Recent Advances in One-Pot Multicomponent Reactions for the Synthesis of Substituted Quinazolin-4(3H)-ones
Abstract
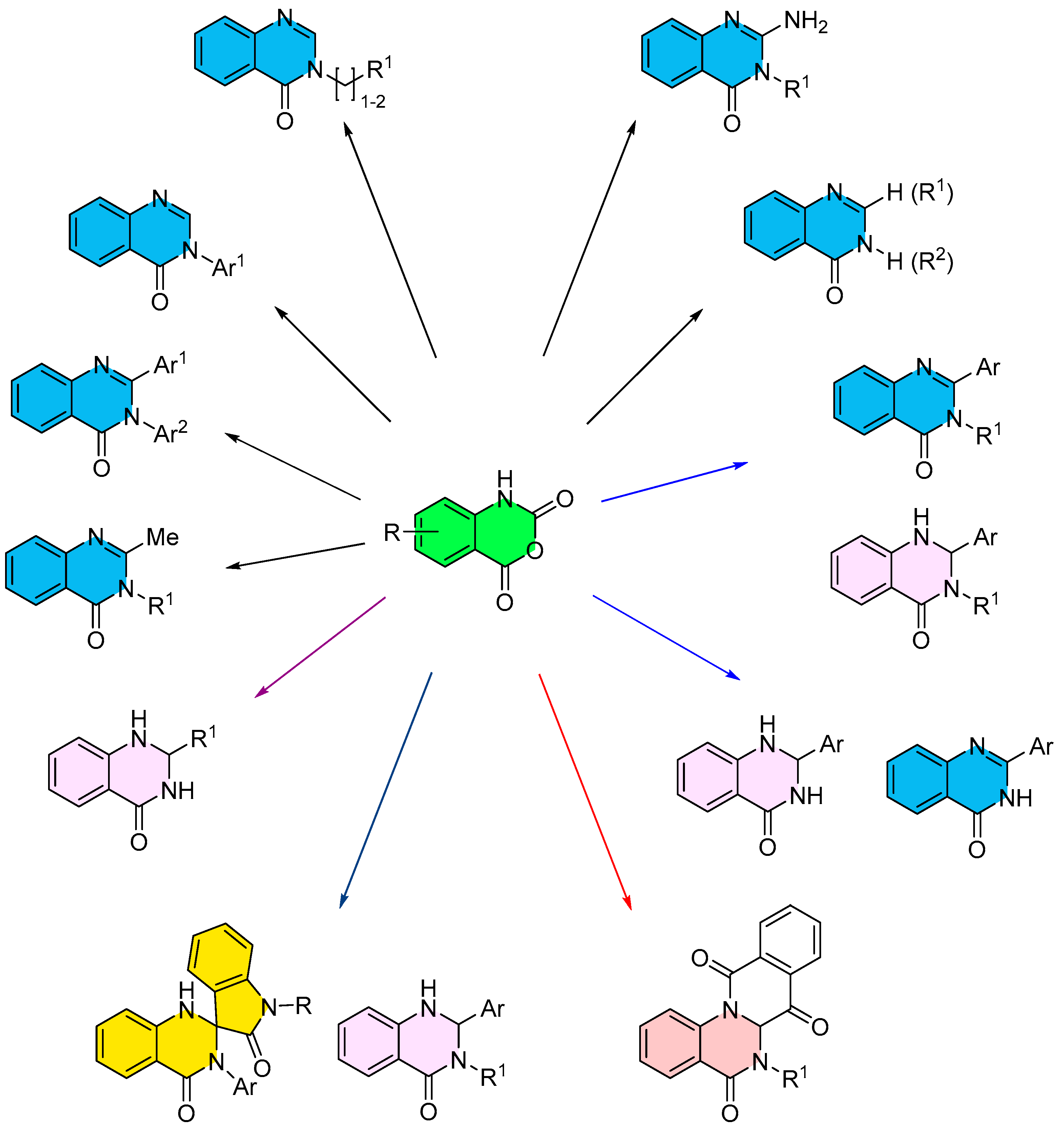
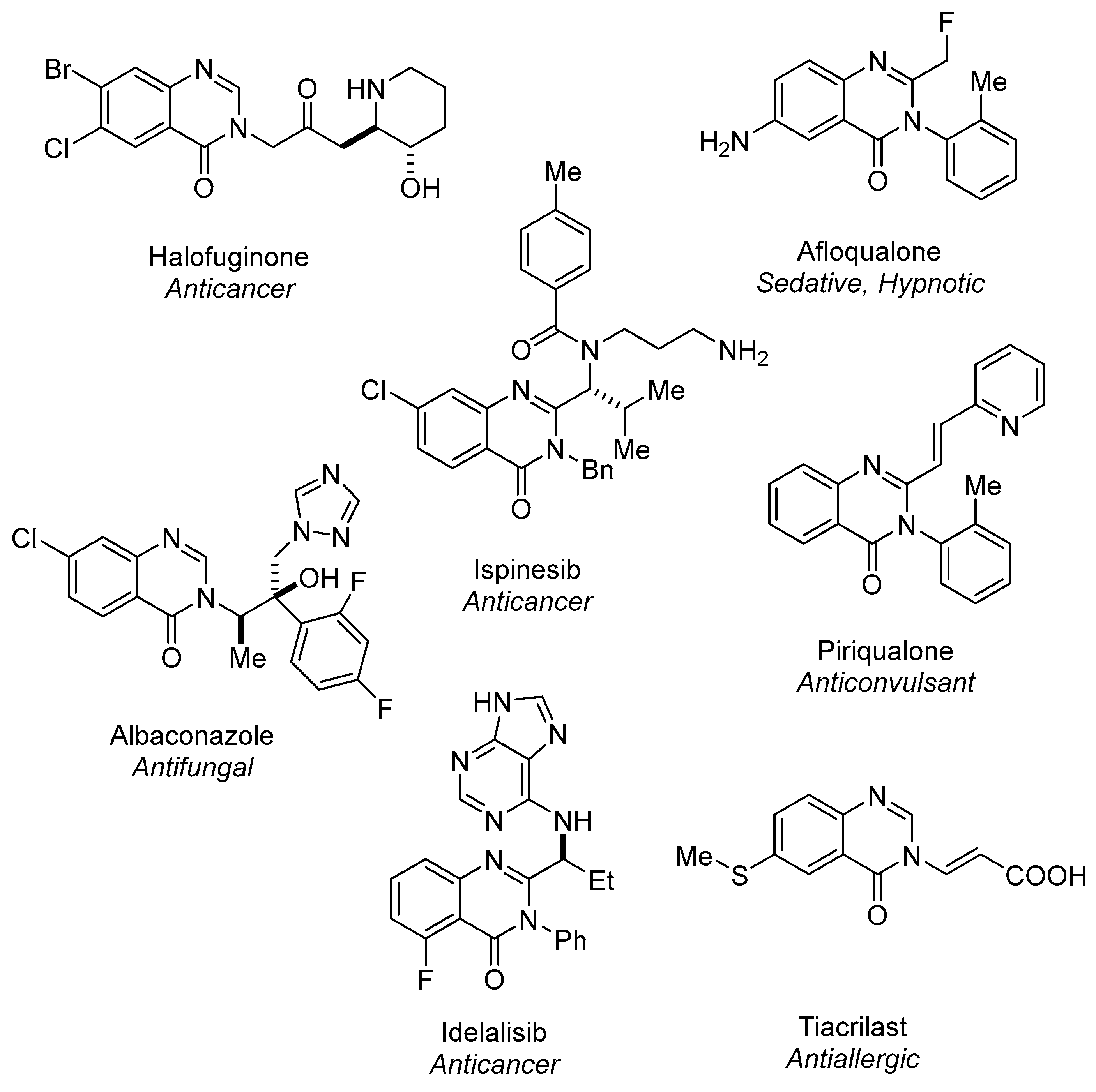
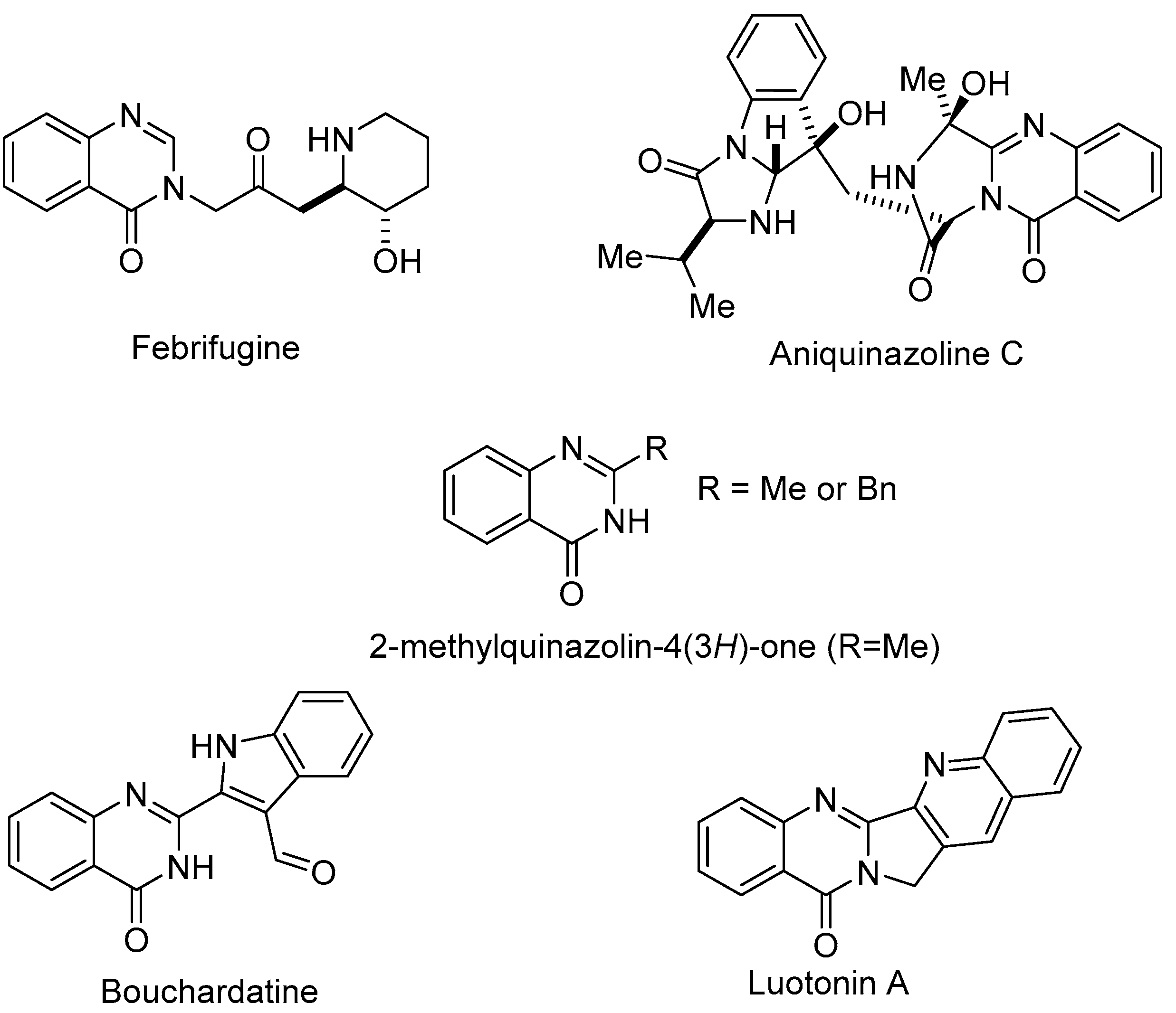
1. Introduction
- (1)
- Metal-catalyzed reactions, employing catalysts, such as palladium, copper, ruthenium, cobalt, and iridium;
- (2)
- Isatoic-anhydride-based strategies, utilizing isatoic anhydride as a key starting material for the construction of quinazolinone ring;
- (3)
- Alternative synthetic approaches involving, among others, the utilization of N-(2-aminobenzoyl)benzotriazoles or aryldiazonium salts as efficient building blocks.
2. Metal (Palladium, Copper, Ruthenium, Cobalt, Iridium)-Catalyzed Reactions (Scheme 1)
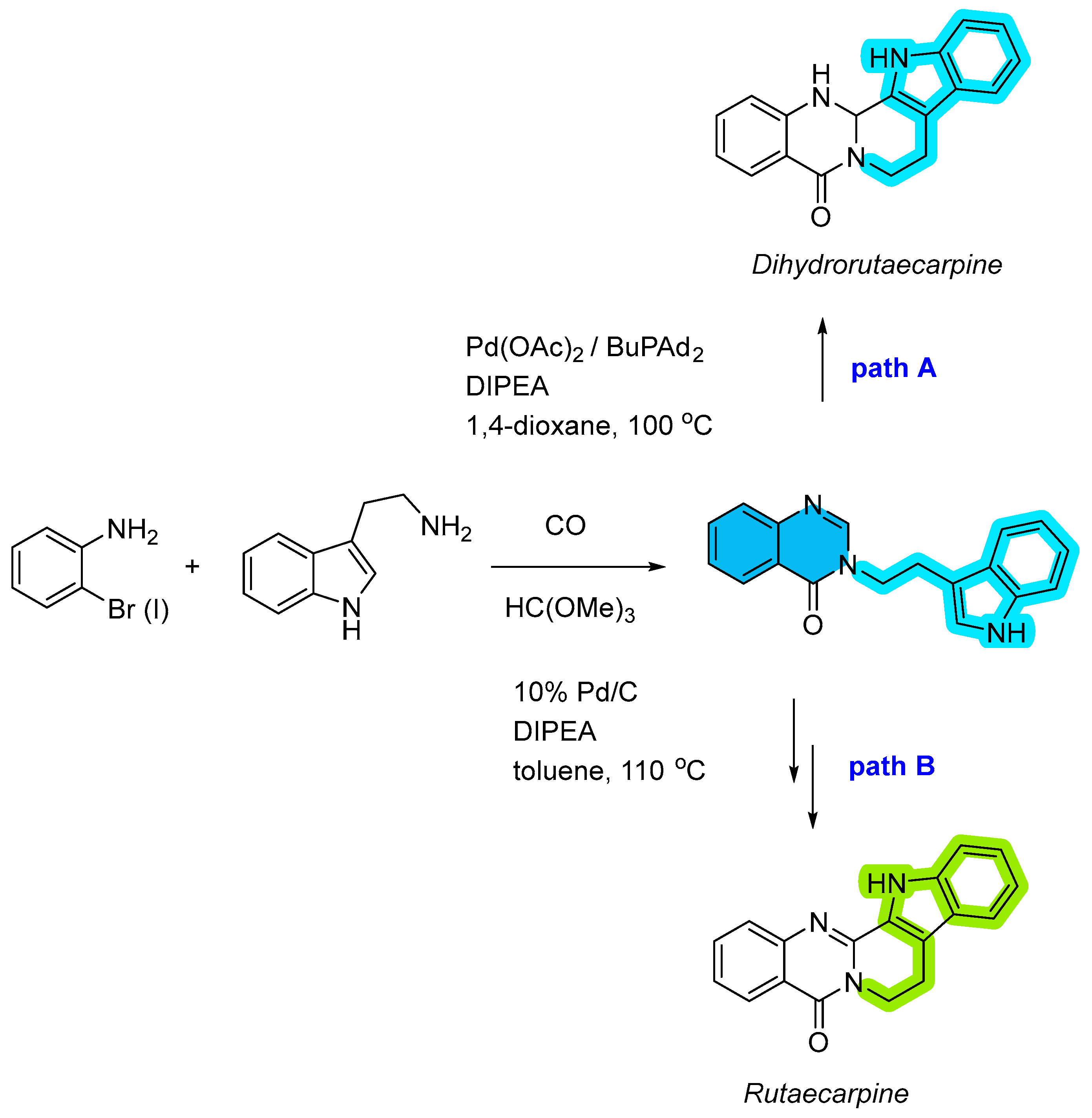
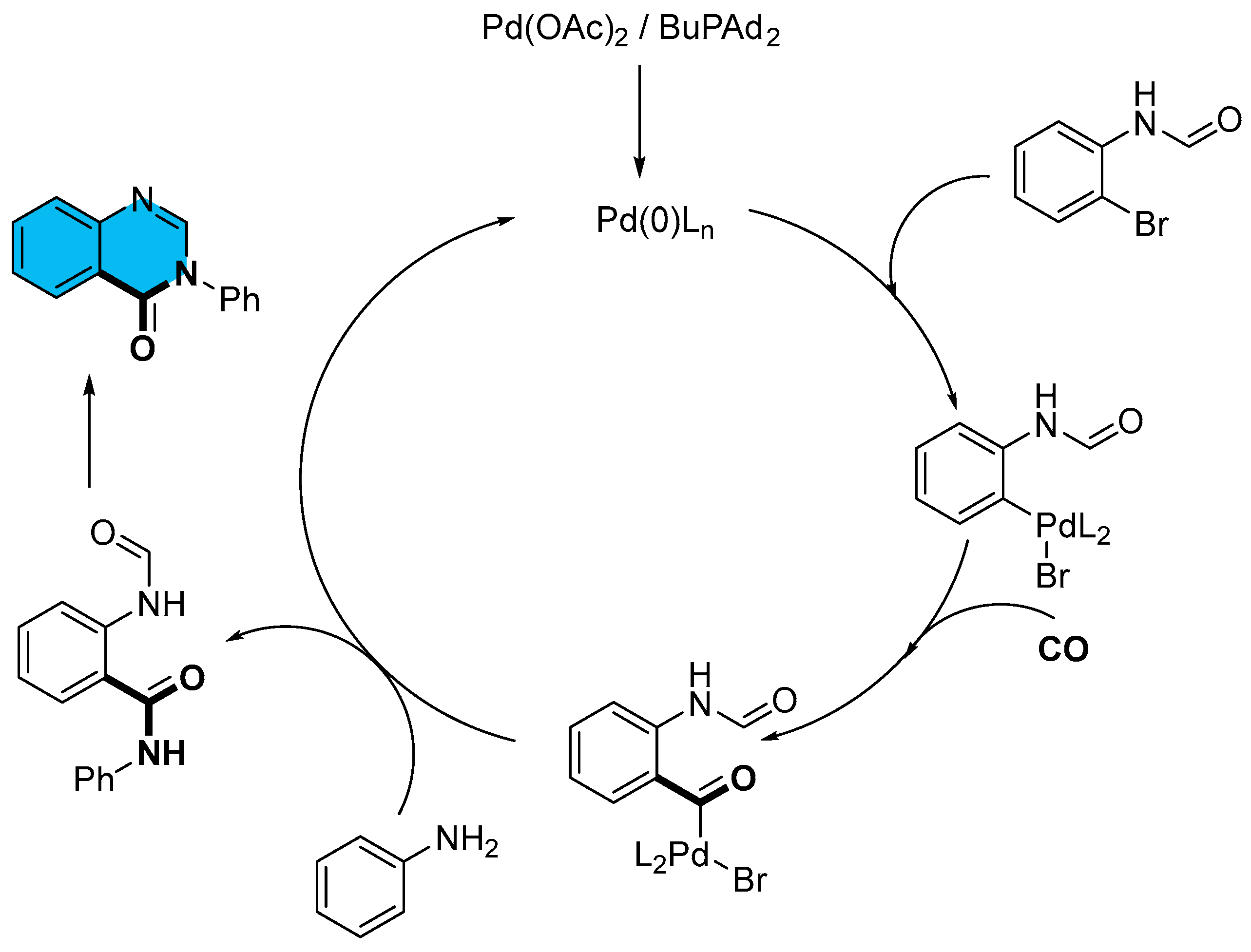
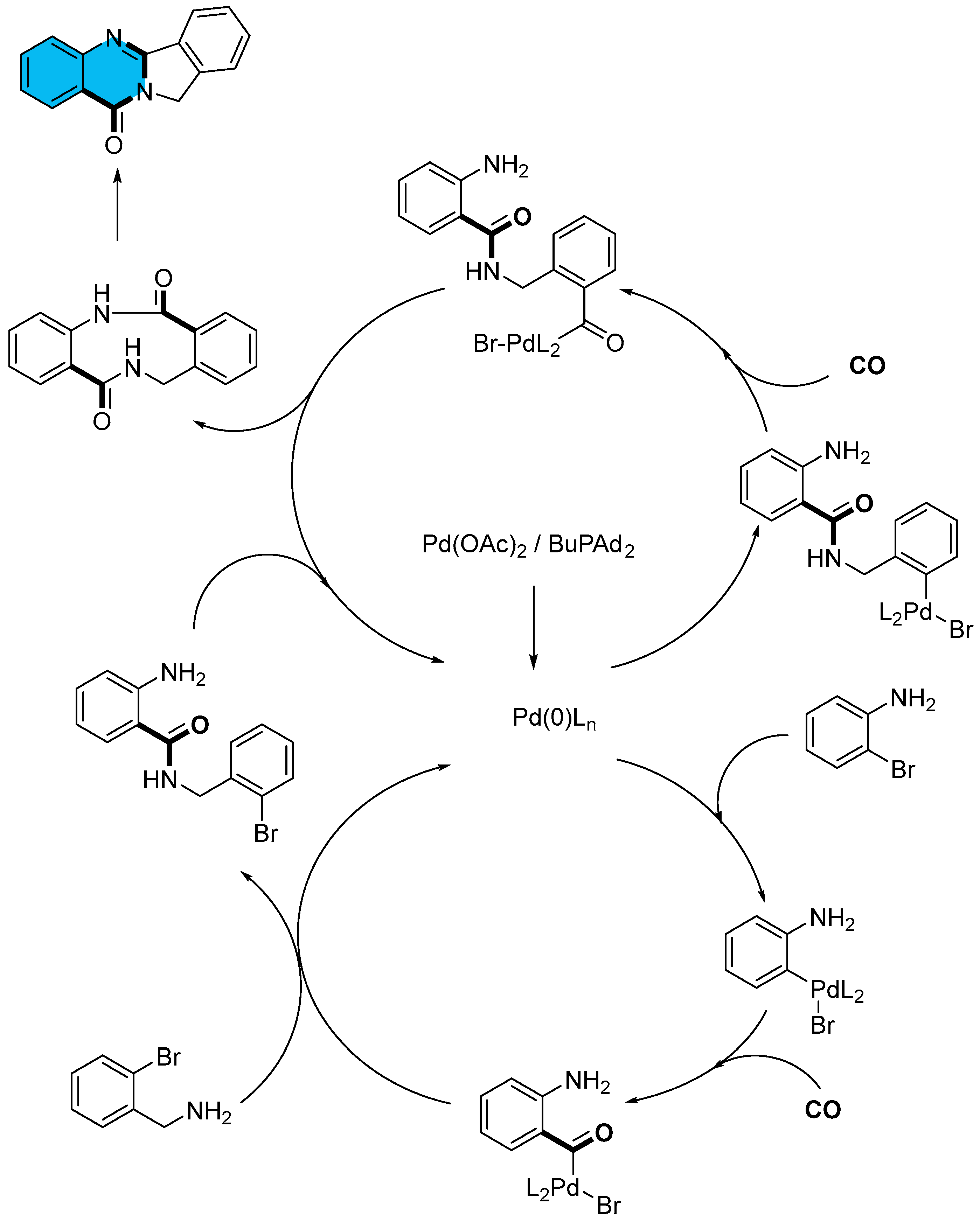
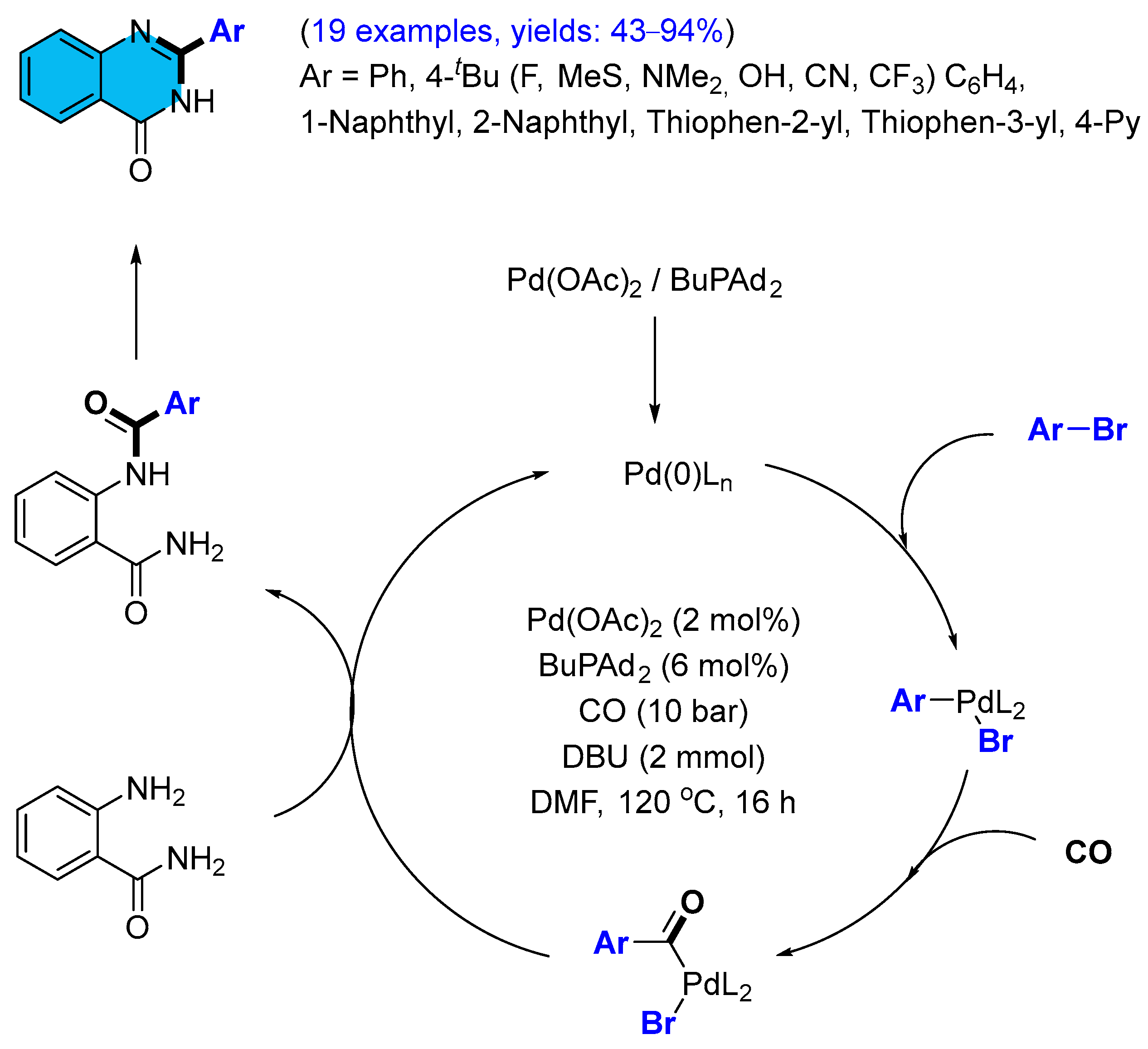
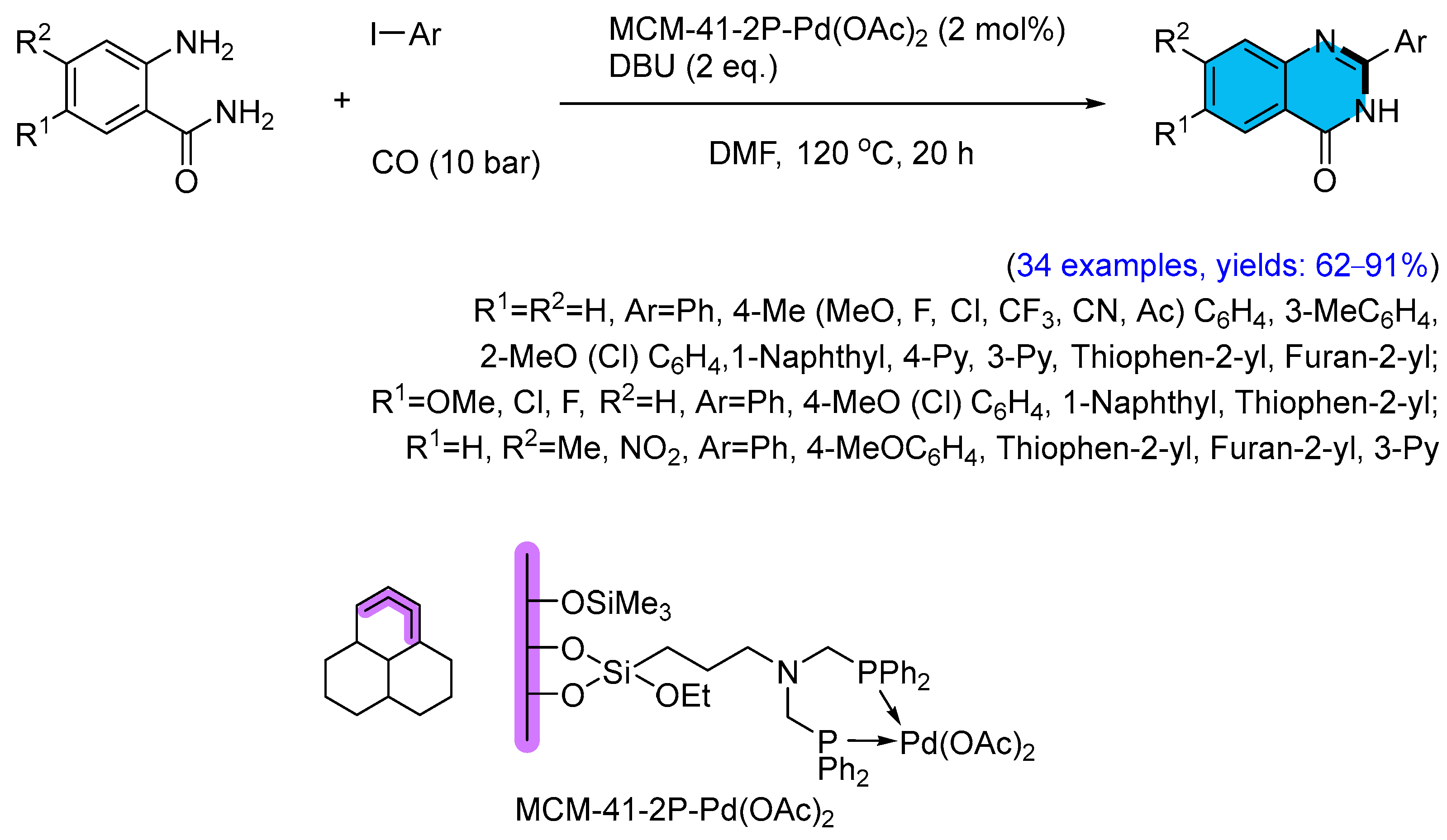
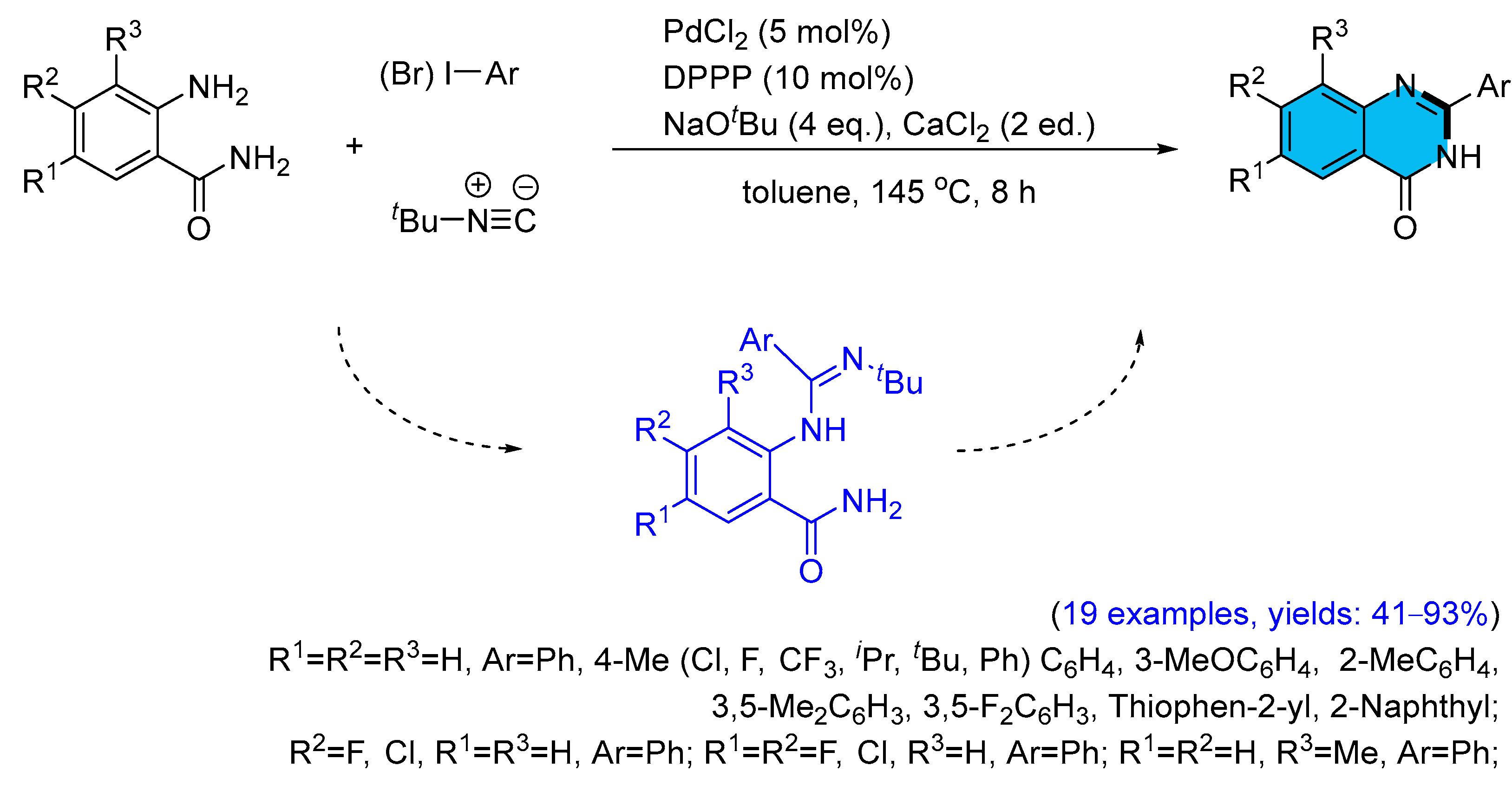
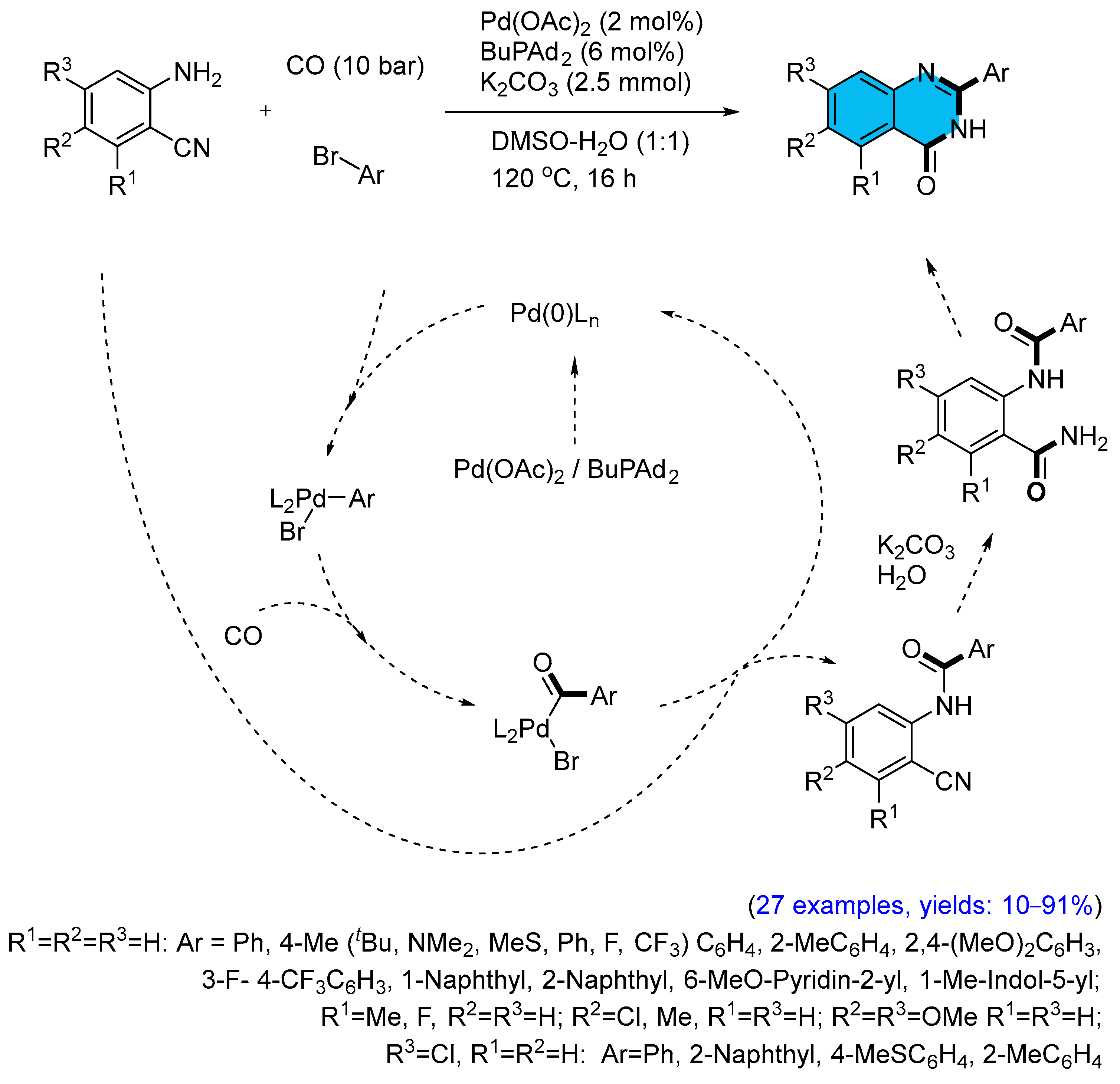
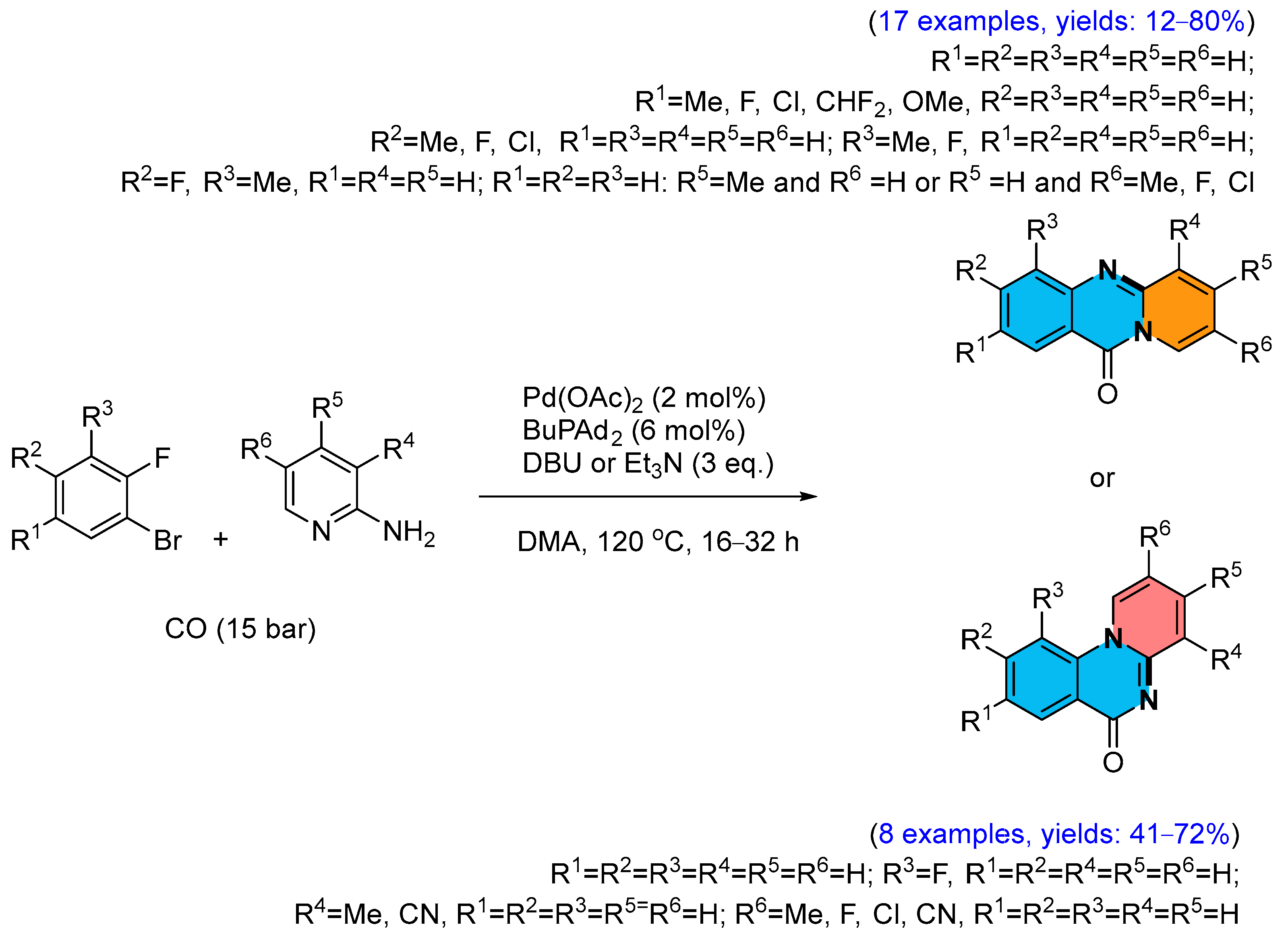
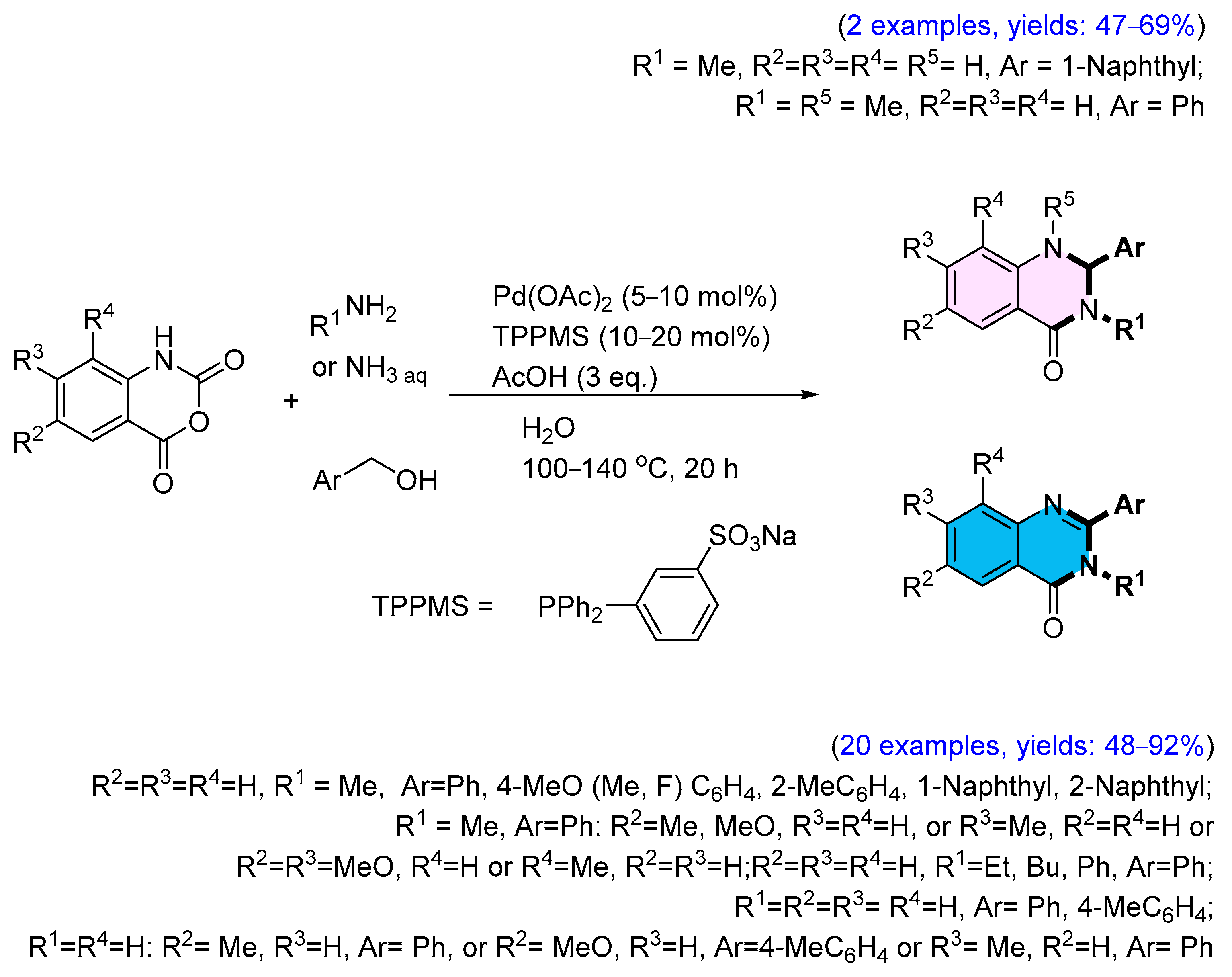
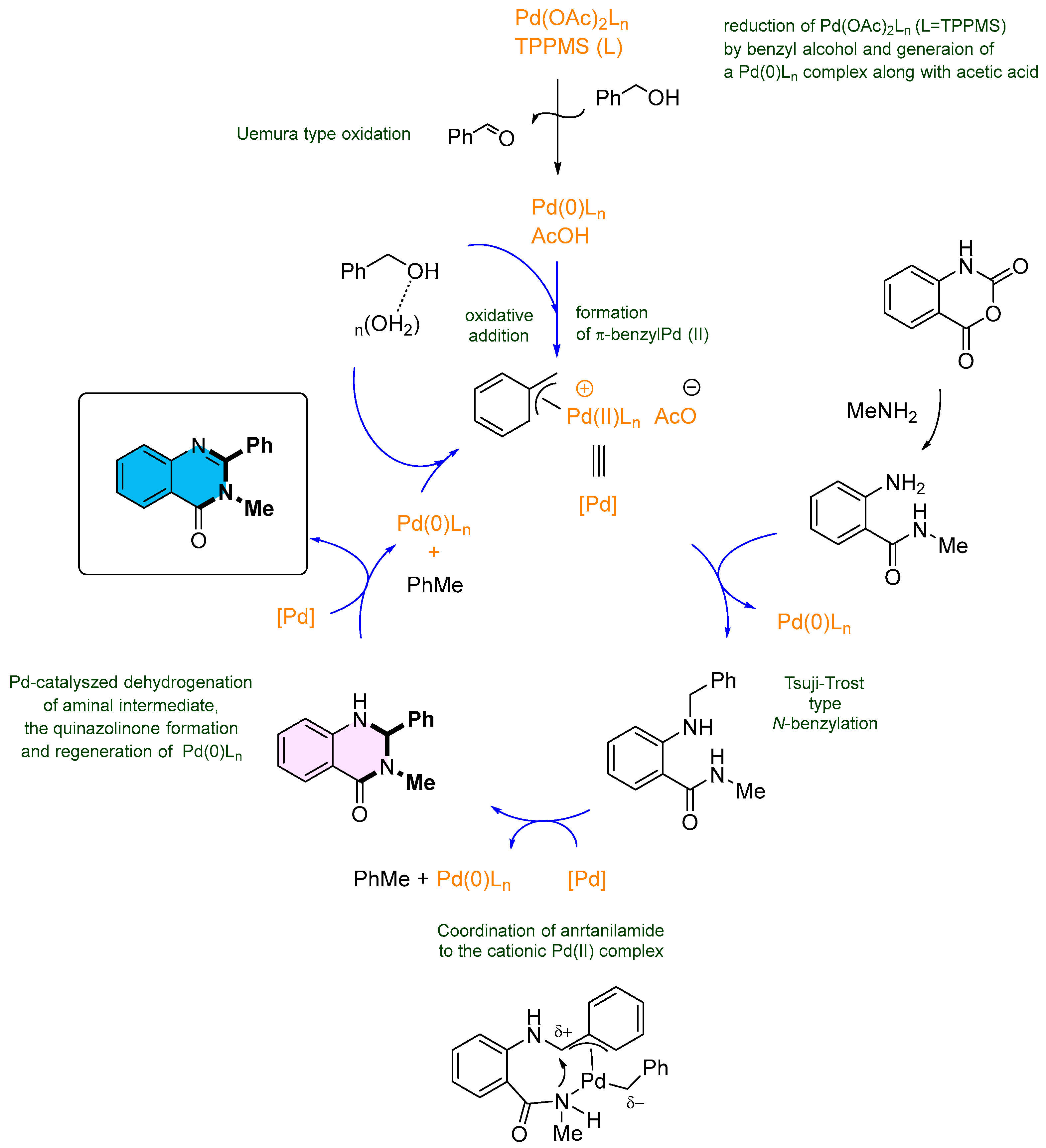
2.1. Palladium-Catalyzed Multicomponent Synthesis of Quinazolinones
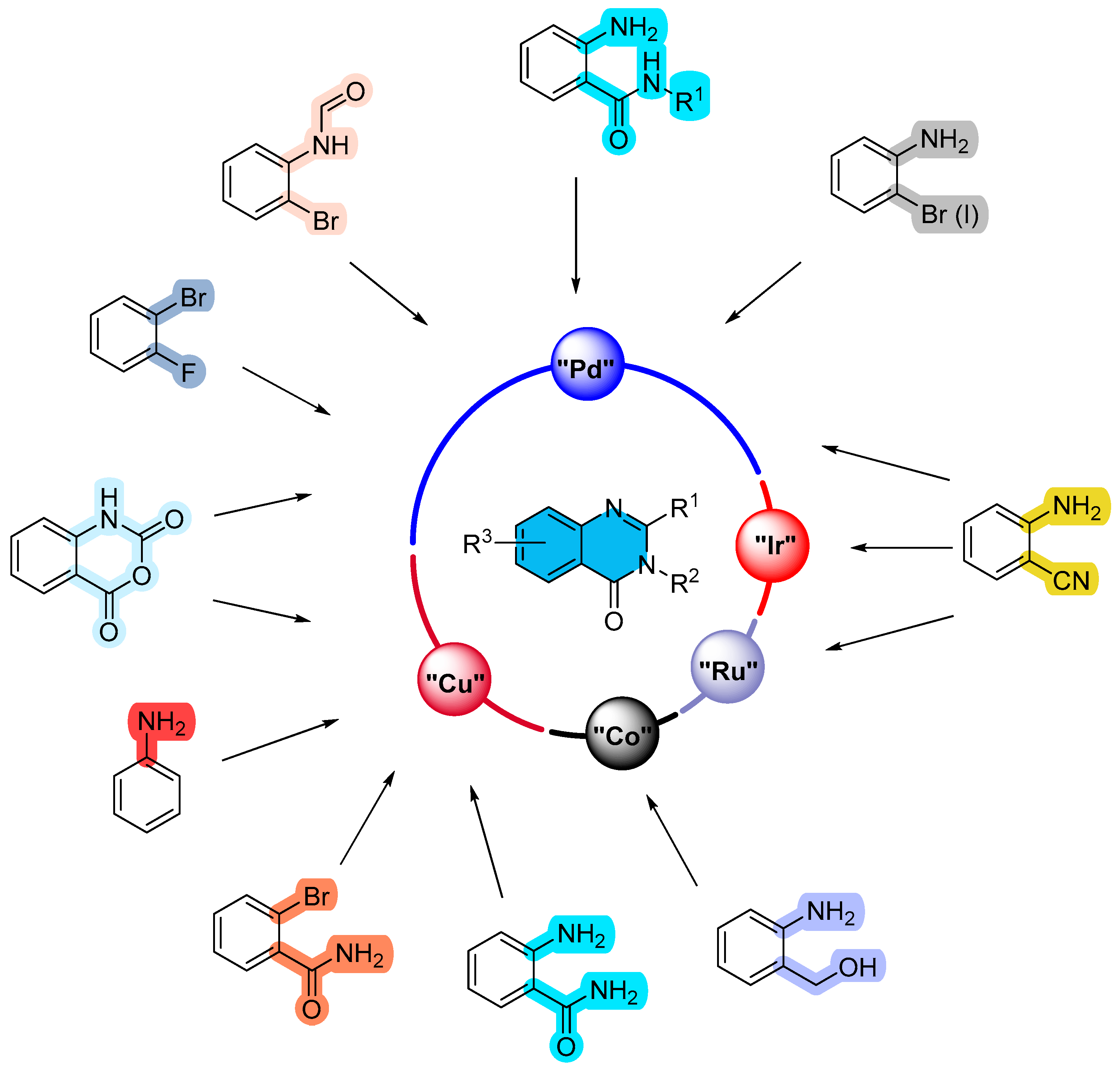
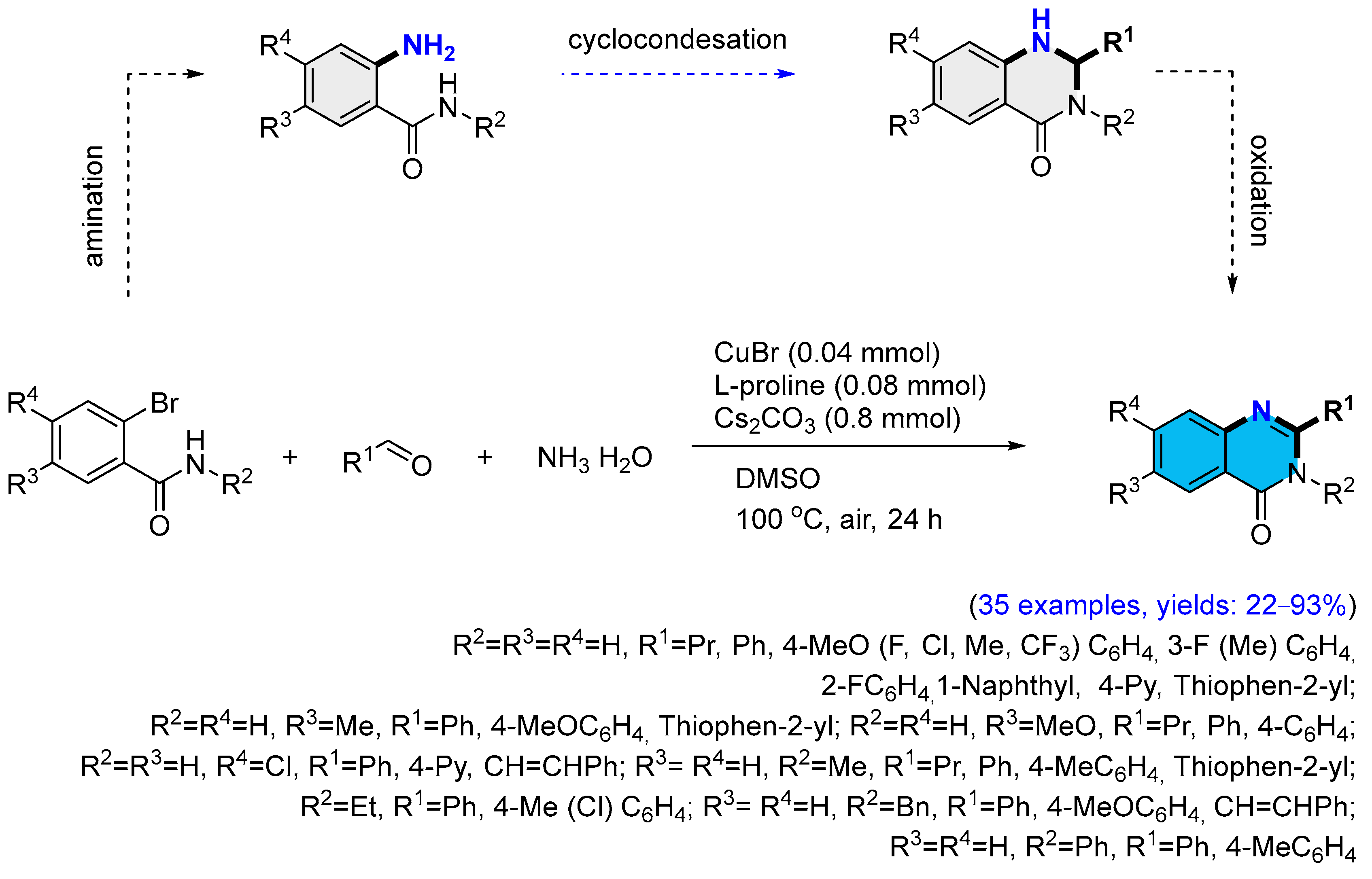
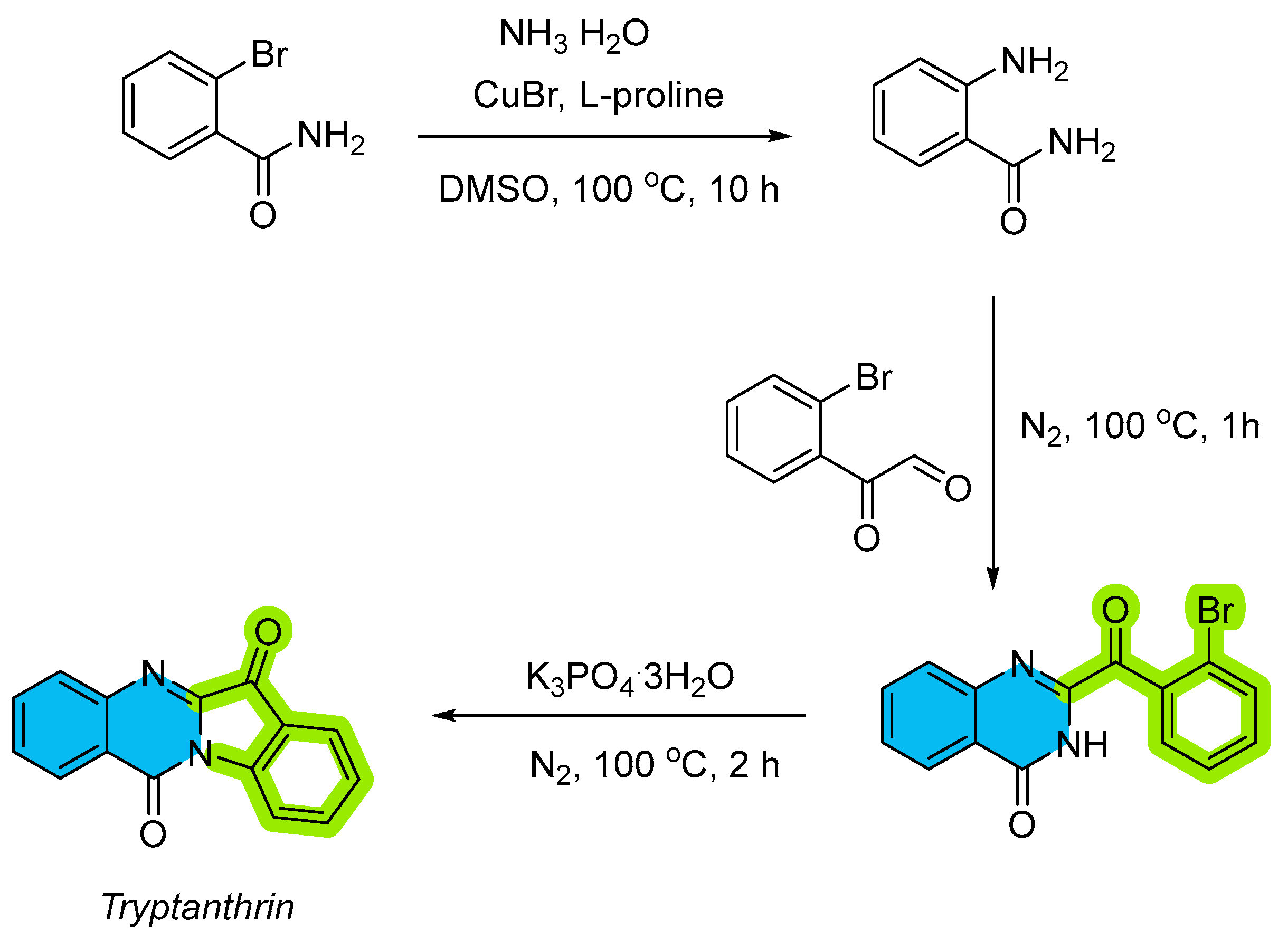
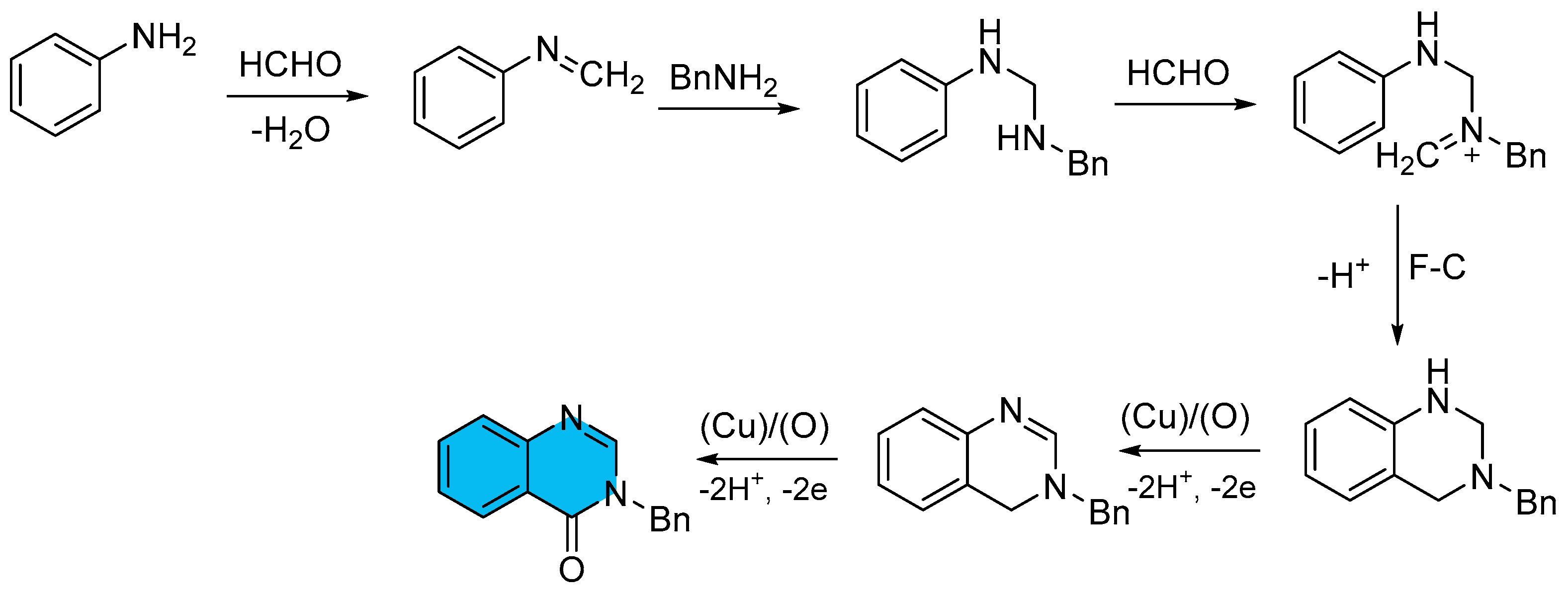
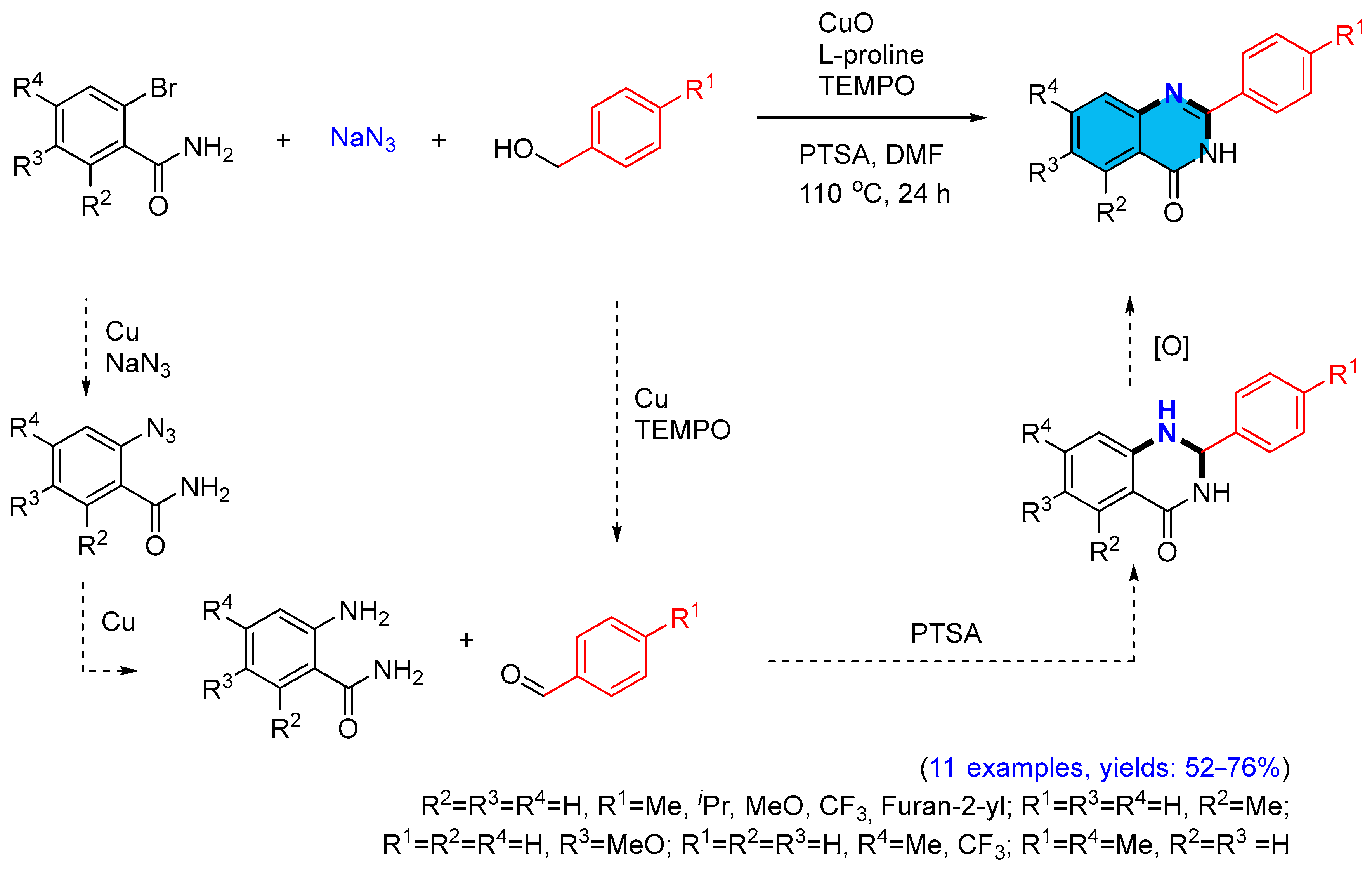
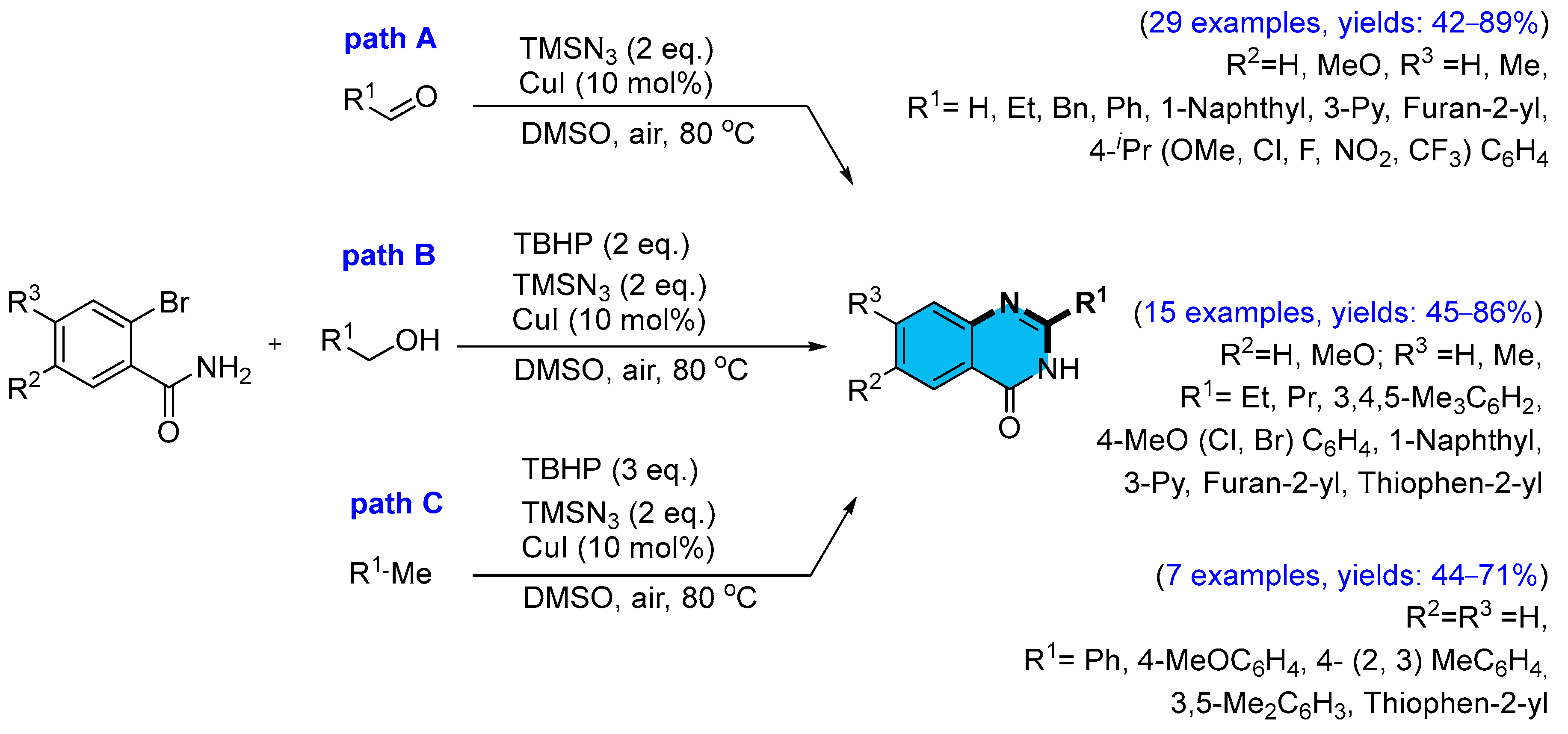
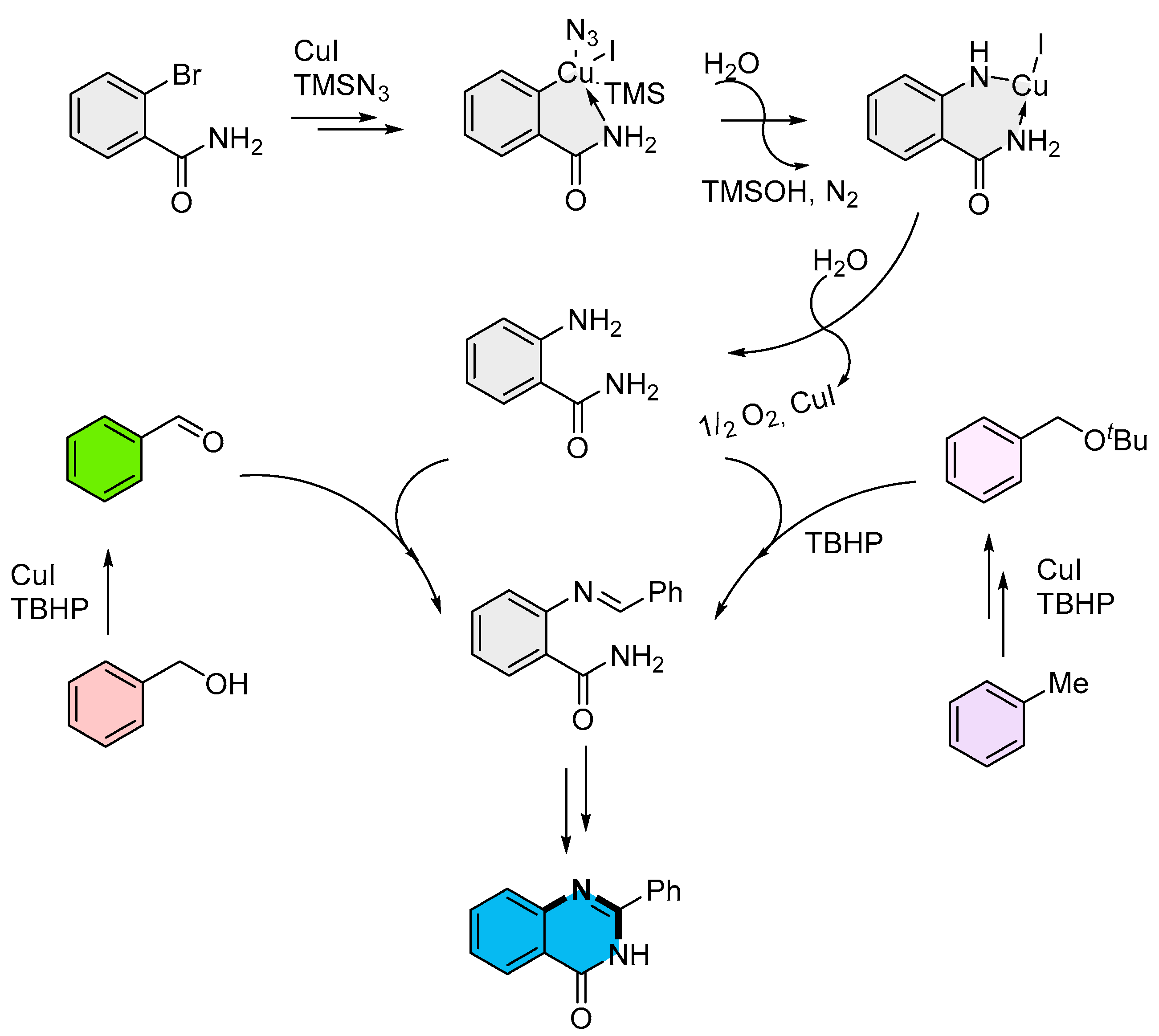
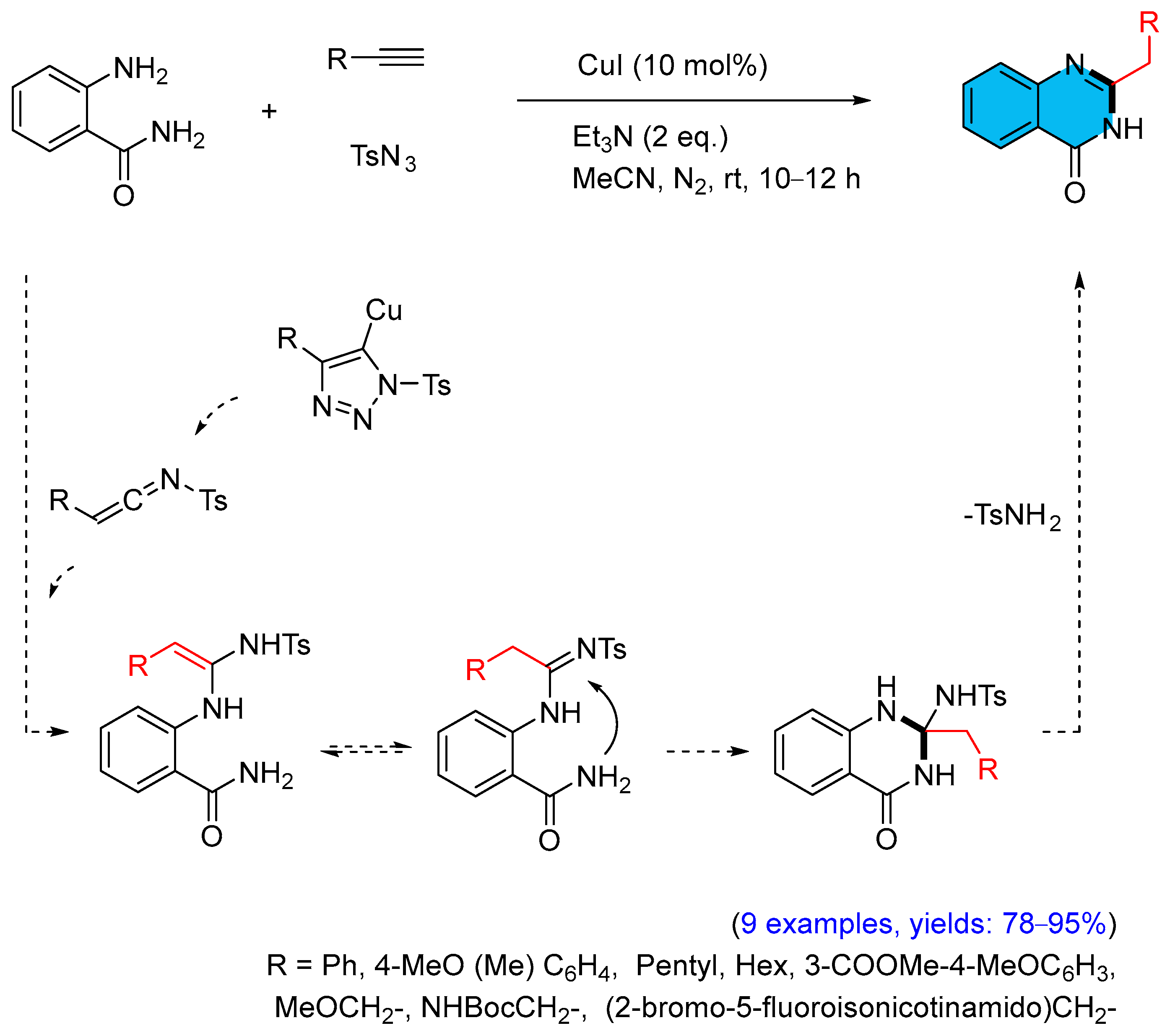
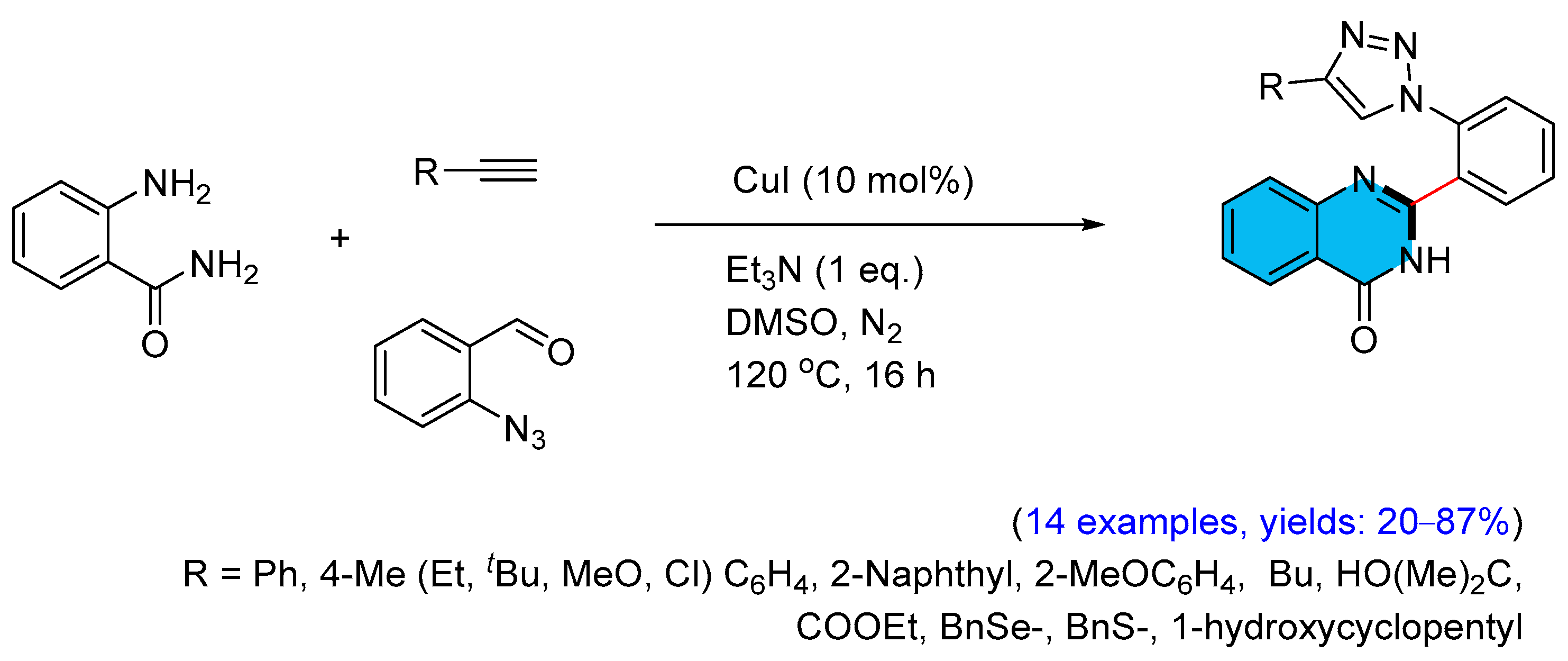
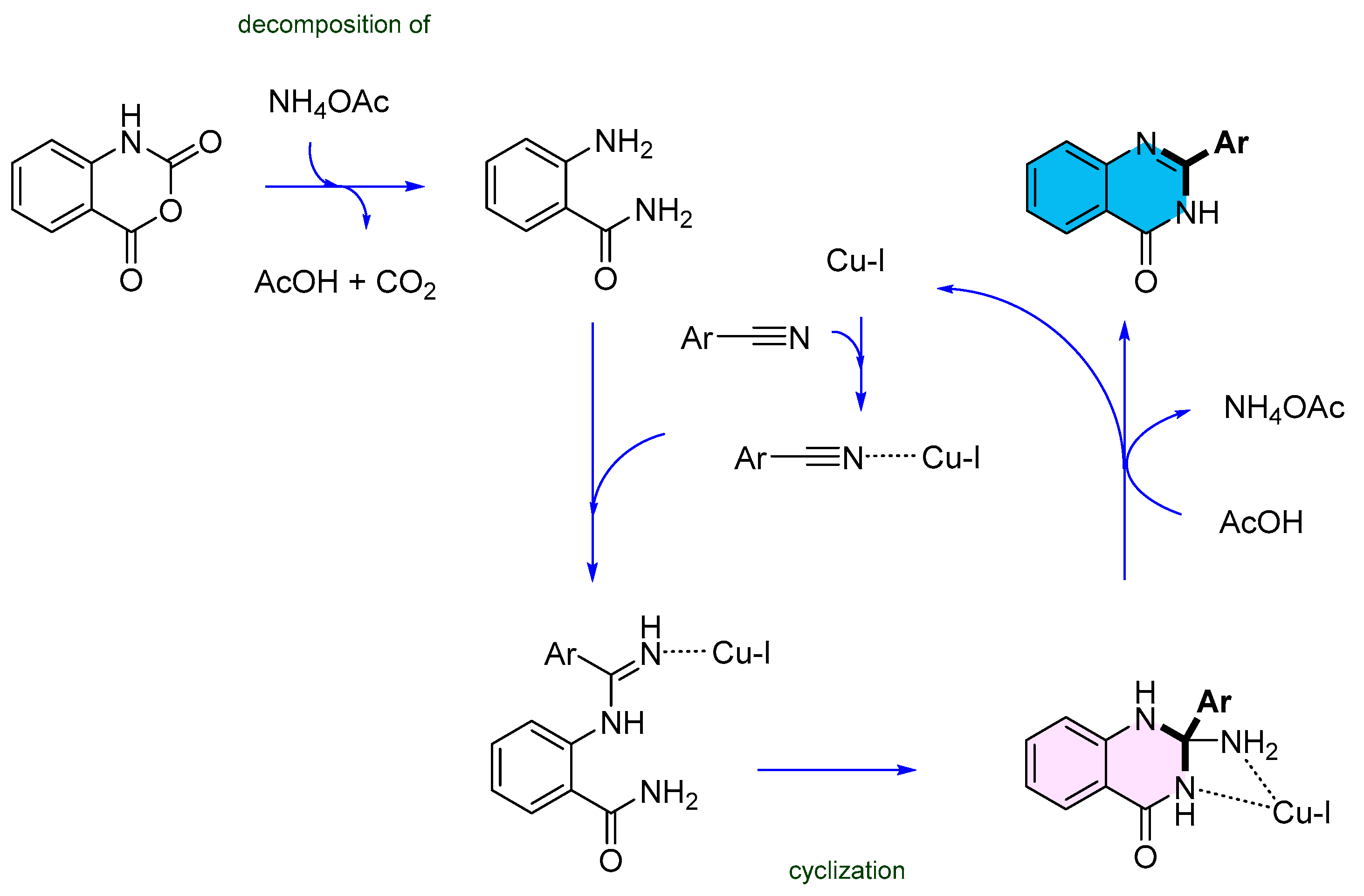
2.2. Copper-Catalyzed Multicomponent Synthesis of Quinazolinones
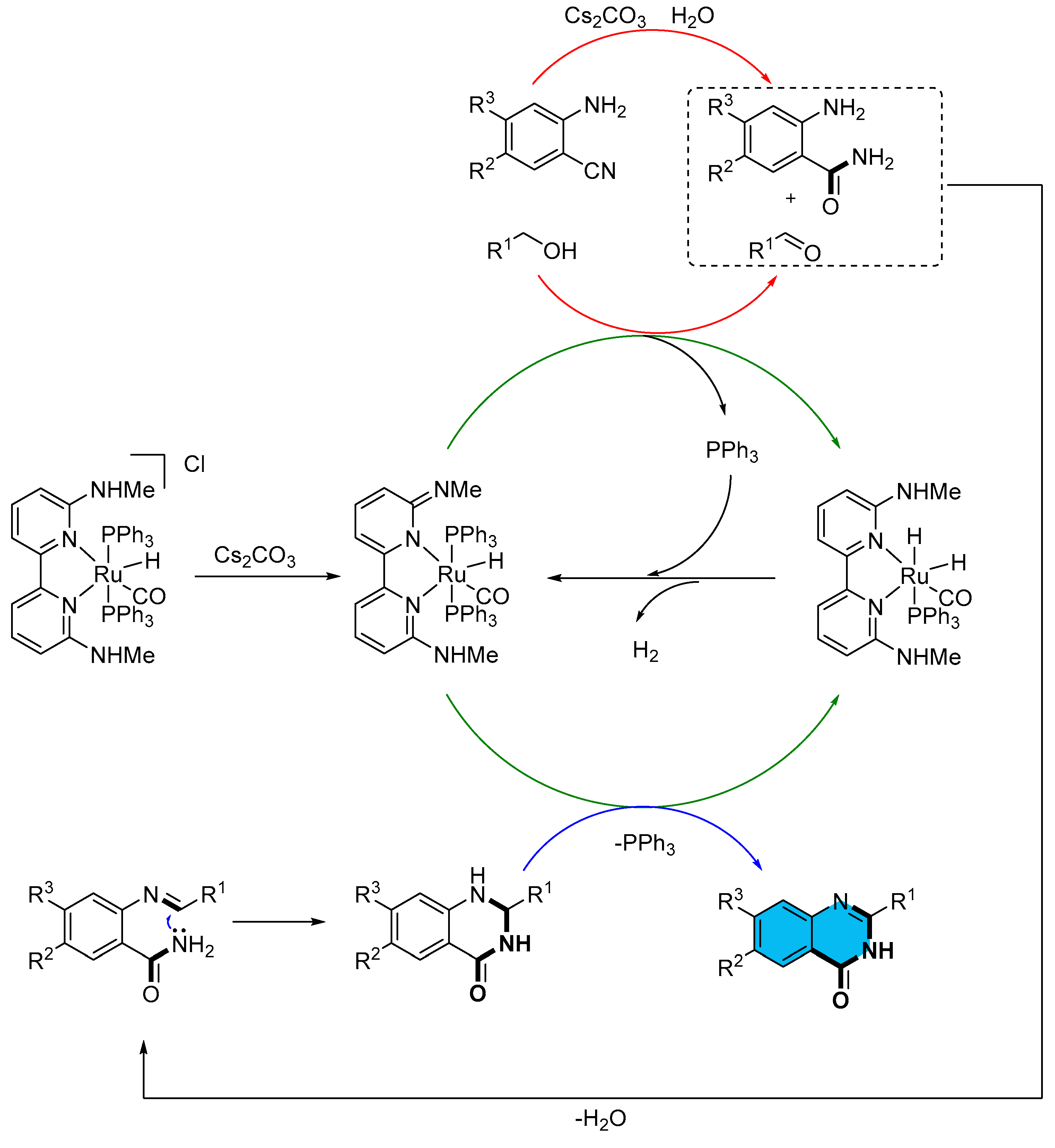
2.3. Ruthenium-Catalyzed Multicomponent Synthesis of Quinazolinones
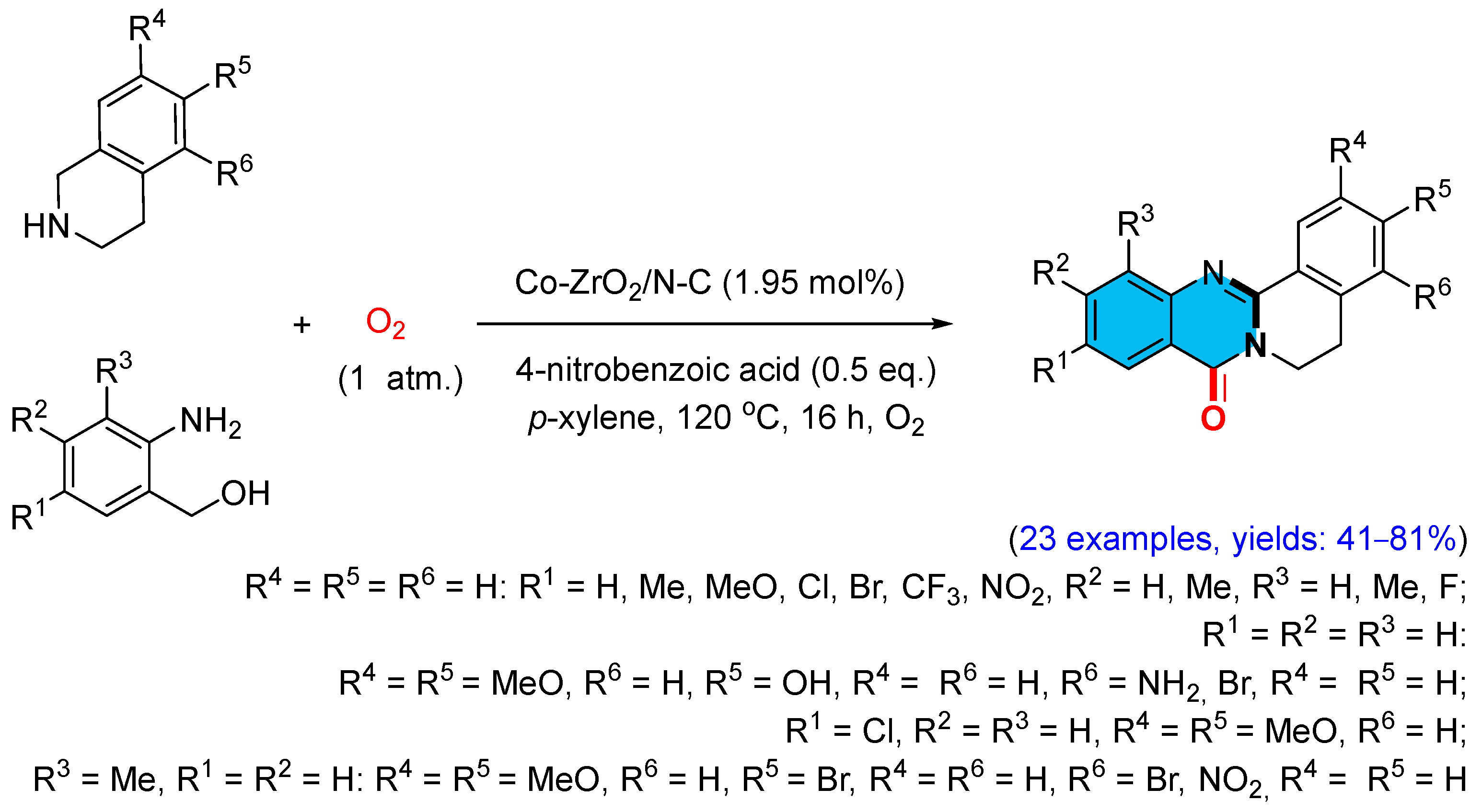
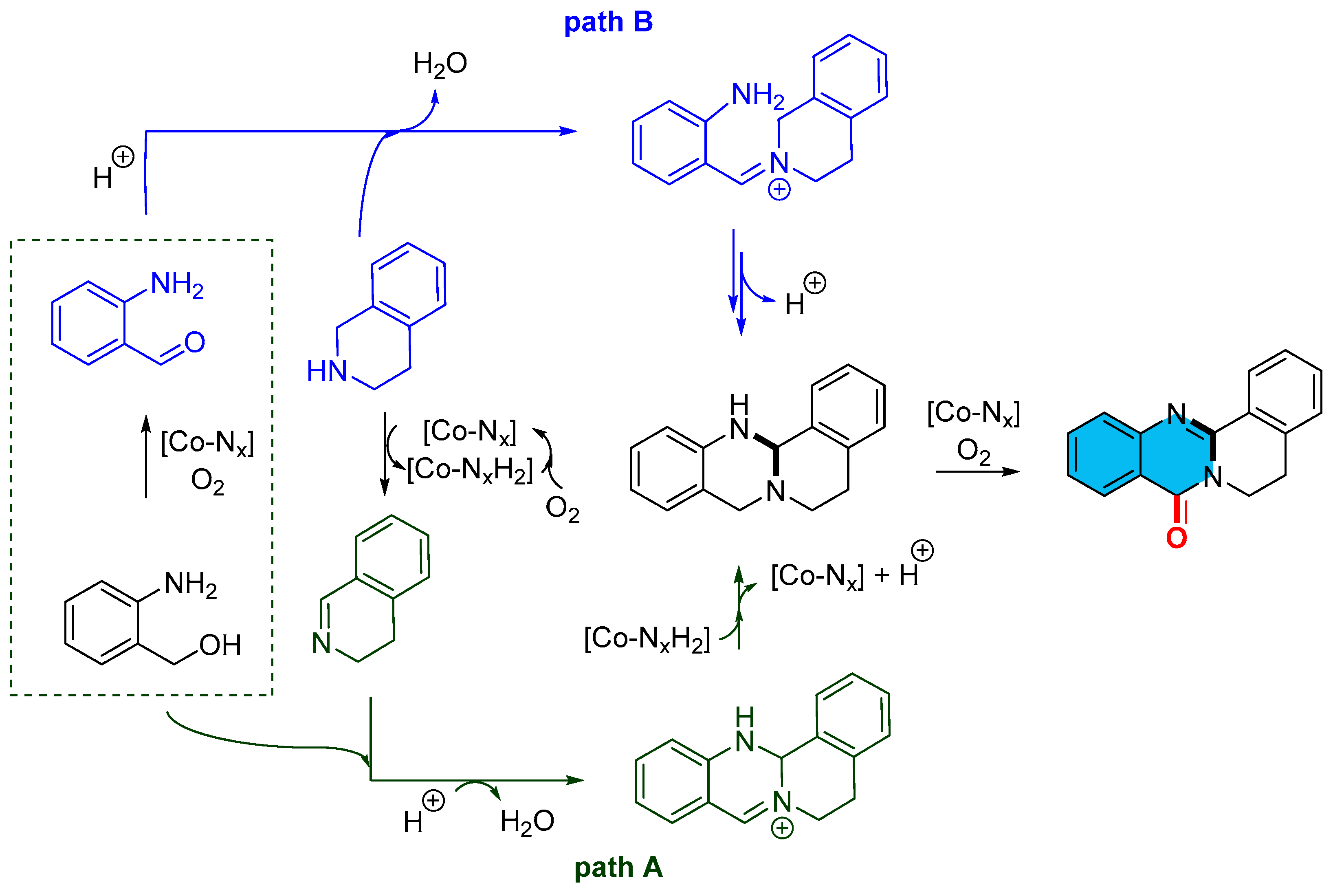
2.4. Cobalt-Catalyzed Multicomponent Synthesis of Quinazolinones
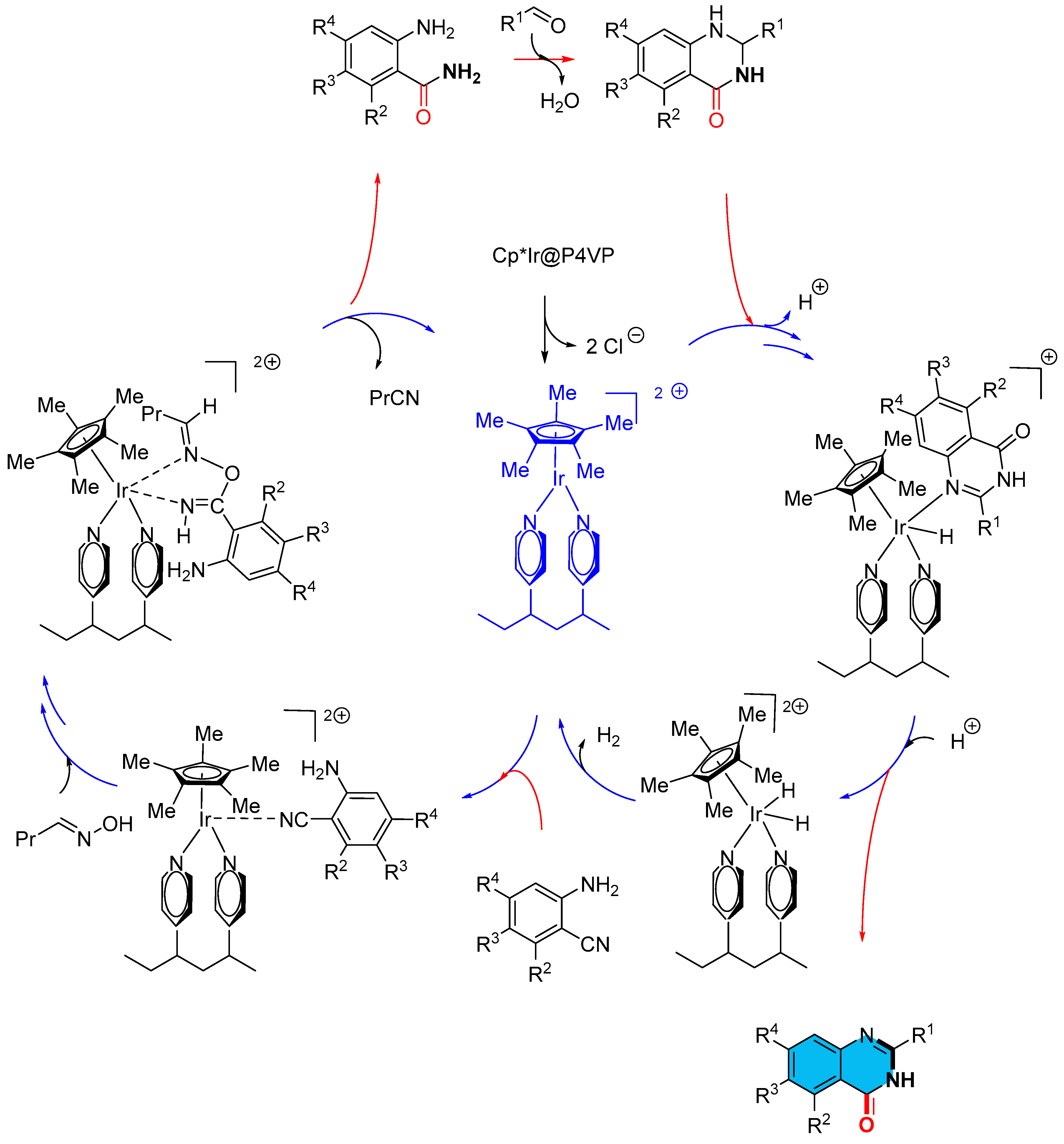
2.5. Iridium-Catalyzed Multicomponent Synthesis of Quinazolinones
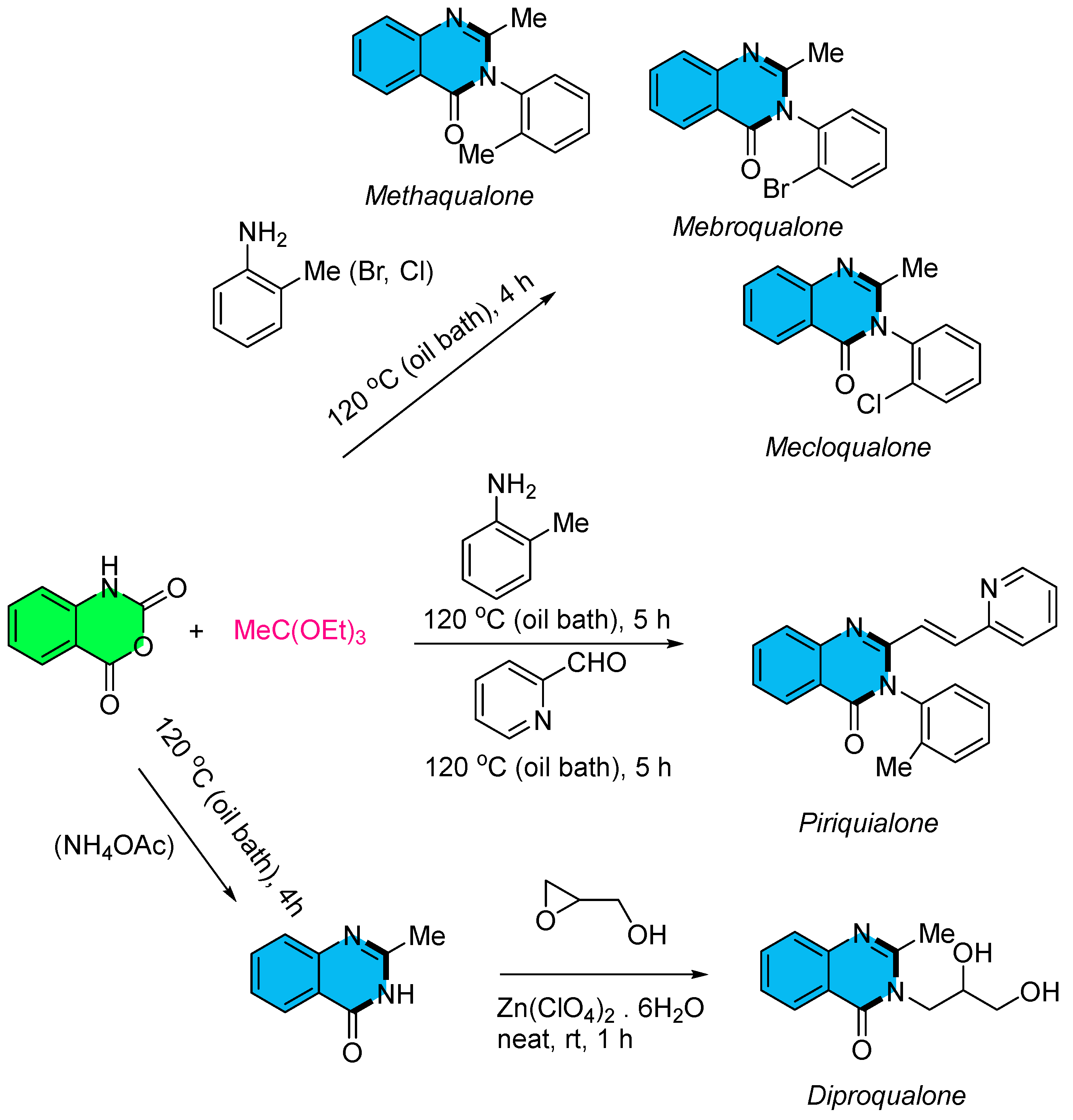
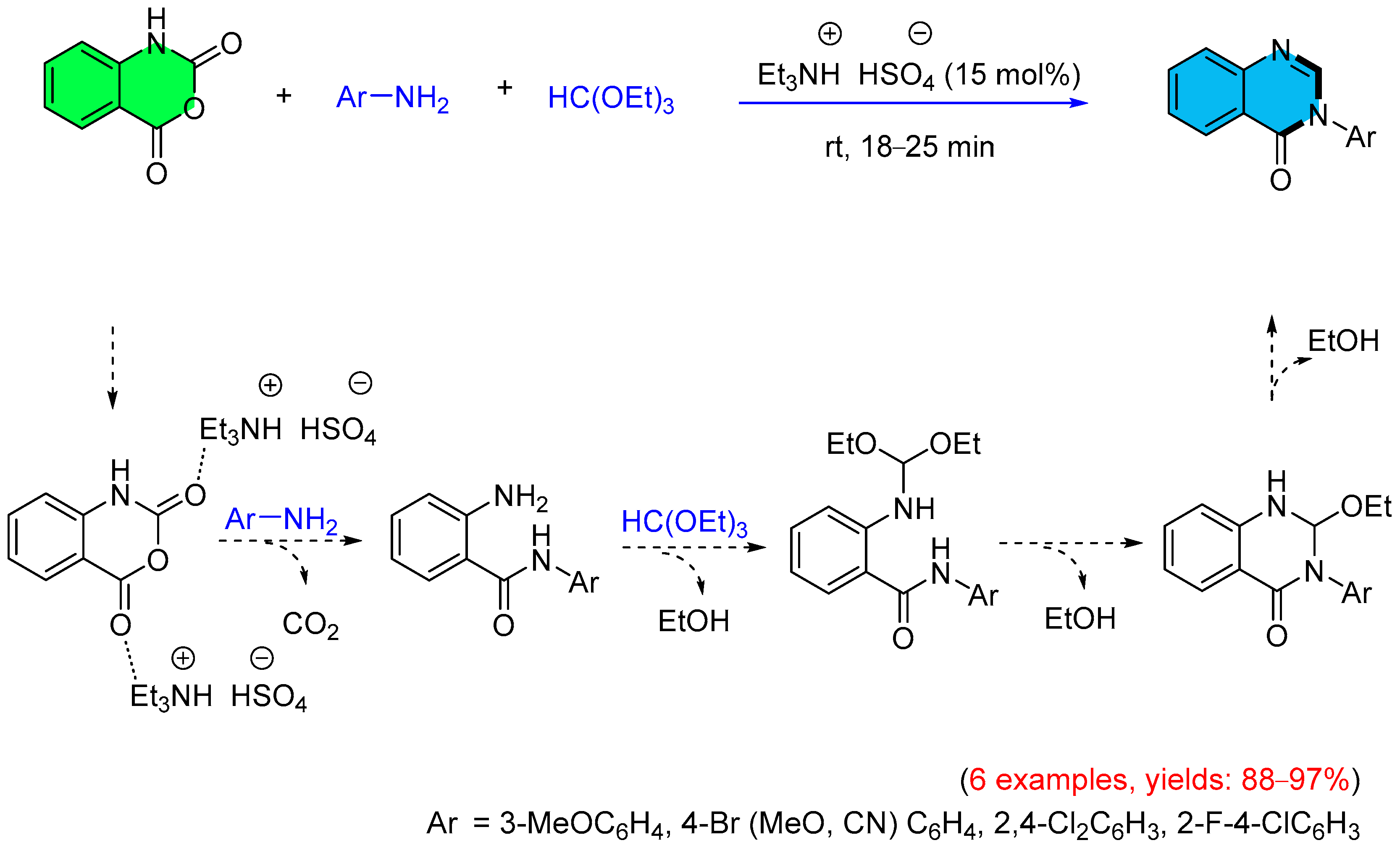
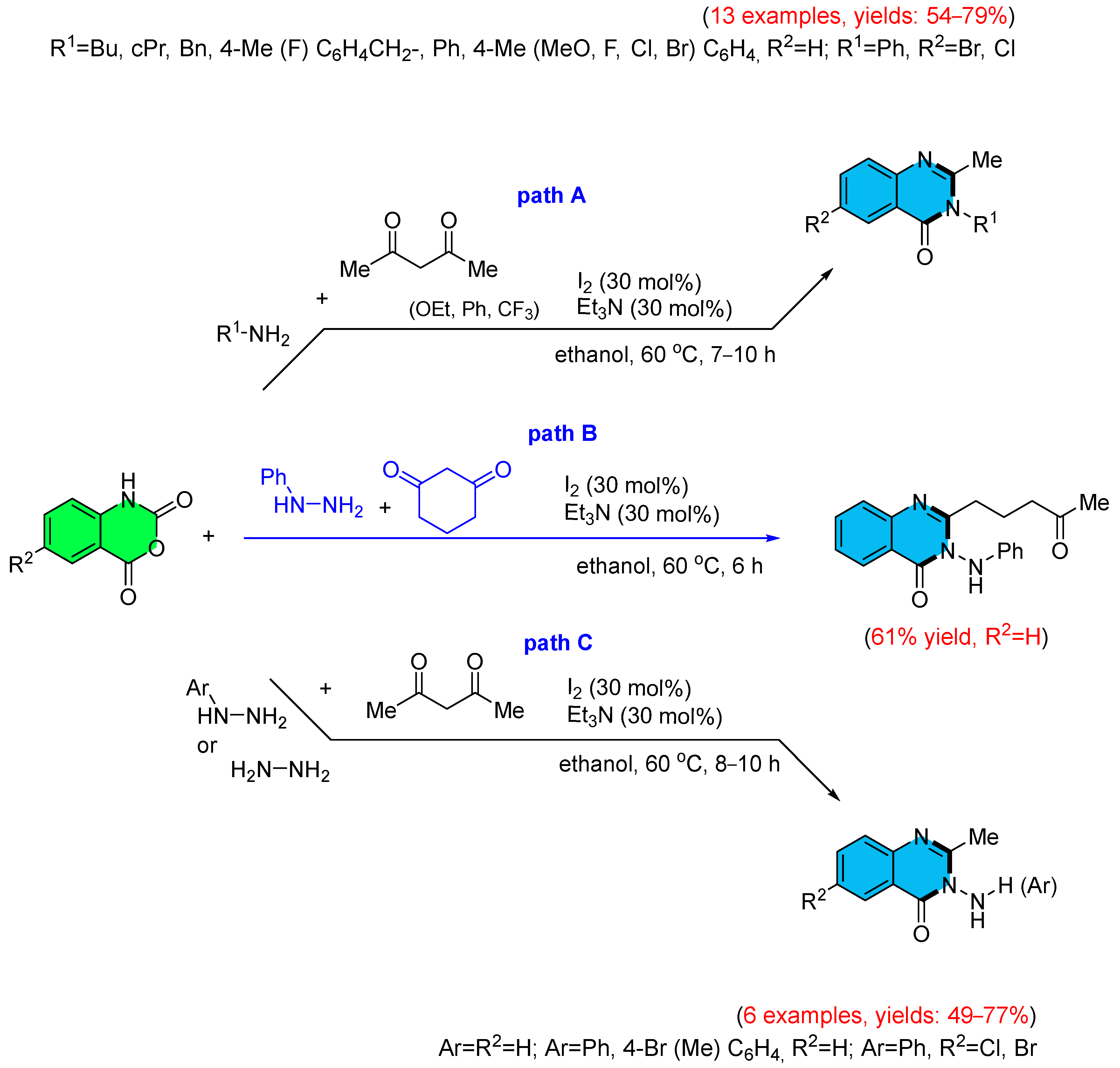
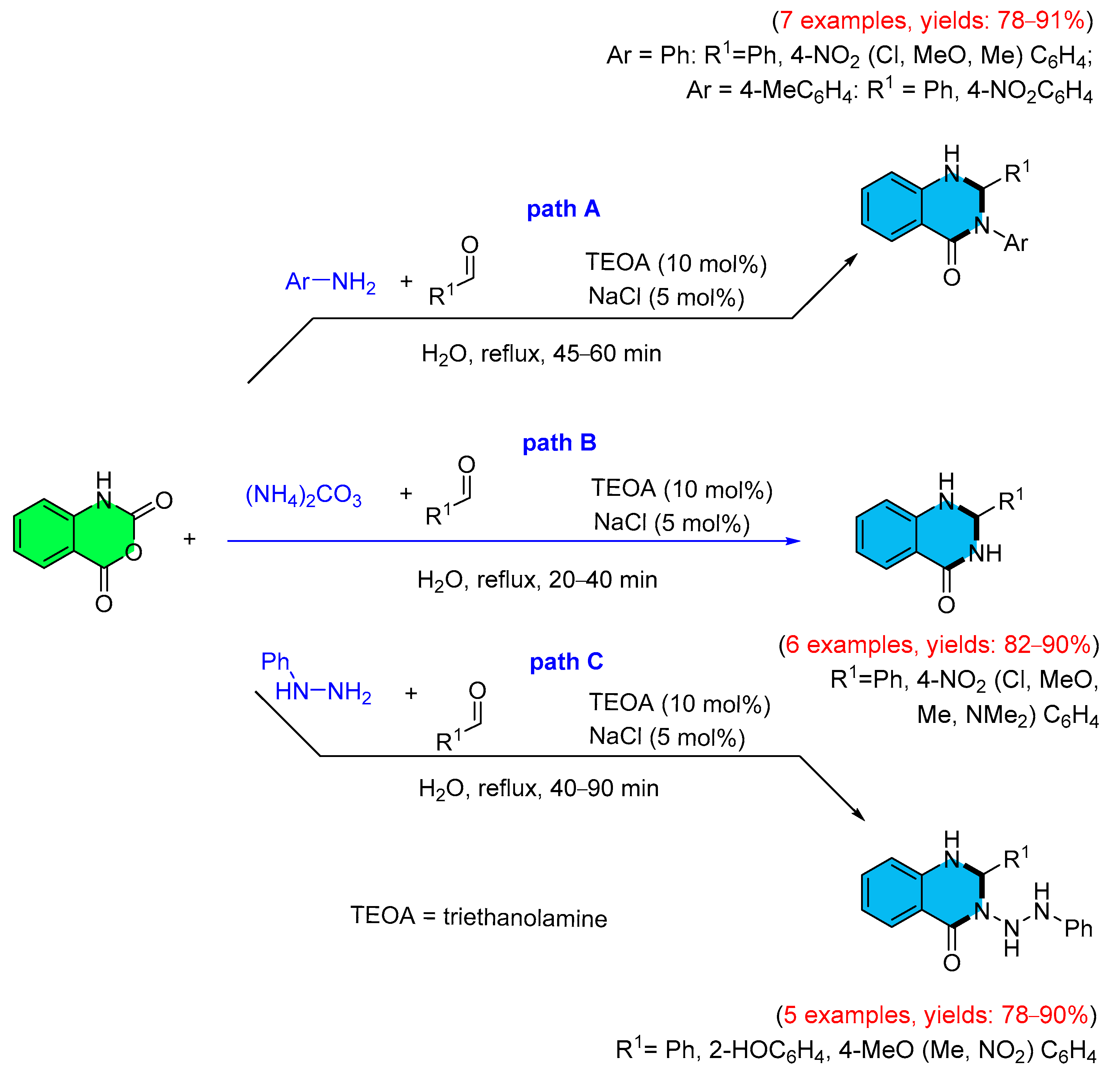
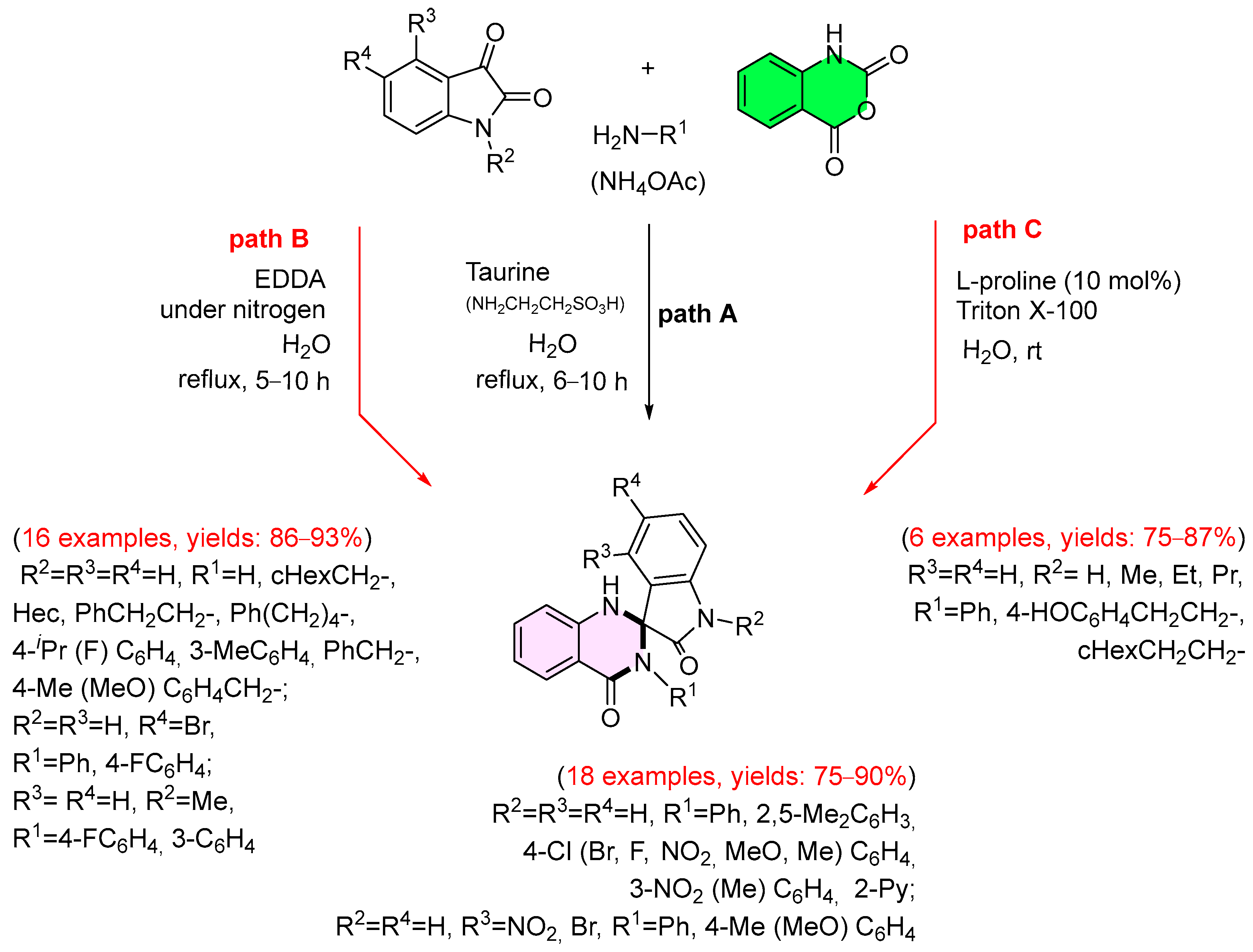
3. Strategies for the Synthesis of Quinazolinones Involving Isatoic Anhydride (Scheme 33)
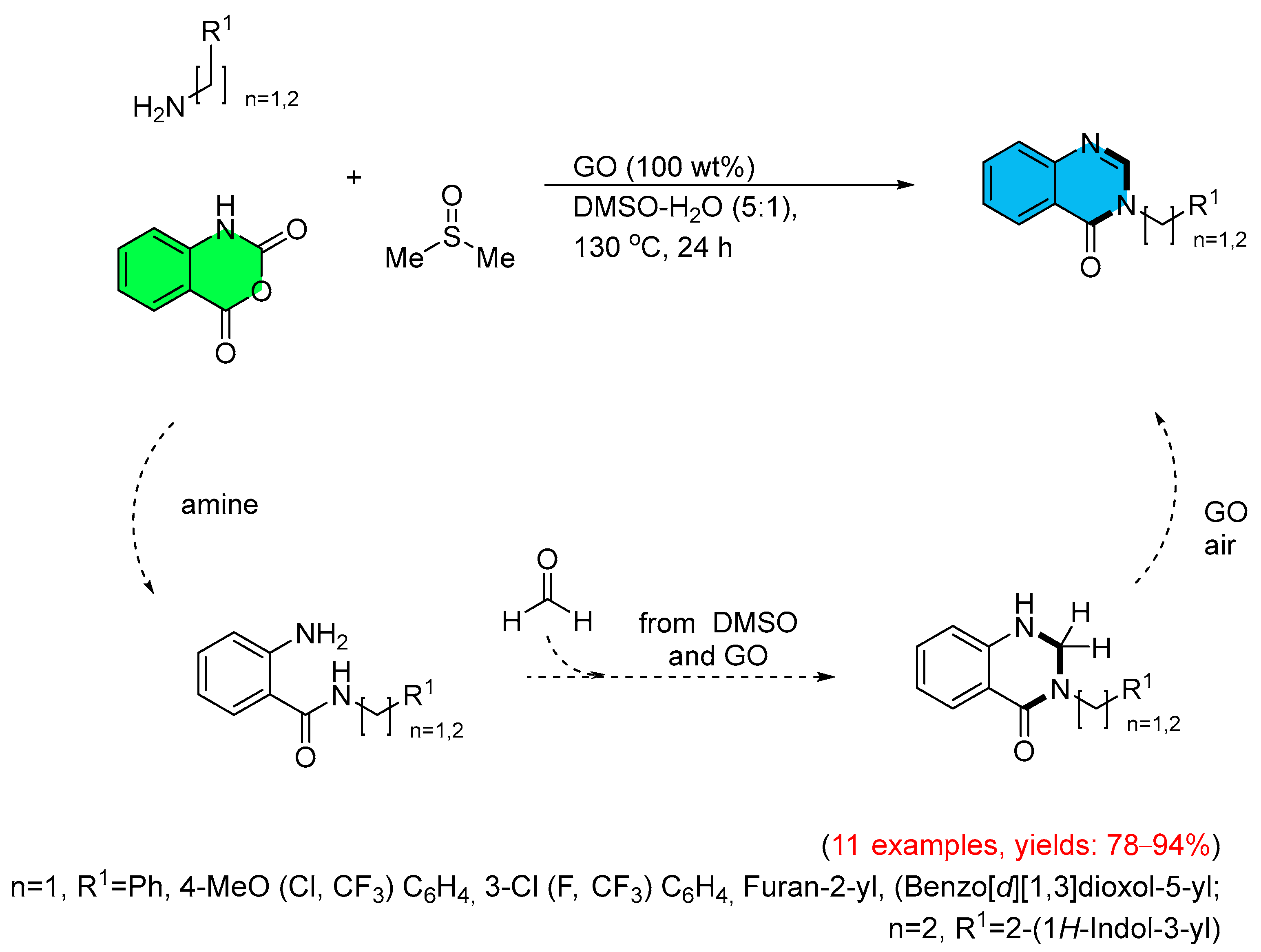
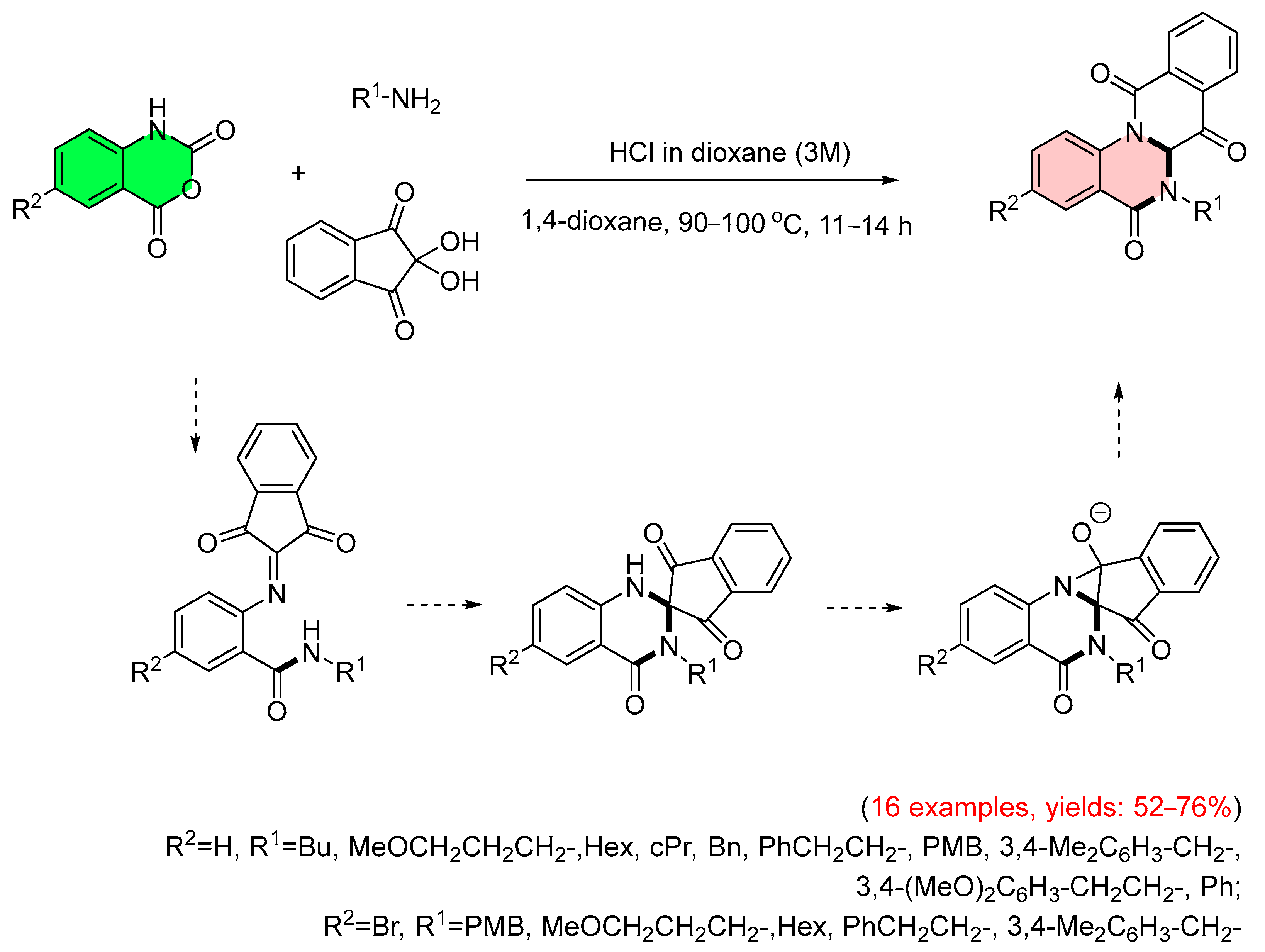
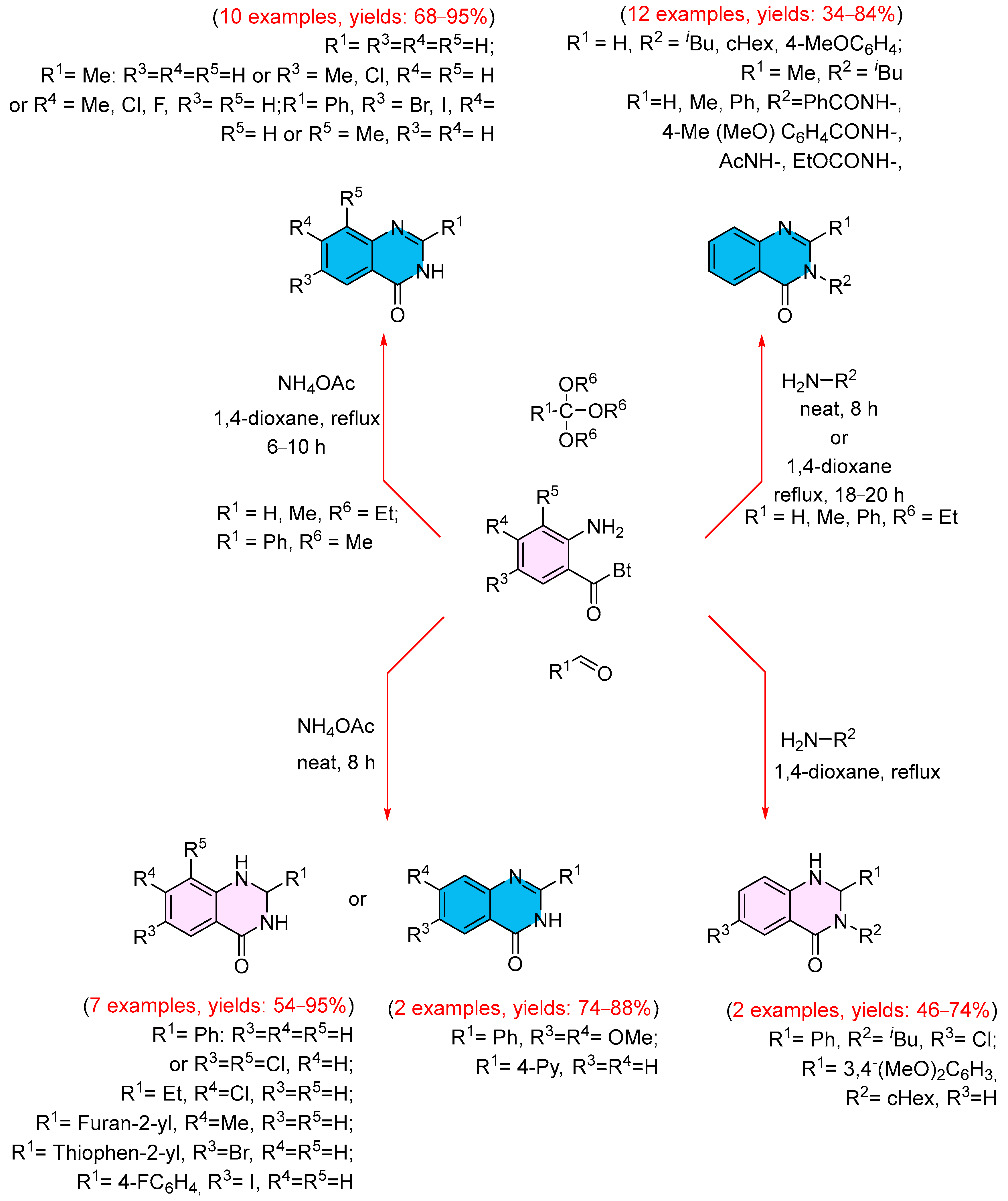
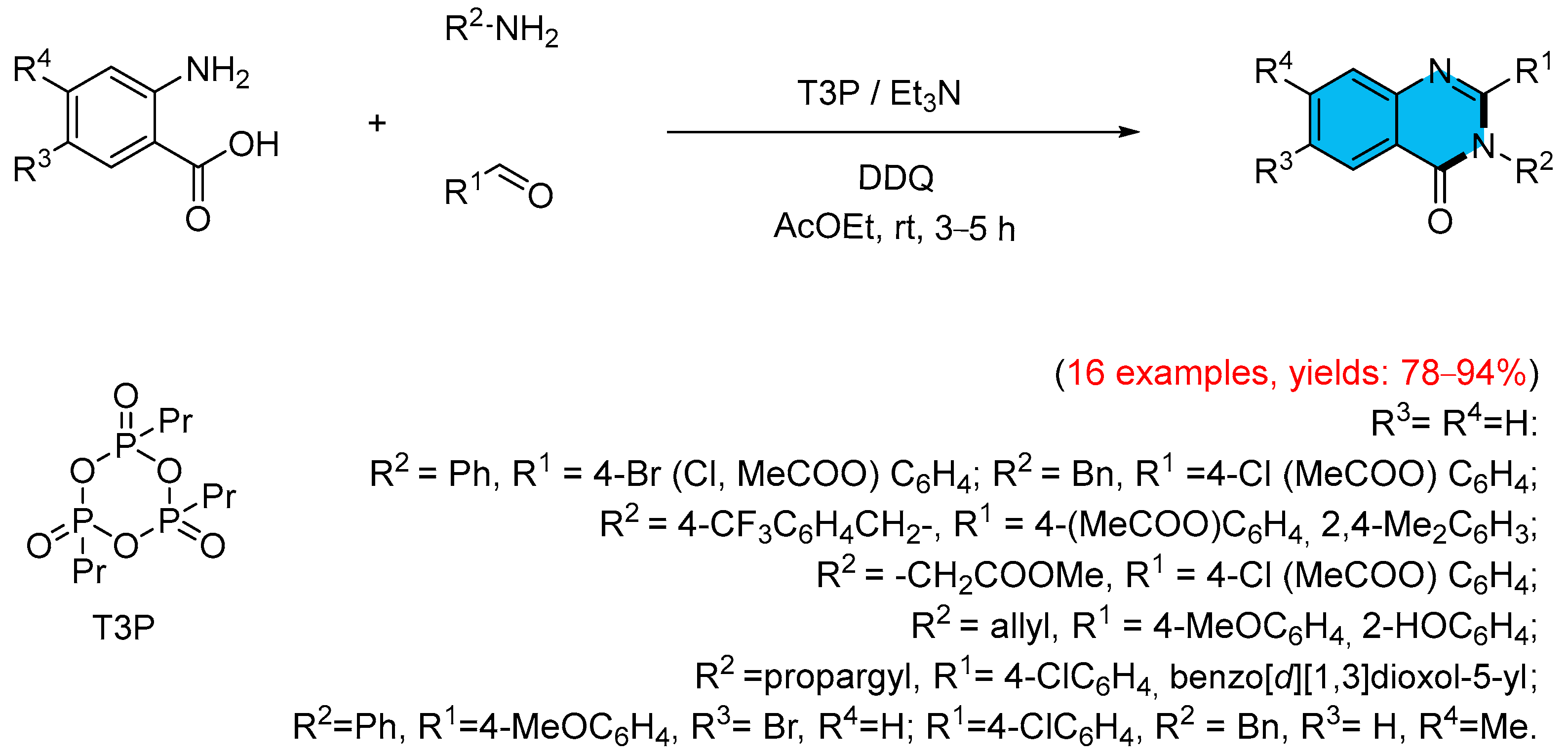
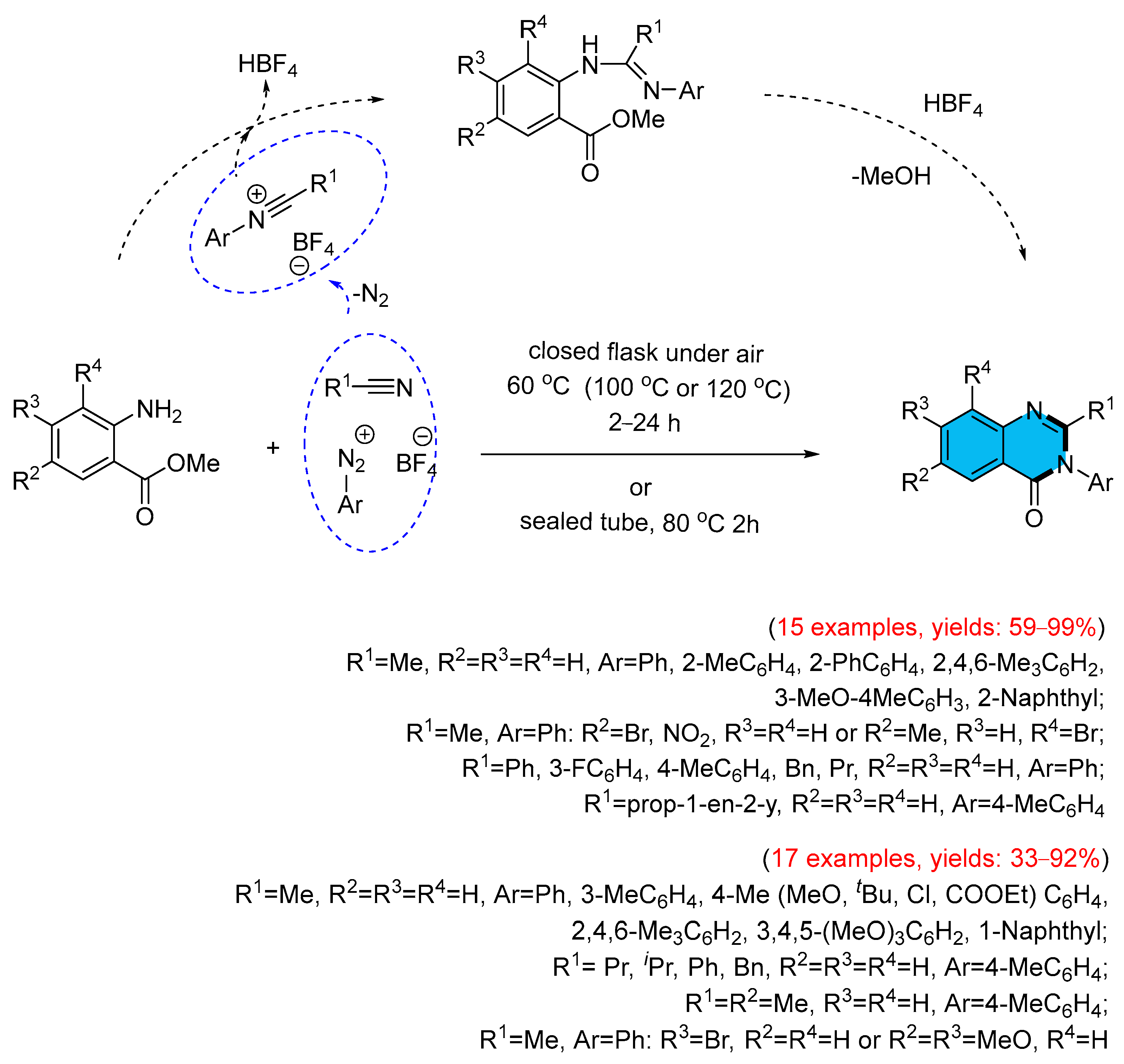
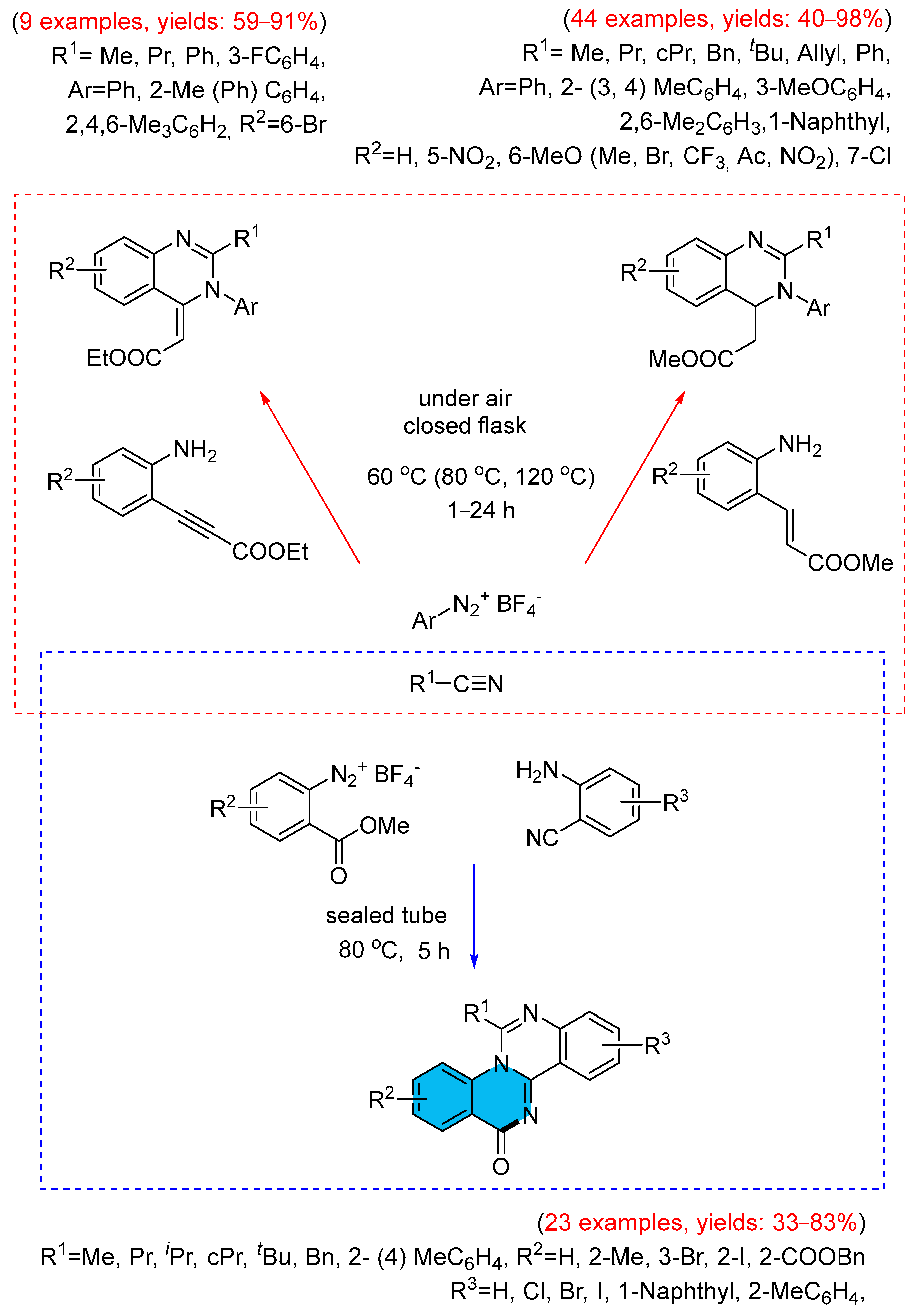
4. Alternative Synthetic Approaches
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| Ac | acetyl |
| AcOH | acetic acid |
| Bn | benzyl |
| Boc | tert-butoxycarbonyl |
| BuPAd2 | di-1-adamantyl-n-butylphosphine |
| Bu | n-butyl |
| iBu | isobutyl |
| tBu | tert-butyl |
| cBu | cyclobutyl |
| NaOtBu | sodium tert-butoxide |
| Bt | benzotriazol-1-yl |
| Cu(OAc)2 | copper(II) acetate |
| Cu(OTf)2 | copper(II) trifluoromethanesulfonate, copper(II) triflate |
| CMC | critical micelle concentration |
| DCE | 1,2-dichloroethane |
| DIPEA | N,N-diisopropylethylamine |
| DMAc | N,N-dimethylacetamide |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| DDQ | 2,3-dichloro-5,6-dicyano-1,4-benzoquinone |
| DMF | dimethylformamide |
| DMSO | dimethyl sulfoxide |
| DPPP | 1,3- bis(diphenylphosphino)propane |
| DTBP | di-tert-butyl peroxide |
| EDDA | ethylenediamine diacetate |
| GO | graphene oxide |
| rGO | reduced GO |
| N-rGO | nitrogen-doped reduced graphene oxide |
| LiHMDS, LHMDS | lithium hexamethyldisilazane, lithium bis(trimethylsilyl)amide |
| Me | methyl |
| MeO | methoxy |
| MeS | methylsulfanyl |
| MeCN | acetonitrile |
| NMe2 | dimethylamino |
| Ph | phenyl |
| PPh3 | triphenylphosphine |
| PCy3 | tricyclohexylphosphine |
| Xantphos | 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene |
| DPPB | 1,4-bis(diphenylphosphino)butane |
| BINAP | 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl |
| P4VP | poly(4-vinylpyridine) |
| [Cp*IrCl2]2 | pentamethylcyclopentadienyliridium(III) chloride, dimer |
| DPPF | 1,1′-ferrocenediyl-bis(diphenylphosphine) |
| DCM | dimethylchloromethane |
| Hex | n-hexyl |
| cHex | cyclohexyl |
| HBT | N-hydroxybenzotriazole |
| Pentyl | n-pentyl |
| Neopentyl | 2,2-dimethylpropyl |
| PEG-400 | poly(ethylene glycol) 400 |
| PMB | para-methoxybenzyl |
| Pr | n-propyl |
| iPr | isopropyl |
| cPr | cyclopropyl |
| 2-Py | 2-pyridyl |
| 3-Py | 3-pyridyl |
| 4-Py | 4-pyridyl |
| Pd(OAc)2 | palladium(II) acetate |
| PdCl2 | palladium(II) chloride |
| Pd(TFA)2 | palladium(II) trifluoroacetate |
| Pd2(dba)3 CHCl3 | tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct |
| Pd(PPh3)2Cl2 | bis(triphenylphosphine)palladium(II) dichloride |
| PTSA | para-toluenesulfonic acid |
| T3P | propylphosphonic anhydride |
| TPPMS | sodium diphenylphosphinobenzene-3-sulfonate |
| TBHP | tert-butyl hydroperoxide |
| TFA | trifluoroacetic acid |
| TEMPO | 2,2,6,6-tetramethyl-1-piperidinyloxy |
| TEOA | triethanolamine |
| THF | tetrahydrofuran |
| THF-2-yl | tetrahydrofuran-2-yl |
| Tol | p-tolyl |
| 2-MeTHF | 2-methyltetrahydrofuran |
| TMSN3 | trimethylsilyl azide |
| Ts | tosyl (p-CH3C6H4SO2) |
| TsN3 | p-toluenesulfonyl azide, tosyl azide |
| TsNH2 | p-toluenesulfonamide |
| Triton X-100 | polyethylene glycol tert-octylphenyl ether |
| US | ultrasound irradiation |
| MW | microwave irradiation |
References
- Boddapati, S.N.M.; Bollikolla, H.B.; Bhavani, K.G.; Saini, H.S.; Ramesh, N.; Jonnalagadda, S.B. Advances in Synthesis and Biological Activities of Quinazoline Scaffold Analogues: A review. Arab. J. Chem. 2023, 16, 105190–105228. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic Approaches, Functionalization and Therapeutic Potential of Quinazoline and Quinazolinone Skeletons: The Advances Continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Analgesic and Anti-Inflammatory Agents. ChemEngineering 2022, 6, 94–112. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Solomon, V.R.; Sheorey, R.V.; Jayakumar, R. 3-(3-Ethylphenyl)-2-substituted Hydrazino-3H-quinazolin-4-one Derivatives: New Class of Analgesic and Anti-Inflammatory Agents. Chem. Biol. Drug Des. 2009, 73, 471–479. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, L.; Bian, Y.; Li, Y.; Qu, J.; Song, F. The Antibacterial Activity of Quinazoline and Quinazolinone Hybrids. Curr. Top. Med. Chem. 2022, 22, 1035–1044. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. CNS Depressant and Anticonvulsant Activities of some Novel 3-[5-Substituted 1,3,4-thiadiazole-2-yl]-2-styrylquinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 1945–1954. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Shamel, M.; Satar, M.; Khalid, Y.; Mohamad, A.B. Antioxidant and Antimicrobial Activities of Novel Quinazolinones. Med. Chem. Res. 2014, 23, 236–242. [Google Scholar] [CrossRef]
- Gatadi, S.; Pulivendala, G.; Gour, J.; Malasala, S.; Bujji, S.; Parupalli, R.; Shaikh, M.; Godugu, C.; Nanduri, S. Synthesis and Evaluation of New 4(3H)-Quinazolinone Derivatives as Potential Anticancer Agents. J. Mol. Struct. 2020, 1200, 127097–127110. [Google Scholar] [CrossRef]
- Dohle, W.; Jourdan, F.L.; Menchon, G.; Prota, A.E.; Foster, P.A.; Mannion, P.; Hamel, E.; Thomas, M.P.; Kasprzyk, P.G.; Ferrandis, E.; et al. Quinazolinone-Based Anticancer Agents: Synthesis, Antiproliferative SAR, Antitubulin Activity, and Tubulin Co-Crystal Structure. J. Med. Chem. 2018, 61, 1031–1044. [Google Scholar] [CrossRef]
- Deng, Z.; Li, J.; Zhu, P.; Wang, J.; Kong, Y.; Hu, Y.; Cai, J.; Dong, C. Quinazolinones as Potential Anticancer Agents: Synthesis and Action Mechanisms. Biomolecules 2025, 15, 210–247. [Google Scholar] [CrossRef]
- Koepfli, J.B.; Mead, J.F.; Brockman, J.A., Jr. An Alkaloid with High Antimalarial Activity from Dichroa febrifuga. J. Am. Chem. Soc. 1947, 69, 1837. [Google Scholar] [CrossRef] [PubMed]
- An, C.-Y.; Li, X.-M.; Li, C.-S.; Wang, M.-H.; Xu, G.-M.; Wang, B.-G. Aniquinazolines A–D, Four New Quinazolinone Alkaloids from Marine-Derived Endophytic Fungus Aspergillus nidulans. Mar. Drugs 2013, 11, 2682–2694. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Fu, H.; Zhong, H.; Hong, K.; Zhu, W. Antifungal Quinazolinones from Marine-Derived Bacillus Cereus and Their Preparation. Bioorganic Med. Chem. Lett. 2011, 21, 4005–4007. [Google Scholar] [CrossRef]
- Vergotena, G.; Bailly, C. Molecular Modeling of Alkaloids Bouchardatine and Orirenierine Binding to Sirtuin-1 (SIRT1). Digit. Chin. Med. 2022, 5, 276–285. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, K.; Wang, Z.; Liu, Y.; Ma, D.; Wang, Q. Luotonin A and Its Derivatives as Novel Antiviral and Antiphytopathogenic Fungus Agents. J. Agric. Food Chem. 2020, 68, 8764–8773. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, J.; Wu, X.-F. Recent Advances in 4(3H)-Quinazolinone Syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- Liu, H.-L.; Li, X.-T.; Tian, H.-Z.; Sun, S.-W. Unexpected Insertion of Nitrogen into a C−C Bond: Access to 2,3-Disubstituted Quinazolinone Scaffolds. Org. Lett 2021, 23, 4579–4583. [Google Scholar] [CrossRef]
- Luo, J.; Wan, J.; Wu, L.; Yang, L.; Wang, T. tert-Butyl Hydroperoxide Promoted the Reaction of Quinazoline-3-oxides with Primary Amines Affording Quinazolin-4(3H)-ones. J. Org. Chem. 2022, 87, 9864–9874. [Google Scholar] [CrossRef]
- Lu, C.; Gong, C.; Zhao, B.; Hu, L.; Yao, Y. RE[N(SiMe3)2]3-Catalyzed Guanylation/Cyclization of Amino Acid Esters and Carbodiimides. J. Org. Chem. 2018, 83, 1154–1159. [Google Scholar] [CrossRef]
- Javahershenas, R.; Makarem, A.; Klika, K.D. Recent Advances in Microwave-Assisted Multicomponent Synthesis of Spiro Heterocycles. RSC Adv. 2024, 14, 5547–5565. [Google Scholar] [CrossRef]
- Nandi, S.; Jamatia, R.; Sarkar, R.; Sarkar, F.K.; Alam, S.; Pal, A.K. One-Pot Multicomponent Reaction: A Highly Versatile Strategy for the Construction of Valuable Nitrogen-Containing Heterocycles. ChemistrySelect 2022, 7, e202201901–e202201937. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, K.; Wang, W. Chemistry & Biology Of Multicomponent Reactions. Chem Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Abdou, I.M.; Al-Neyadi, S.S. Synthesis of Quinazolines and Quinazolinones via Palladium-Mediated Approach. Heterocycl. Commun. 2015, 21, 115–132. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Neumann, H.; Beller, M.; Wu, X.-F. Highly Efficient Four-Component Synthesis of 4(3H)-Quinazolinones: Palladium-Catalyzed Carbonylative Coupling Reactions. Angew. Chem. Int. Ed. 2014, 53, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Natte, K.; Neumann, H.; Wu, X.-F. Pd/C as an Efficient Heterogeneous Catalyst for Carbonylative Four-Component Synthesis of 4(3H)-Quinazolinones. Catal. Sci. Technol. 2015, 5, 4474–4480. [Google Scholar] [CrossRef]
- Shen, C.; Man, N.Y.T.; Stewart, S.; Wu, X.-F. Palladium-Catalyzed Dicarbonylative Synthesis of Tetracycle Quinazolinones. Org. Biomol. Chem. 2015, 13, 4422–4425. [Google Scholar] [CrossRef]
- Wu, X.F.; He, L.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylative Synthesis of Quinazolinones from 2-Aminobenzamide and Aryl Bromides. Chem. Eur. J. 2013, 19, 12635–12638. [Google Scholar] [CrossRef]
- You, S.; Huang, B.; Yan, T.; Cai, M. A Highly Efficient Heterogeneous Palladium-Catalyzed Carbonylative Annulation of 2-Aminobenzamides with Aryl Iodides Leading to Quinazolinones. J. Organomet. Chem. 2018, 875, 35–45. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, T.; Wang, J.-M.; Chen, Z.; Zhu, Y.-M.; Ji, S.-J. Palladium-Catalyzed One-Pot Synthesis of Quinazolinones via tert-Butyl Isocyanide Insertion. J. Org. Chem. 2014, 79, 5082–5087. [Google Scholar] [CrossRef]
- Qian, C.; Liu, K.; Tao, S.-W.; Zhang, F.-L.; Zhu, Y.-M.; Yang, S.-L. Palladium-Catalyzed Oxidative Three-Component Coupling of Anthranilamides with Isocyanides and Arylboronic Acids: Access to 2,3-Disubstituted Quinazolinones. J. Org. Chem. 2018, 83, 9201–9209. [Google Scholar] [CrossRef]
- Li, H.; He, L.; Neumann, H.; Beller, M.; Wu, X.-F. Cascade Synthesis of Quinazolinones from 2-Aminobenzonitriles and Aryl Bromides via Palladium-Catalyzed Carbonylation Reaction. Green Chem. 2014, 16, 1336–1343. [Google Scholar] [CrossRef]
- Chen, J.; Natte, K.; Spannenberg, A.; Neumann, H.; Langer, P.; Beller, M.; Wu, X.-F. Base-Controlled Selectivity in the Synthesis of Linear and Angular Fused Quinazolinones by a Palladium-Catalyzed Carbonylation/ Nucleophilic Aromatic Substitution Sequence. Angew. Chem. Int. Ed. 2014, 53, 7579–7583. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sharif, M.; Neumann, H.; Beller, M.; Wu, X.-F. A Convenient Palladium-Catalyzed Carbonylative Synthesis of 4(3H)-Quinazolinones from 2-Bromoformanilides and Organo Nitros with Mo(CO)6 as a Multiple Promoter. Green Chem. 2014, 16, 3763–3767. [Google Scholar] [CrossRef]
- Hikawa, H.; Nakayama, T.; Takahashi, M.; Kikkawa, S.; Azumaya, I. Direct Use of Benzylic Alcohols for Multicomponent Synthesis of 2-Aryl Quinazolinones Utilizing the π-Benzylpalladium(II) System in Water. Adv. Synth. Catal. 2021, 363, 4075–4084. [Google Scholar] [CrossRef]
- Hikawa, H.; Ino, Y.; Suzuki, H.; Yokoyama, Y. Pd-Catalyzed Benzylic C-H Amidation with Benzyl Alcohols in Water: A Strategy to Construct Quinazolinones. J. Org. Chem. 2012, 77, 7046–7051. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hikawa, H.; Enda, T.; Shoko Kikkawa, S.; Azumaya, I. Synthesis of 1-Aryl-β-Carbolines via a Pd-Catalyzed Oxidative Pictet-Spengler Reaction/Aromatization Cascade Using Benzylic Alcohols in Water. Eur. J. Org. Chem. 2025, 28, e202401269–e202401275. [Google Scholar] [CrossRef]
- Deutsch, C.; Krause, N.; Lipshutz, B.H. CuH-Catalyzed Reactions. Chem. Rev. 2008, 108, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W. Copper-Catalyzed C–H Functionalization Reactions: Efficient Synthesis of Heterocycles. Chem. Rev. 2015, 115, 1622–1651. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, Y.; Tao, L.; Zhang, W.; Fan, X. Rapid Assembly of Quinazolinone Scaffold via Copper-Catalyzed Tandem Reaction of 2-Bromobenzamides with Aldehydes and Aqueous Ammonia: Application to the Synthesis of the Alkaloid Tryptanthrin. RSC Adv. 2014, 4, 59289–59296. [Google Scholar] [CrossRef]
- Liang, Y.; Tan, Z.; Jiang, H.; Zhu, Z.; Zhang, M. Copper-Catalyzed Oxidative Multicomponent Annulation Reaction for Direct Synthesis of Quinazolinones via an Imine-Protection Strategy. Org. Lett. 2019, 21, 4725–4728. [Google Scholar] [CrossRef]
- Shinde, M.H.; Kshirsagar, U.A. A Copper Catalyzed Multicomponent Cascade Redox Reaction for the Synthesis of Quinazolinones. RSC Adv. 2016, 6, 52884–52887. [Google Scholar] [CrossRef]
- Upadhyaya, K.; Thakur, R.K.; Shukla, S.K.; Tripathi, R.T. One-Pot Copper(I)-Catalyzed Ligand/Base-Free Tandem Cyclooxidative Synthesis of Quinazolinones. J. Org. Chem. 2016, 81, 5046–5055. [Google Scholar] [CrossRef]
- Srishyalam, V.; Devanna, N.; Rao, M.V.B.; Mulakayala, N. Novel One Pot Synthesis of Substituted Quinazolin-4(3H)-ones and Pyrazolo[4,3-d]pyrimidin-7(6H)-ones using Copper Iodide, Azides and Terminal Alkynes. Tetrahedron Lett. 2017, 58, 2889–2892. [Google Scholar] [CrossRef]
- Sacramento, M.; Piúma, L.P.A.; Nascimento, J.E.R.; Cargnelutti, R.; Raquel, G.; Jacob, R.G.J.; Lenardão, E.J.; Alves, D. Selective Synthesis of 2-(1,2,3-Triazoyl) Quinazolinones through Copper-Catalyzed Multicomponent Reaction. Catalysts 2021, 11, 1170–1180. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Solomon, V.R.; Murugan, M. Synthesis and Pharmacological Investigation of Novel 4-Benzyl-1-substituted- 4H-[1,2,4]triazolo[4,3-a]quinazolin-5-ones as new Class of H1-Antihistaminic Agents. Bioorg. Med. Chem. 2007, 15, 4009–4015. [Google Scholar] [CrossRef]
- Driowya, M.; Leclercq, J.; Verones, V.; Barczyk, A.; Lecoeur, M.; Renault, N.; Flouquet, N.; Ghinet, A.; Berthelot, P.; Lebegue, N. Synthesis of Triazoloquinazolinone Based Compounds as Tubulin Polymerization Inhibitors and Vascular Disrupting Agents. Eur. J. Med. Chem. 2016, 115, 393–405. [Google Scholar] [CrossRef]
- Bertelli, L.; Biagi, G.; Giorgi, I.; Livi, O.; Manera, C.; Scartoni, V.; Lucacchini, A.; Giannaccini, G.; Barili, P.L. Substituted 1,2,3-triazolo[1,5-a]quinazolines: Synthesis and Binding to Benzodiazepine and Adenosine Receptors. Eur. J. Med. Chem. 2000, 35, 333–341. [Google Scholar] [CrossRef]
- Soheilizad, M.; Soroosh, S.; Pashazadeh, R. Solvent-free Copper-catalyzed Three-component Synthesis of 2-Substituted Quinazolin-4(3H)-ones. Monatsh. Chem. 2017, 148, 739–743. [Google Scholar] [CrossRef]
- Roy, B.C.; Samim, S.A.; Panja, D.; Kundu, S. Tandem Synthesis of Quinazolinone Scaffolds from 2-Aminobenzonitriles using Aliphatic Alcohol–Water System. Catal. Sci. Technol. 2019; 9, 6002–6006. [Google Scholar] [CrossRef]
- Xie, F.; Chen, Q.-H.; Xie, R.; Jiang, H.-F.; Zhang, M. MOF-Derived Nanocobalt for Oxidative Functionalization of Cyclic Amines to Quinazolinones with 2-Aminoarylmethanols. ACS Catal. 2018, 8, 5869–5874. [Google Scholar] [CrossRef]
- Hao, S.; Yang, J.; Liu, P.; Xu, J.; Yang, C.; Li, F. Linear-Organic-Polymer-Supported Iridium Complex as a Recyclable Auto-Tandem Catalyst for the Synthesis of Quinazolinones via Selective Hydration/Acceptorless Dehydrogenative Coupling from o-Aminobenzonitriles. Org. Lett. 2021, 23, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, P.; Li, F. Quinazolinones from o-Aminobenzonitriles by One-Pot Sequential Selective Hydration/Condensation/Acceptorless Dehydrogenation Catalyzed by an Iridium Complex. ChemCatChem 2016, 8, 1523–1530. [Google Scholar] [CrossRef]
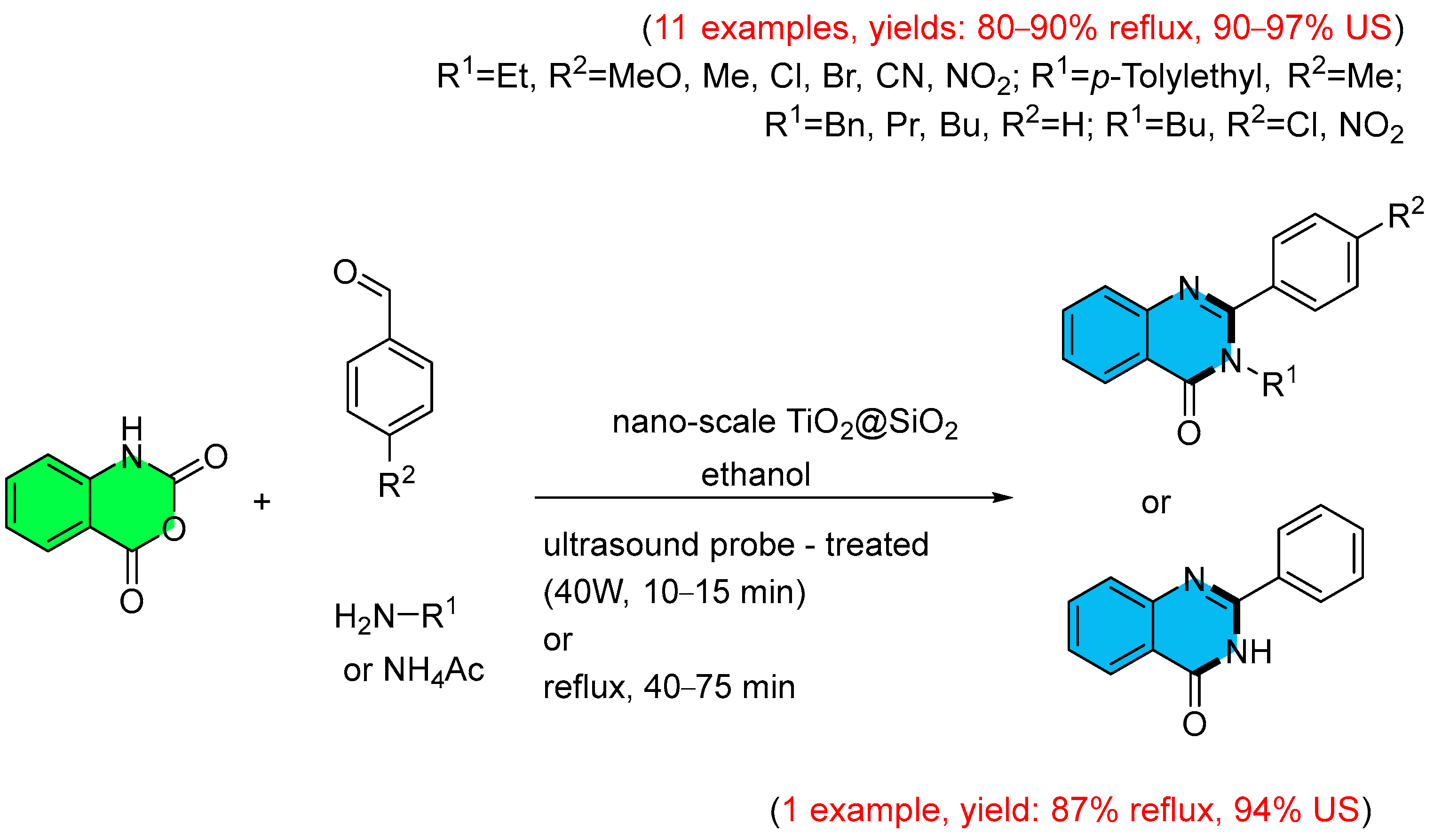
- Farhang, M.R.; Fadaeian, M.; Najafi, G.N.; sadat Sharif, M. Ultrasound Probe-Assisted Fabrication of 2,3-Disubstituted Quinazoline-4 (3H)-one Framework in the Existence of SiO2-Decorated Nano-Scale TiO2 Composite and Investigating Their Antibacterial Attributes via Molecular Docking Simulations. J. Saudi Chem. Soc. 2024, 28, 101942–101952. [Google Scholar] [CrossRef]
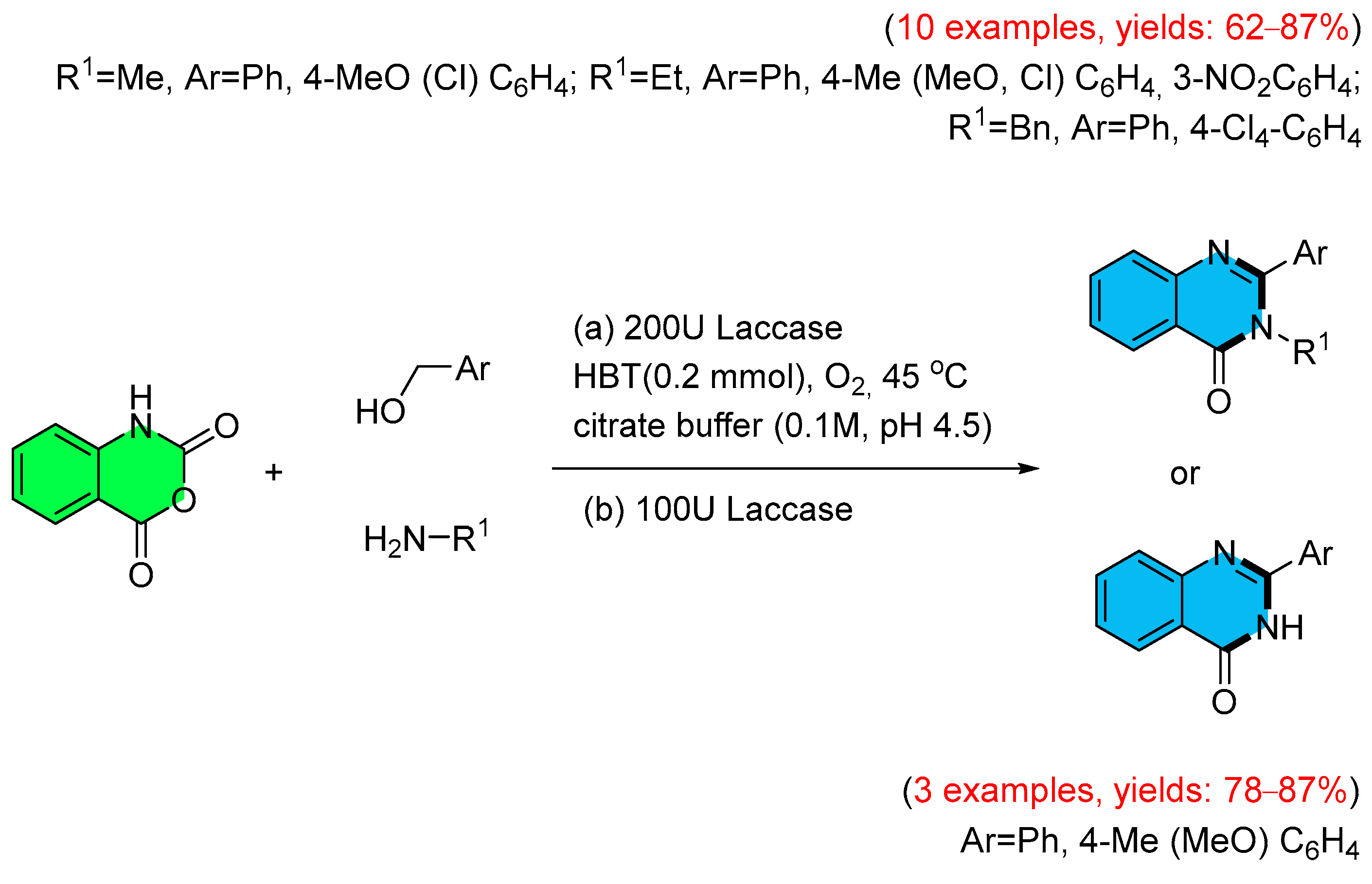
- Heidary, M.; Khoobi, M.; Ghasemi, S.; Habibi, Z.; Faramarzi, M.A. Synthesis of Quinazolinones from Alcohols via Laccase-Mediated Tandem Oxidation. Adv. Synth. Catal. 2014, 356, 1789–1794. [Google Scholar] [CrossRef]
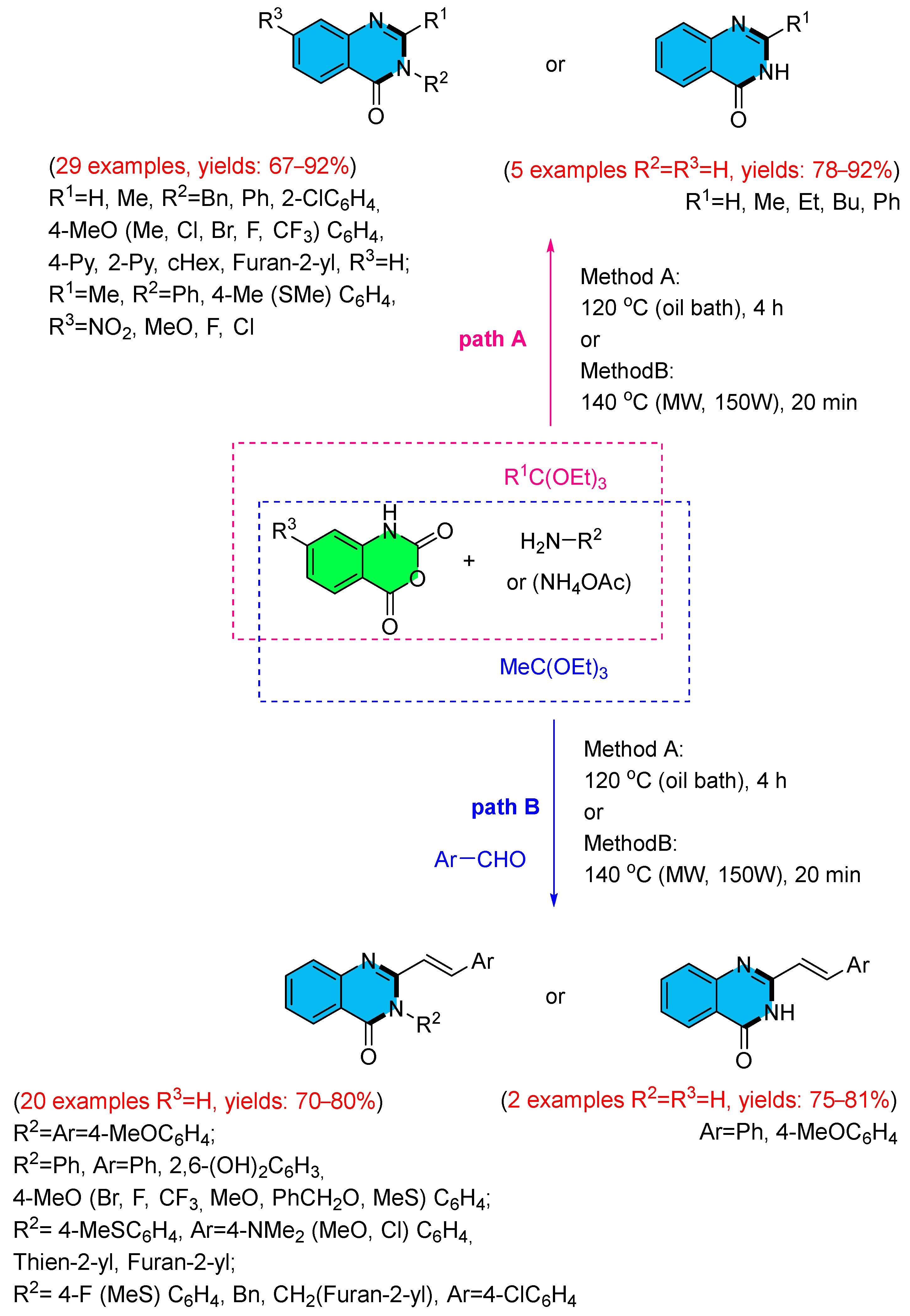
- Kumar, D.; Jadhavar, P.S.; Nautiyal, M.; Sharma, H.; Meena, P.H.; Adane, L.; Pancholia, S.; Chakraborti, A.K. Convenient Synthesis of 2,3-Disubstituted Quinazolin-4(3H)-ones and 2-Styryl-3-substituted Quinazolin-4(3H)-ones: Applications Towards the Synthesis of Drugs. RSC Adv. 2015, 5, 30819–30825. [Google Scholar] [CrossRef]
- Kakati, P.; Singh, P.; Yadav, P.; Awasthi, S.K. Aiding the Versatility of Simple Ammonium Ionic Liquids by the Synthesis of Bioactive 1,2,3,4-Tetrahydropyrimidine, 2-Aminothiazole and Quinazolinone Derivatives. New J. Chem. 2021, 45, 6724–6738. [Google Scholar] [CrossRef]
- Sarkar, S.; Mandal, S.; Pramanik, A. One-Pot Three Component Synthesis of Quinazolin-4(3H)-one Derivatives: Investigation of Photophysical Properties and FRET Application toward Protein Lysozyme. Polycycl. Aromat. Compd. 2024, 44, 1896–1917. [Google Scholar] [CrossRef]
- Khandebharad, A.U.; Sarda, S.R.; Gill, C.H.; Agrawal, B.R. Synthesis of Quinazolinone Derivatives Catalyzed by Triethanolamine/NaCl in Aqueous Media. Polycycl. Aromat. Compd. 2020, 40, 437–445. [Google Scholar] [CrossRef]
- Dadgar, M.; Kalkhorani, N.M. [g-Fe2O3-HAp-(CH2)3-NHSO3H] Nanoparticles As A Highly Efficient and Magnetically Separable Catalyst for Green One-Pot Synthesis of 4(3H)-Quinazolinones. Int. J. Nano Dimens. 2015, 6, 473–478. [Google Scholar] [CrossRef]
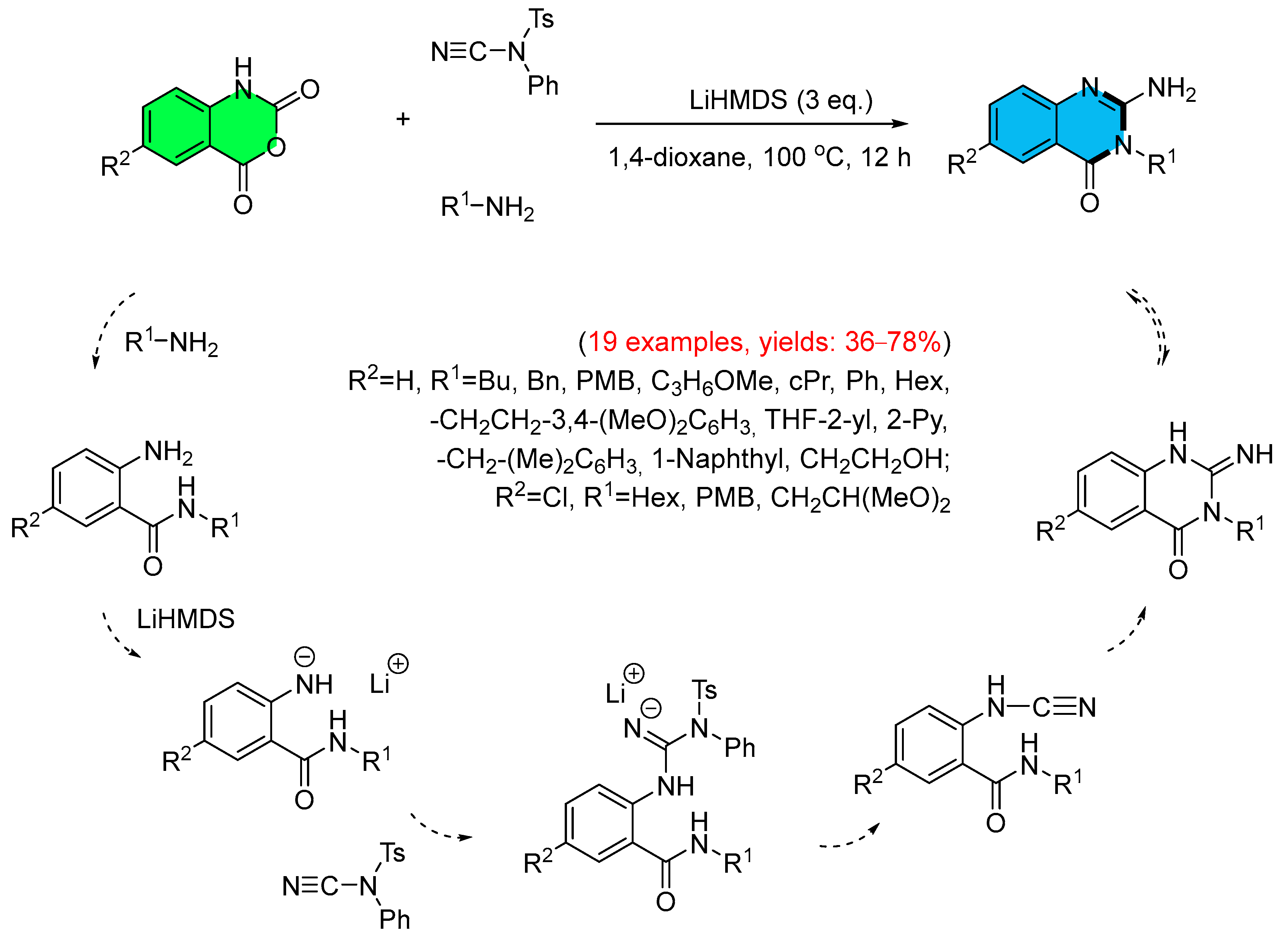
- Murthy, V.N.; Nikumbh, S.P.; Kumar, S.P.; Rao, L.V.; Raghunadh, A. An Efficient One-Pot Synthesis of 2-Aminoquinazolin-4(3H)-one Derivative via MCR Strategy. Tetrahedron Lett. 2015, 56, 5767–5770. [Google Scholar] [CrossRef]
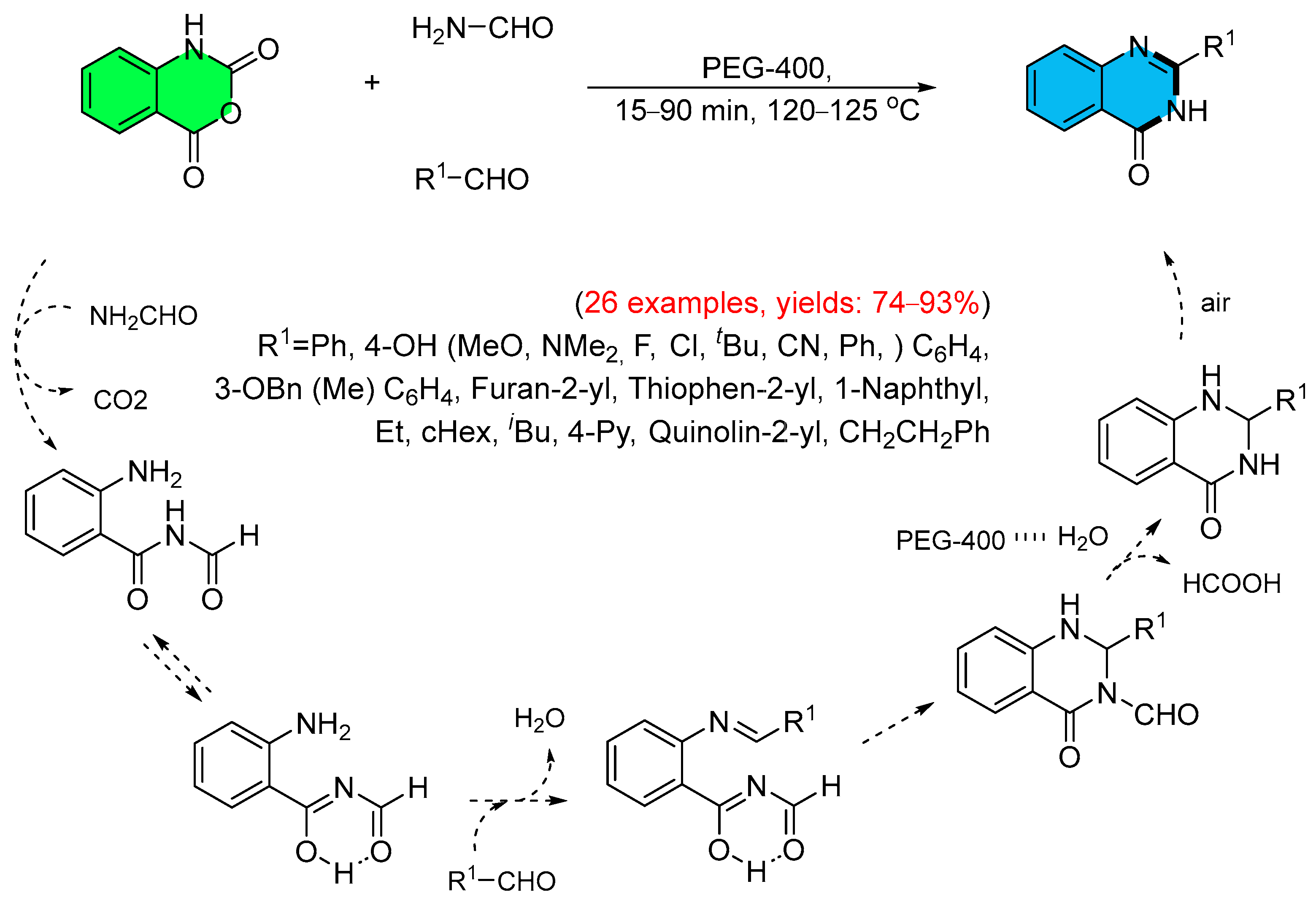
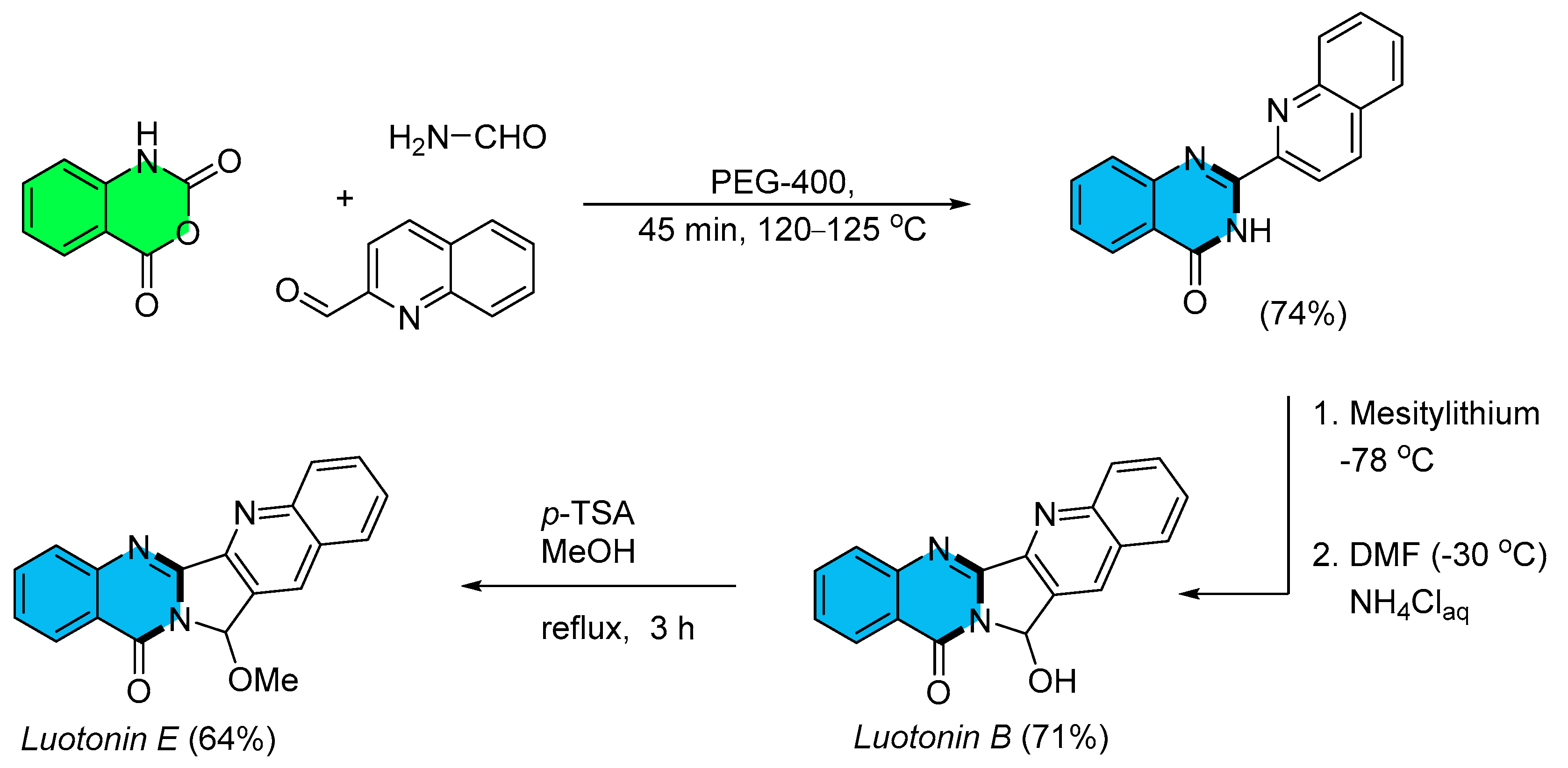
- Rao, K.R.; Mekala, R.; Raghunadh, A.; Meruva, S.B.; Kumar, S.P.; Kalita, D.; Laxminarayana, E.; Prasad, B.; Pal, M. A Catalyst-free Rapid, Practical and General Synthesis of 2-Substituted Quinazolin-4(3H)-ones Leading to Luotonin B and E, Bouchardatine and 8-Norrutaecarpine. RSC Adv. 2015, 5, 61575–61579. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Afsar, S.Y.; Gholamzadeh, P.; Badiei, A. Synthesis of Quinazolinone Derivatives through Multicomponent/Click Reactions. Org. Chem. Res. 2019, 5, 64–72. [Google Scholar] [CrossRef]
- Hada, S.; Zai, M.S.K.Z.; Roat, P.; Verma, V.P.; Shah, A.K.; Yadav, D.K.; Kumari, N. Metal-Free Graphene Oxide Promoted a Novel Multicomponent Reaction for the Synthesis of 3-Substituted Quinazolinones Using DMSO as One Carbon Synthon. ChemistrySelect 2019, 4, 1176–1179. [Google Scholar] [CrossRef]
- Mitobe, Y.; Ito, S.; Mizutani, T.; Nagase, T.; Sato, N.; Tokita, S. Development of a Selective and Potent Radioactive Ligand for Histamine H3 Receptors: A Compound Potentially Useful for Receptor Occupancy Studies. Bioorg. Med. Chem. Lett. 2009, 19, 4075–4078. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Dabiri, M.; Qaraat, H. A Regioselective Three-Component Reaction for Synthesis of Novel 1’H-Spiro[isoindoline-1,2’-quinazoline]-3,4’(3’H)-dione Derivatives. Tetrahedron 2009, 65, 3804–3808. [Google Scholar] [CrossRef]
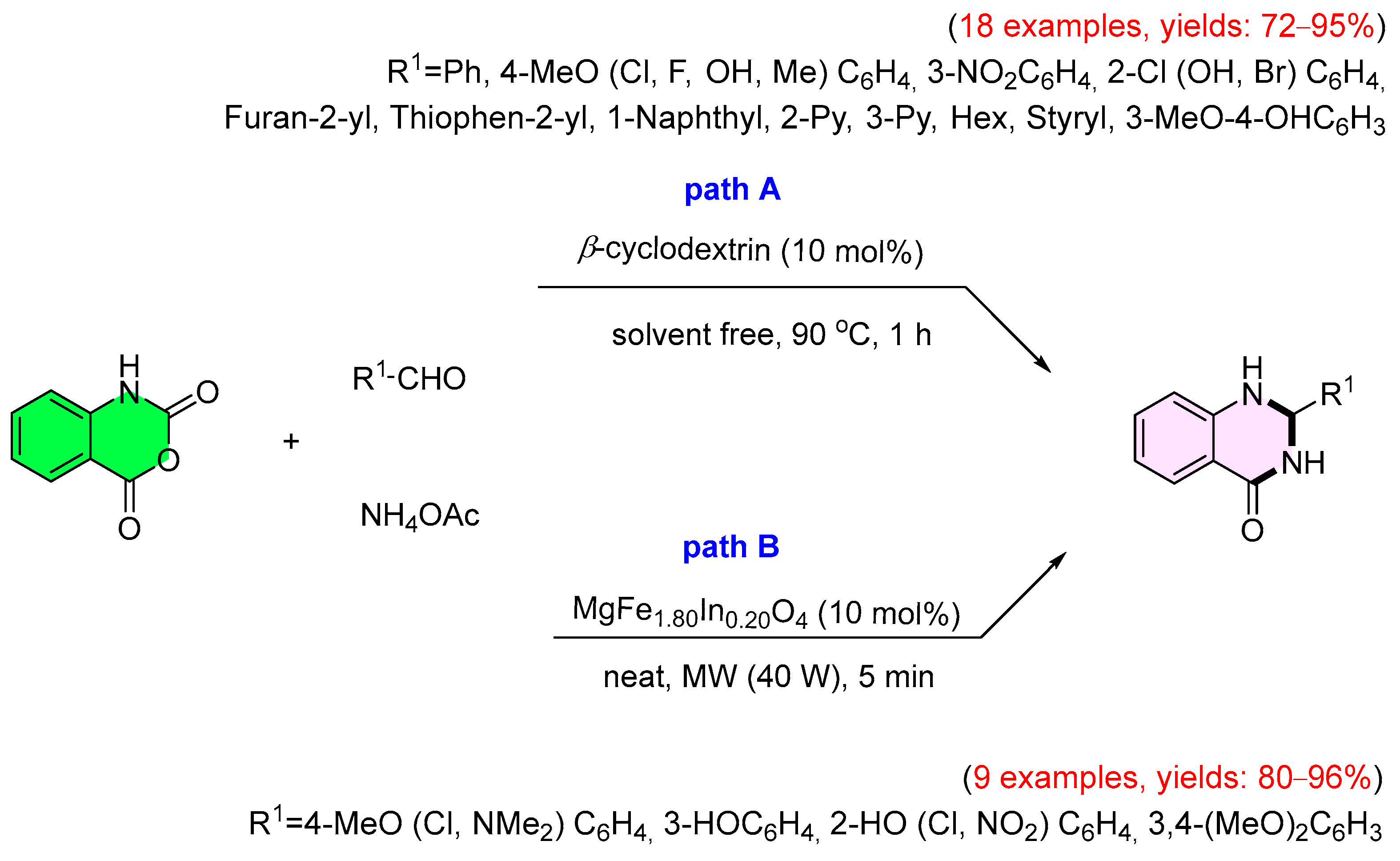
- Mitra, B.; Pariyar, G.C.; Ghosh, P. β-Cyclodextrin: A Supramolecular Catalyst for Metal-Free Approach towards the Synthesis of 2-Amino-4,6-diphenylnicotinonitriles and 2,3-Dihydroquinazolin-4(1H)-one. RSC Adv. 2021, 11, 1271–1281. [Google Scholar] [CrossRef]
- Naik, M.Z.; Rodrigues, L.; Torney, P.S.; Salker, V. In3+ Doped Magnesium Ferrite an Efficient Magnetic Catalyst for the Synthesis of Functionalized Quinazolinone and Henry Reaction. J. Chem. Sci. 2022, 134, 19–27. [Google Scholar] [CrossRef]
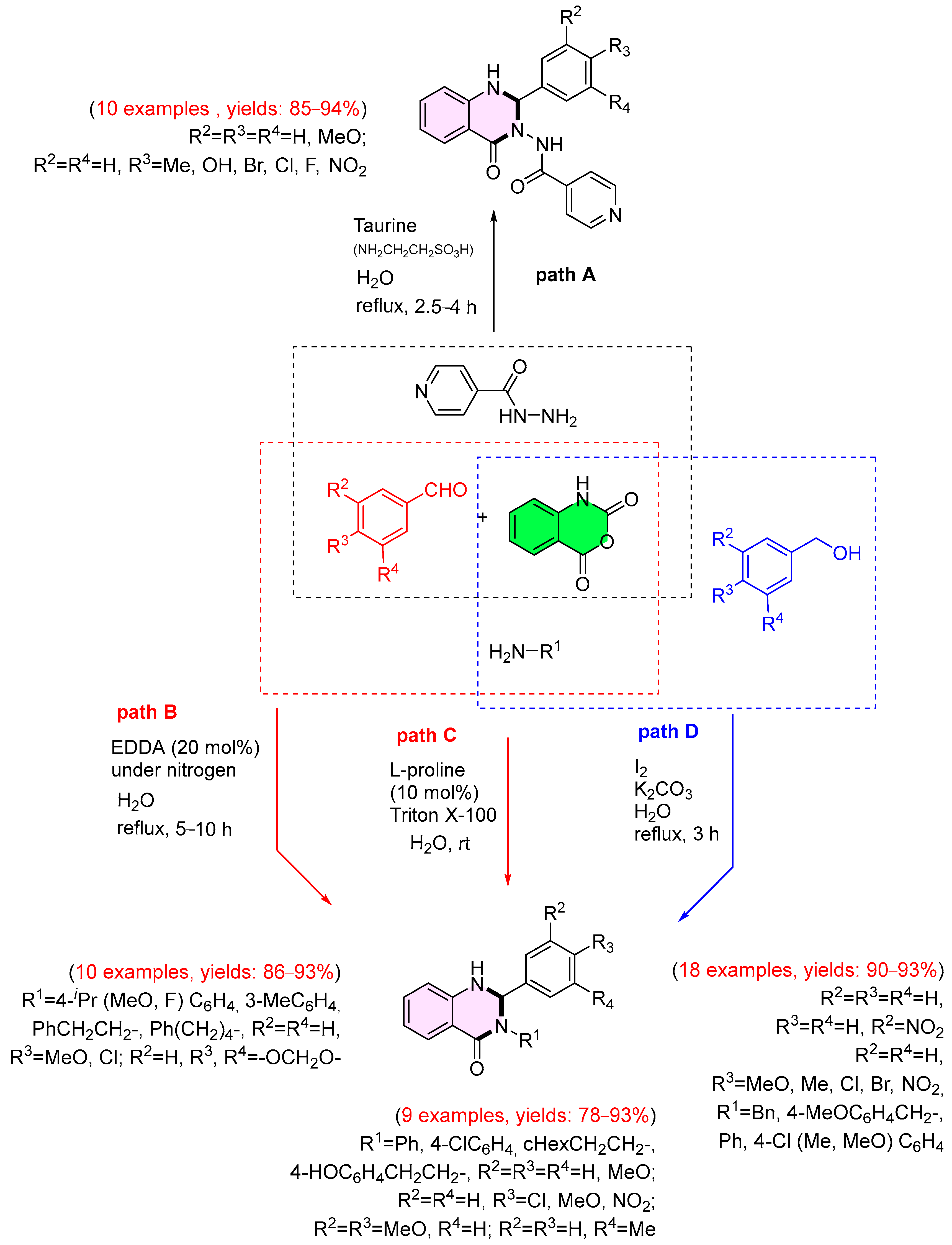
- Chate, A.V.; Rudrawar, P.P.; Bondle, G.M.; Sangeshetti, J.N. 2-Aminoethanesulfonic Acid: An Efficient Organocatalyst for Green Synthesis of Spirooxindole Dihydroquinazolinones and Novel 1,2-(Dihydroquinazolin-3(4H)isonicotinamides in Water. Synth. Commun. 2020, 50, 226–242. [Google Scholar] [CrossRef]
- Kumar, P.; Amber, N.; Tripathi, V.D. A Catalyst Free Surfactant Mediated Multicomponent Synthesis of Quinazolinone Derivatives in Aqueous Media. Eur. J. Adv. Chem. Res. 2023, 4, 1–9. [Google Scholar] [CrossRef]
- Narasimhulu, M.; Lee, Y.R. Ethylenediamine Diacetate-Catalyzed Three-Component Reaction for the Synthesis of 2,3-Dihydroquinazolin-4(1H)-ones and Their Spirooxindole Derivatives. Tetrahedron 2011, 67, 9627–9634. [Google Scholar] [CrossRef]
- Azimi, S.B.; Azizian, J. Reaction of Benzyl Alcohols, Isatoic Anhydride, and Primary Amines Mediated by I2/K2CO3 in Water: A New and Green Approach for the Synthesis of 2,3-Dihydroquinazolin-4(1H)-ones. Tetrahedron Lett. 2016, 57, 181–184. [Google Scholar] [CrossRef]
- Murthy, V.N.; Nikumbh, S.P.; Kumar, S.P.; Chiranjeevi, Y.; Rao, L.V.; Raghunadh, A. A Simple Approach for the Synthesis of Fused Quinazoline-Based Tetracyclic Compounds via a Multicomponent Reaction Strategy. Synlett 2016, 27, 2362–2367. [Google Scholar] [CrossRef]
- Şenol, İ.M.; Çelik, İ.; Avan, İ. One-Pot Synthesis of Quinazolin-4(3H)-ones and 2,3-Dihydroquinazolin-4(1H)-ones Utilizing N -(2-Aminobenzoyl)benzotriazoles. Turk. J. Chem. 2019, 49, 1580–1596. [Google Scholar] [CrossRef]
- Şenol, İ.M.; Gökbulut Satioğlu, S.; Çelik, İ. N-(2-Aminobenzoyl)benzotriazole Mediated Synthesis of 3-Acyl-2-alkyl(aryl)-4-hydroxyquinolines and 3-Acylamino-4(3H) quinazolinones. Turk. J. Chem. 2024, 48, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, G.M.; Kumar, C.S.P.; Suresha, G.P.; Rangappa, K.S.; Mantelingu, K. T3P Catalyzed One Pot Three-Component Synthesis of 2,3-Disubstituted 3H-quinazolin-4-ones. Chin. Chem. Lett. 2015, 26, 963–968. [Google Scholar] [CrossRef]
- Chen, S.; Ji, Y.S.; Choi, Y.; Youn, S.W. One-Pot Three-Component Reaction for the Synthesis of 3,4-Dihydroquinazolines and Quinazolin-4(3H)-ones. J. Org. Chem. 2024, 89, 6428–6443. [Google Scholar] [CrossRef]
- Ramanathan, M.; Liu, S.-T. Preparation of Quinazolinoquinazolinones via a Cascade Approach. J. Org. Chem. 2018, 83, 14138–14145. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Hsu, M.-T.; Liu, S.-T. Preparation of 4(3H)-Quinazolinones from Aryldiazonium Salt, Nitriles and 2-Aminobenzoate via a Cascade Annulation. Tetrahedron 2019, 75, 791–796. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, R.; Srivastava, V.; Singh, P.P. Photocatalysed Eosin Y Mediated Synthesis of Biologically Potent 4 (3H)-Quinazolinone. Tetrahedron Green Chem. 2024, 4, 100053–100061. [Google Scholar] [CrossRef]
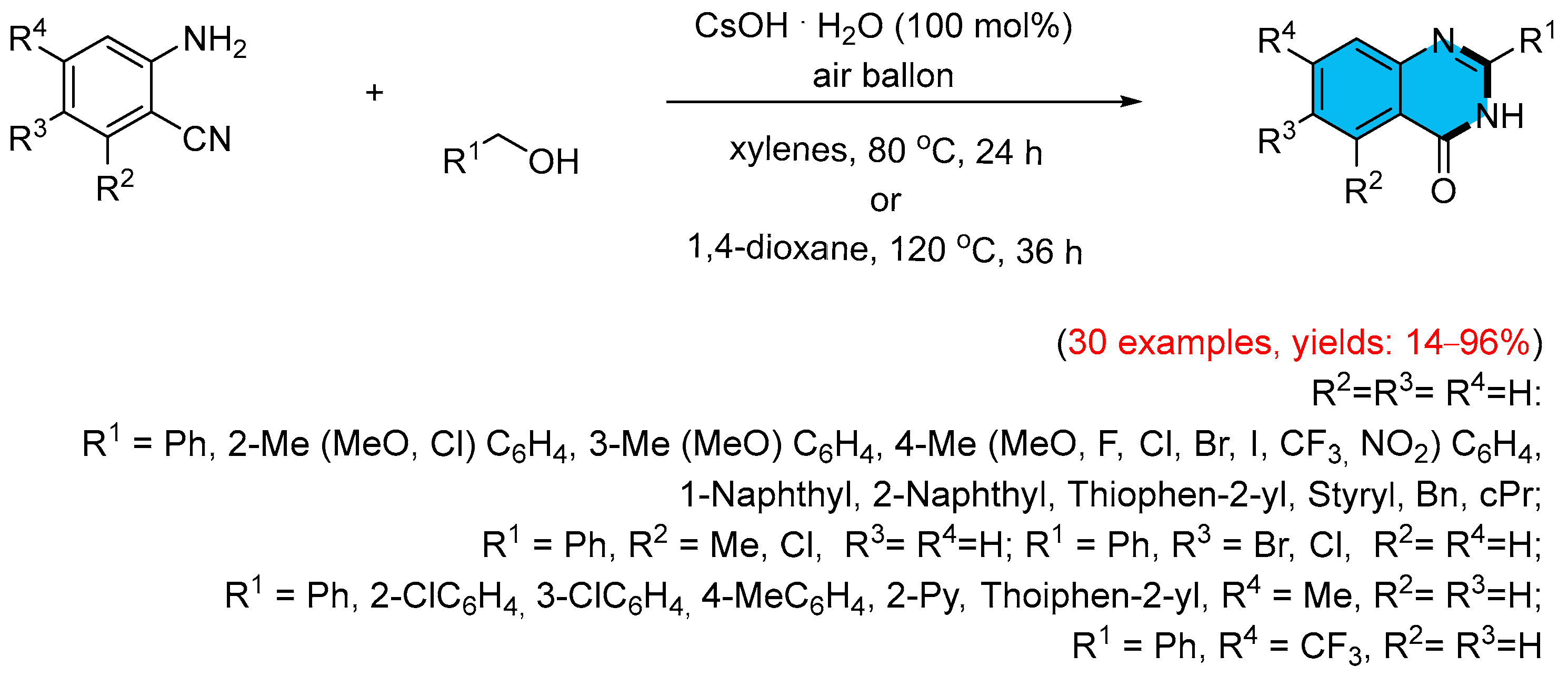
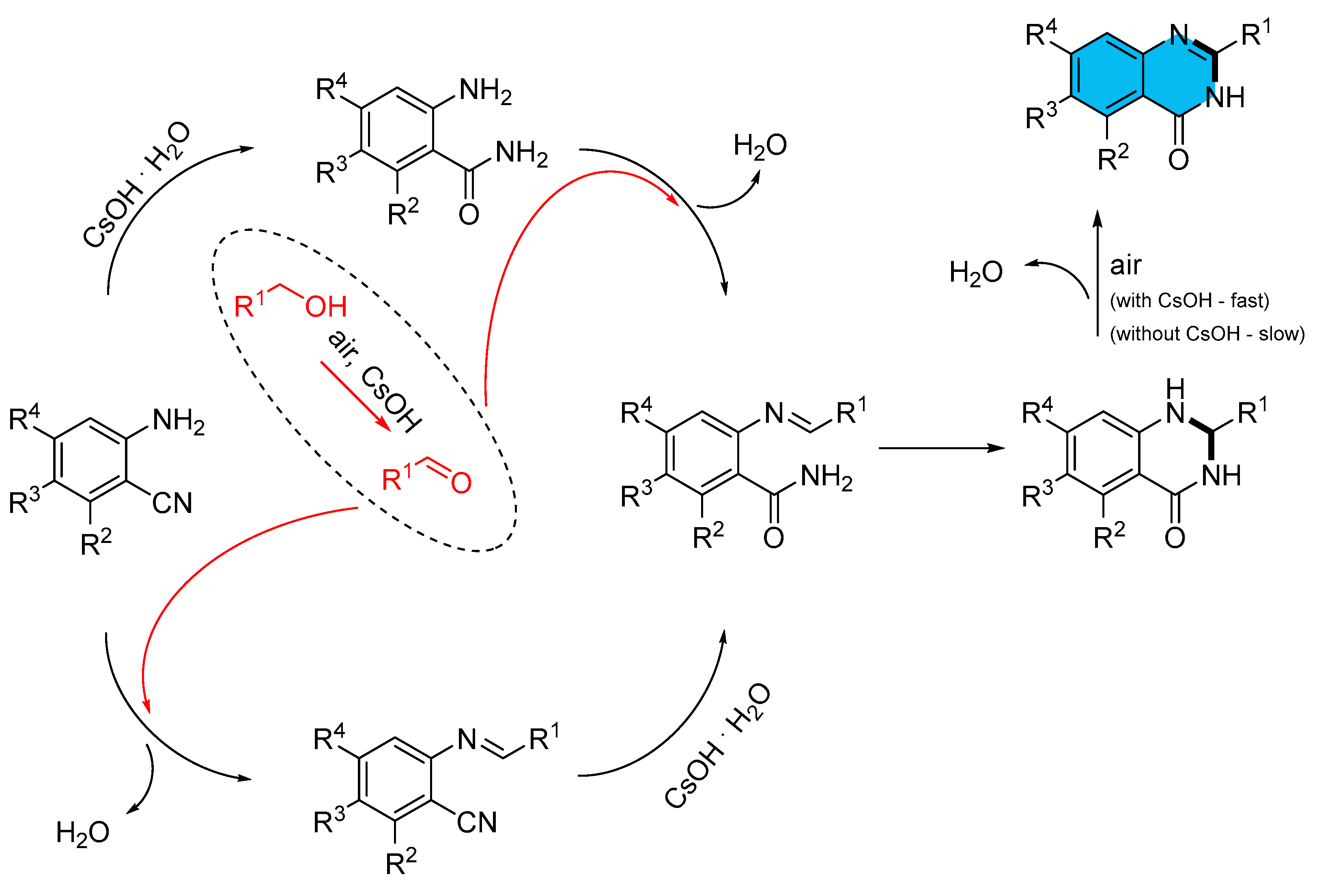
- Wang, Q.; Lv, M.; Liu, J.; Li, Y.; Xu, Q.; Zhang, X.; Cao, H. Efficient Synthesis of Quinazolinones by Transition-Metal-Free Direct Aerobic Oxidative Cascade Annulation of Alcohols with o-Aminoarylnitriles. ChemSusChem 2019, 12, 3043–3048. [Google Scholar] [CrossRef]
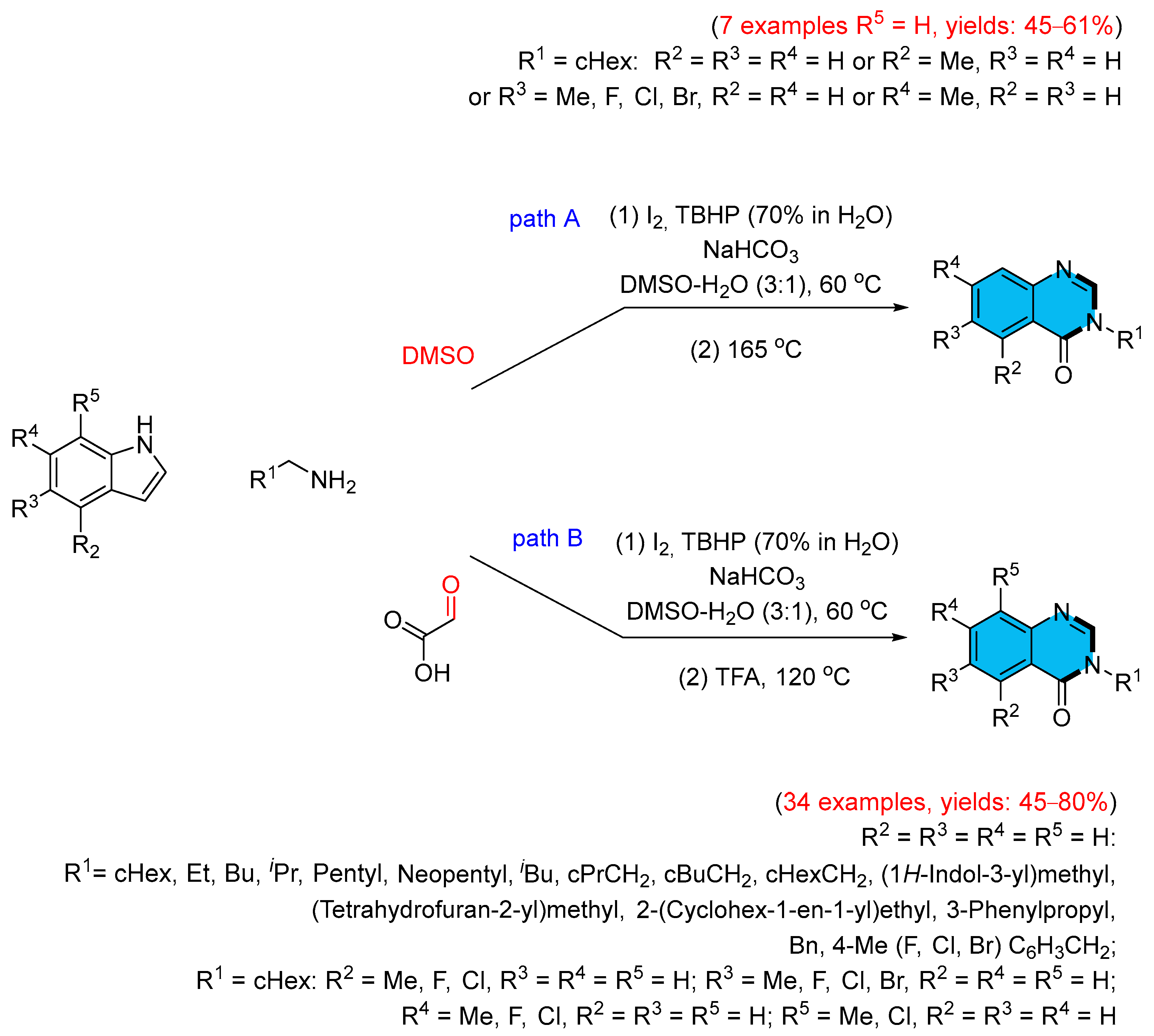
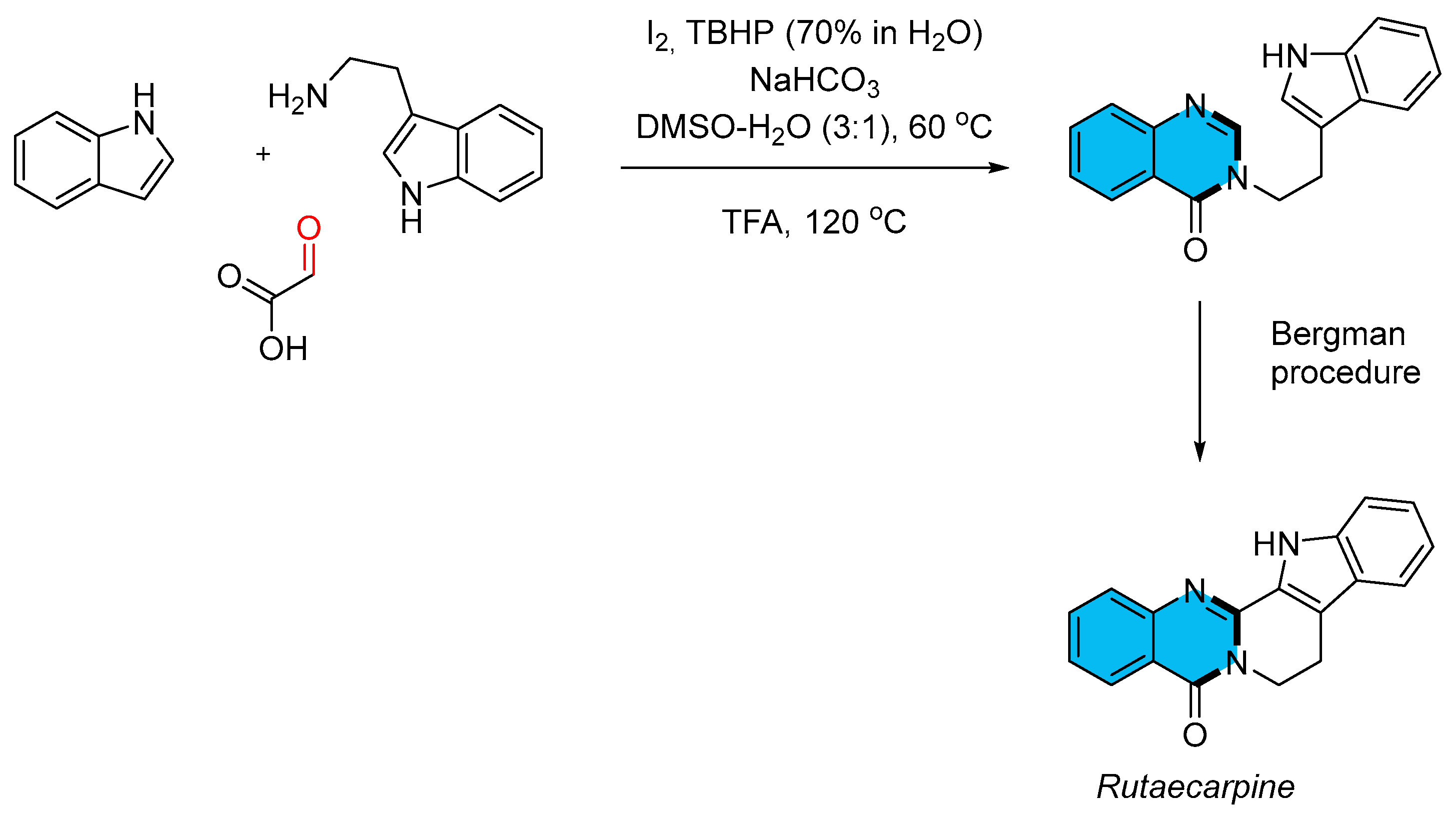
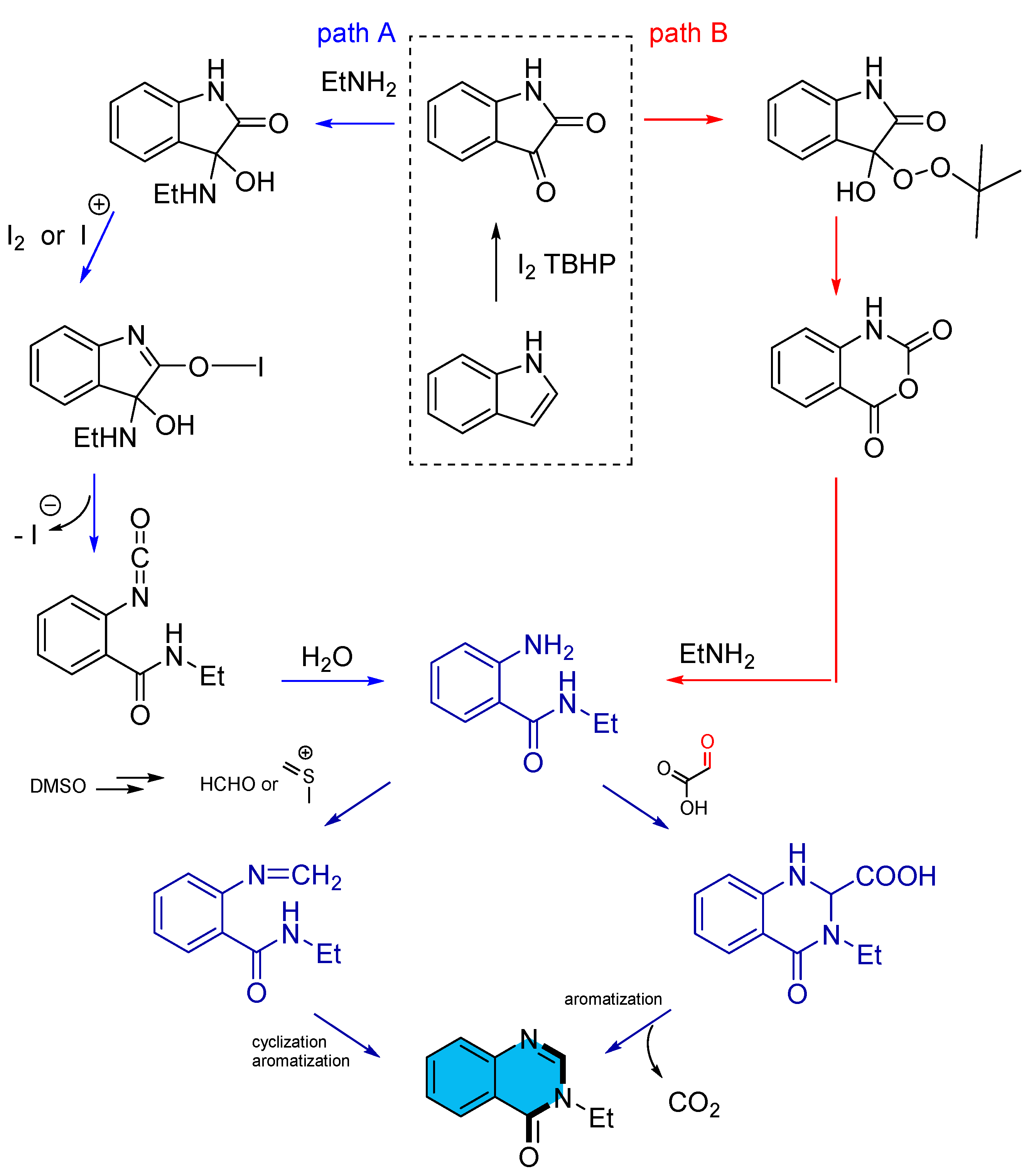
- Zhang, R.-L.; Liu, X.-Y.; Long, Z.-Z.; Zhang, K.-Y.; Chu, X.-L. Iodine-Catalyzed Tandem Cyclization for Efficient Synthesis of Quinazolinones from Indoles and Amines. Eur. J. Org. Chem. 2025, 28, e202401323–e202401329. [Google Scholar] [CrossRef]
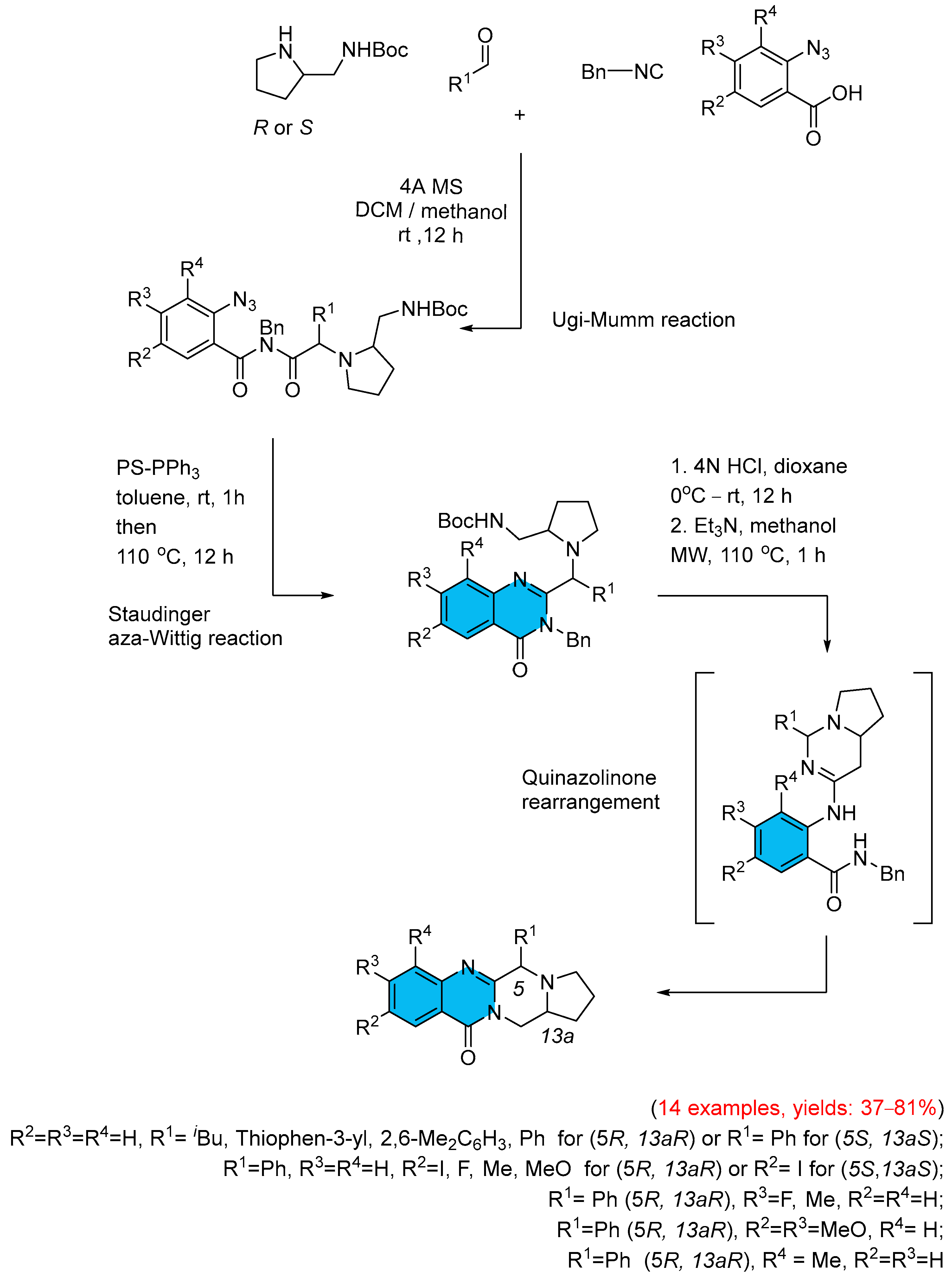
- Jaffett, V.A.; Fitz-Henley, J.N.; Guzei, M.M.K.I.A.; Golden, J.E. Diastereoselective, Multicomponent Synthesis of Pyrrolopyrazinoquinazolinones via a Tandem Quinazolinone Rearrangement/Intramolecular Ring Closure of Tautomeric (Z)-Benzamidines. Org. Lett. 2021, 23, 5799–5803. [Google Scholar] [CrossRef] [PubMed]
- Jaffett, V.A.; Nerurkar, A.; Cao, X.; Guzei, I.A.; Golden, J.E. Telescoped synthesis of C3-functionalized (E)-arylamidines using Ugi–Mumm and regiospecific quinazolinone rearrangements. Org. Biomol. Chem. 2019, 17, 3118–3128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, J.; Sharma, A. Synthesis of Quinazolinones and Benzothiazoles Using α-Keto Acids under Ball Milling. J. Org. Chem. 2024, 89, 5229–5238. [Google Scholar] [CrossRef]
- Kumar, S.; Padala, K.; Maiti, B. H2O2-Mediated Synthesis of a Quinazolin-4(3H)-one Scaffold: A Sustainable Approach. ACS Omega 2023, 8, 33058–33068. [Google Scholar] [CrossRef]
- Cao, L.; Huo, H.; Zeng, H.; Yu, Y.; Lu, D.; Gong, Y. One-Pot Synthesis of Quinazolin-4(3H)-ones through Anodic Oxidation and the Related Mechanistic Studies. Adv. Synth. Catal. 2018, 360, 4764–4773. [Google Scholar] [CrossRef]
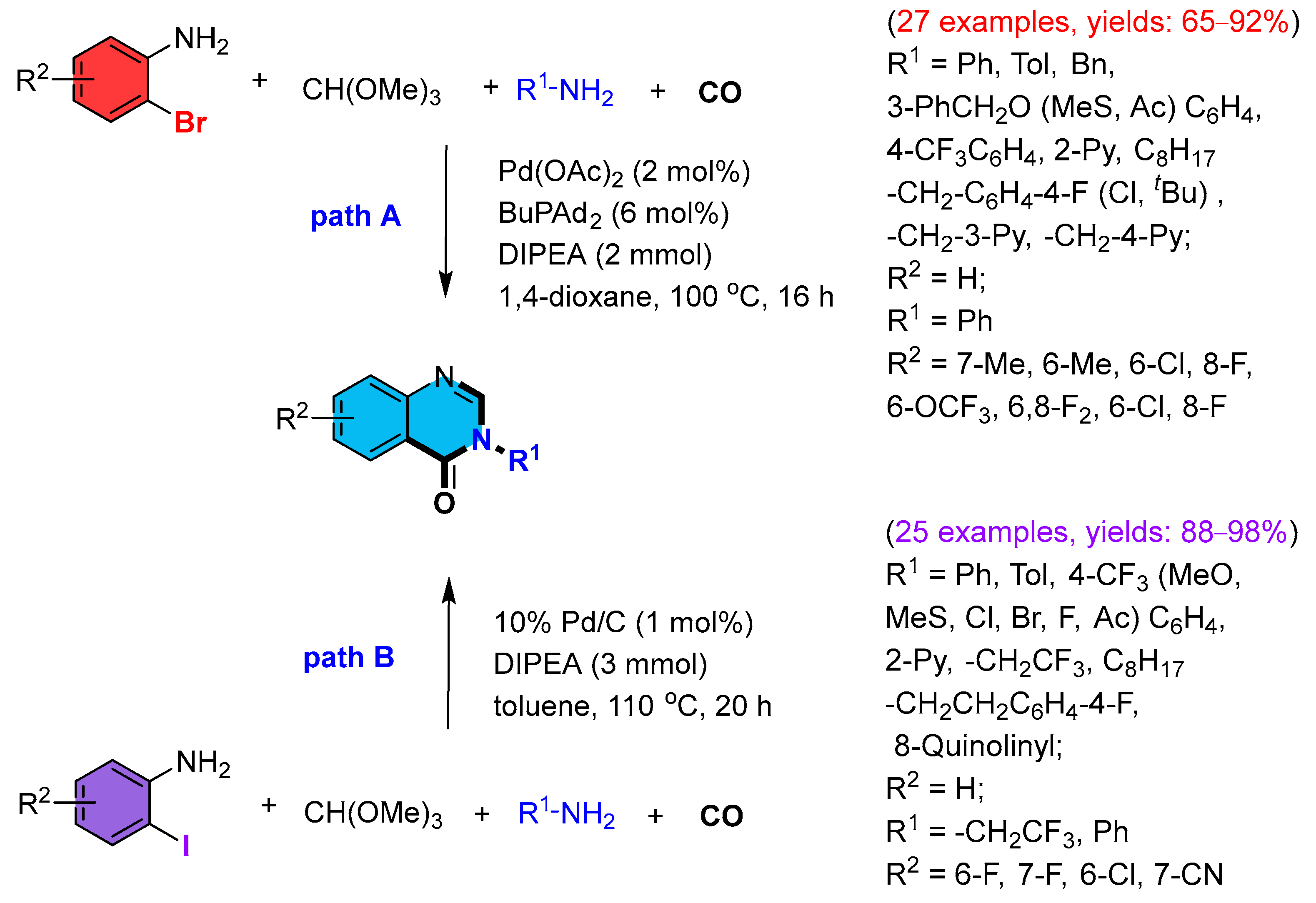
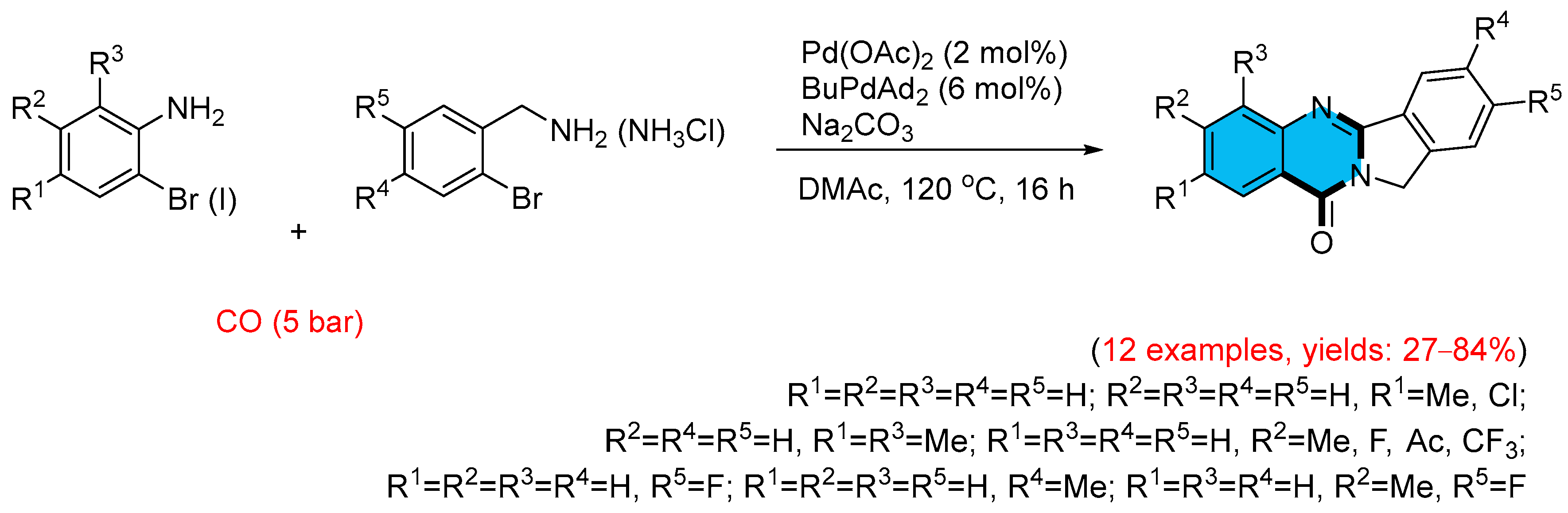
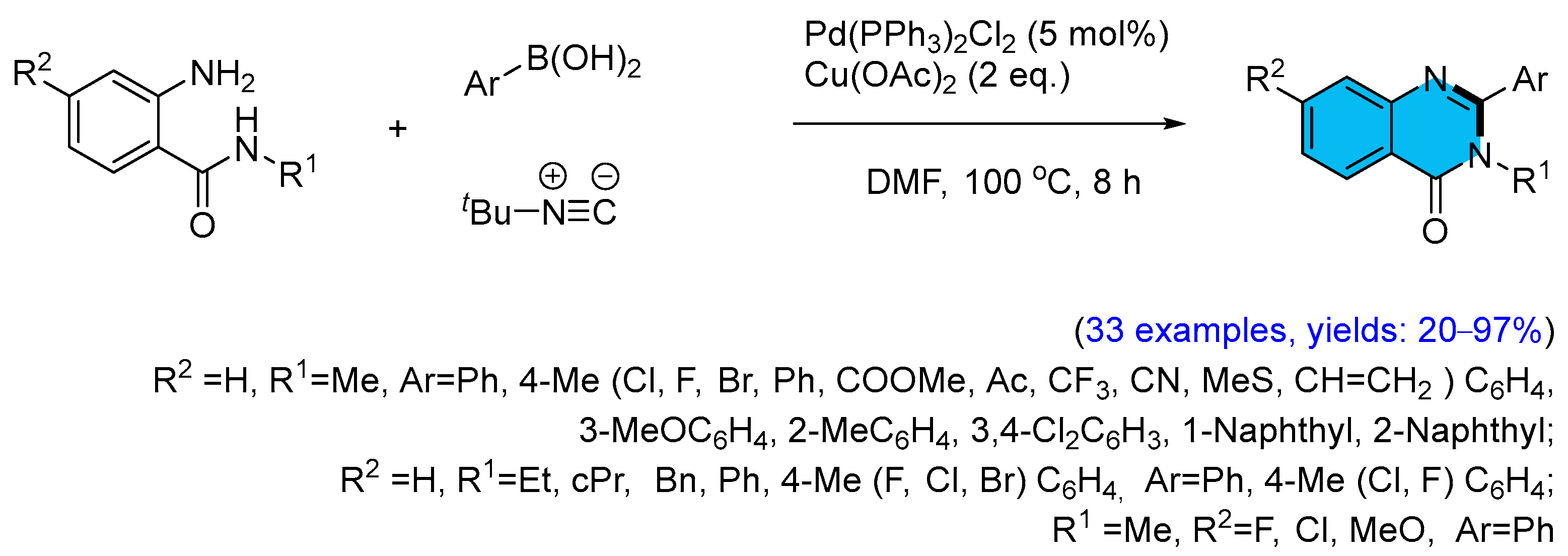
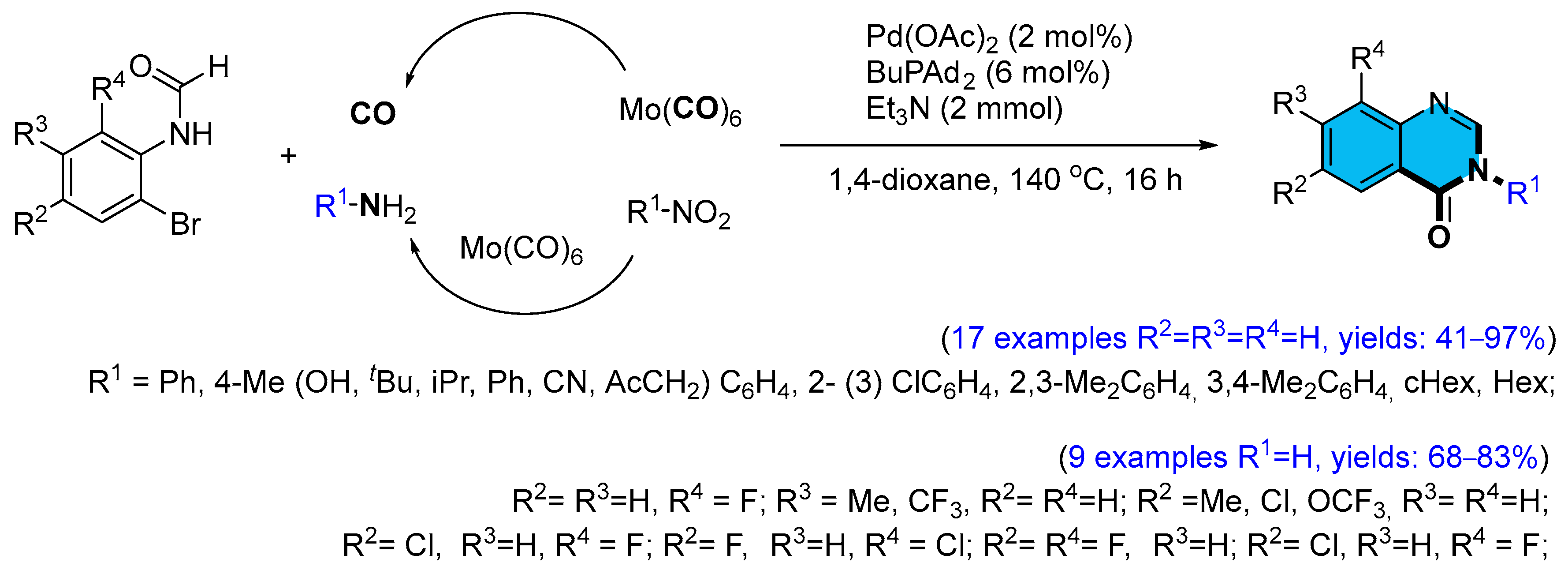
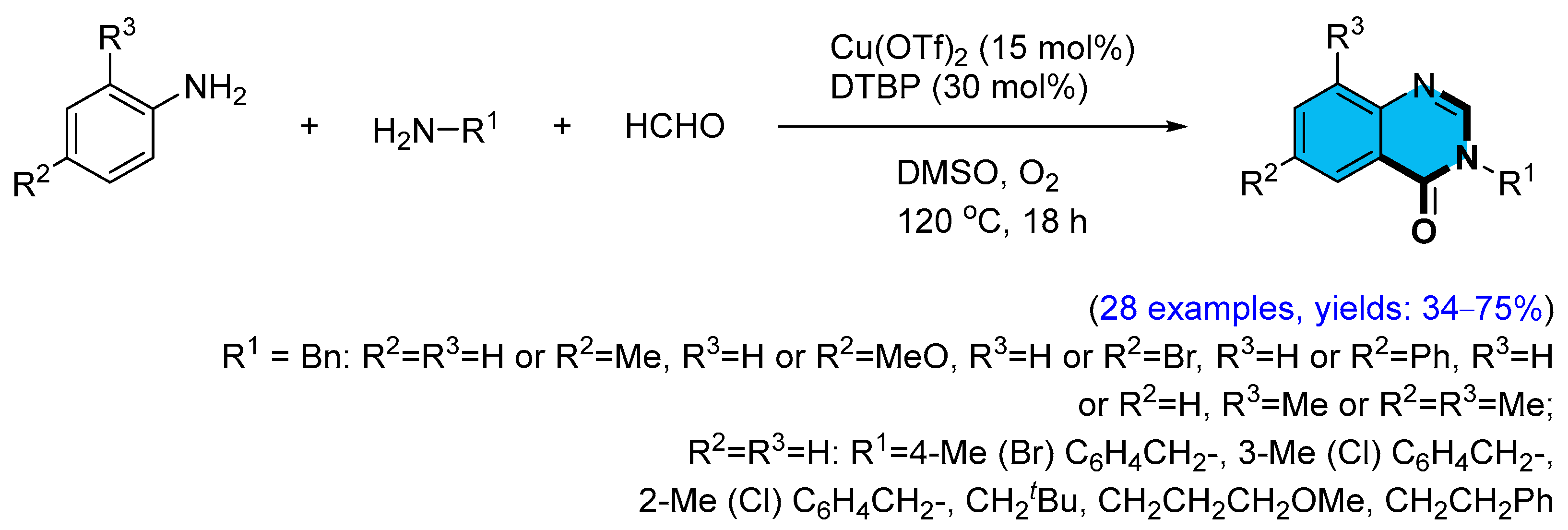
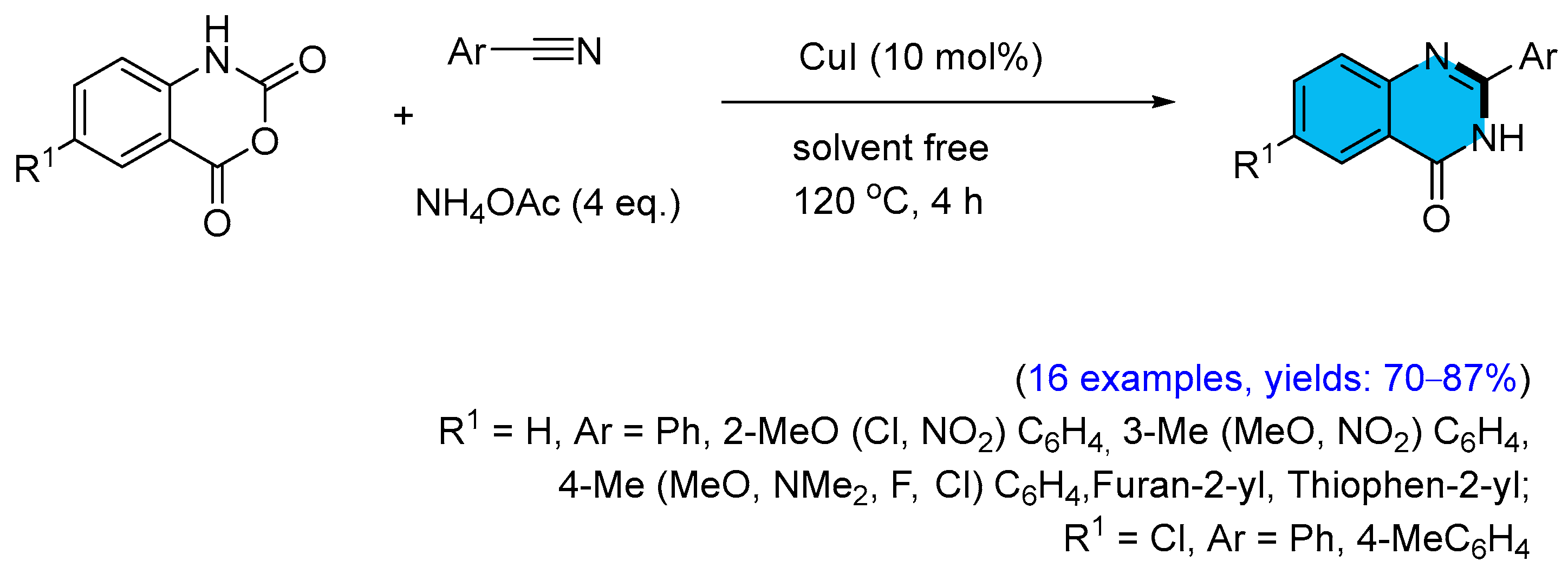
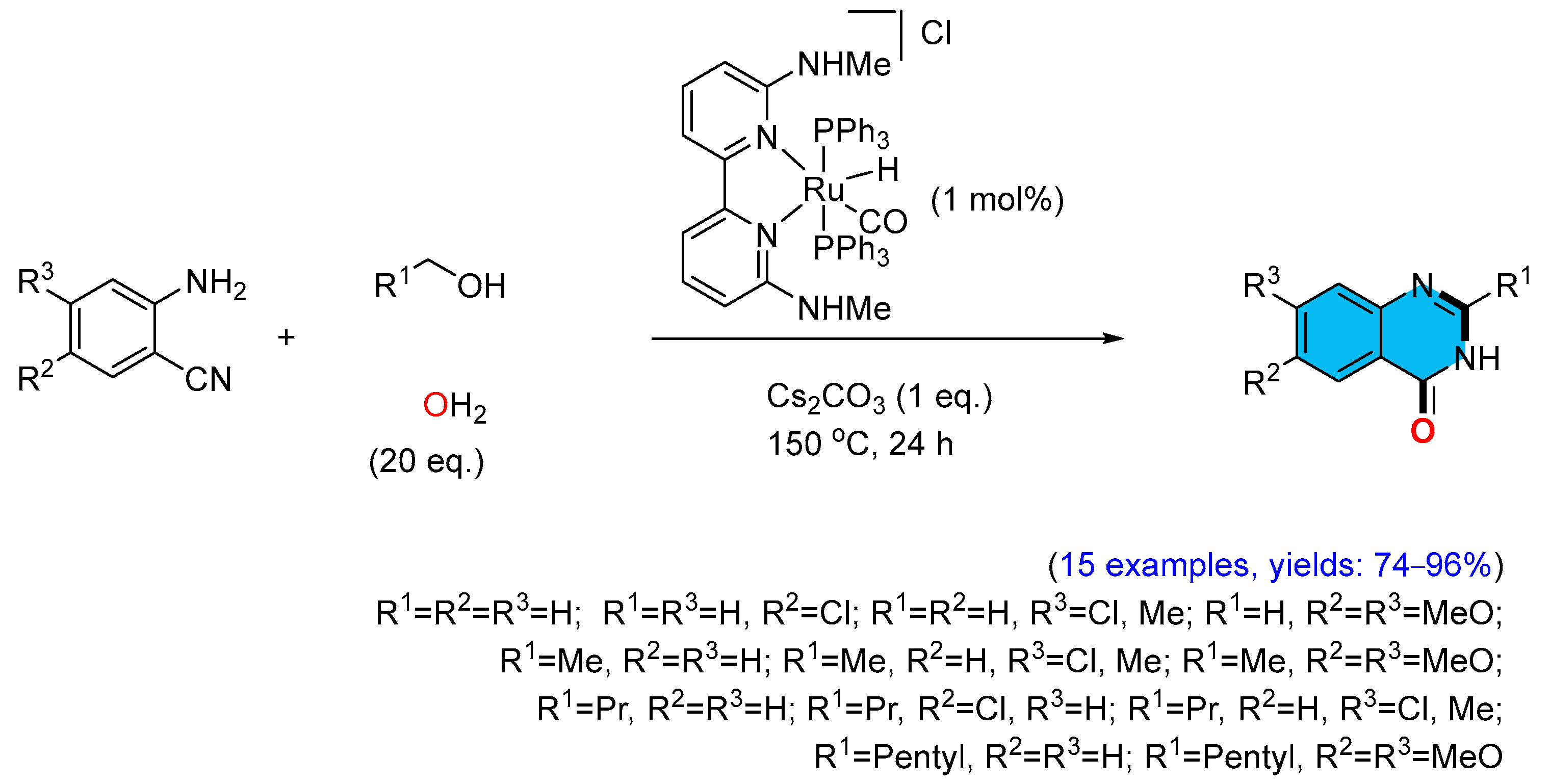
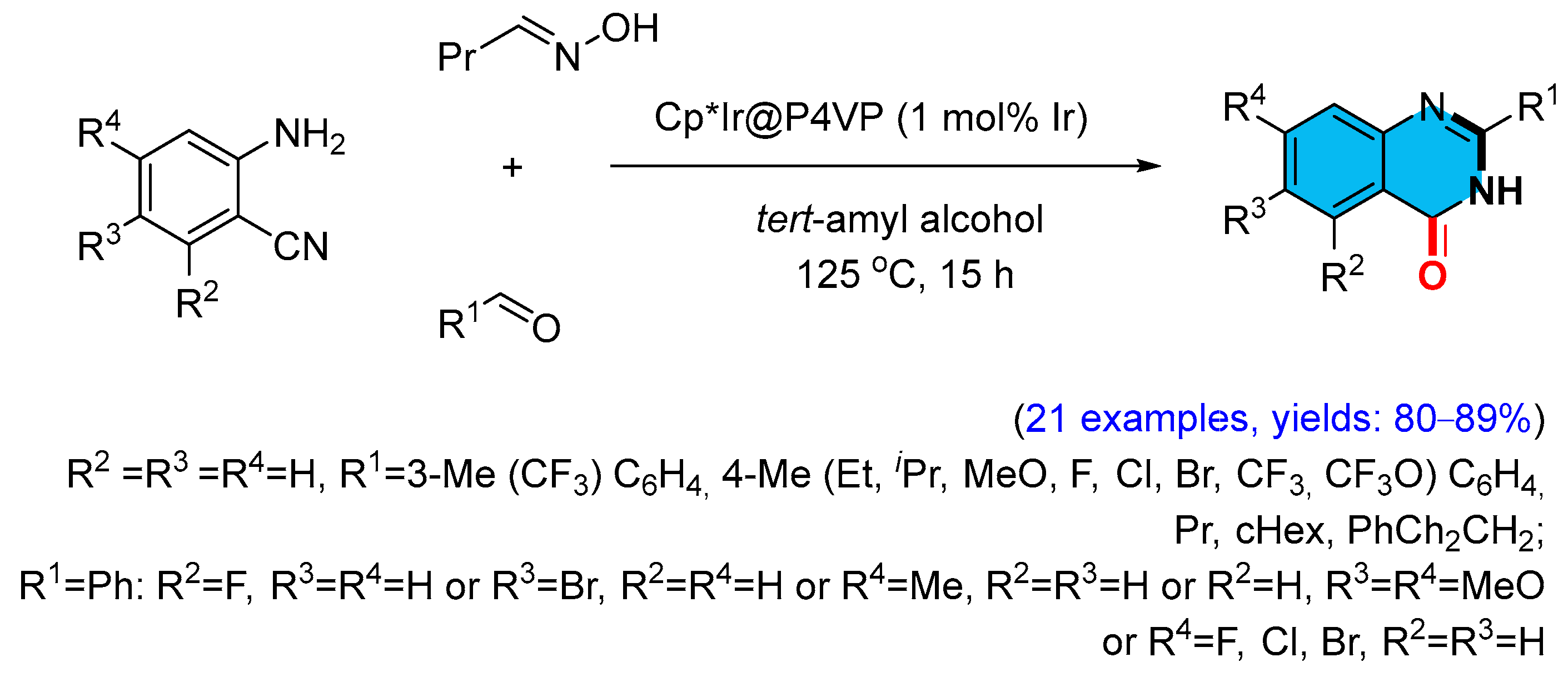
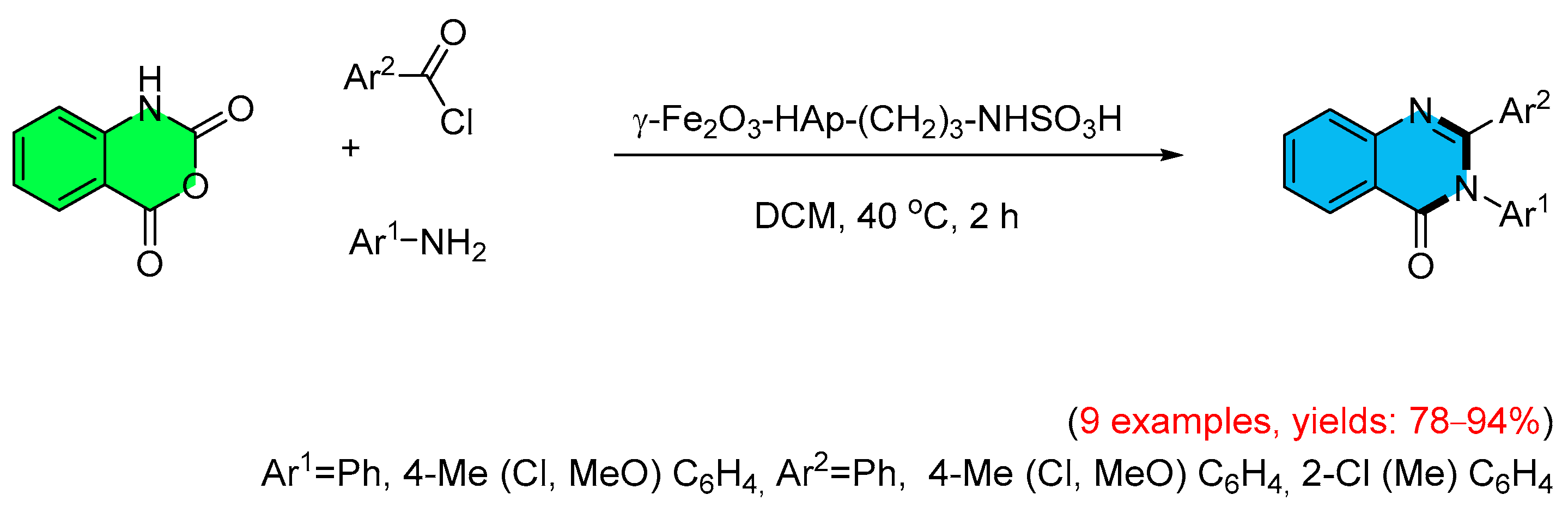
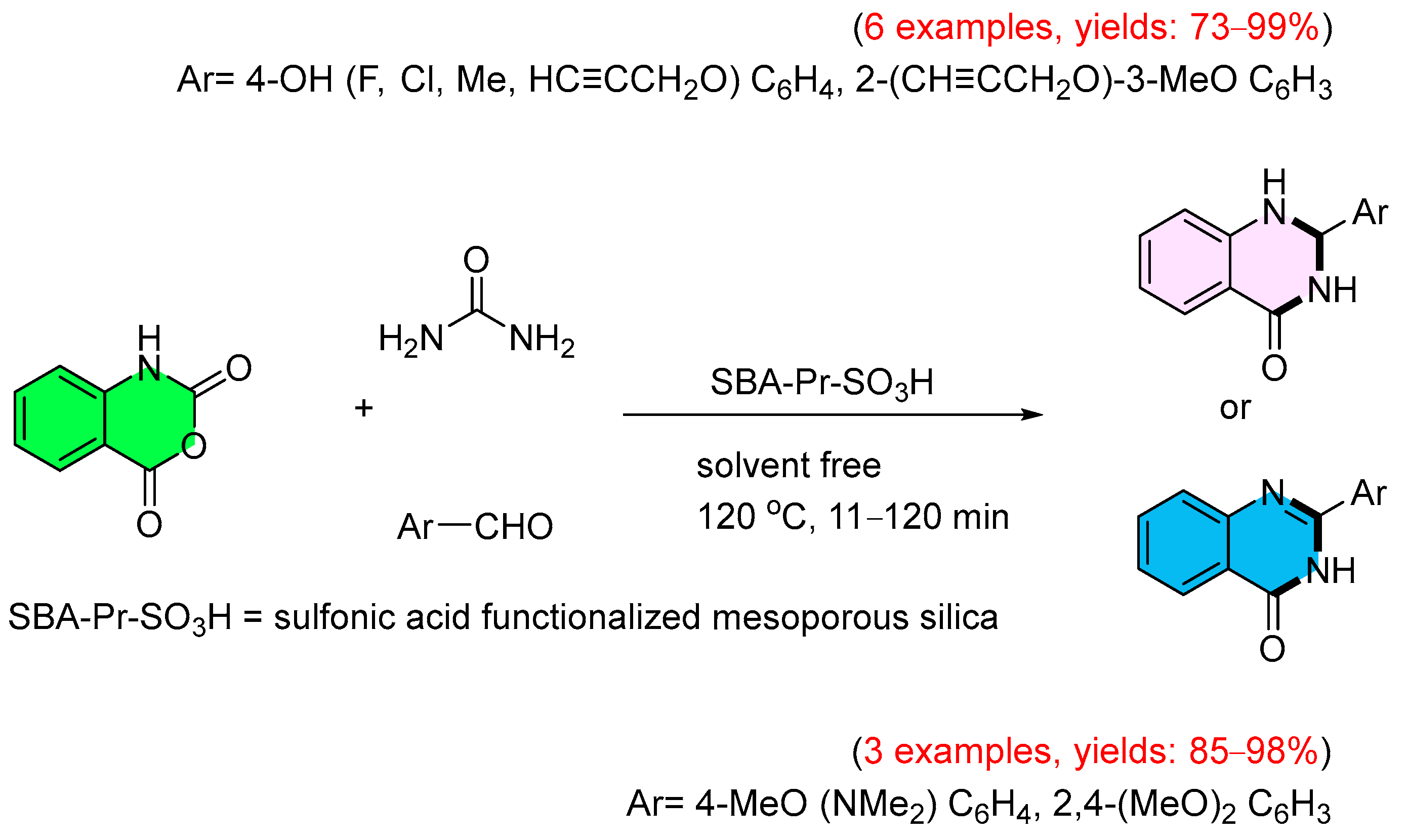
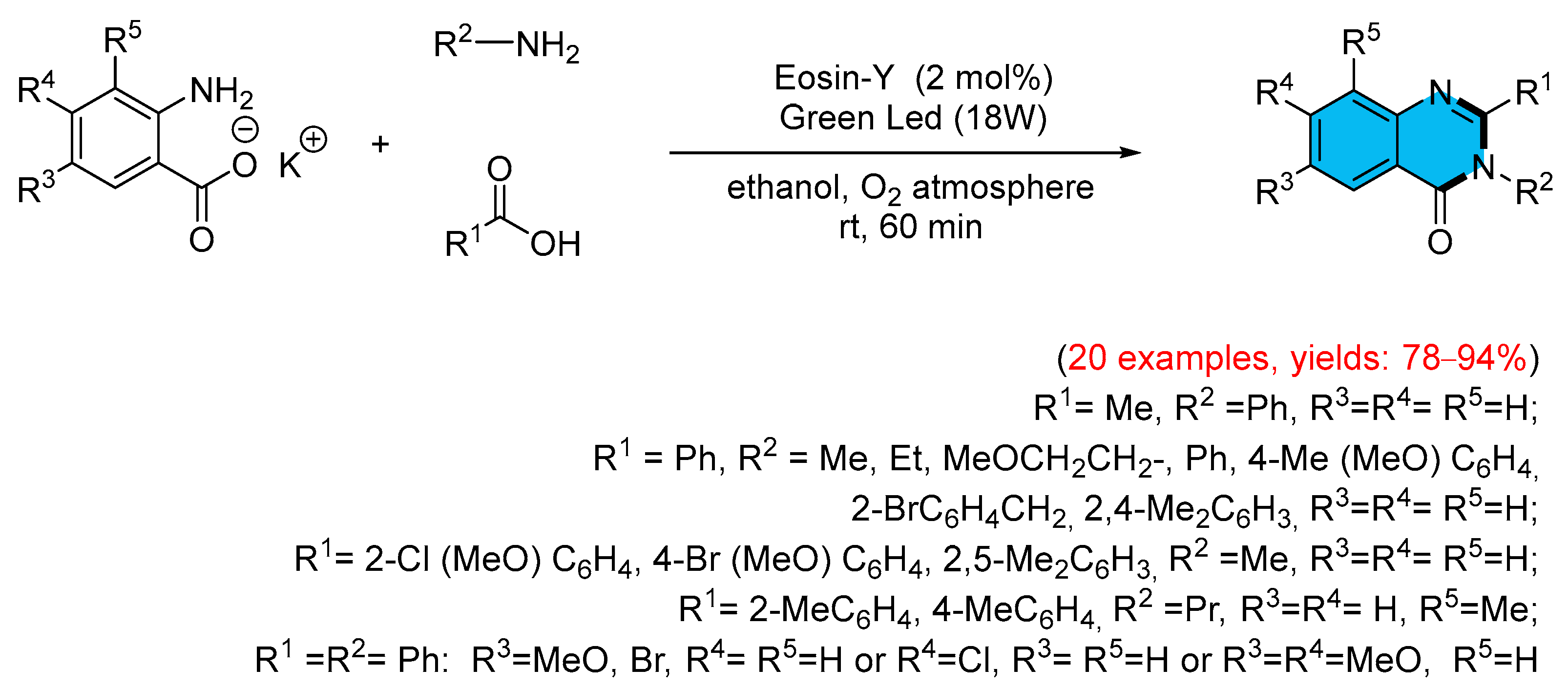
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowski, Z. Recent Advances in One-Pot Multicomponent Reactions for the Synthesis of Substituted Quinazolin-4(3H)-ones. Molecules 2025, 30, 3729. https://doi.org/10.3390/molecules30183729
Malinowski Z. Recent Advances in One-Pot Multicomponent Reactions for the Synthesis of Substituted Quinazolin-4(3H)-ones. Molecules. 2025; 30(18):3729. https://doi.org/10.3390/molecules30183729
Chicago/Turabian StyleMalinowski, Zbigniew. 2025. "Recent Advances in One-Pot Multicomponent Reactions for the Synthesis of Substituted Quinazolin-4(3H)-ones" Molecules 30, no. 18: 3729. https://doi.org/10.3390/molecules30183729
APA StyleMalinowski, Z. (2025). Recent Advances in One-Pot Multicomponent Reactions for the Synthesis of Substituted Quinazolin-4(3H)-ones. Molecules, 30(18), 3729. https://doi.org/10.3390/molecules30183729