Effect of Relative Humidity on Quality and Metabolite Profiles of Perilla frutescens Seed Powder During Storage
Abstract
1. Introduction
2. Results and Discussion
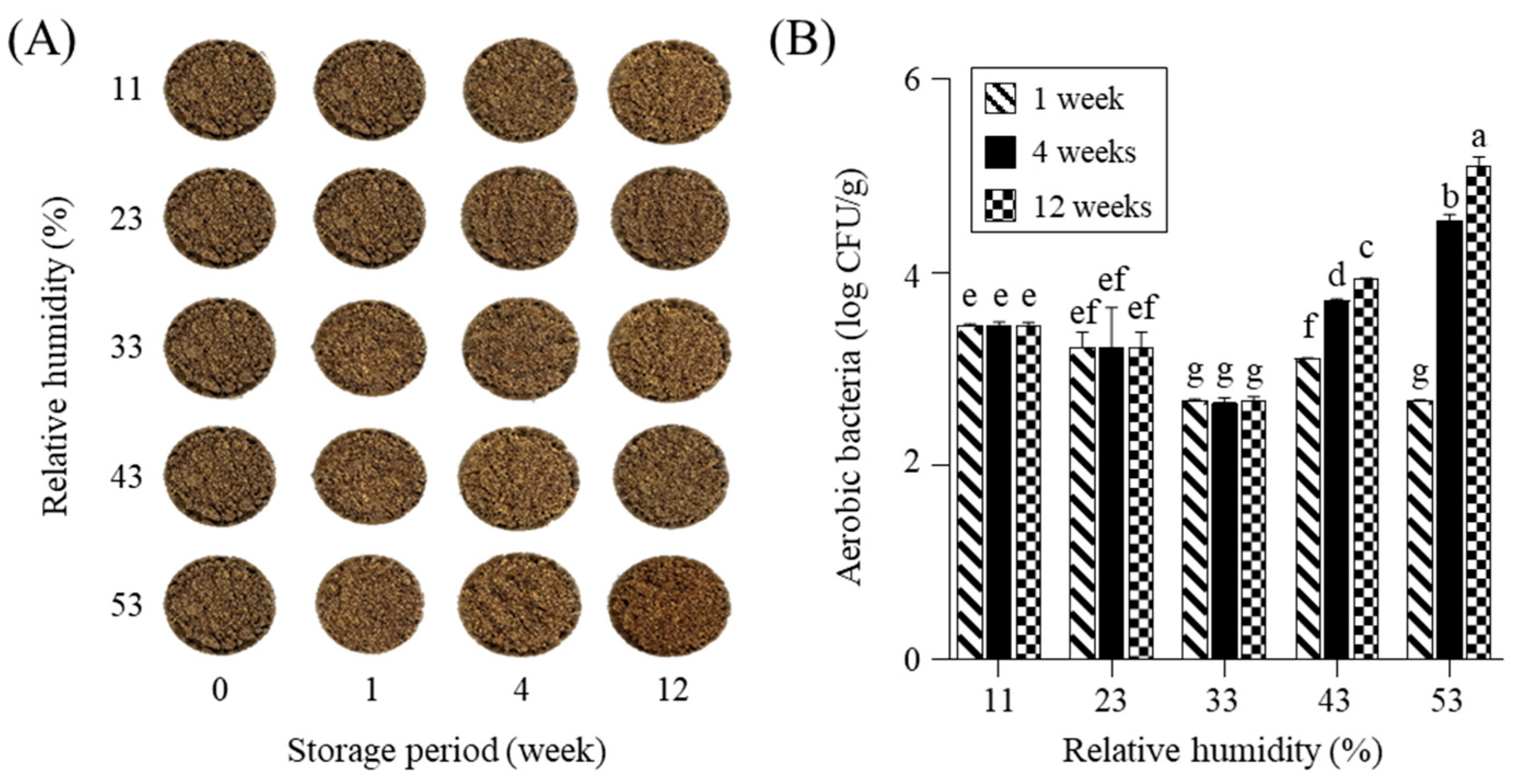
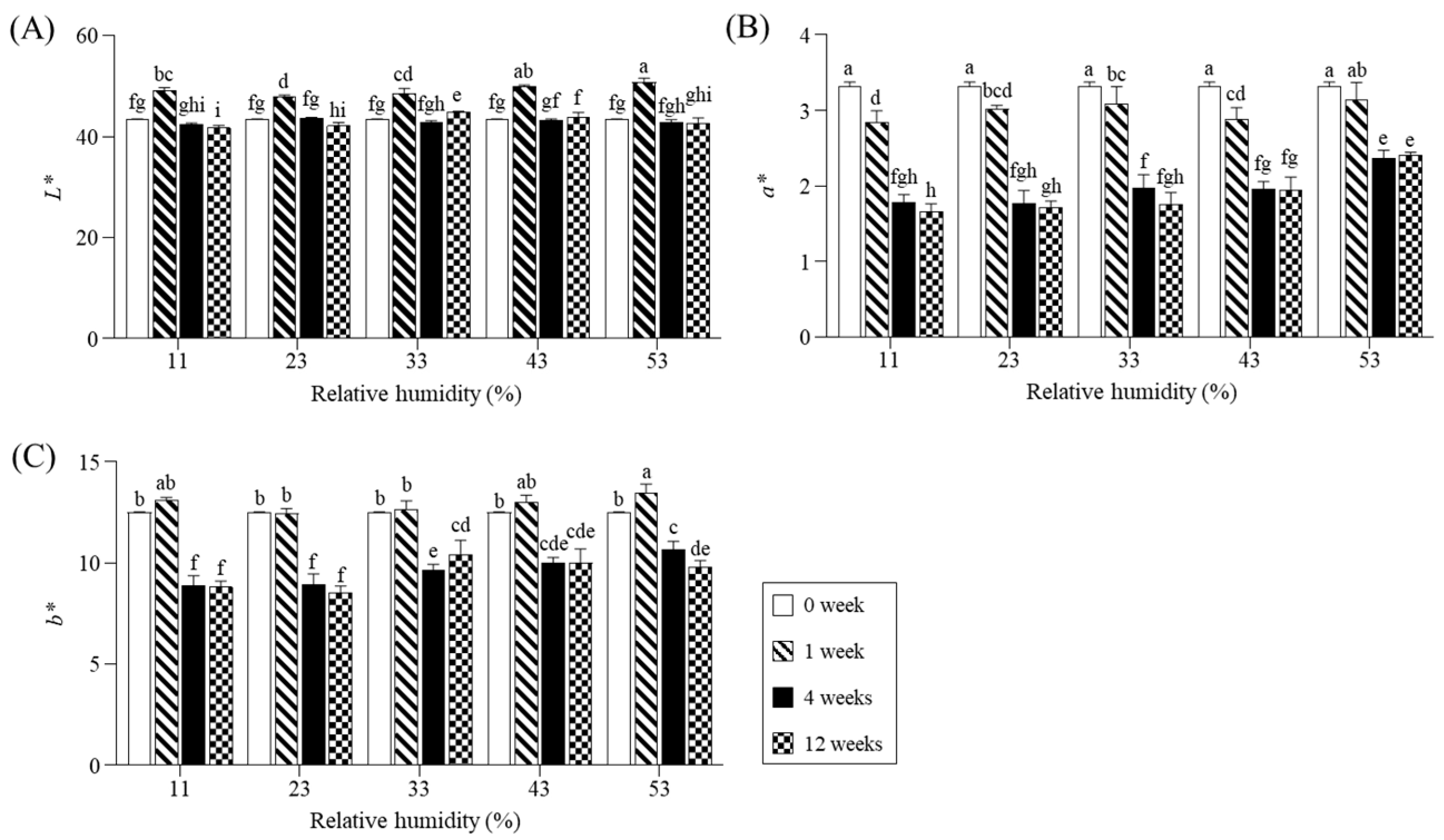
2.1. Appearance Changes and Microbial Populations During Storage
2.2. Lipid Oxidation in Perilla Seed Powders During Storage
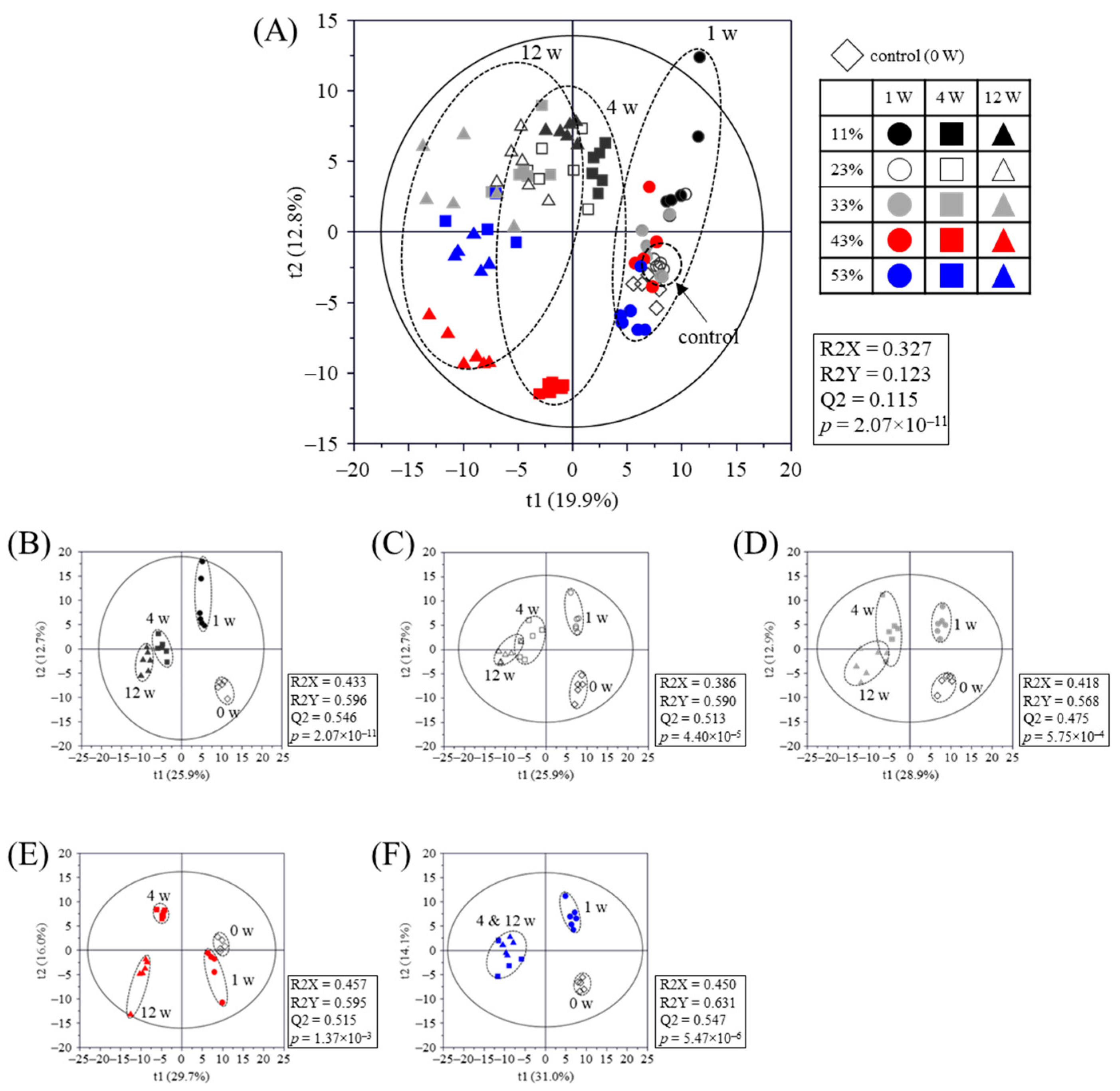
2.3. Metabolomic Analysis
2.4. Metabolomic Pathway and Relative Abundances of Identified Metabolites
2.5. Prediction of Storage RH and Weeks Using Regression Models
3. Materials and Methods
3.1. Sample Preparation
3.2. Color, Microbial Population, and Lipid Oxidation
3.3. GC-MS Analysis
3.4. UPLC-Q-TOF-MS Analysis
3.5. Data Processing
3.6. Prediction Models for Storage Relative Humidity and Duration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adam, G.; Robu, S.; Flutur, M.-M.; Cioanca, O.; Vasilache, I.-A.; Adam, A.-M.; Mircea, C.; Nechita, A.; Harabor, V.; Harabor, A.; et al. Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants 2023, 12, 727. [Google Scholar] [CrossRef]
- Yao, L.; Huang, J.; He, H.; Song, Z.; Wang, X.; Wang, X. Effect of perilla seeds pretreatment on the volatile profile and flavor characteristics of perilla oil: Comparison of oven, infrared, and microwave heating. Eur. J. Lipid Sci. Technol. 2025, 127, e202400106. [Google Scholar] [CrossRef]
- Son, Y.; Lee, K.Y.; Gu, S.; Park, J.Y.; Choi, S.G.; Kim, H.J. Quality changes in perilla seed powder related to storage duration and temperature. J. Food Sci. Technol. 2020, 57, 263–273. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhuo, Y.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; Lin, Y.; Jiang, X.; Zhao, X.; et al. Effects of Multi-Strain Probiotics and Perilla frutescens Seed Extract Supplementation Alone or Combined on Growth Performance, Antioxidant Indices, and Intestinal Health of Weaned Piglets. Animals 2022, 12, 2246. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Zhu, L.; Zhang, X.; Han, Y.; Wang, K.; Ji, N.; Yao, X.; Zhou, Y.; Li, B.; Chen, Q.; et al. Perilla Seed Oil and Protein: Composition, Health Benefits, and Potential Applications in Functional Foods. Molecules 2024, 29, 5258. [Google Scholar] [CrossRef]
- Yi, D.; Wang, Z.; Peng, M. Comprehensive Review of Perilla frutescens: Chemical Composition, Pharmacological Mechanisms, and Industrial Applications in Food and Health Products. Foods 2025, 14, 1252. [Google Scholar] [CrossRef] [PubMed]
- Lia, F.; Baron, B. Analysis of Polyphenolic Composition, Antioxidant Power and Stress-Response Effects of Fractionated Perilla Leaf Extract on Cells In Vitro. Biologics 2025, 5, 2. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, X.; Qu, Z.; Gao, Y.; Li, Q.; Yu, X. Mechanism, indexes, methods, challenges, and perspectives of edible oil oxidation analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 4901–4915. [Google Scholar] [CrossRef]
- Dadlani, M.; Gupta, A.; Sinha, S.N.; Kavali, R. Seed storage and packaging. In Seed Science and Technology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 239–266. [Google Scholar]
- Clemente, A.; Costa, C.A.; Oliveira, G.; Correia, O. Short-term seed storage of two Mediterranean shrubs used in restoration: Simple procedures to reduce seed deterioration. Ecol. Eng. 2024, 202, 107243. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rahman, M.S.; Kim, A.N.; Jeong, E.J.; Kim, B.G.; Lee, M.H.; Kim, H.J.; Choi, S.G. Oil yield, physicochemical characteristics, oxidative stability and microbial safety of perilla seeds stored at different relative humidity. Ind. Crops Prod. 2021, 165, 113431. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Yoon, H.; Shin, M.-J.; Lee, S.; Yi, J.; Jeon, Y.; Wang, X.; Desta, K.T. Nutrient Levels, Bioactive Metabolite Contents, and Antioxidant Capacities of Faba Beans as Affected by Dehulling. Foods 2023, 12, 4063. [Google Scholar] [CrossRef]
- Idrissi, Z.L.; Amakhmakh, M.; Moudden, H.E.; Guezzane, C.E.; Ullah, R.; Bari, A.; Bouyahya, A.; Lee, L.-H.; Harhar, H.; Tabyaoui, M. Thermal oxidative stability and kinetics of lipophilic bioactive compounds degradation during accelerated storage of unroasted and roasted peanut oil. J. Food Compos. Anal. 2024, 131, 106239. [Google Scholar] [CrossRef]
- Ning, K.; Hou, C.; Wei, X.; Zhou, Y.; Zhang, S.; Chen, Y.; Yu, H.; Dong, L.; Chen, S. Metabolomics Analysis Revealed the Characteristic Metabolites of Hemp Seeds Varieties and Metabolites Responsible for Antioxidant Properties. Front. Plant Sci. 2022, 13, 904163. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Liu, A.; Wang, J.; Sun, X.; Deng, J.; Chen, Q.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Dossou, S.S.K.; Xu, F.; You, J.; Zhou, R.; Li, D.; Wang, L. Widely targeted metabolome profiling of different colored sesame (Sesamum indicum L.) seeds provides new insight into their antioxidant activities. Food Res. Int. 2022, 151, 110850. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, L.; Gou, Y.; He, X.; Lu, Q. Effects of deterioration and mildewing on the quality of wheat seeds with different moisture contents during storage. RSC Adv. 2020, 10, 14581–14594. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xu, L.L.; Xue, C.; Jiang, X. Effect of Stored Humidity and Initial Moisture Content on the Qualities and Mycotoxin Levels of Maize Germ and Its Processing Products. Toxins 2020, 12, 535. [Google Scholar] [CrossRef]
- Köhl, J.; Butterbach, P.B.E.; Ehlers, R.; Gaildry, T.; Groenenboom-de Haas, L.; Groot, S.P.C.; Heijden, L.; Houwers, I.; Lange, E.; López, G.; et al. Screening criteria for microbial bioprotectants for seed coating to protect seeds and seedlings from diseases. Biol. Control 2024, 190, 105450. [Google Scholar] [CrossRef]
- Pohndorf, R.S.; Meneghetti, V.L.; Paiva, F.F.; de Oliveira, M.; Elias, M.C. Kinetic evaluation of oxidative stability and physical degradation of soybean grains stored at different conditions. J. Food Process. Preserv. 2018, 42, e13717. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of Secondary and Tertiary Volatile Compounds Resulting from the Lipid Oxidation of Rapeseed Oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef]
- Homma, S.; Fujimaki, M. Effect of Water Activity on Lipid Oxidation and Browning of Kori-tofu. Agric. Biol. Chem. 1982, 46, 301–304. [Google Scholar] [CrossRef]
- Oh, S.; Yi, B.R.; Park, J.W.; Kim, M.J.; Lee, J.W. Effects of relative humidity and neutral emulsifier on oxidative stability of corn oil during room temperature storage. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 521–526. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture food: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, S.; Caboni, M.F.; Miani, M.G.; Pasini, F. Wheat Germ and Lipid Oxidation: An Open Issue. Foods 2022, 11, 1032. [Google Scholar] [CrossRef]
- Luo, K.K.; Huang, G.; Mitchell, A.E. Acceleration of lipid oxidation in raw stored almond kernels in response to postharvest moisture exposure. J. Sci. Food Agric. 2021, 102, 1155–1164. [Google Scholar] [CrossRef]
- Nguyen, K.; Hennebelle, M.; Duynhoven, J.P.M.; Dubbelboer, A.; Boerkamp, V.J.P.; Wierenga, P.A. Mechanistic kinetic modelling of lipid oxidation in vegetable oils to estimate shelf-life. Food Chem. 2023, 433, 137266. [Google Scholar] [CrossRef] [PubMed]
- Mitcham, E.; Adkison, C.; Llingga, N.; Bikoba, V. Storage Temperature, Relative humidity, and time effects on the organoleptic profile of walnut kernels. J. Amer. Soc. Hort. Sci. 2022, 147, 291–299. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Litsenburg, M.J.; Kodde, J.; Hall, R.D.; Vos, R.C.H.; Mumm, R. Analyses of metabolic activity in peanuts under hermetic storage at different relative humidity levels. Food Chem. 2022, 373, 131020. [Google Scholar] [CrossRef]
- Bu, M.; Fan, W.; Li, R.; He, B.; Cui, P. Lipid Metabolism and Improvement in Oilseed Crops: Recent Advances in Multi-Omics Studies. Metabolites 2023, 13, 1170. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Adkison, C.; Richmond, K.; Lingga, N.; Bikoba, V.; Mitcham, E. Optimizing Walnut Storage Conditions: Effects of Relative Humidity, Temperature, and Shelling on Quality after Storage. HortScience 2021, 56, 1244–1250. [Google Scholar] [CrossRef]
- Gorina, S.S.; Egorova, A.M.; Lantsova, N.V.; Toporkova, Y.Y.; Grechkin, A.N. Discovery of α-linolenic acid 16(S)-lipoxygenase: Cucumber (Cucumis sativus L.) vegetative lipoxygenase 3. Int. J. Mol. Sci. 2023, 24, 12977. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Xu, Y.; An, Y.; Hu, Z.; Xiong, A.; Wang, G. Effects of Jasmonic Acid on Stress Response and Quality Formation in Vegetable Crops and Their Underlying Molecular Mechanisms. Plants 2024, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Seem, K.; Ali, A.; Jaiswal, S.; Gumachanamardi, P.; Kaur, G.; Singh, N.; Touthang, L.; Singh, S.K.; Bhardwaj, R.; et al. A comprehensive review on nutritional, nutraceutical, and industrial perspectives of perilla (Perilla frutscens L.) seeds—An orphan oilseed crop. Heliyon 2024, 10, e33281. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, R.; Cheng, S.; Wang, K.; Qin, L. Moisture absorption and dynamic flavor changes in hydrolysed and freeze-dried pine nut (Pinus koraiensis) by-products during storage. Food Res. Int. 2018, 103, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Takemoto, H.; Koeduka, T.; Ohnishi, T. 1-Octen-3-ol is formed from its glycoside during processing of soybean [Glycine max (L.) Merr.] seeds. J. Agric. Food Chem. 2018, 66, 7409–7416. [Google Scholar] [CrossRef]
- Suresh, K.; Chetna, C.; Mandal, R.K.; Prakash, D.; Dora, K.A.; Kavana, J.M. The Role of Volatile Organic Compounds (VOCs) in Determining Seed Physiological Quality: A Review. Int. J. Enviorn. Clim. Change 2024, 14, 124–138. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, P.; Cui, Y.; Li, K.; Qiao, X.; Zhang, Y.T.; Li, S.M.; Cox, R.J.; Wu, B.; Ye, M.; et al. Regio- and stereospecific O-glycosylation of phenolic compounds catalyzed by a fungal glycosyltransferase from Mucor hiemalis. Adv. Synth. Catal. 2017, 359, 995–1006. [Google Scholar] [CrossRef]
- Azeem, M.; Kharl, H.; Niazi, S.; Ameer, N.; Fayyaz, F.; Huda, N.; Zehra, T.; Aftab, M. An overview of anti-inflammatory, antioxidant, anti-cancer, anti-hyperlipidemic, neuroprotective and muscle relaxant effects of natural flavonoid, apigenin; a review. Biol. Clin. Sci. Res. J. 2024, 2024, 644. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Neagu, D.; Trundle, P. Evaluation of k-nearest neighbour classifier performance for heterogeneous data sets. SN Appl. Sci. 2019, 1, 1559. [Google Scholar] [CrossRef]
- Taheri, S.; Andrade, J.C.; Conte-Junior, C.A. Emerging perspectives on analytical techniques and machine learning for food metabolomics in the era of industry 4.0: A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, L.; Yang, N.; Fisk, I.D.; Wei, W.; Wang, L.; Li, J.; Sun, Q.; Zeng, R. Integration of hyperspectral imaging, non-targeted metabolomics and machine learning for vigour prediction of naturally and accelerated aged sweetcorn seeds. Food Control 2023, 153, 109930. [Google Scholar] [CrossRef]
- Do, E.; Kim, M.; Ko, D.Y.; Lee, M.; Lee, C.; Ku, K.M. Machine learning for storage duration based on volatile organic compounds emitted from ‘Jukhyang’ and ‘Merry Queen’ strawberries during post-harvest storage. Postharvest Biol. Technol. 2024, 211, 112808. [Google Scholar] [CrossRef]
- Kim, A.N.; Kim, H.J.; Kerr, W.L.; Choi, S.G. The effect of grinding at various vacuum levels on the color, phenolics, and antioxidant properties of apple. Food Chem. 2017, 216, 234–242. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; pp. 987–1009. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Lee, K.-Y.; Park, J.Y.; Son, Y.; Gu, S.; Choi, S.-G.; Lee, M.-H.; Kim, H.-J. Effect of Relative Humidity on Quality and Metabolite Profiles of Perilla frutescens Seed Powder During Storage. Molecules 2025, 30, 3662. https://doi.org/10.3390/molecules30183662
Kim D-S, Lee K-Y, Park JY, Son Y, Gu S, Choi S-G, Lee M-H, Kim H-J. Effect of Relative Humidity on Quality and Metabolite Profiles of Perilla frutescens Seed Powder During Storage. Molecules. 2025; 30(18):3662. https://doi.org/10.3390/molecules30183662
Chicago/Turabian StyleKim, Dong-Shin, Kyo-Yeon Lee, Ji Yeong Park, Yejin Son, Suyeon Gu, Sung-Gil Choi, Myoung-Hee Lee, and Hyun-Jin Kim. 2025. "Effect of Relative Humidity on Quality and Metabolite Profiles of Perilla frutescens Seed Powder During Storage" Molecules 30, no. 18: 3662. https://doi.org/10.3390/molecules30183662
APA StyleKim, D.-S., Lee, K.-Y., Park, J. Y., Son, Y., Gu, S., Choi, S.-G., Lee, M.-H., & Kim, H.-J. (2025). Effect of Relative Humidity on Quality and Metabolite Profiles of Perilla frutescens Seed Powder During Storage. Molecules, 30(18), 3662. https://doi.org/10.3390/molecules30183662




