Structural Speciation of Hybrid Ti(IV)-Chrysin Systems—Biological Profiling and Antibacterial, Anti-Inflammatory, and Tissue-Specific Anticancer Activity
Abstract
1. Introduction
2. Results
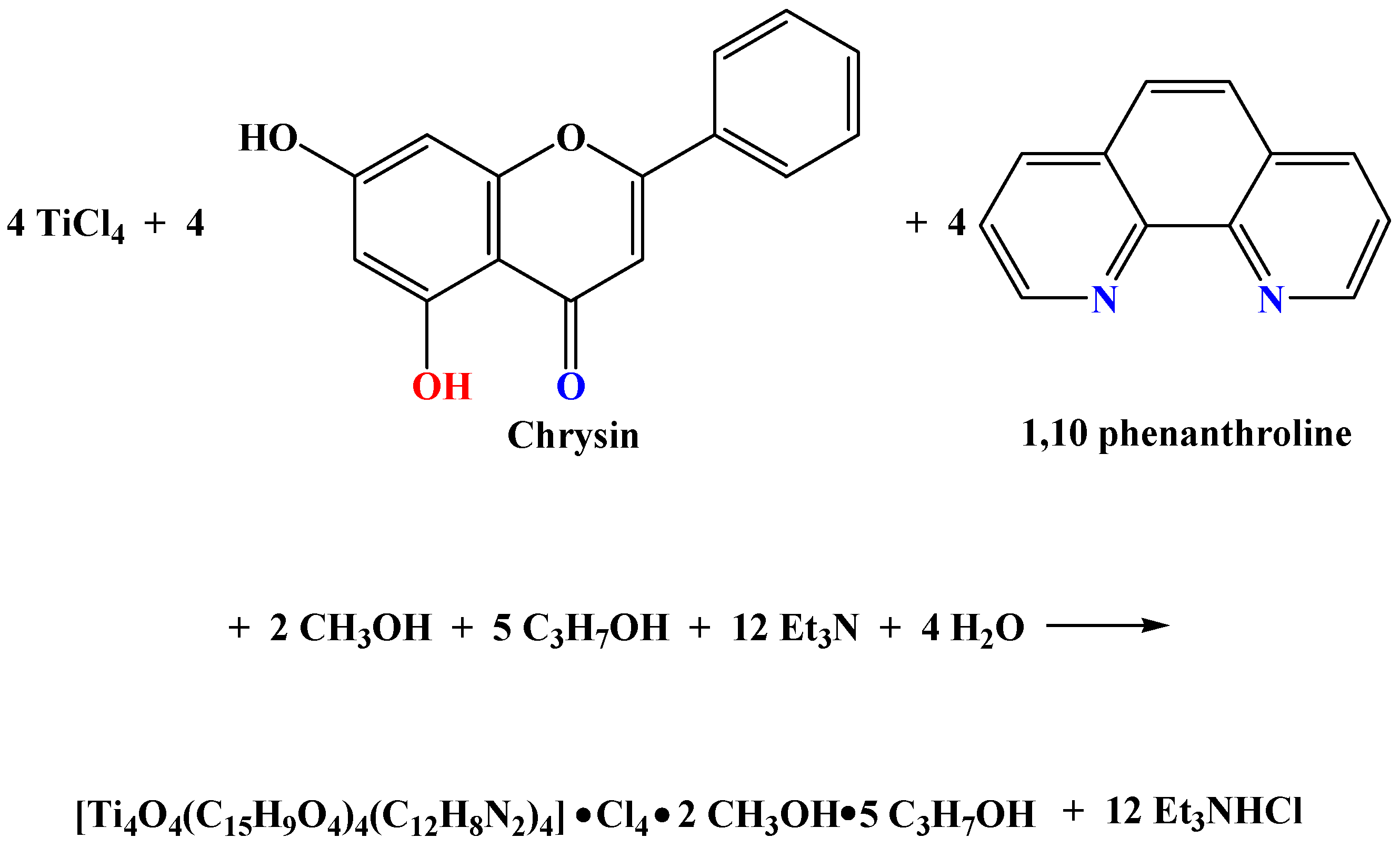
2.1. Synthesis of Binary and Ternary Materials
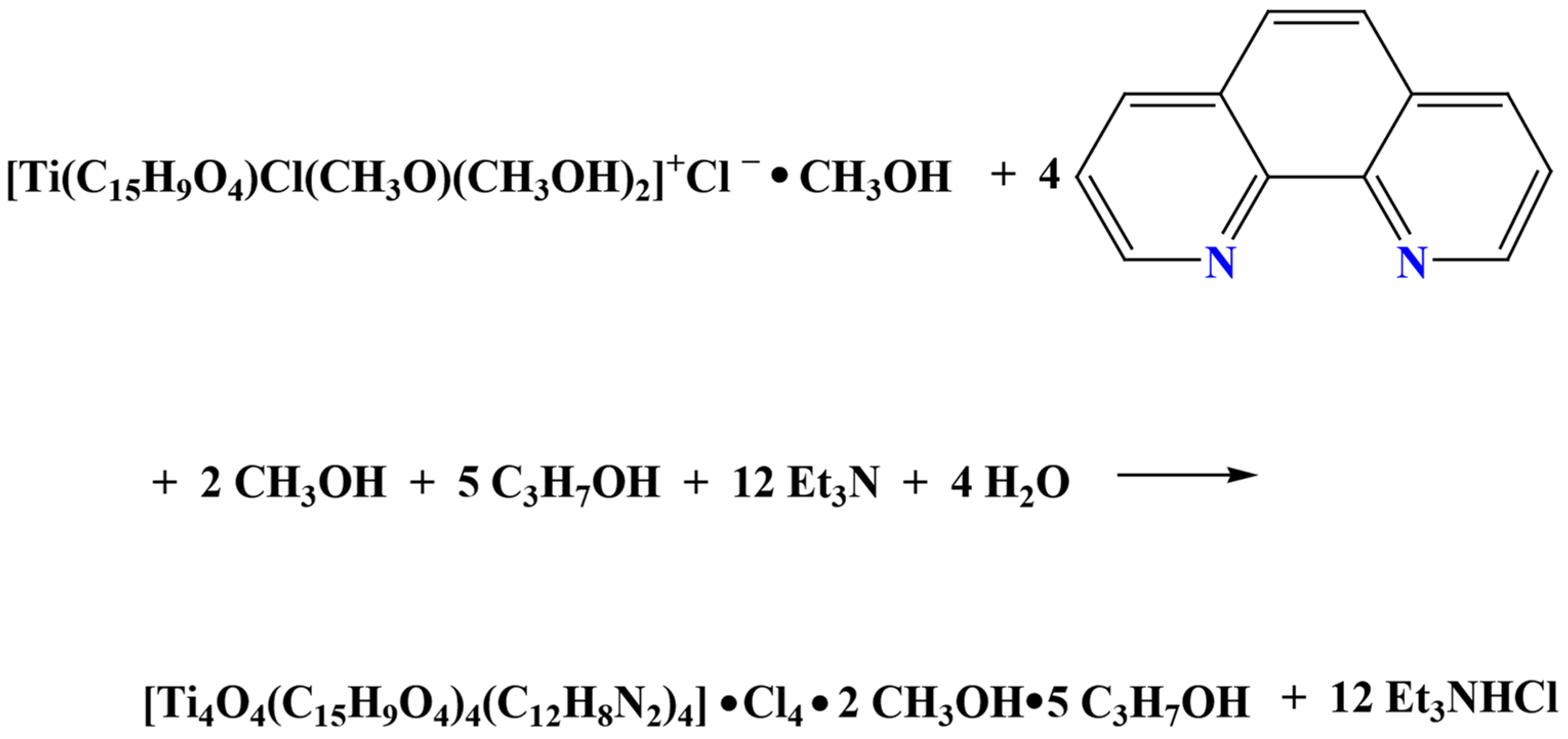
2.2. Transformation of 1 to 2
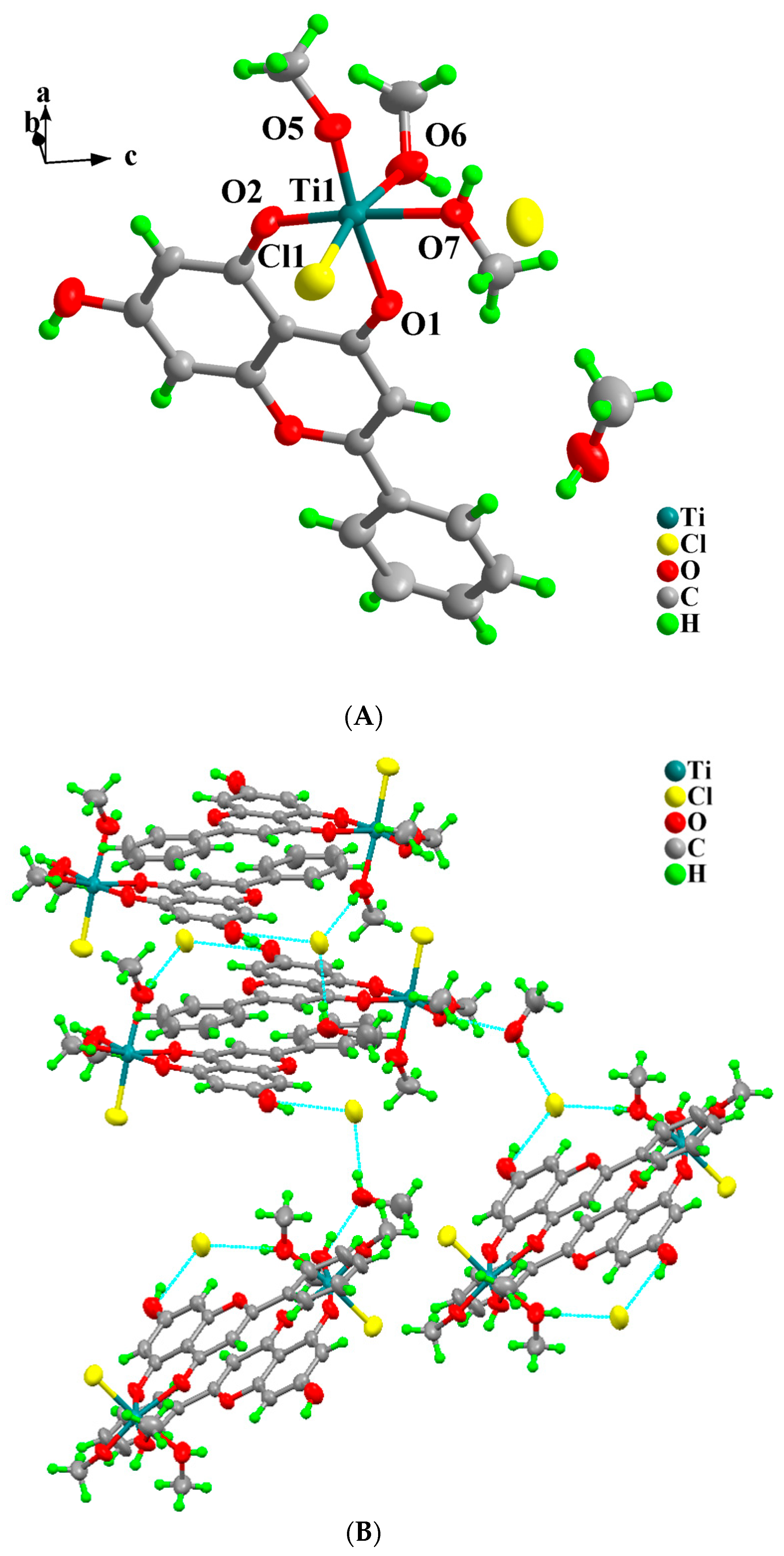
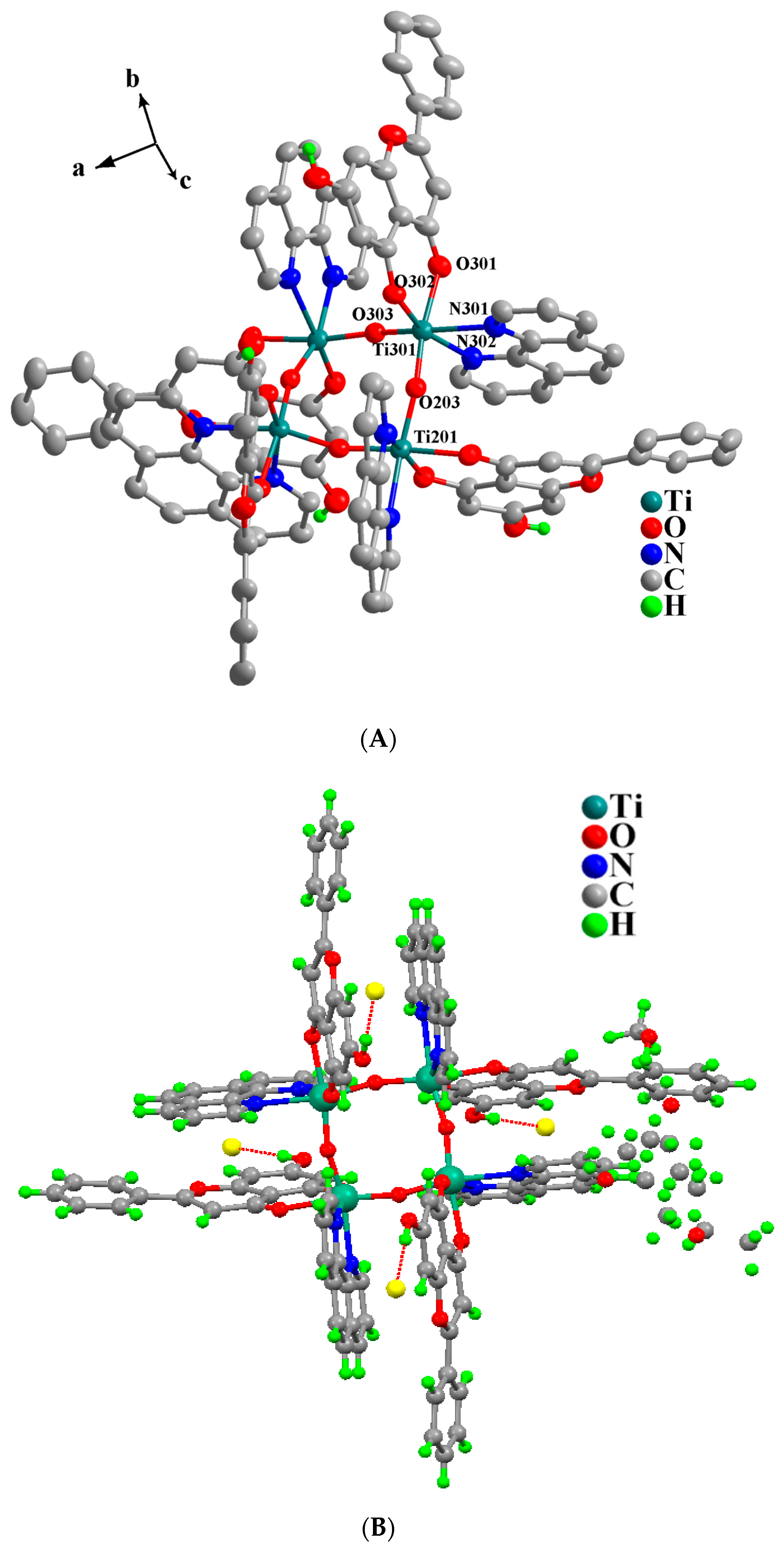
2.3. Description of X-Ray Crystallographic Structures
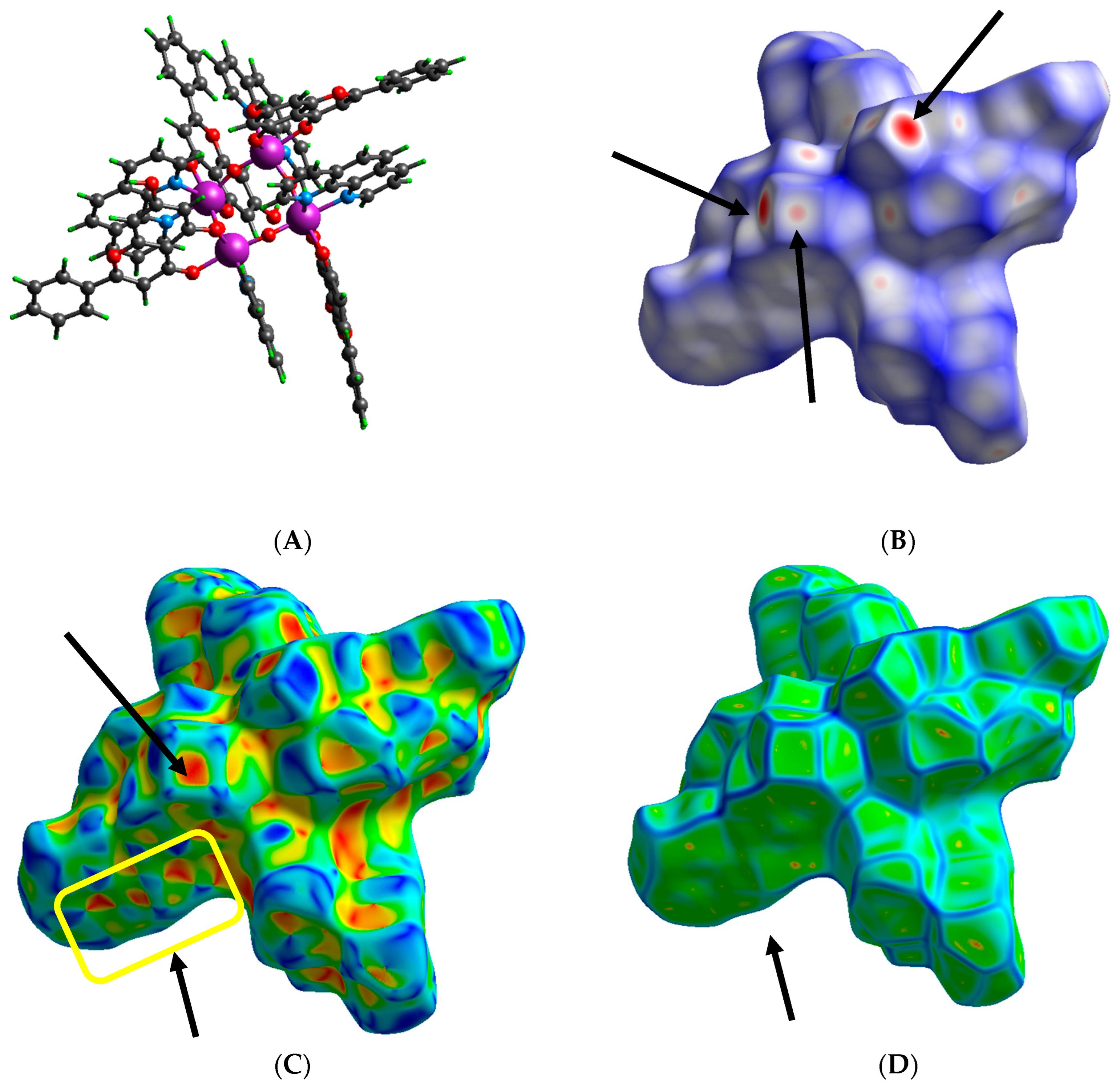
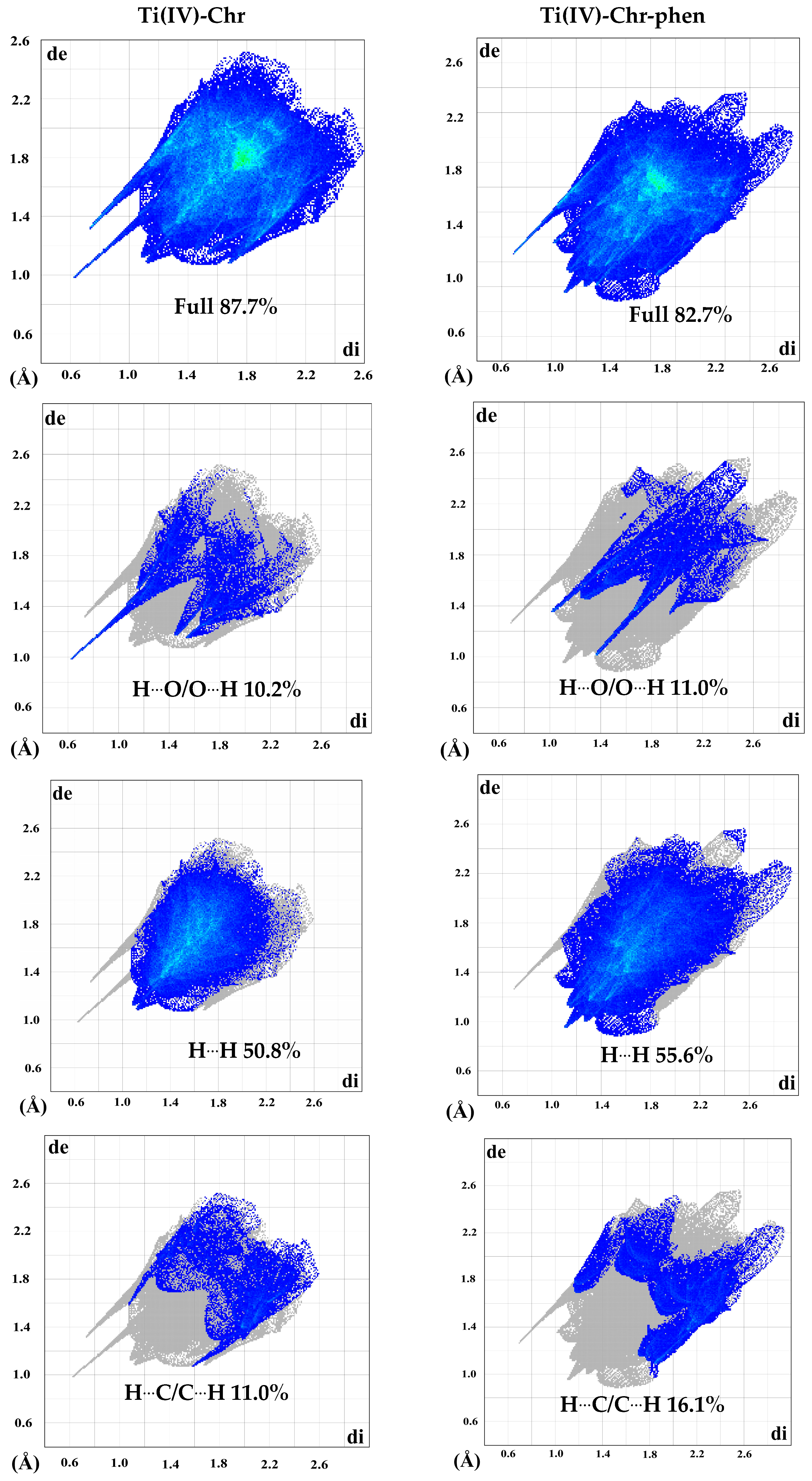
2.4. Hirshfeld Surface Analysis
2.5. FT-IR Spectroscopy
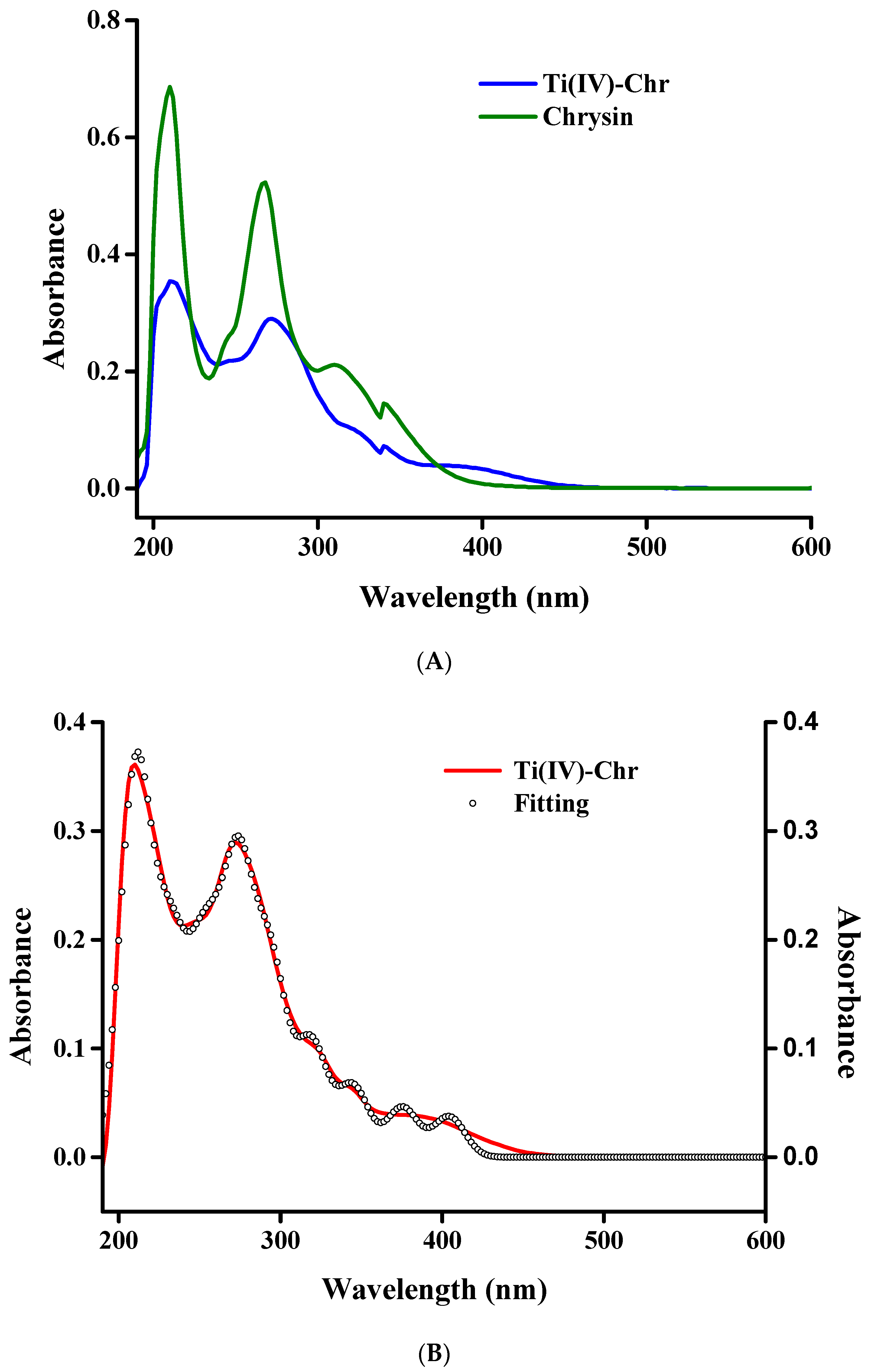
2.6. UV–Visible Spectroscopy
2.7. ESI-MS Analysis
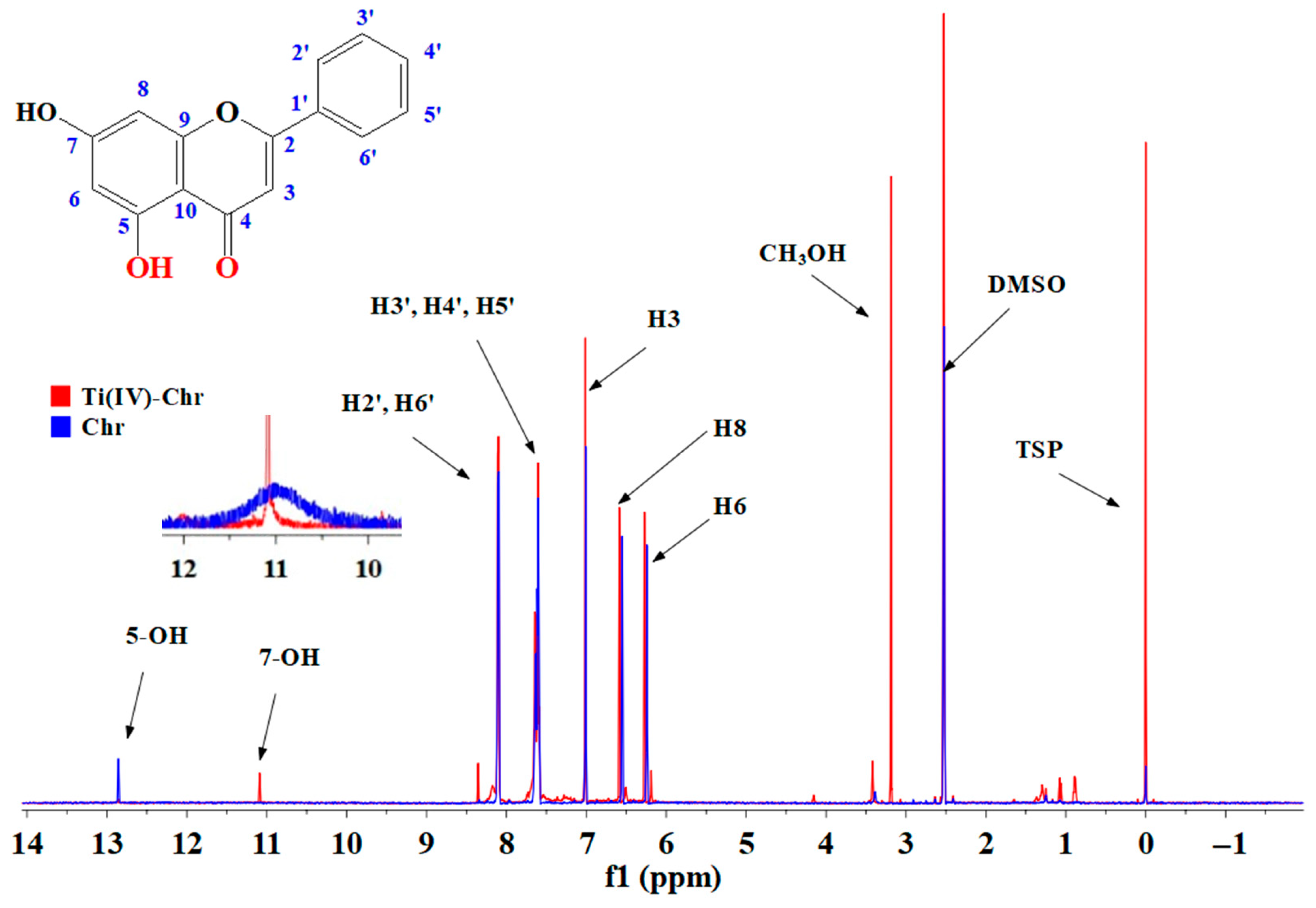
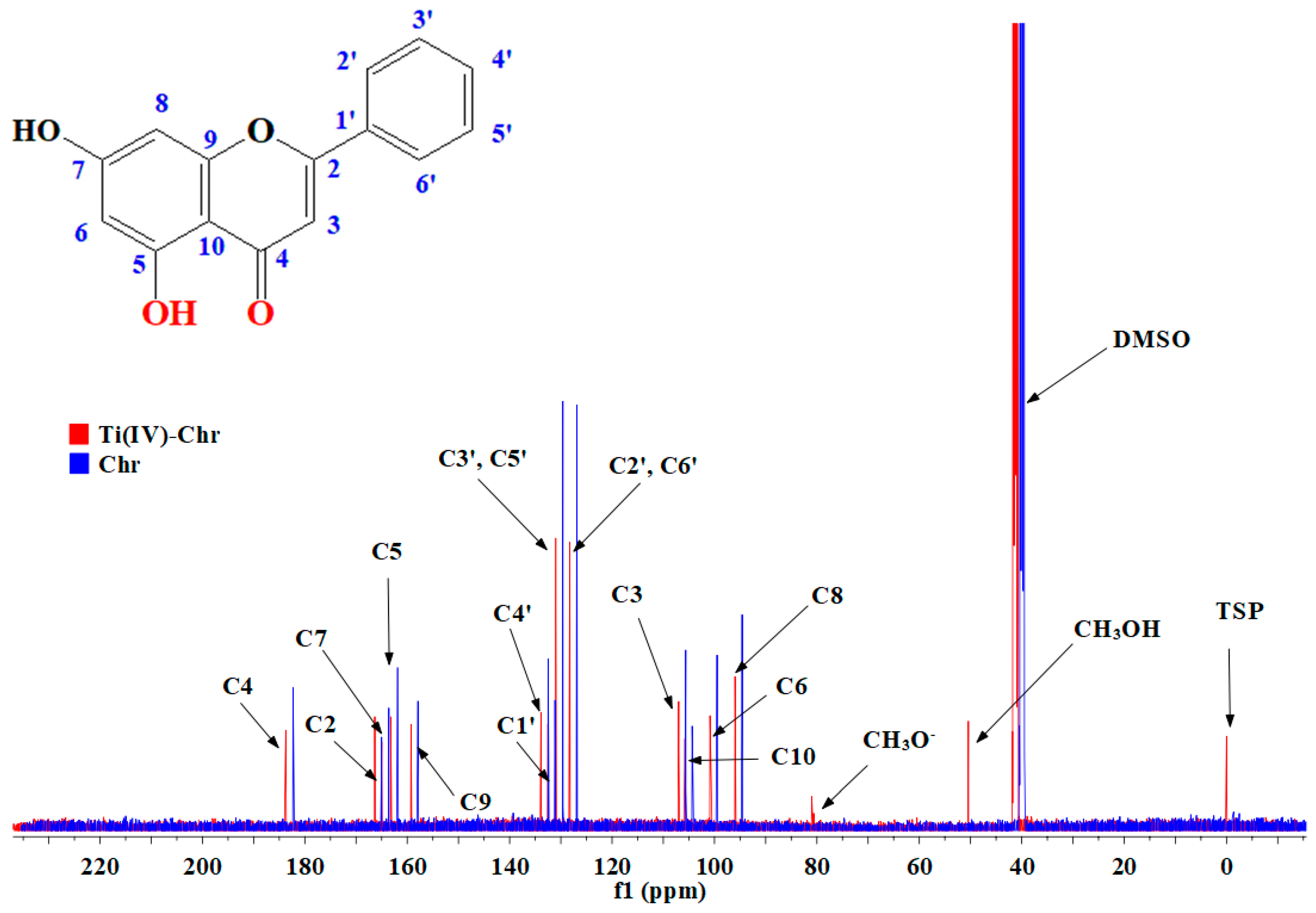
2.8. NMR Spectroscopy
2.8.1. 1D 1H-, 13C-NMR
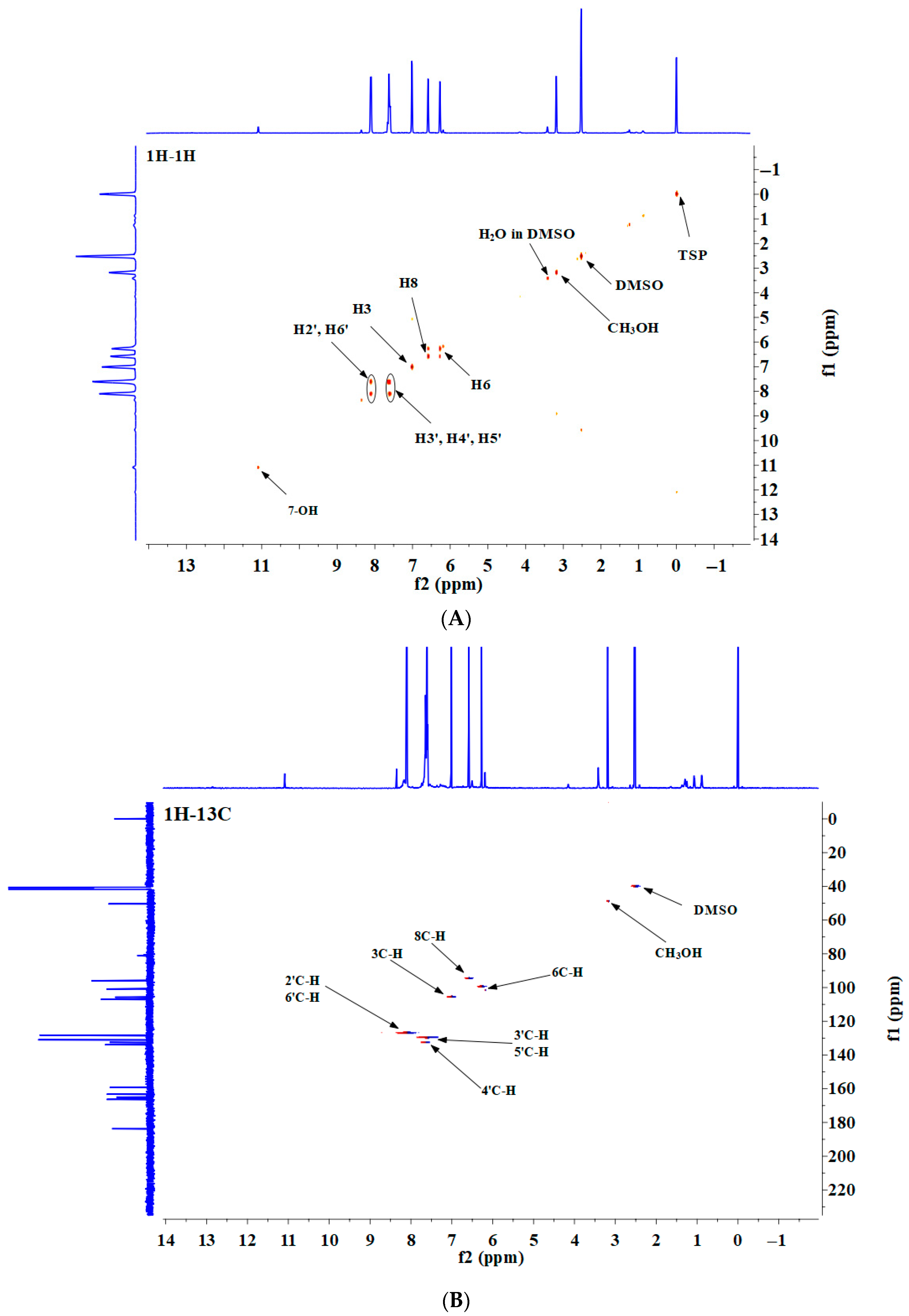
2.8.2. 2D gCOSY and gHSQC NMR
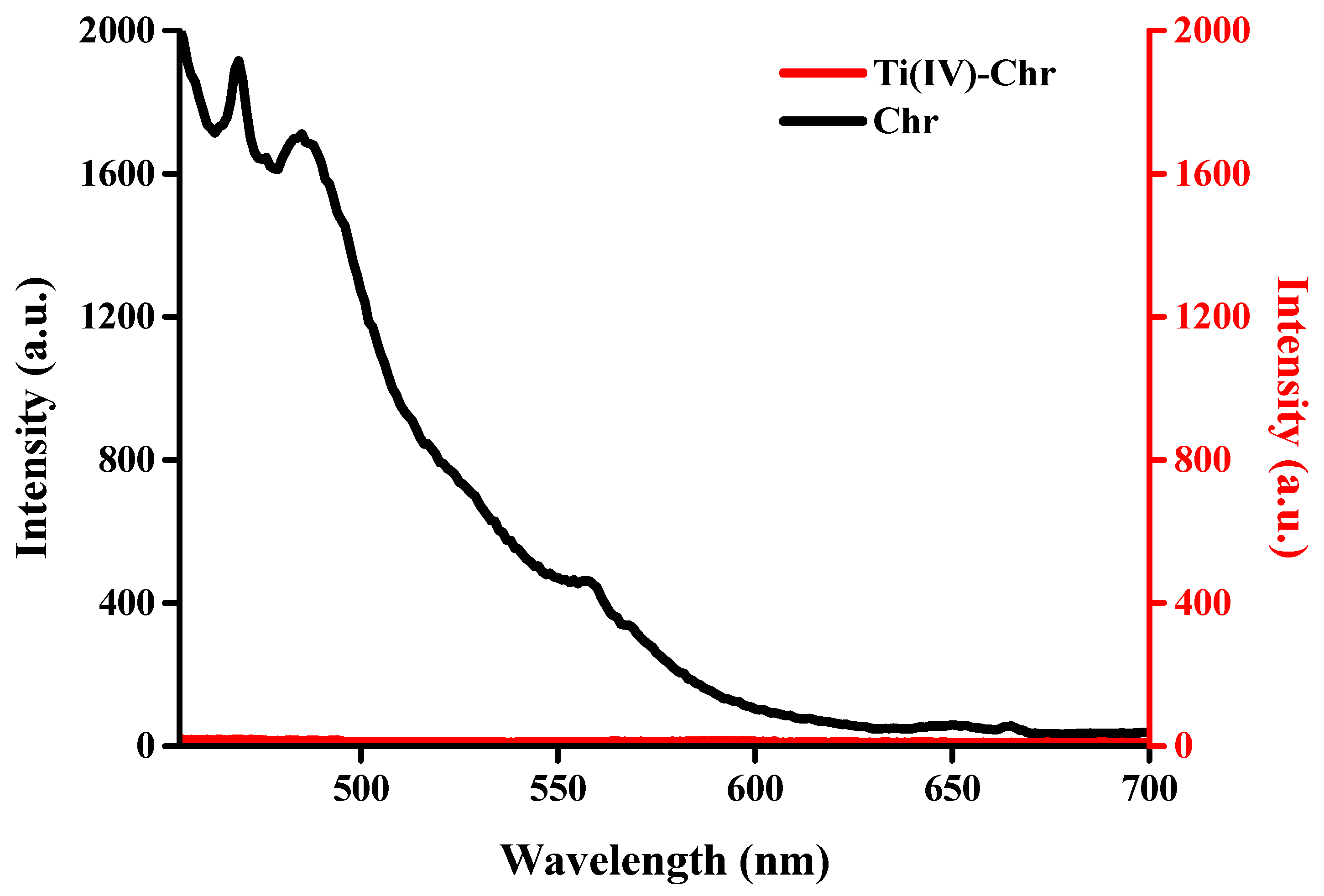
2.9. Luminescence Studies
2.10. Antibacterial Properties
2.11. Anti-Inflammatory Activity
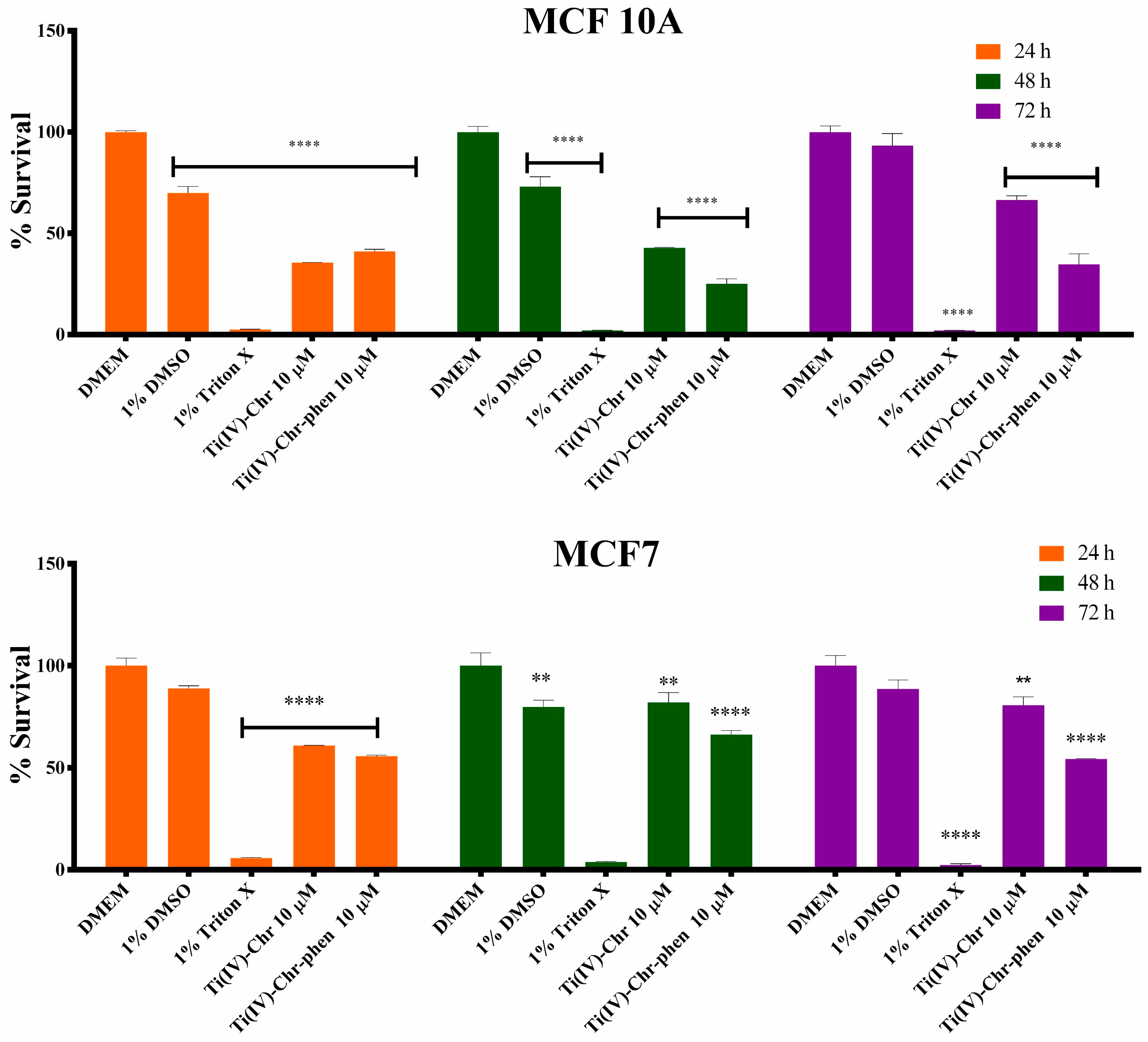
2.12. Cytotoxicity Results
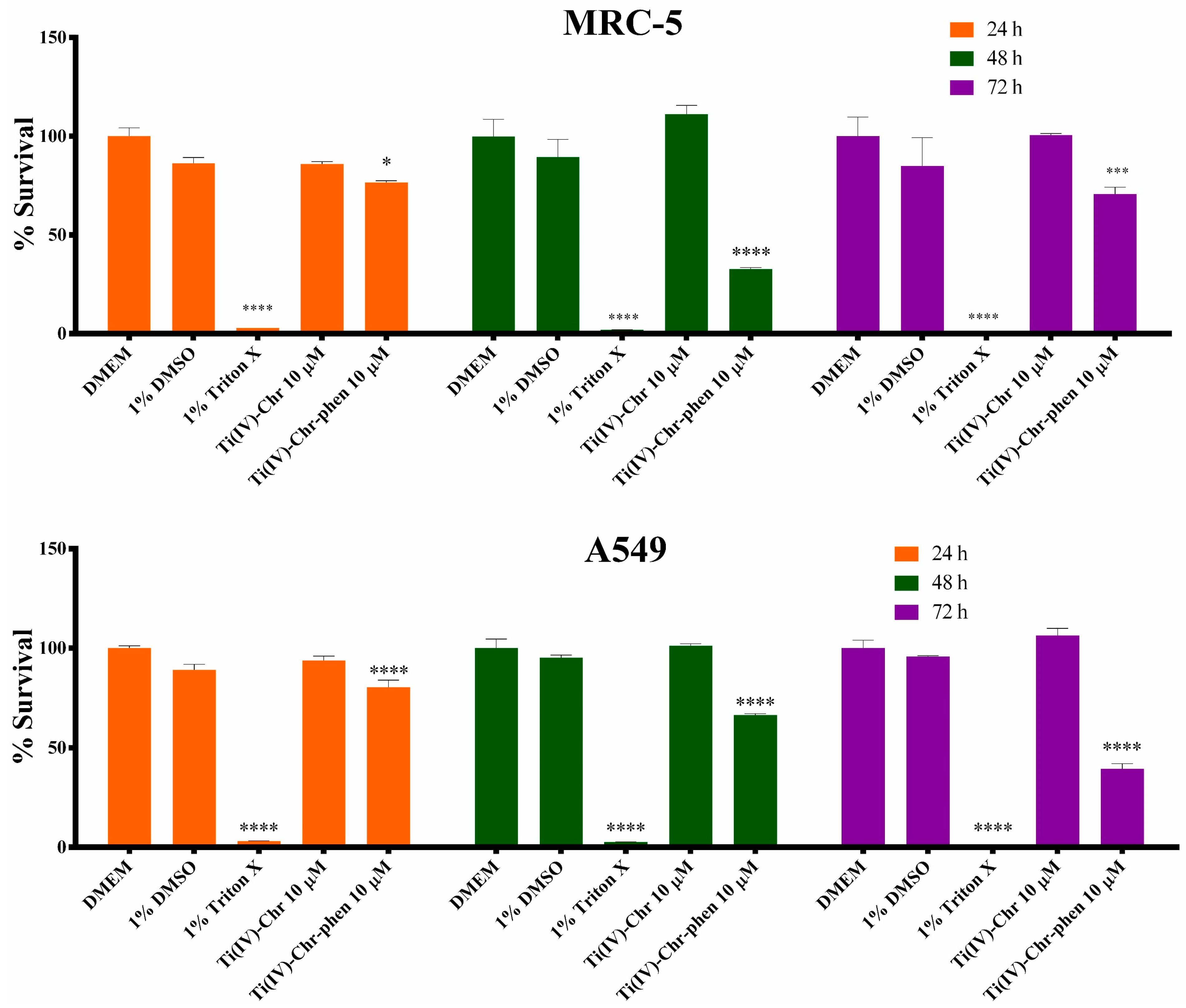
2.12.1. Cell Viability
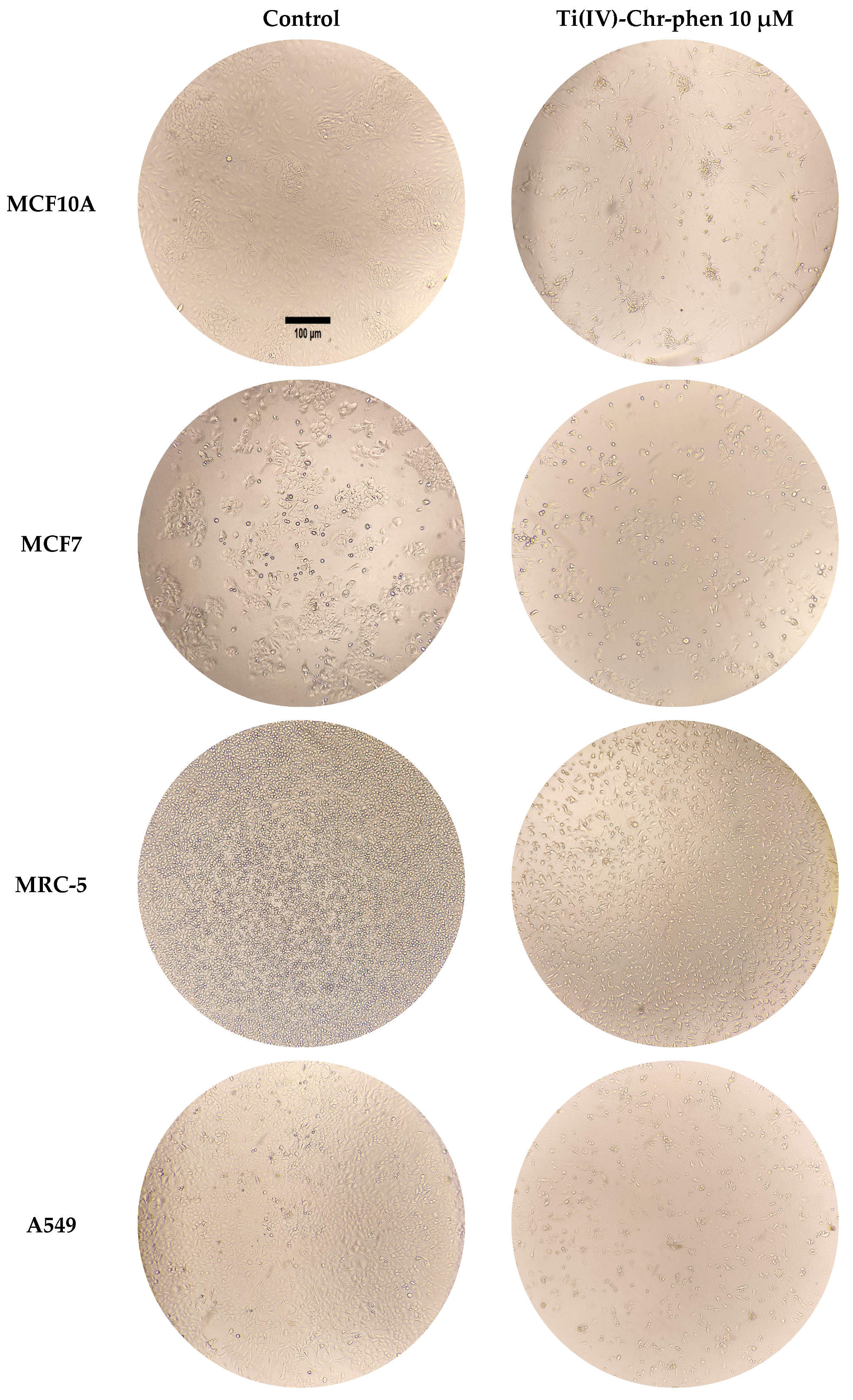
2.12.2. Cell Morphology Studies
3. Discussion
3.1. Structural Speciation of Ti(IV)-Flavonoid Compounds
3.2. Theoretical Interactions and Solid-Solution Properties
3.3. Antibacterial and Anti-Inflammatory Properties
3.4. In Vitro Biological Activity in Breast and Lung Tissues
4. Materials and Methods
4.1. Reagents and General Procedures
4.2. Physical Measurements
4.2.1. ESI-MS Spectrometry
4.2.2. Solution 1H-, 13C-NMR and 2D NMR
4.2.3. Photoluminescence
4.3. Bond Valence Sum
4.4. Hirshfeld Surface Investigation
4.5. Synthesis
4.6. X-Ray Structural Determination
4.7. Antibacterial Properties In Vitro
4.8. Anti-Inflammatory Properties
4.9. In Vitro Toxicity Profile Through Physiological and Cancer Cell Lines
4.9.1. Cell Cultures
4.9.2. Cell Viability Assay
4.9.3. Cell Morphology
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BVS | Bond Valence Sum |
| BSA | Bovine Serum Albumin |
| Chr | Chrysin |
| gCOSY | Gradient Correlation Spectroscopy |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ESI-MS | Electron Spray Ionization Mass Spectrometry |
| FBS | Fetal Bovine Serum |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| IC50 | Half-maximal Inhibitory Concentration |
| gHSQC | Gradient Heteronuclear Single Quantum Coherence |
| MIC | Minimum Inhibitory Concentration |
| NMR | Nuclear Magnetic Resonance Spectroscopy |
| phen | 1,10-Phenanthroline |
| PBS | Phosphate Buffer Saline |
| PRESAT | Presaturation |
| SD | Standard deviation |
| SEM | Standard error mean |
| Et3N | Triethylamine |
| UV–Visible | Ultraviolet–Visible Spectroscopy |
| XTT | 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide |
| ZOI | Zone Of Inhibition |
References
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best practice & research. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Zhang, W.; Ruan, J.; Cheng, J.; Wang, Y.; Zheng, Y.; Lin, M.; Zhang, Y.; Wang, T. In vitro anti-inflammatory terpenoid glycosides from the seeds of dolichos lablab. Molecules 2025, 30, 1779. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131–134. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Goldkind, L.; Laine, L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: Lessons learned from the bromfenac experience. Pharmacoepidemiol. Drug Saf. 2006, 15, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Acharya, Y.; Taneja, K.K.; Haldar, J. Dual functional therapeutics: Mitigating bacterial infection and associated inflammation. RSC Med. Chem. 2023, 14, 1410–1428. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, absorption, metabolism and advanced pharmaceutical technology for improving bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Sokal, A.; Mruczek, P.; Niedoba, M.; Dewalska, A.; Stocerz, K.; Kadela-Tomanek, M. Anticancer activity of ether derivatives of chrysin. Molecules 2025, 30, 960. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef] [PubMed]
- Loginova, N.V.; Harbatsevich, H.I.; Osipovich, N.P.; Ksendzova, G.A.; Koval’chuk, T.V.; Polozov, G.I. Metal complexes as promising agents for biomedical applications. Current Med. Chem. 2020, 27, 5213–5249. [Google Scholar] [CrossRef] [PubMed]
- Yasir, K.H.; Parveen, S.; Yousuf, I.; Tabassum, S.; Arjmand, F. Metal complexes of NSAIDs as potent anti-tumor chemotherapeutics: Mechanistic insights into cytotoxic activity via multiple pathways primarily by inhibition of COX–1 and COX–2 enzymes. Coord. Chem. Rev. 2022, 453, 214316. [Google Scholar] [CrossRef]
- Halevas, E.; Matsia, S.; Hatzidimitriou, A.; Geromichalou, E.; Papadopoulos, T.A.; Katsipis, G.; Pantazaki, A.; Litsardakis, G.; Salifoglou, A. A unique ternary Ce(III)-quercetin-phenanthroline assembly with antioxidant and anti-inflammatory properties. J. Inorg. Biochem. 2022, 235, 111947. [Google Scholar] [CrossRef]
- Matsia, S.; Papadopoulos, A.; Hatzidimitriou, A.; Schumacher, L.; Koldemir, A.; Pöttgen, R.; Panagiotopoulou, A.; Chasapis, C.T.; Salifoglou, A. Hybrid lanthanide metal–organic compounds with flavonoids: Magneto-optical properties and biological activity profiles. Int. J. Mol. Sci. 2025, 26, 1198. [Google Scholar] [CrossRef]
- Tsave, O.; Salifoglou, A. Biomimetic activity of soluble, well-defined, aqueous Ti(IV)-citrate species toward adipogenesis. An in vitro study. J. Inorg. Biochem. 2021, 214, 111290. [Google Scholar] [CrossRef] [PubMed]
- Elie, B.T.; Fernández-Gallardo, J.; Curado, N.; Cornejo, M.A.; Ramos, J.W.; Contel, M. Bimetallic titanocene-gold phosphane complexes inhibit invasion, metastasis, and angiogenesis-associated signaling molecules in renal cancer. Eur. J. Med. Chem. 2019, 161, 310–322. [Google Scholar] [CrossRef]
- Etsè, K.S.; Harrad, M.A.; Etsè, K.D.; Zaragoza, G.; Demonceau, A.; Mouithys-Mickalad, A. Free radical scavenging activity and inhibition of enzyme-catalyzed oxidation by trans-aryl-Palladium complexes. Molecules 2025, 30, 1122. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. B Struct. Sci. 2004, 60 Pt 6, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A. DFT and 1H NMR molecular spectroscopic studies on biologically anti-oxidant active paramagnetic lanthanide(III)-chrysin complexes. Main. Group. Chem. 2008, 7, 43–56. [Google Scholar] [CrossRef]
- Matsia, S.; Lazopoulos, G.; Hatzidimitriou, A.; Reimann, M.K.; Pöttgen, R.; Salifoglou, A. Chemical reactivity profile of rare earth metal ions with flavonoids. From structural speciation to magneto-optical properties. Polyhedron 2023, 234, 116231. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Pelecanou, M.; Hatzidimitriou, A. Structurally characterized zinc complexes of flavonoids chrysin and quercetin with antioxidant potential. Inorg. Chim. Acta 2021, 523, 14–25. [Google Scholar] [CrossRef]
- Ystenes, M.; Rytter, E. Fourier transform infrared spectra of three titanium tetrachloride-ethyl benzoate complexes. Assignment based on five isotopic homologues and extension of the ethyl benzoate force field. Spectrochim. Acta Part. A Mol. Spectrosc. 1992, 48, 543–555. [Google Scholar] [CrossRef]
- Kaushal, R.; Kumar, N.; Chaudhary, A.; Arora, S.; Awasthi, P. Synthesis, spectral characterization, and antiproliferative studies of mixed ligand titanium complexes of adamantylamine. Bioinorg. Chem. Appl. 2014, 2014, 142828. [Google Scholar] [CrossRef]
- Alem, M.B.; Desalegn, T.; Damena, T.; Bayle, E.A.; Koobotse, M.O.; Ngwira, K.J.; Ombito, J.O.; Zachariah, M.; Demissie, T.B. Organic-inorganic hybrid salt and mixed ligand Cr(III) complexes containing the natural flavonoid chrysin: Synthesis, characterization, computational, and biological studies. Front. Chem. 2023, 11, 1173604. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Antonoglou, O.; Hatzidimitriou, A.; Sagnou, M.; Pantazaki, N.; Litsardakis, G.; Pelecanou, M. Structurally characterized gallium-chrysin complexes with anticancer potential. Dalton Trans. 2020, 49, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Khitrov, G.A.; Strouse, G.F.; Gaumet, J.-J. Characterization of Ti6O4(O2C4H5)8(OCH2CH3)8 by electrospray time of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 260–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, S.H.; Kim, H.Y.; Zuo, G.; Wang, Z.; Lee, J.-Y.; Lim, S.S. Anti-glycation, carbonyl trapping and anti-inflammatory activities of chrysin derivatives. Molecules 2018, 23, 1752. [Google Scholar] [CrossRef]
- Oggero, J.; Gasser, F.B.; Zacarías, S.M.; Burns, P.; Baravalle, M.E.; Renna, M.S.; Ortega, H.H.; Vaillard, S.E.; Vaillard, V.A. PEGylation of Chrysin improves its water solubility while preserving the in vitro biological activity. J. Agric. Food Chem. 2023, 71, 19817–19831. [Google Scholar] [CrossRef]
- Shrestha, A.; Pandey, R.P.; Dhakal, D.; Parajuli, P.; Sohnget, J.K. Biosynthesis of flavone C-glucosides in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 1251–1267. [Google Scholar] [CrossRef] [PubMed]
- Pusz, J. The Titanium(IV), Iron(III) and Manganese(II) complexes of Chrysin-4′-sulfonate. Pol. J. Chem. 2001, 75, 795–801. [Google Scholar]
- Dimitrov, G.D.; Atanassova, M.S. Synthesis and spectroscopic characterization of a complex of 1,10-phenanthroline with magnesium. Z. Anorg. Allg. Chem. 2003, 629, 12–14. [Google Scholar] [CrossRef]
- Pazderski, L.; Pawlak, T.; Sitkowski, J.; Kozerski, L.; Szłyk, E. 1H NMR assignment corrections and 1H, 13C, 15N NMR coordination shifts structural correlations in Fe(II), Ru(II) and Os(II) cationic complexes with 2,2′-bipyridine and 1,10-phenanthroline. Magn. Reson. Chem. 2010, 48, 450–457. [Google Scholar] [CrossRef]
- Meléndez, E. Titanium complexes in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 309–315. [Google Scholar] [CrossRef]
- Petanidis, S.; Kioseoglou, E.; Hadzopoulou-Cladaras, M.; Salifoglou, A. Novel ternary vanadium-betaine-peroxido species suppresses H-ras and matrix metalloproteinase-2 expression by increasing reactive oxygen species-mediated apoptosis in cancer cells. Cancer Lett. 2013, 335, 387–396. [Google Scholar] [CrossRef]
- Halevas, E.; Tsave, O.; Yavropoulou, M.P.; Hatzidimitriou, A.; Yovos, J.G.; Psycharis, V.; Gabriel, C.; Salifoglou, A. Design, synthesis and characterization of novel binary V(V)-Schiff base materials linked with insulin-mimetic vanadium-induced differentiation of 3T3-L1 fibroblasts to adipocytes. Structure–function correlations at the molecular level. J. Inorg. Biochem. 2015, 147, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Venetis, J.; Kaliva, M.; Raptopoulou, C.P.; Terzis, A.; Drouza, C.; Meier, B.; Voyiatzis, G.; Potamitis, C.; Salifoglou, A. Probing for missing links in the binary and ternary V(V)–citrate–(H2O2) systems: Synthetic efforts and in vitro insulin mimetic activity studies. J. Inorg. Biochem. 2009, 103, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Tsave, O.; Yavropoulou, M.P.; Kafantari, M.; Gabriel, C.; Yovos, J.G.; Salifoglou, A. Comparative assessment of metal-specific adipogenic activity in zinc and vanadium-citrates through associated gene expression. J. Inorg. Biochem. 2018, 186, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Faraji, F.; Jafarpour, S.; Faraji, F.; Rasoulpoor, S.; Dokaneheifard, S.; Mohammadi, M. Anti-cancer activity of Chrysin in cancer therapy: A systematic review. Indian. J. Surg. Oncol. 2022, 13, 681–690. [Google Scholar] [CrossRef]
- Fu, B.; Xue, J.; Li, Z.; Shi, X.; Jiang, B.-H.; Fang, J. Chrysin inhibits expression of hypoxia-inducible factor-1α through reducing hypoxia-inducible factor-1α stability and inhibiting its protein synthesis. Mol. Cancer Ther. 2007, 6, 220–226. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, X.; Long, J.; Li, R.; Liu, Y.; Yang, Z.; Zheng, X. Fluorine-containing chrysin derivatives: Synthesis and biological activity. Nat. Prod. Commun. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Moghadam, E.R.; Ang, H.L.; Asnaf, S.E.; Zabolian, A.; Saleki, H.; Yavari, M.; Esmaeili, H.; Zarrabi, A.; Ashrafizadeh, M.; Kumar, A.P. Broad-spectrum preclinical antitumor activity of chrysin: Current trends and future perspectives. Biomolecules 2020, 10, 1374. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. Microbial carcinogenic toxins and dietary anti-cancer protectants. Cell. Mol. Life Sci. 2017, 74, 2627–2643. [Google Scholar] [CrossRef]
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Jair, B.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Hadar, K.; et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res. Ther. 2015, 6, 87. [Google Scholar] [CrossRef]
- Fukata, M.; Michelsen, K.S.; Eri, R.; Thomas, L.S.; Hu, B.; Lukasek, K.; Nast, C.C.; Lechago, J.; Xu, R.; Naiki, Y.; et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1055–G1065. [Google Scholar] [CrossRef]
- Guo, M.; Guo, Z.; Sadler, P.J. Titanium(IV) targets phosphoesters on nucleotides: Implications for the mechanism of action of the anticancer drug titanocene dichloride. J. Biol. Inorg. Chem. 2001, 6, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, A.; Claffey, J.; Tacke, M. Binding and hydrolysis studies of antitumoural titanocene dichloride and Titanocene Y with phosphate diesters. J. Inorg. Biochem. 2010, 104, 390–396. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, J.; Xiao, K.; Tian, Z. Enrichment of intact phosphoproteins using immobilized titanium(IV) affinity chromatography microspheres. Sep. Sci. Plus 2018, 1, 93–99. [Google Scholar] [CrossRef]
- Christodoulou, C.V.; Eliopoulos, A.G.; Young, L.S.; Hodgkins, L.; Ferry, D.R.; Kerr, D.J. Anti-proliferative activity and mechanism of action of titanocene dichloride. Br. J. Cancer 1998, 77, 2088–2097. [Google Scholar] [CrossRef]
- Olszewski, U.; Deally, A.; Tacke, M.; Hamilton, G. Alterations of phosphoproteins in NCI-H526 small cell lung cancer cells involved in cytotoxicity of cisplatin and titanocene Y. Neoplasia 2012, 14, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Cryst. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17.5; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Clausen, H.F.; Chevallier, M.S.; Spackman, M.A.; Iversen, B.B. Three new co-crystals of hydroquinone: Crystal structures and Hirshfeld surface analysis of intermolecular interactions. New J. Chem. 2010, 34, 193–199. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Comm. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Parkin, A.; Barr, G.; Dong, W.; Gilmore, C.J.; Jayatilaka, D.; McKinnon, J.J.; Spackman, M.A.; Wilson, C.C. Comparing entire crystal structures: Structural genetic fingerprinting. Cryst. Eng. Comm. 2007, 9, 648–652. [Google Scholar] [CrossRef]
- Rohl, A.L.; Moret, M.; Kaminsky, W.; Claborn, K.; McKinnon, J.J.; Kahr, B. Hirshfeld surfaces identify inadequacies in computations of intermolecular interactions in crystals: Pentamorphic 1,8-dihydroxyanthraquinone. Cryst. Growth Des. 2008, 8, 4517–4525. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R.J. Tables of bond lengths determined by X-Ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Bruker Analytical X–Ray Systems, Inc. Apex2, Version 2 User Manual; M86–E01078; Bruker: Madison, WI, USA, 2006. [Google Scholar]
- Siemens Industrial Automation Inc. SADABS: Area–Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Crystallogr. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Elmehrath, S.; Ahsan, K.; Munawar, N.; Alzamly, A.; Nguyen, H.L.; Greish, Y. Antibacterial efficacy of copper-based metal–organic frameworks against Escherichia coli and Lactobacillus. RSC Adv. 2024, 14, 15821–15831. [Google Scholar] [CrossRef]
- Munir, M.T.; Pailhories, H.; Eveillard, M.; Irle, M.; Aviat, F.; Dubreil, L.; Federighi, M.; Belloncle, C. Testing the antimicrobial characteristics of wood materials: A Review of methods. Antibiotics 2020, 9, 225. [Google Scholar] [CrossRef]
- Smeriglio, A.; D’Angelo, V.; Cacciola, A.; Ingegneri, M.; Raimondo, F.M.; Trombetta, D.; Germanò, M.P. New insights on phytochemical features and biological properties of Alnus glutinosa Stem Bark. Plants 2022, 11, 2499. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and anti-inflammatory activity of citrus flavanones mix and its stability after in vitro simulated digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef]
- Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-inflammatory and wound healing properties of leaf and rhizome extracts from the medicinal plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules 2022, 27, 4271. [Google Scholar] [CrossRef]
- Cornara, L.; Ambu, G.; Alberto, A.; Trombetta, D.; Smeriglio, A. Characterization of ingredients incorporated in the traditional mixed-salad of the capuchin monks. Plants 2022, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, anti-inflammatory and anti-angiogenic properties of citrus lumia juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef] [PubMed]
- Lazopoulos, G.; Matsia, S.; Maroulis, M.; Salifoglou, A. Cornus mas L. extracts exhibit neuroprotective properties, further enhanced by metal-bound energy-linked organic substrates. Int. J. Mol. Sci. 2025, 26, 1159. [Google Scholar] [CrossRef] [PubMed]



| Compound | 1 | 2 |
|---|---|---|
| Chemical formula | C19H24Cl2O8Ti | C125H116Cl4N8O27Ti4 |
| Mr | 499.20 | 2495.74 |
| Crystal system, space group | Monoclinic P21/n | Tetragonal P42/n |
| Temperature (K) | 295 | 295 |
| a (Å) b (Å) c (Å) | 7.5536 (6) 13.4847 (10) 22.4709 (17) | 22.231 (6) 22.231 (6) 13.361 (5) |
| β (°) | 90.322 (3) | 90 |
| V (Å3) | 2288.8 (3) | 6603 (4) |
| Z | 4 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 0.65 | 0.39 |
| Crystal size (mm) | 0.19 × 0.18 × 0.12 | 0.16 × 0.04 × 0.03 |
| Data collection | ||
| Diffractometer | Bruker Kappa Apex2 | Bruker Kappa Apex2 |
| Absorption correction | Numerical | Numerical |
| Tmin, Tmax | 0.89, 0.93 | 0.98, 0.99 |
| No. of reflections measured independent observed [I > 2.0σ(I)] | 19,253 4344 3556 | 33,909 6381 3889 |
| Rint | 0.019 | 0.036 |
| (sin θ/λ)max (Å−1) | 0.612 | 0.615 |
| Refinement | ||
| R[F2 > 2σ(F2)] wR(F2) S | 0.034 0.053 1.00 | 0.056 0.104 1.00 |
| No. of reflections | 3556 | 3889 |
| No. of parameters | 271 | 381 |
| No. of restraints | - | 11 |
| H-atom treatment | H-atom parameters constrained | |
| Δρmax, Δρmin (e Å−3) | 0.26, −0.25 | 0.72, −0.42 |
| 1 | 2 | |||
|---|---|---|---|---|
| Distances (Å) | Ti(1)—Cl(1) | 2.2957 (8) | Ti(1)—O(3) i | 1.804 (3) |
| Ti(1)—O(1) | 2.0351 (14) | Ti(1)—O(1) | 2.087 (3) | |
| Ti(1)—O(2) | 1.8638 (15) | Ti(1)—O(2) | 1.889 (2) | |
| Ti(1)—O(5) | 1.7397 (15) | Ti(1)—O(3) | 1.809 (2) | |
| Ti(1)—O(6) | 2.1005 (17) | Ti(1)—N(1) | 2.234 (3) | |
| Ti(1)—O(7) | 2.0628 (15) | Ti(1)—N(2) | 2.183 (3) | |
| Bond angles (o) | Cl(1)—Ti(1)—O(1) | 91.49 (5) | O(3) i—Ti(1)—O(1) | 172.47 (11) |
| Cl(1)—Ti(1)—O(2) | 95.19 (6) | O(3) i—Ti(1)—O(2) | 98.00 (11) | |
| O(1)—Ti(1)—O(2) | 86.56 (6) | O(1)—Ti(1)—O(2) | 86.25 (11) | |
| Cl(1)—Ti(1)—O(5) | 98.91 (6) | O(3) i—Ti(1)—O(3) | 100.17 (15) | |
| O(1)—Ti(1)—O(5) | 169.56 (7) | O(1)—Ti(1)—O(3) | 85.04 (11) | |
| O(2)—Ti(1)—O(5) | 93.31 (7) | O(2)—Ti(1)—O(3) | 100.88 (11) | |
| Cl(1)—Ti(1)—O(6) | 168.31 (5) | O(3) i—Ti(1)—N(1) | 88.30 (11) | |
| O(1)—Ti(1)—O(6) | 79.20 (7) | O(1)—Ti(1)—N(1) | 85.20 (11) | |
| O(2)—Ti(1)—O(6) | 91.33 (7) | O(2)—Ti(1)—N(1) | 93.84 (12) | |
| O(5)—Ti(1)—O(6) | 90.37 (7) | O(3)—Ti(1)—N(1) | 161.74 (11) | |
| Cl(1)—Ti(1)—O(7) | 88.84 (5) | O(3) i—Ti(1)—N(2) | 92.02 (11) | |
| O(1)—Ti(1)—O(7) | 86.09 (6) | O(1)—Ti(1)—N(2) | 82.43 (11) | |
| O(2)—Ti(1)—O(7) | 171.71 (7) | O(2)—Ti(1)—N(2) | 163.06 (12) | |
| O(5)—Ti(1)—O(7) | 93.22 (7) | O(3)—Ti(1)—N(2) | 90.68 (11) | |
| O(6)—Ti(1)—O(7) | 83.53 (7) | N(1)—Ti(1)—N(2) | 72.74 (11) | |
| Ti(1)—O(3)—Ti(1) ii | 162.50 (14) | |||
| Mass Content (mg)/Mass Content/Unit Area (mg/cm2) ZOI (mm) | Mass Content (mg)/Mass Content/Unit Area (mg/cm2) ZOI (mm) | |||
| E. coli | Ti(IV)-Chr | 0.50 mg (1.8 mg/cm2) 31.5 ± 0.1 | Ti(IV)-Chr-phen | 0.080 mg (0.28 mg/cm2) 27.5 ± 0.1 |
| TiO2 | 0.080 mg (0.28 mg/cm2) 29.7 ± 0.2 | TiO2 | 0.010 mg (0.035 mg/cm2) 26.5 ± 0.1 | |
| Chr | 0.25 mg (0.88 mg/cm2) n.e.z. | Chr | 0.040 mg (0.14 mg/cm2) n.e.z. | |
| phen | 0.030 mg (0.11 mg/cm2) 20.5 ± 0.3 | |||
| Mass Content (mg)/Mass Content/Unit Area (mg/cm2) ZOI (mm) | Mass Content (mg)/Mass Content/Unit Area (mg/cm2) ZOI (mm) | |||
| S. aureus | Ti(IV)-Chr | 10 mg (35 mg/cm2) 13.5 ± 0.2 | Ti(IV)-Chr-phen | 10 mg (35 mg/cm2) 15.3 ± 0.1 |
| TiO2 | 1.5 mg (5.3 mg/cm2) n.e.z. | TiO2 | 1.5 mg (5.3 mg/cm2) n.e.z. | |
| Chr | 5.0 mg (17 mg/cm2) n.e.z. | Chr | 4.6 mg (16 mg/cm2) n.e.z. | |
| phen | 3.3 mg (12 mg/cm2) 30.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsia, S.; Lazopoulos, G.; Hatzidimitriou, A.; Salifoglou, A. Structural Speciation of Hybrid Ti(IV)-Chrysin Systems—Biological Profiling and Antibacterial, Anti-Inflammatory, and Tissue-Specific Anticancer Activity. Molecules 2025, 30, 3667. https://doi.org/10.3390/molecules30183667
Matsia S, Lazopoulos G, Hatzidimitriou A, Salifoglou A. Structural Speciation of Hybrid Ti(IV)-Chrysin Systems—Biological Profiling and Antibacterial, Anti-Inflammatory, and Tissue-Specific Anticancer Activity. Molecules. 2025; 30(18):3667. https://doi.org/10.3390/molecules30183667
Chicago/Turabian StyleMatsia, Sevasti, Georgios Lazopoulos, Antonios Hatzidimitriou, and Athanasios Salifoglou. 2025. "Structural Speciation of Hybrid Ti(IV)-Chrysin Systems—Biological Profiling and Antibacterial, Anti-Inflammatory, and Tissue-Specific Anticancer Activity" Molecules 30, no. 18: 3667. https://doi.org/10.3390/molecules30183667
APA StyleMatsia, S., Lazopoulos, G., Hatzidimitriou, A., & Salifoglou, A. (2025). Structural Speciation of Hybrid Ti(IV)-Chrysin Systems—Biological Profiling and Antibacterial, Anti-Inflammatory, and Tissue-Specific Anticancer Activity. Molecules, 30(18), 3667. https://doi.org/10.3390/molecules30183667










