Abstract
Different cultures worldwide have attributed particular healing abilities to various plants for a long time. After decades of studies, research has demonstrated that their bioactivity is associated mainly with the presence of natural products, including short protein fragments known as peptides. These molecules may occur naturally in plants or be generated from plant protein through enzyme hydrolysis. In recent years, a growing body of evidence has linked plant-derived peptides to diverse biological activities, underscoring the importance of their structural and physicochemical features in determining functionality. Compared with peptides of animal or microbial origin, plant peptides stand out for their high abundance in sustainable sources, low allergenic potential, and distinctive structural traits- such as enrichment in hydrophobic and aromatic residues- that influence their stability, mechanisms of action, and biological functions. This review compiles and analyzes current literature to provide insights into how amino acid composition, secondary structure, net charge, and hydrophobicity influence peptide bioactivity. In addition, the review highlights the mechanisms of action most frequently described for plant peptides. Finally, the article discusses the current landscape and prospects of peptide-based drugs.
1. Introduction
For a long time, popular culture has established that certain foods and plants help alleviate various chronic ailments. However, in recent years, the role of specific dietary compounds in preventing diseases and improving human quality of life has become evident. In recent decades, the search for bioactive molecules has intensified, linking their activity with specific mechanisms of action [1]. Peptides are one of the most reported natural compounds with nutraceutical potential. They are linear chains composed of fewer than 40 amino acids joined by covalent bonds and released from a more complex protein structure called the parent protein [2]. Due to their size, peptides may be absorbed in the intestine, accessing the bloodstream, allowing these molecules to exert a physiological effect in vivo. They have been found to impact body functions and influence health positively, boosting the quality of human health, and therefore have been considered bioactive molecules, i.e., nutraceuticals [3]. These molecules are sometimes called cryptides, bioactive peptide fragments encrypted within larger protein sequences and released through enzymatic hydrolysis [4]. Furthermore, peptides have minimal side effects since they are not accumulated in organs and are absent from immunoreactions [5]. Over 5300 bioactive peptides have been cataloged in the BIOPEP-UWMtm database [6].
Protein-rich foods are considered optimal sources for the extraction of peptides. Within this category, animal-derived foods are particularly prominent, encompassing milk, eggs, cheese, meat, fish industry by-products, and even bovine blood [7]. In recent years, bioactive peptide research has focused on alternative sources, such as those derived from protein-rich plants. This shift is mainly because producing plant-based proteins is more economical and sustainable than animal sources. Additionally, plants are plentiful, and the demand for plant-derived products continues to rise, as their consumption is linked to healthier dietary choices [8]. Moreover, agri-food byproducts have emerged as promising reservoirs of bioactive peptides, offering an opportunity to valorize waste streams while supporting circular economy approaches [9]. Beyond these advantages, plant peptides display distinctive features compared to animal- or microbe-derived peptides. They are generally associated with lower allergenic potential, which enhances their safety in nutraceutical and functional food applications [10]. Structurally, they often show a higher prevalence of hydrophobic and aromatic residues—particularly at the C-terminal—that improve their stability, antioxidant potential, and interactions with molecular targets [11]. These differences, combined with their sustainability and accessibility, highlight plant peptides as a unique and promising class of bioactive molecules. In this context, plant peptides have been found to display anti-thrombotic, antimicrobial, antihypertensive, immunomodulatory, antioxidative, and anticancer activities, among many others [12].
Bioactive peptides are mainly produced from plant proteins through enzymatic hydrolysis, gastrointestinal digestion, and microbial fermentation [13,14,15]. Enzymatic hydrolysis of proteins is the most common and effective method for producing bioactive peptides. This process involves using either a single enzyme or a combination of different enzymes. The most frequently used proteinases are digestive enzymes, such as pepsin, trypsin, chymotrypsin, and pancreatic enzymes. Additionally, enzymes derived from fungal and bacterial sources, such as alcalase and plant-based enzymes like papain, are also utilized in this process [16]. Each enzyme has a particular active site within the protein; consequently, the degree of hydrolysis of a specific enzyme depends on controllable and optimizable factors such as hydrolysis time, enzyme concentration, and enzyme combination [17]. Once produced, peptides require purification and characterization. Ultrafiltration and chromatographic methods are the principal strategies for isolation, whereas advanced techniques such as liquid chromatography coupled with UV detection or tandem mass spectrometry (MS/MS) enable structural identification [13,14,15,16,17]. These technological improvements have greatly expanded the discovery of new bioactive peptides and insights regarding their structure-function [18,19,20]. Research has shown that the activity of peptides is closely related to their chemical structure and the conformation of their amino acids. This review seeks to analyze the structure–activity relationship of plant peptides based on our current knowledge and analyses. Understanding this relationship is essential for developing therapeutic peptides, which offer an innovative, effective, and promising alternative for treating prevalent diseases in today’s population.
2. Structure-Function Relationship of Bioactive Plant Peptides
2.1. Relation Between Plant Peptide Structure and Its Antioxidant Activity
Oxidative stress is an imbalance caused by a higher concentration of oxidants than antioxidants. This phenomenon breaks redox signaling, promoting cellular damage [21]. Reactive oxygen species (ROS), such as O2−, HO2, H2O2, and OH−, damage biomolecules, including DNA, proteins, and lipids; the damage caused by oxidative stress can both promote the onset of chronic diseases as well as their complications [22]. Over the past few decades, considerable interest has been focused on antioxidants, particularly those of natural origin [23]. Plant peptides are among the most extensively studied natural antioxidant molecules, emerging as a trending and prominent research area. Since 2010, 98 research articles have been published evaluating the antioxidant potential of plant peptides, with around 40 of these papers identifying the plant peptide sequences. Note that the primary methods used to determine the antioxidant activity of peptides are chemical-based, iron chelating activity, and radical scavenging (ABTS and DPPH). Cell-based methods have also been developed, such as cellular antioxidant activity assays using various cell lines like HepG2 and Caco-2 [24]. In Table 1, the antioxidant activity of plant peptides is presented. After our analysis, we determined that the peptides with higher antioxidant activity repeat key amino acids such as glutamic acid, aspartic acid, glycine, alanine, leucine, and phenylalanine. Glutamic acid and aspartic acid are negatively charged amino acids; since they have excess electrons, they have free radical quenching activity. Also, due to their charged residues, they chelate metals and inhibit metal-mediated oxidation [25,26]. On the other hand, these peptides show the most potent lipid peroxidation activity [27].

Table 1.
Summarization of plant peptides with antioxidant activity.
Glycine, alanine, and leucine are aliphatic amino acids that provide structural flexibility and contribute to peptide–lipid interactions, enhancing cellular uptake [11,75]. In contrast, antioxidant capacity is primarily attributed to residues with aromatic or sulfur-containing side chains—such as tyrosine, tryptophan, phenylalanine, cysteine, and methionine—which can donate electrons or hydrogen atoms to neutralize free radicals. Histidine, with its imidazole group, also plays a key role in radical scavenging. Importantly, the position of these within the peptide sequence is critical: antioxidant activity is enhanced when hydrophobic or aromatic amino acids are located at the C-or N-terminal nds, improving solubility in lipid systems and protecting membranes from peroxyl radical–mediated oxidation [76,77].
For example, the hydrophobic amino acid leucine reduces Fe3+, mainly when located at the N and C-terminal, increasing DPPH radical scavenging activity. Leucine also contributes to antioxidant activity through its long aliphatic side chain, which interacts with the acyl chains of fatty acids [56]. Regarding phenylalanine, it has been reported that this aromatic amino acid provides protons from its benzene group, acting directly as a radical scavenger and promoting high values on the assays ABTS and ORAC [78]. Tyrosine contains a phenolic group, which acts as a hydrogen atom donor, providing protons to electron-deficient free radicals and quenching them in in vitro methods such as the DPPH assay. Also, the most effective position for tyrosine has been reported in the C-terminal. This amino acid also scavenges the peroxyl radicals generated during the AAPH assay. It has also been associated with high CAA values since it can remove peroxyl radicals more easily than other amino acids [29]. It has been reported that peptides containing tyrosine have twice the antioxidant activity as those without it in their structure [62].
The sulfur-containing amino acids, methionine and cysteine, have a nucleophilic character and have been found to contribute to the scavenging activity of peptides by sulfur-hydrogen donation [71]. Cysteine is another important amino acid since it directly interacts with free radicals because its thiol group protects cellular molecules from oxidation with high TEAC assay values [30,38,62]. Also, peptides containing cysteine possess higher ferric antioxidant power [57].
Nevertheless, since plant protein is deficient in sulfur-containing amino acids, they are not commonly found in all plant-derived peptides [41]. Another amino acid recognized for its antioxidant activity is histidine, a radical scavenger due to its imidazole group proton-donating ability [41]. Histidine’s contribution to peptides’ antioxidant activity is higher when located in the C-terminus position. Finally, valine at the N-terminal has been related to enhancing the activity of antioxidant peptides in oil systems [36].
Besides particular amino acids interacting with free radicals, particular peptides may influence gene expression. One is SOD-3, a crucial endogenous antioxidant enzyme, which is the case for the peptide FDPAL obtained from soybean [69]. In addition, other soy peptides were able to activate the Keap1/Nrf2 pathway, also increasing the expression of antioxidant and phase II enzymes such as SOD1, TrxR1, NQO1, and GR [70].
A relationship between peptides having a secondary structure and their antioxidant activity has been established, being higher with a lower random coil content [51]. Also, lower α-helix content has been reported to be linked with increased antioxidant capacity [41]. As for the length, antioxidant plant peptides have been reported to have 2 to 21 amino acids. We found that up to 66% of the identified antioxidant plant peptides have from 2 to 10 amino acids. This is very convenient because smaller peptides can easily pass through cell membranes. Also, short peptides may pass through the intestinal barrier and exert their effect in the body. The reported peptides have been found not to cause damage to healthy cells; cytotoxic and hemolytic assays have seen this. Plant peptides should be utilized as medicinal antioxidants and as nutraceuticals for functional foods to increase antioxidant activities and prevent food oxidation reactions, increasing shelf life, for example, in high lipid foods. Antioxidant plant peptides are an alternative to synthetic antioxidants considered harmful, such as BHA, BHT, and TBHQ, which affect the spleen, lung, and liver [74].
2.2. Relation Between Plant Peptides Structure and Its Antiproliferative Activity
Cancer is a process involving an uncontrolled division of the body’s cells. It is one of the most relevant health problems globally, one of the leading causes of death. The short size of peptides allows them to penetrate cell membranes, where they can interact with oncogenic proteins inside the cell [79]. Also, they are efficacious signaling molecules that bind to specific cell surface receptors, such as ion channels. These actions trigger cell cycle arrest, suppress cancer cell growth, and inhibit invasion through various mechanisms, including cytoplasmic membrane disruption, mitochondrial membrane depolarization, DNA damage, and autophagy-like cell death [80,81].
Bowman-Birk trypsin inhibitor is an 8 kDa naturally occurring peptide isolated from various legumes and highlighted for its antiproliferative activity, which is attributed to its ability to inhibit serine proteases. This function is due to the presence of two inhibitory domains in its structure: CTKSNPPTC and CAYSNPPKC. The specific residues responsible for this activity are lysine (in the first domain) and tyrosine (in the second domain). Additionally, the high abundance of cysteine in this peptide facilitates the formation of disulfide bonds, enhancing its stability and resistance [82].
It has been established that the amino acids that repeat the most among plant peptides reported with anticancer activity are glutamic acid, leucine, serine, phenylalanine, and alanine (Table 2). As reported in the literature, associations between these amino acids and anticancer activity in peptides are detailed below. As for glutamic acid, it is a negatively charged amino acid and has been recognized to have antiproliferative activity on tumor cells [83,84]. Leucine and alanine are hydrophobic amino acids, improving peptide cell penetration [85]. Although the serine anticancer mechanism is not fully understood, Chatupheeraphat et al. [79] reported that removing serine from anticancer peptide sequences significantly reduced bioactivity. Peptides containing phenylalanine have a higher affinity for cancer cell membranes because phenylalanine contributes to the hydrophobicity of these peptides [83]. Hydrophobic and hydrophilic amino acids in the C or N-terminal promote an amphiphilic character, which has been reported to be beneficial. While hydrophobic amino acids are necessary to invade cancer cell membranes, hydrophilic ones give the peptide stability and a high possibility of interaction inside the cell [86]. Peptides containing hydrophobic amino acids (>30%) and also amino acids that give the peptide a positive charge (greater than or equal to 1), for example, lysine and arginine, bind to the membranes of cancer cells, which have a net negative charge associated with the presence of the phosphatidylserine phospholipid, which accounts for 9% of the total amount of phospholipids in the human cell membrane. Usually, this phospholipid faces the cytoplasm, but it goes to the outer part of the membrane in cancer cells, giving them a net negative charge. Once they attach to the membrane, peptides with these characteristics create pores through electrostatic interactions. This process leads to the leakage of intracellular components, resulting in necrosis. In the case of non-cationic peptides, the presence of hydrophobic amino acids enables them to penetrate the cell interior and interact with cytoplasmic regulatory proteins [79,87].

Table 2.
Summarization of plant peptides with antiproliferative activity.
Other important peptide amino acids with anticancer activity include proline, histidine, tryptophan, and glycine. The presence of proline increases the flexibility of peptides [99]. Histidine enables peptides to induce cancer cytotoxicity by increasing membrane permeability. Tryptophan has been found to enter cancer cells by the endocytic pathway and bind to the major groove of nuclear DNA [83]. On the other hand, glycine-rich peptides have been found to stimulate NK cell activation, enhancing antitumor activity in both animal and human models, although the exact mechanism has not been established [106].
Amino acids described in the antioxidant section are also essential in the structure of anticancer peptides, as they prevent cells from being in an oxidative stress state and play a role in anti-tumor activity [97,107]. It has been previously reported that antioxidant peptides can potentially prevent and treat ROS-related diseases, particularly certain types of cancer. These peptides can act as anticancer compounds by inhibiting oxidative stress and reducing genetic alterations, such as mutations and chromosomal rearrangements [96]. As for the secondary structure, β-pleated sheet peptides usually have two or even more disulfide bonds and therefore are more stable than α-helical peptides [106]. As for the length of the identified anticancer plant peptides, they range from 3 to 158 amino acids.
Nevertheless, we have determined that approximately 63% of the identified anticancer plant peptides contain between 3 and 10 amino acids. Small peptides exhibit greater molecular mobility compared to those of longer length, and they have high diffusivity across membranes and a higher probability of interacting with cancer cell molecules within the cells. Peptides longer than 20 amino acids have been found to possess less cytotoxic effect on cancer cells. Molecular docking studies have shown that specific peptides interact with the active site of enzymes involved in regulating gene expression, such as HDAC1 and MDM2. In this context, the quinoa-derived peptide HYNPYFPG forms hydrogen bonds with the active site of HDAC1, thereby inhibiting the enzyme’s activity. Histidine and aspartic acid were identified as the key amino acids responsible for this inhibitory effect [96]. Regarding MDM2, the rapeseed-derived peptide NDGNQPL exhibited high affinity for the enzyme’s active site, promoting its inhibition primarily through establishing hydrophobic interactions [98].
Different mechanisms have been associated with plant peptides, such as the mitochondrial apoptotic pathway (rapeseed, sweet potato, pecan, soybean, and maize) and autophagic cell death (walnut peptides). On the other hand, bean peptides specifically affected gene expression in colon cancer cells HCT116 and RKO. They mainly upregulated transcriptionally activated genes that encode for antioxidant enzymes related to NRF-2, associated with cancer prevention. Also, it affected genes involved in MAPK signaling, having a role in proliferation and apoptosis [88]. Bean peptides also promote DNA damage through PARP cleavage and cell cycle arrest through nuclear translocation of p53 [81]. Another reported mechanism is for Pombalia calceolaria peptides, which inhibited cancer cells’ migration [95].
2.3. Relation Between Plant Peptides Structure and Its Angiotensin-Converting Enzyme Inhibitory Activity
The feasibility of using bioactive peptides as potential hypotensive drugs depends on their bioavailability and bioactivity, Plant bioactive peptides can act as hypotensive drugs by known mechanisms. However, one of the most studied is their capacity to inhibit the angiotensin-converting enzyme (ACE). The renin-angiotensin and the kinin-nitric oxide systems are the hypotensive activity mechanisms that have been studied the most. However, the most well-studied bioactive peptides are the renin-angiotensin system, where the angiotensin-converting enzyme is one of the main protagonists [108,109]. The angiotensin-converting-enzyme, a glycosylated membrane-bound zinc metalloprotease, is the main protagonist of the renin-angiotensin system; in normal conditions, its primary metabolic function begins after renin acts on angiotensinogen to angiotensin I, an inactive peptide which is later catalyzed by the ACE to angiotensin II and then binds to vascular wall receptors to cause the contraction of the blood vessels. However, during hypertensive conditions, the abnormal conditions cause the renin-angiotensin system to function excessively, leading to high levels of angiotensin II, thus causing hypertension [110,111]. Therefore, the angiotensin-converting enzyme inhibition can help lower the blood pressure in hypertensive patients [112,113]. Moreover, most recent studies have shown that bioinformatic predictions can help to model peptides with specific bioactivities like antihypertensive biopeptides [114].
Previous studies have elucidated a correlation between the amino acid sequences and their ACE-inhibitory capacity (Table 3) [109]. In another related subject, one mode of action of bioactive peptides in the ACE inhibition is competition of the peptides with the ACE substrate for the union with the enzyme’s catalytic site. This site contains three specific pockets, and the interaction between peptides and ACE involves various types of molecular forces, such as van der Waals interactions, hydrophobic contacts, and electrostatic attractions. Among these, hydrogen bonds are predominant in stabilizing the peptide–ACE complex [115]. According to studies employing molecular docking tools, amino acids such as isoleucine, leucine, and proline enhance the antihypertensive activity of peptides. This effect is attributed to their ability to establish specific interactions with residues like histidine, alanine, and arginine, respectively, which are located within the binding pockets of the ACE. These interactions hinder the enzyme’s catalytic activity, thereby contributing to its inhibition [116,117]. On the other hand, A net negative charge resulting from the incorporation of strongly acidic amino acids, such as aspartic acid and glutamic acid, can potentially interfere with ACE activity by binding to the zinc ion essential for its catalytic function [118].

Table 3.
Summarization of plant peptides with ACE-inhibitory activity.
The above is not always the case, for some bioactive peptides like LW and IY, a noncompetitive inhibition has been reported. In the case of the peptides IW and FY, they have been cataloged as uncompetitive inhibitors [109,118]. It is worth noting that competitive ACE inhibitors have a practical limitation, as they must compete with the high physiological concentration of the natural substrate, angiotensin I. In this regard, noncompetitive and uncompetitive peptides like those mentioned above, which do not rely on substrate competition, may offer greater effectiveness [111]. The different mechanisms by which plant-derived peptides inhibit ACE are illustrated in a schematic representation in Figure 1.
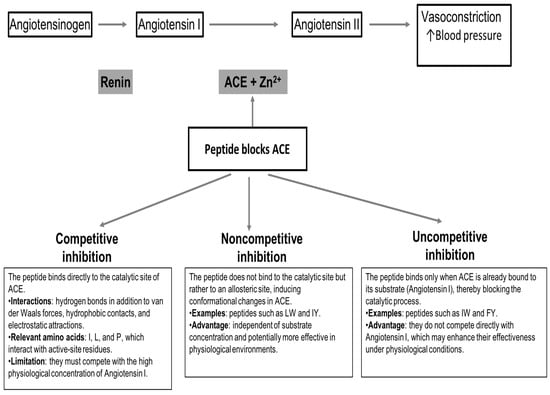
Figure 1.
Schematic representation of the inhibitory mechanisms of plant-derived peptides on ACE.
Hydrophobic and aliphatic amino acids are also necessary for peptides’ hypotensive activity [109]. Most ACE-inhibitory peptides are characterized by the presence of hydrophobic or branched-chain amino acids at the N-terminus. At the same time, residues with cyclic or aromatic rings—such as tyrosine, phenylalanine, tryptophan, and proline—are commonly found at the C-terminus. In fact, structural analyses of hypotensive peptides have shown that approximately 40% contain tryptophan at the C-terminal end [115]. One of the main reasons for the prevalence of hydrophobic amino acids in these bioactive peptides is the hydrophobic nature of the binding pockets in the ACE, which enhances interactions and binding affinity with peptides possessing such characteristics [112,117,118].
Peptide size also plays a critical role in determining bioactivity. In this regard, it has been documented that smaller peptides, particularly those composed of fewer than ten amino acids, exhibit a more potent antihypertensive effect compared to larger peptides. One contributing factor is that shorter peptides—especially dipeptides and tripeptides—can be absorbed intact through the gastrointestinal tract and reach the blood circulatory system. Additionally, specific amino acid residues, such as proline, phenylalanine at the C-terminal, and leucine at the N-terminal, have been shown to confer resistance to peptidase degradation. These residues also enhance affinity for peptide transporters, further facilitating absorption. Moreover, the smaller size of these peptides increases their likelihood of effectively interacting with the active site of ACE [121].
Moreover, it is essential to mention that unless there is sufficient and significant data regarding the pharmacological potential of bioactive peptides from plant origin, absorption, distribution, metabolism, and excretion studies are important. Unfortunately, this approach is often neglected, whether by instrumental or budget restrictions. Also, studies should focus on mimicking physiological conditions and concentrations of peptides, because the aleatory use of peptide concentrations in in vitro studies might lead to an overestimation of their antihypertensive potential [128,129]. Thus, an experimental design considering the abovementioned factors is needed for more physiologically relevant results.
2.4. Relation Between Plant Peptides Structure and Its Hypolipidemic Activity
Dyslipidemia is a metabolic disorder characterized by abnormal concentrations of lipids and lipoproteins in the bloodstream, most commonly involving elevated levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, or decreased levels of high-density lipoprotein cholesterol (HDL-C). It represents a significant risk factor for cardiovascular diseases and is influenced by genetic, dietary, and lifestyle factors, as well as enzymatic activity involved in lipid metabolism and various underlying pathological conditions.
Peptides isolated from soybean and lupine have been shown to affect 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCoAR), since they act as competitive inhibitors of the enzyme. This effect is due to characteristics such as the number, position, and type of residues of amino acids, as well as peptide hydrophobicity. For example, proline and valine have been reported as crucial, and the inhibitory activity is higher when they are located within the first and the fourth N-terminal positions and when they are flanked by leucine, phenylalanine, alanine, and glycine [130,131].
By Molecular docking and in vitro studies, lupine and rapeseed peptides (Table 4) were found to inhibit HMGCoAR, low-density lipoprotein receptor (LDLR), and proprotein convertase subtilisin/kexin type-9 (PCSK9).

Table 4.
Summarization of plant peptides with hypolipidemic activity.
As for PCSK9 inhibition, it was reported that the amino acid leucine within the peptide LPKHSDAD (Table 4) was inserted in the hydrophobic pocket of PCSK9, forming a stable peptide-PCSK9 union stabilized by hydrogen bonds [142,145]. Plant peptides containing at least four hydrophobic amino acids have a hypocholesterolemic effect [151]. Mainly if one is located at the C- or N-terminal [152,153,154,155]. Hydrophobic amino acids establish hydrophobic interactions with lipids and with the non-polar molecules of bile acids, which, although weak, promote the reduction in blood cholesterol levels [131,156,157,158]. On the other hand, peptides with cationic amino acids can also capture bile acids because the cationic residues interact with the carboxyl groups of bile acids. Some plant peptide sequences have been modified to increase their hypocholesterolemic activity by adding polar amino acids like serine residues to the C-terminal, improving their solubility in aqueous systems [155,159]. We determined that the amino acids that repeat the most among peptides with hypolipidemic activity are lysine, threonine, valine, glutamic acid, and isoleucine. These peptides are mainly made up of 2 to 20 amino acids.
2.5. Relation Between Plant Peptides Structure and Its Hypoglycemic Activity
Diabetes is a degenerative pathology that causes chronic hyperglycemia due to apoptosis of the pancreatic β-cells. If it is not controlled, cardiovascular diseases and other health complications can develop [160,161]. Peptides with hypoglycemic activity are characterized by inhibiting carbohydrate metabolism enzymes such as α-amylase, α-glucosidase, and DPP-IV. In addition, they can also act by inhibiting the glucose transporter system and acting as insulin-like molecules [162,163]. Table 5 shows plant sources of hypoglycemic peptides.

Table 5.
Summarization of plant peptides with hypoglycemic activity.
Notably, in the inhibition of α-glucosidase, the most potent plant peptides correspond to those containing three to six amino acids [174]. Similarly, another relevant factor is the properties of the amino acid residues at the N-terminal and C-terminal. In this regard, the analysis of several articles has led to the conclusion that α-glucosidase inhibitory activity is higher when arginine, tyrosine, lysine, threonine, or serine is located at the final N-terminal residue and when methionine or alanine is situated at the final C-terminus [175]. Another outstanding characteristic that has been observed is the presence of proline. Some investigations have documented that proline and a basic amino acid, such as lysine and arginine, can increase the bioactivity against α-glucosidase.
Regarding their net charge, peptides with 0 or +1 are the most effective in inhibiting α-glucosidase [191,192,193]. Docking simulation has shown that a glycine-serine-arginine sequence inhibits α-glucosidase by attaching to the pocket of the enzyme due to van der Waals forces [175,189,190]. The enzymes with which the hydrolysis is carried out play an important role in defining the biological activity of the peptides. Plant hydrolysates obtained with alkaline protease show the highest α-glucosidase inhibition rate [126]. It is worth mentioning that the most studied peptide inhibitors of α-glucosidase are between 5 and 6 amino acids; the tetrapeptides are the most potent inhibitors of this enzyme [194].
Plant peptides containing proline or alanine residues inhibited DPP-IV enzyme through competitive inhibition for its active site [195,196,197]. Regarding DPP-IV inhibition, the amino acids alanine and leucine have the most potential to interact with the catalytic site through hydrogen bonds and polar and non-polar interactions [171,184]. Also, peptides with high inhibition of DPP-IV contain isoleucine in the N1 position. This may be because isoleucine favors the formation of α-helix [172].
On the other hand, amino acids such as serine, threonine, and tyrosine interact with the α-amylase active site due to their hydroxyl group, inhibiting it. Furthermore, higher α-amylase inhibitory activities have been reported when these amino acids are located at the N-terminal position. On the other hand, proline or alanine near or at the C-terminal position promotes the formation of hydrogen bonds and electrostatic interactions of plant peptides with the catalytic site of α-amylase [187]. Our literature research found that the reports associate the amino acids glycine, alanine, valine, threonine, and proline with the most abundant amino acids in peptides with hypoglycemic activity.
2.6. Relation Between Plant Peptide Structure and Its Antimicrobial Activity
Medicinal plants used in traditional medicine are attractive sources of bioactive proteins and peptides that demonstrate a broad spectrum of activities, including antimicrobial [198]. Usually, antimicrobial peptides (AMPs) are broad-spectrum agents that act on bacteria, fungi, metazoans, and other parasites [199]. Most AMPs are short protein fragments composed of 10 to 50 amino acids with a net positive charge from +2 to +11. It has been observed that 50% of AMPs contain hydrophobic residues, which allows them to have a strong affinity to net negatively charged microbial membranes [200].
The enormous variety of plant AMPs causes difficulty in their classification. Considering the secondary structures, AMPs are classified into “α” family (with α-helical structures), “β” family (containing β-sheet structures stabilized by disulfide bonds), α-hairpinin (with a motif formed by antiparallel α-helices that are stabilized by two disulfide bridges), and “αβ” family (having both “α” and “β” structures) [201]. On the other hand, peptides with extended/combined conformation [202].
AMPs are classified considering their similarity to protein sequence, cysteine motifs, and distinctive patterns of disulfide bonds, determining the peptide folding. Therefore, they are commonly grouped as thionins, defensins, heveins, knottins, lipid transfer proteins, and cyclotides. Thionins are a family of antimicrobial peptides with low molecular weight (about 5 kDa), rich in arginine, lysine, and cysteine residues. Their structure includes two antiparallel α-helices and an antiparallel double-stranded β-sheet with three or four conserved disulfide linkages. They are positively charged with a neutral pH. Their toxic effect was postulated to arise from the lysis of the membranes of attaching cells. However, the precise mechanism underlying toxicity remains unknown [203]. Plant defensins consist of three antiparallel β-sheets and an α-helix parallel to them. They possess a variety of biological functions, such as inhibiting microbial growth and inhibiting enzyme activity [204]. The hevein family consists of positively charged peptides of 29 to 45 amino acids, with abundant glycine (6) and cysteine (8–10) and aromatic residues. They have a coil-β1-β2-coil-β3 structure that occurs by variations with the secondary structural motif in the presence of turns in two long coils in the β3 chain. Hevein domains bind to chitin, which is their primary target; usually, their action modes include degradation and disruption of the cell wall and plasma membrane due to their hydrolytic action, causing extravasation of plasma particles, so heveins have good antifungal activity [201]. Plant knottins contain approximately 30 amino acids. Their antimicrobial activity has been attributed to alterations in functional components of the plasma membrane. The typical structure of knottins involves conserved disulfide bonds between multiple cysteine pairs, forming a cystine knot [204]. The lipid transfer proteins (LTPs) family comprises cationic proteins of approximately 70 and 90 amino acids with eight cysteine residues. They share a defining structural feature, a conserved inner hydrophobic cavity surrounded by α-helices. They bind to a wide range of lipids, including fatty acids, phospholipids, prostaglandin B2, lyso-derivatives, and acyl-coenzyme A. Plant LTPs inhibit bacterial and fungal pathogens’ growth by promoting pathogen membrane permeabilization [205]. Finally, cyclotides are ultra-stable peptides. They are around 30 amino acids and are disulfide-rich peptides from plants with a head-to-tail cyclic backbone and cystine knot arrangement of three conserved disulfide bonds [206].
Huan (2020) proposed that AMPs can also be classified based on amino acid-rich species, proline, tryptophan, arginine, histidine, and glycine-rich peptides [207,208]. Therefore, the data presented in Table 6 indicate that the most frequent residues were alanine, arginine, glycine, valine, and cysteine. Furthermore, an average isoelectric point of 8.5 and an average hydropathy of −0.15, showing the cationic and hydrophilic nature of most AMPs. Beyond these classical families of plant AMPs, recent studies have also pointed out that enzyme-derived cryptic peptides (cryptides) may represent an additional reservoir of antimicrobial molecules. For instance, ribosome-inactivating proteins (PD-L1/2) from Phytolacca dioica have been reported to release bioactive cryptides with antimicrobial and anti-biofilm activity [209]. Remarkably, these peptides exhibit structural plasticity, shifting between α-helix and β-sheet conformations depending on their environment (e.g., TFE, SDS, alginate, or LPS), directly influencing their bioactivity. More broadly, enzymes have been proposed as reservoirs of host defense peptides, underscoring the importance of considering cryptides as a relevant but underexplored source of plant-derived AMPs [210].

Table 6.
Summarization of plant peptides with antimicrobial activity.
Antimicrobial mechanisms of these small amino acid fragments are as heterogeneous as their structure, and some are based on breaking the membrane to cause lysis of bacterial cells [226]. Cationic AMPs generally exhibit a balance between hydrophobic and positively charged amino acid residues, allowing them to adopt an amphipathic conformation, which allows greater interaction with negatively charged bacterial membranes, which helps promote their insertion [227]. There are four models of interaction between an AMPs and the cell membrane. In (I) Barrel-stave model, AMPs insert vertically into the plasma membrane to form transmembrane pores. Here, hydrophobic regions of AMPs align with lipid tails. In (II) Carpet model, peptides are adsorbed parallel to the lipid bilayer to cover the cell surface. Here, peptides disrupt the membrane in a detergent-like manner, breaking the lipid bilayer into separate micelles. (III) The toroidal pore model is an intermediate type between the carpet and the barrel. Finally, (IV) in the disordered toroidal pore model, the pore formation is more random and involves fewer peptides, but additional peptides must stabilize the opening (Figure 2) [203,204,207,228].
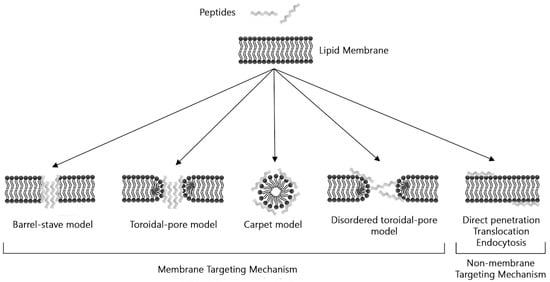
Figure 2.
Schematic illustration of models of action by AMPs.
2.7. Relation Between Plant Peptides Structure and Its Antiviral Activity
Recent evidence highlights that some AMPs also present activity against various viruses, thus being called antiviral peptides (AVPs). Several properties may influence the antiviral activity of peptides, such as the topology, amino acid composition, charge, and many other chemical and structural characteristics. The overall biochemical features of AVPs are cationic and amphipathic characteristics and positive net charges. It has been observed that AVPs act primarily by directly inhibiting the viral particle, competing for the receptor on the target cell, and blocking its interaction/adsorption. However, they may also act at other levels of the viral cycle [229]. Miscellaneous AVPs target various steps in the viral cycle from receptor binding to replication and may be virucidal. In addition, some peptides can also be translocated into the cell cytoplasm and interact with intracellular targets, interfering with physiological and chemical functions, such as nucleic acid or protein synthesis [199]. We performed a bioinformatic analysis with the AVPs, as shown in Table 7. We observe that the most frequent residues in AVPs were cysteine, proline, glycine, isoleucine, and aspartic acid. In contrast, asparagine, proline, aspartic acid, isoleucine, and valine are found in more peptides. Likewise, we observed an average isoelectric point of 5.18 and an average hydropathy of −0.23.

Table 7.
Summarization of plant peptides with antiviral activity.
To synthesize the information discussed in Section 2.1, Section 2.2, Section 2.3, Section 2.4, Section 2.5, Section 2.6 and Section 2.7, Table 8 summarizes the main structural and physicochemical features of plant-derived peptides associated with seven major biological activities. Table 8 integrates evidence on peptide length, net charge, secondary-structure tendencies, hydrophobicity patterns, and recurrent amino acids, highlighting general trends and mechanisms reported in the literature. This integrative overview provides a practical framework to connect peptide structural features with their observed bioactivities.

Table 8.
Summary of structural and physicochemical features of plant-derived bioactive peptides by biological activity.
3. New Plant Sources for Bioactive Peptides
For a long time, legumes and cereals were the primary sources of bioactive peptides, particularly from legumes. They represent 27% of the world’s primary agricultural production, supplying around 15% of the protein worldwide [236]. However, obtaining peptides from legumes has become a concern, as it has been observed that these peptides may retain the allergenic sequence of various legume proteins [237]. As for cereal proteins, the trend of consuming gluten-free products has diminished their demand, even for gluten-free cereals such as maize, buckwheat, rice, millet, quinoa, etc., since it is known that they can be contaminated with gluten during processing, transportation, and handling [238]. For this reason, in recent years, peptides have been obtained from plant-derived sources such as leaves (e.g., Moringa oleifera, Camelia sinensis, Spinaca oleracea) or fruits (e.g., Cucumis melo, Citrus species, Stenocereus pruinosus), which have demonstrated bioactivities including antioxidant, antihypertensive, and anticancer effects, thus representing a safer alternative for consumers [239,240,241].
4. Current Scenario and Future Perspectives of Peptide-Based Drugs
Currently, the bioactive properties of peptides are often evaluated at non-physiological concentrations, which can lead to overestimating their antioxidant, anti-inflammatory, hypotensive, hypolipidemic, antimicrobial, and antiviral activities. For example, many in vitro studies report antioxidant activity at concentrations ranging from 0.1 to 1 mM. In contrast, physiological plasma concentrations after oral intake are typically in the low micromolar or even nanomolar range (≤10 µM). Similarly, antihypertensive peptides derived from food proteins are commonly tested at doses of 0.5–5 mg/mL, which far exceed the levels likely to be achieved systemically in vivo [242]. This discrepancy underscores the importance of validating bioactivities under conditions that mimic physiological environments. In this sense, future research should prioritize the use of physiologically relevant concentrations and incorporate pharmacokinetic studies addressing absorption, distribution, metabolism, and excretion (ADME) to assess these molecules’ bioavailability accurately. The application of in vivo models is also essential to determine therapeutic doses, safe administration periods, and potential toxicity profiles. Additionally, computational tools such as molecular docking and Density Functional Theory (DFT) simulations have become invaluable for screening thousands of peptide sequences, allowing researchers to identify candidates with higher chances of in vivo efficacy and pharmacological stability [242,243].
Beyond these aspects, other translational challenges also require attention. Purification remains a critical step, as many studies rely on different isolation and fractionation strategies—such as ultrafiltration and various chromatographic techniques—which makes results difficult to compare. The lack of standardized protocols for peptide production, purification, and characterization further complicates reproducibility across laboratories [244]. Moreover, peptide stability under physiological conditions is often compromised due to susceptibility to proteolytic degradation [245]. This highlights the need for innovative formulation strategies, such as microencapsulation, liposomes, or nanocarriers, which can improve stability, protect peptides from degradation, and enhance controlled release [246]. At the same time, the ability to target relevant tissues remains limited. Most bioactive peptides are studied in general systemic contexts, but specific delivery to organs such as the heart, liver, or brain will be essential for their therapeutic translation [247]. Recent work on peptide-based nanoparticles also emphasizes challenges related to stability, scalability, and tissue selectivity [248].
While bioactive peptides are generally considered safe, more systematic assessments of their safety profiles are required to confirm the absence of long-term or context-dependent adverse effects. Notably, evidence suggests that peptide activity can vary with factors such as dose, cellular context, or extracellular conditions, highlighting the need for deeper mechanistic studies under physiologically relevant scenarios [249].
These considerations highlight that while plant-derived peptides hold substantial promise as future therapeutic agents, further efforts in standardization, formulation, mechanistic elucidation, and safety assessment are necessary to bridge the gap between laboratory findings and clinical application. Together with the increasing consumer demand for plant-derived biopeptides, these strategies point toward a promising future for peptide-based drugs, provided that translational challenges are addressed through realistic experimental approaches [233,234].
5. Conclusions
Numerous diseases worldwide significantly impact the quality of life of large population groups. Consequently, searching for natural-origin molecules with therapeutic and preventive potential has gained considerable attention. In this context, peptides have emerged as promising agents against communicable and non-communicable diseases. Among these, plant-derived peptides stand out as an attractive option due to their vast botanical diversity, sustainability, and alignment with circular economy strategies. Compared to animal-derived peptides, they often present distinct structural and functional characteristics, such as lower allergenic potential and specific sequence–activity relationships. Recent scientific advances have established a clear relationship between the bioactivity of these molecules and their structural characteristics. This has underscored the importance of peptide purification and structural identification, which are now essential in studies evaluating their biological potential. These developments represent a significant step forward in peptide-based therapeutics. Nevertheless, critical challenges remain: the stability of peptides under physiological conditions, their bioavailability and tissue targeting, and the lack of standardized protocols for production and characterization continue to limit translation into real-world applications. Furthermore, depending on concentration, cell type, or extracellular environment, peptides may exert different or opposite effects, highlighting the need for deeper mechanistic studies under physiologically relevant conditions.
Integrating advanced purification methods, computational screening, and in vivo validation will be crucial to fully exploit the therapeutic promise of plant peptides. If these barriers are addressed, plant-derived bioactive peptides could evolve from experimental findings into reliable components of functional foods and peptide-based drugs, ultimately contributing to both human health and sustainable innovation.
Author Contributions
Conceptualization, S.A.-G.; formal analysis, S.A.-G., J.B.H. and E.P.G.-G.; investigation, S.A.-G., I.G.-A., L.A.J.-O., E.P.G.-G. and J.B.H.; writing—original draft preparation, S.A.-G., I.G.-A., L.A.J.-O., E.P.G.-G. and J.B.H.; editing, S.A.-G., J.B.H. and E.P.G.-G.; funding acquisitions, S.A.-G. and J.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT scholarship 597872 and CONFIE Sinaloa (PAIPC 2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon reasonable request from the author (S.A.-G.).
Acknowledgments
We thank the Office of Graduate Studies at the Centro de Investigación en Alimentación y Desarrollo (CIAD).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- González, S. Dietary Bioactive Compounds and Human Health and Disease. Nutrients 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.M.; Iegre, J.; O’Donovan, D.H.; Halvarsson, M.Ö.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, D.C.; Lebrun, I. Cryptides: Buried secrets in proteins. Peptides 2007, 28, 2403–2410. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Escamilla Rosales, M.F.; Olvera Rosales, L.; Jara Gutiérrez, C.E.; Jaimez Ordaz, J.; Santana Sepúlveda, P.A.; González Olivares, L.G. Proteins of milk, egg, and fish as a source of antioxidant peptides: Production, mechanism of action, and health benefits. Food Rev. Int. 2023, 40, 1600–1620. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Loa, J.; Rojas-Avelizapa, N. Agroindustrial plant wastes: Novel source of antimicrobial peptides. Circ. Econ. Sustain. 2025, 5, 2431–2465. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; He, Z.; Du, R. Plant-derived as alternatives to animal-derived bioactive peptides: A review of the preparation, bioactivities, structure–activity relationships, and applications in chronic diseases. Nutrients 2024, 16, 3277. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z.; Li, Y.; Fan, Y.; Zhou, Y.; Song, K.; Meng, L. Antioxidant function and application of plant-derived peptides. Antioxidants 2024, 13, 1203. [Google Scholar] [CrossRef]
- Nirmal, N.; Khanashyam, A.C.; Shah, K.; Awasti, N.; Babu, K.S.; Ucak, I.; Afreen, M.; Hassoun, A.; Tuanthong, A. Plant protein-derived peptides: Frontiers in sustainable food systems and applications. Front. Sustain. Food Syst. 2024, 8, 1292297. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Heng, X.; Chen, H.; Lu, C.; Fen, T.; Li, K.; Gao, E. Study on synergistic fermentation of bean dregs and soybean meal by multiple strains and proteases. LWT 2022, 154, 112626. [Google Scholar] [CrossRef]
- Farid, M.S.; Anjum, R.; Yang, Y.; Tu, M.; Zhang, T.; Pan, D.; Sun, Y.; Wu, Z. Recent trends in fermented plant-based analogues and products, bioactive peptides, and novel technologies-assisted fermentation. Trends Food Sci. Technol. 2024, 149, 104529. [Google Scholar] [CrossRef]
- de Souza, T.S.P.; de Andrade, C.J.; Koblitz, M.G.B.; Fai, A.E.C. Microbial peptidase in food processing: Current state of the art and future trends. Catal. Lett. 2023, 153, 114–137. [Google Scholar] [CrossRef]
- Kullman, W. Enzymatic Peptide Synthesis; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Li, W.; Huang, J.; Zheng, L.; Liu, W.; Fan, L.; Sun, B.; Su, G.; Xu, J.; Zhao, M. A fast stop-flow two-dimensional liquid chromatography tandem mass spectrometry and its application in food-derived protein hydrolysates. Food Chem. 2023, 406, 135000. [Google Scholar] [CrossRef]
- Léonil, J.; Gagnaire, V.; Mollé, D.; Pezennec, S.; Bouhallab, S. Application of chromatography and mass spectrometry to the characterization of food proteins and derived peptides. J. Chromatogr. A 2000, 881, 1–21. [Google Scholar] [CrossRef]
- Noor, Z.; Ahn, S.B.; Baker, M.S.; Ranganathan, S.; Mohamedali, A. Mass spectrometry–based protein identification in proteomics—A review. Brief. Bioinform. 2021, 22, 1620–1638. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2015, 16, 193–217. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ju, X.; Yuan, J.; Wang, L.; Girgih, A.T.; Aluko, R.E. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Res. Int. 2012, 49, 432–438. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; del Mar Contreras, M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-P.; Lai, S.-J.; Chang, C.-R.; Yu, R.C.; Hsieh, J.-F.; Chen, H.-M. Peptidomic analysis of low molecular weight antioxidative peptides prepared by lotus (Nelumbo nucifera Gaertn.) seed protein hydrolysates. LWT 2021, 144, 111138. [Google Scholar] [CrossRef]
- Huang, C.; Tang, X.; Liu, Z.; Huang, W.; Ye, Y. Enzymes-dependent antioxidant activity of sweet apricot kernel protein hydrolysates. LWT 2022, 154, 112825. [Google Scholar] [CrossRef]
- Montone, C.M.; Zenezini Chiozzi, R.; Marchetti, N.; Capriotti, A.L.; Cerrato, A.; Laganà, A.; Fai, P.B.S.; Cavaliere, C. Peptidomic approach for the identification of peptides with potential antioxidant and anti-hyperthensive effects derived from asparagus by-products. Molecules 2019, 24, 3627. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jiménez-Aliaga, K.; Guzmán, F.; Pérez-Rea, D.; Cárdenas, M.; Zavaleta, A.I. Novel antioxidant peptides obtained by alcalase hydrolysis of Erythrina edulis (pajuro) protein. J. Sci. Food Agric. 2019, 99, 2420–2427. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Extraction of antioxidative and antihypertensive bioactive peptides from Parkia speciosa seeds. Food Chem. 2013, 141, 3435–3442. [Google Scholar] [CrossRef]
- Zhu, S.; Du, C.; Yu, T.; Wu, T.; Zhu, H.; Li, S. Antioxidant activity of selenium-enriched peptides from the protein hydrolysate of Cardamine violifolia. J. Food Sci. 2019, 84, 3504–3511. [Google Scholar] [CrossRef]
- García, M.C.; Endermann, J.; Gonzalez-Garcia, E.; Marina, M.L. HPLC-Q-TOF-MS identification of antioxidant and antihypertensive peptides recovered from cherry (Prunus cerasus L.) subproducts. J. Agric. Food Chem. 2015, 63, 1514–1520. [Google Scholar] [CrossRef]
- Wali, A.; Mijiti, Y.; Yanhua, G.; Maimaitituersun, W.; Abudureheman, B. Isolation and Identification of a Novel Antioxidant Peptide from Chickpea (Cicer arietinum L.) Sprout Protein Hydrolysates. Int. J. Pept. Res. Ther. 2020, 27, 219–227. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Miao, M.; Jiang, B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011, 128, 28–33. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Sila, A.; Przybylski, R.; Ghorbel, R.; Bougatef, A.; Cheba, B.A.; Nedjar-Arroume, N. Purification and identification of novel antioxidant peptides from enzymatic hydrolysate of chickpea (Cicer arietinum L.) protein concentrate. J. Funct. Foods 2015, 12, 516–525. [Google Scholar] [CrossRef]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, H.; Wang, H. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT-Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Jin, D.-X.; Liu, X.-L.; Zheng, X.-Q.; Wang, X.-J.; He, J.-F. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, M.; Yang, R.; Zhang, S.; Lin, S. Preparation, identification, and activity evaluation of antioxidant peptides from protein hydrolysate of corn germ meal. J. Food Process Preserv. 2019, 43, e14160. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, X.; Wang, J.; Liu, H.; Li, H.; Zhang, Z. Isolation and identification of a novel peptide from zein with antioxidant and antihypertensive activities. Food Funct. 2015, 6, 3799–3806. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Food Res. Int. 2018, 105, 836–844. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- Tang, X.; He, Z.; Dai, Y.; Zheng, F.; Tan, M.; Li, L. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.-K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; He, R.; Malomo, S.A.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Lu, R.-R.; Qian, P.; Sun, Z.; Zheng, X.Q.; Qi, G.J. Hempseed protein derived antioxidative peptides: Purification, identification and protection from hydrogen peroxide-induced apoptosis in PC12 cells. Food Chem. 2010, 123, 1210–1218. [Google Scholar] [CrossRef]
- Gao, J.; Li, T.; Chen, D.; Gu, H.; Mao, X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. LWT 2021, 147, 111453. [Google Scholar] [CrossRef]
- Samaei, S.P.; Martini, S.; Tagliazucchi, D.; Gianotti, A.; Babini, E. Antioxidant and angiotensin I-converting enzyme (ACE) inhibitory peptides obtained from alcalase protein hydrolysate fractions of hemp (Cannabis sativa L.) bran. J. Agric. Food Chem. 2021, 69, 9220–9228. [Google Scholar] [CrossRef]
- Peng, L.; Kong, X.; Wang, Z.; Ai-lati, A.; Ji, Z.; Mao, J. Baijiu vinasse as a new source of bioactive peptides with antioxidant and anti-inflammatory activity. Food Chem. 2021, 339, 128159. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Mahaki, H.; Zare-Zardini, H. Antioxidant activity of protein hydrolysates and purified peptides from Zizyphus jujuba fruits. J. Funct. Foods 2013, 5, 62–70. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Lin, S.; Zhang, Z.; Chen, F. Identification of novel peptides from 3 to 10 kDa pine nut (Pinus koraiensis) meal protein, with an exploration of the relationship between their antioxidant activities and secondary structure. Food Chem. 2017, 219, 311–320. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Bonache, M.A.; González-Miret, M.L.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; León-Félix, J.; Jiménez-Nevárez, Y.B.; Gutiérrez-Grijalva, E.P.; Araiza-Macias, M.J.; Heredia, J.B. Antioxidant and anti-inflammatory properties of novel peptides from Moringa oleifera Lam. leaves. S. Afr. J. Bot. 2021, 141, 466–473. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Ren, Y.; Yang, D.; Lu, W.; Xiong, W. Novel Antioxidant Peptides Purified from Mulberry (Morus atropurpurea Roxb.) Leaf Protein Hydrolysates with Hemolysis Inhibition Ability and Cellular Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 7650–7659. [Google Scholar] [CrossRef]
- Kusumah, J.; Real Hernandez, L.M.; de Mejia, E.G. Antioxidant potential of mung bean (Vigna radiata) albumin peptides produced by enzymatic hydrolysis analyzed by biochemical and in silico methods. Foods 2020, 9, 1241. [Google Scholar] [CrossRef]
- Chunkao, S.; Youravong, W.; Yupanqui, C.T.; Alashi, A.M.; Aluko, R.E. Structure and Function of Mung Bean Protein-Derived Iron-Binding Antioxidant Peptides. Foods 2020, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Sonklin, C.; Alashi, A.M.; Laohakunjit, N.; Aluko, R.E. Functional characterization of mung bean meal protein-derived antioxidant peptides. Molecules 2021, 26, 1515. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Song, H.; Huang, K.; Li, S.; Guan, X. Purification and characterization of antioxidant peptides from enzymatic hydrolysate of mungbean protein. J. Food Sci. 2020, 85, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Saari, N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014, 62, 726–734. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Li, H.; Li, J.; Zhao, F.; Jiang, L. In vitro and in silico antioxidant activity of novel peptides prepared from Paeonia ostii ‘Feng Dan’ hydrolysate. Antioxidants 2019, 8, 433. [Google Scholar] [CrossRef]
- Yang, J.; Hu, L.; Cai, T.; Li, P.; Zheng, Z.; Cheng, X.; Liu, J.; Zhou, H.; Li, H.; Fu, H. Purification and identification of two novel antioxidant peptides from perilla (Perilla frutescens L. Britton) seed protein hydrolysates. PLoS ONE 2018, 13, e0200021. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Liceaga, A.M.; Yoon, K.Y. Purification and identification of an antioxidant peptide from perilla seed (Perilla frutescens) meal protein hydrolysate. Food Sci. Nutr. 2019, 7, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.-J.; Huang, L.-H.; Sun, Q.; Jiang, Z.-Q.; Wu, X. Isolation, identification and synthesis of four novel antioxidant peptides from rice residue protein hydrolyzed by multiple proteases. Food Chem. 2015, 179, 290–295. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Ren, L.-K.; Yang, Y.; Ma, C.-M.; Liu, D.-Y.; Wu, K.; Wang, T.-W.; Feng, W.-H.; Li, Y. Identification and in silico analysis of novel antioxidant peptides in broken rice protein hydrolysate and its cytoprotective effect against H2O2-induced 2BS cell model. Food Res. Int. 2022, 162, 112108. [Google Scholar] [CrossRef]
- Garzón, A.G.; Veras, F.F.; Brandelli, A.; Drago, S.R. Purification, identification and in silico studies of antioxidant, antidiabetogenic and antibacterial peptides obtained from sorghum spent grain hydrolysate. LWT 2022, 153, 112414. [Google Scholar] [CrossRef]
- Ma, H.; Liu, R.; Zhao, Z.; Song, D.; Yang, J.; Ni, Y. A novel peptide from soybean protein isolate significantly enhances resistance of the organism under oxidative stress. PLoS ONE 2016, 11, e0159938. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Grinzato, A.; Fiorese, F.; Folda, A.; Scalcon, V.; Marin, O.; Bindoli, A.; Feller, E.; Bellamio, M.; et al. Fermented Soy-Derived Bioactive Peptides Selected by a Molecular Docking Approach Show Antioxidant Properties Involving the Keap1/Nrf2 Pathway. Antioxidants 2020, 9, 1306. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, T.-H.; Sun, M.-J. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J. Funct. Foods 2014, 7, 191–200. [Google Scholar] [CrossRef]
- Meshginfar, N.; Mahoonak, A.S.; Hosseinian, F.; Ghorbani, M.; Tsopmo, A. Production of antioxidant peptide fractions from a by-product of tomato processing: Mass spectrometry identification of peptides and stability to gastrointestinal digestion. J. Food Sci. Technol. 2018, 55, 3498–3507. [Google Scholar] [CrossRef]
- Ce, N.; Tianhong, C.; Min, C.; Jianing, D.; Xueli, Z.; Juanjuan, L. Enzymatic hydrolysis preparation and activity analysis of antioxidant peptides derived from walnut dregs. Food Mach. 2024, 40, 51–61. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Q.; Lu, Q. Purification, structural analysis, and stability of antioxidant peptides from purple wheat bran. BMC Chem. 2020, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chao, W.; Qiu, L. Therapeutic peptides: Chemical strategies fortify peptides for enhanced disease treatment efficacy. Amino Acids 2025, 57, 25. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liu, H.; Zhang, Y.; Liao, M.; Wang, A.; Wang, J. Review on plant-derived bioactive peptides: Biological activities, mechanism of action and utilizations in food development. J. Future Foods 2022, 2, 143–159. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Shen, Y.; Xiao, H.; Li, C.; Wang, S.; Chen, J.; Liu, G. Research Progress on Antioxidant Peptides from Fish By-Products: Purification, Identification, and Structure-Activity Relationship. Metabolites 2024, 14, 561. [Google Scholar] [CrossRef]
- Selamassakul, O.; Laohakunjit, N.; Kerdchoechuen, O.; Yang, L.; Maier, C.S. Isolation and characterisation of antioxidative peptides from bromelain-hydrolysed brown rice protein by proteomic technique. Process Biochem. 2018, 70, 179–187. [Google Scholar] [CrossRef]
- Chatupheeraphat, C.; Roytrakul, S.; Phaonakrop, N.; Rattanabunyong, S.; Viyanant, V.; Rinaldi, G.; Brindley, P.J.; Berriman, M.; Hongeng, S.; Loukas, A.; et al. A novel peptide derived from ginger induces apoptosis through the modulation of p53, BAX, and BCL2 expression in leukemic cell lines. Planta Medica 2021, 87, 560–569. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; de Mejía, E.G.; Loarca-Piña, G. Selective mechanism of action of dietary peptides from common bean on HCT116 human colorectal cancer cells through loss of mitochondrial membrane potential and DNA damage. J. Funct. Foods 2016, 23, 24–39. [Google Scholar] [CrossRef]
- Clemente, A.; Marín-Manzano, M.C.; Jiménez, E.; Arques, M.C.; Domoney, C. The anti-proliferative effect of TI1B, a major Bowman–Birk isoinhibitor from pea (Pisum sativum L.), on HT29 colon cancer cells is mediated through protease inhibition. Br. J. Nutr. 2012, 108, S135–S144. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application. Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Lin, J.; Peng, D.; Lin, L.; Yang, B.; Lin, Z. Peptide fractions from Sacha inchi induced apoptosis in HepG2 cells via P53 activation and a mitochondria-mediated pathway. J. Sci. Food Agric. 2023, 103, 7621–7630. [Google Scholar] [CrossRef] [PubMed]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. ACPred: A computational tool for the prediction and analysis of anticancer peptides. Molecules 2019, 24, 1973. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huang, D.; Zhai, M.; Li, D.; Wang, Z.; Duan, H.; Ji, H. Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells. BMC Complement. Altern. Med. 2015, 15, 413. [Google Scholar] [CrossRef]
- Gupta, N.; Bhagyawant, S.S. Bioactive peptide of Cicer arietinum L. induces apoptosis in human endometrial cancer via DNA fragmentation and cell cycle arrest. 3 Biotech 2021, 11, 63. [Google Scholar] [CrossRef]
- Vital, D.A.L.; Loarca-Piña, G.; Dia, V.P.; de Mejía, E.G. Peptides extracted from common bean (Phaseolus vulgaris L.) non-digestible fraction caused differential gene expression of HCT116 and RKO human colorectal cancer cells. Food Res. Int. 2014, 62, 193–204. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T. Isolation and characterization of an antifungal peptide with antiproliferative activity from seeds of Phaseolus vulgaris cv. ‘Spotted Bean’. Appl. Microbiol. Biotechnol. 2007, 74, 125–130. [Google Scholar] [CrossRef]
- Kuerban, A.; Al-Malki, A.L.; Kumosani, T.A.; Yagoub, A.E.A.; Abuzinadah, M.F.; Al-Jaouni, S.K.; Kamal, M.A.; Al-Ghamdi, S.N.; Hassan, H.M. Identification, protein antiglycation, antioxidant, antiproliferative, and molecular docking of novel bioactive peptides produced from hydrolysis of Lens culinaris. J. Food Biochem. 2020, 44, e13494. [Google Scholar] [CrossRef]
- Caccialupi, P.; Ceci, L.R.; Siciliano, R.A.; Duranti, M.; Morale, C.U.; Zacheo, G. Bowman-Birk inhibitors in lentil: Heterologous expression, functional characterisation and anti-proliferative properties in human colon cancer cells. Food Chem. 2010, 120, 1058–1066. [Google Scholar] [CrossRef]
- Ye, N.; Hu, P.; Xu, S.; Xu, S.; Liu, L.; Wang, K.; Li, J.; Wang, J. Preparation and characterization of antioxidant peptides from carrot seed protein. J. Food Qual. 2018, 2018, 8579094. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; de Mejia, E.G.; García-Lara, S.; Oseguera-Toledo, M.E. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. J. Funct. Foods 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Chu, K.T.; Ng, T.B. First report of a glutamine-rich antifungal peptide with immunomodulatory and antiproliferative activities from family Amaryllidaceae. Biochem. Biophys. Res. Commun. 2004, 325, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.E.F.; Najas, J.Z.; Magalhães, L.G.; Cilli, E.M.; de Souza, J.; Raddi, M.S.G.; de Azevedo, R.A.; de Camargo, M.S.; Said, S.; de Oliveira, L.S.; et al. Inhibition of breast cancer cell migration by cyclotides isolated from Pombalia calceolaria. J. Nat. Prod. 2018, 81, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, H.; Teng, C.; Yang, X.; Wang, Z.; Li, Y.; Zhang, Z. Anti-colon cancer activity of novel peptides isolated from in vitro digestion of quinoa protein in Caco-2 cells. Foods 2022, 11, 194. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Yuan, Q.; Sun, Q.; Yao, W.; Guo, H. Separation and purification of an anti-tumor peptide from rapeseed (Brassica campestris L.) and the effect on cell apoptosis. Food Funct. 2016, 7, 2239–2248. [Google Scholar] [CrossRef]
- Ma, K.; Wang, Z.; Ju, X.; Huang, J.; He, R. Rapeseed peptide inhibits HepG2 cell proliferation by regulating the mitochondrial and P53 signaling pathways. J. Sci. Food Agric. 2023, 103, 1474–1483. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.S.; Lay, J.O.; Liyanage, R. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from rice bran. Peptides 2010, 31, 1629–1634. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Santhanam, R.K.; Lim, S.H.E.; Kim, H. Bioactive peptide with antioxidant and anticancer activities from black soybean [Glycine max (L.) Merr.] byproduct: Isolation, identification and molecular docking study. Eur. Food Res. Technol. 2019, 245, 677–689. [Google Scholar] [CrossRef]
- Gao, C.; Sun, R.; Xie, Y.-R.; Fan, S.; Ma, G.; Ma, F.; Hou, B. The soy-derived peptide Vglycin inhibits the growth of colon cancer cells in vitro and in vivo. Exp. Biol. Med. 2017, 242, 1034–1043. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Silván, J.M.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem. 2018, 242, 75–82. [Google Scholar] [CrossRef]
- Rayaprolu, S.J.; Hettiarachchy, N.S.; Horax, R.; Satchithanandam, E.; Chen, P.; Mauromoustakos, A. Purification and characterization of a peptide from soybean with cancer cell proliferation inhibition. J. Food Biochem. 2017, 41, e12374. [Google Scholar] [CrossRef]
- Cruz-Huerta, E.; Fernández-Tomé, S.; Arques, M.C.; Clemente, A.; Amigo, L.; Recio, I.; Hernández-Ledesma, B. The protective role of the Bowman-Birk protease inhibitor in soybean lunasin digestion: The effect of released peptides on colon cancer growth. Food Funct. 2015, 6, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.H.-S.; Yang, D.H.-A.; Lin, H.-H.; Hsu, J.-C.; Liu, Y.-C.; Hsu, C.-L. IbACP, a sixteen-amino-acid peptide isolated from Ipomoea batatas leaves, induces carcinoma cell apoptosis. Peptides 2013, 47, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Akbarmehr, A.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Sarabandi, K. Physicochemical, antioxidant, antimicrobial, and in vitro cytotoxic activities of corn pollen protein hydrolysates obtained by different peptidases. Food Sci. Nutr. 2023, 11, 2403–2417. [Google Scholar] [CrossRef]
- Kaur, A.; Kehinde, B.A.; Sharma, P.; Sharma, D.; Kaur, S. Recently isolated food-derived antihypertensive hydrolysates and peptides: A review. Food Chem. 2021, 346, 128719. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Aluko, R.E. Structure and function of plant protein-derived antihypertensive peptides. Curr. Opin. Food Sci. 2015, 4, 44–50. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, F.; Chen, H.; Shu, G. Preparation and identification of novel angiotensin-I-converting enzyme inhibitory peptides from Moringa oleifera leaf. LWT 2024, 205, 116472. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure−Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive protein/peptides of flaxseed: A review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Health relevance of antihypertensive peptides in foods. Curr. Opin. Food Sci. 2018, 19, 8–14. [Google Scholar] [CrossRef]
- Tang, S.; Chen, D.; Shen, H.; Guo, X.; Yuan, Y.; Jiang, X.; Zheng, B.; Zeng, S.; Huang, M. Discovery of two novel ACE inhibitory peptides from soybeans: Stability, molecular interactions, and in vivo antihypertensive effects. Int. J. Biol. Macromol. 2025, 308, 142247. [Google Scholar] [CrossRef]
- Wu, J.-S.; Li, J.-M.; Lo, H.-Y.; Hsiang, C.-Y.; Ho, T.-Y. Anti-hypertensive and angiotensin-converting enzyme inhibitory effects of Radix Astragali and its bioactive peptide AM-1. J. Ethnopharmacol. 2020, 254, 112724. [Google Scholar] [CrossRef]
- Wei, D.; Fan, W.-l.; Xu, Y. Identification of water-soluble peptides in distilled spent grain and its angiotensin converting enzyme (ACE) inhibitory activity based on UPLC-Q-TOF-MS and proteomics analysis. Food Chem. 2021, 353, 129521. [Google Scholar] [CrossRef]
- Sangiorgio, S.; Vidović, N.; Boschin, G.; Arnoldi, A.; Lammi, C. Preparation, characterization and in vitro stability of a novel ACE-inhibitory peptide from soybean protein. Foods 2022, 11, 2667. [Google Scholar] [CrossRef]
- Aiello, G.; Lammi, C.; Boschin, G.; Zanoni, C.; Arnoldi, A. Exploration of Potentially Bioactive Peptides Generated from the Enzymatic Hydrolysis of Hempseed Proteins. J. Agric. Food Chem. 2017, 65, 10174–10184. [Google Scholar] [CrossRef]
- Nuchprapha, A.; Paisansak, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Two novel ACE inhibitory peptides isolated from longan seeds: Purification, inhibitory kinetics and mechanisms. RSC Adv. 2020, 10, 12711–12720. [Google Scholar] [CrossRef]
- Ma, K.; Wang, Y.; Wang, M.; Wang, J.; Guo, C.; Yang, Y. Antihypertensive activity of the ACE–renin inhibitory peptide derived from Moringa oleifera protein. Food Funct. 2021, 12, 8994–9006. [Google Scholar] [CrossRef]
- Esteve, C.; Marina, M.L.; Garcia, M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Extraction, identification, and structure-activity relationship of antioxidative and α-amylase inhibitory peptides from cumin seeds (Cuminum cyminum). J. Funct. Foods 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, E.; Marina, M.L.; Garcia, M. Plum (Prunus domestica L.) by-product as a new and cheap source of bioactive peptides: Extraction method and peptides characterization. J. Funct. Foods 2014, 11, 428–437. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, J.; Guo, J.; Liu, S.; Wang, R.; Ding, Y. Two novel angiotensin I-converting enzyme (ACE) inhibitory peptides from rice (Oryza sativa L.) bran protein. J. Agric. Food Chem. 2023, 71, 4153–4162. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of alpha-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, C.; Sun-Waterhouse, D.; Li, D.; Zhao, W.; Su, G.; Ma, H. Identification of post-digestion angiotensin-I converting enzyme (ACE) inhibitory peptides from soybean protein Isolate: Their production conditions and in silico molecular docking with ACE. Food Chem. 2021, 345, 128855. [Google Scholar] [CrossRef]
- Foltz, M.; van der Pijl, P.C.; Duchateau, G. Current In Vitro Testing of Bioactive Peptides Is Not Valuable. J. Nutr. 2010, 140, 117–118. [Google Scholar] [CrossRef]
- Shen, W.; Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Foods 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Ye, H.; Xu, Y.; Sun, Y.; Ye, S.; Tang, H.; Wei, X. Purification, identification and hypolipidemic activities of three novel hypolipidemic peptides from tea protein. Food Res. Int. 2023, 165, 112450. [Google Scholar] [CrossRef]
- Soares, R.A.M.; Mendonça, S.; de Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major peptides from Amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Lima, S.L.S.; Alves, N.E.G.; Silva, A.G.M.; Morais, H.A.; Pimenta, D.C.; Vidigal, P.V.T.; Arêas, J.A.G. Common bean protein hydrolysate modulates lipid metabolism and prevents endothelial dysfunction in BALB/c mice fed an atherogenic diet. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 141–150. [Google Scholar] [CrossRef]
- Garzón, A.G.; Cian, R.E.; Aquino, M.E.; Drago, S.R. Isolation and identification of cholesterol esterase and pancreatic lipase inhibitory peptides from brewer’s spent grain by consecutive chromatography and mass spectrometry. Food Funct. 2020, 11, 4994–5003. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Míguez, R.; Marina, M.L.; Castro-Puyana, M. High resolution liquid chromatography tandem mass spectrometry for the separation and identification of peptides in coffee silverskin protein hydrolysates. Microchem. J. 2019, 149, 103951. [Google Scholar] [CrossRef]
- Marques, M.R.; Fontanari, G.G.; Pimenta, D.C.; Soares-Freitas, R.M.; Arêas, J.A.G. Proteolytic hydrolysis of cowpea proteins is able to release peptides with hypocholesterolemic activity. Food Res. Int. 2015, 77, 43–48. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed Peptides Exert Hypocholesterolemic Effects with a Statin-Like Mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Y.; Li, W.; Yang, B.; Wang, X.; Tian, Z.; Zhang, X. Hemp seed protein exerts its hypoglycemic and hypolipidemic effects through degradation into short peptides. Food Chem. 2025, 484, 144406. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Aiello, G. YDFYPSSTKDQQS (P3), a peptide from lupin protein, absorbed by Caco-2 cells, modulates cholesterol metabolism in HepG2 cells via SREBP-1 activation. J. Food Biochem. 2018, 42, e12757. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Lecca, D.; Pia Abbracchio, M.; Arnoldi, A. Lupin peptide T9 (GQEQSHQDEGVIVR) modulates the mutant PCSK9D374Y pathway: In vitro characterization of its dual hypocholesterolemic behavior. Nutrients 2019, 11, 1665. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Dellafiora, L.; Galaverna, G.; Vistoli, G.; Arnoldi, A. Assessment of the multifunctional behavior of lupin peptide P7 and its metabolite using an integrated strategy. J. Agric. Food Chem. 2020, 68, 13179–13188. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Bollati, C.; Vistoli, G.; Abbracchio, M.P.; Arnoldi, A. Trans-epithelial transport, metabolism and biological activity assessment of the multi-target lupin peptide lilpkhsdad (P5) and its metabolite lpkhsdad (p5-met). Nutrients 2021, 13, 863. [Google Scholar] [CrossRef]
- Prados, I.M.; Plaza, M.; Marina, M.L.; García, M.C. Evaluation of the relationship between the peptide profiles and the lipid-lowering properties of olive seed hydrolysates as a tool for tuning hypocholesterolemic functionality. Food Funct. 2020, 11, 4973–4981. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.-Y.; Choi, S.B.; Gan, C.-Y. The potential roles of Pinto bean (Phaseolus vulgaris cv. Pinto) bioactive peptides in regulating physiological functions: Protease activating, lipase inhibiting and bile acid binding activities. J. Funct. Foods 2017, 33, 67–75. [Google Scholar] [CrossRef]
- Yang, F.; Huang, J.; He, H.; Li, Y.; Zhang, Z.; Chen, Z. Study on the hypolipidemic activity of rapeseed protein-derived peptides. Food Chem. 2023, 423, 136315. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.T.; Ju, Z.; Qiu, J.; Zhang, H.L.; Wang, Y.Q.; Wang, F. Peptide GEQQQQPGM derived from rice α-globulin reduces the risk of atherosclerosis in hamsters by improving vascular endothelial cells injury. RSC Adv. 2017, 7, 49194–49203. [Google Scholar] [CrossRef]
- Wakasa, Y.; Tamakoshi, C.; Ohno, T.; Hayashi, S.; Ozawa, K.; Takaiwa, F. The hypocholesterolemic activity of transgenic rice seed accumulating lactostatin, a bioactive peptide derived from bovine milk β-lactoglobulin. J. Agric. Food Chem. 2011, 59, 3845–3850. [Google Scholar] [CrossRef]
- Aiello, G.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Vistoli, G.; Arnoldi, A.; Lammi, C. Behavior of three hypocholesterolemic peptides from soy protein in an intestinal model based on differentiated Caco-2 cell. J. Funct. Foods 2018, 45, 363–370. [Google Scholar] [CrossRef]
- Lammi, C.; Arnoldi, A.; Aiello, G. Soybean peptides exert multifunctional bioactivity modulating 3-hydroxy-3-methylglutaryl-CoA reductase and dipeptidyl peptidase-IV targets in vitro. J. Agric. Food Chem. 2019, 67, 4824–4830. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Two peptides from soy β-conglycinin induce a hypocholesterolemic effect in HepG2 cells by a statin-like mechanism: Comparative in vitro and in silico modeling studies. J. Agric. Food Chem. 2015, 63, 7945–7951. [Google Scholar] [CrossRef]
- Yao, S.; Agyei, D.; Udenigwe, C.C. Structural basis of bioactivity of food peptides in promoting metabolic health. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 84, pp. 145–181. [Google Scholar]
- Karami, Z.; Akbari-adergani, B. Bioactive food derived peptides: A review on correlation between the structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Nagaoka, S. Structure-function properties of hypolipidemic peptides. J. Food Biochem. 2019, 43, e12539. [Google Scholar] [CrossRef] [PubMed]
- Orona-Tamayo, D.; Valverde, M.E.; Paredes-López, O. Bioactive peptides from selected Latin American food crops–A nutraceutical and molecular approach. Crit. Rev. Food Sci. Nutr. 2019, 59, 1949–1975. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Marmiroli, M.; Marmiroli, N. Bioactive peptides in plant-derived foodstuffs. J. Proteom. 2016, 147, 140–155. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- García, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef]
- Meena, S.; Kanthaliya, B.; Joshi, A.; Khan, F.; Arora, J. Biologia futura: Medicinal plants-derived bioactive peptides in functional perspective—A review. Biol. Futur. 2020, 71, 195–208. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-Derived Bioactive Peptides: A Treatment to Cure Diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef]
- Marya; Khan, H.; Nabavi, S.M.; Habtemariam, S. Anti-diabetic potential of peptides: Future prospects as therapeutic agents. Life Sci. 2018, 193, 153–158. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U. Identification of potential inhibitory peptides of enzymes involved in the metabolic syndrome obtained by simulated gastrointestinal digestion of fermented bean (Phaseolus vulgaris L.) seeds. Food Res. Int. 2017, 100, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Luna-Vital, D.A.; González de Mejía, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension, and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Pan, F.F.C.; Hsieh, C.S. McIRBP-19 of bitter melon peptide effectively regulates diabetes mellitus (DM) patients’ blood sugar levels. Nutrients 2020, 12, 1252. [Google Scholar] [CrossRef]
- Jin, J.; Huang, W.-L.; Rao, G.-L.; Zhang, X.-Q.; Xu, D.-Q.; Ye, H.-L. Total Synthesis of MC2, a Peptide from Momordica Charantia and Its Hypoglycemic Activity. Chin. J. Nat. Med. 2011, 9, 58–60. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Zhang, C.; Deng, Y.; Zhang, Z.; Rao, Z.; Ji, A.; Xu, L. Design, synthesis and biological evaluation of novel peptide MC2 analogues from Momordica charantia as potential anti-diabetic agents. Org. Biomol. Chem. 2015, 13, 4551–4561. [Google Scholar] [CrossRef]
- Lo, H.Y.; Li, C.C.; Ho, T.Y.; Hsiang, C.Y. Identification of the bioactive and consensus peptide motif from Momordica charantia insulin receptor-binding protein. Food Chem. 2016, 204, 298–305. [Google Scholar] [CrossRef]
- Mojica, L.; De Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Cermeño, M.; Connolly, A.; O’Keeffe, M.B.; Flynn, C.; Alashi, A.M.; Aluko, R.E.; FitzGerald, R.J. Identification of bioactive peptides from brewers’ spent grain and contribution of Leu/Ile to bioactive potency. J. Funct. Foods 2019, 60, 103455. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Luna-Vital, D.; de Mejia, E.G. Identification and comparison of peptides from chickpea protein hydrolysates using either bromelain or gastrointestinal enzymes and their relationship with markers of type 2 diabetes and bitterness. Nutrients 2020, 12, 3843. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, G.; Liang, R.; Zhu, Y.; Li, Y.; Liu, B. Identification and function analysis of Novel hypoglycemic and antioxidant peptides from Chickpea. Plant Foods Hum. Nutr. 2024, 79, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wu, S.; Jia, C.; Cui, C.; Sun-Waterhouse, D. Active peptides with hypoglycemic effect obtained from hemp (Cannabis sativa L.) protein through identification, molecular docking, and virtual screening. Food Chem. 2023, 429, 136912. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Bollati, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Canali, R.; Virgili, F.; Arnoldi, A. Soybean-and lupin-derived peptides inhibit DPP-IV activity on in situ human intestinal Caco-2 cells and ex vivo human serum. Nutrients 2018, 10, 1082. [Google Scholar] [CrossRef]
- Jha, S.; Gupta, S.K.; Bhattacharyya, P.; Ghosh, A.; Mandal, P. In vitro Antioxidant and Antidiabetic Activity of Oligopeptides Derived from Different Mulberry (Morus alba L.) Cultivars. Pharmacogn. Res. 2018, 10, 361–367. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Yu, T.; Zhang, B.; Tian, S.; Fan, J.; Mao, F. Oat globulin peptides regulate antidiabetic drug targets and glucose transporters in Caco-2 cells. J. Funct. Foods 2018, 42, 12–20. [Google Scholar] [CrossRef]
- Wang, F.; Yu, G.; Zhang, Y.; Zhang, B.; Fan, J. Dipeptidyl peptidase IV inhibitory peptides derived from oat (Avena sativa L.), buckwheat (Fagopyrum esculentum), and highland barley (Hordeum vulgare trifurcatum (L.) Trofim) proteins. J. Agric. Food Chem. 2015, 63, 9543–9549. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Mora, L.; Aristoy, M.-C.; Toldrá, F.; Mahoonak, A.S.; Ghorbani, M. Impact of simulated gastrointestinal digestion on the biological activity of an alcalase hydrolysate of orange seed (Siavaraze, Citrus sinensis) by-products. Foods 2020, 9, 1217. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Mahoonak, A.S.; Mora, L.; Aristoy, M.-C.; Ghorbani, M.; Toldrá, F. Pepsin hydrolysis of orange by-products for the production of bioactive peptides with gastrointestinal resistant properties. Foods 2021, 10, 679. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Tye, G.J.; Gan, C.-Y. The investigation of α-amylase inhibitory activity of selected Pinto bean peptides via preclinical study using AR42J cell. J. Funct. Foods 2017, 35, 641–647. [Google Scholar] [CrossRef]
- Asokan, S.M.; Wang, T.; Su, W.T.; Lin, W.T. Antidiabetic effects of a short peptide of potato protein hydrolysate in STZ-induced diabetic mice. Nutrients 2019, 11, 779. [Google Scholar] [CrossRef]
- Mudgil, P.; Kilari, B.P.; Kamal, H.; Ghuman, B.S.; Maqsood, S. Multifunctional bioactive peptides derived from quinoa protein hydrolysates: Inhibition of α-glucosidase, dipeptidyl peptidase-IV and angiotensin I converting enzymes. J. Cereal Sci. 2020, 96, 103130. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from vegetable protein sources. Food Chem. 2021, 354, 129473. [Google Scholar] [CrossRef] [PubMed]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-glucosidase enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- Jiang, H.; Feng, J.; Du, Z.; He, X.; Zhang, C.; Gao, C.; Yang, M.; Sun, R.; Ma, G.; Xie, Y. Oral administration of soybean peptide Vglycin normalizes fasting glucose and restores impaired pancreatic function in Type 2 diabetic Wistar rats. J. Nutr. Biochem. 2014, 25, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Z.; Yan, H.; He, R.H.; Ma, Y.K. Purification and a molecular docking study of alpha-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005. [Google Scholar] [CrossRef]
- Wang, T.; Bo, N.; Sha, G.; Li, Y.; Sun, Q.; Huang, W.; Wang, Z.; Li, Z.; Ma, C. Identification and molecular mechanism of novel hypoglycemic peptide in ripened pu-erh tea: Molecular docking, dynamic simulation, and cell experiments. Food Res. Int. 2024, 194, 114930. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.H.; Gaspar, A.R.M. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018, 91, 370–379. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, J.; Yang, R.; Zhao, W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019, 54, 1909–1919. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Antidiabetic food-derived peptides for functional feeding: Production, functionality and in vivo evidences. Foods 2020, 9, 983. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. Nutr. 2020, 62, 2158–2171. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Jiménez-Morales, K.; Quintal-Novelo, C.; Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Enzymatic protein hydrolysates and ultrafiltered peptide fractions from Cowpea Vigna unguiculata L bean with in vitro antidiabetic potential. J. Iran. Chem. Soc. 2019, 16, 1773–1781. [Google Scholar] [CrossRef]
- Liu, R.; Cheng, J.; Wu, H. Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: A review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Sawasdee, N.; Songprakhon, P.; Tragoolpua, Y.; Rotarayanont, S.; Choowongkomon, K.; Yenchitsomanus, P.T. A Synthetic Bioactive Peptide Derived from the Asian Medicinal Plant Acacia catechu Binds to Dengue Virus and Inhibits Cell Entry. Viruses 2020, 12, 1267. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Tarallo, R.; Vitiello, M.; Raiola, J.; Galdiero, M.; Vitiello, G. Peptide inhibitors against herpes simplex virus infections. J. Pept. Sci. 2013, 19, 148–158. [Google Scholar] [CrossRef]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef]
- dos Santos-Silva, C.A.; Zupin, L.; Oliveira-Lima, M.; Ricci-Azevedo, R.; Guimarães, A.G.; da Cunha, M.; da Silva, A.A. Plant Antimicrobial Peptides: State of the Art, In Silico Prediction and Perspectives in the Omics Era. Bioinform. Biol. Insights 2020, 14, 1177932220952739. [Google Scholar] [CrossRef]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-Membrane Permeabilizing Modes of Action of Antimicrobial Peptides on Bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. 2014, 59, 181–196. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant antimicrobial peptides: Structures, functions, and applications. Bot. Stud. 2021, 62, 5. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Craik, D.J.; Du, J. Cyclotides as drug design scaffolds. Curr. Opin. Chem. Biol. 2017, 38, 8–16. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 2559. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Enan, G.; Al-Mohammadi, A.-R.; Badr, A.; El-Didamony, G.; El-Gazzar, N.; El-Badry, M. Antibacterial peptides produced by Alcalase from cowpea seed proteins. Antibiotics 2021, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, E.; Pane, K.; Bosso, A.; Gaglione, R.; Di Maro, A.; Chambery, A.; Parente, A.; Arciello, A. Novel bioactive peptides from PD-L1/2, a type 1 ribosome inactivating protein from Phytolacca dioica L. Evaluation of their antimicrobial properties and anti-biofilm activities. Biochim. Biophys. Acta (BBA)—Biomembr. 2018, 1860, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Bosso, A.; Di Maro, A.; Cafaro, V.; Gaglione, R.; Pizzo, E.; Varcamonti, M.; Parente, A.; Arciello, A. Enzymes as a reservoir of host defence peptides. Curr. Top. Med. Chem. 2020, 20, 1310–1323. [Google Scholar] [CrossRef]
- Kobbi, S.; Nedjar, N.; Chihib, N.; Guesmi, A.; Bachère, E.; Arroume, N. Synthesis and antibacterial activity of new peptides from Alfalfa RuBisCO protein hydrolysates and mode of action via a membrane damage mechanism against Listeria innocua. Microb. Pathog. 2018, 115, 41–49. [Google Scholar] [CrossRef]
- Kaewklom, S.; Wongchai, M.; Petvises, S.; Hanpithakphong, W.; Aunpad, R. Structural and biological features of a novel plant defensin from Brugmansia × candida. PLoS ONE 2018, 13, e0201668. [Google Scholar] [CrossRef]
- Farkas, A.; Maróti, G.; Dürgő, H. Medicago truncatula symbiotic peptide NCR247 contributes to bacteroid differentiation through multiple mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 5183. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Chen, C.-Y.; Ravinath, D.M.; Lin, C.-H.; Chuang, C.-C.; Chen, J.-Y.; Tzen, T.C. Functional characterization of chitin-binding lectin from Solanum integrifolium containing anti-fungal and insecticidal activities. BMC Plant Biol. 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Rogozhin, E.A.; Musolyamov, A.K.; Andreev, Y.A.; Oparin, P.B.; Berkut, A.A.; Egorov, T.A.; Grishin, E.V.; Odintsova, T.I. Novel antifungal α-hairpinin peptide from Stellaria media seeds: Structure, biosynthesis, gene structure and evolution. Plant Mol. Biol. 2014, 84, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Diz, M.S.; Carvalho, A.O.; Ribeiro, S.F.F.; Da Cunha, M.; Gadelha, C.A.; Gomes, V.M. Characterisation, immunolocalisation and antifungal activity of a lipid transfer protein from chili pepper (Capsicum annuum) seeds with novel α-amylase inhibitory properties. Physiol. Plant. 2011, 142, 233–246. [Google Scholar] [CrossRef]
- Schmidt, M.; Arendt, E.K.; Thery, T.L.C. Isolation and characterisation of the antifungal activity of the cowpea defensin Cp-thionin II. Food Microbiol. 2019, 82, 504–514. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides 2005, 26, 1120–1126. [Google Scholar] [CrossRef]
- Lee, J.-K.; Gopal, R.; Seo, C.H.; Cheong, H.; Park, Y. Isolation and Purification of a Novel Deca-Antifungal Peptide from Potato (Solanum tuberosum L. cv. Jopung) Against Candida albicans. Int. J. Mol. Sci. 2012, 13, 4021–4032. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kameda, M.; Namae, T.; Ochiai, A.; Saitoh, E.; Tanaka, T. Identification and characterization of multifunctional cationic peptides derived from peptic hydrolysates of rice bran protein. J. Funct. Foods 2017, 34, 287–296. [Google Scholar] [CrossRef]
- Takayama, S.; Hashimoto, K.; Kokubu, E.; Ishihara, K.; Saito, K. Inhibitory effects of a novel cationic dodecapeptide [CL(14–25)] derived from cyanate lyase of rice on endotoxic activities of LPSs from Escherichia coli and periodontopathic Aggregatibacter actinomycetemcomitans. Microb. Pathog. 2016, 94, 2–11. [Google Scholar] [CrossRef]
- Pu, C.; Tang, W. The antibacterial and antibiofilm efficacies of a liposomal peptide originating from rice bran protein against Listeria monocytogenes. Food Funct. 2017, 8, 4159–4169. [Google Scholar] [CrossRef]
- Cunsolo, V.; Schicchi, R.; Chiaramonte, M.; Salmeri, M.; Vella, F.M.; Foti, S.; Geraci, C. Identification of New Antimicrobial Peptides from Mediterranean Medical Plant Charybdis pancration (Steinh.) Speta. Antibiotics 2020, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Dhayakaran, R.; Neethirajan, S.; Weng, X. Investigation of the antimicrobial activity of soy peptides by developing a high throughput drug screening assay. Biochem. Biophys. Rep. 2016, 6, 149–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freitas, C.S.; Vericimo, M.A.; da Silva, M.L.; Motta, M.C.; da Silva, A.F.; Leite, S.G.; Rozental, S.; de Alencar, A.C.; Uchoa, A.F.; de Freitas, C.M.; et al. Encrypted antimicrobial and antitumoral peptides recovered from a protein-rich soybean (Glycine max) by-product. J. Funct. Foods 2019, 54, 187–198. [Google Scholar] [CrossRef]
- Rashid, R.; Veleba, M.; Kline, K.A. Focal Targeting of the Bacterial Envelope by Antimicrobial Peptides. Front. Cell Dev. Biol. 2016, 4, 55. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Shwaiki, L.N.; Lynch, K.M.; Arendt, E.K. Future of antimicrobial peptides derived from plants in food application—A focus on synthetic peptides. Trends Food Sci. Technol. 2021, 112, 312–324. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef]
- Panya, A.; Yongpitakwattana, P.; Budchart, P.; Sawasdee, N.; Songprakhon, P.; Choowongkomon, K.; Yenchitsomanus, P.T. Novel bioactive peptides demonstrating anti-dengue virus activity isolated from the Asian medicinal plant Acacia Catechu. Chem. Biol. Drug Des. 2019, 93, 100–109. [Google Scholar] [CrossRef]
- Moulin, M.M.; Rodrigues, R.; Ribeiro, S.F.F.; Nogueira, F.C.; de Freitas, R.G.; Soares, M.R.; da Cunha, M.; Aragão, F.J.; Gomes, V.M. Trypsin inhibitors from Capsicum baccatum var. pendulum leaves involved in Pepper yellow mosaic virus resistance. Genet. Mol. Res. 2014, 13, 9229–9243. [Google Scholar] [CrossRef]
- Nguyen, P.Q.T.; Ooi, J.S.G.; Nguyen, N.T.K.; He, W.S.; Tan, T.Y.T.; Fang, C.M.; Chen, W.N.; Tam, J.P.; Ooi, L.S.M. Antiviral Cystine Knot α-Amylase Inhibitors from Alstonia scholaris. J. Biol. Chem. 2015, 290, 31138–31150. [Google Scholar] [CrossRef]
- Henriques, S.T.; Huang, Y.-H.; Rosengren, K.J.; Franquelim, H.G.; Carvalho, F.A.; Johnson, A.; Sonza, S.; Tachedjian, G.; Castanho, M.A.; Daly, N.L.; et al. Decoding the membrane activity of the cyclotide kalata B1: The importance of phosphatidylethanolamine phospholipids and lipid organization on hemolytic and anti-HIV activities. J. Biol. Chem. 2011, 286, 24231–24241. [Google Scholar] [CrossRef] [PubMed]
- Nawae, W.; Hannongbua, S.; Ruengjitchatchawalya, M. Molecular dynamics exploration of poration and leaking caused by Kalata B1 in HIV-infected cell membrane compared to host and HIV membranes. Sci. Rep. 2017, 7, 3638. [Google Scholar] [CrossRef] [PubMed]
- Camargo Filho, I.; Cortez, D.A.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Antiviral activity and mode of action of a peptide isolated from Sorghum bicolor. Phytomedicine 2008, 15, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Nucara, A. Legume proteins and peptides as compounds in nutraceuticals: A structural basis for dietary health effects. Nutrients 2022, 14, 1188. [Google Scholar] [CrossRef]
- Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Safety Assessment of Plant-Derived Proteins and Peptides as Used in Cosmetics; Cosmetic Ingredient Review: Washington, DC, USA, 2017. [Google Scholar]
- Bustamante, M.Á.; Fernández-Gil, M.P.; Churruca, I.; Pinto, Á.; Sarria, B.; Rodriguez, M.A.; Landa, B.B. Evolution of gluten content in cereal-based gluten-free products: An overview from 1998 to 2016. Nutrients 2017, 9, 21. [Google Scholar] [CrossRef]
- Yuan, H.; Luo, Z.; Ban, Z.; Liu, D.; Wu, S.; Cheng, Z.; Li, Y.; Chen, M. Bioactive peptides of plant origin: Distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Contreras-Angulo, L.A.; García-Aguiar, I.; Heredia, J.B. Moringa oleifera Lam. leaf peptides: Antioxidant and antiproliferative activity in human colon cancer Caco-2 cell line. Antioxidants 2024, 13, 1367. [Google Scholar] [CrossRef]
- Rasouli, H.; Parvaneh, S.; Mahnam, A.; Kiamari, A.M.; Barzegar, A. Anti-angiogenic potential of trypsin inhibitor purified from Cucumis melo seeds: Homology modeling and molecular docking perspective. Int. J. Biol. Macromol. 2017, 96, 118–128. [Google Scholar] [CrossRef]
- Nardo, A.E.; Suárez, S.; Quiroga, A.V.; Añón, M.C. Amaranth as a source of antihypertensive peptides. Front. Plant Sci. 2020, 11, 578631. [Google Scholar] [CrossRef]
- Gao, X.; Bu, F.; Yi, D.; Shi, H.; Yang, Y.; Jiang, X.; Zheng, S. Molecular docking and antihypertensive effects of a novel angiotensin-I converting enzyme inhibitory peptide from yak bone. Front. Nutr. 2022, 9, 993744. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, J.; Chandrapala, J.; Liu, Z.; Wang, S.; Chen, J.; Liu, G. Recent progress of food-derived bioactive peptides: Extraction, purification, function, and encapsulation. Food Front. 2024, 5, 1240–1264. [Google Scholar] [CrossRef]
- Pérez-Pérez, V.; Jiménez-Martínez, C.; González-Escobar, J.L.; Corzo-Ríos, L.J. Exploring the impact of encapsulation on the stability and bioactivity of peptides extracted from botanical sources: Trends and opportunities. Front. Chem. 2024, 12, 1423500. [Google Scholar] [CrossRef]
- Atma, Y.; Murray, B.S.; Sadeghpour, A.; Goycoolea, F.M. Encapsulation of short-chain bioactive peptides (BAPs) for gastrointestinal delivery: A review. Food Funct. 2024, 15, 3959–3979. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jiang, W.; Chen, Z.; Hang, Z.; Liu, C.; Han, K.; Li, J.; Zhang, S.; Wei, D.; Wang, T.; et al. Advance in peptide-based drug development: Delivery platforms, therapeutics and vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Thongpon, P.; Tang, M.; Cong, Z. Peptide-based nanoparticle for tumor therapy. Biomedicines 2025, 13, 1415. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; McClements, D.J.; Taghizadeh, M.S.; Niazi, A.; Garcia-Vaquero, M. Strategies for oral delivery of bioactive peptides with focus on debittering and masking. NPJ Sci. Food 2023, 7, 22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).