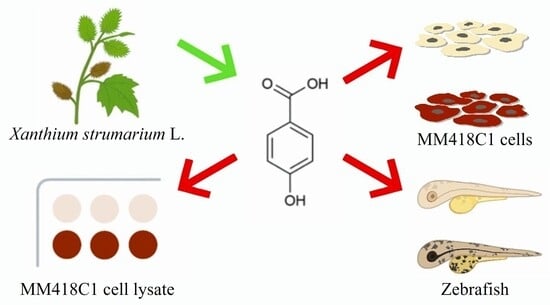
Isolation and Biological Evaluation of Human Tyrosinase Inhibitors from the Fruit of Xanthium strumarium L.
Abstract
1. Introduction
2. Results
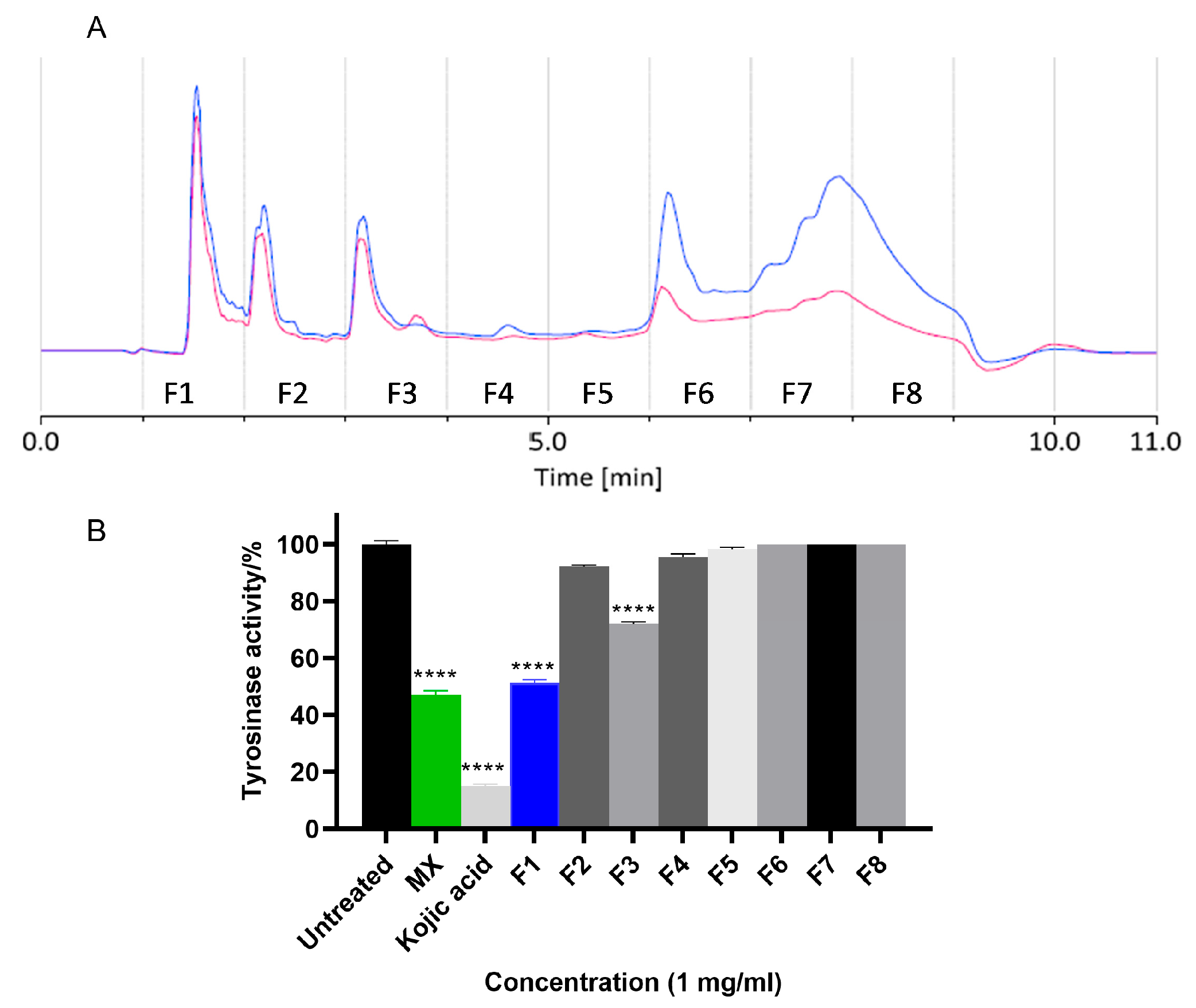
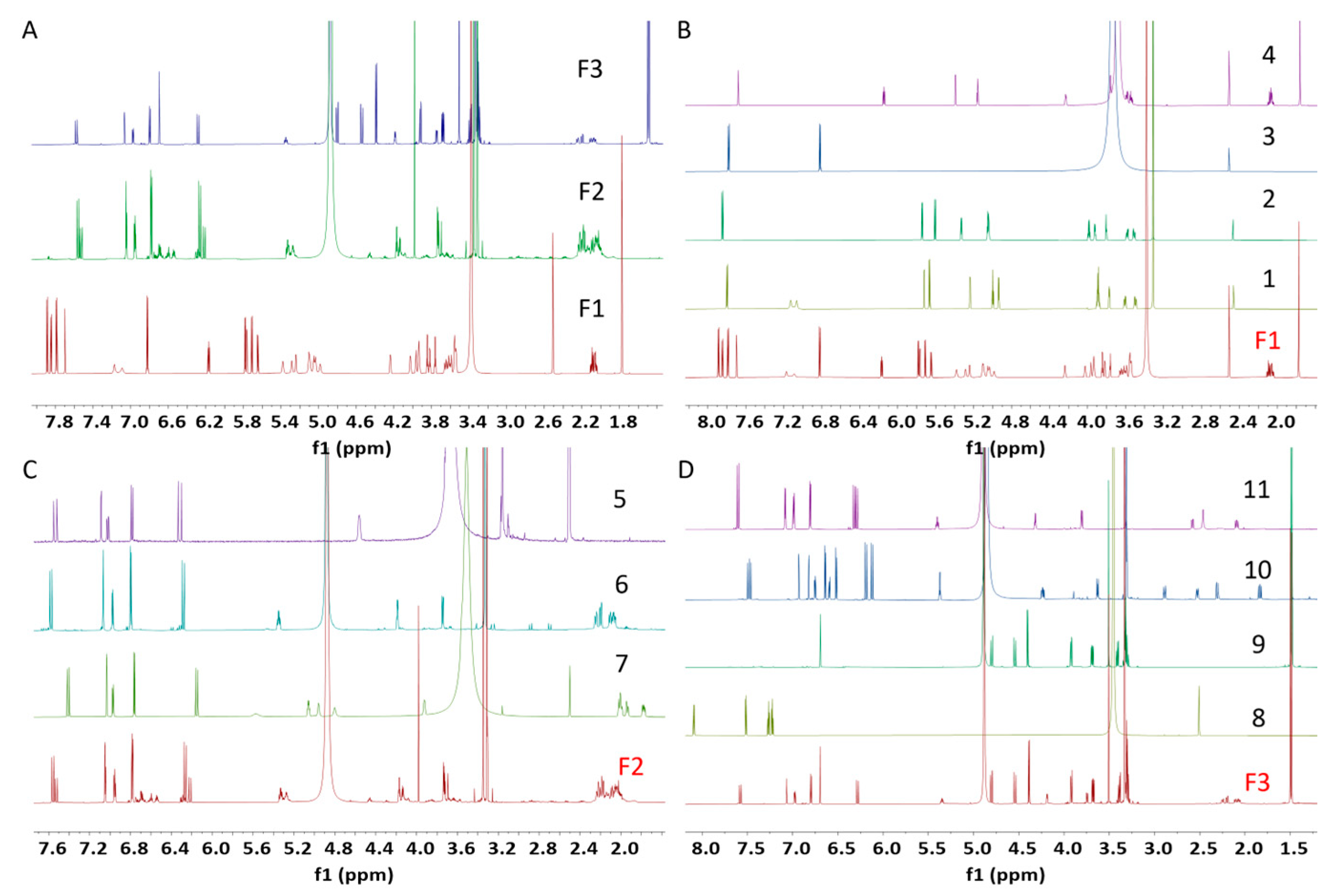
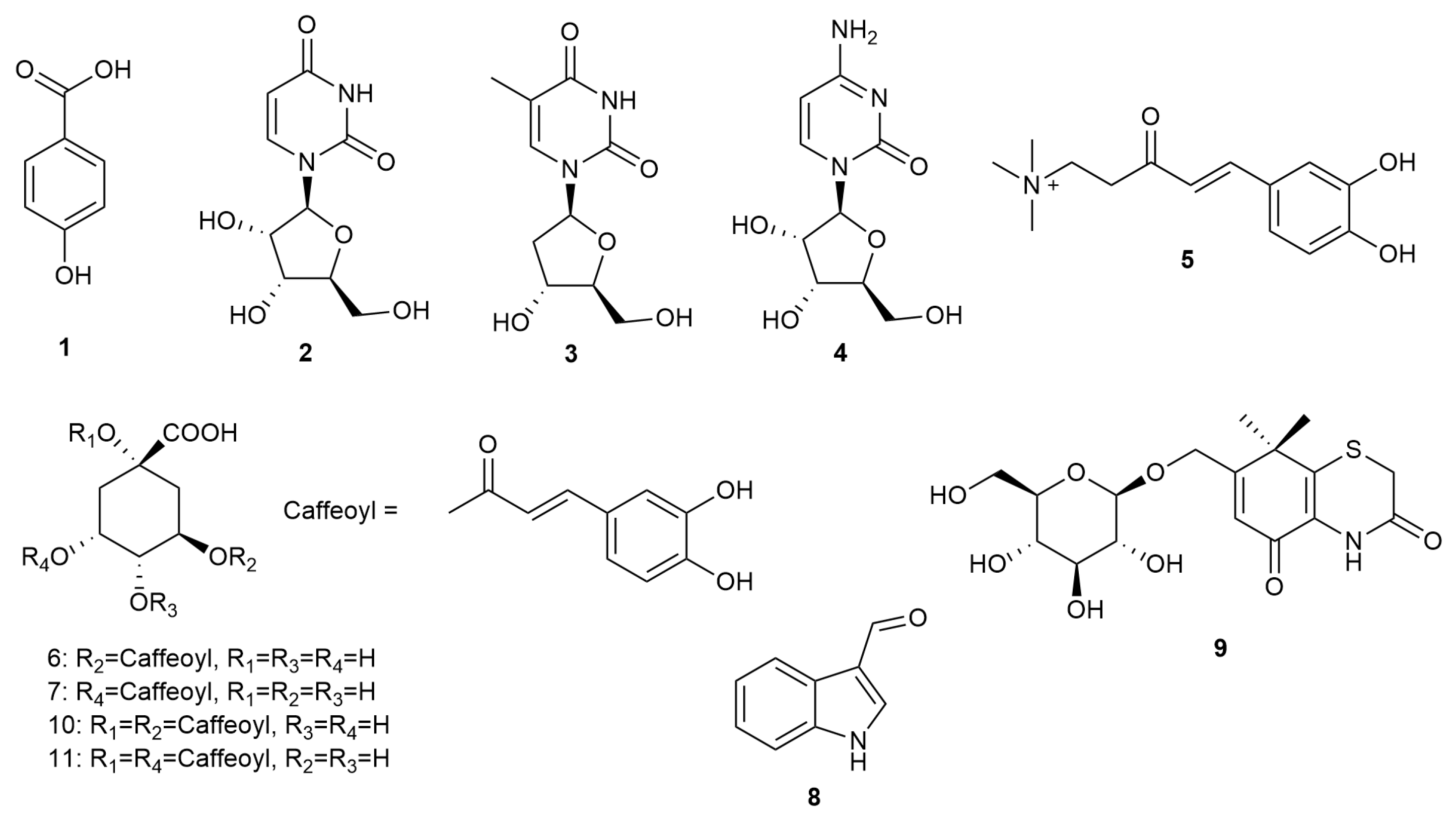
2.1. Isolation of Natural Products from the Methanol Extract of X. strumarium Fruit
2.2. Biological Activity of MX
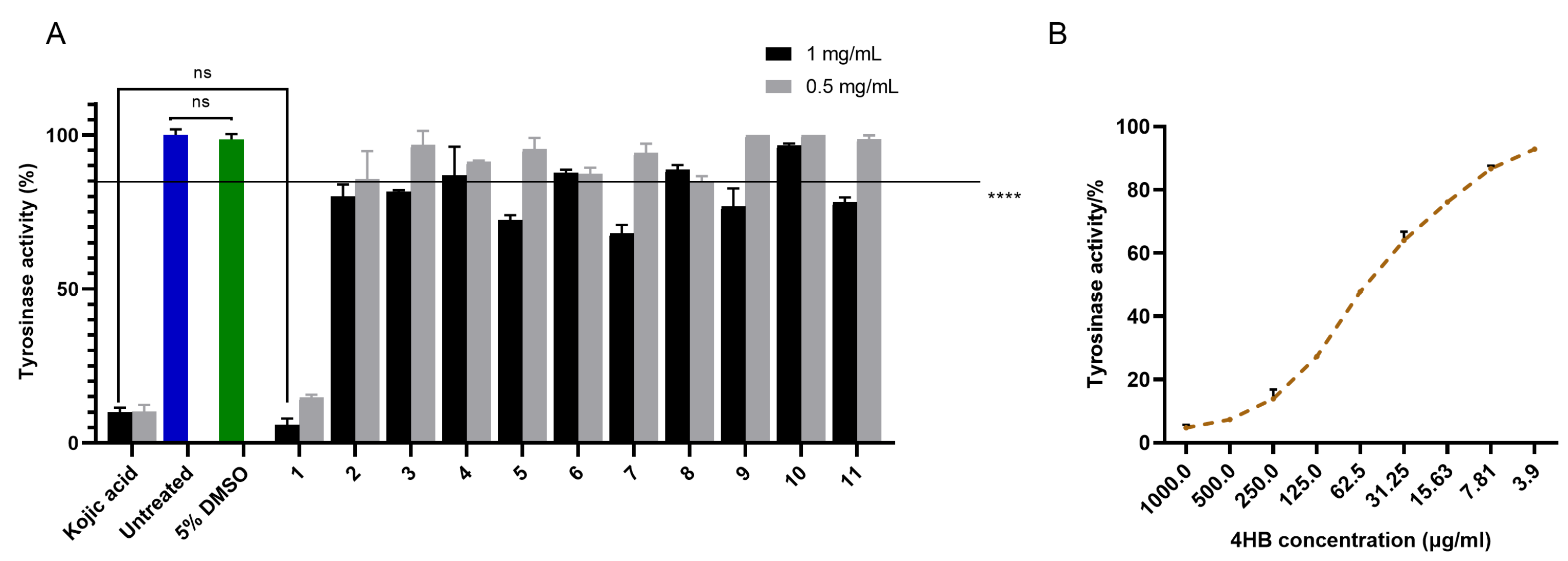
2.2.1. Inhibitory Activity of MX Against hsTYR
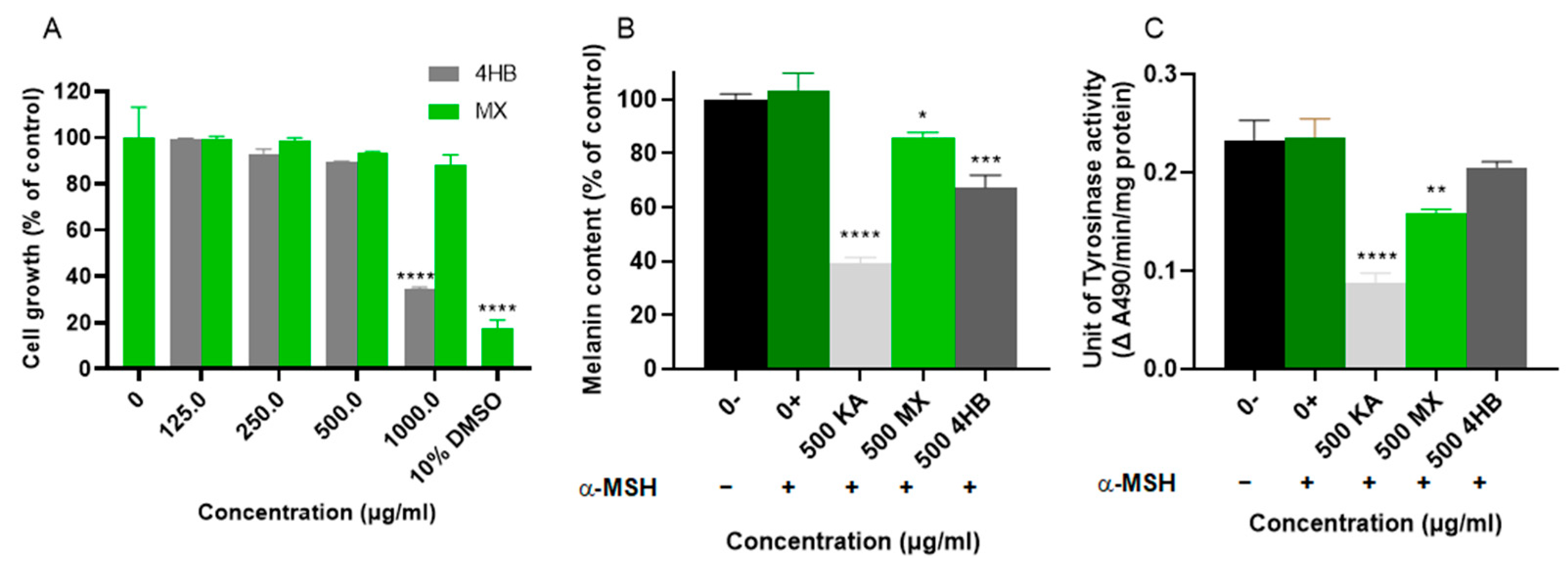
2.2.2. Cell Proliferation Test for MX and 4-Hydroxybenzoic Acid (4HB)
2.2.3. Cell-Based hsTYR Assay and Melanin Assay for MX and 4HB
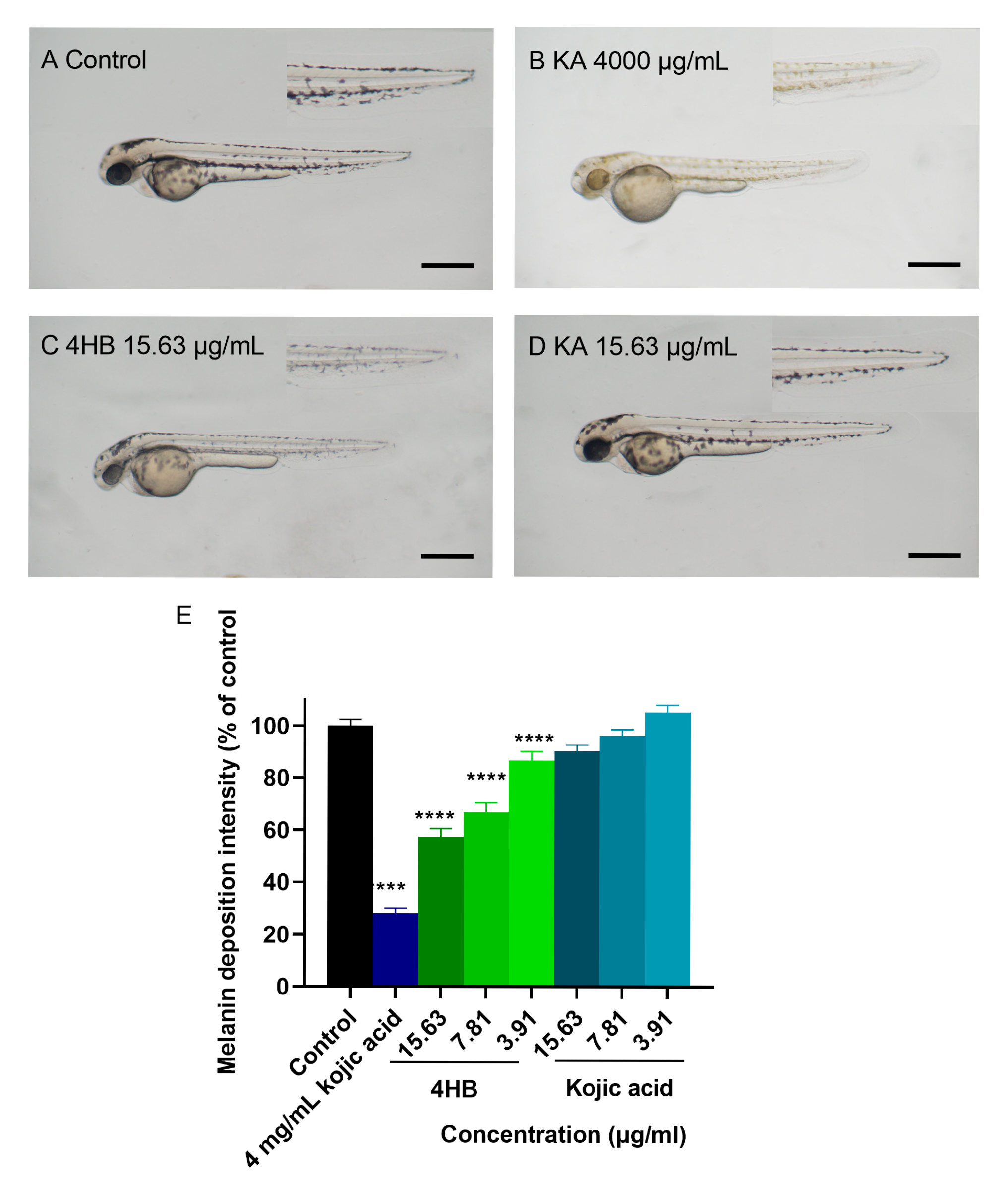
2.3. Zebrafish Pigmentation Assays for MX and 4HB
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Extraction
4.3. Isolation and Purification
4.4. Cell Culture
4.5. Protein Estimation
4.6. Cell Lysate Based hsTYR Assay
4.7. Resazurin Cell Proliferation Assay
4.8. Melanin Assay
4.9. Cellular Tyrosinase Assay
4.10. Zebrafish Maintenance and Depigmentation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Xiong, B.; Xing, S.; Chen, Y.; Liao, Q.; Mo, J.; Chen, Y.; Li, Q.; Sun, H. Medicinal prospects of targeting tyrosinase: A feature review. Curr. Med. Chem. 2023, 30, 2638–2671. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and function of human tyrosinase and tyrosinase-related proteins. Chem.–A Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Solano, F. On the metal cofactor in the tyrosinase family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef]
- Jergil, B.; Lindbladh, C.; Rorsman, H.; Rosengren, E. Dopa oxidation and tyrosine oxygenation by human melanoma tyrosinase. Acta Derm.-Venereol. 1983, 63, 468–475. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Moolla, S.; Miller-Monthrope, Y. Dermatology: How to manage facial hyperpigmentation in skin of colour. Drugs Context 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Panteri, E.; Rogiers, V.; et al. SCCS OPINION on the Safety of Alpha-Arbutin and Beta-Arbutin in Cosmetic Products-SCCS/1642/22–Final Opinion; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Ayuhastuti, A.; Syah, I.S.K.; Megantara, S.; Chaerunisaa, A.Y. A Comprehensive review of the cosmetic application of kojic acid dipalmitate: Kojic acid derivative with improved properties. arXiv 2023. [Google Scholar] [CrossRef]
- Roulier, B.; Pérès, B.; Haudecoeur, R. Advances in the design of genuine human tyrosinase inhibitors for targeting melanogenesis and related pigmentations. J. Med. Chem. 2020, 63, 13428–13443. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Feng, Y.; Tonissen, K.F. Development of a human tyrosinase activity inhibition assay using human melanoma cell lysate. BioTechniques 2024, 76, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Min, Y.S.; Park, K.-C.; Kim, D.-S. Inhibition of melanogenesis by Xanthium strumarium L. Biosci. Biotechnol. Biochem. 2012, 76, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.F.; Liu, M.-C.; Iram, A.; Feng, Y.-L.; Xu, F. Effects of the invasive plant Xanthium strumarium on diversity of native plant species: A competitive analysis approach in North and Northeast China. PLoS ONE 2020, 15, e0228476. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-S.; Liu, Y.-B.; Ma, S.-G.; Li, Y.; Qu, J.; Li, L.; Yuan, S.-P.; Hou, Q.; Li, Y.-H.; Jiang, J.-D.; et al. Bioactive sesquiterpenes and lignans from the fruits of Xanthium sibiricum. J. Nat. Prod. 2015, 78, 1526–1535. [Google Scholar] [CrossRef]
- Han, T.; Zhang, H.; Li, H.-L.; Zhang, Q.-Y.; Zheng, H.-C.; Qin, L.-P. Composition of supercritical fluid extracts of some Xanthium species from China. Chem. Nat. Compd. 2008, 44, 814–816. [Google Scholar] [CrossRef]
- Karmakar, U.K.; Ishikawa, N.; Toume, K.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Sesquiterpenes with TRAIL-resistance overcoming activity from Xanthium strumarium. Bioorg. Med. Chem. 2015, 23, 4746–4754. [Google Scholar] [CrossRef]
- Shi, Y.-S.; Li, L.; Liu, Y.-B.; Ma, S.-G.; Li, Y.; Qu, J.; Liu, Q.; Shen, Z.-F.; Chen, X.-G.; Yu, S.-S. A new thiophene and two new monoterpenoids from Xanthium sibiricum. J. Asian Nat. Prod. Res. 2015, 17, 1039–1047. [Google Scholar] [CrossRef]
- Hwang, S.H.; Wang, Z.; Na Yoon, H.; Lim, S.S. Xanthium strumarium as an inhibitor of α-glucosidase, protein tyrosine phosphatase 1β, protein glycation and ABTS+ for diabetic and its complication. Molecules 2016, 21, 1241. [Google Scholar] [CrossRef]
- Han, T.; Li, H.; Zhang, Q.; Zheng, H.; Qin, L. New thiazinediones and other components from Xanthium strumarium. Chem. Nat. Compd. 2006, 42, 567–570. [Google Scholar] [CrossRef]
- Ingawale, A.S.; Sadiq, M.B.; Nguyen, L.T.; Ngan, T.B. Optimization of extraction conditions and assessment of antioxidant, α-glucosidase inhibitory and antimicrobial activities of Xanthium strumarium L. fruits. Biocatal. Agric. Biotechnol. 2018, 14, 40–47. [Google Scholar] [CrossRef]
- Sultana, A. Phytochemical Studies on the Chemical Constituents of Xanthium strumarium Linn., Synthesis in Addition Bioactivities of 2, 3-Diaminonaphthalenimidazole Derivatives and Amides of Piperic Acid. Ph.D. Thesis, Federal Urdu University of Arts, Science and Technology, Karachi, Pakistan, 2014. [Google Scholar]
- Kan, S.; Chen, G.; Han, C.; Chen, Z.; Song, X.; Ren, M.; Jiang, H. Chemical constituents from the roots of Xanthium sibiricum. Nat. Prod. Product Res. 2011, 25, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Agata, I.; Goto, S.; Hatano, T.; Nishibe, S.; Okuda, T. 1, 3, 5-Tri-O-caffeoylquinic acid from Xanthium strumarium. Phytochemistry 1993, 33, 508–509. [Google Scholar] [CrossRef]
- Yin, R.-H.; Bai, X.; Feng, T.; Dong, Z.-J.; Li, Z.-H.; Liu, J.-K. Two new compounds from Xanthium strumarium. J. Asian Nat. Prod. Res. 2016, 18, 354–359. [Google Scholar] [CrossRef]
- Pandey, D.; Rather, M.A. Isolation and identification of phytochemicals from Xanthium strumarium. Int. J. Chem Tech Res. 2012, 4, 266–271. [Google Scholar]
- Jiang, H.; Yang, L.; Liu, C.; Hou, H.; Wang, Q.; Wang, Z.; Yang, B.; Kuang, H. Four new glycosides from the fruit of Xanthium sibiricum Patr. Molecules 2013, 18, 12464–12473. [Google Scholar] [CrossRef]
- Craig, J.C., Jr.; Mole, M.L.; Billets, S.; El-Feraly, F. Isolation and identification of the hypoglycemic agent, carboxyatracrylate, from Xanthium strumarium. Phytochemistry 1976, 15, 1178. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Kuang, H.-X.; Jiang, H.; Wang, X.-J.; Yang, L.; Zhang, J.-X.; Hou, A.-J.; Man, W.-J.; Wang, S.; Yang, B.-Y.; et al. The fruits of Xanthium sibiricum Patr: A review on phytochemistry, pharmacological activities, and toxicity. World J. Tradit. Chin. Med. 2020, 6, 408–422. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Satti, N.K.; Sharma, V.K.; Dutt, P.; Suri, K.A.; Bani, S. Amelioration of inflammatory responses by chlorogenic acid via suppression of pro-inflammatory mediators. J. Appl. Pharm. Sci. 2011, 1, 67–75. [Google Scholar]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Filip, G.A.; Vlase, L.; Crișan, G. Influences of different extraction techniques and their respective parameters on the phytochemical profile and biological activities of Xanthium spinosum L. extracts. Plants 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Chemical constituents from Xanthii fructus. Chin. Tradit. Herb. Drugs 2013, 44, 1717–1720. [Google Scholar]
- Jiang, H. Chemical constituents from fruits of Xanthium sibiricum. Chin. Tradit. Herb. Drugs 2017, 48, 47–51. [Google Scholar]
- Chinnakannu, E.; Sankar, M.; Chandran, S.; Thamotharan, K.; Manickam, S. Crystal growth, structural, DFT, optical and Z-scan analyses of 4-hydroxybenzoic acid-1H-imidazole crystal. J. Mater. Sci. Mater. Electron. 2023, 34, 1187. [Google Scholar] [CrossRef]
- Lomozik, L.; Jastrzab, R. Interference of copper (II) ions with non-covalent interactions in uridine or uridine 5′-monophosphate systems with adenosine, cytidine, thymidine and their monophosphates in aqueous solution. J. Solut. Chem. 2007, 36, 357–374. [Google Scholar] [CrossRef]
- Mouterde, L.M.M.; Peru, A.A.M.; Mention, M.M.; Brunissen, F.; Allais, F. Sustainable straightforward synthesis and evaluation of the antioxidant and antimicrobial activity of sinapine and analogues. J. Agric. Food Chem. 2020, 68, 6998–7004. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, X.-W.; Yang, Y.; Liu, W.-W.; Xu, Z.-H.; Zhao, Q.-C. Isolation, structure elucidation and neuroprotective effects of caffeoylquinic acid derivatives from the roots of Arctium lappa L. Phytochemistry 2020, 177, 112432. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Hadi, J.; Al-Ansari, H. A new series of Schiff bases derived from sulfa drugs and indole-3-carboxaldehyde: Synthesis, characterization, spectral and DFT computational studies. J. Mol. Struct. 2013, 1039, 37–45. [Google Scholar] [CrossRef]
- Thody, A.J.; Graham, A. Does α-MSH have a role in regulating skin pigmentation in humans? Pigment. Cell Res. 1998, 11, 265–274. [Google Scholar] [CrossRef]
- Chung, S.; Lim, G.J.; Lee, J.Y. Quantitative analysis of melanin content in a three-dimensional melanoma cell culture. Sci. Rep. 2019, 9, 780. [Google Scholar] [CrossRef]
- Wang, W.; Yang, L.; Wang, W.; Zhang, J.; Engelhardt, U.H.; Jiang, H. Inhibitory activities of samples on tyrosinases were affected by enzyme species and sample addition methods. Int. J. Mol. Sci. 2023, 24, 6013. [Google Scholar] [CrossRef]
- Goenka, S. Cyclocurcumin, a minor curcuminoid, is a novel candidate for hypopigmentary skin disorders with melanogenesis-stimulating capacity. Drugs Drug Candidates 2024, 3, 410–436. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Xu, Y.-L.; Yan, C.; Gao, R.-Y. Preparation and characterization of molecularly imprinted monolithic column based on 4-hydroxybenzoic acid for the molecular recognition in capillary electrochromatography. Anal. Chim. Acta 2004, 523, 243–250. [Google Scholar] [CrossRef]
- Sander, K.; Kottke, T.; Weizel, L.; Stark, H. Kojic acid derivatives as histamine H3 receptor ligands. Chem. Pharm. Bull. 2010, 58, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Cleal, M.; Gibbon, A.; Fontana, B.D.; Parker, M.O. The importance of pH: How aquarium water is affecting behavioural responses to drug exposure in larval zebrafish. Pharmacol. Biochem. Behav. 2020, 199, 173066. [Google Scholar] [CrossRef]
- Safarova, I.R. Hydroxybenzoic acid derivatives and their biological activity. Process. Petrochem. Oil Refin. 2022, 23, 134–147. [Google Scholar]
- Sharma, A.; Churungu, D.; Giri, A.; Bhardwaj, P. Cosmeceuticals significance of hydroxybenzoic acids. In Specialized Plant Metabolites as Cosmeceuticals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 99–118. [Google Scholar]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Park, J.; Park, J.H.; Suh, H.-J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Wu, B.; Han, J.; Tan, N. Phytochemical and pharmacological properties of Xanthium species: A review. Phytochem. Rev. 2024, 24, 773–844. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, W.; Li, Z.; Zhou, C.; Fan, H.; Yang, Z.; Lin, X.; Li, C. TCM herbal prescription recommendation model based on multi-graph convolutional network. J. Ethnopharmacol. 2022, 297, 115109. [Google Scholar] [CrossRef]
- Clark, J.; Grabs, A.J.; Parsons, P.G.; Smithers, B.M.; Addison, R.S.; Roberts, M.S. Melphalan uptake, hyperthermic synergism and drug resistance in a human cell culture model for the isolated limb perfusion of melanoma. Melanoma Res. 1994, 4, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1α, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar] [PubMed]
- Fechner, G.A.; Michel, J.; Sturm, R.A.; Jacobs, J.J.; Parsons, P.G. Reduction of DNA synthesis, pigment synthesis, pigmentation gene mRNA and resistance to UVB in human melanoma cells treated with analogues of a histamine (H2) agonist. Biochem. Pharmacol. 1994, 48, 121–130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Lu, Y.; Zhang, Y.; Zheng, K.; Giacomotto, J.; Tonissen, K.F.; Feng, Y. Isolation and Biological Evaluation of Human Tyrosinase Inhibitors from the Fruit of Xanthium strumarium L. Molecules 2025, 30, 3689. https://doi.org/10.3390/molecules30183689
Shi G, Lu Y, Zhang Y, Zheng K, Giacomotto J, Tonissen KF, Feng Y. Isolation and Biological Evaluation of Human Tyrosinase Inhibitors from the Fruit of Xanthium strumarium L. Molecules. 2025; 30(18):3689. https://doi.org/10.3390/molecules30183689
Chicago/Turabian StyleShi, Gengxuan, Yaoying Lu, Yougang Zhang, Ke Zheng, Jean Giacomotto, Kathryn F. Tonissen, and Yunjiang Feng. 2025. "Isolation and Biological Evaluation of Human Tyrosinase Inhibitors from the Fruit of Xanthium strumarium L." Molecules 30, no. 18: 3689. https://doi.org/10.3390/molecules30183689
APA StyleShi, G., Lu, Y., Zhang, Y., Zheng, K., Giacomotto, J., Tonissen, K. F., & Feng, Y. (2025). Isolation and Biological Evaluation of Human Tyrosinase Inhibitors from the Fruit of Xanthium strumarium L. Molecules, 30(18), 3689. https://doi.org/10.3390/molecules30183689