
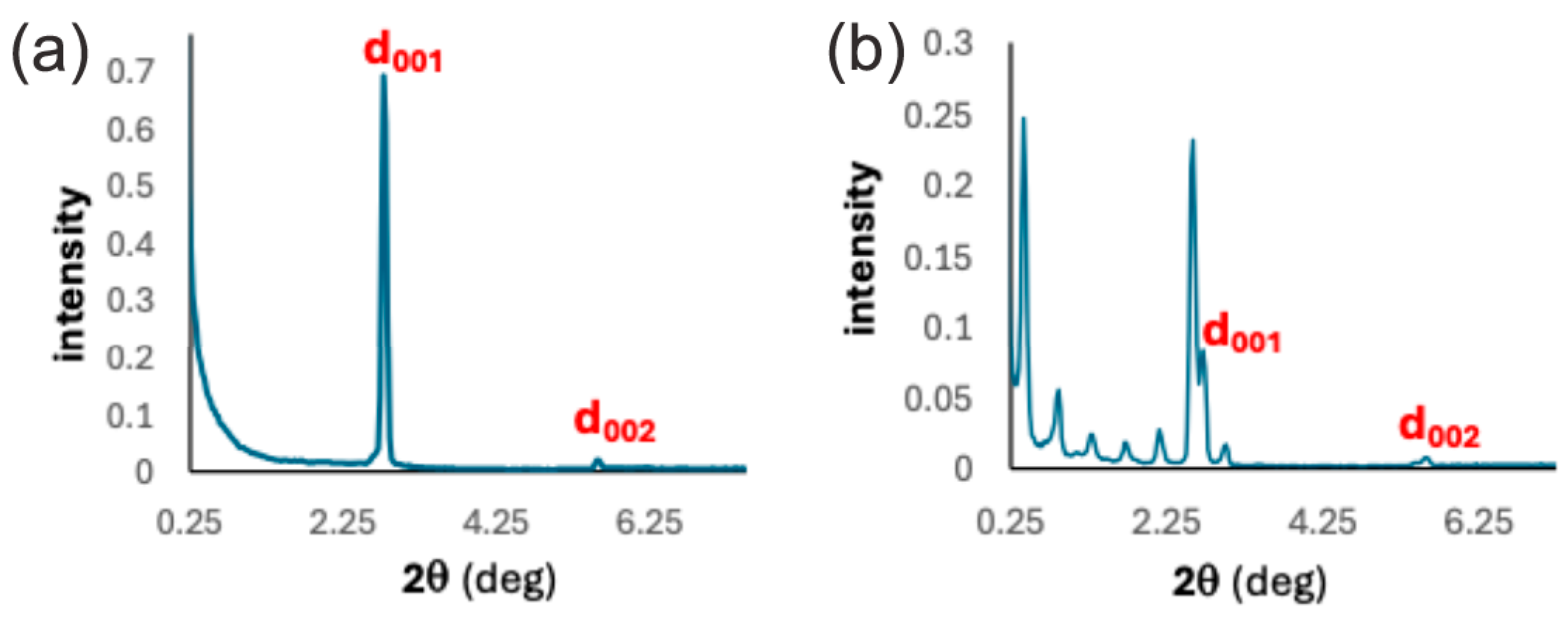
2.2. Mesomorphic Properties
3. Materials and Methods
3.1. Methods
3.2. Chemical Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albuquerque, H.M.T.; Santos, C.M.M.; Silva, A.M.S. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules 2018, 24, 116. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A. Liquid Crystal Dimers and Higher Oligomers: Between Monomers and Polymers. Chem. Soc. Rev. 2007, 36, 2096–2124. [Google Scholar] [CrossRef] [PubMed]
- Mallia, V.A.; Tamaoki, N. Design of Chiral Dimesogens Containing Cholesteryl Groups; Formation of New Molecular Organizations and Their Application to Molecular Photonics. Chem. Soc. Rev. 2004, 33, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yelamaggad, C.V.; Shanker, G.; Hiremath, U.S.; Prasad, S.K. Cholesterol-Based Nonsymmetric Liquid Crystal Dimers: An Overview. J. Mater. Chem. 2008, 18, 2927–2949. [Google Scholar] [CrossRef]
- Frizon, T.E.A.; Vieira, A.A.; Giacomelli, F.C.; Cercená, R.; Dal Bó, A.; Zapp, E.; Junca, E.; Chepluki, A.A.; Tomasi, C.D.; Ribeiro, L.B.; et al. Synthesis of Cholesterol Containing Unsymmetrical Dimers: A New Series of Liquid Crystals. Liq. Cryst. 2022, 49, 758–768. [Google Scholar] [CrossRef]
- Hiremath, U.S.; Nair, G.G.; Rao, D.S.S. Supramolecular Non-Symmetric Dimers Derived from Cholesterol: Synthesis and Phase Transitional Properties. Liq. Cryst. 2016, 43, 711–728. [Google Scholar] [CrossRef]
- Huang, H.; Cong, Y.; Du, A.; Hu, B.; Li, J.; Zhang, B. Liquid Crystalline Dimers Containing a Cholesteryl Group: Chiral Smectic Phases, and Re-Entrant TGBA* Phase. J. Mol. Liq. 2022, 346, 117920. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Alshargabi, A.; Mahmood, W.A.K.; Han, C.-C.; Lin, H.-C.; Santo, M.; Ito, M.M. Synthesis, Characterization and Molecular Organization for Induced Smectic Phase of Triazole Ring in Non-Symmetric Liquid Crystalline Dimer. Tetrahedron 2015, 71, 3939–3945. [Google Scholar] [CrossRef]
- Champagne, P.-L.; Ester, D.; Aldosari, S.; Williams, V.E.; Ling, C.-C. Synthesis and Comparison of Mesomorphic Behaviour of a Cholesterol-Based Liquid Crystal Dimer and Analogous Monomers. Liq. Cryst. 2018, 45, 1164–1176. [Google Scholar] [CrossRef]
- Hiremath, U.S.; Menezes, H.M.; Nair, G.G.; Rao, D.S.S.; Prasad, S.K. Observation of a Chiral Smectic C Phase over a Wide Thermal Range with Novel Phase Sequences in Rigid, Bulky Chiral Dimers. J. Mater. Chem. C 2013, 1, 5799–5806. [Google Scholar] [CrossRef]
- Yadav, N.; Panov, V.P.; Swaminathan, V.; Sreenilayam, S.P.; Vij, J.K.; Perova, T.S.; Dhar, R.; Panov, A.; Rodriguez-Lojo, D.; Stevenson, P.J. Chiral smectic-A and smectic-C Phases with de Vries Characteristics. Phys. Rev. E 2017, 95, 062704. [Google Scholar] [CrossRef]
- Mali, H.; Sharma, V.S.; Rathod, S.L.; Sharma, A.S.; Shrivastav, P.S.; Prajapati, H.R. Synthesis, Characterization and Photophysical Study of Cholesterol Functionalized Azo-Based Room Temperature Liquid Crystals. J. Mol. Struct. 2023, 1289, 135776. [Google Scholar] [CrossRef]
- Pradhan, B.; Chakraborty, N.; Gupta, R.K.; Shanker, G.; Achalkumar, A.S. Nonsymmetrical Cholesterol Dimers Constituting Regioisomeric Oxadiazole and Thiadiazole Cores: An Investigation of the Structure–Property Correlation. New J. Chem. 2017, 41, 879–888. [Google Scholar] [CrossRef]
- Kanakala, M.B.; Yelamaggad, C.V. Exceptionally Wide Thermal Range Enantiotropic Existence of a Highly Complex Twist Grain Boundary Phase in a Pure, Single-Component Liquid Crystal Chiral Dimer. ACS Omega 2021, 6, 11556–11562. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.C.; Chakravorty, S.; Pal, N.; Sinha, R.K. Synthesis and Characterization of Cholesterol-Based Nonsymmetric Dimers Terminated with Ferrocenyl Core. Tetrahedron 2009, 65, 7998–8006. [Google Scholar] [CrossRef]
- Yelamaggad, C.V.; Shashikala, I.S.; Liao, G.; Rao, D.S.S.; Prasad, S.K.; Li, Q.; Jakli, A. Blue Phase, Smectic Fluids, and Unprecedented Sequences in Liquid Crystal Dimers. Chem. Mater. 2006, 18, 6100–6102. [Google Scholar] [CrossRef]
- Yelamaggad, C.V.; Bhat, S.A. Cholesterol-Based Nonsymmetric Dimers Comprising Phenyl 4-(Benzoyloxy)Benzoate Core: The Occurrence of Frustrated Phases. Liquid Crystals 2022, 49, 995–1009. [Google Scholar] [CrossRef]
- Chen, X.; Korblova, E.; Dong, D.; Wei, X.; Shao, R.; Radzihovsky, L.; Glaser, M.A.; Maclennan, J.E.; Bedrov, D.; Walba, D.M.; et al. First-Principles Experimental Demonstration of Ferroelectricity in a Thermotropic Nematic Liquid Crystal: Polar Domains and Striking Electro-Optics. Proc. Natl. Acad. Sci. USA 2020, 117, 14021–14031. [Google Scholar] [CrossRef]
- Guo, Q.; Yan, K.; Chigrinov, V.; Zhao, H.; Tribelsky, M. Ferroelectric Liquid Crystals: Physics and Applications. Crystals 2019, 9, 470. [Google Scholar] [CrossRef]
- Jiang, H.-H.; Zhang, N.; Mao, W.-X.; Lan, J.-F.; Zhou, L.-X.; Xu, H.-M.; Zhang, H.-Y.; Liao, W.-Q. Modulating the Ferroelectric Phases in Cholesteryl-Based Organic Compounds with Perfluoroalkyl Tail Engineering. Chem. Commun. 2024, 60, 4322–4325. [Google Scholar] [CrossRef]
- Mihara, T.; Uedaira, T.; Koide, N. Fixation of a Cholesteric Helical Structure by the Photopolymerization of a Cholesteryl Derived Monomer. Liq. Cryst. 2002, 29, 855–861. [Google Scholar] [CrossRef]
- Guo, J.; Sun, J.; Li, K.; Cao, H.; Yang, H. Reflectance Properties of Polymer-stabilised Cholesteric Liquid Crystals Cells with Cholesteryl Compounds of Different Functionality. Liq. Cryst. 2008, 35, 87–97. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Zhang, J.; Liu, Y.-H.; Guo, Y.; Yu, X.-Q.; Huang, Z. Zinc(II)-Cyclen Coordinative Amphiphiles for Enhanced Gene Delivery. RSC Adv. 2020, 10, 39842–39853. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, D.; Wang, Z.-G.; Zschoche, S.; Zhuang, R.-C.; Häussler, L.; Friedel, P.; Simon, F.; Jehnichen, D.; Grundke, K.; Eichhorn, K.-J.; et al. Bulk and Surface Properties of Maleimide Copolymers: Effect of Fluorinated Side Chains. Macromolecules 2005, 38, 1655–1664. [Google Scholar] [CrossRef]
| Compound | Phase a | T (°C) [ΔH (kJ/mol)] b | Phase a | T (°C) [ΔH (kJ/mol)] b | Phase a |
|---|---|---|---|---|---|
| 1 | Cr | 83.8 [1.1] 76.6 [−0.8] | SmA* | 145.9 [1.1] 146.4 [−1.3] | Iso |
| 2 | Cr | 134.9 [13.0] 122.6 [−13.4] | SmA* | 171.1 [5.7] 169.2 [−5.7] | Iso |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, A.; Zellmann-Parrotta, C.O.; Ghaseminezhad, H.; Duong, J.; Williams, V.E.; Ling, C.-C. Synthesis and Characterization of Cholesterol-Based Liquid Crystals Linked with Perfluorinated Alkyl Chains. Molecules 2025, 30, 3731. https://doi.org/10.3390/molecules30183731
Che A, Zellmann-Parrotta CO, Ghaseminezhad H, Duong J, Williams VE, Ling C-C. Synthesis and Characterization of Cholesterol-Based Liquid Crystals Linked with Perfluorinated Alkyl Chains. Molecules. 2025; 30(18):3731. https://doi.org/10.3390/molecules30183731
Chicago/Turabian StyleChe, Austin, Carson O. Zellmann-Parrotta, Homayoun Ghaseminezhad, Jessica Duong, Vance E. Williams, and Chang-Chun Ling. 2025. "Synthesis and Characterization of Cholesterol-Based Liquid Crystals Linked with Perfluorinated Alkyl Chains" Molecules 30, no. 18: 3731. https://doi.org/10.3390/molecules30183731
APA StyleChe, A., Zellmann-Parrotta, C. O., Ghaseminezhad, H., Duong, J., Williams, V. E., & Ling, C.-C. (2025). Synthesis and Characterization of Cholesterol-Based Liquid Crystals Linked with Perfluorinated Alkyl Chains. Molecules, 30(18), 3731. https://doi.org/10.3390/molecules30183731







