Synthetic Protein-Assisted Co-Assembly of Zeolitic Imidazolate Framework-8 and Novosphingobium capsulatum for Enhanced Saline–Alkali Resistance of Wheat
Abstract
1. Introduction
2. Results
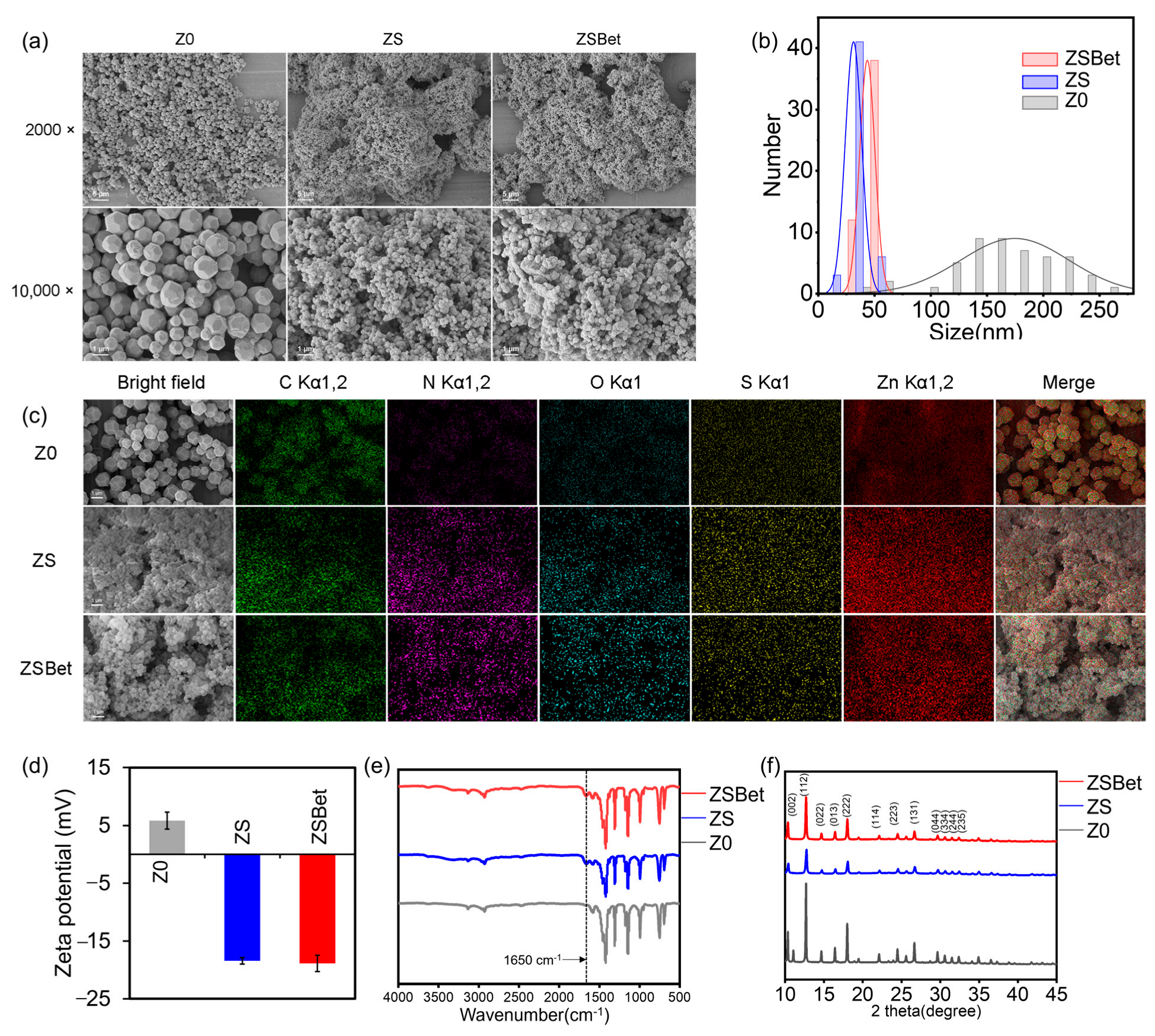
2.1. Characterization of the Synthesized ZIF-8
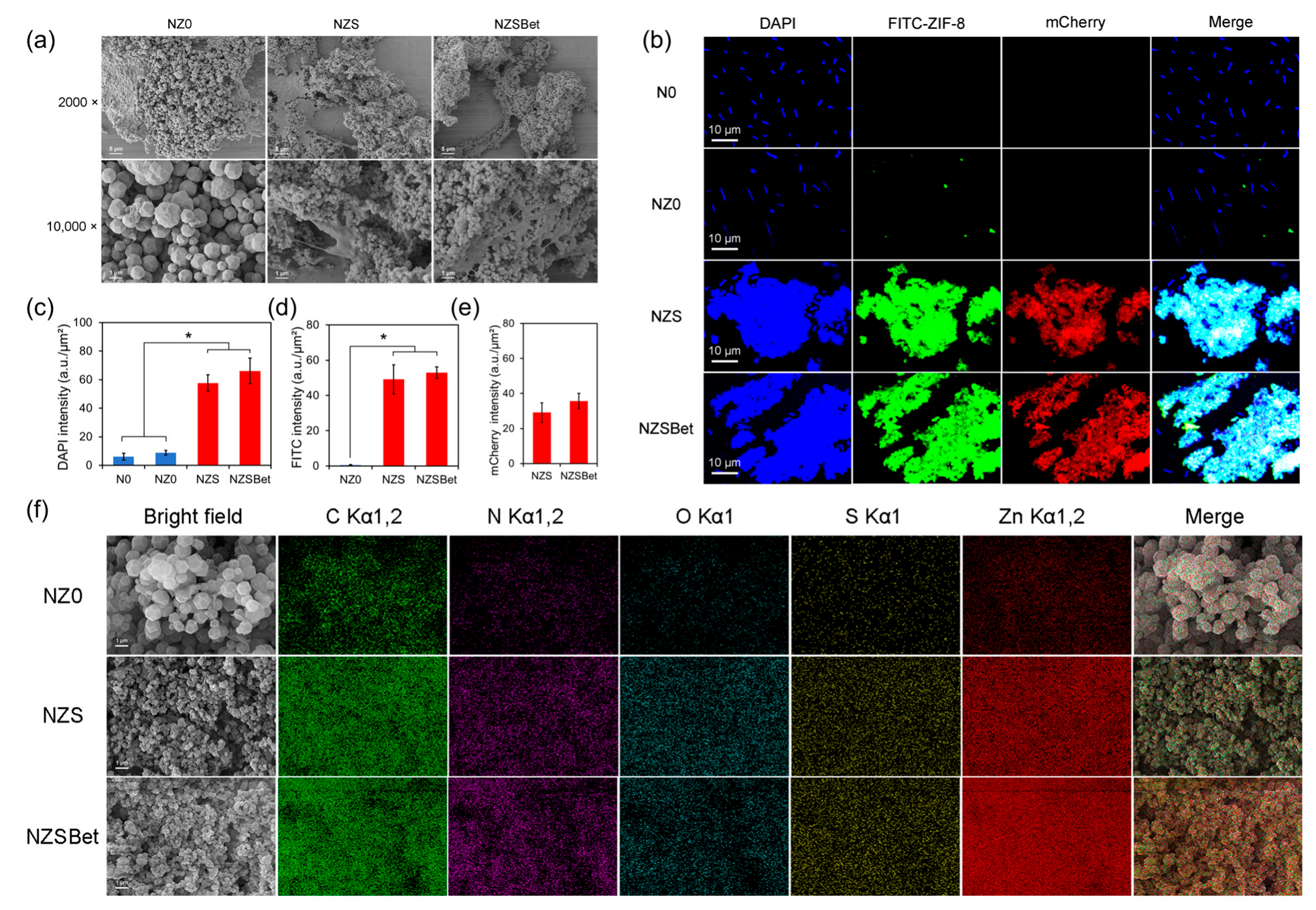
2.2. The SPBP-Mediated Binding and Co-Assembly of ZS and ZSBet with Probiotics
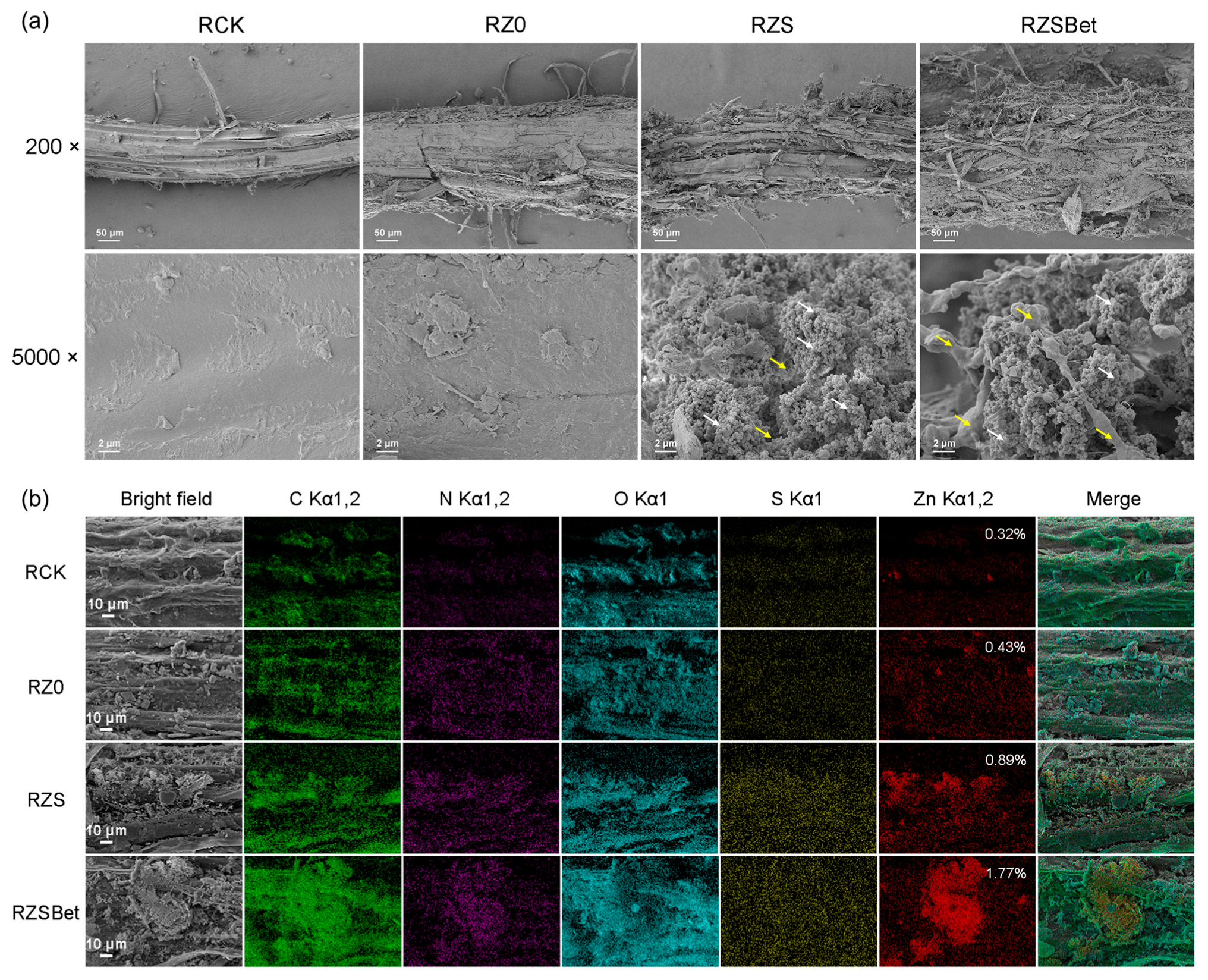
2.3. The SPBP-Mediated Root Targeting of ZS and ZSBet Co-Assemblies with Probiotic Bacteria
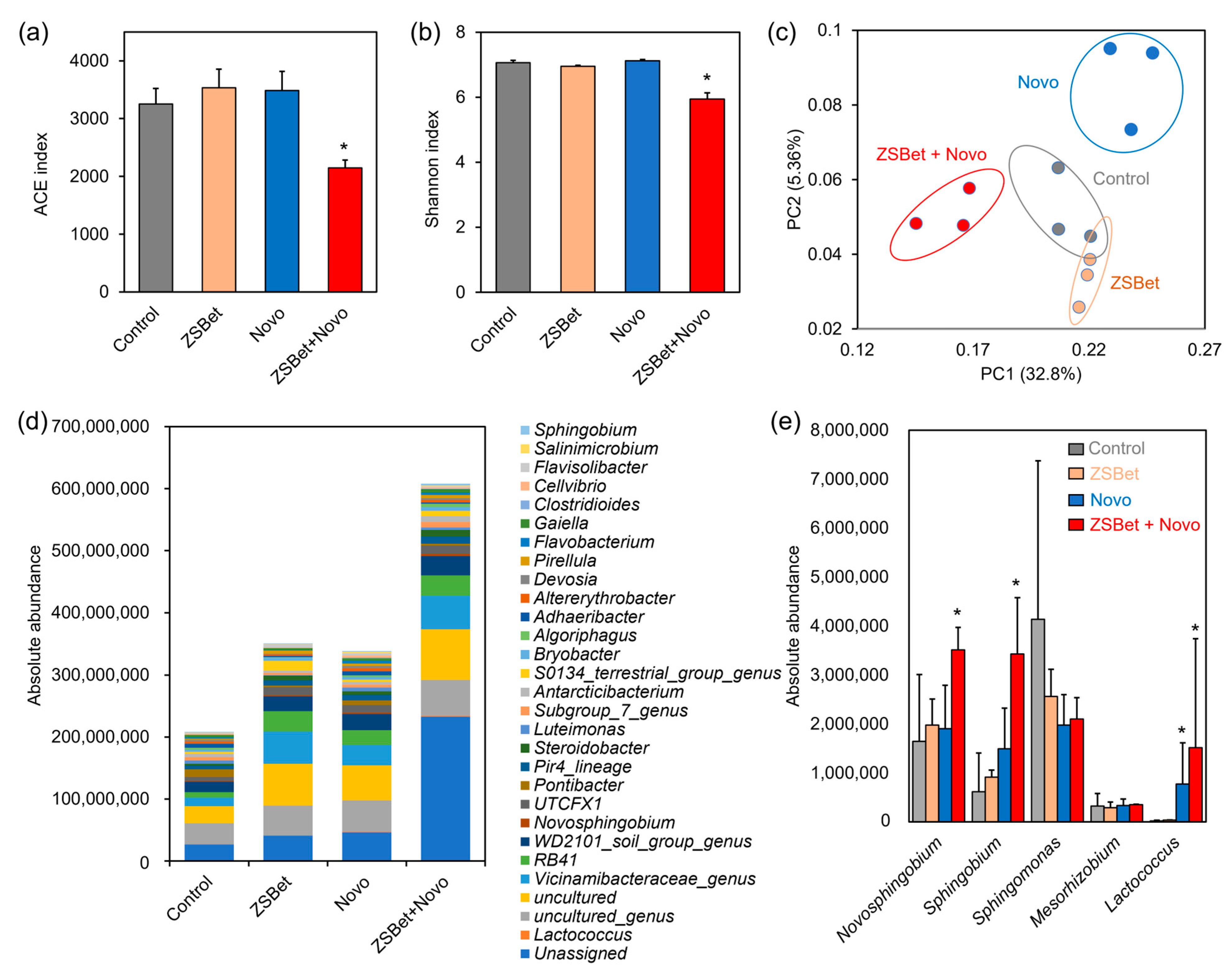
2.4. The Co-Assembly of ZSBet and Novo Modifies the Rhizosphere Microbiome
2.5. The Co-Assembly of ZSBet and Novo Improves the Quality of Saline–Alkali Soil
2.6. The Co-Assembly of ZSBet and Novo Boosts Crop Resistance in Saline–Alkali Soil
3. Discussion
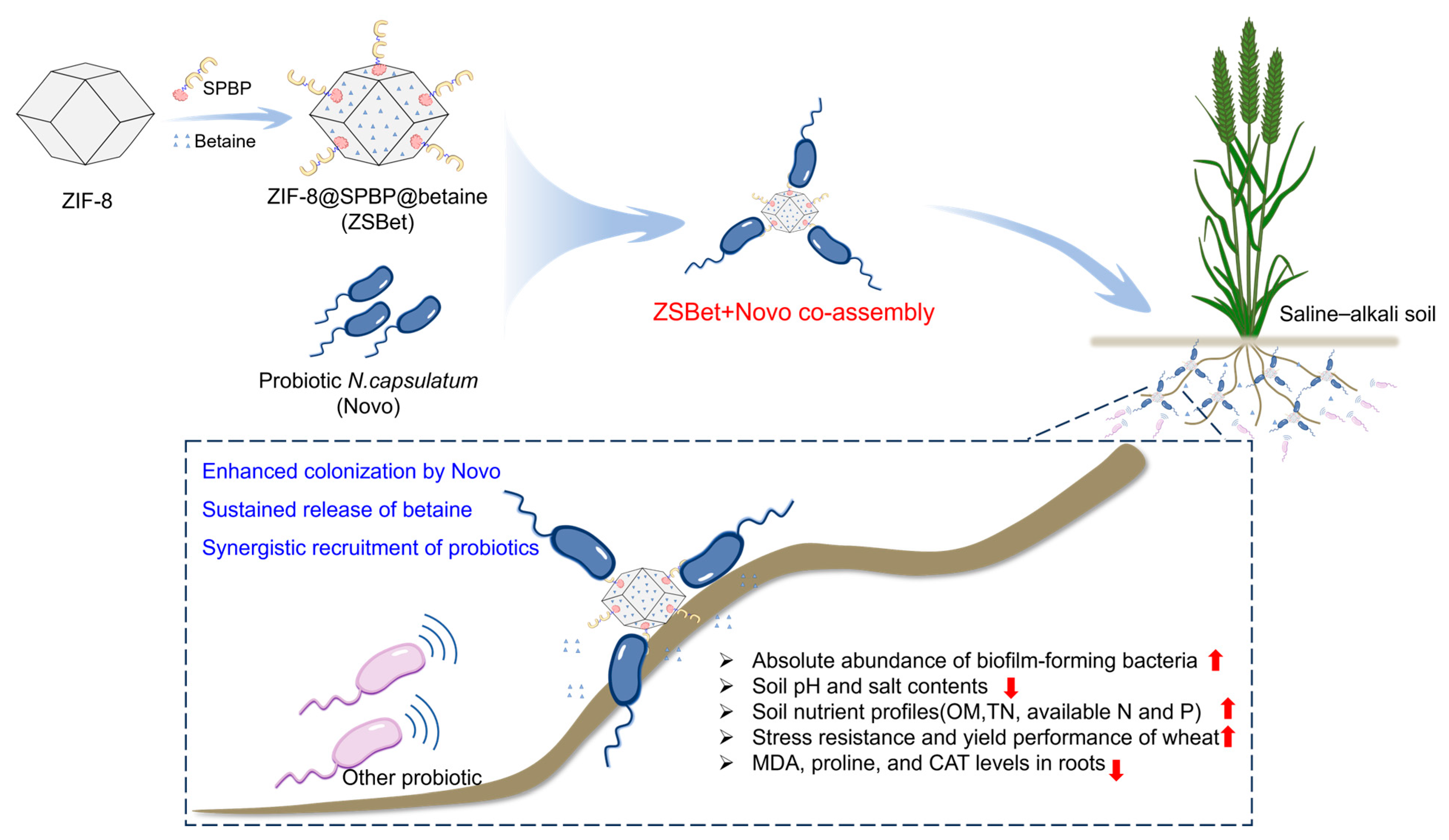
3.1. Construction of the Nanocarrier ZIF-8@SPBP@betaine
3.2. SPBP Mediates the Co-Assembly of ZIF-8 and Probiotics for Enhancing Their Targeted Localization to Plant Roots
3.3. The Co-Assembly of ZSBet and Novo Reshaped the Rhizosphere Microbial Community in Saline–Alkali Soil
3.4. The Co-Assembly of ZSBet and Novo Promoted the Systematic Improvement in Soil Physicochemical Properties and Crop Stress Resistance
3.5. Future Perspectives
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of ZIF-8-Based Materials
4.3. Microbial Recruitment Assay
4.4. Root-Binding Assay
4.5. Field Experiment Site and Design
4.6. Microbial Diversity Analysis
4.7. Determination of Rhizosphere Soil Physicochemical Properties
4.8. Measurement of Plant Growth and Root Biochemical Indicators
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munir, N.; Hasnain, M.; Roessner, U.; Abideen, Z. Strategies in Improving Plant Salinity Resistance and Use of Salinity Resistant Plants for Economic Sustainability. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2150–2196. [Google Scholar] [CrossRef]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective Strategies for Reclamation of Saline-Alkali Soil and Response Mechanisms of the Soil-Plant System. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef] [PubMed]
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-Scale Soil Salinization Dynamics From Global to Pore Scale: A Review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, G.; Feng, B.; Wang, C.; Luo, Y.; Li, F.; Shen, C.; Ma, D.; Zhang, C.; Zhang, J. Saline-Alkali Land Reclamation Boosts Topsoil Carbon Storage by Preferentially Accumulating Plant-Derived Carbon. Sci. Bull. 2024, 69, 2948–2958. [Google Scholar] [CrossRef]
- Javed, S.A.; Shahzad, S.M.; Ashraf, M.; Kausar, R.; Arif, M.S.; Albasher, G.; Rizwana, H.; Shakoor, A. Interactive Effect of Different Salinity Sources and Their Formulations on Plant Growth, Ionic Homeostasis and Seed Quality of Maize. Chemosphere 2022, 291, 132678. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into Plant Salt Stress Signaling and Tolerance. J. Genet. Genomics 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Singh, A.; Rajput, V.D.; Sharma, R.; Ghazaryan, K.; Minkina, T. Salinity Stress and Nanoparticles: Insights into Antioxidative Enzymatic Resistance, Signaling, and Defense Mechanisms. Environ. Res. 2023, 235, 116585. [Google Scholar] [CrossRef]
- Raza, A.; Zaman, Q.U.; Shabala, S.; Tester, M.; Munns, R.; Hu, Z.; Varshney, R.K. Genomics-assisted Breeding for Designing Salinity-smart Future Crops. Plant Biotechnol. J. 2025, 23, 3119–3151. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, D.; Zhang, X.; Lv, X.; Li, B. Current Progress in Deciphering the Molecular Mechanisms Underlying Plant Salt Tolerance. Curr. Opin. Plant Biol. 2025, 83, 102671. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim Yahya, K.; Jia, Z.; Luo, W.; YuanChun, H.; Ame, M.A. Enhancing Salt Leaching Efficiency of Saline-Sodic Coastal Soil by Rice Straw and Gypsum Amendments in Jiangsu Coastal Area. Ain Shams Eng. J. 2022, 13, 101721. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Y.P.; Sun, J.; Huang, G.H. Optimizing Water Resources Allocation and Soil Salinity Control for Supporting Agricultural and Environmental Sustainable Development in Central Asia. Sci. Total Environ. 2020, 704, 135281. [Google Scholar] [CrossRef]
- Melino, V.; Tester, M. Salt-Tolerant Crops: Time to Deliver. Annu. Rev. Plant Biol. 2023, 74, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Feng, C.; Wang, Y.; Yun, C.; Zou, X.; Cheng, N.; Zhang, W.; Jing, Y.; Li, H. Understanding of Plant Salt Tolerance Mechanisms and Application to Molecular Breeding. Int. J. Mol. Sci. 2024, 25, 10940. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, Y.; Liu, X.; Guo, M.; Gao, J.; Yang, M.; Liu, X.; Wang, W.; Jin, Y.; Qu, J. Screening of Saline-Alkali Tolerant Microorganisms and Their Promoting Effects on Rice Growth under Saline-Alkali Stress. J. Clean. Prod. 2024, 481, 144176. [Google Scholar] [CrossRef]
- Benito, P.; Celdrán, M.; Bellón, J.; Arbona, V.; González-Guzmán, M.; Porcel, R.; Yenush, L.; Mulet, J.M. The Combination of a Microbial and a Non-microbial Biostimulant Increases Yield in Lettuce (Lactuca sativa) under Salt Stress Conditions by Up-regulating Cytokinin Biosynthesis. J. Integr. Plant Biol. 2024, 66, 2140–2157. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, X.; Wang, X.; Tang, Y.; Zhang, H.; Yuan, Z.; Zhou, J.; Han, Y.; Li, T. Bioremediation of a Saline-Alkali Soil Polluted with Zn Using Ryegrass Associated with Fusarium incarnatum. Environ. Pollut. 2022, 312, 119929. [Google Scholar] [CrossRef]
- Wang, C.; Pei, J.; Li, H.; Zhu, X.; Zhang, Y.; Wang, Y.; Li, W.; Wang, Z.; Liu, K.; Du, B.; et al. Mechanisms on Salt Tolerant of Paenibacillus polymyxa SC2 and Its Growth-Promoting Effects on Maize Seedlings under Saline Conditions. Microbiol. Res. 2024, 282, 127639. [Google Scholar] [CrossRef]
- Jaiswal, S.; Tripathi, D.K.; Gupta, R.; Corpas, F.J.; Singh, V.P. The Uneven Molecular Distribution: A Connection with Plant Functioning and Stress Resilience. Phytochem. Rev. 2025, 24, 151–163. [Google Scholar] [CrossRef]
- Yin, W.; Dong, N.; Li, X.; Yang, Y.; Lu, Z.; Zhou, W.; Qian, Q.; Chu, C.; Tong, H. Understanding Brassinosteroid-centric Phytohormone Interactions for Crop Improvement. J. Integr. Plant Biol. 2025, 67, 563–581. [Google Scholar] [CrossRef]
- Dong, N.; Lin, H. Contribution of Phenylpropanoid Metabolism to Plant Development and Plant-Environment Interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Muthusamy, M.; Lee, S.I. Abiotic Stress-Induced Secondary Metabolite Production in Brassica: Opportunities and Challenges. Front. Plant Sci. 2024, 14, 1323085. [Google Scholar] [CrossRef]
- Jiang, Z.; Van Zanten, M.; Sasidharan, R. Mechanisms of Plant Acclimation to Multiple Abiotic Stresses. Commun. Biol. 2025, 8, 655. [Google Scholar] [CrossRef]
- Yang, H.; Fang, R.; Luo, L.; Yang, W.; Huang, Q.; Yang, C.; Hui, W.; Gong, W.; Wang, J. Uncovering the Mechanisms of Salicylic Acid-Mediated Abiotic Stress Tolerance in Horticultural Crops. Front. Plant Sci. 2023, 14, 1226041. [Google Scholar] [CrossRef]
- Yang, L.; Qian, X.; Zhao, Z.; Wang, Y.; Ding, G.; Xing, X. Mechanisms of Rhizosphere Plant-Microbe Interactions: Molecular Insights into Microbial Colonization. Front. Plant Sci. 2024, 15, 1491495. [Google Scholar] [CrossRef] [PubMed]
- Teotia, P.; Kumar, M.; Prasad, R.; Sharma, S.; Kumar, V. Endophytic Probiotics and Plant Health: Toward a Balanced Accost. In Probiotics and Plant Health; Springer: Singapore, 2017; pp. 383–399. [Google Scholar]
- Yin, H.; Chen, Y.; Feng, Y.; Feng, L.; Yu, Q. Synthetic Physical Contact-Remodeled Rhizosphere Microbiome for Enhanced Phytoremediation. J. Hazard. Mater. 2022, 433, 128828. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Gao, W.; Wang, R.; Yang, W.; Zhang, H.; Huang, L.; Guo, L. Dynamic Metabolites: A Bridge between Plants and Microbes. Sci. Total Environ. 2023, 899, 165612. [Google Scholar] [CrossRef]
- Sun, L.; Hou, C.; Wei, N.; Tan, Y.; Liang, Q.; Feng, J. pH/Cellulase Dual Environmentally Responsive Nano-Metal Organic Frameworks for Targeted Delivery of Pesticides and Improved Biosafety. Chem. Eng. J. 2023, 478, 147294. [Google Scholar] [CrossRef]
- Wang, C.Y.; Qin, J.C.; Yang, Y.W. Multifunctional Metal-Organic Framework (MOF)-Based Nanoplatforms for Crop Protection and Growth Promotion. J. Agric. Food Chem. 2023, 71, 5953–5972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, H.; Zhou, T.; Chen, X.; Li, W.; Pang, H. Metal-Organic Frameworks and Their Composites for Environmental Applications. Adv. Sci. 2022, 9, 2204141. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, L.; Liu, T.; Chen, H.; Li, Y. pH-Responsive Copper-Doped ZIF-8 MOF Nanoparticles for Enhancing the Delivery and Translocation of Pesticides in Wheat Plants. Environ. Sci. Nano 2023, 10, 2578–2590. [Google Scholar] [CrossRef]
- Hu, S.; Yang, L.-L.; Yan, C.; Xiao, Y.; Jin, Z.; Gan, X.; Zhang, B.; Wu, W. pH and Redox Dual-Responsive ZIF-8-Based Nanoplatform for Targeted Pathogens and Environmental Protection. Chem. Eng. J. 2024, 498, 154844. [Google Scholar] [CrossRef]
- Khosravi, A.; Habibpour, R.; Ranjbar, M. Enhanced Adsorption and Removal of Cd(II) from Aqueous Solution by Amino-Functionalized ZIF-8. Sci. Rep. 2024, 14, 10736. [Google Scholar] [CrossRef]
- Channab, B.-E.; El Idrissi, A.; Ammar, A.; Akil, A.; White, J.C.; Zahouily, M. ZIF-8 Metal Organic Framework, Carboxymethylcellulose and Polyvinyl Alcohol Bio-Nanocomposite Controlled-Release Phosphorus Fertilizer: Improved P Management and Tomato Growth. Chem. Eng. J. 2024, 495, 153610. [Google Scholar] [CrossRef]
- Ma, N.; Cai, K.; Zhao, J.; Liu, C.; Li, H.; Tan, P.; Li, Y.; Li, D.; Ma, X. Mannosylated MOF Encapsulated in Lactobacillus Biofilm for Dual-Targeting Intervention Against Mammalian Escherichia coli Infections. Adv. Mater. 2025, 37, 2503056. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, Y.; Zhao, H.; Wang, X. Magnetic Microrobots with Folate Targeting for Drug Delivery. Cyborg Bionic Syst. 2023, 4, 0019. [Google Scholar] [CrossRef]
- Oh, J.Y.; Jana, B.; Seong, J.; An, E.-K.; Go, E.M.; Jin, S.; Ok, H.W.; Seu, M.-S.; Bae, J.; Lee, C.; et al. Unveiling the Power of Cloaking Metal–Organic Framework Platforms via Supramolecular Antibody Conjugation. ACS Nano 2024, 18, 15790–15801. [Google Scholar] [CrossRef]
- Masoudifar, R.; Pouyanfar, N.; Liu, D.; Ahmadi, M.; Landi, B.; Akbari, M.; Moayeri-Jolandan, S.; Ghorbani-Bidkorpeh, F.; Asadian, E.; Shahbazi, M.-A. Surface Engineered Metal-Organic Frameworks as Active Targeting Nanomedicines for Mono- and Multi-Therapy. Appl. Mater. Today 2022, 29, 101646. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, X.; Wang, Y.; Huang, Q.; Chen, D.; Hou, C.; Mu, Y.; Shen, J. Enhanced Anaerobic Reduction of Nitrobenzene at High Salinity by Betaine Acting as Osmoprotectant and Regulator of Metabolism. Water Res. 2022, 223, 118982. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, Q.; Jiang, X.; Wang, Y.; Guo, S.; Chen, D.; Mu, Y.; Shen, J. Enhanced Anaerobic Reduction of Nitrobenzene under Hypersaline Fluctuation: Glycine Betaine Metabolism and Microbial Osmoadaptation Mechanisms. Chem. Eng. J. 2024, 500, 157460. [Google Scholar] [CrossRef]
- Yu, C.; Hu, Y.; Zhang, Y.; Luo, W.; Zhang, J.; Xu, P.; Qian, J.; Li, J.; Yu, J.; Liu, J.; et al. Concurrent Enhancement of Biomass Production and Phycocyanin Content in Salt-Stressed Arthrospira platensis: A Glycine Betaine-Supplementation Approach. Chemosphere 2024, 353, 141387. [Google Scholar] [CrossRef]
- Islam, S.; Mohammad, F.; Shakeel, A.; Corpas, F.J. Glycine Betaine: A Multifaceted Protectant against Salt Stress in Indian Mustard through Ionic Homeostasis, ROS Scavenging and Osmotic Regulation. Physiol. Plant. 2024, 176, e14530. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Asaf, S.; Imran, M.; Al-Harrasi, A.; Lee, I.J. Efficacy of Endophytic SB10 and Glycine Betaine Duo in Alleviating Phytotoxic Impact of Combined Heat and Salinity in Glycine max L. via Regulation of Redox Homeostasis and Physiological and Molecular Responses. Environ. Pollut. 2023, 316, 120658. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Shi, Z.; Zhu, H.; Li, M.; Yu, Q. Pathogen Infection-Responsive Nanoplatform Targeting Macrophage Endoplasmic Reticulum for Treating Life-Threatening Systemic Infection. Nano Res. 2022, 15, 6243–6255. [Google Scholar] [CrossRef]
- Zhao, L.; Bai, T.; Wei, H.; Gardea-Torresdey, J.L.; Keller, A.; White, J.C. Nanobiotechnology-Based Strategies for Enhanced Crop Stress Resilience. Nat. Food 2022, 3, 829–836. [Google Scholar] [CrossRef]
- Yan, H.; Hao, L.; Chen, H.; Zhou, X.; Ji, H.; Zhou, H. Salicylic Acid Functionalized Zein for Improving Plant Stress Resistance and as a Nanopesticide Carrier with Enhanced Anti-Photolysis Ability. J. Nanobiotechnol. 2023, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Zhao, Y.Y.; Xue, A.R.; Song, C.G.; Zhang, M.Z.; Qin, J.C.; Yang, Y.W. Metal-Organic Framework-Based Dual Function Nanosystems for Aluminum Detoxification and Plant Growth in Acidic Soil. J. Control. Release 2025, 377, 106–115. [Google Scholar] [CrossRef]
- Sahu, K.P.; Singh, S.; Singh, A.K.; Azad, U.P.; Yadav, D.; Kumar, V.; Singh, S.K. Nanomaterials via ZIF-8: Preparations, Catalytic and Drug Delivery Applications. Chem. Eng. J. 2025, 508, 160663. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Haponiuk, J.; Saeb, M.R.; Rabiee, N.; Bencherif, S.A. Mitigating Metal-Organic Framework (MOF) Toxicity for Biomedical Applications. Chem. Eng. J. 2023, 471, 144400. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.X.; Shang, H.B. Synthetic Methods, Properties and Controlling Roles of Synthetic Parameters of Zeolite Imidazole Framework-8: A Review. J. Solid State Chem. 2021, 297, 122040. [Google Scholar] [CrossRef]
- Bhowmick, K.; Roy, D.; Rana, D.; Ghosh, A.; Sadhukhan, S.; Chakraborty, M.; Chattopadhyay, D.; Ghosh, T.K. Potential Microbes in Bioremediation: A Review. Mater. Today Sustain. 2024, 28, 101032. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, R.; Yang, S.; Dai, Z.; Di, N.; Yang, H.; He, Z.; Wei, M. A Salt-Tolerant Growth-Promoting Phyllosphere Microbial Combination from Mangrove Plants and Its Mechanism for Promoting Salt Tolerance in Rice. Microbiome 2024, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Wang, X.; Feng, Z.; Yang, E.; Wu, M.; Jiang, Y.; Huang, J.; Gao, Z.; Du, Y. The Synergistic Effect of Extracellular Polysaccharide-Producing Salt-Tolerant Bacteria and Biochar Promotes Grape Growth under Saline-Alkaline Stress. Environ. Technol. Innov. 2025, 38, 104070. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, A.; Chen, Y.; Xu, Z.; Liu, Y.; Yao, Y.; Wang, Y.; Jia, B. Beneficial Microorganisms: Regulating Growth and Defense for Plant Welfare. Plant Biotechnol. J. 2025, 23, 986–998. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, D.; Sun, Y.; Xin, L.; Li, H.; Zeng, X. Antioxidant Activity of Phosphorylated Exopolysaccharide Produced by Lactococcus Lactis Subsp. Lactis. Carbohydr. Polym. 2013, 97, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Menon, R.R.; Likhitha; Busse, H.J.; Tanaka, N.; Krishnamurthi, S.; Rameshkumar, N. Novosphingobium pokkalii Sp Nov, a Novel Rhizosphere-Associated Bacterium with Plant Beneficial Properties Isolated from Saline-Tolerant Pokkali Rice. Res. Microbiol. 2017, 168, 113–121. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Gurav, R.; Choi, Y.K.; Choi, T.R.; Kim, H.; Song, H.S.; Mi Lee, S.; Lee Park, S.; Soo Lee, H.; Kim, Y.G.; et al. Bioprospecting of Exopolysaccharide from Marine Sphingobium yanoikuyae BBL01: Production, Characterization, and Metal Chelation Activity. Bioresour. Technol. 2021, 324, 124674. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Proline Alleviates Abiotic Stress Induced Oxidative Stress in Plants. J. Plant Growth Regul. 2023, 42, 4629–4651. [Google Scholar] [CrossRef]
- Riseh, R.S.; Fathi, F.; Vatankhah, M.; Kennedy, J.F. Catalase-Associated Immune Responses in Plant-Microbe Interactions: A Review. Int. J. Biol. Macromol. 2024, 280, 135859. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Sun, Y.; Xie, L.; Liu, S.; Li, M.; Yu, Q. Combined Microbe-Plant Remediation of Cadmium in Saline-Alkali Soil Assisted by Fungal Mycelium-Derived Biochar. Environ. Res. 2024, 240, 117424. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, Y.; Sun, Q.; Liang, D.; Yang, X.; Zhao, W.; Zhang, Y.; Liu, H.; Tang, J.; et al. Halophyte Inspired Solar-Driven Salt Extractor for Saline Soil Remediating. Chem. Eng. J. 2025, 514, 162748. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, B.D.; Raj, R. A Review on the Application of Biopolymers (Xanthan, Agar and Guar) for Sustainable Improvement of Soil. Discov. Appl. Sci. 2024, 6, 393. [Google Scholar] [CrossRef]
- Sultan, H.; Li, Y.; Ahmed, W.; Yixue, M.; Shah, A.; Faizan, M.; Ahmad, A.; Abbas, H.M.M.; Nie, L.; Khan, M.N. Biochar and Nano Biochar: Enhancing Salt Resilience in Plants and Soil While Mitigating Greenhouse Gas Emissions: A Comprehensive Review. J. Environ. Manag. 2024, 355, 120448. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.M.M.; Rais, U.; Altaf, M.M.; Rasul, F.; Shah, A.; Tahir, A.; Nafees-Ur-Rehman, M.; Shaukat, M.; Sultan, H.; Zou, R.; et al. Microbial-Inoculated Biochar for Remediation of Salt and Heavy Metal Contaminated Soils. Sci. Total Environ. 2024, 954, 176104. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, S.; Gao, S.; Wang, S.; Gao, Z.; He, Z. Effect of a Superabsorbent Polymer (Poly-Gamma-Glutamic Acid) on Water and Salt Transport in Saline Soils under the Influence of Multiple Factors. Polymers 2022, 14, 4056. [Google Scholar] [CrossRef]
- Dos Reis, R.A.; Fernández-Baldo, M.A.; Nunes, R.S.; Seabra, A.B. Metal-Organic Frameworks: An Overview of a Possible Solution for Modern Agriculture. Microporous Mesoporous Mater. 2025, 391, 113609. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, Y.; Zhang, X.; Zhou, L.; Song, Z.; Nezamzadeh-Ejhieh, A.; Huang, Y. Application Progress of Nano-Platforms Based on Metal-Organic Frameworks (MOFs) in Modern Agriculture. J. Environ. Chem. Eng. 2025, 13, 116870. [Google Scholar] [CrossRef]
- Wu, K.; Xu, X.; Ma, F.; Du, C. Fe-Based Metal-Organic Frameworks for the Controlled Release of Fertilizer Nutrients. ACS Omega 2022, 7, 35970–35980. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Y.; Tsyusko, O.V.; Wang, J.; Unrine, J.M.; Wei, G.; Chen, C. Metal-Organic Framework-Enabled Sustainable Agrotechnologies: An Overview of Fundamentals and Agricultural Applications. J. Agric. Food Chem. 2024, 72, 8890–8905. [Google Scholar] [CrossRef] [PubMed]
- Rogowska-van Der Molen, M.A.; Berasategui-Lopez, A.; Coolen, S.; Jansen, R.S.; Welte, C.U. Microbial Degradation of Plant Toxins. Environ. Microbiol. 2023, 25, 2988–3010. [Google Scholar] [CrossRef]
- Manikandan, V.; Vinoth Kumar, J.; Elango, D.; Subash, V.; Jayanthi, P.; Dixit, S.; Singh, S. Metal-Organic Frameworks (MOFs): Multifunctional Platforms for Environmental Sustainability. Chem. Rec. 2025, 25, e202400257. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Jia, H.; Yao, Y.; Song, J.; Dong, H.; Cao, Y.; Zhu, F.; Huo, Z. Pectin Functionalized Metal-Organic Frameworks as Dual-Stimuli-Responsive Carriers to Improve the Pesticide Targeting and Reduce Environmental Risks. Colloids Surf. B Biointerfaces 2022, 219, 112796. [Google Scholar] [CrossRef] [PubMed]
- Dok, A.R.; Radhakrishnan, S.; De Jong, F.; Becquevort, E.; Deschaume, O.; Chandran, C.V.; De Coene, Y.; Bartic, C.; Van Der Auweraer, M.; Thielemans, W.; et al. Amorphous-to-Crystalline Transformation: How Cluster Aggregation Drives the Multistep Nucleation of ZIF-8. J. Am. Chem. Soc. 2025, 147, 8455–8466. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Luo, L.; Chen, Y.; Palta, J.A.; Wang, Z. Zinc Agronomic Biofortification in Wheat and Its Drivers: A Global Meta-Analysis. Nat. Commun. 2025, 16, 3913. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Verma, N.; Tewari, R.K. Micronutrient Deficiency-Induced Oxidative Stress in Plants. Plant Cell Rep. 2024, 43, 213. [Google Scholar] [CrossRef]
- Wang, H.; Jian, M.; Qi, Z.; Li, Y.; Liu, R.; Qu, J.; Zhang, X. Specific Anion Effects on the Stability of Zeolitic Imidazolate Framework-8 in Aqueous Solution. Microporous Mesoporous Mater. 2018, 259, 171–177. [Google Scholar] [CrossRef]
- Nazir, M.A.; Ullah, S.; Shahid, M.U.; Hossain, I.; Najam, T.; Ismail, M.A.; Rehman, A.U.; Karim, M.R.; Shah, S.S.A. Zeolitic Imidazolate Frameworks (ZIF-8 & ZIF-67): Synthesis and Application for Wastewater Treatment. Sep. Purif. Technol. 2025, 356, 129828. [Google Scholar] [CrossRef]
- Shah, B.A.; Malhotra, H.; Papade, S.E.; Dhamale, T.; Ingale, O.P.; Kasarlawar, S.T.; Phale, P.S. Microbial Degradation of Contaminants of Emerging Concern: Metabolic, Genetic and Omics Insights for Enhanced Bioremediation. Front. Bioeng. Biotechnol. 2024, 12, 1470522. [Google Scholar] [CrossRef]
- Yin, Z.; Yuan, Y.; Zhang, R.; Gan, J.; Yu, L.; Qiu, X.; Chen, R.; Wang, Q. Understanding Bacillus Response to Salt Stress: Growth Inhibition, Enhanced EPS Secretion, and Molecular Adaptation Mechanisms. Process Biochem. 2024, 146, 412–422. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, Á.T. Bacillus Subtilis Biofilm Formation and Social Interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; De Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis Biofilm Matrix Components Target Seed Oil Bodies to Promote Growth and Anti-Fungal Resistance in Melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, Y.; Du, Y.; Miao, S.; Liu, J.; Li, Y.; Caiyin, Q.; Qiao, J. Quantitative Proteomics of Lactococcus lactis F44 under Cross-Stress of Low pH and Lactate. J. Dairy Sci. 2018, 101, 6872–6884. [Google Scholar] [CrossRef]
- Li, N.; Geng, A.; Tu, Z.; Fan, Y.; Xie, R.; Li, X.; Sun, J. Isolation of Lactococcus Sp. X1 from Termite Gut, and Its Application in Lactic Acid Production. Fermentation 2023, 9, 85. [Google Scholar] [CrossRef]
- Li, N.; Li, J.; Xie, J.; Rui, W.; Pu, K.; Gao, Y.; Wang, T.; Zhang, M. Glycine Betaine and Plant Abiotic Stresses: Unravelling Physiological and Molecular Responses. Plant Sci. 2025, 355, 112479. [Google Scholar] [CrossRef]
- Spitsyna, A.S.; Poryvaev, A.S.; Sannikova, N.E.; Yazikova, A.A.; Kirilyuk, I.A.; Dobrynin, S.A.; Chinak, O.A.; Fedin, M.V.; Krumkacheva, O.A. Stability of ZIF-8 Nanoparticles in Most Common Cell Culture Media. Molecules 2022, 27, 3240. [Google Scholar] [CrossRef]
- Butonova, S.A.; Ikonnikova, E.V.; Sharsheeva, A.; Chernyshov, I.Y.; Kuchur, O.A.; Mukhin, I.S.; Hey-Hawkins, E.; Vinogradov, A.V.; Morozov, M.I. Degradation Kinetic Study of ZIF-8 Microcrystals with and without the Presence of Lactic Acid. RSC Adv. 2021, 11, 39169–39176. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Guo, L.; Cao, C.; Tan, W.; Li, C. Long-Term Rice-Oilseed Rape Rotation Increases Soil Organic Carbon by Improving Functional Groups of Soil Organic Matter. Agric. Ecosyst. Environ. 2021, 319, 107548. [Google Scholar] [CrossRef]
- Liu, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Root Exudates Shift How N Mineralization and N Fixation Contribute to the Plant-Available N Supply in Low Fertility Soils. Soil Biol. Biochem. 2022, 165, 108541. [Google Scholar] [CrossRef]
- Wang, K.; Ren, T.; Yan, J.; Zhu, D.; Liao, S.; Zhang, Y.; Lu, Z.; Cong, R.; Li, X.; Lu, J. Straw Returning Mediates Soil Microbial Biomass Carbon and Phosphorus Turnover to Enhance Soil Phosphorus Availability in a Rice-Oilseed Rape Rotation with Different Soil Phosphorus Levels. Agric. Ecosyst. Environ. 2022, 335, 107991. [Google Scholar] [CrossRef]
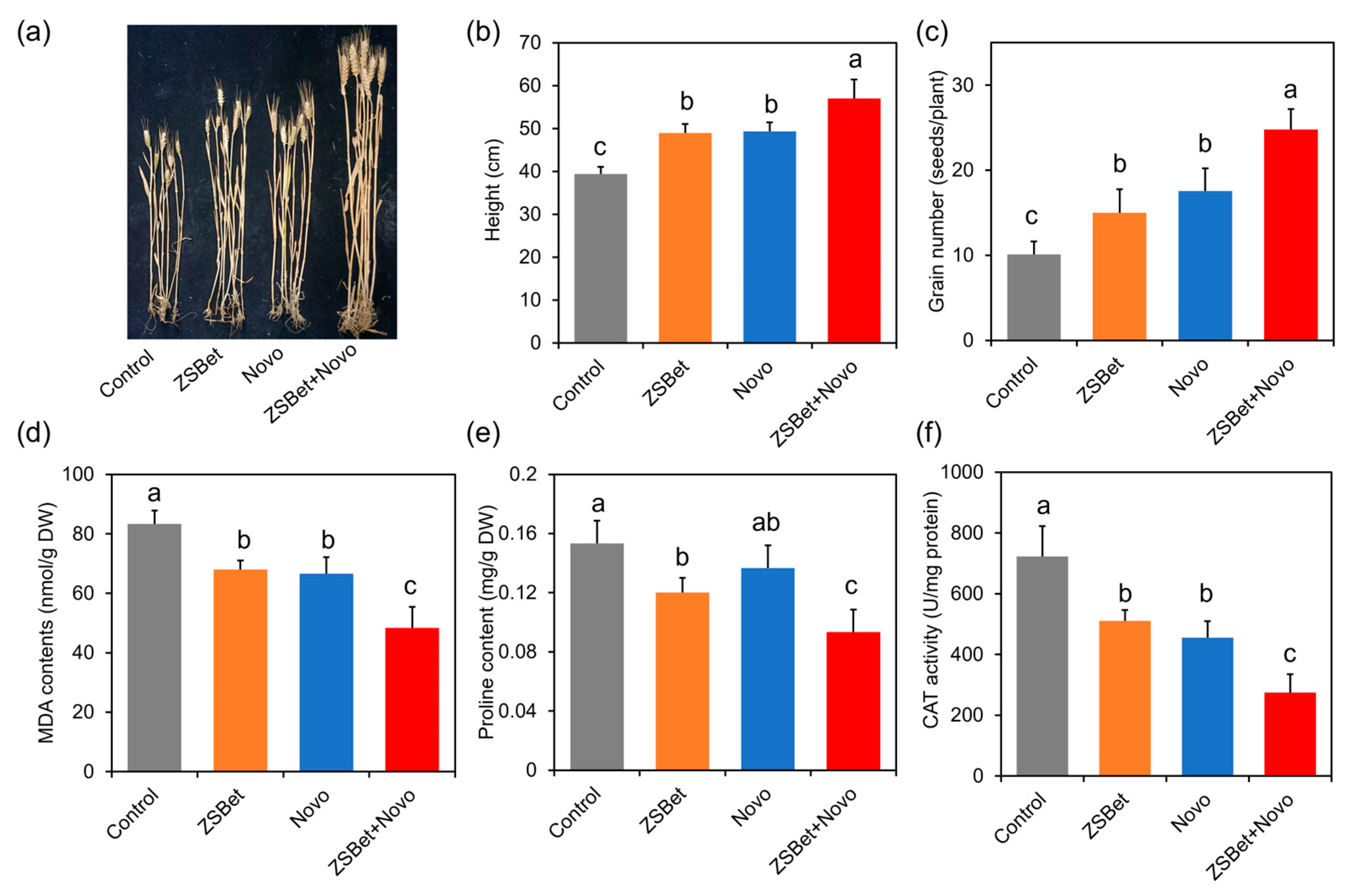
| Control | ZSBet | Novo | ZSBet + Novo | |
|---|---|---|---|---|
| pH | 8.53 ± 0.15 a | 8.10 ± 0.10 b | 8.23 ± 0.12 bc | 7.90 ± 0.10 c |
| Salt content (%) | 0.42 ± 0.02 a | 0.39 ± 0.02 ab | 0.38 ± 0.02 b | 0.31 ± 0.03 c |
| Organic matter (g/kg) | 26.77 ± 1.31 c | 28.70 ± 0.92 bc | 29.33 ± 1.06 ab | 31.00 ± 1.23 a |
| TN (g/kg) | 1.38 ± 0.04 c | 1.54 ± 0.06 b | 1.55 ± 0.07 b | 1.79 ± 0.03 a |
| Available N (mg/kg) | 87.67 ± 5.13 b | 92.33 ± 3.06 b | 91.33 ± 1.53 b | 100.33 ± 4.04 a |
| Available P (mg/kg) | 48.67 ± 2.52 c | 54.67 ± 2.52 b | 55.33 ± 2.52 b | 61.00 ± 2.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Liu, R.; Yu, J.; Liu, Y.; Li, M.; Yu, Q. Synthetic Protein-Assisted Co-Assembly of Zeolitic Imidazolate Framework-8 and Novosphingobium capsulatum for Enhanced Saline–Alkali Resistance of Wheat. Molecules 2025, 30, 3669. https://doi.org/10.3390/molecules30183669
Zhao Z, Liu R, Yu J, Liu Y, Li M, Yu Q. Synthetic Protein-Assisted Co-Assembly of Zeolitic Imidazolate Framework-8 and Novosphingobium capsulatum for Enhanced Saline–Alkali Resistance of Wheat. Molecules. 2025; 30(18):3669. https://doi.org/10.3390/molecules30183669
Chicago/Turabian StyleZhao, Zirun, Rou Liu, Jiawen Yu, Yunlong Liu, Mingchun Li, and Qilin Yu. 2025. "Synthetic Protein-Assisted Co-Assembly of Zeolitic Imidazolate Framework-8 and Novosphingobium capsulatum for Enhanced Saline–Alkali Resistance of Wheat" Molecules 30, no. 18: 3669. https://doi.org/10.3390/molecules30183669
APA StyleZhao, Z., Liu, R., Yu, J., Liu, Y., Li, M., & Yu, Q. (2025). Synthetic Protein-Assisted Co-Assembly of Zeolitic Imidazolate Framework-8 and Novosphingobium capsulatum for Enhanced Saline–Alkali Resistance of Wheat. Molecules, 30(18), 3669. https://doi.org/10.3390/molecules30183669







