Research Progress on Chemical Components of Astragalus membranaceus and Treatment of Metabolic Syndrome
Abstract
1. Introduction
2. Main Components of AM
2.1. Astragalus Flavonoids
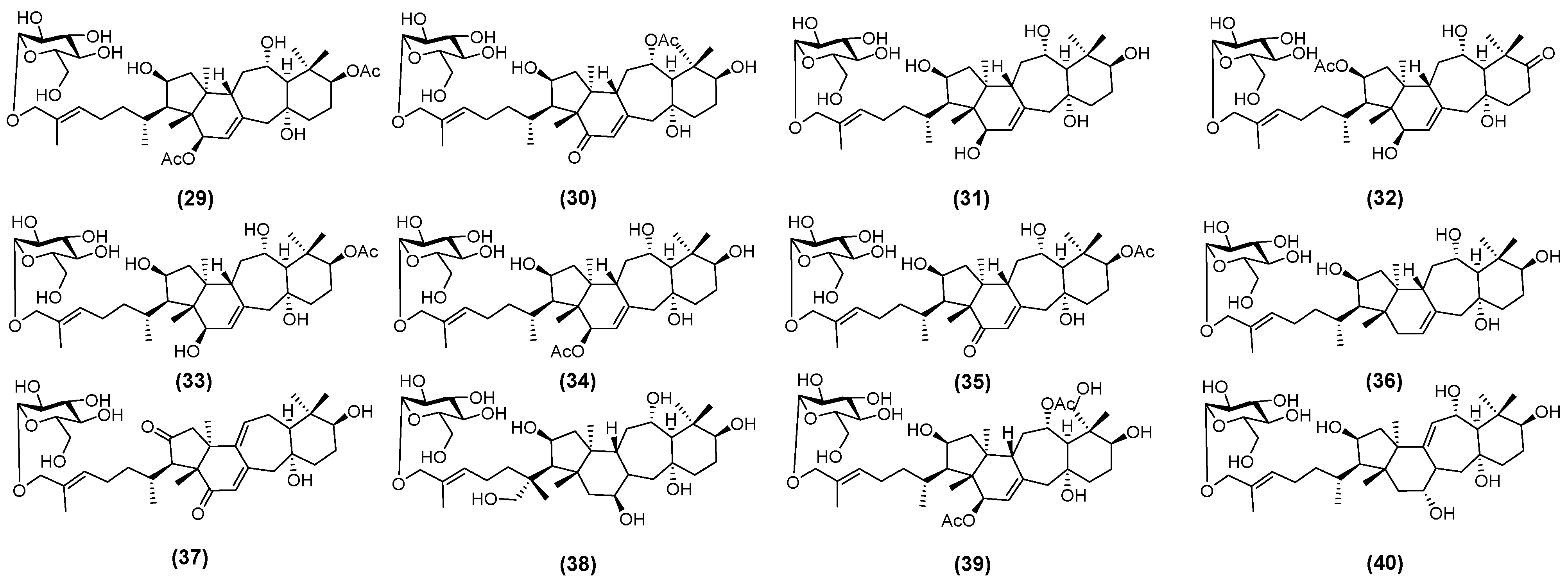
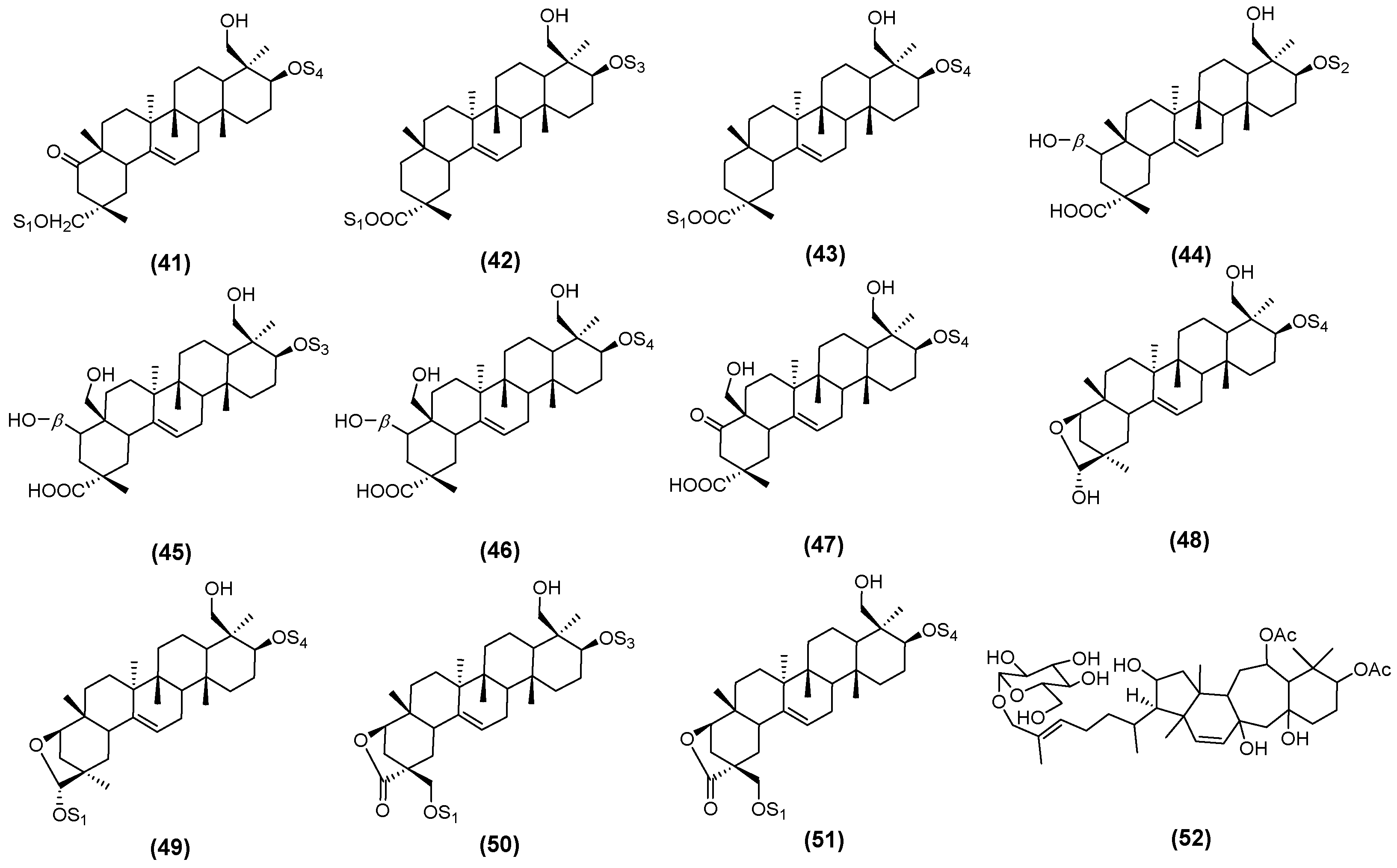
2.2. Astragalus Saponins
2.3. Astragalus Polysaccharides
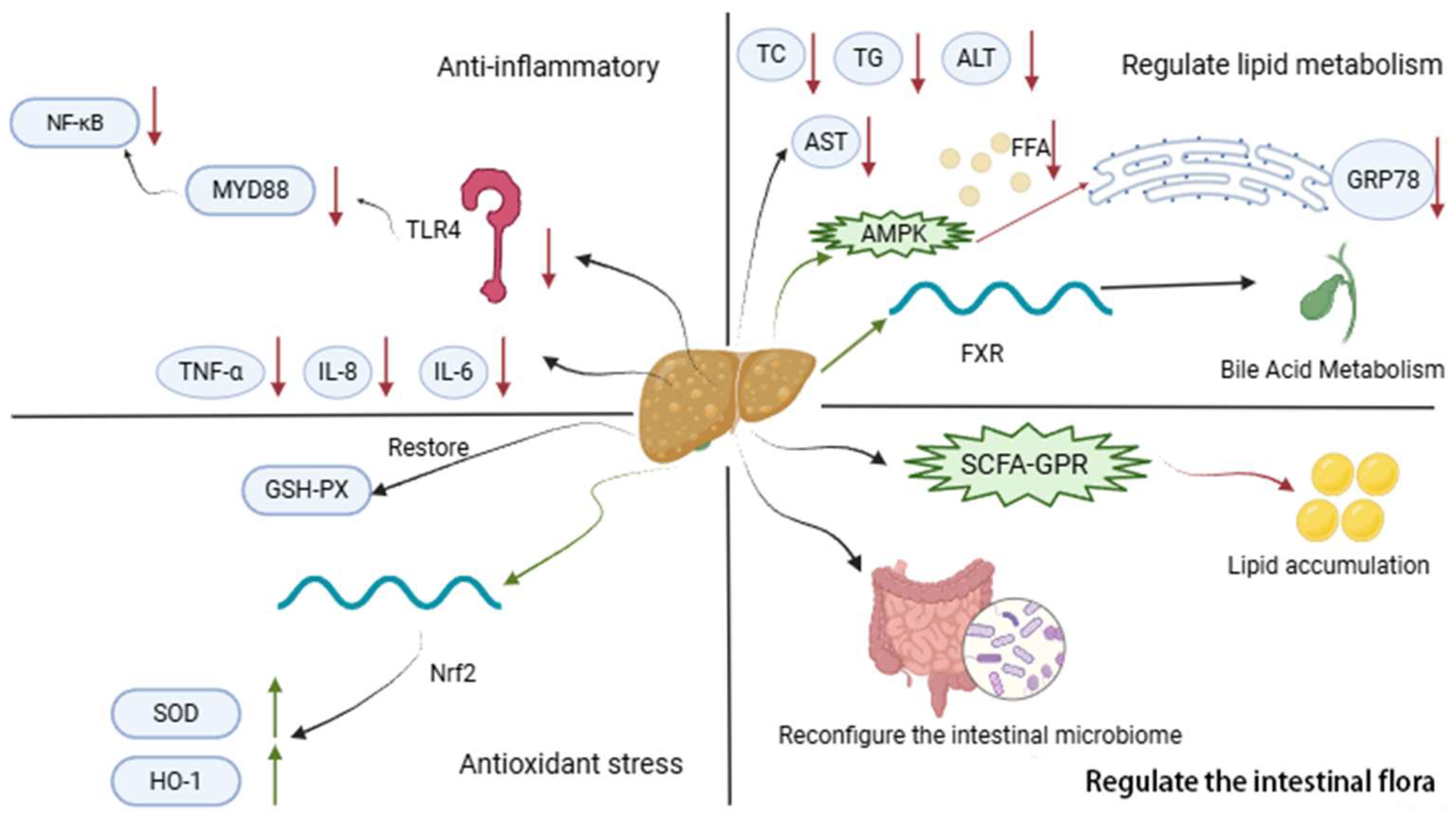
3. Therapeutic Effects of AM and Its Active Ingredients on MetS
3.1. Common Mechanisms of AM in MetS
3.1.1. Antioxidation
3.1.2. Anti-Inflammatory
3.1.3. Regulating Intestinal Flora
3.1.4. Regulating Lipid Metabolism
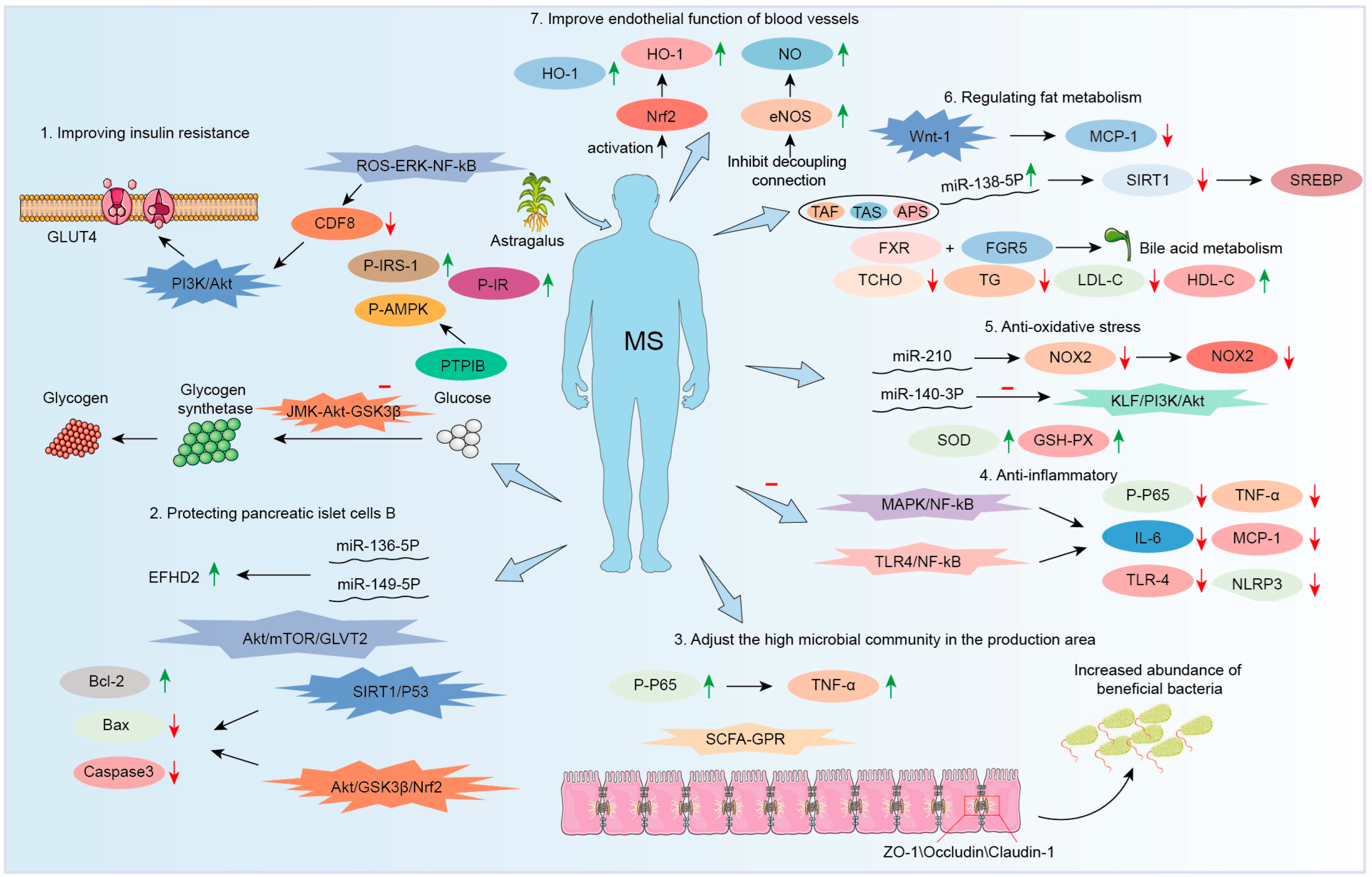
3.2. Therapeutic Effect on Diabetes
3.2.1. Improving Insulin Resistance
| NO | Model | Pathways | Effects * | Ref. |
|---|---|---|---|---|
| 1 | In vivo | / | ↓ PTP1B | [108] |
| 2 | In vitro | / | ↑ Adiponectin ↓ IL-6 | [110] |
| 3 | In vivo | AMPK/PGC1α, IRS/AKT | ↑ PGC-1α, p-AKT, p-AMPK, p-IRS-1 ↓ MCT4 | [111] |
| 4 | In vitro | STAT5/IGF-1 | ↑ IGF-1R, p-AKT/AKT, IGF-1, p-STAT5/STAT5 | [112] |
| 5 | In vivo | ROS-ERK-NF-κB | ↓ myostatin, MDA, NF-κB | [113] |
| 6 | In vivo | ERS | ↑ miR203a-3p ↓ GRP78 | [114] |
| 7 | In vivo | SIRT1-PGC-1α/PPARα-FGF21 | ↑ FGF21, PPARα, SIRT1 ↓NF-κB | [115] |
| 8 | In vivo | / | ↑ IRS-1, PI3K, PDK1, p-AKT ↓ p-GSK-3β | [87] |
| 9 | In vitro | C1q/CTRP3/PI3K/Akt | ↑ p53, CTRP3 ↓ IL-6, TNF-α, | [70] |
| 10 | In vitro/ in vivo | JNK-AKT-GSK3β | ↓ IL-6, TNF-α, | [116] |
| 11 | In vitro/ in vivo | / | ↑ Adiponectin | [117] |
| 12 | In vitro | / | ↑ p-IR, p-IRS-1 ↓ PTP1B | [118] |
| 13 | In vivo | / | ↑ p-AMPK, HDL, ISI ↓ PTP1B, TG, TC, LDL, IS, IRI | [109] |
3.2.2. Protection of Pancreatic β-Cell Function
| NO | Pathways | Effects * | Ref. |
|---|---|---|---|
| 1 | / | ↑ miR-136-5p, miR-149-5p ↓ EFHD2 | [120] |
| 2 | AMPK | ↑ P-AMPKα, P-ACC, GLUT4 | [121] |
| 3 | Akt/mTOR/GLUT2 | ↑ GLUT2, GCK, PDX-1, GSIS, p-Akt, p-mTOR | [122] |
| 4 | PI3K/AKT | ↑ p-Akt, p-AKT/AKT ↓ caspase-3 | [123] |
| 5 | SIRT1/p53 Akt/GSK3β/Nrf2 | ↑ Bcl-2 ↓ caspase-3, Bax | [124] |
3.2.3. Increasing GLP-1 Levels
3.3. Anti-Nonalcoholic Fatty Liver Disease (NAFLD)
| NO | Model | Pathways | Effects * | Ref. |
|---|---|---|---|---|
| 1 | In vitro | / | ↑ AMPK, ACC, SREBP-1c ↓ acc1, fas, scd1, GRP78, CHOP | [132] |
| 2 | In vivo | TLR4/NF-κB | ↓ AST, ALT, TG, TNF-α, IL-6, IL-8, TLR4, MyD88, NF-κB | [74] |
| 3 | In vitro/ in vivo | / | ↑ GSH-Px, Bcl-2, Bax ↓ ROS, MDA, 5-LO, LTB4 | [62] |
| 4 | In vivo | SCFA-GPR | ↑ZO-1, occludin ↓ TLR4, NF-κB, NLRP3, GPR | [75] |
| 5 | In vitro/ in vivo | / | ↑ THDCA, CYP7B1 ↓ CYP7A1, CYP8B1 | [135] |
| 6 | In vivo | FXR | ↓ BG, TG, HBA | [136] |
| 7 | In vitro | AMPK/MAPK/TNF-α, AMPK/ACC/CPT1α | ↑ AMPK, ACC, CPT1α ↓ p-MAPK, TNF-α | [76] |
3.4. Therapeutic Effect on Obesity
3.5. Therapeutic Effect on Hypertension
3.6. Therapeutic Effect on Cardiovascular Disease
3.6.1. Improving Endothelial Function
3.6.2. Autophagy Regulation
| NO | Pharmacology | Diseases | Model | Pathways | Effects * | Ref. |
|---|---|---|---|---|---|---|
| 1 | Anti-atherosclerotic | AS | In vivo | MAPK/NF-κB | ↓ NF-κB, p65, JNK, ERK1/2, p38, iNOS, VCAM-1, IL-6 | [79] |
| 2 | In vitro/ in vivo | miR-33, NFκB | ↑ ABCA1/G1 ↓ CD36, SRA, miR-33, FκB | [80] | ||
| 3 | In vitro | NF-κB | ↓ VCAM-1, ICAM-1, p-iκB, NF-κB | [82] | ||
| 4 | In vivo | NF-κB/PPARγ | ↑ PPAR-γ ↓ oxLDL, TNF-α, IL-6, IL-18, NF-κB, CD36, MMP-9, ICAM-1, VCAM-1, P-p38 | [83] | ||
| 5 | In vivo | / | ↑ GPR78, CHOP, LC3-II;, beclin-1, ATG5 ↓ ER | [66] | ||
| 6 | Anti-myocardial infarction | MI | In vitro/ in vivo | MAPK, EKR, JNK | ↑ EKR ↓ JNK, p38 | [81] |
| 7 | Vascular protection | HHcy | In vivo | / | ↓ MMP-2, MMP-9 | [155] |
| 8 | Anti-heart failure | HF | In vivo | / | ↓ miR-1 | [158] |
| 9 | Activate autophagy | MH | In vivo | / | ↑ LC3-II ↓ NLRP3, IL-1β, p62 | [159] |
| 10 | Protect cardiomyocytes | / | In vitro | / | ↑ GATA-4, Bcl-2, p62 ↓ PARP, Caspase-3, Beclin-1, LC3-II | [160] |
3.7. Therapeutic Effect on Other Diseases
| NO | Pharmacology | Diseases | Model | Pathways | Effects * | Ref. |
|---|---|---|---|---|---|---|
| 1 | Anti-hyperuricemia | HU | In vivo | / | ↓ UA, XOD, CRE, ALT/AST, BUN | [161] |
| 2 | In vitro/ in vivo | PI3K/Akt | ↑ ABCG2 ↓ URAT1, GLUT9 | [164] | ||
| 3 | Anti-hyperinsulinemia | HI | In vivo | / | ↑ TRPC6, GPx, SOD, NOX4 ↓ ERK1/2, MDA, IL-1β, TNF-α, IV Collagen, Laminin | [162] |
| 4 | Anti-PCOS | PCOS | In vivo | / | ↑ CL, IRS1; ↓CF, INS, T | [167] |
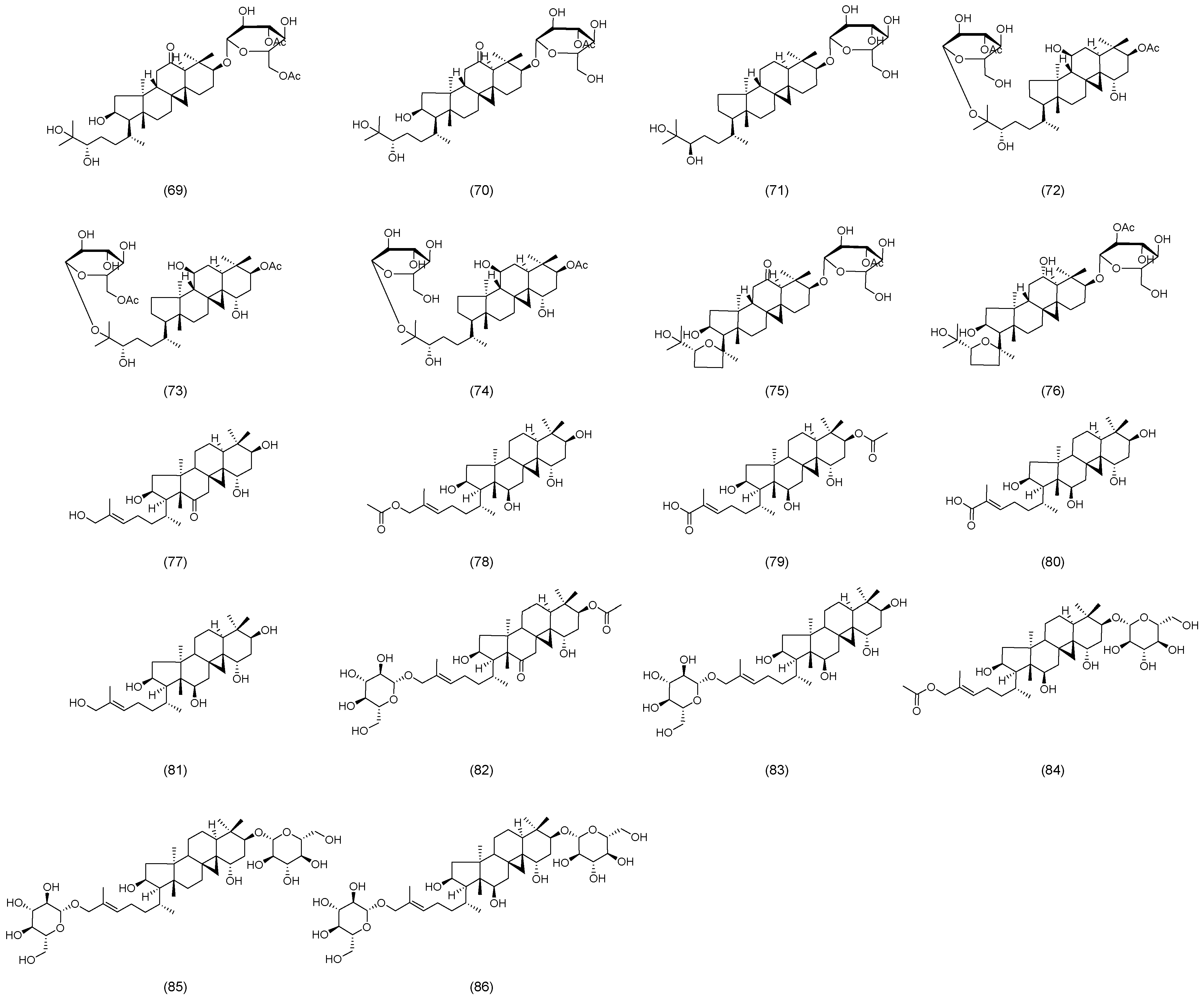
4. Study on the Stems and Leaves of AM
5. Pharmacokinetics Study
6. Safety Evaluation of AM
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pharmacopoeia of the People’s Republic of China. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Tian, H.; An, L.; Wang, P.; Zhang, X.; Gao, W.; Li, X. Review of Astragalus membranaceus polysaccharides: Extraction process, structural features, bioactivities and applications. Chin. Herb. Med. 2025, 17, 56–69. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Liu, Y.; Zhang, Y.Z.; Li, J.C.; Lai, J. Astragalus polysaccharide: A review of its immunomodulatory effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Jiang, J.; Wang, X.; Wang, R.; Han, X. Advancements in the Research of Astragalus membranaceus for the Treatment of Colorectal Cancer. Am. J. Chin. Med. 2025, 53, 119–146. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, P.; Wu, Z.; Zhang, S.; You, F. Mechanisms of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (huang qi) and Angelica sinensis (Oliv.) Diels (dang gui) in Ameliorating Hypoxia and Angiogenesis to Delay Pulmonary Nodule Malignant Transformation. Integr. Cancer Ther. 2025, 24, 15347354241311917. [Google Scholar] [CrossRef]
- Tang, Z.; Tian, X. Astragalus membranaceus: A Traditional Chinese Medicine with Multifaceted Impacts on Breast Cancer Treatment. Biomolecules 2024, 14, 1339. [Google Scholar] [CrossRef]
- Sheng, F.; Yang, S.; Li, M.; Wang, J.; Liu, L.; Zhang, L. Research Progress on the Anti-Cancer Effects of Astragalus membranaceus Saponins and Their Mechanisms of Action. Molecules 2024, 29, 3388. [Google Scholar] [CrossRef]
- Sheng, X.; Yang, L.; Huang, B.; Lin, G.; Wang, Y.; Wu, X.; Lin, R. Efficacy of Astragalus membranaceus (Huang Qi) for Cancer-Related Fatigue: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. Integr. Cancer Ther. 2025, 24, 15347354241313344. [Google Scholar] [CrossRef]
- Luo, Z.H.; Zeng, J.; Yu, H.Y.; Huang, H.Y.; Bao, X.F.; Qin, S.Y.; Chen, G.D.; Zhou, Z.Q.; Zhi, H.; Yao, X.S.; et al. Astramalabaricosides A-T, Highly Oxygenated Malabaricane Triterpenoids with Migratory Inhibitory Activity from Astragalus membranaceus var. mongholicus. J. Nat. Prod. 2022, 85, 2312–2331. [Google Scholar] [CrossRef]
- Bunddulam, P.; Nakamura, M.; Zorig, A.; Hinata, Y.; Takasugi, M.; Feng, C.H.; Sato, T.; Arai, H. Effects of Astragalus membranaceus Leaf Extract on Allergic Inflammation in Immune Cell Lines. Prev. Nutr. Food Sci. 2025, 30, 68–80. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, L.; Luo, X.; Zhu, J.; Yang, H.; Wang, J.; Chen, L.; Zhong, L. Astragalus Polysaccharide Relieves Inflammatory Responses in Guinea Pigs with Allergic Rhinitis via Ameliorating NF-kB-Mediated Treg/Th17 Imbalance. Am. J. Rhinol. Allergy 2022, 36, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Bival Štefan, M. Astragalus membranaceus, Nigella sativa, and Perilla frutescens as Immunomodulators-Molecular Mechanisms and Clinical Effectiveness in Allergic Diseases. Curr. Issues Mol. Biol. 2024, 46, 9016–9032. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Leng, Y.; Shi, Y.; Tan, Z.; Min, X. Astragalus membranaceus as a Drug Candidate for Inflammatory Bowel Disease: The Preclinical Evidence. Am. J. Chin. Med. 2023, 51, 1501–1526. [Google Scholar] [CrossRef]
- Ghabeshi, S.; Mousavizadeh, L.; Ghasemi, S. Enhancing the Antiviral Potential and Anti-inflammatory Properties of Astragalus membranaceus: A Comprehensive Review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2023, 22, 211–219. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Q. Pharmacological Effects and Molecular Protective Mechanisms of Astragalus Polysaccharides on Nonalcoholic Fatty Liver Disease. Front. Pharmacol. 2022, 13, 854674. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zhang, Z.; Leng, Y.; Yang, Y.; Fu, X.; Xie, H.; Gao, H.; Xie, C. The potential of astragalus polysaccharide for treating diabetes and its action mechanism. Front. Pharmacol. 2024, 15, 1339406. [Google Scholar] [CrossRef]
- Chen, C.; Bu, X.; Deng, L.; Xia, J.; Wang, X.; Chen, L.; Li, W.; Huang, J.; Chen, Q.; Wang, C. Astragaloside IV as a promising therapeutic agent for liver diseases: Current landscape and future perspectives. Front. Pharmacol. 2025, 16, 1574154. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Zhang, Y.; Cheng, Z.; Wan, M.; Qin, W.; Li, P.; Feng, J.; Shao, S.; Xue, W.; et al. Recent pharmacological advances in the treatment of cardiovascular events with Astragaloside IV. Biomed. Pharmacother. 2023, 168, 115752. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chen, H.W.; Li, J. Astragaloside IV: An Effective Drug for the Treatment of Cardiovascular Diseases. Drug Des. Dev. Ther. 2020, 14, 3731–3746. [Google Scholar] [CrossRef]
- Wang, D.; Liu, R.; Zeng, J.; Li, C.; Xiang, W.; Zhong, G.; Xia, Z. Preliminary screening of the potential active ingredients in traditional Chinese medicines using the Ussing chamber model combined with HPLC-PDA-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1189, 123090. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, F.; Yang, L.; Wang, B.; Zhou, G. Major Chemical Compounds and Mineral Elements of Astragalus membranaceus Cultivated on the Qinghai-Tibet Plateau with Different Planting Densities. Chem. Biodivers. 2022, 19, e202100778. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, F.; Yang, L.; Xiao, Y.; Zhou, G. A Seasonal Change of Active Ingredients and Mineral Elements in Root of Astragalus membranaceus in the Qinghai-Tibet Plateau. Biol. Trace Elem. Res. 2021, 199, 3950–3959. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Jiang, Y.; Liu, J.; Yang, B. UHPLC-MS/MS Analysis on Flavonoids Composition in Astragalus membranaceus and Their Antioxidant Activity. Antioxidants 2021, 10, 1852. [Google Scholar] [CrossRef]
- Yu, X.; Xia, K.; Wu, S.; Wang, Q.; Cheng, W.; Ji, C.; Yang, W.; Kang, C.; Yuan, Z.; Li, Y. Simultaneous determination and pharmacokinetic study of six components in beagle dog plasma by UPLC-MS/MS after oral administration of Astragalus membranaceus aqueous extract. Biomed. Chromatogr. 2022, 36, e5488. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Li, S.; Liu, C.; Zhuang, S.; Zhou, X.; Li, Y.; Zhang, Y.; Liang, J. Extraction and preparation of 5-lipoxygenase and acetylcholinesterase inhibitors from Astragalus membranaceus stems and leaves. J. Sep. Sci. 2023, 46, e2200812. [Google Scholar] [CrossRef]
- Zhao, C.G.; Li, C.Y.; Yang, S.; Xu, Q.L.; Zheng, Y.F.; Wang, Y.F.; Li, H.Y.; Peng, G.P. Analysis of quality difference based on Astragalus membranaceus var. mongholicus in genuine region. Zhongguo Zhong Yao Za Zhi 2020, 45, 3183–3190. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Chen, L.; Dong, Q.; Yang, D.H.; Zhang, Q.; Zeng, J.; Wang, Y.; Liu, X.; Cui, Y.; et al. Astragali radix (Huangqi): A time-honored nourishing herbal medicine. Chin. Med. 2024, 19, 119. [Google Scholar] [CrossRef]
- Tan, J.Y.; Zhao, J.X.; Zang, Y.; Li, P.; Yang, S.Q.; Li, X.M.; Wang, Y.L.; Cheng, Y.G. New flavonoid glycosides from the stems and leaves of Astragalus membranaceus. Fitoterapia 2025, 180, 106321. [Google Scholar] [CrossRef]
- Wang, Q.X.; Guo, S.; Shen, K.X.; Li, H.W.; Zhang, H.K.; Xie, Y.J.; Shang, E.X.; Duan, J.A. Chemical composition analysis and value evaluation of stems and leaves of Astragalus membranaceus var. mongholicus. Zhongguo Zhong Yao Za Zhi 2023, 48, 6600–6612. [Google Scholar] [CrossRef]
- Li, A.; Cui, W.; Zhao, Y.; Luo, T.; Zhang, Q.; Liu, Y.; Li, K.; Qin, X.; Zhang, L. Exploration of the main effective constituent and the mechanism in Astragali Radix in the treatment for doxorubicin-induced nephropathy by integrating metabolomics and molecular docking. J. Ethnopharmacol. 2023, 305, 116074. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Flowers of Astragalus membranaceus var. mongholicus as a Novel High Potential By-Product: Phytochemical Characterization and Antioxidant Activity. Molecules 2019, 24, 434. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.Q.; Xu, F.; Kuang, H.X.; Shi, X.P.; Cao, F.; Yang, B.Y.; Wang, Z.B. 10-Secocycloartane (=9, 19-cyclo-9, 10-secolanostane) triterpenoid saponins: Huangqiyenins M-X from Astragalus membranaceus (Fisch.) Bge. Phytochemistry 2024, 222, 114072. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Nguyen, V.P.; Yang, S.Y.; Min, B.S.; Kim, J.A. Astraoleanosides E-P, oleanane-type triterpenoid saponins from the aerial parts of Astragalus membranaceus Bunge and their β-glucuronidase inhibitory activity. Bioorg. Chem. 2024, 145, 107230. [Google Scholar] [CrossRef]
- Du, R.; Xu, F.; Wei, D.; Wei, Y.; Wang, Z.; Wang, Z. Pharmacokinetics of two triterpenoid saponins and three flavonoids in Astragalus membranaceus leaves by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2024, 251, 116419. [Google Scholar] [CrossRef]
- Zhao, J.X.; Zang, Y.; Li, P.; Zhang, J.N.; Yang, S.Q.; Li, H.F.; Wang, Y.; Tan, J.Y.; Wang, Y.L.; Cheng, Y.G. A new flavonol glycoside isolated from the stems and leaves of Astragalus membranaceus. Nat. Prod. Res. 2025; 1–6, in press. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Zhang, Q.; Jiang, P.; Zhang, Y.; Luo, Y.; Guan, W.; Li, M.; Chen, Q.; Zhang, L.; et al. New triterpenoid saponins isolated from the leaves of Astragalus membranaceus (Fisch.) Bge. and their neuroprotective effects. Bioorg. Chem. 2025, 156, 108149. [Google Scholar] [CrossRef]
- Xu, W.X.; Liu, Z.Y.; Bu, Q.Y.; Jiang, P.; Zhang, Y.Q.; Luo, Y.M.; Guan, W.; Ma, X.C.; Li, M.M.; Chen, Q.S.; et al. Eight new triterpenoid saponins from the leaves of Astragalus membranaceus (Fisch.) Bunge Their Neuroprotective effects. Fitoterapia 2025, 183, 106559. [Google Scholar] [CrossRef]
- Cheng, Y.G.; Li, J.L.; Li, P.; Yang, S.Q.; Zang, Y.; Wang, Y.; Yu, Y.T.; Xie, X.; Li, H.F.; Hao, X.L.; et al. Neuroprotective triterpenoids from Astragalus membranaceus stems and leaves: Anti-inflammatory and anti-apoptotic mechanisms for memory improvement via in vivo and in vitro models. Bioorg. Chem. 2025, 160, 108492. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, G. Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed. Pharmacother. 2022, 150, 113015. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Liu, K.; Li, M.; Jin, Y.; Gu, L.; Yu, X.A.; Wang, S.; Wang, P.; Wang, B.; et al. A Rapid and Accurate UHPLC Method for Determination of Monosaccharides in Polysaccharides of Different Sources of Radix Astragali and Its Immune Activity Analysis. Molecules 2024, 29, 2287. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Du, Y.; Qin, X. Screening and structure study of active components of Astragalus polysaccharide for injection based on different molecular weights. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1152, 122255. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, X.; Zhao, M.; Ma, S.; Zhang, Y. Pharmacological potential of Astragali Radix for the treatment of kidney diseases. Phytomedicine 2024, 123, 155196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Sun, D.; Ye, N.; Chen, T.; Yang, M.; Zhou, L.; Zou, H. Research progress of astragaloside IV in treating acute kidney injury. Int. Urol. Nephrol. 2024, 56, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Zhang, T.; Li, X.; Wu, C.; Zhao, Z.; Tang, J.; Tan, X.; Hu, Q.; Liao, W. Astragaloside IV attenuates cadmium induced nephrotoxicity in rats by activating Nrf2. Sci. Rep. 2025, 15, 2028. [Google Scholar] [CrossRef]
- Borowicz, K.K.; Jach, M.E. Astragalus Membranaceus—Can It Delay Cellular Aging? Nutrients 2025, 17, 1229. [Google Scholar] [CrossRef]
- Chen, K.; Qin, C.; Ji, C.; Yu, Y.; Wu, Y.; Xu, L.; Jiang, Y.; Zou, G. Astragalus polysaccharide alleviates oxidative stress and senescence in chondrocytes in osteoarthritis via GCN2/ATF4/TXN axis. Int. J. Biol. Macromol. 2025, 310, 143285. [Google Scholar] [CrossRef]
- Son, S.R.; Kim, K.S.; Jun, M.; Jang, D.S.; Lee, S. Effects of Astraflavonoid A and Astraside C from the Aerial Part of Astragalus membranaceus on TNF-α-Induced Human Dermal Fibroblasts. Plants 2025, 14, 1358. [Google Scholar] [CrossRef]
- Wang, S.W.; Li, P.; Liu, S.Y.; Huang, D.L.; Zhang, S.J.; Zeng, X.X.; Lan, T.; Mao, K.L.; Gao, Y.; Cheng, Y.F.; et al. Astragaloside IV inhibits retinal pigment epithelial cell senescence and reduces IL-1β mRNA stability by targeting FTO-mediated m(6)A methylation. Phytomedicine 2025, 138, 156408. [Google Scholar] [CrossRef]
- Miao, X.; Rong, L.; Fu, B.; Cui, S.; Gu, Z.; Hu, F.; Lu, Y.; Yan, S.; Sun, B.; Jiang, W.; et al. Astragalus polysaccharides attenuate rat aortic endothelial senescence via regulation of the SIRT-1/p53 signaling pathway. BMC Complement. Med. Ther. 2024, 24, 80. [Google Scholar] [CrossRef]
- Zaman, Q.; Zhang, D.; Reddy, O.S.; Wong, W.T.; Lai, W.F. Roles and Mechanisms of Astragaloside IV in Combating Neuronal Aging. Aging Dis. 2022, 13, 1845–1861. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Z.; Chen, Y.; Wang, S.D.; Yang, Y.M.; Cai, L.Q.; Zhao, J.X.; Huang, W.J.; Xiao, Y.H. Radix Astragali and Its Representative Extracts for Diabetic Nephropathy: Efficacy and Molecular Mechanism. J. Diabetes Res. 2024, 2024, 5216113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J. Possible mechanism for the protective effect of active ingredients of astragalus membranaceus on diabetes nephropathy. J. Asian Nat. Prod. Res. 2024, 26, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Di, Y.M.; May, B.; Zhang, A.L.; Zhang, L.; Chen, J.; Wang, R.; Liu, X.; Xue, C.C. Renal protective effects and mechanisms of Astragalus membranaceus for diabetic kidney disease in animal models: An updated systematic review and meta-analysis. Phytomedicine 2024, 129, 155646. [Google Scholar] [CrossRef]
- Liu, P.Y.; Hong, K.F.; Liu, Y.D.; Sun, Z.Y.; Zhao, T.T.; Li, X.L.; Lao, C.C.; Tan, S.F.; Zhang, H.Y.; Zhao, Y.H.; et al. Total flavonoids of Astragalus protects glomerular filtration barrier in diabetic kidney disease. Chin. Med. 2024, 19, 27. [Google Scholar] [CrossRef]
- Zou, X.; Liu, X.; Qu, W.; Zhang, X.; Zou, Y.; Lin, X.; Hu, W.; Gao, R.; He, Y.; Zhou, S.; et al. Astragaloside IV Relieves Mitochondrial Oxidative Stress Damage and Dysfunction in Diabetic Mice Endothelial Progenitor Cells by Regulating the GSK-3β/Nrf2 Axis. Appl. Biochem. Biotechnol. 2025, 197, 3964–3981. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, X.; Zou, X.L.; Peng, S.; Lei, H.J.; Liu, X.N.; Zhao, L.; Huang, Z.X. Exosomes Derived from Astragaloside IV-pretreated Endothelial Progenitor Cells (AS-IV-Exos) Alleviated Endothelial Oxidative Stress and Dysfunction Via the miR-210/Nox2/ROS Pathway. Curr. Mol. Med. 2024, 25, 320–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, X.D.; Cao, A.L.; Chu, S.; Li, Z.J.; Wang, Y.M.; Peng, W.; Wang, L.; Wang, H. Astragaloside IV prevents endothelial dysfunction by improving oxidative stress in streptozotocin-induced diabetic mouse aortas. Exp. Ther. Med. 2021, 22, 1197. [Google Scholar] [CrossRef]
- Leng, B.; Li, C.; Sun, Y.; Zhao, K.; Zhang, L.; Lu, M.L.; Wang, H.X. Protective Effect of Astragaloside IV on High Glucose-Induced Endothelial Dysfunction via Inhibition of P2X7R Dependent P38 MAPK Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 5070415. [Google Scholar] [CrossRef]
- Liang, X.Y.; Hong, F.F.; Yang, S.L. Astragaloside IV Alleviates Liver Inflammation, Oxidative Stress and Apoptosis to Protect Against Experimental Non-Alcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 1871–1883. [Google Scholar] [CrossRef]
- Yuan, Q.F.; Tang, S.M.; Chen, S.Y.; Yang, Z.M. Therapeutic effect of astragalus polysaccharides on nonalcoholic fatty liver disease in rats. Acad. J. Nav. Med. Univ. 2018, 39, 573–578. [Google Scholar] [CrossRef]
- Li, X.Z.; Ding, Y.Z.; Wu, H.F.; Bian, Z.P.; Xu, J.D.; Gu, C.R.; Chen, X.J.; Yang, D. Astragaloside IV Prevents Cardiac Remodeling in the Apolipoprotein E-Deficient Mice by Regulating Cardiac Homeostasis and Oxidative Stress. Cell Physiol. Biochem. 2017, 44, 2422–2438. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhuang, Y.; Tian, Y.; Thomas, G.N.; Ying, M.; Tomlinson, B. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid. Med. Cell Longev. 2012, 2012, 282383. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, T.; Zhang, Y.; Pan, T.; Yao, S.; Yu, H.; Ma, K.; Wang, S. Astragaloside IV protects against C/EBP homologous protein-mediated apoptosis in oxidized low-density lipoprotein-treated macrophages by promoting autophagy. Eur. J. Pharmacol. 2022, 923, 174912. [Google Scholar] [CrossRef]
- Luo, L.F.; Qin, L.Y.; Wang, J.X.; Guan, P.; Wang, N.; Ji, E.S. Astragaloside IV Attenuates the Myocardial Injury Caused by Adriamycin by Inhibiting Autophagy. Front. Pharmacol. 2021, 12, 669782. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qin, Y. Effect and mechanism of astragalus polysaccharide on lipid metabolism of macrophages. J. Xi’an Jiaotong Univ. (Med. Sci.) 2019, 40, 640–645. [Google Scholar] [CrossRef]
- Chan, K.W.; Kwong, A.S.K.; Tsui, P.N.; Chan, G.C.W.; Choi, W.F.; Yiu, W.H.; Cheung, S.C.Y.; Wong, M.M.Y.; Zhang, Z.J.; Tan, K.C.B.; et al. Add-on astragalus in type 2 diabetes and chronic kidney disease: A multi-center, assessor-blind, randomized controlled trial. Phytomedicine 2024, 130, 155457. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Huang, B.; Chen, D.; Ye, R. Astragaloside IV Regulates Insulin Resistance and Inflammatory Response of Adipocytes via Modulating CTRP3 and PI3K/AKT Signaling. Diabetes Ther. 2022, 13, 1823–1834. [Google Scholar] [CrossRef]
- Hoo, R.L.; Wong, J.Y.; Qiao, C.; Xu, A.; Xu, H.; Lam, K.S. The effective fraction isolated from Radix Astragali alleviates glucose intolerance, insulin resistance and hypertriglyceridemia in db/db diabetic mice through its anti-inflammatory activity. Nutr. Metab. 2010, 7, 67. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, R.; Yang, S.; Zhang, Y.; Shi, D. Astragaloside IV alleviates placental oxidative stress and inflammation in GDM mice. Endocr. Connect. 2020, 9, 939–945. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Xing, B.; Zhao, J.; Zhang, P.; Shi, D.; Yang, F. Astragaloside IV attenuates gestational diabetes mellitus via targeting NLRP3 inflammasome in genetic mice. Reprod. Biol. Endocrinol. 2019, 17, 77. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, Q.Z.; Wang, Y.R.; Fu, L.N.; Han, J.S.; Zhang, J.; Wang, B.M. Astragaloside IV Improves High-Fat Diet-Induced Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Rats by Regulating Inflammatory Factors Level via TLR4/NF-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 605064. [Google Scholar] [CrossRef]
- Zhong, M.; Yan, Y.; Yuan, H.; A, R.; Xu, G.; Cai, F.; Yang, Y.; Wang, Y.; Zhang, W. Astragalus mongholicus polysaccharides ameliorate hepatic lipid accumulation and inflammation as well as modulate gut microbiota in NAFLD rats. Food Funct. 2022, 13, 7287–7301. [Google Scholar] [CrossRef]
- Fu, L.; Wu, Z.; Chu, Y.; Chen, W.; Gao, L.; Mu, S.; Zhao, J. Explore the Mechanism of Astragalus mongholicus Bunge against Nonalcoholic Fatty Liver Disease Based on Network Pharmacology and Experimental Verification. Gastroenterol. Res. Pract. 2022, 2022, 4745042. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ma, D.; Wang, X.; Wang, Y.; Bi, Y.; Yang, J.; Wang, X.; Li, X. Astragaloside IV Prevents Obesity-Associated Hypertension by Improving Pro-Inflammatory Reaction and Leptin Resistance. Mol. Cells 2018, 41, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, M.; Wang, J.; Liu, P. Astragaloside IV Relieves Atherosclerosis and Hepatic Steatosis via MAPK/NF-κB Signaling Pathway in LDLR(-/-) Mice. Front. Pharmacol. 2022, 13, 828161. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Yang, S.; Hua, Y.; Su, J.; Shang, Y.; Wang, Z.; Feng, K.; Zhang, J.; Yang, X.; et al. Astragalus Flavone Ameliorates Atherosclerosis and Hepatic Steatosis Via Inhibiting Lipid-Disorder and Inflammation in apoE(-/-) Mice. Front. Pharmacol. 2020, 11, 610550. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, G.; Wang, T.; Ren, H.; An, H.; Lian, C.; Liu, J.; Guo, L.; Li, W. Astragaloside IV Ameliorates Myocardial Infarction Induced Apoptosis and Restores Cardiac Function. Front. Cell Dev. Biol. 2021, 9, 671255. [Google Scholar] [CrossRef]
- You, Y.; Duan, Y.; Liu, S.W.; Zhang, X.L.; Zhang, X.L.; Feng, J.T.; Yan, C.H.; Han, Y.L. Anti-atherosclerotic function of Astragali Radix extract: Downregulation of adhesion molecules in vitro and in vivo. BMC Complement. Altern. Med. 2012, 12, 54. [Google Scholar] [CrossRef]
- Sun, B.; Rui, R.; Pan, H.; Zhang, L.; Wang, X. Effect of Combined Use of Astragaloside IV (AsIV) and Atorvastatin (AV) on Expression of PPAR-γ and Inflammation-Associated Cytokines in Atherosclerosis Rats. Med. Sci. Monit. 2018, 24, 6229–6236. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Zhang, X. Astragaloside IV Protects Against Oxidized Low-Density Lipoprotein (ox-LDL)-Induced Endothelial Cell Injury by Reducing Oxidative Stress and Inflammation. Med. Sci. Monit. 2019, 25, 2132–2140. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Thomas, M.S.; Lemos, B.S.; DiMarco, D.M.; Missimer, A.; Melough, M.; Chun, O.K.; Murillo, A.G.; Alyousef, H.M.; Medina-Vera, I. TA-65, A Telomerase Activator improves Cardiovascular Markers in Patients with Metabolic Syndrome. Curr. Pharm. Des. 2018, 24, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Fu, X. Hypoglycemic effect of the polysaccharides from Astragalus membranaceus on type 2 diabetic mice based on the “gut microbiota-mucosal barrier”. Food Funct. 2022, 13, 10121–10133. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; La, X.; Zhang, B.; Xu, W.; Tian, C.; Fu, Q.; Wang, M.; Wu, C.; Chen, Z.; Chang, H.; et al. Total Astragalus saponins can reverse type 2 diabetes mellitus-related intestinal dysbiosis and hepatic insulin resistance in vivo. Front. Endocrinol. 2023, 14, 1190827. [Google Scholar] [CrossRef]
- Xu, G.; Yuan, H.; Liu, J.; Wang, X.; Ma, L.; Wang, Y.; Dong, G. Astragalus mongholicus Polysaccharides Alleviate Kidney Injury in Rats with Type 2 Diabetes Through Modulation of Oxidation, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2025, 26, 1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiong, Z.; Lin, M.; Yang, Y.; Chen, Y.; Zeng, J.; Jia, X.; Feng, L. Astragalus polysaccharides alleviate type 1 diabetes via modulating gut microbiota in mice. Int. J. Biol. Macromol. 2023, 234, 123767. [Google Scholar] [CrossRef]
- Song, Q.; Cheng, S.W.; Li, D.; Cheng, H.; Lai, Y.S.; Han, Q.; Wu, H.Y.; Shaw, P.C.; Zuo, Z. Gut microbiota mediated hypoglycemic effect of Astragalus membranaceus polysaccharides in db/db mice. Front. Pharmacol. 2022, 13, 1043527. [Google Scholar] [CrossRef]
- Song, Q.; Zou, J.; Li, D.; Cheng, S.W.; Li, K.L.S.; Yang, X.; Shaw, P.C.; Zuo, Z. Gastrointestinal metabolism of Astragalus membranaceus polysaccharides and its related hypoglycemic mechanism based on gut microbial transformation. Int. J. Biol. Macromol. 2024, 280, 135847. [Google Scholar] [CrossRef]
- Gong, P.; Xiao, X.; Wang, S.; Shi, F.; Liu, N.; Chen, X.; Yang, W.; Wang, L.; Chen, F. Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway. J. Ethnopharmacol. 2021, 281, 114558. [Google Scholar] [CrossRef]
- He, X.Y.; He, J.J.; Zheng, N.N.; Wang, S.C.; Li, H.K. Study on Anti-Obesity Effect and Modulation of Gut Microbiota by Astragalus Polysaccharides in Mice. World Chin. Med. 2016, 11, 2379–2384+2388. [Google Scholar] [CrossRef]
- Mohammad, N.H.L.; Mohamed, A.S.R.; Mohamed, S.M.; Abdelsalam, H.M.; Abdel-Wahab, M.D.; Maher, S.M. Diet, Serum Short Chain Fatty Acids, and Potential Molecular Associations in Obesity. QJM 2021, 114, pi52. [Google Scholar] [CrossRef]
- Zhao, W.; Duan, C.; Liu, Y.; Lu, G.; Lyu, Q.; Liu, X.; Zheng, J.; Zhao, X.; Wang, S.; Zhao, H. Modulating effects of Astragalus polysaccharide on immune disorders via gut microbiota and the TLR4/NF-κB pathway in rats with syndrome of dampness stagnancy due to spleen deficiency. J. Zhejiang Univ. Sci. B 2023, 24, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.Y. Mechanism of Astragaloside IV Alleviating Diet-Induced Obesity by Regulating Intestinal Flora. Master’s Thesis, Hebei University, Baoding, China, 2022. [Google Scholar]
- Wang, L.; Dong, X.L.; Qin, X.M.; Li, Z.Y. Investigating the inter-individual variability of Astragali Radix against cisplatin-induced liver injury via 16S rRNA gene sequencing and LC/MS-based metabolomics. Phytomedicine 2022, 101, 154107. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Dong, X.; Wu, Y.; Li, B.; Kuang, B.; Chen, G.; Zhang, L. Astragaloside IV attenuates myocardial dysfunction in diabetic cardiomyopathy rats through downregulation of CD36-mediated ferroptosis. Phytother. Res. 2023, 37, 3042–3056. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.G.; Feng, X.C.; Wang, Y.H.; Lei, X.L. Effects Mechanism of Astragalus Polysaccharide on the Disorder of Glucose and Lipid Metabolism in Diabetes Rats via Wnt1 Signaling. J. Liaoning Univ. Chin. Med. 2024, 26, 43–51. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.Y.; Yang, Z.M. Chinese Journal of Experimental Traditional Medical Formulae Intestine of Rat Model Induced by High-sugar and High-fat Diet. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 64–68. [Google Scholar] [CrossRef]
- Guo, S.; Ma, B.; Jiang, X.; Li, X.; Jia, Y. Astragalus Polysaccharides Inhibits Tumorigenesis and Lipid Metabolism Through miR-138-5p/SIRT1/SREBP1 Pathway in Prostate Cancer. Front. Pharmacol. 2020, 11, 598. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.L.; Hong, K.F.; Zhao, T.T.; Dong, R.X.; Wang, W.M.; Li, Y.T.; Zhang, G.L.; Lin, J.; Gui, D.K.; et al. Total flavonoids of Astragalus Ameliorated Bile Acid Metabolism Dysfunction in Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2021, 2021, 6675567. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsay, H.J.; Lu, M.K.; Lin, C.H.; Yeh, C.W.; Liu, H.K.; Shiao, Y.J. Astragalus membranaceus-Polysaccharides Ameliorates Obesity, Hepatic Steatosis, Neuroinflammation and Cognition Impairment without Affecting Amyloid Deposition in Metabolically Stressed APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2017, 18, 2746. [Google Scholar] [CrossRef]
- Li, B.; Hong, Y.; Gu, Y.; Ye, S.; Hu, K.; Yao, J.; Ding, K.; Zhao, A.; Jia, W.; Li, H. Functional Metabolomics Reveals that Astragalus Polysaccharides Improve Lipids Metabolism through Microbial Metabolite 2-Hydroxybutyric Acid in Obese Mice. Engineering 2022, 9, 111–122. [Google Scholar] [CrossRef]
- Wu, H.; Gao, Y.; Shi, H.L.; Qin, L.Y.; Huang, F.; Lan, Y.Y.; Zhang, B.B.; Hu, Z.B.; Wu, X.J. Astragaloside IV improves lipid metabolism in obese mice by alleviation of leptin resistance and regulation of thermogenic network. Sci. Rep. 2016, 6, 30190. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, W.; Guo, F.; Liao, S.; Hu, M.; Yu, T.; Yu, S.; Shi, Q. Astragaloside IV alleviates neuronal ferroptosis in ischemic stroke by regulating fat mass and obesity-associated-N6-methyladenosine-acyl-CoA synthetase long-chain family member 4 axis. J. Neurochem. 2023, 166, 328–345. [Google Scholar] [CrossRef]
- Dhondge, R.H.; Agrawal, S.; Patil, R.; Kadu, A.; Kothari, M. A Comprehensive Review of Metabolic Syndrome and Its Role in Cardiovascular Disease and Type 2 Diabetes Mellitus: Mechanisms, Risk Factors, and Management. Cureus 2024, 16, e67428. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ou-Yang, J.P.; Wu, K.; Wang, Y.; Zhou, Y.F.; Wen, C.Y. Hypoglycemic effect of Astragalus polysaccharide and its effect on PTP1B. Acta Pharmacol. Sin. 2005, 26, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shen, H.; Chen, M.; Qian, M. Effect of astragalus polysaccharide nanoliposomes on lipid metabolism and insulin resistance in pregnant rats with gestational diabetes by regulating AMPK pathway via protein-tyrosine phosphatase 1B inhibition. Mater. Express 2022, 12, 831–838. [Google Scholar] [CrossRef]
- Liu, H.; Bai, J.; Weng, X.; Wang, T.; Li, M. Amelioration of insulin resistance in rat cells by Astragalus polysaccharides and associated mechanisms. Exp. Ther. Med. 2014, 7, 1599–1604. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y.; Wang, M.; Chen, R.; Sun, X. Comparison of chemical composition between imitation wild and transplanted Astragali Radix and their therapeutic effects on heart failure. J. Ethnopharmacol. 2025, 337, 118827. [Google Scholar] [CrossRef]
- Yue, X.; Hao, W.; Wang, M.; Fu, Y. Astragalus polysaccharide ameliorates insulin resistance in HepG2 cells through activating the STAT5/IGF-1 pathway. Immun. Inflamm. Dis. 2023, 11, e1071. [Google Scholar] [CrossRef]
- Liu, M.; Qin, J.; Hao, Y.; Liu, M.; Luo, J.; Luo, T.; Wei, L. Astragalus polysaccharide suppresses skeletal muscle myostatin expression in diabetes: Involvement of ROS-ERK and NF-κB pathways. Oxid. Med. Cell Longev. 2013, 2013, 782497. [Google Scholar] [CrossRef]
- Wei, Z.; Weng, S.; Wang, L.; Mao, Z. Mechanism of Astragalus polysaccharides in attenuating insulin resistance in Rats with type 2 diabetes mellitus via the regulation of liver microRNA-203a-3p. Mol. Med. Rep. 2018, 17, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zeng, Y.; Tang, Z.; Wang, C.; He, Y.; Feng, X.; Zhou, L. Astragalus polysaccharides affect insulin resistance by regulating the hepatic SIRT1-PGC-1α/PPARα-FGF21 signaling pathway in male Sprague Dawley rats undergoing catch-up growth. Mol. Med. Rep. 2015, 12, 6451–6460. [Google Scholar] [CrossRef]
- Ye, X.; Xing, X.; Yuan, K.; Wang, D.; Wu, D.; Chen, J.; Yu, J. Astragaloside IV ameliorates insulin induced insulin resistance in HepG2 cells through reactive oxygen species mediated c-Jun N-terminal kinase pathway. J. Tradit. Chin. Med. 2023, 43, 60–67. [Google Scholar] [CrossRef]
- Xu, A.; Wang, H.; Hoo, R.L.; Sweeney, G.; Vanhoutte, P.M.; Wang, Y.; Wu, D.; Chu, W.; Qin, G.; Lam, K.S. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology 2009, 150, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.L.; Tang, W.J.; Tang, B. Astragaloside IV inhibits protein tyrosine phosphatase 1B and improves insulin resistance in insulin-resistant HepG2 cells and triglyceride accumulation in oleic acid (OA)-treated HepG2 cells. J. Ethnopharmacol. 2021, 268, 113556. [Google Scholar] [CrossRef]
- Dutta, B.; Loo, S.; Kam, A.; Tam, J.P. Plant-derived cell-penetrating microprotein α-astratide aM1 targets Akt signaling and alleviates insulin resistance. Cell Mol. Life Sci. 2023, 80, 293. [Google Scholar] [CrossRef]
- Deng, S.; Yang, L.; Ma, K.; Bian, W. Astragalus polysaccharide improve the proliferation and insulin secretion of mouse pancreatic β cells induced by high glucose and palmitic acid partially through promoting miR-136-5p and miR-149-5p expression. Bioengineered 2021, 12, 9872–9884. [Google Scholar] [CrossRef]
- Zou, F.; Mao, X.Q.; Wang, N.; Liu, J.; Ou-Yang, J.P. Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta Pharmacol. Sin. 2009, 30, 1607–1615. [Google Scholar] [CrossRef]
- Ren, X.; Dai, Y.; Shan, M.; Zheng, J.; Zhang, Z.; Shen, T. Astragalus polysaccharide restores insulin secretion impaired by lipopolysaccharides through the protein kinase B/mammalian target of rapamycin/glucose transporter 2 pathway. BMC Complement. Med. Ther. 2023, 23, 358. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, G.; Meng, L.; Tang, Y.; Yang, M.; Ni, C. Protective Effects of Astragaloside IV on Uric Acid-Induced Pancreatic β-Cell Injury through PI3K/AKT Pathway Activation. Evid. Based Complement. Altern. Med. 2022, 2022, 2429162. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, Y.; Zheng, X.; Zhang, J.; Liu, J.; Wu, G. Astragaloside IV Ameliorates Streptozotocin Induced Pancreatic β-Cell Apoptosis and Dysfunction Through SIRT1/P53 and Akt/GSK3β/Nrf2 Signaling Pathways. Diabetes Metab. Syndr. Obes. 2022, 15, 131–140. [Google Scholar] [CrossRef]
- Huang, C.; Yang, H.F. Effect of Astragaloside IV on GLP-1 secretion in type 2 diabetic rats. J. Tradit. Chin. Vet. Med. 2022, 41, 1–6. [Google Scholar] [CrossRef]
- Yang, Z.M.; Wang, Y.; Chen, S.Y. Astragalus polysaccharide alleviates type 2 diabetic rats by reversing the glucose transporters and sweet taste receptors/GLP-1/GLP-1 receptor signaling pathways in the intestine-pancreatic axis. J. Funct. Foods 2021, 76, 104310. [Google Scholar] [CrossRef]
- Li, X.; Zhao, T.; Gu, J.; Wang, Z.; Lin, J.; Wang, R.; Duan, T.; Li, Z.; Dong, R.; Wang, W.; et al. Intake of flavonoids from Astragalus membranaceus ameliorated brain impairment in diabetic mice via modulating brain-gut axis. Chin. Med. 2022, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, W.L.; Liu, S.; Cao, Z.M.; Xu, L.; Chen, j.; Li, J.M. Mechanism of Astragali Radix in treatment of metabolic associated fatty liver disease based on network pharmacology. Drug Eval. Res. 2021, 44, 89–97. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, D.L.; Wei, X.H.; Zhong, R.Y.; Xu, J.; Sun, L. Astragaloside IV attenuates free fatty acid-induced ER stress and lipid accumulation in hepatocytes via AMPK activation. Acta Pharmacol. Sin. 2017, 38, 998–1008. [Google Scholar] [CrossRef]
- Wei, X.H.; Yang, X.Y.; Hu, F.; Liang, A.M.; Guo, Y.F.; Sun, L. Astragaloside IV Activates AMPK to Alleviate Liver Lipid Deposition of Nonalcoholic Fatty Liver Disease in Mice. Drug Eval. 2021, 18, 1230–1234. [Google Scholar] [CrossRef]
- Yang, X.Y.; Hu, F.; Wei, X.H.; HEAN, P.; Sun, L. Effect of astragaloside IV on liver lipid metabolism of nonalcoholic fatty liver disease in mice. J. Pract. Med. 2018, 34, 4064–4067+4071. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, H.; Zhu, W.; Li, Y.; Li, H. Astragalus polysaccharide attenuates nonalcoholic fatty liver disease through THDCA in high-fat diet-fed mice. J. Ethnopharmacol. 2024, 320, 117401. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhang, S.; Zhao, Y.; Huang, J.; Wang, Y.; Li, Y.; Fan, S.; Yang, L.; Ji, G.; Tong, Q.; et al. Cycloastragenol improves hepatic steatosis by activating farnesoid X receptor signalling. Pharmacol. Res. 2017, 121, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Ma, D.; Wu, T.; Qu, Y.; Yang, J.; Cai, L.; Li, X.; Wang, Y. Astragalus polysaccharide prevents heart failure-induced cachexia by alleviating excessive adipose expenditure in white and brown adipose tissue. Lipids Health Dis. 2023, 22, 9. [Google Scholar] [CrossRef]
- Zhang, S.H. Effects of Astragalus Polysacharins on Brown Adipogenesis in C3H10T 1/2 Cells. Master’s Thesis, Shanxi Agricultural University, Taiyuan, China, 2021. [Google Scholar]
- Cao, Y.; Deng, B.; Zhang, S.; Gao, H.; Song, P.; Zhang, J.; Zhao, J. Astragalus polysaccharide regulates brown adipogenic differentiation through miR-1258-5p-modulated cut-like homeobox 1 expression. Acta Biochim. Biophys. Sin. 2021, 53, 1713–1722. [Google Scholar] [CrossRef]
- Nie, T.; Zhao, S.; Mao, L.; Yang, Y.; Sun, W.; Lin, X.; Liu, S.; Li, K.; Sun, Y.; Li, P.; et al. The natural compound, formononetin, extracted from Astragalus membranaceus increases adipocyte thermogenesis by modulating PPARγ activity. Br. J. Pharmacol. 2018, 175, 1439–1450. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, X.X.; Chen, T.; Gao, Y.; Tian, H.M. Radix Astragali Improves Dysregulated Triglyceride Metabolism and Attenuates Macrophage Infiltration in Adipose Tissue in High-Fat Diet-Induced Obese Male Rats through Activating mTORC1-PPARγ Signaling Pathway. PPAR Res. 2014, 2014, 189085. [Google Scholar] [CrossRef]
- Kim, J.T.; Chen, J.; Zhou, Y.; Son, M.J.; Jeon, D.H.; Kwon, J.W.; Lee, G.Y.; Lee, H.J. Cycloastragenol inhibits adipogenesis and fat accumulation in vitro and in vivo through activating Hedgehog signaling. Food Sci. Biotechnol. 2024, 33, 711–720. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Vetrani, C.; Barrea, L.; Savastano, S.; Colao, A. Obesity: A gender-view. J. Endocrinol. Investig. 2024, 47, 299–306. [Google Scholar] [CrossRef]
- Litwin, M.; Kułaga, Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr. Nephrol. 2021, 36, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Li, X. The effect of astragalus extract tablets on cardiac and vascular function in patients with hypertension and metabolic syndrome. J. Hypertens. 2018, 36, pe141. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Li, J. The antihypertensive and lipid-lowering functions of the active components of Astragalus membranaceus. Chin. J. Gerontol. 2015, 35, 4800–4801. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, T.; Tang, Q.Z.; Li, S.; Qin, J.; Chen, F. Astragalus Polysaccharide Reduces Blood Pressure, Renal Damage, and Dysfunction Through the TGF-β1-ILK Pathway. Front. Pharmacol. 2021, 12, 706617. [Google Scholar] [CrossRef]
- Li, X.; Ma, D.; Wang, X.; Wang, Y.; Yang, J.; Jiang, P. Astragaloside IV Ameliorates Obesity-Associated Hypertension by Up-Regulating α7 Nicotinic Acetylcholine Receptor. J. Am. Coll. Cardiol. 2017, 70, C43. [Google Scholar] [CrossRef]
- Li, N.Y.; Li, X.L.; Zhai, X.P.; Wang, Q.Y.; Zhang, X.W.; Zhao, F.; Wang, X.F.; Fan, J.Y.; Bai, F.; Yu, J. Effects of Mongolia Astragali Radix in protecting early cardiac and nephritic functions of patients of hypertension with metabolic syndrome. Zhongguo Zhong Yao Za Zhi 2016, 41, 4051–4059. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Li, H.; Ye, X.; Qin, J.J. Changes of metabolic syndrome status alter the risks of cardiovascular diseases, stroke and all cause mortality. Sci. Rep. 2025, 15, 5448. [Google Scholar] [CrossRef]
- Li, M.R.; Yu, Y.R.; Deng, G. Astragalus membranaceus improves endothelial-dependent vasodilator function in obese rats. J. South. Med. Univ. 2010, 30, 7–10. [Google Scholar] [CrossRef]
- Meng, P.; Yang, R.; Jiang, F.; Guo, J.; Lu, X.; Yang, T.; He, Q. Molecular Mechanism of Astragaloside IV in Improving Endothelial Dysfunction of Cardiovascular Diseases Mediated by Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 1481236. [Google Scholar] [CrossRef]
- Lyu, X.; Yi, Z.; He, Y.; Zhang, C.; Zhu, P.; Liu, C. Astragaloside IV induces endothelial progenitor cell angiogenesis in deep venous thrombosis through inactivation of PI3K/AKT signaling. Histol. Histopathol. 2024, 39, 1149–1157. [Google Scholar] [CrossRef]
- Qiu, L.H.; Zhang, B.Q.; Lian, M.J.; Xie, X.J.; Chen, P. Vascular protective effects of Astragalus membranaceus and its main constituents in rats with chronic hyperhomocysteinemia. Exp. Ther. Med. 2017, 14, 2401–2407. [Google Scholar] [CrossRef]
- Li, N.Y.; Yu, H.; Li, X.L.; Wang, Q.Y.; Zhang, X.W.; Ma, R.X.; Zhao, Y.; Xu, H.; Liang, W.; Bai, F.; et al. Astragalus membranaceus Improving Asymptomatic Left Ventricular Diastolic Dysfunction in Postmenopausal Hypertensive Women with Metabolic Syndrome: A Prospective, Open-Labeled, Randomized Controlled Trial. Chin. Med. J. 2018, 131, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Ren, J. Autophagic Regulation of Lipid Homeostasis in Cardiometabolic Syndrome. Front. Cardiovasc. Med. 2018, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, X.; Song, Y.; Sun, X.; Li, W.; Zhang, L.; Hu, X.; Wang, H.; Zhao, N.; Zhuang, R.; et al. Astragaloside IV-targeting miRNA-1 attenuates lipopolysaccharide-induced cardiac dysfunction in rats through inhibition of apoptosis and autophagy. Life Sci. 2021, 275, 119414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, H.; Lu, M.; Zhao, K.; Yin, J.; Liu, Y.; Sun, Y. Astragaloside IV prevents myocardial hypertrophy induced by mechanical stress by activating autophagy and reducing inflammation. Am. J. Transl. Res. 2020, 12, 5332–5342. [Google Scholar]
- Yang, J.J.; Zhang, X.H.; Ma, X.H.; Duan, W.J.; Xu, N.G.; Chen, Y.J.; Liang, L. Astragaloside IV enhances GATA-4 mediated myocardial protection effect in hypoxia/reoxygenation injured H9c2 cells. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 829–842. [Google Scholar] [CrossRef]
- Deng, S.; Cai, K.; Pei, C.; Zhang, X.; Xiao, X.; Chen, Y.; Chen, Y.; Liang, R.; Chen, Y.; Li, P.; et al. 16S rRNA and Metagenomics Combined with UPLC-Q/TOF-MS Metabolomics Analysis Reveals the Potential Mechanism of Radix Astragali Against Hyperuricemia in Mice. Drug Des. Devel. Ther. 2023, 17, 1371–1386. [Google Scholar] [CrossRef]
- He, K.Q.; Li, W.Z.; Chai, X.Q.; Yin, Y.Y.; Jiang, Y.; Li, W.P. Astragaloside IV prevents kidney injury caused by iatrogenic hyperinsulinemia in a streptozotocin-induced diabetic rat model. Int. J. Mol. Med. 2018, 41, 1078–1088. [Google Scholar] [CrossRef]
- Wang, R.; Lin, F.; Ye, C.; Aihemaitijiang, S.; Halimulati, M.; Huang, X.; Jiang, Z.; Li, L.; Zhang, Z. Multi-omics analysis reveals therapeutic effects of Bacillus subtilis-fermented Astragalus membranaceus in hyperuricemia via modulation of gut microbiota. Food Chem. 2023, 399, 133993. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Sun, K.X.; Guo, X.; Chen, Y.Y.; Feng, C.Y.; Chen, J.S.; Barreira, J.C.M.; Prieto, M.A.; Sun, J.Y.; Zhang, J.D.; et al. The antihyperuricemia activity of Astragali Radix through regulating the expression of uric acid transporters via PI3K/Akt signalling pathway. J. Ethnopharmacol. 2023, 317, 116770. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, Y.; Zhang, J. Multi metabolomics-based analysis of application of Astragalus membranaceus in the treatment of hyperuricemia. Front. Pharmacol. 2022, 13, 948939. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y.; Cui, Y.; Lan, X.; Song, S.; Zhang, Z.; Wang, S.; Yang, A.; Zhang, J. Integrated metabolomics and transcriptomics to study mechanisms of Paecilomyces cicadae-fermented Radix astragali in the treatment of hyperuricemia. Food Biosci. 2024, 59, 104136. [Google Scholar] [CrossRef]
- Nejati, A.; Shahri, M.P.K.; Farahvash, T. Astragalus hamosus Acts as an Insulin Sensitizer in the Treatment of Polycystic Ovary Syndrome Rat Models by Affecting IRS1 Expression. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, R.; Huang, Y.; Li, D.; Ma, X.; Yang, Y. Astragalus polysaccharide alleviates polycystic ovary syndrome by reducing insulin resistance and oxidative stress and increasing the diversity of gut microbiota. Endocrine 2024, 83, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Chen, W.; Zhou, Q.; Dou, X. Astragaloside IV regulates autophagy-mediated proliferation and apoptosis in a rat model of PCOS by activating the PPARγ pathway. Iran. J. Basic. Med. Sci. 2022, 25, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ma, Z.; Wang, D.; Niu, Y. Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves. Ultrason. Sonochemistry 2022, 90, 106190. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Li, W.; Ma, H.; Guo, S.; Wang, D.; Niu, Y. Inhibitory activity of flavonoids fraction from Astragalus membranaceus Fisch. ex Bunge stems and leaves on Bacillus cereus and its separation and purification. Front. Pharmacol. 2023, 14, 1183393. [Google Scholar] [CrossRef]
- Samuel, A.O.; Huang, B.T.; Chen, Y.; Guo, F.X.; Yang, D.D.; Jin, J.Q. Antioxidant and antibacterial insights into the leaves, leaf tea and medicinal roots from Astragalus membranaceus (Fisch.) Bge. Sci. Rep. 2021, 11, 19625. [Google Scholar] [CrossRef]
- Rao, T.; Gong, Y.F.; Peng, J.B.; Wang, Y.C.; He, K.; Zhou, H.H.; Tan, Z.R.; Lv, L.Z. Comparative pharmacokinetic study on three formulations of Astragali Radix by an LC-MS/MS method for determination of formononetin in human plasma. Biomed. Chromatogr. 2019, 33, e4563. [Google Scholar] [CrossRef]
- Xiang, L.H.; Guo, X.Y.; Feng, M.G.; Zhang, J.; Zhang, J.; Feng, Z.M.; Li, Y.; Lv, Y.; Liu, G.X.; Cai, S.Q.; et al. Phenomenological pharmacokinetics of traditional Chinese medicines: A proof-of-concept study of the Paeoniae Radix Rubra and Astragali Radix-Saposhnikoviae Radix herb pair. J. Ethnopharmacol. 2025, 348, 119875. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, P.X.; Gong, T.T.; Lu, Y.Q.; Yang, B.; Wang, X.T. Pharmacokinetics and tissue distribution of fluorescent-labeled Astragalus polysaccharides in mice. Zhongguo Zhong Yao Za Zhi 2025, 50, 1959–1968. [Google Scholar] [CrossRef]
- Du, W.; Hu, J.; Liang, J.; Yang, X.; Fang, B.; Ma, G. Effect of Astragali radix extract on pharmacokinetic behavior of dapagliflozin in healthy and type 2 diabetic rats. Front. Pharmacol. 2023, 14, 1214658. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Tang, X.H.; Jiang, S.L.; Pang, J.Q.; Xu, Y.B.; Yuan, D.D.; Zhang, L.L.; Liu, H.M.; Fan, Q. Astragaloside IV improves the pharmacokinetics of febuxostat in rats with hyperuricemic nephropathy by regulating urea metabolism in gut microbiota. Front. Pharmacol. 2022, 13, 1031509. [Google Scholar] [CrossRef]
- Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Gawel, K.; Gaweł-Bęben, K.; Khurelbat, D.; Boguszewska-Czubara, A. Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor-From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. Int. J. Mol. Sci. 2023, 24, 9152. [Google Scholar] [CrossRef]
- Wu, Y.J.; Li, Z.H.; Li, J.Y.; Zhou, Y.; Wang, R.Y.; Chen, X.Y.; Qing, L.S.; Luo, P. Elucidation of the binding mechanism of astragaloside IV derivative with human serum albumin and its cardiotoxicity in zebrafish embryos. Front. Pharmacol. 2022, 13, 987882. [Google Scholar] [CrossRef]
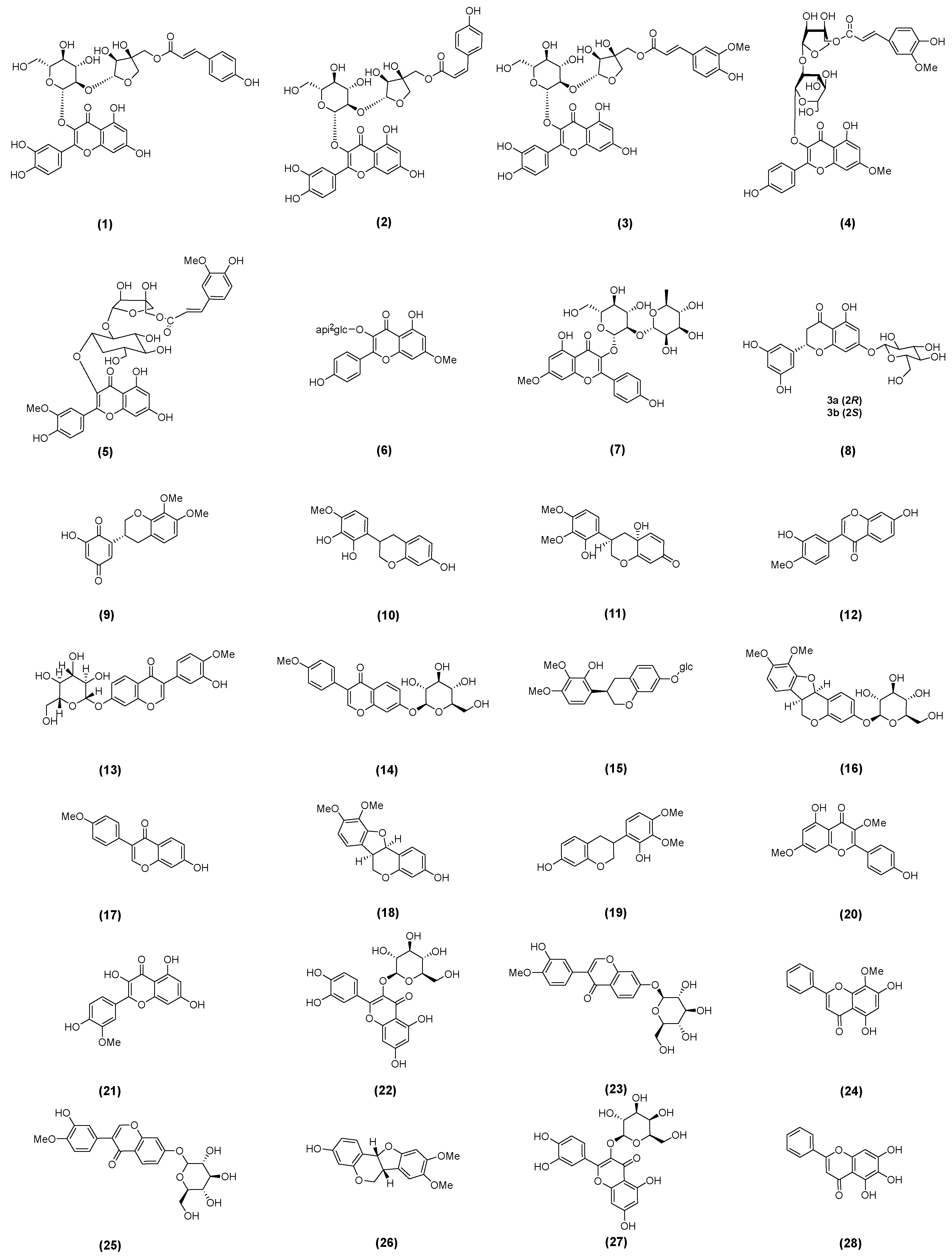


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Sun, Y.; Zhao, X. Research Progress on Chemical Components of Astragalus membranaceus and Treatment of Metabolic Syndrome. Molecules 2025, 30, 3721. https://doi.org/10.3390/molecules30183721
Liu T, Sun Y, Zhao X. Research Progress on Chemical Components of Astragalus membranaceus and Treatment of Metabolic Syndrome. Molecules. 2025; 30(18):3721. https://doi.org/10.3390/molecules30183721
Chicago/Turabian StyleLiu, Taiyu, Yumu Sun, and Xueying Zhao. 2025. "Research Progress on Chemical Components of Astragalus membranaceus and Treatment of Metabolic Syndrome" Molecules 30, no. 18: 3721. https://doi.org/10.3390/molecules30183721
APA StyleLiu, T., Sun, Y., & Zhao, X. (2025). Research Progress on Chemical Components of Astragalus membranaceus and Treatment of Metabolic Syndrome. Molecules, 30(18), 3721. https://doi.org/10.3390/molecules30183721






