Hydrogel Beads Loaded with Glucosinolate-Rich Brassicaceae Extract as a Controlled-Release Alternative to Biofumigation
Abstract
1. Introduction
2. Results and Discussion
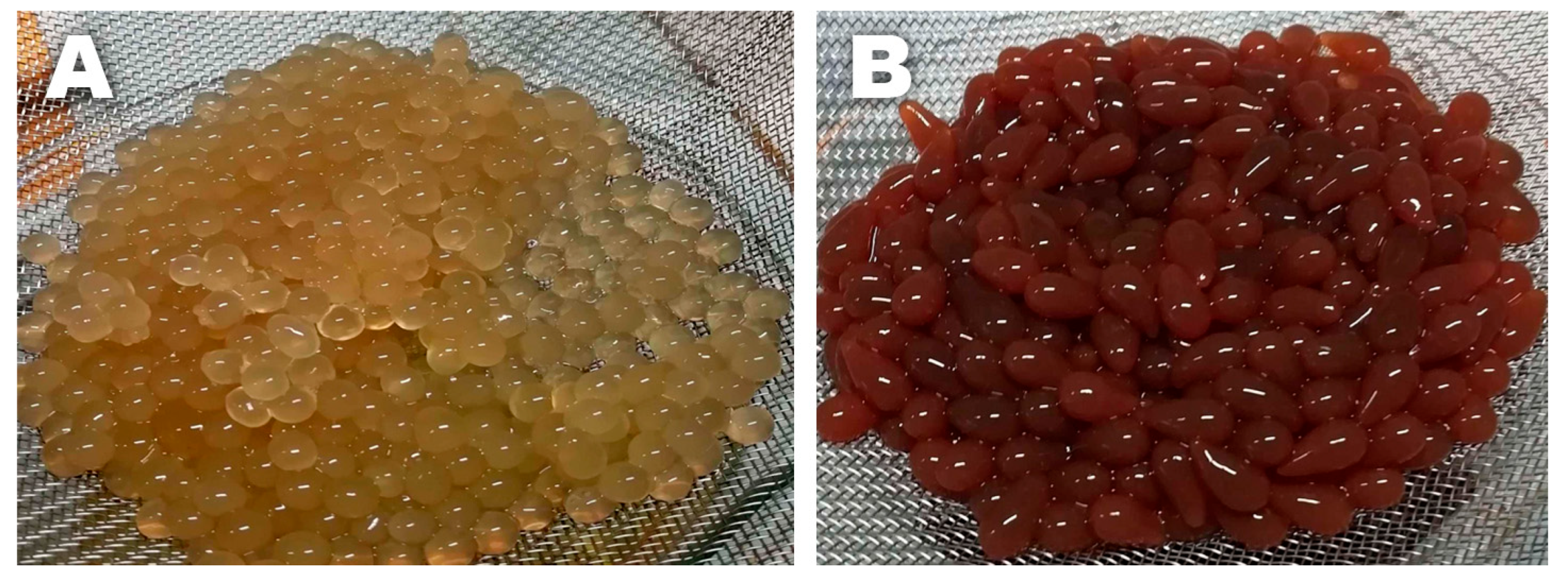
2.1. Brassica Oleracea Extract Characterization
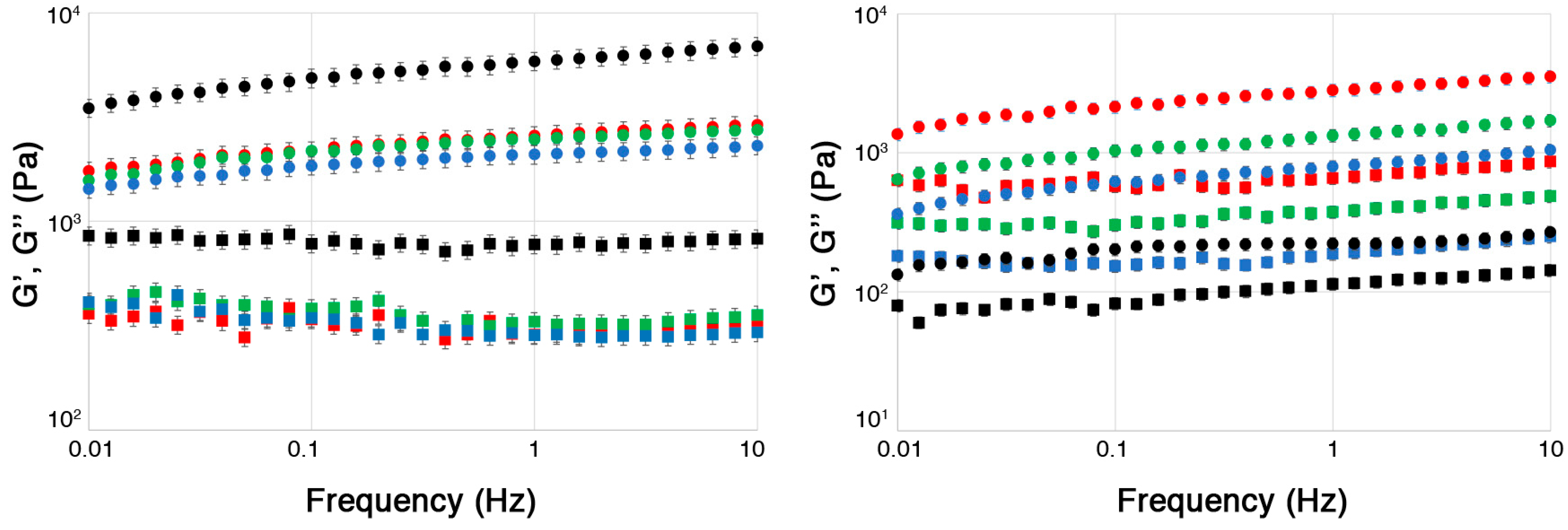
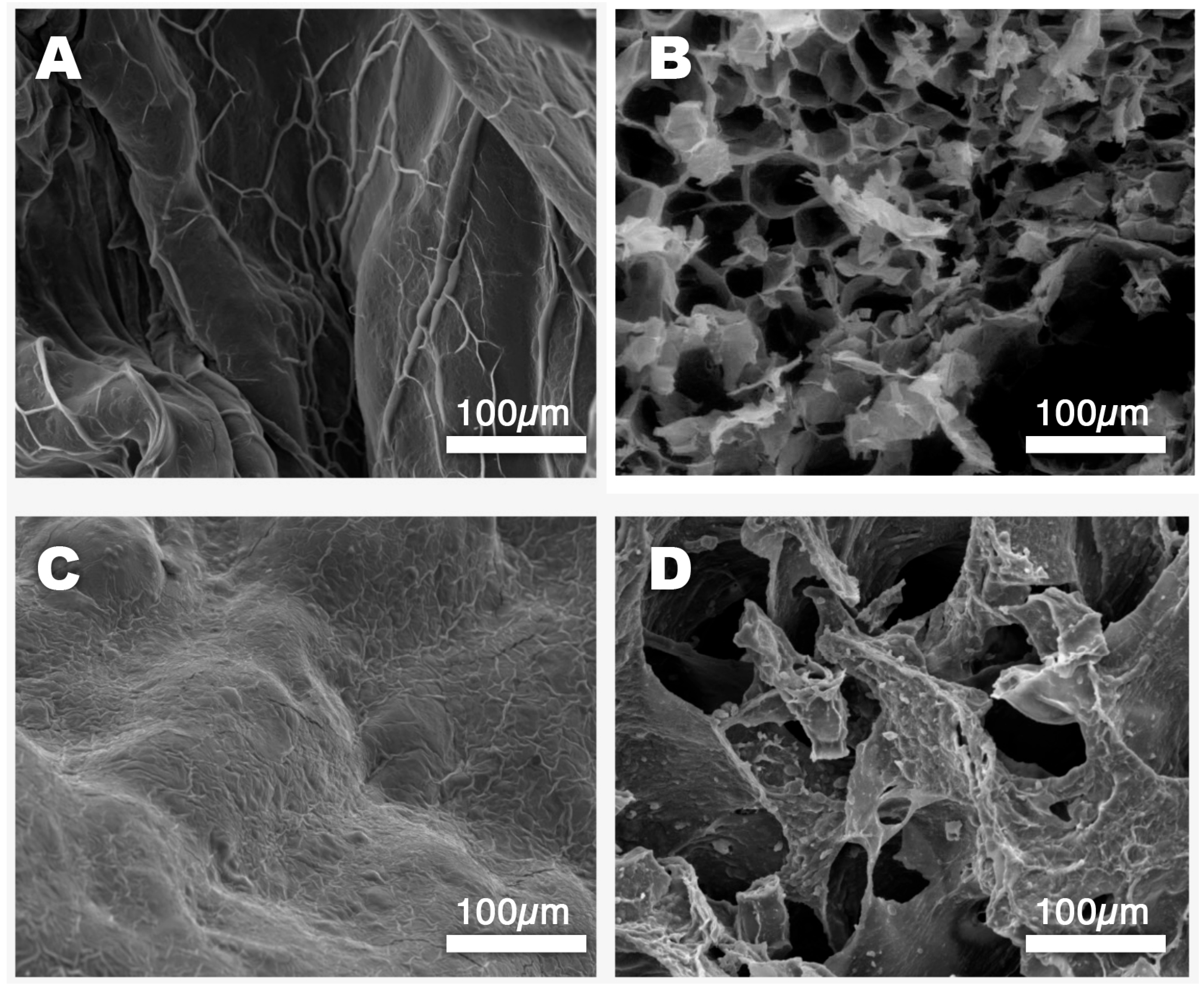
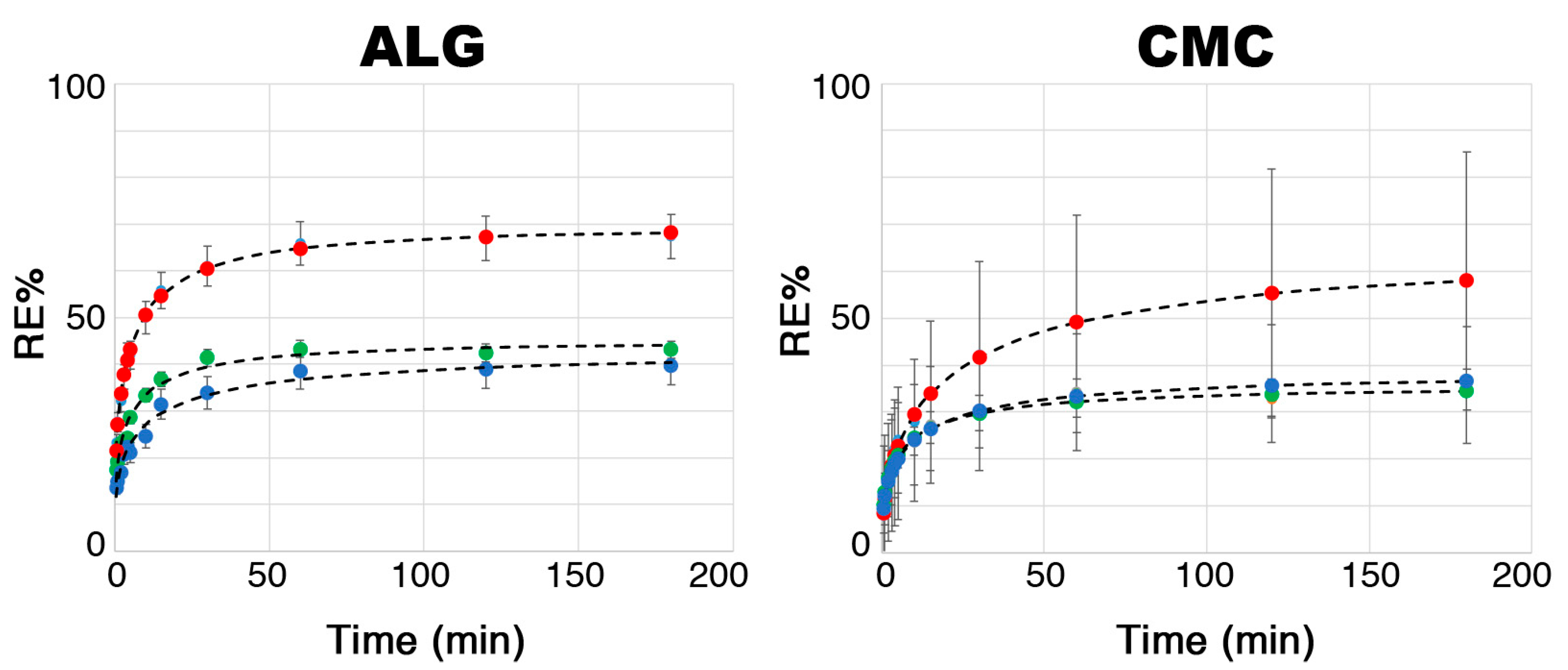
2.2. Physico-Chemical Characterization of ALG and CMC Hydrogels
3. Materials and Methods
3.1. Chemicals
3.2. Hydrogel Synthesis
3.3. Brassica Oleracea Extract Characterization
3.4. Equilibrium Water Content (EWC)
3.5. Differential Scanning Calorimetry (DSC) and Free Water Index (FWI)
3.6. Scanning Electron Microscopy (SEM)
3.7. Rheometry on Polymer Solutions and Hydrogels
3.8. Encapsulation Efficiency and Release Profiles
3.8.1. Encapsulation Efficiency (EE%)
3.8.2. Release Kinetics in Water and Release Efficiency (RE%)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, Distribution Pathways and Effects on Human Health—A Review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Toppari, J.; Larsen, J.C.; Christiansen, P.; Giwercman, A.; Grandjean, P.; Guillette, L.J.; Jégou, B.; Jensen, T.K.; Jouannet, P.; Keiding, N.; et al. Male Reproductive Health and Environmental Xenoestrogens. Environ. Health Perspect. 1996, 104, 741–803. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How Sustainable Agriculture Can Address the Environmental and Human Health Harms of Industrial Agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef]
- Devkota, P.; Norsworthy, J.K.; Rainey, R. Comparison of Allyl Isothiocyanate and Metam Sodium with Methyl Bromide for Weed Control in Polyethylene-Mulched Bell Pepper. Weed Technol. 2013, 27, 468–474. [Google Scholar] [CrossRef]
- Stephen, B.; Pruett Deborah, E.; Keil, L.P.M. Toxicology of metam sodium. J. Toxicol. Environ. Health Part B 2001, 4, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Sederholm, M.R.; Schmitz, B.W.; Barberán, A.; Pepper, I.L. Effects of Metam Sodium Fumigation on the Abundance, Activity, and Diversity of Soil Bacterial Communities. Appl. Soil. Ecol. 2018, 124, 27–33. [Google Scholar] [CrossRef]
- Zheng, W.; Yates, S.R.; Papiernik, S.K.; Nunez, J. Conversion of Metam Sodium and Emission of Fumigant from Soil Columns. Atmos. Environ. 2006, 40, 7046–7056. [Google Scholar] [CrossRef]
- Authority, E.F.S. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Metam. EFSA J. 2011, 9, 2334. [Google Scholar] [CrossRef][Green Version]
- Kirkegaard, J.A.; Sarwar, M. Biofumigation Potential of Brassicas: I. Variation in Glucosinolate Profiles of Diverse Field-Grown Brassicas. Plant Soil 1998, 201, 71–89. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Biofumigation Potential of Brassicas: III. In Vitro Toxicity of Isothiocyanates to Soil-Borne Fungal Pathogens. Plant Soil 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Winkelmann, T. Biofumigation for Fighting Replant Disease—A Review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolate and Isothiocyanate Concentration in Soil Following Incorporation of Brassica Biofumigants. Soil Biol. Biochem. 2006, 38, 2255–2264. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and Biofumigation: Fate of Glucosinolates and Their Hydrolysis Products in Soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. CRC Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Pardini, A.; Tamasi, G.; De Rocco, F.; Bonechi, C.; Consumi, M.; Leone, G.; Magnani, A.; Rossi, C. Kinetics of Glucosinolate Hydrolysis by Myrosinase in Brassicaceae Tissues: A High-Performance Liquid Chromatography Approach. Food Chem. 2021, 355, 129634. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J.; McCaffrey, J.P.; Auld, D.L.; Williams, L. Allelochemicals Produced during Glucosinolate Degradation in Soil. J. Chem. Ecol. 1991, 17, 2021–2034. [Google Scholar] [CrossRef]
- Rosa, E.A.S.; Heaney, R.K.; Fenwick, G.R.; Portas, C.A.M. Glucosinolates in Crop Plants. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996; pp. 99–215. ISBN 9780470650622. [Google Scholar]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of Glucosinolate-Derived Isothiocyanates on Fungi: A Comprehensive Review on Direct Effects, Mechanisms, Structure-Activity Relationship Data and Possible Agricultural Applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Mari, M.; Leoni, O.; Bernardi, R.; Neri, F.; Palmieri, S. Control of Brown Rot on Stonefruit by Synthetic and Glucosinolate-Derived Isothiocyanates. Postharvest Biol. Technol. 2008, 47, 61–67. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The Antibacterial Properties of Isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef]
- Lazzeri, L.; Leoni, O.; Manici, L.M. Biocidal Plant Dried Pellets for Biofumigation. Ind. Crops Prod. 2004, 20, 59–65. [Google Scholar] [CrossRef]
- Morris, E.K.; Fletcher, R.; Veresoglou, S.D. Effective Methods of Biofumigation: A Meta-Analysis. Plant Soil. 2020, 446, 379–392. [Google Scholar] [CrossRef]
- Mun, A.; Simaan Yameen, H.; Edelbaum, G.; Seliktar, D. Alginate Hydrogel Beads Embedded with Drug-Bearing Polycaprolactone Microspheres for Sustained Release of Paclobutrazol. Sci. Rep. 2021, 11, 10877. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in Controlled Release Pesticide Formulations: Prospects to Safer Integrated Pest Management and Sustainable Agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous Materials for Pesticide Formulation and Delivery in the Agricultural Sector. J. Control. Release 2022, 343, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan Nanoparticle Based Delivery Systems for Sustainable Agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; Smith, A.; Kavanagh, K.; Devereux, M.; Colleran, J.; Breslin, C.; Richards, K.G.; McCann, M.; Rooney, A.D. Preparation and Antimicrobial Properties of Alginate and Serum Albumin/Glutaraldehyde Hydrogels Impregnated with Silver(I) Ions. Chemistry 2021, 3, 672–686. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of Plant Growth Promoting Rhizobacteria—Prospects and Potential in Agricultural Sector: A Review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- Bravo Cadena, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing Cinnamon Essential Oil Activity by Nanoparticle Encapsulation to Control Seed Pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating Polymer Networks Polysaccharide Hydrogels for Drug Delivery and Tissue Engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Mikula, K.; Skrzypczak, D.; Ligas, B.; Witek-Krowiak, A. Preparation of Hydrogel Composites Using Ca2+ and Cu2+ Ions as Crosslinking Agents. SN Appl. Sci. 2019, 1, 643. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Aguirre Calvo, T.R.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Alginate Beads Containing Lactase: Stability and Microstructure. Biomacromolecules 2017, 18, 1785–1792. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Gu, B.K.; Kim, C.-H. Ionically Crosslinked Alginate–Carboxymethyl Cellulose Beads for the Delivery of Protein Therapeutics. Appl. Surf. Sci. 2012, 262, 28–33. [Google Scholar] [CrossRef]
- Neri-Badang, M.C.; Chakraborty, S. Carbohydrate Polymers as Controlled Release Devices for Pesticides. J. Carbohydr. Chem. 2019, 38, 67–85. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From Waste to Health: Sustainable Exploitation of Grape Pomace Seed Extract to Manufacture Antioxidant, Regenerative and Prebiotic Nanovesicles within Circular Economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef]
- Basta, A.H.; Lotfy, V.F. The Synergistic Route for Enhancing Rice By-Product Derived Nanoparticles in Sustained Release of Bioactive Compound. Cellulose 2023, 30, 11473–11491. [Google Scholar] [CrossRef]
- Clemente, I.; Bonechi, C.; Rodolfi, L.; Bacia-Verloop, M.; Rossi, C.; Ristori, S. Lipids from Algal Biomass Provide New (Nonlamellar) Nanovectors with High Carrier Potentiality for Natural Antioxidants. Eur. J. Pharm. Biopharm. 2021, 158, 410–416. [Google Scholar] [CrossRef]
- Clemente, I.; D’Aria, F.; Giancola, C.; Bonechi, C.; Slouf, M.; Pavlova, E.; Rossi, C.; Ristori, S. Structuring and De-Structuring of Nanovectors from Algal Lipids. Part 1: Physico-Chemical Characterization. Colloids Surf. B Biointerfaces 2022, 220, 112939. [Google Scholar] [CrossRef]
- Bonechi, C.; Tamasi, G.; Donati, A.; Bisozzi, F.; Baglioni, M.; Andreassi, M.; Ietta, F.; Leone, G.; Magnani, A.; Rossi, C. Cationic Liposomes as Carriers of Natural Compounds from Plant Extract. Biophys. Chem. 2025, 318, 107381. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Clemente, I.; Tamasi, G.; Bisozzi, F.; Costantini, S.; Fattori, G.; Gentile, M.; Rossi, C. Isothiocyanate-Based Microemulsions Loaded into Biocompatible Hydrogels as Innovative Biofumigants for Agricultural Soils. Molecules 2024, 29, 3935. [Google Scholar] [CrossRef]
- Rochfort, S.J.; Trenerry, V.C.; Imsic, M.; Panozzo, J.; Jones, R. Class Targeted Metabolomics: ESI Ion Trap Screening Methods for Glucosinolates Based on MSn Fragmentation. Phytochemistry 2008, 69, 1671–1679. [Google Scholar] [CrossRef]
- Nandasiri, R.; Eskin, N.A.M.; Komatsu, E.; Perreault, H.; Thiyam-Holländer, U. Valorization of Canola By-Products: Concomitance of Flavor-Active Bitter Phenolics Using Pressurized Heat Treatments. LWT 2021, 138, 110397. [Google Scholar] [CrossRef]
- Mouterde, L.M.M.; Peru, A.A.M.; Mention, M.M.; Brunissen, F.; Allais, F. Sustainable Straightforward Synthesis and Evaluation of the Antioxidant and Antimicrobial Activity of Sinapine and Analogues. J. Agric. Food Chem. 2020, 68, 6998–7004. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.E.B.; Yu, H.C.; Noel, M.G. Identification of Major Glucosinolates in Broccoli (Brassica Oleracea Var. Italica) by Liquid Chromatography—Mass Spectrometry (LC-MS) and Determination of Anticancer Properties of Broccoli Extracts. In Proceedings of the DLSU 2013 Research Congress—Commitment to Serve: Lasallian Research fo the Next 100 Years, Manila, Philippines, 7–9 March 2013. [Google Scholar]
- Bellostas, N.; Sørensen, A.D.; Sørensen, J.C.; Sørensen, H. Fe2+-Catalyzed Formation of Nitriles and Thionamides from Intact Glucosinolates. J. Nat. Prod. 2008, 71, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Massana Roquero, D.; Othman, A.; Melman, A.; Katz, E. Iron(Iii)-Cross-Linked Alginate Hydrogels: A Critical Review. Mater. Adv. 2022, 3, 1849–1873. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic Nanocellulose Alginate Hydrogel Beads as Potential Drug Delivery System. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the Morphology of Hydrophilic Polymeric Matrices on the Diffusion and Release of Water Soluble Drugs. J. Memb. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Caccavo, D. An Overview on the Mathematical Modeling of Hydrogels’ Behavior for Drug Delivery Systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the Use of the Weibull Function for the Discernment of Drug Release Mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Clemente, I.; Baglioni, M.; Bonechi, C.; Bisozzi, F.; Rossi, C.; Tamasi, G. Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils. Polymers 2023, 15, 4455. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Skrodzka, M.; Sicińska, H.; Michalak, I.; Detyna, J. Influence of Cross-Linking Conditions on Drying Kinetics of Alginate Hydrogel. Gels 2023, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogels Based on Semi-Interpenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
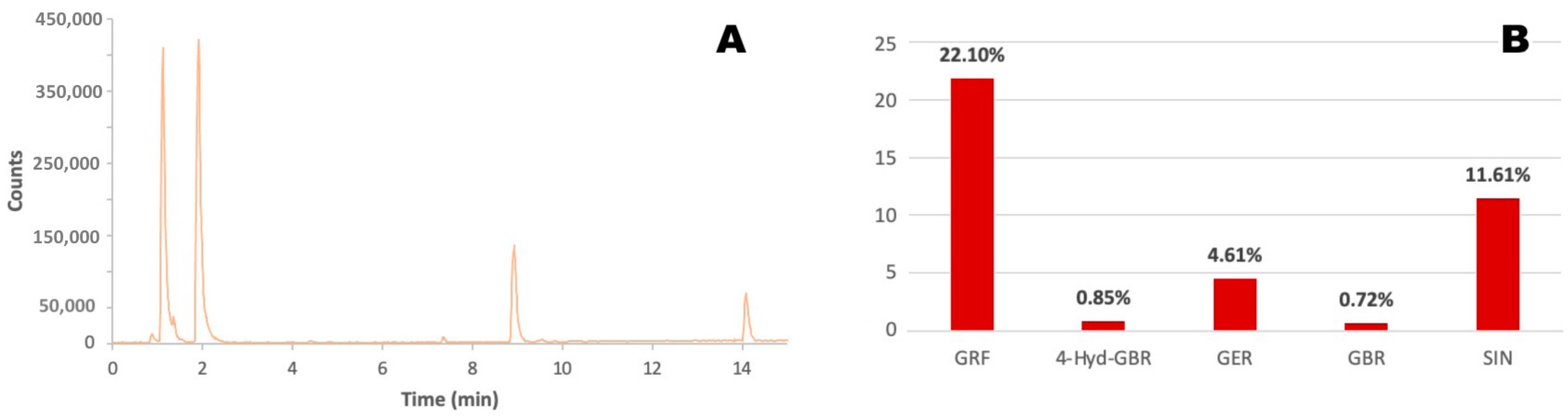


| Analyte | Retention Time (min) | [M − H]− | Fragment Ions |
|---|---|---|---|
| Glucoraphanin | 1.91 | 436 | 292, 275, 259, 194, 130 |
| 4-Hydroxyglucobrassicin | 4.4 | 463 | 383, 285, 275, 267, 259, 240 |
| Glucoerucin | 8.93 | 420 | 340, 291, 275, 259, 242, 227, 224, 195, 178 |
| Glucobrassicin | 9.55 | 447 | 275, 259, 251, 205 |
| Sinapine | 14.09 | 354 | 294, 279, 264, 223, 208 |
| Sample | ΔHexp (J/g) | FWI (%) |
|---|---|---|
| ALG | 238 ± 12 | 77 ± 4 |
| ALG-1 | 242 ± 12 | 78 ± 4 |
| ALG-2-5 | 279 ± 14 | 90 ± 5 |
| ALG-5 | 288 ± 14 | 93 ± 5 |
| CMC | 265 ± 13 | 86 ± 4 |
| CMC-1 | 217 ± 11 | 70 ± 4 |
| CMC-2.5 | 179 ± 9 | 58 ± 3 |
| CMC-5 | 165 ± 8 | 53 ± 3 |
| System | K | a | b | Max Glucoraphanin Released a |
|---|---|---|---|---|
| CMC 1% | 62% | 0.16 | 0.48 | 0.6 ± 0.1 |
| CMC 2.5% | 39% | 0.46 | 0.41 | 1.4 ± 1.0 |
| CMC 5% | 35% | 0.38 | 0.42 | 3.2 ± 1.0 |
| ALG 1% | 73% | 0.50 | 0.42 | 0.7 ± 0.1 |
| ALG 2.5% | 46% | 0.53 | 0.41 | 1.5 ± 1.0 |
| ALG 5% | 44% | 0.42 | 0.38 | 3.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baglioni, M.; Clemente, I.; Nardin, R.; Bisozzi, F.; Costantini, S.; Fattori, G.; Tamasi, G.; Rossi, C. Hydrogel Beads Loaded with Glucosinolate-Rich Brassicaceae Extract as a Controlled-Release Alternative to Biofumigation. Molecules 2025, 30, 3660. https://doi.org/10.3390/molecules30183660
Baglioni M, Clemente I, Nardin R, Bisozzi F, Costantini S, Fattori G, Tamasi G, Rossi C. Hydrogel Beads Loaded with Glucosinolate-Rich Brassicaceae Extract as a Controlled-Release Alternative to Biofumigation. Molecules. 2025; 30(18):3660. https://doi.org/10.3390/molecules30183660
Chicago/Turabian StyleBaglioni, Michele, Ilaria Clemente, Raffaello Nardin, Flavia Bisozzi, Sara Costantini, Giacomo Fattori, Gabriella Tamasi, and Claudio Rossi. 2025. "Hydrogel Beads Loaded with Glucosinolate-Rich Brassicaceae Extract as a Controlled-Release Alternative to Biofumigation" Molecules 30, no. 18: 3660. https://doi.org/10.3390/molecules30183660
APA StyleBaglioni, M., Clemente, I., Nardin, R., Bisozzi, F., Costantini, S., Fattori, G., Tamasi, G., & Rossi, C. (2025). Hydrogel Beads Loaded with Glucosinolate-Rich Brassicaceae Extract as a Controlled-Release Alternative to Biofumigation. Molecules, 30(18), 3660. https://doi.org/10.3390/molecules30183660







