The Presence of Micro- and Nanoplastics in Food and the Estimation of the Amount Consumed Depending on Dietary Patterns
Abstract
1. Introduction
2. Sources of MNPs in the Environment and Pathways of Contamination
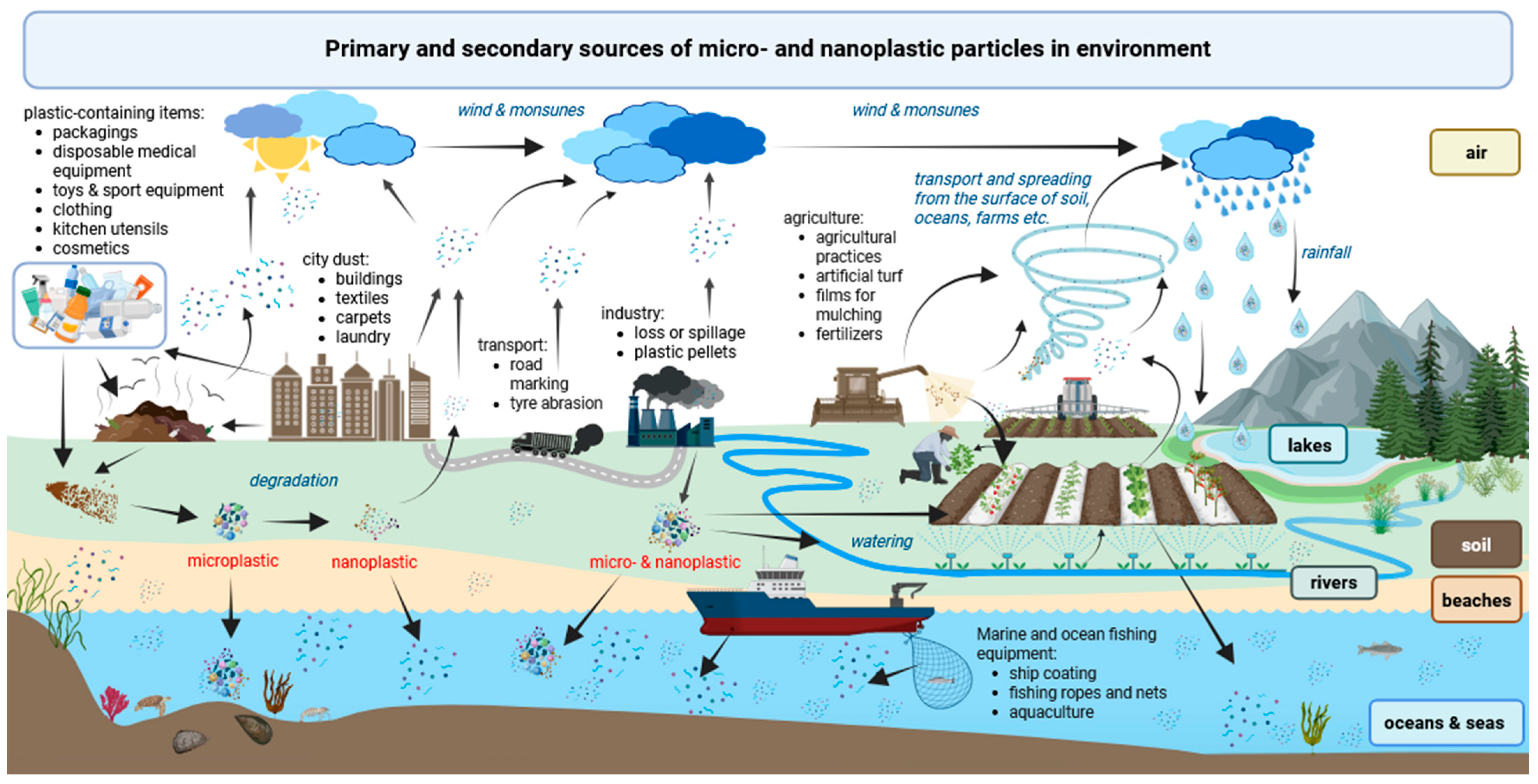
- Mechanical fragmentation of plastic products during normal, daily use or as a result of weathering, abrasion, and pouring of particular objects, especially when improperly recycled (synthetic shoe soles, toys, synthetic kitchen utensils, medical devices, infrastructure components, buildings, equipment, carpets, furniture) [99,121,122,123,124],
- Broken plastic fishing ropes and nets [126],
3. Entry Routes of MNPs into the Human Body
3.1. Inhalation of Airborne Plastic Particles
3.2. Dermal Penetration of Nanoplastic Particles
3.3. Ingestion of MNPs
4. Mechanisms of Food Contamination
4.1. Kitchen Accessories, Food Preparation, and Food Packaging as a Source of MNPs in Meals
4.2. Plastics Particles Identified in Various Types of Food
4.3. Plastic-Associated Chemicals and Other Contaminants
5. Food Pyramid as a Graphical Representation of FDBG and Various Dietary Patterns
- Western Europe (WE): Austria, Belgium, France, Germany, Ireland, Luxembourg, Liechtenstein, the Netherlands, Switzerland, the United Kingdom (Table S1 in Supplementary Material);
- Nordic and Baltic countries (NBC): Denmark, Finland, Sweden, Iceland, Norway, Estonia, Latvia, and Lithuania (Table S2);
- Central and Eastern Europe (CEE): Belarus, Bulgaria, Czech Republic, Hungary, Poland, Romania, Slovakia, Serbia, North Macedonia, Moldova, Slovenia, Bosnia and Herzegovina, Ukraine (Table S3);
- Southern Europe (SE): Albania, Andorra, Greece, Italy, Malta, Monaco, Portugal, Spain, Croatia, Montenegro, San Marino, and Turkey (Table S4).
6. Assessment of the MNP Intake Depending on Dietary Patterns
6.1. Methodology Used for the Assessment
6.2. MNP Intake According to Three Different Dietary Patterns in Europe

| No | Food Group | Daily Amount | Estimated Energy (kcal) | Energy Percentage (%) | Justification/Comments |
|---|---|---|---|---|---|
| 1 | Vegetables | 400 g | 100 | 5.0 | Minimum 4 servings of raw and cooked vegetables per day, which have high volume, but low calories. |
| 2 | Fruit | 200 g | 90 | 4.5 | 2–3 servings (½ cup per portion) per day of fresh, preferably seasonal, colourful fruits, mainly berries. |
| 3 | Cereals and grain products | 250 g | 825 | 41.2 | Main source of energy included whole-grain bread, rice, and pasta. |
| 4 | Fats | 35 g | 309 | 15.5 | The fats consumed are olive oil and other vegetable oils that are rich in monounsaturated fatty acids. |
| 5 | Fish and seafood | 35 g | 100 | 5.0 | It is a source of high-quality protein: 2–3 servings (200–300 g) per week are recommended. |
| 6 | Legumes and nuts | 30 g + 20 g | 90 + 100 | 9.5 | A source of protein and fibre, as well as healthy fats from nuts, is recommended at 2–3 servings per day. |
| 7 | Dairy | 300 g | 225 | 11.2 | It is a source of calcium and protein: 2–3 servings per day, mainly milk, yoghurt, curd, and ripened cheese, but for ripened cheeses, only one slice per day is recommended. |
| 8 | Eggs | 55 g | 75 | 3.8 | Few eggs per week are recommended, including those in pasta or bread. |
| 9a | Meat | 43 g | 86 | 4.3 | Limited consumption is recommended several times a week (no more than 500 g/week), with poultry and lean meat being preferable to red meat and processed meat. |
| 10a | Water (tap water) | 1000 dm3 | 0 | 0 | No energy, but a carrier of MNPs. |
| 10b | Water (bottled) | 1000 dm3 | 0 | 0 | No energy, but a carrier of MNPs. |
| 11 | Salt | 5 g | 0 | 0 | The recommended amount is less than 5 g of salt (2 g of Na) per day. |
| 12 | Sugar | 0 g | 0 | 0 | There is no added sugar in the diet; only carbohydrates that are present naturally in the raw materials. |
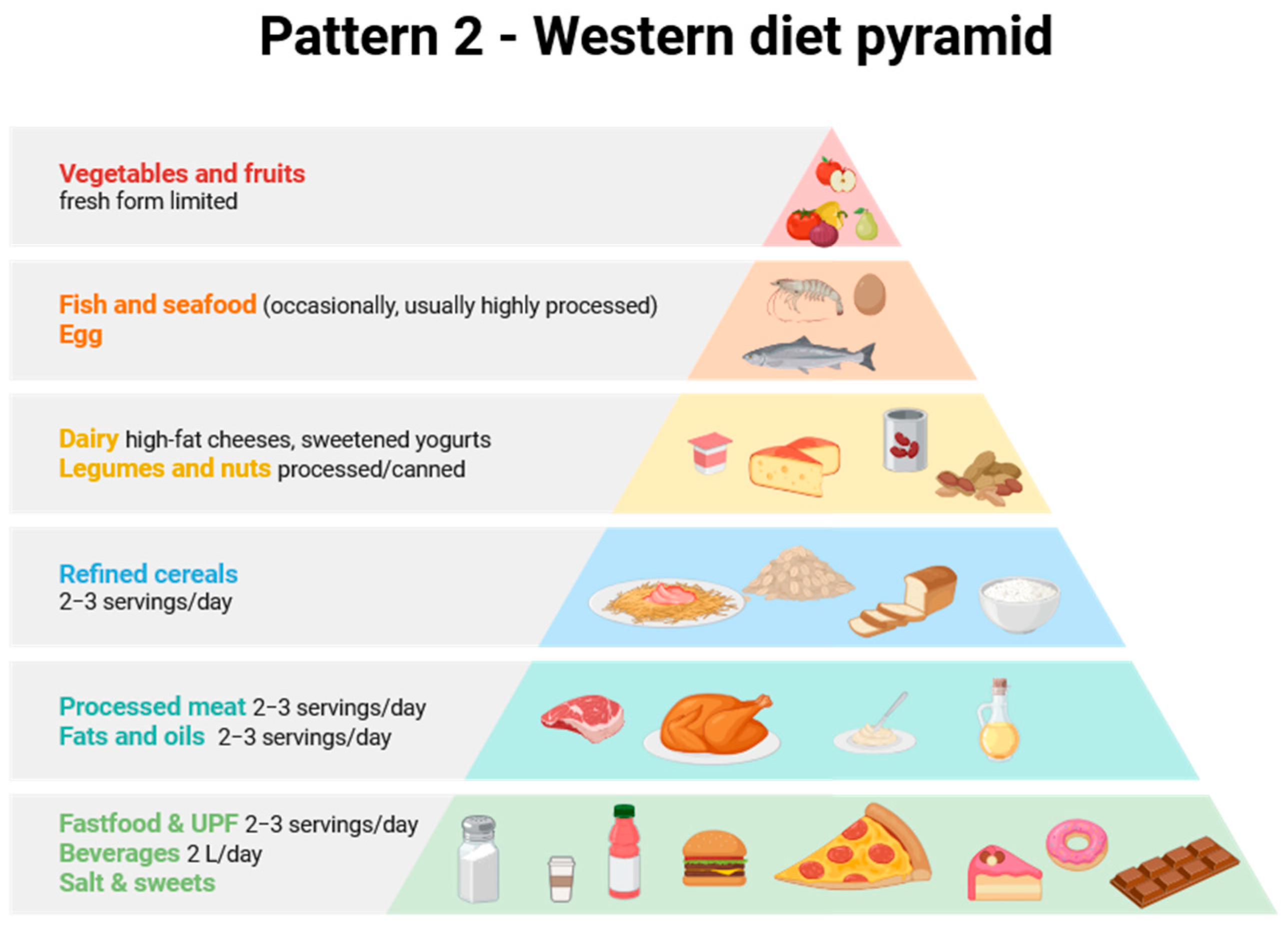
| No | Food Group | Daily Amount | Estimated Energy (kcal) | Energy Percentage (%) | Justification/Comments |
|---|---|---|---|---|---|
| 1 | Vegetables | 50 g | 12.5 | 0.6 | Very small quantities, often negligible, consumption significantly below recommendations. |
| 2 | Fruit | 50 g | 22.5 | 1.1 | Very small quantities, often negligible, provided mainly in the form of processed fruit (canned or dried). Fruit juices have been included in group 12. |
| 3 | Cereals and grain products | 80 g | 160 | 8.0 | Mainly in the form of refined grains, white (wheat) rolls/bread, white rice, pasta, and sweet breakfast cereals. |
| 4 | Fats | 70 g | 560 | 28.0 | The fats consumed are mainly butter, animal fats, frying fat, and palm oil, which are rich in saturated and trans fatty acids. Can provide up to 40% of energy. |
| 5 | Fish and seafood | 6,4 | 18 | 0.9 | Very small quantities, often negligible in daily diet. Often in the form of canned fish. |
| 6 | Legumes and nuts | 10 g + 10 g | 30 + 50 | 4.0 | Mainly in the form of processed foods (canned) and sweet/salted snacks. |
| 7 | Dairy | 40 g | 140 | 7.0 | Mainly high-fat melt-type cheese, cream, ready-made sweetened yoghurt, or milk powder. It is a source of calcium, protein, and fat. |
| 8 | Eggs | 25 g | 35 | 1.8 | Average consumption of ½ egg per day, usually eaten in processed foods or breakfast. |
| 9b | Meat and UPF | 260 g | 650 | 32.5 | High consumption of red meat and processed meats (e.g., sausages and bacon), as well as fast food, takeaways, and ready meals. This is a significant source of fat and salt. Meat and UPFs can provide up to 60% of energy. |
| 10a | Water (tap water) | 1000 dm3 | 0 | 0 | No energy, but a carrier of MNPs. |
| 10c | Bottled beverages | 1000 dm3 | 0 | 0 | Includes fruit juices, carbonated and energy drinks, takeaway coffee and tea, sugar-free and sweetened (sugar was reported in group 12). |
| 11 | Salt | 10 g | 0 | 0 | High consumption as a result of processed food, salted snacks, and meat consumption. |
| 12 | Sugar | 80.5 g | 322 | 16.1 | Sugar added to sweets, energy snacks, chocolate bars, ice cream, and additives. |
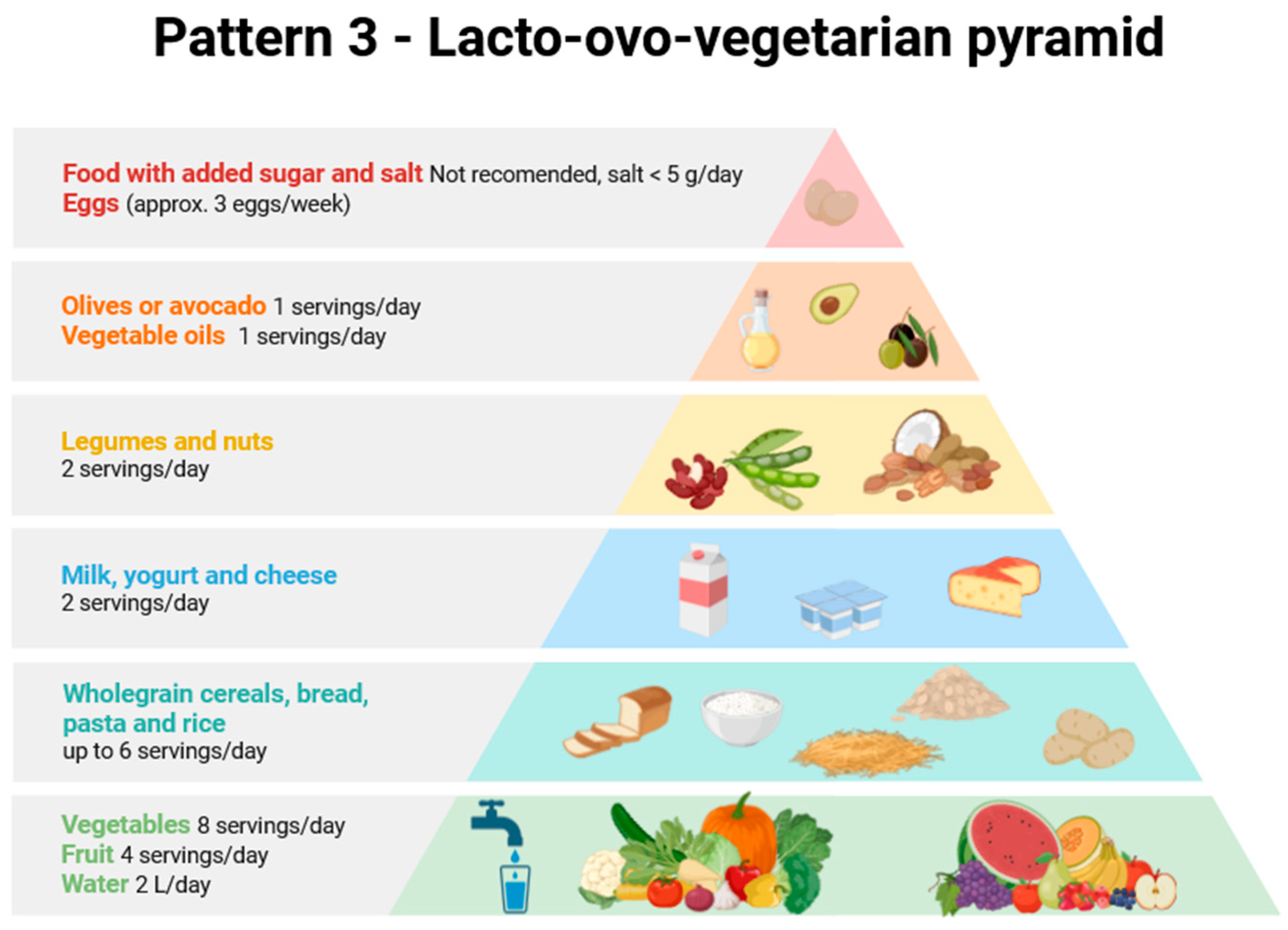
| No | Food Group | Daily Amount | Estimated Energy (kcal) | Energy Percentage (%) | Justification/Comments |
|---|---|---|---|---|---|
| 1 | Vegetables | 400 g | 100.0 | 5.0 | Low calorie, high volume; approx. 8 servings (½ cup each) of raw and cooked vegetables per day. |
| 2 | Fruit | 500 g | 225 | 11.2 | Approx. 4 servings per day such as 1 cup of fresh and seasonal, colourful fruits, mainly berries; ¾ cup of fruit juice; or ¼ cup of dried fruits. |
| 3 | Cereals and grain products | 180 g | 594 | 29.7 | Main source of energy. Mainly in the form of whole-grain bread, whole-grain pasta, groats, brown rice, and dry cereal. Six servings per day (approx. 30 g each) are recommended. |
| 4 | Fats | 44 g | 396 | 19.8 | Main source of fat; 2 servings per day of vegetable oils rich in monounsaturated fatty acids (14 g per serving) or avocado (50 g) or olives (100 g). |
| 5 | Fish and seafood | - | - | - | - |
| 6 | Legumes and nuts | 100 g + 30 g | 300 + 150 | 22.5 | Main source of protein and fibre, and healthy fats from nuts. |
| 7 | Dairy | 300 g | 200.0 | 10.0 | Source of calcium and protein; 2 servings per day, mainly milk, yoghurt, white and curd cheese, and rennet-free ripened cheeses. |
| 8 | Eggs | 25 g | 35 | 1.8 | Provide a small amount of animal protein (½ an egg per day). |
| 9 | Meat | - | - | - | - |
| 10a | Water (tap water) | 1000 dm3 | 0 | 0 | No energy, but a carrier of MNPs. |
| 10b | Water (bottled) | 1000 dm3 | 0 | 0 | No energy, but a carrier of MNPs. |
| 11 | Salt | 5 g | 0 | 0 | The recommended amount is less than 5 g of salt (2 g of Na) per day. |
| 12 | Sugar | 0 g | 0 | 0 | There is no added sugar in the diet; only carbohydrates that are present naturally in the raw materials. |
7. Impact of MNPs on Human Health
8. Recommendations for Food Producers and Consumers
- (1)
- In agriculture and horticulture, especially with regard to soils where food crops are grown, we should strive to move away from using plastic film for mulching and sewage sludge for fertilisation. We should also avoid covering fertilisers with plastic.
- (2)
- Vegetables and fruit should be thoroughly rinsed with tap water before consumption.
- (3)
- Whenever possible, one should avoid drinking bottled water and beverages (both in glass and plastic containers), replacing them with tap water, as well as coffee and tea brewed directly in a glass or ceramic cup. Using tea bags or coffee capsules and drinking from plastic takeaway cups should be limited to exceptional situations, such as travelling.
- (4)
- For cooking, baking, and frying, cookware that is suitable for high temperatures and has an undamaged non-stick coating should be used.
- (5)
- Wherever possible, plastic kitchen accessories such as cutting boards, spatulas, beaters, spoons, funnels, strainers, whisks, various bowls, kettles, blenders, and mixers should be replaced with glass, cast iron, metal, or wooden ones.
- (6)
- Ready meals and raw ingredients should only be heated or defrosted in containers designed for use at high temperatures, such as in a microwave oven.
- (7)
- When storing food at home, glass containers are preferable to plastic boxes and bags.
- (8)
- Where possible, it is better to prepare meals yourself than to use takeaways or so-called ‘box diets’, as these generate huge amounts of single-use plastic packaging.
- (9)
- Food producers should stop wrapping individual fruits and vegetables in plastic film or foil.
- (10)
- Retail chains offering fresh goods such as fruit, vegetables, and bread should reduce self-service in these departments to limit the use of individual packaging. Where this is not possible, they should replace plastic packaging with paper or fabric alternatives.
- (11)
- As soon as possible, countries and law-making institutions should introduce a simple system of paid collection of glass and plastic packaging (e.g., vending machines or collection points), with plastic packaging being collected based on the weight of the returned items, without the need to scan each individual item. This will encourage consumers to return plastic bottles to collection points instead of throwing them away, which will increase the recovery of this packaging from the market, facilitate its transport for recycling, and reduce environmental pollution.
- (12)
- Every manufacturer, particularly those of food products, should strive to abandon plastic packaging in favour of paper or glass. If these materials do not provide adequate protection for the product, they should replace non-degradable plastic packaging with biodegradable packaging (e.g., made of polyhydroxyalkanoates or polylactic acid) as soon as possible.
- (13)
- In everyday life, people should focus on buying good-quality clothes that will last as long as possible and thus reduce the number of clothes they own. It is also recommended that dishwashers and washing machines be used efficiently. Increasing the load capacity of washing machines and dishwashers, eliminating pre-washing, and shortening the rinsing cycle are effective ways to reduce the number of MNPs released into the water.
- (14)
- We recommend air-drying clothes instead of using tumble dryers, as these release large amounts of microplastics into the air. This will also help to keep your clothes in good condition for longer and reduce your energy consumption.
- (15)
- Parents and grandparents are advised to limit the number of plastic toys their children have, replacing them with healthier wooden toys and activities that promote children’s development, such as physical activity and family games, and play.
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB | acrylonitrile butadiene |
| ABS | acrylonitrile–butadiene–styrene, acrylonitrile butadiene styrene |
| CA | cellulose acetate |
| CMFs | cellulose microfibres |
| CP | cellophane |
| CPE | chlorinated polyethylene |
| EPS | expanded polystyrene = Styrofoam |
| EVA | ethylene-vinyl acetate |
| FNS | poly(fumaronitrile:styrene) |
| HDPE | high-density polyethylene |
| HDPET | high-density polyethylene terephthalate |
| LDPE | low-density polyethylene |
| MNP/MNPs | micro- and nanoplastic/micro- and nanoplastic particles |
| MP/MPs | microplastic/microplastic particles |
| NP/NPs | nanoplastic/nanoplastic particles |
| PA | polyamide |
| PA6 | polyamide 6, poly(caprolactame) = Nylon 6 |
| PA66 | polyamide 66 = Nylon 66 |
| PAC | polyacrylate |
| PAK | polyalkene |
| PAM | polyacrylamide |
| PAN | polyacrylonitrile |
| PB | polybutadiene |
| PBA | poly(butyl acrylate) |
| PC | polycarbonate |
| PCL | polycaprolactone |
| PE | polyethylene |
| PEA | poly(ethyl acrylate) |
| PEAs | poly(ester-amide)s |
| PFCAs | perfluoroalkyl carboxylic acids |
| PEO | polyethylene oxide |
| PES | generic polyester (textiles) |
| PESU | polyethersulfone |
| PET | polyethylene terephthalate |
| PEVA | poly(ethylene-co-vinyl acetate) |
| PLA | polylactic acid |
| PMMA | poly(methyl methacrylate), polymethyl methacrylate = acrylic glass |
| POM | polyoxymethylene |
| PP | polypropylene |
| PPS | polyphenylene sulfide |
| PS | polystyrene |
| PSU or PSF | polysulfone |
| PTFE | polytetrafluoroethylene = Teflon |
| PTT | polytrimethylene terephthalate |
| PU | polyurethane |
| PVA or PVOH | polyvinyl alcohol |
| PVC | poly(vinyl chloride) = polyvinyl chloride |
| PVS | polyvinyl stearate |
| rPET | recycled PET |
| RY | Rayon |
| SAN | styrene–acrylonitrile |
| SEBS | styrene-ethylene-butylene-styrene |
| TPE | thermoplastic elastomer |
| UPF | ultra-processed food |
| XPS | extruded polystyrene |
References
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Jadhav, E.B.; Sankhla, M.S.; Bhat, R.A.; Bhagat, D.S. Microplastics from Food Packaging: An Overview of Human Consumption, Health Threats, and Alternative Solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100608. [Google Scholar] [CrossRef]
- Dokl, M.; Copot, A.; Krajnc, D.; Fan, Y.V.; Vujanović, A.; Aviso, K.B.; Tan, R.R.; Kravanja, Z.; Čuček, L. Global Projections of Plastic Use, End-of-Life Fate and Potential Changes in Consumption, Reduction, Recycling and Replacement with Bioplastics to 2050. Sustain. Prod. Consum. 2024, 51, 498–518. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful Effects of the Microplastic Pollution on Animal Health: A Literature Review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef]
- Yang, J.; Tu, C.; Li, L.; Li, R.; Feng, Y.; Luo, Y. The Fate of Micro(Nano)Plastics in Soil–Plant Systems: Current Progress and Future Directions. Curr. Opin. Environ. Sci. Health 2023, 32, 100438. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/Nanoplastic Toxicity in Plants: An Imminent Concern. Environ. Monit. Assess. 2023, 195, 27. [Google Scholar] [CrossRef]
- Qin, F.; Du, J.; Gao, J.; Liu, G.; Song, Y.; Yang, A.; Wang, H.; Ding, Y.; Wang, Q. Bibliometric Profile of Global Microplastics Research from 2004 to 2019. Int. J. Environ. Res. Public Health 2020, 17, 5639. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An Overview on Separation, Identification and Characterization of Microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Rani, A. Types and Sources of Microplastics; The Ubiquitous Environment Contaminant: A Review. J. Polym. Mater. 2022, 39, 17–35. [Google Scholar] [CrossRef]
- Vitali, C.; Peters, R.J.B.; Janssen, H.-G.; Nielen, M.W.F. Microplastics and Nanoplastics in Food, Water, and Beverages; Part I. Occurrence. TrAC Trends Anal. Chem. 2023, 159, 116670. [Google Scholar] [CrossRef]
- Mathew, J.T.; Inobeme, A.; Adetuyi, B.O.; Adetunji, C.O.; Popoola, O.A.; Olaitan, F.Y.; Akinbo, O.; Shahnawaz, M.; Oyewole, O.A.; Eniola, K.I.T.; et al. General Introduction of Microplastic: Uses, Types, and Generation. In Microplastic Pollution; Shahnawaz, M., Adetunji, C.O., Dar, M.A., Zhu, D., Eds.; Springer Nature: Singapore, 2024; pp. 3–21. ISBN 978-981-99-8356-8. [Google Scholar]
- Vdovchenko, A.; Resmini, M. Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7074. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Saravana Kumar, B.K.; Vetrivel, A.; Michael, R.J.; Babu, N.; Nallamuthu, S.S.; Saravanan, K.; Venkatachalam, S.; R J, N.K.; Selvaraju, G.D.; et al. Nanoplastics in Heat-Sensitive Food Packaging: A Review of Migration, Detection, Health, and Environmental Impacts. Food Control 2025, 169, 111002. [Google Scholar] [CrossRef]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the Human Body: Exposure, Detection, and Risk of Carcinogenesis: A State-of-the-Art Review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Jo, H.H.; Nam, J.; Kim, Y.U.; Kim, S. Microplastic: A Particulate Matter(PM) Generated by Deterioration of Building Materials. J. Hazard. Mater. 2022, 437, 129290. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-Sized Microplastics and Pigmented Particles in Bottled Mineral Water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Moraczewska-Majkut, K.; Nocoń, W.K. Microplastic in Tap Water—Preliminary Tests. Desalination Water Treat. 2022, 275, 116–121. [Google Scholar] [CrossRef]
- Shaw, D.B.; Li, Q.; Nunes, J.K.; Deike, L. Ocean Emission of Microplastic. PNAS Nexus 2023, 2, pgad296. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Piñas, F.; Leganes, F.; Gómez, M.; Martínez, I.; Herrera, A.; Hernández-Sánchez, C.; González-Sálamo, J.; Borges, J.H.; López-Castellanos, J.; et al. A Nationwide Monitoring of Atmospheric Microplastic Deposition. Sci. Total Environ. 2023, 905, 166923. [Google Scholar] [CrossRef]
- Klein, M.; Bechtel, B.; Brecht, T.; Fischer, E.K. Spatial Distribution of Atmospheric Microplastics in Bulk-Deposition of Urban and Rural Environments—A One-Year Follow-up Study in Northern Germany. Sci. Total Environ. 2023, 901, 165923. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as Pollutants in Agricultural Soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Lin, S.Y.; Dungan, A.M.; Colwell, J.M.; Ede, S.; Huerta Lwanga, E.; Meng, K.; Geissen, V.; Blackall, L.L.; Chen, D. Microplastic Pollution Alters Forest Soil Microbiome. J. Hazard. Mater. 2021, 409, 124606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, C.; Gu, Y.; Shi, Y.; Gao, X. Microplastics in Plant-Soil Ecosystems: A Meta-Analysis. Environ. Pollut. 2022, 308, 119718. [Google Scholar] [CrossRef]
- Moeck, C.; Davies, G.; Krause, S.; Schneidewind, U. Microplastics and Nanoplastics in Agriculture—A Potential Source of Soil and Groundwater Contamination? Grundwasser 2023, 28, 23–35. [Google Scholar] [CrossRef]
- Nicole, W. Microplastics in Seafood: How Much Are People Eating? Environ. Health Perspect. 2021, 129, 034001. [Google Scholar] [CrossRef]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar Uptake and Leaf-to-Root Translocation of Nanoplastics with Different Coating Charge in Maize Plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef]
- Gopal, J.; Sivanesan, I.; Muthu, M.; Oh, J.-W. Overviewing the Ground Reality of Microplastic Effects on Seafoods, Including Fish, Shrimps and Crabs: Future Research Directions. Foods 2022, 11, 3976. [Google Scholar] [CrossRef]
- Cáceres-Farias, L.; Espinoza-Vera, M.M.; Orós, J.; Garcia-Bereguiain, M.A.; Alfaro-Núñez, A. Macro and Microplastic Intake in Seafood Variates by the Marine Organism’s Feeding Behaviour: Is It a Concern to Human Health? Heliyon 2023, 9, e16452. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Mo, A.; Jiang, J.; Zhang, Y.; Gao, W.; He, D. Micro(Nano)Plastics in Plant-Derived Food: Source, Contamination Pathways and Human Exposure Risks. TrAC Trends Anal. Chem. 2023, 165, 117138. [Google Scholar] [CrossRef]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food Chain Microplastics Contamination and Impact on Human Health: A Review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Kim, D.; An, S.; Kim, L.; Byeon, Y.M.; Lee, J.; Choi, M.-J.; An, Y.-J. Translocation and Chronic Effects of Microplastics on Pea Plants (Pisum sativum) in Copper-Contaminated Soil. J. Hazard. Mater. 2022, 436, 129194. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and Genotoxicity of Polystyrene Microplastics on Higher Plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Uptake of Microplastics by Carrots in Presence of As (III): Combined Toxic Effects. J. Hazard. Mater. 2021, 411, 125055. [Google Scholar] [CrossRef]
- Yu, Y.; Kumar, M.; Bolan, S.; Padhye, L.P.; Bolan, N.; Li, S.; Wang, L.; Hou, D.; Li, Y. Various Additive Release from Microplastics and Their Toxicity in Aquatic Environments. Environ. Pollut. 2024, 343, 123219. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, H.; Liu, Y.; Jin, L.; Peng, R. Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish. Toxics 2023, 11, 490. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive Toxicity of Polystyrene Microplastics: In Vivo Experimental Study on Testicular Toxicity in Mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liang, Y.; Cheng, Y.; Peng, C.; Sun, Y.; Liu, K.; Li, Y.; Lou, Y.; Jiang, X.; Zhang, A.; et al. Microplastics Cause Reproductive Toxicity in Male Mice through Inducing Apoptosis of Spermatogenic Cells via P53 Signaling. Food Chem. Toxicol. 2023, 179, 113970. [Google Scholar] [CrossRef] [PubMed]
- Djouina, M.; Vignal, C.; Dehaut, A.; Caboche, S.; Hirt, N.; Waxin, C.; Himber, C.; Beury, D.; Hot, D.; Dubuquoy, L.; et al. Oral Exposure to Polyethylene Microplastics Alters Gut Morphology, Immune Response, and Microbiota Composition in Mice. Environ. Res. 2022, 212, 113230. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Genotoxicity of Microplastics on Living Organisms: Effects on Chromosomes, DNA and Gene Expression. Environments 2025, 12, 10. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Asgari Lajayer, B.; Biglari, Z.; Nayeri, S.; Ahmadabadi, M.; Taghipour, L.; Senapathi, V.; Astatkie, T.; Price, G.W. Micro (Nano) Plastics Uptake, Toxicity and Detoxification in Plants: Challenges and Prospects. Ecotoxicol. Environ. Saf. 2023, 268, 115676. [Google Scholar] [CrossRef]
- Ge, J.; Li, H.; Liu, P.; Zhang, Z.; Ouyang, Z.; Guo, X. Review of the Toxic Effect of Microplastics on Terrestrial and Aquatic Plants. Sci. Total Environ. 2021, 791, 148333. [Google Scholar] [CrossRef]
- Preda, O.-T.; Vlasceanu, A.-M.; Andreescu, C.V.; Tsatsakis, A.; Mezhuev, Y.; Negrei, C.; Baconi, D.L. Health Implications of Widespread Micro- and Nanoplastic Exposure: Environmental Prevalence, Mechanisms, and Biological Impact on Humans. Toxics 2024, 12, 730. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the Mass of Microplastics Ingested—A Pivotal First Step towards Human Health Risk Assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. Microplastics Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Anal. Health Toxicol. 2020, 35, e2020004. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding Plastic Degradation and Microplastic Formation in the Environment: A Review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Edo, C.; Velázquez, D.; Casero-Chamorro, M.C.; Leganés, F.; Quesada, A.; Fernández-Piñas, F.; Rosal, R. First Detection of Microplastics in the Freshwater of an Antarctic Specially Protected Area. Mar. Pollut. Bull. 2020, 161, 111811. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Zhao, Y.; Wang, J. Greenland Sea Gyre Increases Microplastic Pollution in the Surface Waters of the Nordic Seas. Sci. Total Environ. 2020, 712, 136484. [Google Scholar] [CrossRef]
- Kelly, A.; Lannuzel, D.; Rodemann, T.; Meiners, K.M.; Auman, H.J. Microplastic Contamination in East Antarctic Sea Ice. Mar. Pollut. Bull. 2020, 154, 111130. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic Abundance, Distribution and Composition in the Mid-West Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Liu, K.; Zhu, L.; Wei, N.; Zong, C.; Li, D. Pelagic Microplastics in Surface Water of the Eastern Indian Ocean during Monsoon Transition Period: Abundance, Distribution, and Characteristics. Sci. Total Environ. 2021, 755, 142629. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, S.; Berezina, A.; Lusher, A.L.; Zhdanov, I.; Silvestrova, K.; Zavialov, P.; Van Bavel, B.; Yakushev, E. Microplastic Variability in Subsurface Water from the Arctic to Antarctica. Environ. Pollut. 2022, 298, 118808. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, M.; Chen, S.; Dasgupta, S.; Xu, H.; Ta, K.; Du, M.; Li, J.; Guo, Z.; Bai, S. Microplastics Contaminate the Deepest Part of the World’s Ocean. Geochem. Perspect. Lett. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Carbery, M.; Herb, F.; Reynes, J.; Pham, C.K.; Fong, W.-K.; Lehner, R. How Small Is the Big Problem? Small Microplastics <300 Μm Abundant in Marine Surface Waters of the Great Barrier Reef Marine Park. Mar. Pollut. Bull. 2022, 184, 114179. [Google Scholar] [CrossRef]
- Samandra, S.; Johnston, J.M.; Jaeger, J.E.; Symons, B.; Xie, S.; Currell, M.; Ellis, A.V.; Clarke, B.O. Microplastic Contamination of an Unconfined Groundwater Aquifer in Victoria, Australia. Sci. Total Environ. 2022, 802, 149727. [Google Scholar] [CrossRef]
- Aytan, U.; Valente, A.; Senturk, Y.; Usta, R.; Esensoy Sahin, F.B.; Mazlum, R.E.; Agirbas, E. First Evaluation of Neustonic Microplastics in Black Sea Waters. Mar. Environ. Res. 2016, 119, 22–30. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Manna, R.K.; Behera, B.K.; Samanta, S. Spatial Distribution of Meso and Microplastics in the Sediments of River Ganga at Eastern India. Sci. Total Environ. 2019, 694, 133712. [Google Scholar] [CrossRef]
- Lisina, A.A.; Platonov, M.M.; Lomakov, O.I.; Sazonov, A.A.; Shishova, T.V.; Berkovich, A.K.; Frolova, N.L. Microplastic Abundance In Volga River: Results Of A Pilot Study In Summer 2020. Geogr. Environ. Sustain. 2021, 14, 82–93. [Google Scholar] [CrossRef]
- Sekudewicz, I.; Dąbrowska, A.M.; Syczewski, M.D. Microplastic Pollution in Surface Water and Sediments in the Urban Section of the Vistula River (Poland). Sci. Total Environ. 2021, 762, 143111. [Google Scholar] [CrossRef]
- Khedre, A.M.; Ramadan, S.A.; Ashry, A.; Alaraby, M. Abundance and Risk Assessment of Microplastics in Water, Sediment, and Aquatic Insects of the Nile River. Chemosphere 2024, 353, 141557. [Google Scholar] [CrossRef] [PubMed]
- Saad, D.; Alamin, H. The First Evidence of Microplastic Presence in the River Nile in Khartoum, Sudan: Using Nile tilapia Fish as a Bio-Indicator. Heliyon 2024, 10, e23393. [Google Scholar] [CrossRef] [PubMed]
- Stanković, J.; Milošević, D.; Paunović, M.; Jovanović, B.; Popović, N.; Tomović, J.; Atanacković, A.; Radulović, K.; Lončarević, D.; Raković, M. Microplastics in the Danube River and Its Main Tributaries—Ingestion by Freshwater Macroinvertebrates. Water 2024, 16, 962. [Google Scholar] [CrossRef]
- Stratmann, C.N.; Dris, R.; Gasperi, J.; Buschman, F.A.; Markus, A.A.; Guerin, S.; Vethaak, A.D.; Tassin, B. Monitoring Microplastics in the Seine River in the Greater Paris Area. Front. Earth Sci. 2024, 12, 1386547. [Google Scholar] [CrossRef]
- Piskuła, P.; Astel, A.M. Microplastics Occurrence in Two Mountainous Rivers in the Lowland Area—A Case Study of the Central Pomeranian Region, Poland. Microplastics 2022, 1, 167–185. [Google Scholar] [CrossRef]
- Prikler, B.; Svigruha, R.; Háhn, J.; Harkai, P.; Fodor, I.; Kaszab, E.; Kriszt, B.; Tóth, G.; Szabó, I.; Csenki, Z.; et al. Spatial Variations in Microplastics in the Largest Shallow Lake of Central Europe and Its Protecting Wetland Area. Water 2024, 16, 1014. [Google Scholar] [CrossRef]
- Bowszys, M. Microplastics in the Waters of European Lakes and Their Potential Effect on Food Webs: A Review. Pol. J. Nat. Sci. 2022, 37, 345–364. [Google Scholar]
- Emberson-Marl, H.; Coppock, R.L.; Cole, M.; Godley, B.J.; Mimpriss, N.; Nelms, S.E.; Lindeque, P.K. Microplastics in the Arctic: A Transect through the Barents Sea. Front. Mar. Sci. 2023, 10, 1241829. [Google Scholar] [CrossRef]
- Materić, D.; Kjær, H.A.; Vallelonga, P.; Tison, J.-L.; Röckmann, T.; Holzinger, R. Nanoplastics Measurements in Northern and Southern Polar Ice. Environ. Res. 2022, 208, 112741. [Google Scholar] [CrossRef] [PubMed]
- Jones-Williams, K.; Rowlands, E.; Primpke, S.; Galloway, T.; Cole, M.; Waluda, C.; Manno, C. Microplastics in Antarctica—A Plastic Legacy in the Antarctic Snow? Sci. Total Environ. 2025, 966, 178543. [Google Scholar] [CrossRef]
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Ambrosini, R.; Azzoni, R.S.; Pittino, F.; Diolaiuti, G.; Franzetti, A.; Parolini, M. First Evidence of Microplastic Contamination in the Supraglacial Debris of an Alpine Glacier. Environ. Pollut. 2019, 253, 297–301. [Google Scholar] [CrossRef]
- Abbasi, S. Microplastics Washout from the Atmosphere during a Monsoon Rain Event. J. Hazard. Mater. Adv. 2021, 4, 100035. [Google Scholar] [CrossRef]
- Hinojosa, I.A.; Thiel, M. Floating Marine Debris in Fjords, Gulfs and Channels of Southern Chile. Mar. Pollut. Bull. 2009, 58, 341–350. [Google Scholar] [CrossRef]
- Matias, R.S.; Gomes, S.; Barboza, L.G.A.; Almeida, C.M.R.; Marques, A.; Guilhermino, L.; Valente, L.M.P. Occurrence of Microplastics and Metals in European Seabass Produced in Different Aquaculture Systems: Implications for Human Exposure, Risk, and Food Safety. Sci. Total Environ. 2024, 929, 172535. [Google Scholar] [CrossRef]
- Donohue, M.J.; Timmers, M.A.; Kistner, C.A. Marine Debris of the Northwestern Hawaiian Islands: Ghost Net Identification. 2005. Report Number: UNIHI-SEAGRANT-AR-05-01, University of Hawaii, Sea Grant College Program, U.S. National Oceanic and Atmospheric Administration. Available online: https://www.researchgate.net/publication/280238108_Marine_Debris_of_the_Northwestern_Hawaiian_Islands_Ghost_Net_Identification?channel=doi&linkId=55aead5d08ae98e661a6ef54&showFulltext=true#fullTextFileContent (accessed on 5 September 2025).
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The Contribution of Washing Processes of Synthetic Clothes to Microplastic Pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Belzagui, F.; Gutiérrez-Bouzán, C.; Álvarez-Sánchez, A.; Vilaseca, M. Textile Microfibers Reaching Aquatic Environments: A New Estimation Approach. Environ. Pollut. 2020, 265, 114889. [Google Scholar] [CrossRef]
- Akyildiz, S.H.; Fiore, S.; Bruno, M.; Sezgin, H.; Yalcin-Enis, I.; Yalcin, B.; Bellopede, R. Release of Microplastic Fibers from Synthetic Textiles during Household Washing. Environ. Pollut. 2024, 357, 124455. [Google Scholar] [CrossRef] [PubMed]
- Klinkhammer, K.; Kolbe, S.; Brandt, S.; Meyer, J.; Ratovo, K.; Bendt, E.; Rabe, M. Release of Fibrous Microplastics from Functional Polyester Garments through Household Washing. Front. Environ. Sci. 2024, 12, 1330922. [Google Scholar] [CrossRef]
- Bashir, S.M.; Kimiko, S.; Mak, C.-W.; Fang, J.K.-H.; Gonçalves, D. Personal Care and Cosmetic Products as a Potential Source of Environmental Contamination by Microplastics in a Densely Populated Asian City. Front. Mar. Sci. 2021, 8, 683482. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Oanh, L.T.K.; Chi, N.D.T. The Presence of Microplastics in Personal Care and Cosmetic Products (PCCPs) Commonly Used in Ho Chi Minh City. IOP Conf. Ser. Earth Environ. Sci. 2024, 1349, 012012. [Google Scholar] [CrossRef]
- Rehm, R.; Zeyer, T.; Schmidt, A.; Fiener, P. Soil Erosion as Transport Pathway of Microplastic from Agriculture Soils to Aquatic Ecosystems. Sci. Total Environ. 2021, 795, 148774. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s Something in the Air: A Review of Sources, Prevalence and Behaviour of Microplastics in the Atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne Microplastics in Indoor and Outdoor Environments of a Coastal City in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef]
- Abbasi, S.; Jaafarzadeh, N.; Zahedi, A.; Ravanbakhsh, M.; Abbaszadeh, S.; Turner, A. Microplastics in the Atmosphere of Ahvaz City, Iran. J. Environ. Sci. 2023, 126, 95–102. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Zheng, L.; Dai, D.; Li, F. Atmospheric Microplastics in the Northwestern Pacific Ocean: Distribution, Source, and Deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef]
- Caracci, E.; Vega-Herrera, A.; Dachs, J.; Berrojalbiz, N.; Buonanno, G.; Abad, E.; Llorca, M.; Moreno, T.; Farré, M. Micro(Nano)Plastics in the Atmosphere of the Atlantic Ocean. J. Hazard. Mater. 2023, 450, 131036. [Google Scholar] [CrossRef]
- Cabrera, M.; Moulatlet, G.M.; Valencia, B.G.; Maisincho, L.; Rodríguez-Barroso, R.; Albendín, G.; Sakali, A.; Lucas-Solis, O.; Conicelli, B.; Capparelli, M.V. Microplastics in a Tropical Andean Glacier: A Transportation Process across the Amazon Basin? Sci. Total Environ. 2022, 805, 150334. [Google Scholar] [CrossRef]
- Sommer, F.; Dietze, V.; Baum, A.; Sauer, J.; Gilge, S.; Maschowski, C.; Gieré, R. Tire Abrasion as a Major Source of Microplastics in the Environment. Aerosol Air Qual. Res. 2018, 18, 2014–2028. [Google Scholar] [CrossRef]
- Yang, T.; Gao, M.; Nowack, B. Formation of Microplastic Fibers and Fibrils during Abrasion of a Representative Set of 12 Polyester Textiles. Sci. Total Environ. 2023, 862, 160758. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.D.; Jo, H.H.; Kim, Y.U.; Kim, S. Degradation of Floor Finishing Materials Owing to Continuous Gait: A Comprehensive Study on the Generation and Impact of Microplastics. J. Hazard. Mater. 2025, 484, 136726. [Google Scholar] [CrossRef] [PubMed]
- Bullard, J.E.; Ockelford, A.; O’Brien, P.; McKenna Neuman, C. Preferential Transport of Microplastics by Wind. Atmos. Environ. 2021, 245, 118038. [Google Scholar] [CrossRef]
- Ferrero, L.; Scibetta, L.; Markuszewski, P.; Mazurkiewicz, M.; Drozdowska, V.; Makuch, P.; Jutrzenka-Trzebiatowska, P.; Zaleska-Medynska, A.; Andò, S.; Saliu, F.; et al. Airborne and Marine Microplastics from an Oceanographic Survey at the Baltic Sea: An Emerging Role of Air-Sea Interaction? Sci. Total Environ. 2022, 824, 153709. [Google Scholar] [CrossRef]
- Sa’adu, I.; Farsang, A. Plastic Contamination in Agricultural Soils: A Review. Environ. Sci. Eur. 2023, 35, 13. [Google Scholar] [CrossRef]
- Cusworth, S.J.; Davies, W.J.; McAinsh, M.R.; Gregory, A.S.; Storkey, J.; Stevens, C.J. Agricultural Fertilisers Contribute Substantially to Microplastic Concentrations in UK Soils. Commun. Earth Environ. 2024, 5, 7. [Google Scholar] [CrossRef]
- Xu, J.; Yang, A.; Yan, Y.; Xu, Y.; Tang, M.; Li, P.; Zhao, L.; Zhang, X.; Ren, Z. Quantification and Identification of Microplastics in Organic Fertilizers: The Implication for the Manufacture and Safe Application. Water Air Soil Pollut. 2024, 235, 169. [Google Scholar] [CrossRef]
- Thomas, D.; Bloem, E. Visible Intruders: Tracing (Micro-) Plastic in Organic Fertilizers. Sci. Total Environ. 2024, 947, 174311. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B.; Moeinpour, A. Microplastics and Potentially Toxic Elements in Coastal Sediments of Iran’s Main Oil Terminal (Khark Island). Environ. Pollut. 2017, 220, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the Sediments of a UK Urban Lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; Trigui El Menif, N. Microplastics in Sediments from the Littoral Zone of the North Tunisian Coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Bosker, T.; Guaita, L.; Behrens, P. Microplastic Pollution on Caribbean Beaches in the Lesser Antilles. Mar. Pollut. Bull. 2018, 133, 442–447. [Google Scholar] [CrossRef]
- Hengstmann, E.; Tamminga, M.; Vom Bruch, C.; Fischer, E.K. Microplastic in Beach Sediments of the Isle of Rügen (Baltic Sea)—Implementing a Novel Glass Elutriation Column. Mar. Pollut. Bull. 2018, 126, 263–274. [Google Scholar] [CrossRef]
- Bridson, J.H.; Patel, M.; Lewis, A.; Gaw, S.; Parker, K. Microplastic Contamination in Auckland (New Zealand) Beach Sediments. Mar. Pollut. Bull. 2020, 151, 110867. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Z.; Peng, G.; Luo, L.; Liu, K.; Huang, X.; Xu, Y.; Shen, Q.; Li, D. Microplastic Abundance and Distribution in a Central Asian Desert. Sci. Total Environ. 2021, 800, 149529. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Lin, Q.; Chen, T.; Peng, R. The Hidden Threat of Microplastics in Desert Environments: Environmental Impact, Challenges, and Response Measures. Sustainability 2025, 17, 1897. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2017; ISBN 978-2-8317-1827-9. [Google Scholar]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Goßmann, I.; Halbach, M.; Scholz-Böttcher, B.M. Car and Truck Tire Wear Particles in Complex Environmental Samples—A Quantitative Comparison with “Traditional” Microplastic Polymer Mass Loads. Sci. Total Environ. 2021, 773, 145667. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ho, S.S.H.; Niu, X.; Xu, H.; Qu, L.; Shen, Z.; Cao, J.; Chuang, H.-C.; Ho, K.-F. Explorations of Tire and Road Wear Microplastics in Road Dust PM2.5 at Eight Megacities in China. Sci. Total Environ. 2022, 823, 153717. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic Fibers in Atmospheric Fallout: A Source of Microplastics in the Environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Duda, A. Created in BioRender. 2025. Available online: https://BioRender.Com/v9ihwlv (accessed on 5 September 2025).
- Enfrin, M.; Lee, J.; Gibert, Y.; Basheer, F.; Kong, L.; Dumée, L.F. Release of Hazardous Nanoplastic Contaminants Due to Microplastics Fragmentation under Shear Stress Forces. J. Hazard. Mater. 2020, 384, 121393. [Google Scholar] [CrossRef]
- Jander, J.; Hummel, D.; Stürmer, S.; Monteleone, A.; Neumaier, T.; Broghammer, F.; Lewin-Kretzschmar, U.; Brock, T.; Knoll, M.; Fath, A.S. Release of Microplastics from Reusable Kitchen Plasticware and Generation of Thermal Potential Toxic Degradation Products in the Oven. Appl. Sci. 2022, 12, 2535. [Google Scholar] [CrossRef]
- Yu, Y.; Craig, N.; Su, L. A Hidden Pathway for Human Exposure to Micro- and Nanoplastics—The Mechanical Fragmentation of Plastic Products during Daily Use. Toxics 2023, 11, 774. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Li, H.; Liu, H.; Bi, L.; Chen, Q.; Peng, R. A Systematic Review of Microplastics Emissions in Kitchens: Understanding the Links with Diseases in Daily Life. Environ. Int. 2024, 188, 108740. [Google Scholar] [CrossRef]
- Luo, Y.; Naidu, R.; Fang, C. Toy Building Bricks as a Potential Source of Microplastics and Nanoplastics. J. Hazard. Mater. 2024, 471, 134424. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and Road Wear Particles (TRWP)—A Review of Generation, Properties, Emissions, Human Health Risk, Ecotoxicity, and Fate in the Environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Okoffo, E.D.; O’Brien, J.W.; Ribeiro, F.; Wang, X.; Wright, S.L.; Samanipour, S.; Rauert, C.; Toapanta, T.Y.A.; Albarracin, R.; et al. Airborne Emissions of Microplastic Fibres from Domestic Laundry Dryers. Sci. Total Environ. 2020, 747, 141175. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kwon, M.; Park, M.-J.; Kim, J. Analysis of Microplastics Released from Plain Woven Classified by Yarn Types during Washing and Drying. Polymers 2021, 13, 2988. [Google Scholar] [CrossRef]
- De Haan, W.P.; Quintana, R.; Vilas, C.; Cózar, A.; Canals, M.; Uviedo, O.; Sanchez-Vidal, A. The Dark Side of Artificial Greening: Plastic Turfs as Widespread Pollutants of Aquatic Environments. Environ. Pollut. 2023, 334, 122094. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Ma, J.; An, Y.; Liu, Q.; Yang, S.; Qu, Y.; Chen, H.; Zhao, W.; Tian, Y. Microplastics Pollution in the Soil Mulched by Dust-Proof Nets: A Case Study in Beijing, China. Environ. Pollut. 2021, 275, 116600. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Pan, X.; Zhang, D. The Persistently Breaking Trade-Offs of Three-Decade Plastic Film Mulching: Microplastic Pollution, Soil Degradation and Reduced Cotton Yield. J. Hazard. Mater. 2022, 439, 129586. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. Accumulation of Microcapsules Derived from Coated Fertilizer in Paddy Fields. Chemosphere 2021, 267, 129185. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Jiang, L.; Chen, X.; Zhao, Y.; Shi, W.; Xing, Z. From Organic Fertilizer to the Soils: What Happens to the Microplastics? A Critical Review. Sci. Total Environ. 2024, 919, 170217. [Google Scholar] [CrossRef]
- Burghardt, T.E.; Pashkevich, A.; Babić, D.; Mosböck, H.; Babić, D.; Żakowska, L. Microplastics and Road Markings: The Role of Glass Beads and Loss Estimation. Transp. Res. Part Transp. Environ. 2022, 102, 103123. [Google Scholar] [CrossRef]
- Klavins, M.; Klavins, L.; Stabnikova, O.; Stabnikov, V.; Marynin, A.; Ansone-Bertina, L.; Mezulis, M.; Vaseashta, A. Interaction between Microplastics and Pharmaceuticals Depending on the Composition of Aquatic Environment. Microplastics 2022, 1, 520–535. [Google Scholar] [CrossRef]
- Nagato, E.G.; Noothalapati, H.; Kogumasaka, C.; Kakii, S.; Hossain, S.; Iwasaki, K.; Takai, Y.; Shimasaki, Y.; Honda, M.; Hayakawa, K.; et al. Differences in Microplastic Degradation in the Atmosphere and Coastal Water Environment from Two Island Nations: Japan and New Zealand. Environ. Pollut. 2023, 333, 122011. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of Conventional Plastic Wastes in the Environment: A Review on Current Status of Knowledge and Future Perspectives of Disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Z.; Xiang, S.; Yan, H.; Tian, H. Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers 2024, 16, 2807. [Google Scholar] [CrossRef]
- Delre, A.; Goudriaan, M.; Morales, V.H.; Vaksmaa, A.; Ndhlovu, R.T.; Baas, M.; Keijzer, E.; de Groot, T.; Zeghal, E.; Egger, M.; et al. Plastic Photodegradation under Simulated Marine Conditions. Mar. Pollut. Bull. 2023, 187, 114544. [Google Scholar] [CrossRef]
- Lee, S.; Alam, M.B.; Lee, S.-H.; Jung, M.-J.; Shim, W.J.; Kim, S. Identification and Quantification of Photodegradation Products of Disposed Expanded Polystyrene Buoy Used in Aquaculture. Mar. Pollut. Bull. 2023, 192, 114998. [Google Scholar] [CrossRef]
- Zha, F.; Dai, J.; Han, Y.; Liu, P.; Wang, M.; Liu, H.; Guo, X. Release of Millions of Micro(Nano)Plastic Fragments from Photooxidation of Disposable Plastic Boxes. Sci. Total Environ. 2023, 858, 160044. [Google Scholar] [CrossRef]
- Subair, A.; G, M.; Chellappan, S.; Chinglenthoiba, C.; S, I.M. Techniques for Removal and Degradation of Microplastics. In Environmental Chemistry for a Sustainable World; Springer Nature: Cham, Switzerland, 2023; pp. 127–153. ISBN 978-3-031-36350-4. [Google Scholar]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire Wear Particles in the Aquatic Environment—A Review on Generation, Analysis, Occurrence, Fate and Effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Santo, N.; Ortenzi, M.A.; Bolzoni, E.; Bacchetta, R.; Tremolada, P. Does Mechanical Stress Cause Microplastic Release from Plastic Water Bottles? Water Res. 2019, 166, 115082. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Checon, H.H.; Leonardi, B.; Baruque-Ramos, J. Laundering and Textile Parameters Influence Fibers Release in Household Washings. Environ. Pollut. 2020, 257, 113553. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-J.; Qin, Y.; Zhang, T.-L.; Zhu, Y.-Y.; Wang, Z.-J.; Zhou, Z.-S.; Xie, T.-Z.; Luo, X.-D. Migration of (Non-) Intentionally Added Substances and Microplastics from Microwavable Plastic Food Containers. J. Hazard. Mater. 2021, 417, 126074. [Google Scholar] [CrossRef]
- Xu, C.; Hu, J.; Dong, B.; Lin, Q.; Wu, S.; Chen, J.; Wang, J.; Li, D.; Zhong, H. Safety Assessment of Polypropylene Self-Heating Food Container: The Release of Microplastics and Volatile Organic Compounds. Food Packag. Shelf Life 2024, 44, 101307. [Google Scholar] [CrossRef]
- Jin, T.; Liu, Y.; Lyu, H.; He, Y.; Sun, H.; Tang, J.; Xing, B. Plastic Takeaway Food Containers May Cause Human Intestinal Damage in Routine Life Usage: Microplastics Formation and Cytotoxic Effect. J. Hazard. Mater. 2024, 475, 134866. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Islam, Z.; Hasan, R.; Islam Molla Jamal, A.S. Acidic Hydrolysis of Recycled Polyethylene Terephthalate Plastic for the Production of Its Monomer Terephthalic Acid. Prog. Rubber Plast. Recycl. Technol. 2023, 39, 12–25. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- De Oliveira, C.R.S.; Da Silva Júnior, A.H.; Mulinari, J.; Ferreira, A.J.S.; Da Silva, A. Fibrous Microplastics Released from Textiles: Occurrence, Fate, and Remediation Strategies. J. Contam. Hydrol. 2023, 256, 104169. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, P.; Zhao, Z.; Wu, Y.; Sun, H.; Liu, C. Microplastics Release from Face Masks: Characteristics, Influential Factors, and Potential Risks. Sci. Total Environ. 2024, 921, 171090. [Google Scholar] [CrossRef]
- Bhat, M.A. Unravelling the Microplastic Contamination: A Comprehensive Analysis of Microplastics in Indoor House Dust. Indoor Built Environ. 2024, 33, 1519–1541. [Google Scholar] [CrossRef]
- Catarino, A.I.; León, M.C.; Li, Y.; Lambert, S.; Vercauteren, M.; Asselman, J.; Janssen, C.R.; Everaert, G.; De Rijcke, M. Micro- and Nanoplastics Transfer from Seawater to the Atmosphere through Aerosolization under Controlled Laboratory Conditions. Mar. Pollut. Bull. 2023, 192, 115015. [Google Scholar] [CrossRef]
- Lambert, S.; Vercauteren, M.; Catarino, A.I.; Li, Y.; Van Landuyt, J.; Boon, N.; Everaert, G.; De Rijcke, M.; Janssen, C.R.; Asselman, J. Aerosolization of Micro- and Nanoplastics via Sea Spray: Investigating the Role of Polymer Type, Size, and Concentration, and Potential Implications for Human Exposure. Environ. Pollut. 2024, 351, 124105. [Google Scholar] [CrossRef]
- Van Den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage Sludge Application as a Vehicle for Microplastics in Eastern Spanish Agricultural Soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef] [PubMed]
- Hatinoğlu, M.D.; Sanin, F.D. Sewage Sludge as a Source of Microplastics in the Environment: A Review of Occurrence and Fate during Sludge Treatment. J. Environ. Manag. 2021, 295, 113028. [Google Scholar] [CrossRef] [PubMed]
- Qaiser, Z.; Aqeel, M.; Sarfraz, W.; Fatima Rizvi, Z.; Noman, A.; Naeem, S.; Khalid, N. Microplastics in Wastewaters and Their Potential Effects on Aquatic and Terrestrial Biota. Case Stud. Chem. Environ. Eng. 2023, 8, 100536. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic Contamination in an Urban Area: A Case Study in Greater Paris. Environ. Chem. 2015, 12, 592. [Google Scholar] [CrossRef]
- Militký, J.; Novotná, J.; Wiener, J.; Křemenáková, D.; Venkataraman, M. Microplastics and Fibrous Fragments Generated during the Production and Maintenance of Textiles. Fibers 2024, 12, 51. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Tehrani-Bagha, A. A Review on Microplastic Emission from Textile Materials and Its Reduction Techniques. Polym. Degrad. Stab. 2022, 199, 109901. [Google Scholar] [CrossRef]
- Tao, D.; Zhang, K.; Xu, S.; Lin, H.; Liu, Y.; Kang, J.; Yim, T.; Giesy, J.P.; Leung, K.M.Y. Microfibers Released into the Air from a Household Tumble Dryer. Environ. Sci. Technol. Lett. 2022, 9, 120–126. [Google Scholar] [CrossRef]
- Wieland, S.; Balmes, A.; Bender, J.; Kitzinger, J.; Meyer, F.; Ramsperger, A.F.; Roeder, F.; Tengelmann, C.; Wimmer, B.H.; Laforsch, C.; et al. From Properties to Toxicity: Comparing Microplastics to Other Airborne Microparticles. J. Hazard. Mater. 2022, 428, 128151. [Google Scholar] [CrossRef]
- Morawska, L.; Buonanno, G. The Physics of Particle Formation and Deposition during Breathing. Nat. Rev. Phys. 2021, 3, 300–301. [Google Scholar] [CrossRef]
- Prietl, B.; Meindl, C.; Roblegg, E.; Pieber, T.R.; Lanzer, G.; Fröhlich, E. Nano-Sized and Micro-Sized Polystyrene Particles Affect Phagocyte Function. Cell Biol. Toxicol. 2014, 30, 1–16. [Google Scholar] [CrossRef]
- Jayavel, S.; Govindaraju, B.; Michael, J.R.; Viswanathan, B. Impacts of Micro and Nanoplastics on Human Health. Bull. Natl. Res. Cent. 2024, 48, 110. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin Penetration and Distribution of Polymeric Nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jatana, S.; Callahan, L.; Pentland, A.; DeLouise, L. Impact of Cosmetic Lotions on Nanoparticle Penetration through Ex Vivo C57BL/6 Hairless Mouse and Human Skin: A Comparison Study. Cosmetics 2016, 3, 6. [Google Scholar] [CrossRef]
- Singh, A.; Mishra, B.K. Microbeads in Personal Care Products: An Overlooked Environmental Concern. J. Clean. Prod. 2023, 427, 139082. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, C.-Y.; Zhong, L.-S.; Bao, L.-J.; Zeng, E.Y. Particle Transfer Mediates Dermal Exposure of Consumers to Plasticizers in Eraser and Pen Accessories. Environ. Int. 2023, 180, 108191. [Google Scholar] [CrossRef]
- Sykes, E.A.; Dai, Q.; Tsoi, K.M.; Hwang, D.M.; Chan, W.C.W. Nanoparticle Exposure in Animals Can Be Visualized in the Skin and Analysed via Skin Biopsy. Nat. Commun. 2014, 5, 3796. [Google Scholar] [CrossRef]
- Baroli, B.; Ennas, M.G.; Loffredo, F.; Isola, M.; Pinna, R.; Arturo López-Quintela, M. Penetration of Metallic Nanoparticles in Human Full-Thickness Skin. J. Investig. Dermatol. 2007, 127, 1701–1712. [Google Scholar] [CrossRef]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and Their Interactions with the Dermal Barrier. Dermatoendocrinol. 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Labouta, H.I.; El-Khordagui, L.K.; Kraus, T.; Schneider, M. Mechanism and Determinants of Nanoparticle Penetration through Human Skin. Nanoscale 2011, 3, 4989. [Google Scholar] [CrossRef]
- Menichetti, A.; Mordini, D.; Montalti, M. Penetration of Microplastics and Nanoparticles Through Skin: Effects of Size, Shape, and Surface Chemistry. J. Xenobiotics 2024, 15, 6. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Wu, C.-L.; Hsu, C.-T.; Lo, W.; Chiang, S.-J.; Lin, S.-J.; Dong, C.-Y.; Chen, C.-C. Chemical Enhancer Induced Changes in the Mechanisms of Transdermal Delivery of Zinc Oxide Nanoparticles. Biomaterials 2009, 30, 3002–3008. [Google Scholar] [CrossRef]
- Madhumitha, C.T.; Karmegam, N.; Biruntha, M.; Arun, A.; Al Kheraif, A.A.; Kim, W.; Kumar, P. Extraction, Identification, and Environmental Risk Assessment of Microplastics in Commercial Toothpaste. Chemosphere 2022, 296, 133976. [Google Scholar] [CrossRef] [PubMed]
- Chengappa S, K.; Rao, A.; K S, A.; Jodalli, P.S.; Shenoy Kudpi, R. Microplastic Content of Over-the-Counter Toothpastes—A Systematic Review. F1000Research 2023, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Gopalan, S.; Zhang, X.; Xu, L.; Niu, J.; Naidu, R. Raman Imaging to Identify Microplastics Released from Toothbrushes: Algorithms and Particle Analysis. Environ. Pollut. 2023, 337, 122510. [Google Scholar] [CrossRef] [PubMed]
- Saha, U.; Jena, S.; Simnani, F.Z.; Singh, D.; Choudhury, A.; Naser, S.S.; Lenka, S.S.; Kirti, A.; Nandi, A.; Sinha, A.; et al. The Unseen Perils of Oral-Care Products Generated Micro/Nanoplastics on Human Health. Ecotoxicol. Environ. Saf. 2025, 290, 117526. [Google Scholar] [CrossRef]
- Novotna, K.; Cermakova, L.; Pivokonska, L.; Cajthaml, T.; Pivokonsky, M. Microplastics in Drinking Water Treatment—Current Knowledge and Research Needs. Sci. Total Environ. 2019, 667, 730–740. [Google Scholar] [CrossRef]
- Brancaleone, E.; Mattei, D.; Fuscoletti, V.; Lucentini, L.; Favero, G.; Cecchini, G.; Frugis, A.; Gioia, V.; Lazzazzara, M. Microplastic in Drinking Water: A Pilot Study. Microplastics 2024, 3, 31–45. [Google Scholar] [CrossRef]
- Li, H.; Zhu, L.; Ma, M.; Wu, H.; An, L.; Yang, Z. Occurrence of Microplastics in Commercially Sold Bottled Water. Sci. Total Environ. 2023, 867, 161553. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Socas-Hernández, C.; Miralles, P.; González-Sálamo, J.; Hernández-Borges, J.; Coscollà, C. Assessment of Anthropogenic Particles Content in Commercial Beverages. Food Chem. 2024, 447, 139002. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Fennell, T.R.; Johnson, L.M. Unintended Human Ingestion of Nanoplastics and Small Microplastics through Drinking Water, Beverages, and Food Sources. NanoImpact 2021, 21, 100302. [Google Scholar] [CrossRef] [PubMed]
- Nocoń, W.; Moraczewska-Majkut, K.; Wiśniowska, E. Microplastics in Bottled Water and Bottled Soft Drinks. Desalination Water Treat. 2023, 312, 64–69. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; Luo, Y.; Ding, Y.; Huang, J.; Wu, H.; Han, J.; Du, L.; Kang, A.; Jia, M.; et al. Plastic Bottles for Chilled Carbonated Beverages as a Source of Microplastics and Nanoplastics. Water Res. 2023, 242, 120243. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Hensel, F.; Gomiero, A.; Iordachescu, L.; Vianello, A.; Wittgren, H.B.; Vollertsen, J. Drinking Plastics?—Quantification and Qualification of Microplastics in Drinking Water Distribution Systems by µFTIR and Py-GCMS. Water Res. 2021, 188, 116519. [Google Scholar] [CrossRef]
- Kasim, S.; Daud, A.; Birawida, A.B.; Mallongi, A.; Arundana, A.I.; Rasul, A.; Hatta, M. Analysis of Environmental Health Risks from Exposure to Polyethylene Terephthalate Microplastics in Refilled Drinking Water. Glob. J. Environ. Sci. Manag. 2023, 9, 301–318. [Google Scholar] [CrossRef]
- Aleksander-Kwaterczak, U.; Gaj, D.; Stelmach, A.; Wróbel, T.P. Investigating the Content of Microplastics and Other Extraneous Particles in Polish Bottled Water. Geol. Geophys. Environ. 2023, 49, 335–353. [Google Scholar] [CrossRef]
- Prata, J.C.; Paço, A.; Reis, V.; Da Costa, J.P.; Fernandes, A.J.S.; Da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of Microplastics in White Wines Capped with Polyethylene Stoppers Using Micro-Raman Spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef]
- Joseph, A.; Parveen, N.; Ranjan, V.P.; Goel, S. Drinking Hot Beverages from Paper Cups: Lifetime Intake of Microplastics. Chemosphere 2023, 317, 137844. [Google Scholar] [CrossRef]
- Wang, H.-P.; Huang, X.-H.; Chen, J.-N.; Dong, M.; Zhang, Y.-Y.; Qin, L. Pouring Hot Water through Drip Bags Releases Thousands of Microplastics into Coffee. Food Chem. 2023, 415, 135717. [Google Scholar] [CrossRef]
- Ranjan, V.P.; Joseph, A.; Goel, S. Microplastics and Other Harmful Substances Released from Disposable Paper Cups into Hot Water. J. Hazard. Mater. 2021, 404, 124118. [Google Scholar] [CrossRef]
- Shi, Y.; Li, D.; Xiao, L.; Sheerin, E.D.; Mullarkey, D.; Yang, L.; Bai, X.; Shvets, I.V.; Boland, J.J.; Wang, J.J. The Influence of Drinking Water Constituents on the Level of Microplastic Release from Plastic Kettles. J. Hazard. Mater. 2022, 425, 127997. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low Numbers of Microplastics Detected in Drinking Water from Ground Water Sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of Microplastics in Raw and Treated Drinking Water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Pivokonský, M.; Pivokonská, L.; Novotná, K.; Čermáková, L.; Klimtová, M. Occurrence and Fate of Microplastics at Two Different Drinking Water Treatment Plants within a River Catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef] [PubMed]
- Oladoja, N.A.; Unuabonah, I.E. The Pathways of Microplastics Contamination in Raw and Drinking Water. J. Water Process Eng. 2021, 41, 102073. [Google Scholar] [CrossRef]
- Chu, X.; Zheng, B.; Li, Z.; Cai, C.; Peng, Z.; Zhao, P.; Tian, Y. Occurrence and Distribution of Microplastics in Water Supply Systems: In Water and Pipe Scales. Sci. Total Environ. 2022, 803, 150004. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Tang, Y. Fragmentation of Microplastics in the Drinking Water Treatment Process—A Case Study in Yangtze River Region, China. Sci. Total Environ. 2022, 806, 150545. [Google Scholar] [CrossRef]
- Weisser, J.; Beer, I.; Hufnagl, B.; Hofmann, T.; Lohninger, H.; Ivleva, N.P.; Glas, K. From the Well to the Bottle: Identifying Sources of Microplastics in Mineral Water. Water 2021, 13, 841. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.-M.; Janke, M.; Gerdts, G. Plastic Ingestion by Pelagic and Demersal Fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating Microplastics Bioaccumulation and Biomagnification in Seafood from the Persian Gulf: A Threat to Human Health? Food Addit. Contam. Part A 2019, 36, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liang, W.; Liu, Q.-X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish Ingest Microplastics Unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N.; Thomson, K.T. Microplastics in the Edible Tissues of Shellfishes Sold for Human Consumption. Chemosphere 2021, 264, 128554. [Google Scholar] [CrossRef]
- Nalbone, L.; Cincotta, F.; Giarratana, F.; Ziino, G.; Panebianco, A. Microplastics in Fresh and Processed Mussels Sampled from Fish Shops and Large Retail Chains in Italy. Food Control 2021, 125, 108003. [Google Scholar] [CrossRef]
- Lackner, M.; Branka, M. Microplastics in Farmed Animals—A Review. Microplastics 2024, 3, 559–588. [Google Scholar] [CrossRef]
- Vega-Herrera, A.; Savva, K.; Lacoma, P.; Santos, L.H.M.L.M.; Hernández, A.; Marmelo, I.; Marques, A.; Llorca, M.; Farré, M. Bioaccumulation and Dietary Bioaccessibility of Microplastics Composition and Cocontaminants in Mediterranean Mussels. Chemosphere 2024, 363, 142934. [Google Scholar] [CrossRef]
- Aydın, R.B.; Yozukmaz, A.; Şener, İ.; Temiz, F.; Giannetto, D. Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers. Life 2023, 13, 1686. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Song, Z.; Wei, N.; Li, D. Terrestrial Plants as a Potential Temporary Sink of Atmospheric Microplastics during Transport. Sci. Total Environ. 2020, 742, 140523. [Google Scholar] [CrossRef]
- Yu, Z.; Song, S.; Xu, X.; Ma, Q.; Lu, Y. Sources, Migration, Accumulation and Influence of Microplastics in Terrestrial Plant Communities. Environ. Exp. Bot. 2021, 192, 104635. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Pirsaheb, M.; Amin, A.A.; Kianpour, S.; Hossini, H. Microplastic Pollution in Table Salt and Sugar: Occurrence, Qualification and Quantification and Risk Assessment. J. Food Compos. Anal. 2023, 119, 105261. [Google Scholar] [CrossRef]
- Snekkevik, V.K.; Cole, M.; Gomiero, A.; Haave, M.; Khan, F.R.; Lusher, A.L. Beyond the Food on Your Plate: Investigating Sources of Microplastic Contamination in Home Kitchens. Heliyon 2024, 10, e35022. [Google Scholar] [CrossRef]
- Choi, H.S.; Ashitate, Y.; Lee, J.H.; Kim, S.H.; Matsui, A.; Insin, N.; Bawendi, M.G.; Semmler-Behnke, M.; Frangioni, J.V.; Tsuda, A. Rapid Translocation of Nanoparticles from the Lung Airspaces to the Body. Nat. Biotechnol. 2010, 28, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Hirn, S.; Schleh, C. Nanoparticles in the Lung. Nat. Biotechnol. 2010, 28, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Marcos, R. Pathways of Human Exposure to Microplastics, and Estimation of the Total Burden. Curr. Opin. Food Sci. 2021, 39, 144–151. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in Take-out Food Containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- Cole, M.; Gomiero, A.; Jaén-Gil, A.; Haave, M.; Lusher, A. Microplastic and PTFE Contamination of Food from Cookware. Sci. Total Environ. 2024, 929, 172577. [Google Scholar] [CrossRef]
- Brenz, F.; Linke, S.; Simat, T. Linear and Cyclic Oligomers in Polybutylene Terephthalate for Food Contact Materials. Food Addit. Contam. Part A 2018, 35, 583–598. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Song, X.-C.; Nerin, C.; Goshawk, J.; Dreolin, N. The Use of Ion Mobility Time-of-Flight Mass Spectrometry to Assess the Migration of Polyamide 6 and Polyamide 66 Oligomers from Kitchenware Utensils to Food. Food Chem. 2021, 350, 129260. [Google Scholar] [CrossRef]
- Kaseke, T.; Lujic, T.; Cirkovic Velickovic, T. Nano- and Microplastics Migration from Plastic Food Packaging into Dairy Products: Impact on Nutrient Digestion, Absorption, and Metabolism. Foods 2023, 12, 3043. [Google Scholar] [CrossRef]
- Kappenstein, O.; Ebner, I.; Förster, C.; Richter, S.; Weyer, J.; Pfaff, K.; Luch, A. Validation and Application of an LC-MS/MS Method for the Determination of Cyclic Oligomers Originating from Polyamide 6 and Polyamide 66 in Food Simulant. Food Addit. Contam. Part A 2018, 35, 1410–1420. [Google Scholar] [CrossRef]
- Luo, Y.; Chuah, C.; Amin, M.A.; Khoshyan, A.; Gibson, C.T.; Tang, Y.; Naidu, R.; Fang, C. Assessment of Microplastics and Nanoplastics Released from a Chopping Board Using Raman Imaging in Combination with Three Algorithms. J. Hazard. Mater. 2022, 431, 128636. [Google Scholar] [CrossRef]
- Habib, R.Z.; Kindi, R.A.; Salem, F.A.; Kittaneh, W.F.; Poulose, V.; Iftikhar, S.H.; Mourad, A.-H.I.; Thiemann, T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. Int. J. Environ. Res. Public Health 2022, 19, 13442. [Google Scholar] [CrossRef]
- Yadav, H.; Khan, M.R.H.; Quadir, M.; Rusch, K.A.; Mondal, P.P.; Orr, M.; Xu, E.G.; Iskander, S.M. Cutting Boards: An Overlooked Source of Microplastics in Human Food? Environ. Sci. Technol. 2023, 57, 8225–8235. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.A.; Romanova, S.; Okur, I.; Zhang, D.; Kuebler, J.; Huang, X.; Wang, B.; Fernandez-Ballester, L.; Lu, Y.; Schubert, M.; et al. Assessing the Release of Microplastics and Nanoplastics from Plastic Containers and Reusable Food Pouches: Implications for Human Health. Environ. Sci. Technol. 2023, 57, 9782–9792. [Google Scholar] [CrossRef] [PubMed]
- Castle, L.; Jickells, S.M.; Gilbert, J.; Harrison, N. Migration Testing of Plastics and Microwave-active Materials for High-temperature Food-use Applications. Food Addit. Contam. 1990, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Rondinella, A.; Andreatta, F.; Turrin, D.; Fedrizzi, L. Degradation Mechanisms Occurring in PTFE-Based Coatings Employed in Food-Processing Applications. Coatings 2021, 11, 1419. [Google Scholar] [CrossRef]
- Luo, Y.; Gibson, C.T.; Chuah, C.; Tang, Y.; Naidu, R.; Fang, C. Raman Imaging for the Identification of Teflon Microplastics and Nanoplastics Released from Non-Stick Cookware. Sci. Total Environ. 2022, 851, 158293. [Google Scholar] [CrossRef]
- Schlummer, M.; Sölch, C.; Meisel, T.; Still, M.; Gruber, L.; Wolz, G. Emission of Perfluoroalkyl Carboxylic Acids (PFCA) from Heated Surfaces Made of Polytetrafluoroethylene (PTFE) Applied in Food Contact Materials and Consumer Products. Chemosphere 2015, 129, 46–53. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M. PTFE-Coated Non-Stick Cookware and Toxicity Concerns: A Perspective. Environ. Sci. Pollut. Res. 2017, 24, 23436–23440. [Google Scholar] [CrossRef]
- Addo Ntim, S.; Norris, S.; Scott, K.; Thomas, T.A.; Noonan, G.O. Consumer Use Effects on Nanoparticle Release from Commercially Available Ceramic Cookware. Food Control 2018, 87, 31–39. [Google Scholar] [CrossRef]
- Luo, Y.; Awoyemi, O.S.; Naidu, R.; Fang, C. Detection of Microplastics and Nanoplastics Released from a Kitchen Blender Using Raman Imaging. J. Hazard. Mater. 2023, 453, 131403. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.C. A Summary of the NBS Literature Reviews on the Chemical Nature and Toxicity of the Pyrolysis and Combustion Products from Seven Plastics: Acrylonitrile–Butadiene–Styrenes (ABS), Nylons, Polyesters, Polyethylenes, Polystyrenes, Poly(Vinyl Chlorides) and Rigid Polyurethane Foams. Fire Mater. 1987, 11, 143–157. [Google Scholar] [CrossRef]
- Lund, K.H.; Petersen, J.H. Migration of Formaldehyde and Melamine Monomers from Kitchen- and Tableware Made of Melamine Plastic. Food Addit. Contam. 2006, 23, 948–955. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, J.; Liu, Z.; Chen, Y.; Chen, Q.; Chen, Z.; Yuan, S.; Yu, H.; Yao, W. Influence of Different Food Matrices on the Abundance, Characterization, Migration Kinetics and Hazards of Microplastics Released from Plastic Packaging (PP and PET). Food Chem. 2025, 478, 143691. [Google Scholar] [CrossRef]
- Sturm, M.T.; Kluczka, S.; Wilde, A.; Schuhen, K. Determination of Particles Produced During Boiling in Differenz Plastic and Glass Kettles via Comparative Dynamic Image Analysis Using FlowCam®. Analytik News. Technical Papers, 2019. Available online: https://analytik.news/en/papers/pdf/wasserdreinull4e.pdf (accessed on 5 September 2025).
- Liu, G.; Wang, J.; Wang, M.; Ying, R.; Li, X.; Hu, Z.; Zhang, Y. Disposable Plastic Materials Release Microplastics and Harmful Substances in Hot Water. Sci. Total Environ. 2022, 818, 151685. [Google Scholar] [CrossRef]
- Alak, G.; Köktürk, M.; Atamanalp, M. Evaluation of Different Packaging Methods and Storage Temperature on MPs Abundance and Fillet Quality of Rainbow Trout. J. Hazard. Mater. 2021, 420, 126573. [Google Scholar] [CrossRef]
- Zangmeister, C.D.; Radney, J.G.; Benkstein, K.D.; Kalanyan, B. Common Single-Use Consumer Plastic Products Release Trillions of Sub-100 Nm Nanoparticles per Liter into Water during Normal Use. Environ. Sci. Technol. 2022, 56, 5448–5455. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, Q.; Tang, P.; Chen, C.; Cheng, X.; Wei, X.-F.; Ma, J.; Liu, B. How Many Microplastics Do We Ingest When Using Disposable Drink Cups? J. Hazard. Mater. 2023, 441, 129982. [Google Scholar] [CrossRef]
- Mei, T.; Wang, J.; Xiao, X.; Lv, J.; Li, Q.; Dai, H.; Liu, X.; Pi, F. Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging. Foods 2022, 11, 2871. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.; Shi, Y.; Hill, C.; Pilliadugula, R.; Page, L.; Wang, J.J.; Boland, J.J.; Xiao, L. High Levels of Microparticles Release from Biodegradable Polylactic Acid Paper Cups Compared with Polyethylene-Lined Cups. Chem. Eng. J. 2023, 468, 143620. [Google Scholar] [CrossRef]
- Banaei, G.; García-Rodríguez, A.; Tavakolpournegari, A.; Martín-Pérez, J.; Villacorta, A.; Marcos, R.; Hernández, A. The Release of Polylactic Acid Nanoplastics (PLA-NPLs) from Commercial Teabags. Obtention, Characterization, and Hazard Effects of True-to-Life PLA-NPLs. J. Hazard. Mater. 2023, 458, 131899. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Kutralam-Muniasamy, G. Migration Testing of Microplastics in Plastic Food-Contact Materials: Release, Characterization, Pollution Level, and Influencing Factors. TrAC Trends Anal. Chem. 2024, 170, 117421. [Google Scholar] [CrossRef]
- El Hayany, B.; Rumpel, C.; Hafidi, M.; El Fels, L. Occurrence, Analysis of Microplastics in Sewage Sludge and Their Fate during Composting: A Literature Review. J. Environ. Manag. 2022, 317, 115364. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.-L.; Liu, L.-Y.; Guo, J.-L.; Zeng, L.-X.; Guo, Y. Microplastics in Take-out Food: Are We over Taking It? Environ. Res. 2022, 215, 114390. [Google Scholar] [CrossRef]
- Fadare, O.O.; Wan, B.; Guo, L.-H.; Zhao, L. Microplastics from Consumer Plastic Food Containers: Are We Consuming It? Chemosphere 2020, 253, 126787. [Google Scholar] [CrossRef]
- Katsara, K.; Viskadourakis, Z.; Kenanakis, G.; Papadakis, V.M. Microplastic Migration from Food Packaging on Cheese. Microplastics 2025, 4, 17. [Google Scholar] [CrossRef]
- Moreno-Gordaliza, E.; Dolores Marazuela, M.; Milagros Gómez-Gómez, M. Risk Assessment of Silver and Microplastics Release from Antibacterial Food Containers under Conventional Use and Microwave Heating. Food Chem. 2023, 420, 136097. [Google Scholar] [CrossRef]
- Akbulut, S.; Akman, P.K.; Tornuk, F.; Yetim, H. Microplastic Release from Single-Use Plastic Beverage Cups. Foods 2024, 13, 1564. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Ziino, G.; Nalbone, L.; Giarratana, F.; Romano, B.; Cincotta, F.; Panebianco, A. Microplastics in Vacuum Packages of Frozen and Glazed Icefish (Neosalanx spp.): A Freshwater Fish Intended for Human Consumption. Ital. J. Food Saf. 2021, 10, 9974. [Google Scholar] [CrossRef]
- Mohamed Hadeed, M.D.; Al-Ahmady, K.K. The Effect of Different Storage Conditions for Refilled Plastic Drink Bottles on the Concentration of Microplastic Release in Water. J. Res. Appl. Sci. Biotechnol. 2022, 1, 71–77. [Google Scholar] [CrossRef]
- Chaïb, I.; Doyen, P.; Merveillie, P.; Dehaut, A.; Duflos, G. Microplastic Contaminations in a Set of Beverages Sold in France. J. Food Compos. Anal. 2025, 144, 107719. [Google Scholar] [CrossRef]
- Gambino, I.; Malitesta, C.; Bagordo, F.; Grassi, T.; Panico, A.; Fraissinet, S.; De Donno, A.; De Benedetto, G.E. Characterization of Microplastics in Water Bottled in Different Packaging by Raman Spectroscopy. Environ. Sci. Water Res. Technol. 2023, 9, 3391–3397. [Google Scholar] [CrossRef]
- Tisler, S.; Christensen, J.H. Non-Target Screening for the Identification of Migrating Compounds from Reusable Plastic Bottles into Drinking Water. J. Hazard. Mater. 2022, 429, 128331. [Google Scholar] [CrossRef]
- Giese, A.; Kerpen, J.; Weber, F.; Prediger, J. A Preliminary Study of Microplastic Abrasion from the Screw Cap System of Reusable Plastic Bottles by Raman Microspectroscopy. ACS EST Water 2021, 1, 1363–1368. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, K.; Zhang, T.; Cen, C.; Zheng, Y.; Shuai, Y. Release Characteristics of Small-Sized Microplastics in Bottled Drinks Using Flow Cytometry Sorting and Nile Red Staining. Water 2024, 16, 1898. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to Microplastics (<10 Μm) Associated to Plastic Bottles Mineral Water Consumption: The First Quantitative Study. Water Res. 2019, 157, 365–371. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of Microplastics in Water by Micro-Raman Spectroscopy: Release of Plastic Particles from Different Packaging into Mineral Water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Luo, Y.; Qi, F.; Gibson, C.T.; Lei, Y.; Fang, C. Investigating Kitchen Sponge-Derived Microplastics and Nanoplastics with Raman Imaging and Multivariate Analysis. Sci. Total Environ. 2022, 824, 153963. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics Generated When Opening Plastic Packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Iacovidou, E. Closing the Loop on Plastic Packaging Materials: What Is Quality and How Does It Affect Their Circularity? Sci. Total Environ. 2018, 630, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and Removal of Microplastics in an Advanced Drinking Water Treatment Plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Amin, A.A.; Karimi, H.; Pirsaheb, M.; Kim, H.; Hossini, H. Occurrence of Microplastic Particles in the Most Popular Iranian Bottled Mineral Water Brands and an Assessment of Human Exposure. J. Water Process Eng. 2021, 39, 101708. [Google Scholar] [CrossRef]
- Oßmann, B.E. Microplastics in Drinking Water? Present State of Knowledge and Open Questions. Curr. Opin. Food Sci. 2021, 41, 44–51. [Google Scholar] [CrossRef]
- Basaran, B.; Özçifçi, Z.; Akcay, H.T.; Aytan, Ü. Microplastics in Branded Milk: Dietary Exposure and Risk Assessment. J. Food Compos. Anal. 2023, 123, 105611. [Google Scholar] [CrossRef]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A.; et al. Detection and Characterization of Small-Sized Microplastics (≥5 Μm) in Milk Products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Kiruba, R.; Preethi, M.; Aganasteen, R.; Rithick Raj, M.; Hannah Thabitha, C.; Monica, P.; Sakthivel, J.; Levince, C.; Naseera Banu, I. Identification of Microplastics as Emerging Contaminant in Branded Milk of Tamil Nadu State, India. Asian J. Biol. Life Sci. 2022, 11, 181–187. [Google Scholar] [CrossRef]
- Li, Y.; Peng, L.; Fu, J.; Dai, X.; Wang, G. A Microscopic Survey on Microplastics in Beverages: The Case of Beer, Mineral Water and Tea. Analyst 2022, 147, 1099–1105. [Google Scholar] [CrossRef]
- Wiesheu, A.C.; Anger, P.M.; Baumann, T.; Niessner, R.; Ivleva, N.P. Raman Microspectroscopic Analysis of Fibers in Beverages. Anal. Methods 2016, 8, 5722–5725. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First Study of Its Kind on the Microplastic Contamination of Soft Drinks, Cold Tea and Energy Drinks—Future Research and Environmental Considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human Exposure to Micro/Nano-Plastics through Vegetables, Fruits, and Grains—A Predictive Modelling Approach. J. Hazard. Mater. 2024, 480, 135900. [Google Scholar] [CrossRef]
- Bai, Y.; Song, Y.; He, X.; He, W.; Chen, Y.; Zhao, M.; Zhang, J.; Han, W.; Bai, W. Evidence of Microplastic Accumulation on the Surface of Lettuce and Analysis of Contamination Sources. J. Hazard. Mater. 2025, 492, 138201. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Farooq, M.A.; Shafiq, M.; Arshad, M.; Din, A.A.; Alazba, A.A. Quantification and Polymeric Characterization of Microplastics in Composts and Their Accumulation in Lettuce. Chemosphere 2024, 361, 142520. [Google Scholar] [CrossRef] [PubMed]
- Canha, N.; Jafarova, M.; Grifoni, L.; Gamelas, C.A.; Alves, L.C.; Almeida, S.M.; Loppi, S. Microplastic Contamination of Lettuces Grown in Urban Vegetable Gardens in Lisbon (Portugal). Sci. Rep. 2023, 13, 14278. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Guo, T.; Li, J.; Wang, F. Optimize Lettuce Washing Methods to Reduce the Risk of Microplastics Ingestion: The Evidence from Microplastics Residues on the Surface of Lettuce Leaves and in the Lettuce Washing Wastewater. Sci. Total Environ. 2023, 868, 161726. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Y.; Liu, W.; Ma, X.; He, D.; Wang, Y.; Li, J.; Wu, W. Identification and Quantification of Nanoplastics in Different Crops Using Pyrolysis Gas Chromatography-Mass Spectrometry. Chemosphere 2024, 354, 141689. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Solanki, M.B.; Shimada, Y.; Kamble, S.B.; Patole, S.P.; Kolekar, G.B.; Gore, A.H. Microplastic Contamination in Indian Rice: A Comprehensive Characterization and Health Risk Assessment. J. Hazard. Mater. 2024, 480, 136208. [Google Scholar] [CrossRef]
- Rajendran, K.; Rajendiran, R.; Peter, R.; Pasupathi, M.S.; Nazir, S.B.; Kalyanasundaram, P.; Velu, R.K. Validation of Microplastics Accumulation on Edible Fruits and Vegetables. Biol. Forum 2023, 15, 348–355. [Google Scholar]
- Aysha, S.; Sultan, M.B.; Bhuiyan, M.A.R.; Toha, M.; Ria, R.T. Do We Unknowingly Eat Breakfast That Contains Microplastics? Unveiling the Microplastic Contamination in Commercial Flour. Food Humanit. 2024, 3, 100333. [Google Scholar] [CrossRef]
- van der Veen, I.; van Mourik, L.M.; van Velzen, M.J.N.; Groenewoud, Q.R.; Leslie, H.A. Plastic Particles in Livestock Feed, Milk, Meat and Blood: A Pilot Study; Plastic Soup Foundation: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Bilal, M.; Taj, M.; Ul Hassan, H.; Yaqub, A.; Shah, M.; Sohail, M.; Rafiq, N.; Atique, U.; Abbas, M.; Sultana, S.; et al. First Report on Microplastics Quantification in Poultry Chicken and Potential Human Health Risks in Pakistan. Toxics 2023, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Milne, M.H.; De Frond, H.; Rochman, C.M.; Mallos, N.J.; Leonard, G.H.; Baechler, B.R. Exposure of U.S. Adults to Microplastics from Commonly-Consumed Proteins. Environ. Pollut. 2024, 343, 123233. [Google Scholar] [CrossRef]
- Mohsen, M.; Su, F.; Lin, J.; Li, X.; Lu, K.; Zhang, C. Microplastic Contamination in Aquafeed Ingredients Used as Protein and Carbohydrate Sources. Bull. Environ. Contam. Toxicol. 2024, 113, 41. [Google Scholar] [CrossRef]
- Yao, C.; Liu, X.; Wang, H.; Sun, X.; Qian, Q.; Zhou, J. Occurrence of Microplastics in Fish and Shrimp Feeds. Bull. Environ. Contam. Toxicol. 2021, 107, 684–692. [Google Scholar] [CrossRef]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef]
- Rabari, V.; Patel, H.; Ali, D.; Yadav, V.K.; Patel, A.; Sahoo, D.K.; Trivedi, J. Ingestion and Polymeric Risk Assessment of Microplastic Contamination in Commercially Important Brachyuran Crab Portunus sanguinolentus. Front. Mar. Sci. 2023, 10, 1286782. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, T.; Wang, J.; Huang, W.; Wang, R.; Xu, J.; Fu, G.; Gao, G. Spatio-Temporal Features of Microplastics Pollution in Macroalgae Growing in an Important Mariculture Area, China. Sci. Total Environ. 2020, 719, 137490. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the Commercial Seaweed Nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Zhang, Y.; Hong, F.; Mu, J.; Chen, M.; Song, P.; Lin, L.; Lin, H.; Le, F.; et al. Microplastic Contamination in Benthic Organisms from the Arctic and Sub-Arctic Regions. Chemosphere 2018, 209, 298–306. [Google Scholar] [CrossRef]
- Li, H.-X.; Ma, L.-S.; Lin, L.; Ni, Z.-X.; Xu, X.-R.; Shi, H.-H.; Yan, Y.; Zheng, G.-M.; Rittschof, D. Microplastics in Oysters Saccostrea cucullata along the Pearl River Estuary, China. Environ. Pollut. 2018, 236, 619–625. [Google Scholar] [CrossRef]
- Malloggi, C.; Nalbone, L.; Bartalena, S.; Guidi, M.; Corradini, C.; Foti, A.; Gucciardi, P.G.; Giarratana, F.; Susini, F.; Armani, A. The Occurrence of Microplastics in Donax trunculus (Mollusca: Bivalvia) Collected along the Tuscany Coast (Mediterranean Sea). Animals 2024, 14, 618. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and Mesoplastic Contamination in Canned Sardines and Sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Expósito, N.; Barrientos-Riosalido, A.; Santini, S.; Cincinelli, A.; Alcalde, L.; Castell, V.; Nadal, M.; Sierra, J.; Rovira, J. Microplastics Levels in Cultured or Harvested Mollusks Non-Depurated and Commercially Depurated at Different Times. Mar. Pollut. Bull. 2025, 212, 117568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics Contamination in Eggs: Detection, Occurrence and Status. Food Chem. 2022, 397, 133771. [Google Scholar] [CrossRef]
- Guo, X.; Dai, H.; Gukowsky, J.; Tan, X.; He, L. Detection and Quantification of Microplastics in Commercially Bottled Edible Oil. Food Packag. Shelf Life 2023, 38, 101122. [Google Scholar] [CrossRef]
- Battaglini, E.; Miralles, P.; Lotti, N.; Soccio, M.; Fiorini, M.; Coscollà, C. Analysis of Microplastics in Commercial Vegetable Edible Oils from Italy and Spain. Food Chem. 2024, 443, 138567. [Google Scholar] [CrossRef]
- Basaran, B.; Özçifçi, Z.; Kanbur, E.D.; Akçay, H.T.; Gül, S.; Bektaş, Y.; Aytan, Ü. Microplastics in Honey from Türkiye: Occurrence, Characteristic, Human Exposure, and Risk Assessment. J. Food Compos. Anal. 2024, 135, 106646. [Google Scholar] [CrossRef]
- Schiano, M.E.; D’Auria, L.J.; D’Auria, R.; Seccia, S.; Rofrano, G.; Signorelli, D.; Sansone, D.; Caprio, E.; Albrizio, S.; Cocca, M. Microplastic Contamination in the Agri-Food Chain: The Case of Honeybees and Beehive Products. Sci. Total Environ. 2024, 948, 174698. [Google Scholar] [CrossRef]
- Katsara, K.; Kenanakis, G.; Alissandrakis, E.; Papadakis, V. Honey Quality and Microplastic Migration from Food Packaging: A Potential Threat for Consumer Health? Microplastics 2022, 1, 406–427. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Rosazan, M.N.; Mamat, M.I.I.; Anuar, S.T.; Azmi, W.A. Detection of Microplastics in Honey of Stingless Bee (Heterotrigona itama) and Honey Bee (Apis mellifera) from Malaysia. Biodiversitas J. Biol. Divers. 2023, 26, 1271–1278. [Google Scholar] [CrossRef]
- Alma, A.M.; De Groot, G.S.; Buteler, M. Microplastics Incorporated by Honeybees from Food Are Transferred to Honey, Wax and Larvae. Environ. Pollut. 2023, 320, 121078. [Google Scholar] [CrossRef] [PubMed]
- Cledera-Castro, M.M.; Hueso-Kortekaas, K.; Sanchez-Mata, C.; Morales-Polo, C.; Calzada-Funes, J.; Delgado-Mellado, N.; Caro-Carretero, R. An Exploratory Study of Fibre Microplastics Pollution in Different Process Stages of Salt Production by Solar Evaporation in Spain. Heliyon 2024, 10, e31609. [Google Scholar] [CrossRef]
- Gündoğdu, S. Contamination of Table Salts from Turkey with Microplastics. Food Addit. Contam. Part A 2018, 35, 1006–1014. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The Presence of Microplastics in Commercial Salts from Different Countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Naghshbandi, M.; Ghaderzadeh, K.; Mirzabeigi, M.; Yazdanbakhsh, A.; Hossini, H. Micro-Plastic Occurrence in Bottled Vinegar: Qualification, Quantification and Human Risk Exposure. Process Saf. Environ. Prot. 2021, 152, 404–413. [Google Scholar] [CrossRef]
- Zbucki, Ł.; Plażuk, E. Mechanical Grinding of Spices in Grinders with Polymeric Burrs and Transfer of Microplastics to Food. Health Probl. Civiliz. 2024, 18, 339–353. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.A.; Almomani, F. Microplastics as Carriers of Toxic Pollutants: Source, Transport, and Toxicological Effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef]
- Oliveira, Y.M.; Vernin, N.S.; Maia Bila, D.; Marques, M.; Tavares, F.W. Pollution Caused by Nanoplastics: Adverse Effects and Mechanisms of Interaction via Molecular Simulation. PeerJ 2022, 10, e13618. [Google Scholar] [CrossRef]
- Fung, L.T.; Liu, M.; Yang, K.; Cao, Y.; Chen, Y.; Yan, M.; Leung, M.Y.K. Plasticizers Released from PVC Microplastics in Aquaculture Environments: Leaching Behaviors and Ecological Implications. J. Oceanol. Limnol. 2025, 43, 633–643. [Google Scholar] [CrossRef]
- Caruso, G. Microplastics as Vectors of Contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–Toxic Chemical Interaction: A Review Study on Quantified Levels, Mechanism and Implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Shi, Y.; Almuhtaram, H.; Andrews, R.C. Adsorption of Per- and Polyfluoroalkyl Substances (PFAS) and Microcystins by Virgin and Weathered Microplastics in Freshwater Matrices. Polymers 2023, 15, 3676. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and Effects of Microplastics with Heavy Metals in Aquatic and Terrestrial Environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Lerner, M.I.; Bakina, O.V.; Glazkova, E.A.; Lozhkomoev, A.S.; Svarovskaya, N.V.; Psakh’e, S.G. Adsorption of Microorganisms and Bacterial Endotoxin on Modified Polymer Fibers. Inorg. Mater. Appl. Res. 2011, 2, 488–492. [Google Scholar] [CrossRef]
- Yu, F.; Qin, Q.; Zhang, X.; Ma, J. Characteristics and Adsorption Behavior of Typical Microplastics in Long-Term Accelerated Weathering Simulation. Environ. Sci. Process. Impacts 2024, 26, 882–890. [Google Scholar] [CrossRef]
- Fan, X.; Gan, R.; Liu, J.; Xie, Y.; Xu, D.; Xiang, Y.; Su, J.; Teng, Z.; Hou, J. Adsorption and Desorption Behaviors of Antibiotics by Tire Wear Particles and Polyethylene Microplastics with or without Aging Processes. Sci. Total Environ. 2021, 771, 145451. [Google Scholar] [CrossRef]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between Microplastics, Pharmaceuticals and Personal Care Products: Implications for Vector Transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Razanajatovo, R.M.; Ding, J.; Zhang, S.; Jiang, H.; Zou, H. Sorption and Desorption of Selected Pharmaceuticals by Polyethylene Microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Thio, B.J.R.; Lee, J.-H.; Meredith, J.C. Characterization of Ragweed Pollen Adhesion to Polyamides and Polystyrene Using Atomic Force Microscopy. Environ. Sci. Technol. 2009, 43, 4308–4313. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Hu, W.; Zhao, J.; Lu, J.; Zhang, W.; Liu, X.; Lu, S.; Shinichi, Y.; Ebere, E.C.; Wang, Q.; et al. Studies on Adsorption and Synergistic Biological Effects Induced by Microplastic Particles and the Platanus Pollen Allergenic Protein 3(Pla A3). Environ. Pollut. 2025, 373, 126149. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption Behavior and Mechanism of Five Pesticides on Microplastics from Agricultural Polyethylene Films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef] [PubMed]
- Concha-Graña, E.; Moscoso-Pérez, C.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Adsorption of Pesticides and Personal Care Products on Pristine and Weathered Microplastics in the Marine Environment. Comparison between Bio-Based and Conventional Plastics. Sci. Total Environ. 2022, 848, 157703. [Google Scholar] [CrossRef]
- Gkoutselis, G.; Rohrbach, S.; Harjes, J.; Obst, M.; Brachmann, A.; Horn, M.A.; Rambold, G. Microplastics Accumulate Fungal Pathogens in Terrestrial Ecosystems. Sci. Rep. 2021, 11, 13214. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Z.; Ngiam, L.; Guo, J. Microplastics as Potential Carriers of Viruses Could Prolong Virus Survival and Infectivity. Water Res. 2022, 225, 119115. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, M.; Sonne, C.; Lam, S.S.; Kwong, R.W.M.; Jiang, Y.; Zhao, X.; Sun, X.; Zhang, X.; Li, C.; et al. The Hidden Risk of Microplastic-Associated Pathogens in Aquatic Environments. Eco-Environ. Health 2023, 2, 142–151. [Google Scholar] [CrossRef]
- Mammo, F.K.; Amoah, I.D.; Gani, K.M.; Pillay, L.; Ratha, S.K.; Bux, F.; Kumari, S. Microplastics in the Environment: Interactions with Microbes and Chemical Contaminants. Sci. Total Environ. 2020, 743, 140518. [Google Scholar] [CrossRef]
- Thio, B.J.R.; Meredith, J.C. Quantification of E. coli Adhesion to Polyamides and Polystyrene with Atomic Force Microscopy. Colloids Surf. B Biointerfaces 2008, 65, 308–312. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous Hitchhikers? Evidence for Potentially Pathogenic Vibrio Spp. on Microplastic Particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef]
- Jian, M.; Niu, J.; Li, W.; Huang, Y.; Yu, H.; Lai, Z.; Liu, S.; Xu, E.G. How Do Microplastics Adsorb Metals? A Preliminary Study under Simulated Wetland Conditions. Chemosphere 2022, 309, 136547. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, A.; Carcano, A.M.; Crago, J.; Green, M.J.; Shah, S.A.; Cañas-Carrell, J.E. Translocation, Trophic Transfer, Accumulation and Depuration of Polystyrene Microplastics in Daphnia magna and Pimephales promelas. Environ. Pollut. 2020, 259, 113937. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Zhao, X.; Zhao, T.; Wu, X.; Pu, S.; Tang, Z.; Wu, F. The Adsorption of PAHs on Microplastics and Desorption in the Simulated Human Digestive System. Chem. Eng. J. 2023, 473, 145157. [Google Scholar] [CrossRef]
- Li, W.; Zu, B.; Li, L.; Li, J.; Li, J.; Mei, X. Desorption of Bisphenol A from Microplastics under Simulated Gastrointestinal Conditions. Front. Mar. Sci. 2023, 10, 1195964. [Google Scholar] [CrossRef]
- Emecheta, E.E.; Pfohl, P.M.; Wohlleben, W.; Haase, A.; Roloff, A. Desorption of Polycyclic Aromatic Hydrocarbons from Microplastics in Human Gastrointestinal Fluid Simulants—Implications for Exposure Assessment. ACS Omega 2024, 9, 24281–24290. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Data on Sorption of Organic Compounds by Aged Polystyrene Microplastic Particles. Data Brief 2018, 18, 474–479. [Google Scholar] [CrossRef]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Hui, J.; Irvine, J.T.S.; Edwards, C.; Lawton, L.A. Adsorption of Cyanotoxins on Polypropylene and Polyethylene Terephthalate: Microplastics as Vector of Eight Microcystin Analogues. Environ. Pollut. 2022, 303, 119135. [Google Scholar] [CrossRef]
- O’Donovan, S.; Mestre, N.C.; Abel, S.; Fonseca, T.G.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Bebianno, M.J. Ecotoxicological Effects of Chemical Contaminants Adsorbed to Microplastics in the Clam Scrobicularia Plana. Front. Mar. Sci. 2018, 5, 143. [Google Scholar] [CrossRef]
- Wan, X.; Zhao, Y.; Xu, X.; Li, Z.; Xie, L.; Wang, G.; Yang, F. Microcystin Bound on Microplastics in Eutrophic Waters: A Potential Threat to Zooplankton Revealed by Adsorption-Desorption Processes. Environ. Pollut. 2023, 321, 121146. [Google Scholar] [CrossRef]
- Beans, C. Are Microplastics Spreading Infectious Disease? Proc. Natl. Acad. Sci. USA 2023, 120, e2311253120. [Google Scholar] [CrossRef]
- Pham, D.N.; Clark, L.; Li, M. Microplastics as Hubs Enriching Antibiotic-Resistant Bacteria and Pathogens in Municipal Activated Sludge. J. Hazard. Mater. Lett. 2021, 2, 100014. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Wu, J.; Wang, J.; Luo, Y. Potential Risks of Microplastics Combined with Superbugs: Enrichment of Antibiotic Resistant Bacteria on the Surface of Microplastics in Mariculture System. Ecotoxicol. Environ. Saf. 2020, 187, 109852. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.D.; Klaiber, M.; Moreno-Gordaliza, E.; Barata, A.; Gómez-Gómez, M.M. Safety Assessment of Commercial Antimicrobial Food Packaging: Triclosan and Microplastics, a Closer Look. Food Packag. Shelf Life 2022, 31, 100780. [Google Scholar] [CrossRef]
- Smallwood, K. Who Invented the Food Pyramid? Today Found It Feed Your Brain 2013. Available online: https://www.todayifoundout.com/index.php/2013/09/invented-food-pyramid/ (accessed on 7 July 2025).
- Piramida—Food and Nutrition Institute. Available online: https://ncez.pzh.gov.pl/wp-content/uploads/2021/03/piramida-dla-doroslych-opis866.pdf (accessed on 5 September 2025).
- FAO Web Page—Dietary Guidelines. Available online: https://www.fao.org/nutrition/education/dietary-guidelines/home/en/ (accessed on 5 July 2025).
- Food-Based Dietary Guidelines in Europe: Source Documents|Knowledge for Policy. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/food-based-dietary-guidelines-europe-source-documents-food_en (accessed on 13 July 2025).
- FAO Food-Based Dietary Guidelines—China. Available online: http://www.fao.org/nutrition/education/dietary-guidelines/regions/china/en/ (accessed on 8 July 2025).
- FAO Food-Based Dietary Guidelines—Bolivia (Plurinational State of). Available online: http://www.fao.org/nutrition/education/dietary-guidelines/regions/bolivia-plurinational-state-of/en/ (accessed on 8 July 2025).
- Arceño, M.A. On Consuming and Constructing Material and Symbolic Culture: An Anthropology of Pictorial Representations of Food-Based Dietary Guidelines (FBDGs). Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2016. [Google Scholar]
- The Food Pyramid: A Dietary Guideline in Europe. Available online: https://www.eufic.org/en/healthy-living/article/food-based-dietary-guidelines-in-europe (accessed on 8 July 2025).
- Armocida, B.; Klepp, K.-I.; Onder, G.; Louise Farrington, J.; Granlund, L.; Takki, M.; Mikkelsen, B.; Cecchini, M.; Galea, G.; Rakovac, I. Advancing Europe’s Non-Communicable Diseases Agenda through Cross-National Collaboration: Translating WHO-Europe Findings into Actionable Strategies. Lancet Reg. Health Eur. 2025, 55, 101361. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/FBS/visualize (accessed on 14 July 2025).
- World Health Organization. Food-Based Dietary Guidelines in the WHO European Region; World Health Organization: Copenhagen, Denmark, 2003; Available online: https://iris.who.int/handle/10665/107490 (accessed on 5 September 2025).
- FDGB FAO. Available online: http://www.fao.org/nutrition/education/dietary-guidelines/regions/countries/en/ (accessed on 20 August 2025).
- Webb, S.; Ruffell, H.; Marsden, I.; Pantos, O.; Gaw, S. Microplastics in the New Zealand Green Lipped Mussel Perna Canaliculus. Mar. Pollut. Bull. 2019, 149, 110641. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Köşker, A.R. Microplastic Contamination in Canned Fish Sold in Türkiye. PeerJ 2023, 11, e14627. [Google Scholar] [CrossRef]
- Silva, D.M.; Almeida, C.M.R.; Guardiola, F.A.; Pereira, R.; Rodrigues, S.M.; Ramos, S. Uncovering Microplastics Contamination in Canned Seafood. Food Chem. 2024, 448, 139049. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Microplastics in the Edible and Inedible Tissues of Pelagic Fishes Sold for Human Consumption in Kerala, India. Environ. Pollut. 2020, 266, 115365. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Otero, X.L.; Guilhermino, L. Microplastic Contamination in Marine Mussels from the Atlantic Coast of North Portugal and Human Risk of Microplastic Intake through Mussel Consumption. Environ. Pollut. 2024, 352, 124133. [Google Scholar] [CrossRef]
- Rabari, V.; Rakib, M.R.J.; Patel, H.; Idris, A.M.; Malafaia, G.; Trivedi, J. Microplastic Prevalence in Epipelagic Layer: Evidence from Epipelagic Inhabiting Prawns of North-West Arabian Sea. Mar. Pollut. Bull. 2024, 200, 116137. [Google Scholar] [CrossRef] [PubMed]
- Sambolino, A.; Iniguez, E.; Herrera, I.; Kaufmann, M.; Dinis, A.; Cordeiro, N. Microplastic Ingestion and Plastic Additive Detection in Pelagic Squid and Fish: Implications for Bioindicators and Plastic Tracers in Open Oceanic Food Webs. Sci. Total Environ. 2023, 894, 164952. [Google Scholar] [CrossRef] [PubMed]
- Pennino, M.G.; Bachiller, E.; Lloret-Lloret, E.; Albo-Puigserver, M.; Esteban, A.; Jadaud, A.; Bellido, J.M.; Coll, M. Ingestion of Microplastics and Occurrence of Parasite Association in Mediterranean Anchovy and Sardine. Mar. Pollut. Bull. 2020, 158, 111399. [Google Scholar] [CrossRef] [PubMed]
- Fraissinet, S.; Arduini, D.; Martines, A.; De Benedetto, G.E.; Malitesta, C.; Giangrande, A.; Rossi, S. Microplastics Uptake by Four Filter Feeders. J. Mar. Sci. Eng. 2024, 12, 1000. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mahagamage, M.G.Y.L.; Gajanayake, P.; Abeynayaka, A.; Gamaralalage, P.J.D.; Ohgaki, M.; Takenaka, M.; Fukai, T.; Itsubo, N. Microplastics and Potentially Toxic Elements: Potential Human Exposure Pathways through Agricultural Lands and Policy Based Countermeasures. Microplastics 2022, 1, 102–120. [Google Scholar] [CrossRef]
- Meng, F.; Yang, X.; Riksen, M.; Xu, M.; Geissen, V. Response of Common Bean (Phaseolus vulgaris L.) Growth to Soil Contaminated with Microplastics. Sci. Total Environ. 2021, 755, 142516. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; Lee, J.; Choi, M.-J.; Kweon, H.-S.; An, Y.-J. Evidence of Parental Transfer of Nanoplastics in Pea (Pisum sativum) Plants. J. Hazard. Mater. 2024, 465, 133516. [Google Scholar] [CrossRef]
- Slavin, J.; Carlson, J. Carbohydrates1. Adv. Nutr. 2014, 5, 760–761. [Google Scholar] [CrossRef]
- Dessì, C.; Okoffo, E.D.; O’Brien, J.W.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Rauert, C.; Wang, X.; Thomas, K.V. Plastics Contamination of Store-Bought Rice. J. Hazard. Mater. 2021, 416, 125778. [Google Scholar] [CrossRef]
- Masciarelli, E.; Casorri, L.; Di Luigi, M.; Beni, C.; Valentini, M.; Costantini, E.; Aielli, L.; Reale, M. Microplastics in Agricultural Crops and Their Possible Impact on Farmers’ Health: A Review. Int. J. Environ. Res. Public Health 2024, 22, 45. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and Microplastics: A Comprehensive Review on Their Exposure Routes, Translocation, and Fate in Humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Li, H.; Chang, X.; Zhang, J.; Wang, Y.; Zhong, R.; Wang, L.; Wei, J.; Wang, Y. Uptake and Distribution of Microplastics of Different Particle Sizes in Maize (Zea mays) Seedling Roots. Chemosphere 2023, 313, 137491. [Google Scholar] [CrossRef]
- Shi, Y.; Yi, L.; Du, G.; Hu, X.; Huang, Y. Visual Characterization of Microplastics in Corn Flour by near Field Molecular Spectral Imaging and Data Mining. Sci. Total Environ. 2023, 862, 160714. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, S.; Pang, L.; Sun, C.; Chen, L.; Li, F. Potential Risk of Microplastics in Processed Foods: Preliminary Risk Assessment Concerning Polymer Types, Abundance, and Human Exposure of Microplastics. Ecotoxicol. Environ. Saf. 2022, 247, 114260. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, M.; Cüvelek, M.A. Abundance of Microplastics in the Agro-Industrial Product Beet Sugar; Food or Plastifood. Process Saf. Environ. Prot. 2024, 188, 467–479. [Google Scholar] [CrossRef]
- Buyukunal, S.; Rbaibi Zipak, S.; Muratoglu, K. Microplastics in a Traditional Turkish Dairy Product: Ayran. Pol. J. Food Nutr. Sci. 2023, 73, 139–150. [Google Scholar] [CrossRef]
- Adjama, I.; Dave, H.; Balarabe, B.Y.; Masiyambiri, V.; Marycleopha, M. Microplastics in Dairy Products and Human Breast Milk: Contamination Status and Greenness Analysis of Available Analytical Methods. J. Hazard. Mater. Lett. 2024, 5, 100120. [Google Scholar] [CrossRef]
- Mondal, R.; Sarkar, D.J.; Bhattacharyya, S.; Raja, R.; Chaudhuri, P.; Biswas, J.K.; Kumar Das, B. Health Risk Assessment of Microplastics Contamination in the Daily Diet of South Asian Countries. J. Hazard. Mater. 2025, 494, 138527. [Google Scholar] [CrossRef]
- Ribeiro, V.V.; Nobre, C.R.; Moreno, B.B.; Semensatto, D.; Sanz-Lazaro, C.; Moreira, L.B.; Castro, Í.B. Oysters and Mussels as Equivalent Sentinels of Microplastics and Natural Particles in Coastal Environments. Sci. Total Environ. 2023, 874, 162468. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.; Su, L.; Kellar, C.; Craig, N.J.; Keough, M.J.; Pettigrove, V. Identification of Microplastics in Surface Water and Australian Freshwater Shrimp Paratya australiensis in Victoria, Australia. Environ. Pollut. 2020, 259, 113865. [Google Scholar] [CrossRef] [PubMed]
- Reunura, T.; Prommi, T.O. Detection of Microplastics in Litopenaeus vannamei (Penaeidae) and Macrobrachium rosenbergii (Palaemonidae) in Cultured Pond. PeerJ 2022, 10, e12916. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, X.; Ren, T.; Wang, J.; Shan, J. Microplastics Contamination in Food and Beverages: Direct Exposure to Humans. J. Food Sci. 2021, 86, 2816–2837. [Google Scholar] [CrossRef]
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Seafood Intended for Human Consumption: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2020, 128, 126002. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Aleluia, A.A.M.R.A.; Barboza, L.G.A.; Otero, X.L.; Cunha, S.C.; Fernandes, J.O.; Guilhermino, L. Microplastic Contamination and Biological Alterations in Atlantic Wild Fish Populations, and Human Health Risks Associated to Fillet Consumption. Mar. Environ. Res. 2025, 208, 107139. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Nabipour, I.; Tajbakhsh, S.; Darabi, A.H.; Spitz, J. Abundance, Composition, and Potential Intake of Microplastics in Canned Fish. Mar. Pollut. Bull. 2020, 160, 111633. [Google Scholar] [CrossRef]
- Banica, A.L.; Radulescu, C.; Maria, R.; Dulama, I.D.; Bucurica, I.A.; Stanescu, G.; Stirbescu, N.M. Microplastics Contamination of Dairy Products with High-Fat Content—Occurrence and Associated Risks. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2024, 86, 85–108. [Google Scholar]
- Chen, H.; Xu, L.; Yu, K.; Wei, F.; Zhang, M. Release of Microplastics from Disposable Cups in Daily Use. Sci. Total Environ. 2023, 854, 158606. [Google Scholar] [CrossRef]
- Kashfi, F.S.; Mohammadi, A.; Rostami, F.; Savari, A.; De-la-Torre, G.E.; Spitz, J.; Saeedi, R.; Kalantarhormozi, M.; Farhadi, A.; Dobaradaran, S. Microplastics and Phthalate Esters Release from Teabags into Tea Drink: Occurrence, Human Exposure, and Health Risks. Environ. Sci. Pollut. Res. 2023, 30, 104209–104222. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Drinking Water: A Systematic Review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef]
- Altunışık, A. Prevalence of Microplastics in Commercially Sold Soft Drinks and Human Risk Assessment. J. Environ. Manag. 2023, 336, 117720. [Google Scholar] [CrossRef]
- Danopoulos, E.; Jenner, L.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Salt Intended for Human Consumption: A Systematic Review and Meta-Analysis. SN Appl. Sci. 2020, 2, 1950. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, B.; Ye, R.; Yu, N.; Xie, Z.; Hua, Y.; Zhou, R.; Tian, B.; Dai, S. Evidence and Impacts of Nanoplastic Accumulation on Crop Grains. Adv. Sci. 2022, 9, e2202336. [Google Scholar] [CrossRef]
- Sofi, F.; Martini, D.; Angelino, D.; Cairella, G.; Campanozzi, A.; Danesi, F.; Dinu, M.; Erba, D.; Iacoviello, L.; Pellegrini, N.; et al. Mediterranean Diet: Why a New Pyramid? An Updated Representation of the Traditional Mediterranean Diet by the Italian Society of Human Nutrition (SINU). Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103919. [Google Scholar] [CrossRef]
- Vitiello, V.; Germani, A.; Capuzzo Dolcetta, E.; Donini, L.M.; Del Balzo, V. The New Modern Mediterranean Diet Italian Pyramid. Ann. Ig. Med. Prev. E Comunità 2016, 28, 179–186. [Google Scholar] [CrossRef]
- Renzella, J.; Townsend, N.; Jewell, J.; Breda, J.; Roberts, N.; Rayner, M.; Wickramasinghe, K. What National and Subnational Interventions and Policies Based on Mediterranean and Nordic Diets Are Recommended or Implemented in the WHO European Region: And Is There Evidence of Effectiveness in Reducing Noncommunicable Diseases? World Health Organization: Copenhagen, Denmark, 2018; ISBN 978-92-890-5301-3. [Google Scholar]
- Shively, C.A.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Michalson, K.T.; Silverstein-Metzler, M.G.; Register, T.C. Mediterranean versus Western Diet Effects on Caloric Intake, Obesity, Metabolism, and Hepatosteatosis in Nonhuman Primates. Obesity 2019, 27, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Rolands, M.R.; Hackl, L.S.; Bochud, M.; Lê, K.A. Protein Adequacy, Plant Protein Proportion, and Main Plant Protein Sources Consumed Across Vegan, Vegetarian, Pescovegetarian, and Semivegetarian Diets: A Systematic Review. J. Nutr. 2025, 155, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Duster, M.; Roberts, T.; Devinsky, O. United States Dietary Trends Since 1800: Lack of Association Between Saturated Fatty Acid Consumption and Non-Communicable Diseases. Front. Nutr. 2022, 8, 748847. [Google Scholar] [CrossRef]
- Steele, E.M.; O’Connor, L.E.; Juul, F.; Khandpur, N.; Galastri Baraldi, L.; Monteiro, C.A.; Parekh, N.; Herrick, K.A. Identifying and Estimating Ultraprocessed Food Intake in the US NHANES According to the Nova Classification System of Food Processing. J. Nutr. 2023, 153, 225–241. [Google Scholar] [CrossRef]
- Silva, M.G.; Oliveira, M.M.; Peixoto, F. The Impact of Micro-Nanoplastics on Mitochondria in the Context of Diet and Diet-Related Diseases. Stresses 2025, 5, 12. [Google Scholar] [CrossRef]
- Haddad, E.H.; Sabaté, J.; Whitten, C.G. Vegetarian Food Guide Pyramid: A Conceptual Framework. Am. J. Clin. Nutr. 1999, 70, 615S–619S. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.M.; Comeau, M.E.; Swanson, K.; Burbank, M. Modeling Ovo-Vegetarian, Lacto-Vegetarian, Pescatarian, and Vegan USDA Food Patterns and Assessing Nutrient Adequacy for Lactation among Adult Females. Curr. Dev. Nutr. 2023, 7, 102034. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hu, J.; Li, Y.; Yang, S.; Li, X.; Hou, T.; Ning, Z.; Ma, C.; Yuan, X.; Wang, Z.; et al. Lacto-Ovo-Vegetarian Diet Is Inversely Associated with the Osteosarcopenia in Older Adults. BMC Geriatr. 2024, 24, 332. [Google Scholar] [CrossRef]
- Venti, C.A.; Johnston, C.S. Modified Food Guide Pyramid for Lactovegetarians and Vegans. J. Nutr. 2002, 132, 1050–1054. [Google Scholar] [CrossRef]
- Ruggieri, F.; Battistini, B.; Sorbo, A.; Senofonte, M.; Leso, V.; Iavicoli, I.; Bocca, B. From Food-to-Human Microplastics and Nanoplastics Exposure and Health Effects: A Review on Food, Animal and Human Monitoring Data. Food Chem. Toxicol. 2025, 196, 115209. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Chan, H.; Peng, J.; Zhu, P.; Li, J.; Jiang, X.; Zhang, Z.; Wang, Y.; Tan, Z.; et al. Polystyrene Microplastics Induce Size-Dependent Multi-Organ Damage in Mice: Insights into Gut Microbiota and Fecal Metabolites. J. Hazard. Mater. 2024, 461, 132503. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Microplastics in Our Diet: A Growing Concern for Human Health. Sci. Total Environ. 2025, 968, 178882. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M.; Xu, X. Microplastics in Food: Sources, Distribution, Health Impacts, and Regulation. J. Food Compos. Anal. 2025, 140, 107274. [Google Scholar] [CrossRef]
- Garcia, M.A.; Liu, R.; Nihart, A.; El Hayek, E.; Castillo, E.; Barrozo, E.R.; Suter, M.A.; Bleske, B.; Scott, J.; Forsythe, K.; et al. Quantitation and Identification of Microplastics Accumulation in Human Placental Specimens Using Pyrolysis Gas Chromatography Mass Spectrometry. Toxicol. Sci. 2024, 199, 81–88. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of Various Microplastics in Placentas, Meconium, Infant Feces, Breastmilk and Infant Formula: A Pilot Prospective Study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-W.; Lim, J.Y.; Yeoh, Y.K.; Chiou, J.-C.; Zhu, Y.; Lai, K.P.; Li, L.; Chan, P.K.S.; Fang, J.K.-H. Preliminary Findings of the High Quantity of Microplastics in Faeces of Hong Kong Residents. Toxics 2022, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Refosco, A.; Dierkes, J.; Kögel, T.; Dankel, S.N.; Laupsa-Borge, J.; Gomiero, A.; Daniel, D.B. Microplastics in Human Feces: A Pilot Study Exploring Links with Dietary Habits. Microplastics Nanoplastics 2025, 5, 22. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You Are What You Eat: Microplastics in the Feces of Young Men Living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using μFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Exacoustos, O.; Artini, C.; Massardo, S.; Caboni, C.; Pastorino, A.; Chiarenza, S.; Zaza, G.; Stallone, G.; Ghiggeri, G.M.; Angeletti, A.; et al. #6111 First Identification and Characterization of Microplastics in Human Kidney and Urine. Nephrol. Dial. Transplant. 2023, 38, gfad063a_6111. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Ceriello, A.; Pellegrini, V.; La Grotta, R.; Graciotti, L.; Olivieri, F.; Paolisso, P.; D’Agostino, B.; Iovino, P.; Balestrieri, M.L.; et al. Micro-Nanoplastics and Cardiovascular Diseases: Evidence and Perspectives. Eur. Heart J. 2024, 45, 4099–4110. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of Microplastics in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Human Exposure to Microplastics: A Study in Iran. J. Hazard. Mater. 2021, 403, 123799. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Xu, H.; Lin, Y.; Zhang, B.; Yu, Z.; Xie, Y.; Yu, J.; Ma, D. Microplastics Detected in Three Types of Female Reproductive Organs Using Micro-Raman Spectroscopy. Ecotoxicol. Environ. Saf. 2024, 285, 117099. [Google Scholar] [CrossRef] [PubMed]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and Additives in Patients with Preterm Birth: The First Evidence of Their Presence in Both Human Amniotic Fluid and Placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Austin, C.; Chapman, E.; Atherall, C.A.; Liddle, C.R.; Dunstan, T.S.; Blackburn, B.; Mead, A.; Filart, K.; Beeby, E.; et al. Microplastics in Human Urine: Characterisation Using μFTIR and Sampling Challenges Using Healthy Donors and Endometriosis Participants. Ecotoxicol. Environ. Saf. 2024, 274, 116208. [Google Scholar] [CrossRef]
- Barnett, A.N.; Arshad, M.; Nabi, D. A Snapshot into the Invasion of Plastics in Human Urine. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Montano, L.; Giorgini, E.; Notarstefano, V.; Notari, T.; Ricciardi, M.; Piscopo, M.; Motta, O. Raman Microspectroscopy Evidence of Microplastics in Human Semen. Sci. Total Environ. 2023, 901, 165922. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Rajendran, D.; Chandrasekaran, N. Journey of Micronanoplastics with Blood Components. RSC Adv. 2023, 13, 31435–31459. [Google Scholar] [CrossRef]
- Baeza-Martínez, C.; Olmos, S.; González-Pleiter, M.; López-Castellanos, J.; García-Pachón, E.; Masiá-Canuto, M.; Hernández-Blasco, L.; Bayo, J. First Evidence of Microplastics Isolated in European Citizens’ Lower Airway. J. Hazard. Mater. 2022, 438, 129439. [Google Scholar] [CrossRef]
- Wu, D.; Feng, Y.; Wang, R.; Jiang, J.; Guan, Q.; Yang, X.; Wei, H.; Xia, Y.; Luo, Y. Pigment Microparticles and Microplastics Found in Human Thrombi Based on Raman Spectral Evidence. J. Adv. Res. 2023, 49, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Gambardella, L.; Bozzuto, G.; Vona, R.; Caruso, D.; Villari, V.; Cappello, T.; Maisano, M.; Dossena, S.; et al. Internalization of Nano- and Micro-Plastics in Human Erythrocytes Leads to Oxidative Stress and Estrogen Receptor-Mediated Cellular Responses. Free Radic. Biol. Med. 2024, 223, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, C.; Yang, Y.; Du, Z.; Li, L.; Zhang, M.; Ni, S.; Yue, Z.; Yang, K.; Wang, Y.; et al. Microplastics in Three Types of Human Arteries Detected by Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS). J. Hazard. Mater. 2024, 469, 133855. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Jenner, L.C.; Chapman, E.; Bennett, R.T.; Bolanle, I.O.; Loubani, M.; Sadofsky, L.; Hobkirk, J.; Palmer, T.M. Detection of Microplastics in Human Saphenous Vein Tissue Using μFTIR: A Pilot Study. PLoS ONE 2023, 18, e0280594. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, C.; Liu, Y.; Li, W.; Li, S.; Peng, L.; Kang, L.; Ullah, S.; Gong, Z.; Li, Z.; et al. Microplastics Are Detected in Human Gallstones and Have the Ability to Form Large Cholesterol-Microplastic Heteroaggregates. J. Hazard. Mater. 2024, 467, 133631. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Shi, L.; Jia, Y.; Sheng, H. Detection and Quantification of Microplastics in Various Types of Human Tumor Tissues. Ecotoxicol. Environ. Saf. 2024, 283, 116818. [Google Scholar] [CrossRef]
- Deng, C.; Zhu, J.; Fang, Z.; Yang, Y.; Zhao, Q.; Zhang, Z.; Jin, Z.; Jiang, H. Identification and Analysis of Microplastics in Para-Tumor and Tumor of Human Prostate. eBioMedicine 2024, 108, 105360. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-Dependent Proinflammatory Effects of Ultrafine Polystyrene Particles: A Role for Surface Area and Oxidative Stress in the Enhanced Activity of Ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef]
- Wu, H.; Xu, T.; Chen, T.; Liu, J.; Xu, S. Oxidative Stress Mediated by the TLR4/NOX2 Signalling Axis Is Involved in Polystyrene Microplastic-Induced Uterine Fibrosis in Mice. Sci. Total Environ. 2022, 838, 155825. [Google Scholar] [CrossRef]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological Effects, Including Oxidative Stress and Genotoxic Damage, of Polystyrene Nanoparticles in Different Human Hematopoietic Cell Lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Jeong, D.; Choi, J.-W.; Jeong, S.; Kim, H.; Song, B.-W.; Lim, S.; Kim, I.-K.; Lee, S.; Kim, S.W. Microplastic Exposure Linked to Accelerated Aging and Impaired Adipogenesis in Fat Cells. Sci. Rep. 2024, 14, 23920. [Google Scholar] [CrossRef] [PubMed]
- Bang, E.; Hwangbo, H.; Lee, H.; Park, C.; Hong, S.H.; Kim, H.S.; Jung, Y.; Hyun, Y.-M.; Hyun, J.W.; Kim, G.-Y.; et al. Exposure to Polystyrene Nanoplastics Promotes Premature Cellular Senescence through Mitochondrial ROS Production and Dysfunction in Pre-Differentiated Skeletal Myoblasts. Toxicology 2025, 510, 154002. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, Y.; Huang, J.; Xu, W. Polystyrene Microplastics Induces Senescence of Osteocytes by Activating the Cyclooxygenase-2 Signaling Pathway. Ecotoxicol. Environ. Saf. 2025, 300, 118466. [Google Scholar] [CrossRef]
- Christodoulides, A.; Hall, A.; Alves, N.J. Exploring Microplastic Impact on Whole Blood Clotting Dynamics Utilizing Thromboelastography. Front. Public Health 2023, 11, 1215817. [Google Scholar] [CrossRef]
- Huang, H.; Hou, J.; Li, M.; Wei, F.; Liao, Y.; Xi, B. Microplastics in the Bloodstream Can Induce Cerebral Thrombosis by Causing Cell Obstruction and Lead to Neurobehavioral Abnormalities. Sci. Adv. 2025, 11, eadr8243. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Liu, L.; Zhang, Q.; Xu, S.; Guo, M. Polystyrene Microplastics Induced Inflammation with Activating the TLR2 Signal by Excessive Accumulation of ROS in Hepatopancreas of Carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2023, 251, 114539. [Google Scholar] [CrossRef]
- Wang, L.; Pei, W.; Li, J.; Feng, Y.; Gao, X.; Jiang, P.; Wu, Q.; Li, L. Microplastics Induced Apoptosis in Macrophages by Promoting ROS Generation and Altering Metabolic Profiles. Ecotoxicol. Environ. Saf. 2024, 271, 115970. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ye, S.; Tang, S.; Liang, N.; Liang, Y.; Xiao, F. Polystyrene Microplastics Trigger Hepatocyte Apoptosis and Abnormal Glycolytic Flux via ROS-Driven Calcium Overload. J. Hazard. Mater. 2021, 417, 126025. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, D.; Zhang, Y.; Lu, H.; Hou, L.; Guo, T.; Zhao, H.; Xing, M. Polystyrene Microplastics Promote Liver Inflammation by Inducing the Formation of Macrophages Extracellular Traps. J. Hazard. Mater. 2023, 452, 131236. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, J.; Wang, W.; Gonzalez-Gil, G.; Vrouwenvelder, J.S.; Li, Z. Effects of Nano- and Microplastics on Kidney: Physicochemical Properties, Bioaccumulation, Oxidative Stress and Immunoreaction. Chemosphere 2022, 288, 132631. [Google Scholar] [CrossRef] [PubMed]
- Das, A. The Emerging Role of Microplastics in Systemic Toxicity: Involvement of Reactive Oxygen Species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, Y.; Bai, H.; Shimizu, K.; Li, R.; Zhang, C. Investigation of Pulmonary Toxicity Evaluation on Mice Exposed to Polystyrene Nanoplastics: The Potential Protective Role of the Antioxidant N-Acetylcysteine. Sci. Total Environ. 2023, 855, 158851. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene Microplastics Induce Hepatotoxicity and Disrupt Lipid Metabolism in the Liver Organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Yin, J.; Zhu, Q.; Liao, C.; Jiang, G. Neurotoxicities Induced by Micro/Nanoplastics: A Review Focusing on the Risks of Neurological Diseases. J. Hazard. Mater. 2024, 469, 134054. [Google Scholar] [CrossRef]
- Wang, W.; Guan, J.; Feng, Y.; Nie, L.; Xu, Y.; Xu, H.; Fu, F. Polystyrene Microplastics Induced Nephrotoxicity Associated with Oxidative Stress, Inflammation, and Endoplasmic Reticulum Stress in Juvenile Rats. Front. Nutr. 2023, 9, 1059660. [Google Scholar] [CrossRef]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and Cytotoxic Effects of Polyethylene Microplastics on Human Peripheral Blood Lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; Oliveira, E.C.S.D.; Stasi, L.C.D.; Sassaki, L.Y. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A Potential Threat to Human and Animal Health by Interfering with the Intestinal Barrier Function and Changing the Intestinal Microenvironment. Sci. Total Environ. 2021, 785, 147365. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dovizio, M.; Milillo, C.; Aruffo, E.; Pesce, M.; Gatta, M.; Chiacchiaretta, P.; Di Carlo, P.; Ballerini, P. Orally Ingested Micro- and Nano-Plastics: A Hidden Driver of Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 3079. [Google Scholar] [CrossRef]
- Schwarzfischer, M.; Niechcial, A.; Lee, S.S.; Sinnet, B.; Wawrzyniak, M.; Laimbacher, A.; Atrott, K.; Manzini, R.; Morsy, Y.; Häfliger, J.; et al. Ingested Nano- and Microsized Polystyrene Particles Surpass the Intestinal Barrier and Accumulate in the Body. NanoImpact 2022, 25, 100374. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, D.; Zhan, J.; Liu, X.; Li, P.; Ma, X.; Hou, H.; Wang, P. Polystyrene Microplastics Induce Kidney Injury via Gut Barrier Dysfunction and C5a/C5aR Pathway Activation. Environ. Pollut. 2024, 342, 122909. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl Chloride Microplastics Induced Gut Barrier Dysfunction, Microbiota Dysbiosis and Metabolism Disorder in Adult Mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Ghosal, S.; Bag, S.; Rao, S.R.; Bhowmik, S. Exposure to Polyethylene Microplastics Exacerbate Inflammatory Bowel Disease Tightly Associated with Intestinal Gut Microflora. RSC Adv. 2024, 14, 25130–25148. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, R.; Li, Z.; Liu, C.; Chen, Y.; Yu, Q. Microplastics Perturb Colonic Epithelial Homeostasis Associated with Intestinal Overproliferation, Exacerbating the Severity of Colitis. Environ. Res. 2023, 217, 114861. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Ali, W.; Zhuang, T.; Sun, J.; Wang, T.; Song, J.; Ma, Y.; Yuan, Y.; Bian, J.; et al. Co-Exposure of Polyvinyl Chloride Microplastics with Cadmium Promotes Nonalcoholic Fatty Liver Disease in Female Ducks through Oxidative Stress and Glycolipid Accumulation. Poult. Sci. 2024, 103, 104152. [Google Scholar] [CrossRef]
- Hong, Y.; Feng, Y.; Yan, T.; Zhang, L.; Zhao, Q.; Zhao, Q.; Huang, J.; Huang, S.; Zhang, Y. Take-out Food Enhances the Risk of MPs Ingestion and Obesity, Altering the Gut Microbiome in Young Adults. J. Hazard. Mater. 2024, 476, 135125. [Google Scholar] [CrossRef] [PubMed]
- Kuai, Y.; Chen, Z.; Xie, K.; Chen, J.; He, J.; Gao, J.; Yu, C. Long-Term Exposure to Polystyrene Microplastics Reduces Macrophages and Affects the Microbiota–Gut–Brain Axis in Mice. Toxicology 2024, 509, 153951. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, X.; Sun, X.; Huang, L.; Kuang, W.; Ou, J.; Zhang, J.; Zhang, Z.; Li, H.; Tang, H.; et al. Polystyrene Microplastics Induce Anxiety via HRAS Derived PERK-NF-κB Pathway. Environ. Int. 2024, 185, 108543. [Google Scholar] [CrossRef] [PubMed]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood–Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.-K.; Kim, K.-S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.-S.; et al. Maternal Exposure to Polystyrene Nanoplastics Causes Brain Abnormalities in Progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Hoelting, L.; Scheinhardt, B.; Bondarenko, O.; Schildknecht, S.; Kapitza, M.; Tanavde, V.; Tan, B.; Lee, Q.Y.; Mecking, S.; Leist, M.; et al. A 3-Dimensional Human Embryonic Stem Cell (hESC)-Derived Model to Detect Developmental Neurotoxicity of Nanoparticles. Arch. Toxicol. 2013, 87, 721–733. [Google Scholar] [CrossRef]
- Paing, Y.M.M.; Eom, Y.; Song, G.B.; Kim, B.; Choi, M.G.; Hong, S.; Lee, S.H. Neurotoxic Effects of Polystyrene Nanoplastics on Memory and Microglial Activation: Insights from in Vivo and in Vitro Studies. Sci. Total Environ. 2024, 924, 171681. [Google Scholar] [CrossRef]
- Lee, C.-W.; Hsu, L.-F.; Wu, I.-L.; Wang, Y.-L.; Chen, W.-C.; Liu, Y.-J.; Yang, L.-T.; Tan, C.-L.; Luo, Y.-H.; Wang, C.-C.; et al. Exposure to Polystyrene Microplastics Impairs Hippocampus-Dependent Learning and Memory in Mice. J. Hazard. Mater. 2022, 430, 128431. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.-K.; Choi, E.-J.; Lee, H.-J.; Shim, I.; Woo, H.; Choi, J.; Kim, G.-H.; et al. Pre/Post-Natal Exposure to Microplastic as a Potential Risk Factor for Autism Spectrum Disorder. Environ. Int. 2022, 161, 107121. [Google Scholar] [CrossRef]
- Liang, X.; Andrikopoulos, N.; Tang, H.; Wang, Y.; Ding, F.; Ke, P.C. Nanoplastic Stimulates the Amyloidogenesis of Parkinson’s Alpha-Synuclein NACore. Small 2024, 20, e2308753. [Google Scholar] [CrossRef]
- Gou, X.; Fu, Y.; Li, J.; Xiang, J.; Yang, M.; Zhang, Y. Impact of Nanoplastics on Alzheimer ’s Disease: Enhanced Amyloid-β Peptide Aggregation and Augmented Neurotoxicity. J. Hazard. Mater. 2024, 465, 133518. [Google Scholar] [CrossRef]
- Xu, M.; Niu, H.; Wu, L.; Xing, M.; Mo, Z.; Chen, Z.; Li, X.; Lou, X. Impact of Microplastic Exposure on Blood Glucose Levels and Gut Microbiota: Differential Effects under Normal or High-Fat Diet Conditions. Metabolites 2024, 14, 504. [Google Scholar] [CrossRef]
- Barshtein, G.; Livshits, L.; Shvartsman, L.D.; Shlomai, N.O.; Yedgar, S.; Arbell, D. Polystyrene Nanoparticles Activate Erythrocyte Aggregation and Adhesion to Endothelial Cells. Cell Biochem. Biophys. 2016, 74, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental Microplastic and Nanoplastic: Exposure Routes and Effects on Coagulation and the Cardiovascular System. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Holzer, M.; Praetner, M.; Fent, J.; Lerchenberger, M.; Reichel, C.A.; Rehberg, M.; Lakatos, S.; Krombach, F. Single-Walled Carbon Nanotubes Activate Platelets and Accelerate Thrombus Formation in the Microcirculation. Toxicology 2010, 269, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Geppner, L.; Hellner, J.; Henjakovic, M. Effects of Micro- and Nanoplastics on Blood Cells in Vitro and Cardiovascular Parameters in Vivo, Considering Their Presence in the Human Bloodstream and Potential Impact on Blood Pressure. Environ. Res. 2025, 273, 121254. [Google Scholar] [CrossRef]
- Goldsworthy, A.; O’Callaghan, L.A.; Blum, C.; Horobin, J.; Tajouri, L.; Olsen, M.; Van Der Bruggen, N.; McKirdy, S.; Alghafri, R.; Tronstad, O.; et al. Micro-Nanoplastic Induced Cardiovascular Disease and Dysfunction: A Scoping Review. J. Expo. Sci. Environ. Epidemiol. 2025, 35, 746–769. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, M.; Ma, J.; Zhang, S.; Liu, S.; Wang, K.; Tian, H.; Cui, J. Enhanced Hepatic Cytotoxicity of Chemically Transformed Polystyrene Microplastics by Simulated Gastric Fluid. J. Hazard. Mater. 2021, 410, 124536. [Google Scholar] [CrossRef]
- Shengchen, W.; Jing, L.; Yujie, Y.; Yue, W.; Shiwen, X. Polystyrene Microplastics-Induced ROS Overproduction Disrupts the Skeletal Muscle Regeneration by Converting Myoblasts into Adipocytes. J. Hazard. Mater. 2021, 417, 125962. [Google Scholar] [CrossRef]
- Yu, C.-W.; Luk, T.C.; Liao, V.H.-C. Long-Term Nanoplastics Exposure Results in Multi and Trans-Generational Reproduction Decline Associated with Germline Toxicity and Epigenetic Regulation in Caenorhabditis Elegans. J. Hazard. Mater. 2021, 412, 125173. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Yang, N.; Xu, S. Polystyrene-Microplastics and DEHP Co-Exposure Induced DNA Damage, Cell Cycle Arrest and Necroptosis of Ovarian Granulosa Cells in Mice by Promoting ROS Production. Sci. Total Environ. 2023, 871, 161962. [Google Scholar] [CrossRef]
- Ma, M.; Zheng, Z.; Li, J.; He, Y.; Kang, W.; Ye, X. Association between the Gut Microbiota, Inflammatory Factors, and Colorectal Cancer: Evidence from Mendelian Randomization Analysis. Front. Microbiol. 2024, 15, 1309111. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A Review of the Endocrine Disrupting Effects of Micro and Nano Plastic and Their Associated Chemicals in Mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef] [PubMed]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The Emerging Risk of Exposure to Nano(Micro)Plastics on Endocrine Disturbance and Reproductive Toxicity: From a Hypothetical Scenario to a Global Public Health Challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, M.; Poma, A.M.G. Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective. Int. J. Mol. Sci. 2025, 26, 2071. [Google Scholar] [CrossRef]
- Bossio, S.; Ruffolo, S.A.; Lofaro, D.; Perri, A.; La Russa, M.F. Endocrine Toxicity of Micro- and Nanoplastics, and Advances in Detection Techniques for Human Tissues: A Comprehensive Review. Endocrines 2025, 6, 23. [Google Scholar] [CrossRef]
- Saha, U.; Kumari, P.; Ghosh, A.; Sinha, A.; Jena, S.; Kirti, A.; Gupta, A.; Choudhury, A.; Simnani, F.Z.; Nandi, A.; et al. Detrimental Consequences of Micropolymers Associated Plasticizers on Endocrinal Disruption. Mater. Today Bio 2024, 27, 101139. [Google Scholar] [CrossRef]
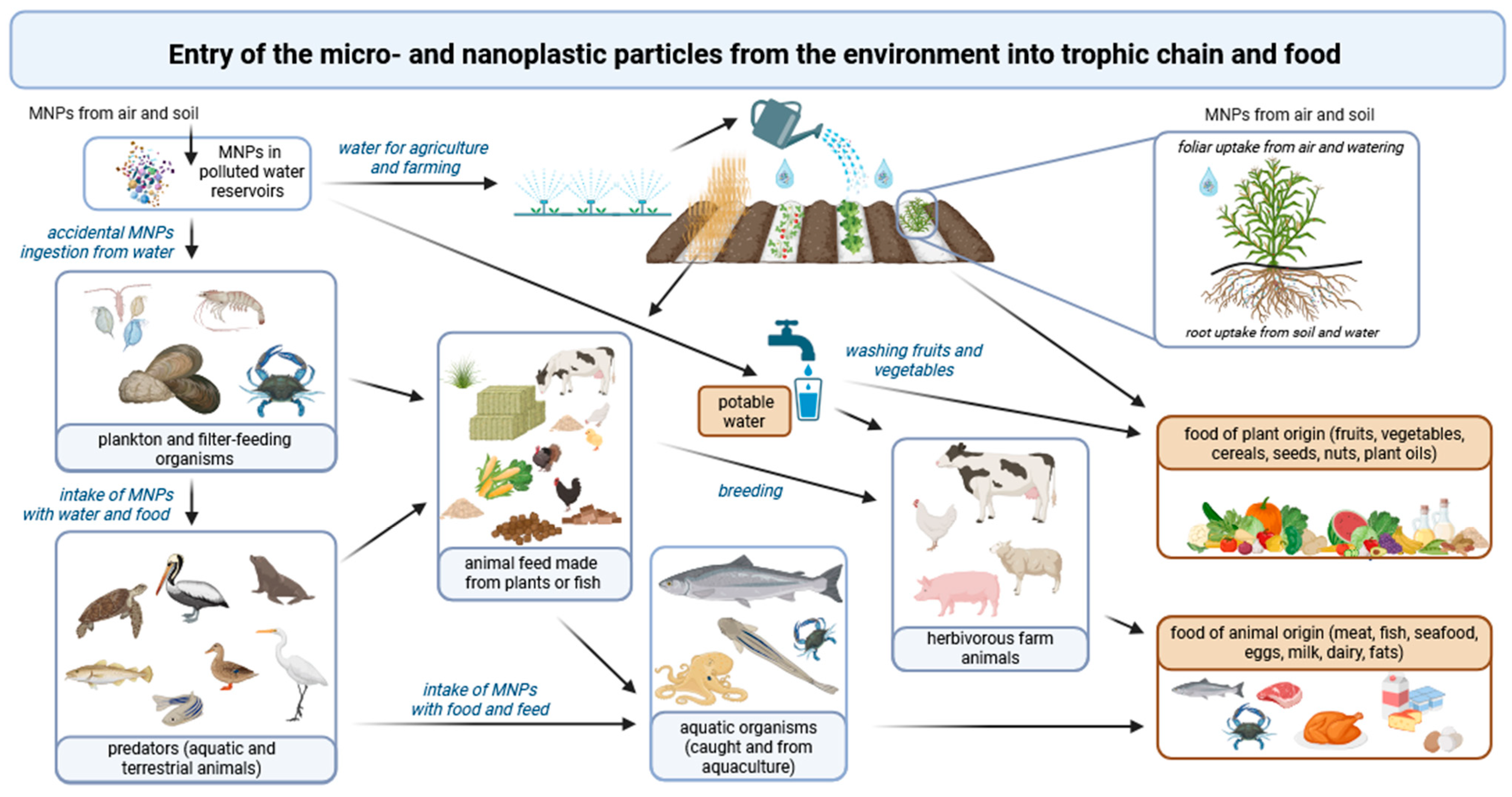

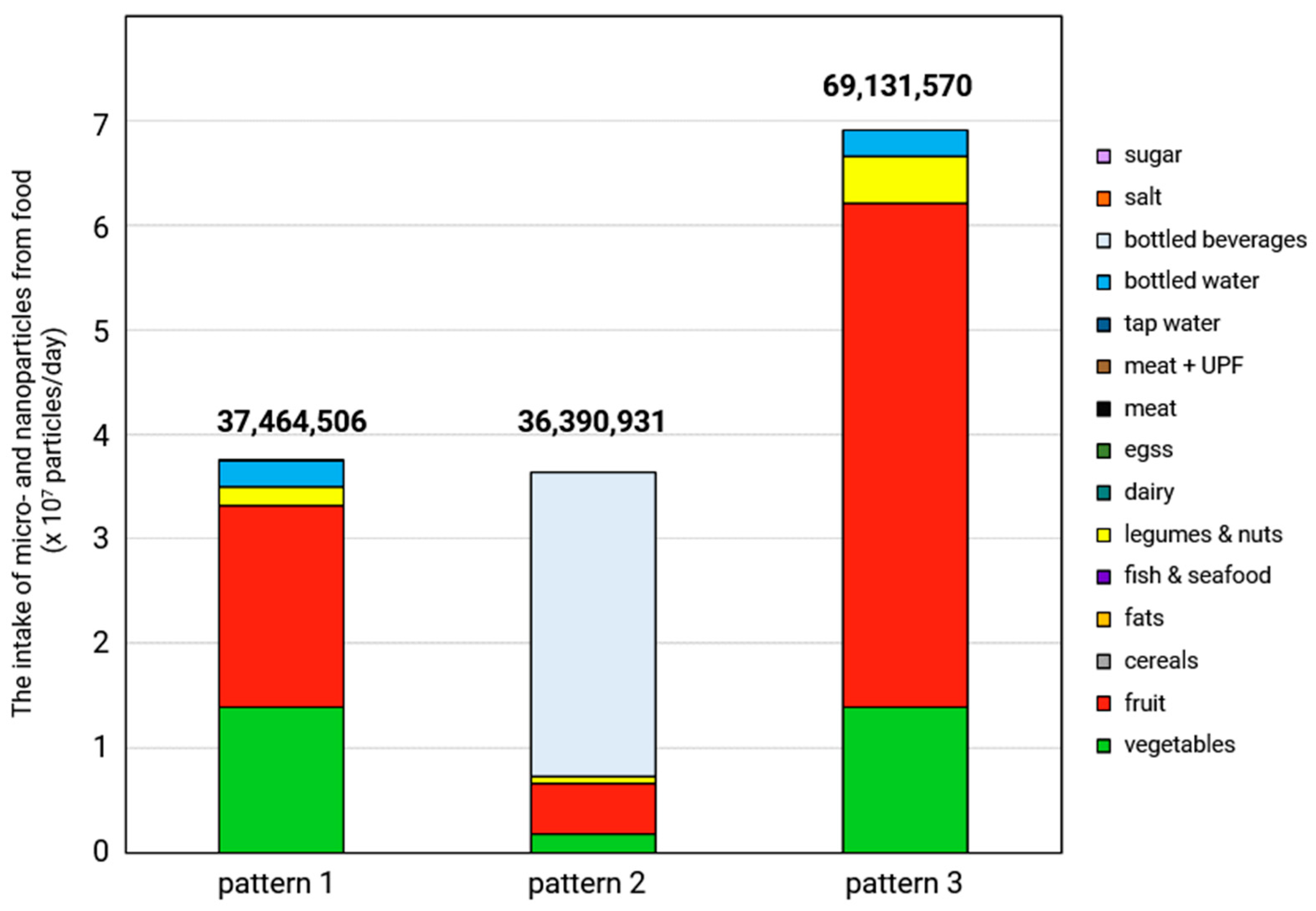
| Food Group/Product | Concentration Range of MNPs | Unit | Reference |
|---|---|---|---|
| Group 1. Vegetables (average value = 34,835 p/g) | |||
| tomato | 3.63 ± 1.39 | p/g | [213] |
| potato | 1.5 ± 1.6 | p/g | [213] |
| cucumber | 3.6 ± 1.8 | p/g | [213] |
| onion | 2.6 ± 1.5 | p/g | [213] |
| lettuce | 50,550 ± 25,011 | p/g | [380] |
| lettuce | 6.3–29.4 | p/g | [283] |
| carrot | 101,950 ± 44,368 | p/g | [380] |
| broccoli | 126,150 ± 80,715 | p/g | [380] |
| Group 2. Fruits (average value = 96,264 p/g) | |||
| apple | 52,600–307,750 | p/g | [380] |
| apple | 3.1 ± 1.2 | p/g | [213] |
| pear | 98,325–302,250 | p/g | [380] |
| pear | 3.1 ± 1.3 | p/g | [213] |
| Group 3. Cereals (average value = 4.0 p/g) | |||
| branded flour non-branded flours | 2747 ± 654 | p/kg | [288] |
| 6409 ± 625 | p/kg | [288] | |
| rice | 0.303 | p/g | [388] |
| Indian rice | 30.3 ± 8.61 | p/100 g | [286] |
| wheat | 4.57 | p/g | [388] |
| Group 4. Fats and oils (average value = 179 p/cm3) | |||
| edible oil | 134,000–580,000 | p/dm3 | [304] |
| vegetable oils | 644–1795 | p/dm3 | [305] |
| Group 5. Fish and seafood (average value = 7.14 p/g) | |||
| mussel | 0.206–0.709 | p/g | [368] |
| oysters | 0.8–44.1 | p/g | [389] |
| frozen and glazed icefish | 0.42 ± 0.28 | p/g | [257] |
| shrimps | 0.11 | p/g | [293] |
| shrimps | 24 ± 31 | p/g | [390] |
| prawns white leg shrimps | 32.66 ± 5.10 | p/g | [391] |
| 10.28 ± 1.19 | p/g | [391] | |
| bivalves | 0–7.2 | p/g | [392] |
| bivalves | 0–10.5 | p/g | [393] |
| crustaceous seafood | 0.1–8.6 | p/g | [393] |
| fish | 0.0–2.9 | p/g | [393] |
| fish | 0.09 ± 0.09 | p/g | [394] |
| fish | 0–20 | p/g | [392] |
| canned fish | 0.13 | p/g | [395] |
| Group 6. Legumes and nuts (average value = 34,835 p/g) * | |||
| Group 7. Dairy (average value = 4.00 p/g or p/cm3) | |||
| milk | 1–14 | p/dm3 | [275] |
| milk | 204–1004 | p/100 cm3 | [274] |
| milk | 1–16 | p/dm3 | [273] |
| milk | 11.1–295.5 | p/dm3 | [388] |
| conventional butter | 942 | p/kg | [396] |
| organic butter | 833 | p/kg | [396] |
| sour cream | 800 | p/kg | [396] |
| skim milk | 134–444 | p/dm3 | [186] |
| various milks | 1–4906 | p/100 cm3 | [387] |
| yoghurt | 20–580 | p/dm3 | [387] |
| cream | 9–596 | p/dm3 | [387] |
| ayran | 18 | p/100 cm3 | [386] |
| Group 8. Eggs (average values = 0.12 p/g—assum. mean egg mass = 55 g) | |||
| eggs | 11.67 ± 3.98 | p/egg | [303] |
| Group 9a. Meat (average value = 0.31 p/g) | |||
| meat | 0.03–1.19 | p/g | [228] |
| packaged meat | 4.0–18.7 | p/kg | [256] |
| Group 9b. Meat + Ultra-processed food (average value = 1.22 p/g) | |||
| Takeaway hot beverages (average value = 2.826 p/cm3) | |||
| coffee in | |||
| - PE-coated cups | 675–5984 | p/dm3 | [397] |
| - PP cup | 781–4951 | p/dm3 | [397] |
| - PS cup | 838–5215 | p/dm3 | [397] |
| beverages in single-use plastic cups: | |||
| - PS cup | 153−1360 | p/dm3 | [255] |
| - PE-coated paper cup | 126–1346 | p/dm3 | [255] |
| - EPS cup | 246−720 | p/dm3 | [255] |
| - PP cup | 126−1420 | p/dm3 | [255] |
| beverages in take-out cups: | |||
| - PP cup | 1612 ± 216 | p/500 cm3 | [245] |
| - PET cup | 1161 ± 393 | p/500 cm3 | [245] |
| - PE cup | 1482 ± 408 | p/500 cm3 | [245] |
| - hot drink (60 °C) | 1905–2204 | p/500 cm3 | [245] |
| tea made from teabags | 55.6–1446.8 | p/250 cm3 | [398] |
| Take-out food (average value = 0.639 p/g) | |||
| Take-out food | 0.639 | p/g | [251] |
| Group 10a. Water—tap water (average value = 0.22 p/cm3) | |||
| European tap water | 0.91–7.73 | p/dm3 | [82] |
| tap water | 0–61 | p/dm3 | [392] |
| tap water | 628 | p/dm3 | [399] |
| Group 10b. Water—bottled water (average value = 2527 p/cm3) | |||
| bottled water (all continents) | 12.5–2277 | p/dm3 | [264] |
| water in | |||
| - single-use plastic bottle | 2–44 | p/dm3 | [266] |
| - returnable plastic bottle | 28–241 | p/dm3 | [266] |
| - glass bottle | 4–156 | p/dm3 | [266] |
| mineral water in | |||
| - single-use PET bottle | 2648 ± 2857 | p/dm3 | [17] |
| - reusable PET bottle | 4889 ± 5432 | p/dm3 | [17] |
| - glass bottle | 6292 ± 10,521 | p/dm3 | [17] |
| bottled water | 0–6292 | p/dm3 | [392] |
| bottled water | 4889 | p/dm3 | [399] |
| bottled water | 0.07–500 | p/dm3 | [388] |
| Group 10c. Bottled non-alcoholic cold beverages (average value = 29,126 p/cm3) | |||
| beverage in cartons | 5–20 | p/dm3 | [266] |
| frozen bottled beverages | 68–4.66 × 108 | p/dm3 | [190] |
| carbonated bottled beverages: | 260.52–281.38 | p/dm3 | [190] |
| - with sugar | 179–218 | p/dm3 | [190] |
| - with additives (sucralose, acesulfame, sunset yellow, and carmine) | 157.81–164.24 | p/dm3 | [190] |
| soft drink | 8.9 ± 2.95 | p/dm3 | [400] |
| soft drink | 0–7 ± 3.21 | p/dm3 | [279] |
| energy drinks | 0–6 ± 1.53 | p/dm3 | [279] |
| refreshing beverage | 68–494 | p/dm3 | [186] |
| Group 11. Salt (average value = 0.303 p/g) | |||
| European sea salt | 66.6–220 | p/kg | [82] |
| European sea salt | 0–284 | p/kg | [392] |
| lake salt | 8–102 | p/kg | [392] |
| rock and well salt | 9–185 | p/kg | [392] |
| sea salt | 0–1674 | p/kg | [401] |
| lake salt | 8–462 | p/kg | [401] |
| rock and well salt | 0–204 | p/kg | [401] |
| salt | 55.2 ± 43.7 (optically) | p/kg | [216] |
| 151 ± 61.8 (Nile Red stain) | p/kg | [216] | |
| salt | 0.303 | p/g | [388] |
| Group 12. Sugar (average value = 58.36 p/g) | |||
| sugar | 11,724–53,464 (av. 29,110) | p/100 g | [385] |
| sugar | 57.7 ± 20.6 (optically) | p/kg | [216] |
| 226 ± 99.5 (Nile Red stain) | p/kg | [216] | |
| sugar | 0.343 | p/g | [388] |
| refined sugar | 217 ± 123 (fibres) | p/kg | [384] |
| 32 ± 7 (fragments) | p/kg | [384] | |
| No. | Food Group | Average MNPs/g (MNPs/cm3) | MNP Intake with Food Depending on Dietary Pattern (MNPs/Food Group) | ||
|---|---|---|---|---|---|
| Pattern 1 | Pattern 2 | Pattern 3 | |||
| 1 | Vegetables | 34,835 | 13,934,000 | 1,741,750 | 13,934,000 |
| 2 | Fruit | 96,264 | 19,252,800 | 4,813,200 | 48,132,000 |
| 3 | Cereals | 4.00 | 1000 | 320 | 720 |
| 4 | Fats | 179 | 6265 | 12,530 | 7876 |
| 5 | Fish/seafood | 7.14 | 249.9 | 45.7 | - |
| 6 | Legumes/nuts | 34,835 | 1,741,750 | 696,700 | 4,528,550 |
| 7 | Dairy | 4.00 | 1200 | 160 | 1200 |
| 8 | Eggs | 0.12 | 6.6 | 3 | 3 |
| 9a | Meat | 0.31 | 13.33 | - | - |
| 9b | Meat + UPF | 1.22 | - | 317.2 | - |
| 10a | Water (tap water) | 0.22 | 220 | 220 | 220 |
| 10b | Water (bottled) | 2527 | 2,527,000 | - | 2,527,000 |
| 10c | Bottled beverages | 29,126 | - | 29,126,000 | - |
| 11 | Salt | 0.20 | 1 | 2 | 1 |
| 12 | Sugar | 58.36 | 0 | 4 698 | 0 |
| total | 37,464,506 | 36,390,931 | 69,131,570 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda, A.; Petka, K. The Presence of Micro- and Nanoplastics in Food and the Estimation of the Amount Consumed Depending on Dietary Patterns. Molecules 2025, 30, 3666. https://doi.org/10.3390/molecules30183666
Duda A, Petka K. The Presence of Micro- and Nanoplastics in Food and the Estimation of the Amount Consumed Depending on Dietary Patterns. Molecules. 2025; 30(18):3666. https://doi.org/10.3390/molecules30183666
Chicago/Turabian StyleDuda, Aleksandra, and Katarzyna Petka. 2025. "The Presence of Micro- and Nanoplastics in Food and the Estimation of the Amount Consumed Depending on Dietary Patterns" Molecules 30, no. 18: 3666. https://doi.org/10.3390/molecules30183666
APA StyleDuda, A., & Petka, K. (2025). The Presence of Micro- and Nanoplastics in Food and the Estimation of the Amount Consumed Depending on Dietary Patterns. Molecules, 30(18), 3666. https://doi.org/10.3390/molecules30183666






