From Mushrooms to Molecules: Exploring Depsidones in Ganoderma lucidum for Antioxidant and Anticancer Applications
Abstract
1. Introduction
2. Results and Discussion
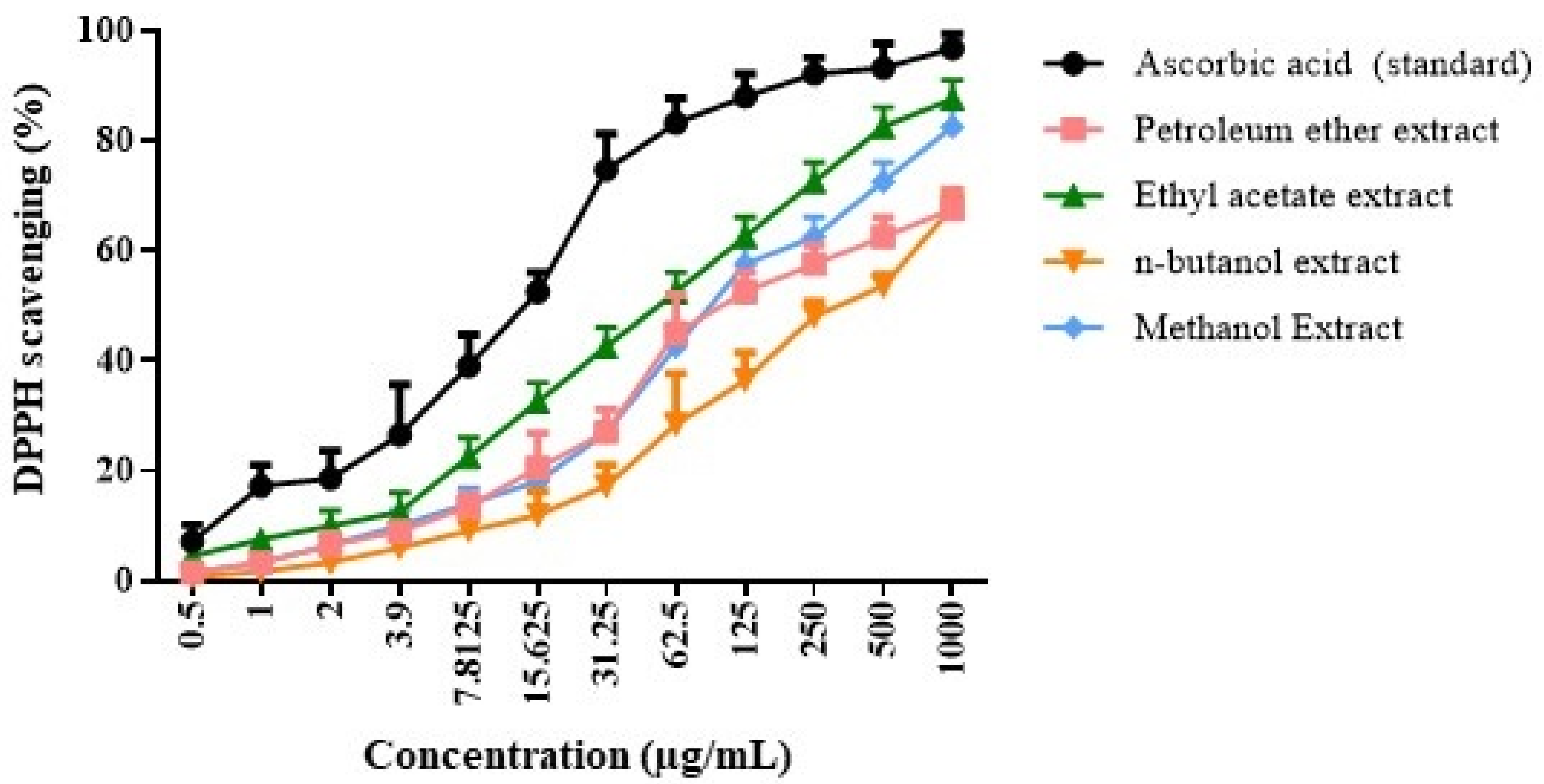
2.1. Qualitative Phytochemical Screening and Antioxidant Potential
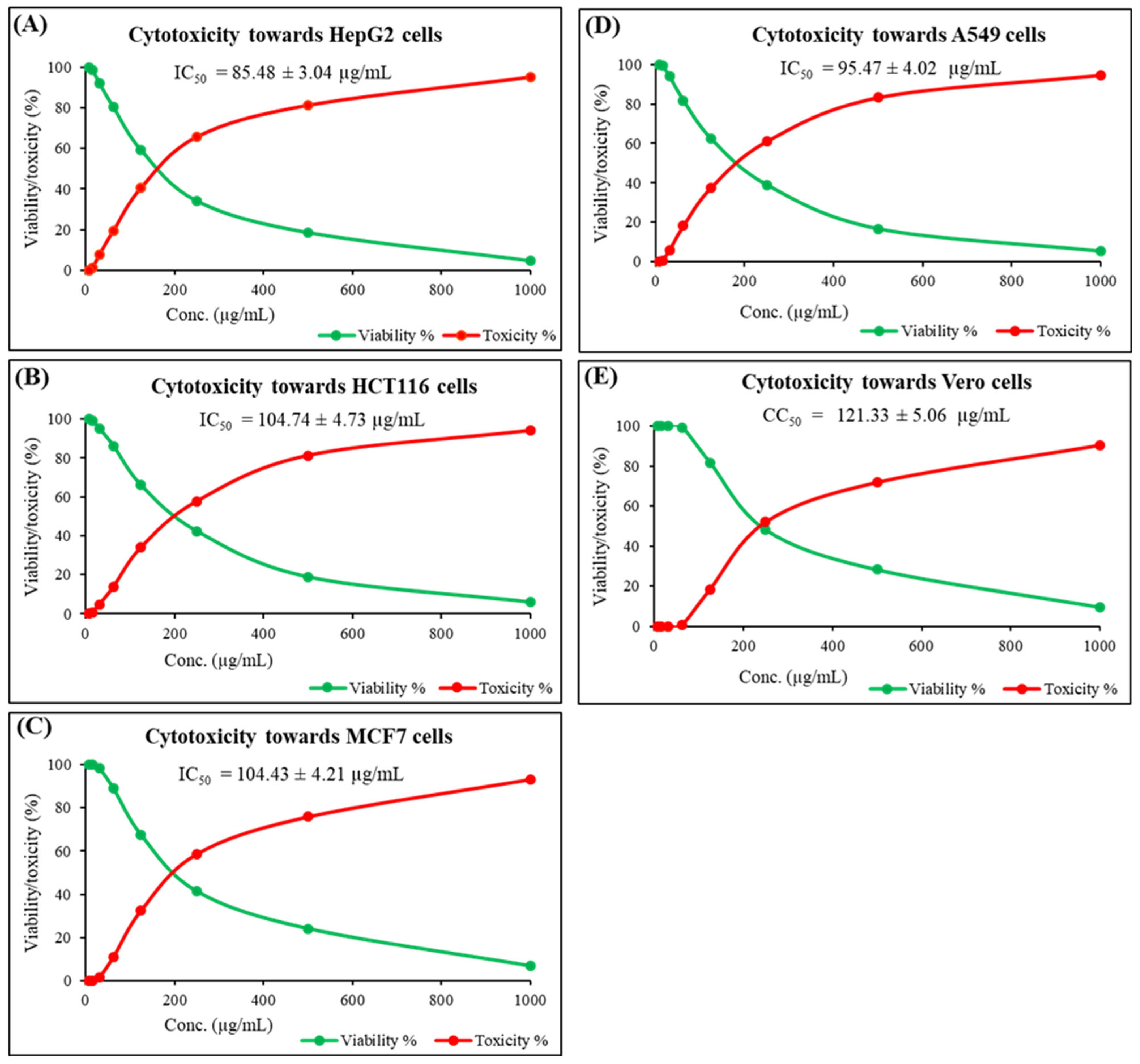
2.2. Cytotoxicity Against Cancer Cell Lines
2.3. UHPLC/Q-TOF-MS-MS Analysis
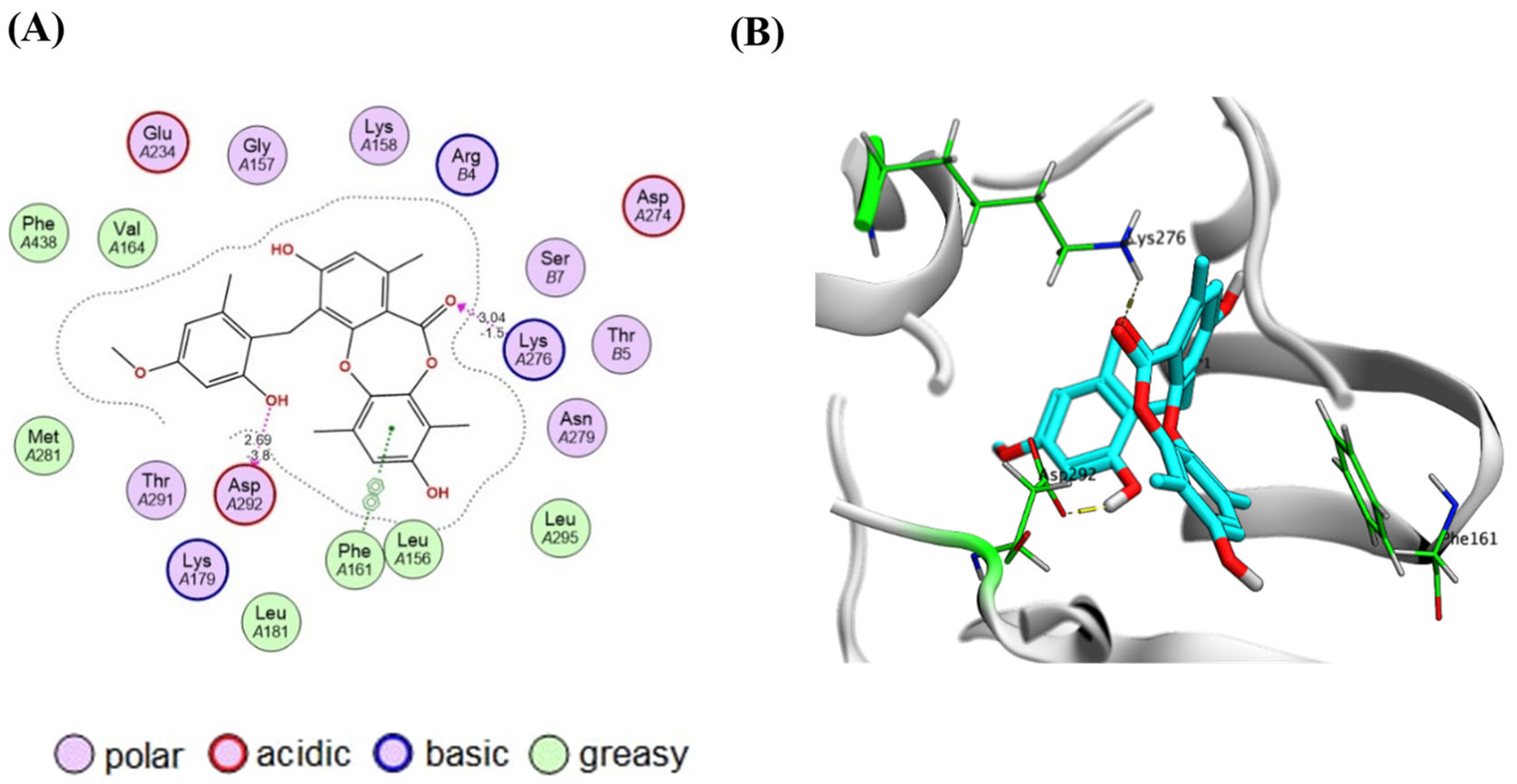
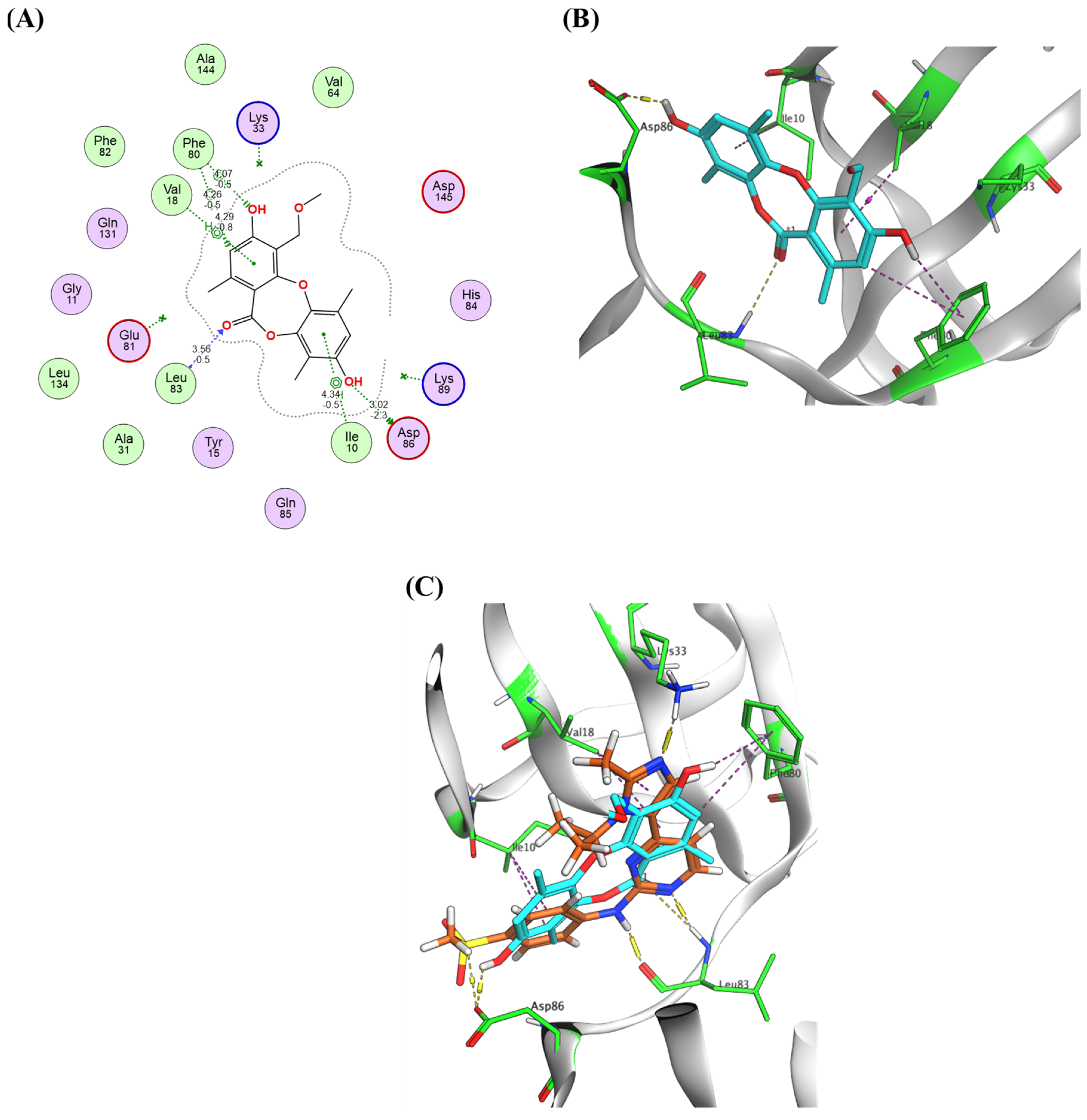
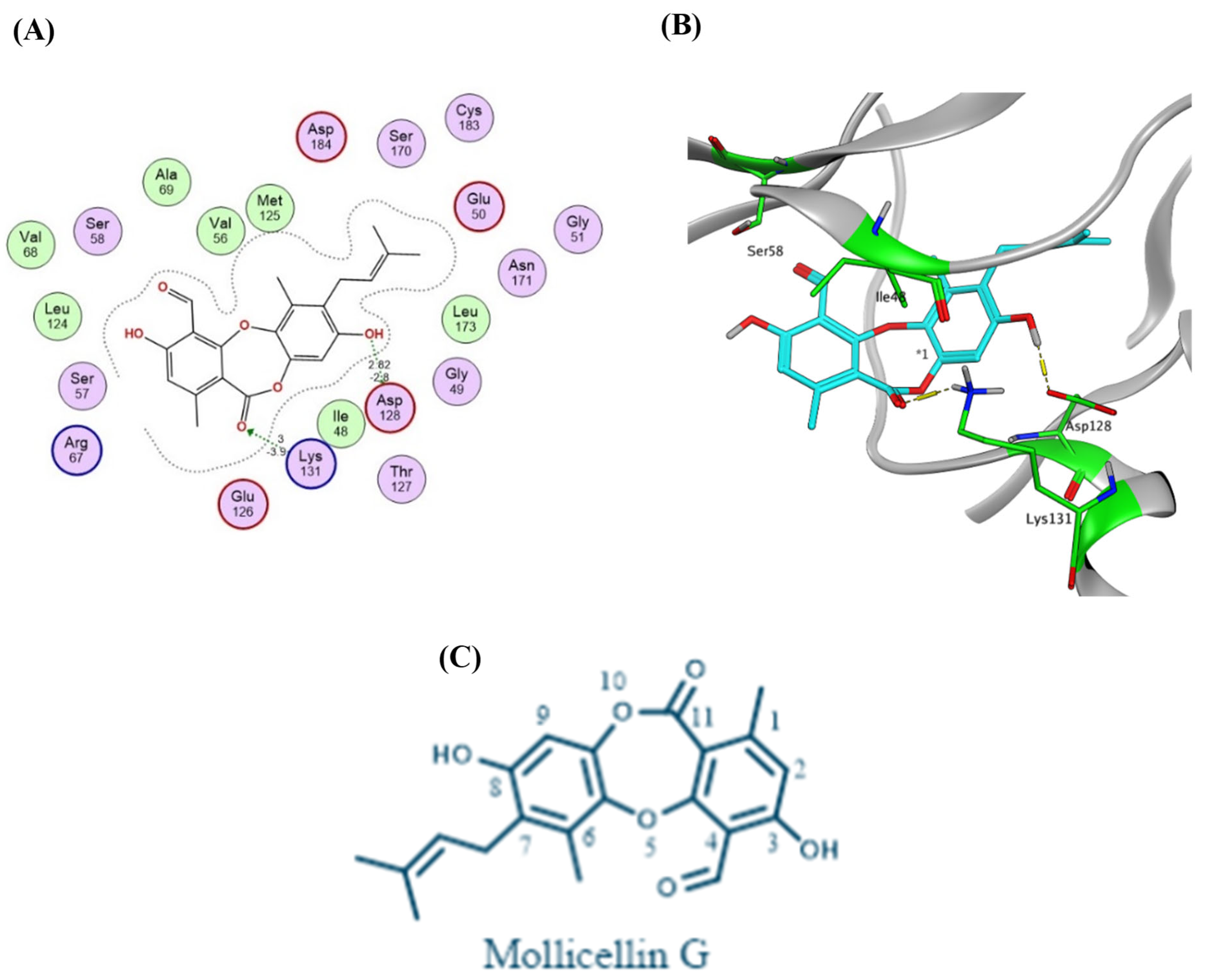
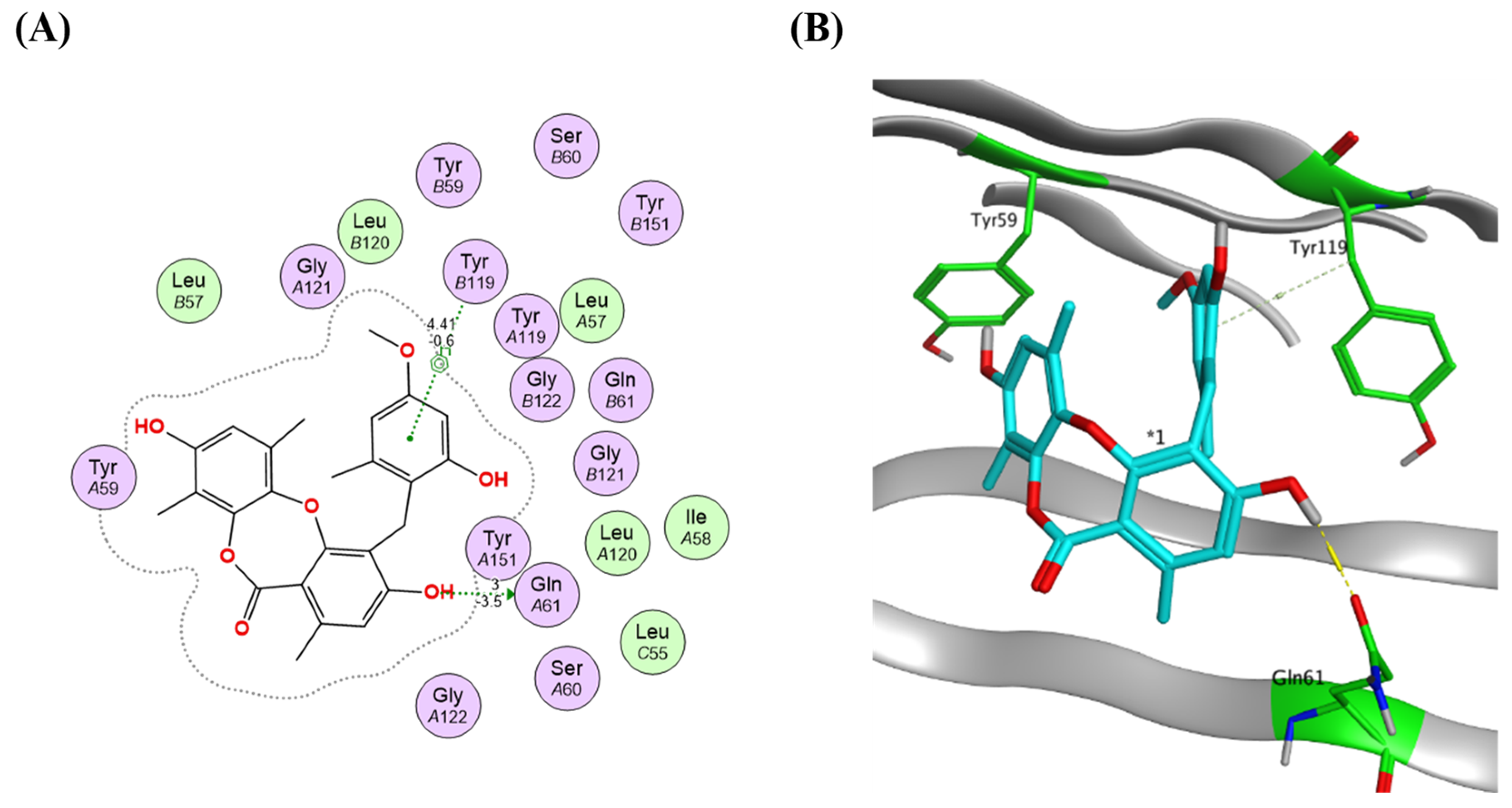
2.4. Virtual Screening and Protein–Ligand Interactions
3. Materials and Methods
3.1. Acquisition of Mushrooms
3.2. Production of the Mushroom Extracts
3.3. Qualitative Screening of Phytochemicals and Total Phenolics in Various Prepared Extracts
3.4. Antioxidant Assay of Different Extracts
3.5. Cytotoxicity Examination
3.6. UHPLC/Q-TOF-MS-MS Testing
3.7. Molecular Docking and Protein–Ligand Interactions
3.8. Statistical Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| G. lucidum | Ganoderma lucidum |
| FFB | Fresh fruiting bodies |
| LC-MS/MS | Liquid chromatography–mass spectrometry/mass spectrometry |
| UHPLC/Q-TOF-MS-MS | Ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| PBS | Phosphate-buffered saline |
| MOE | Molecular Operating Environment |
| RMSD | Root mean square deviation |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| AKT1 | RAC-alpha serine/threonine-protein kinase 1 |
| CDK2 | Cyclin-dependent kinase 2 |
| ERK1 | Extracellular signal-regulated kinase 1 |
| TNFα | Tumor necrosis factor alpha |
| Vero | African green monkey kidney cells (normal cell line) |
| IC50 | Half maximal inhibitory concentration |
| CC50 | 50% cytotoxic concentration |
| PDB | Protein Data Bank |
References
- Oke, M.A.; Afolabi, F.J.; Oyeleke, O.O.; Kilani, T.A.; Adeosun, A.R.; Olanbiwoninu, A.A.; Adebayo, E.A. Ganoderma lucidum: Unutilized natural medicine and promising future solution to emerging diseases in Africa. Front. Pharmacol. 2022, 13, 952027. [Google Scholar] [CrossRef]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A Review of Ganoderma Triterpenoids and Their Bioactivities. Biomolecules 2023, 13, 24. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2023, 13, 568–596. [Google Scholar] [CrossRef]
- Pascale, C.; Sirbu, R.; Cadar, E. Therapeutical Properties of Bioactive Compounds Extracted from Ganoderma lucidum Species on Acute and Chronic Diseases. Eur. J. Nat. Sci. Med. 2023, 6, 73–86. [Google Scholar] [CrossRef]
- Yu, F.; Teng, Y.; Li, J.; Yang, S.; Zhang, Z.; He, Y.; Yang, H.; Ding, C.-F.; Zhou, P. Effects of a Ganoderma lucidum Proteoglycan on Type 2 Diabetic Rats and the Recovery of Rat Pancreatic Islets. ACS Omega 2023, 8, 17304–17316. [Google Scholar] [CrossRef] [PubMed]
- Kou, F.; Ge, Y.; Wang, W.; Mei, Y.; Kao, L.; Wei, X.; Xiao, H.; Wu, X. A review of Ganoderma lucidum polysaccharides: Health benefit, structure-activity relationship, modification, and nanoparticle encapsulation. Int. J. Biol. Macromol. 2023, 243, 125199. [Google Scholar] [CrossRef]
- Pathak, M.P.; Pathak, K.; Saikia, R.; Gogoi, U.; Ahmad, M.Z.; Patowary, P.; Das, A. Immunomodulatory effect of mushrooms and their bioactive compounds in cancer: A comprehensive review. Biomed. Pharmacother. 2022, 149, 112901. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Alsayegh, A.A.; Ahmad, F.A.; Akhtar, M.S.; Alavudeen, S.S.; Bantun, F.; Wahab, S.; Ahmed, A.; Ali, M.; Elbendary, E.Y.; et al. Ganoderma lucidum: Insight into antimicrobial and antioxidant properties with development of secondary metabolites. Heliyon 2024, 10, e25607. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.M.; Proestos, C.; Brennan, C.; Zeng, M.; Tomasevic, I.; Elobeid, T.; Çadırcı, K.; Bayrak, M.; et al. Exploring the Potential Medicinal Benefits of Ganoderma lucidum: From Metabolic Disorders to Coronavirus Infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef]
- Campos Ziegenbein, F.; Hanssen, H.P.; König, W.A. Secondary metabolites from Ganoderma lucidum and Spongiporus leucomallellus. Phytochemistry 2006, 67, 202–211. [Google Scholar] [CrossRef]
- Cheng, C.R.; Yang, M.; Yu, K.; Guan, S.H.; Wu, X.H.; Wu, W.Y.; Sun, Y.; Li, C.; Ding, J.; Guo, D.A. Metabolite identification of crude extract from Ganoderma lucidum in rats using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 941, 90–99. [Google Scholar] [CrossRef]
- Murata, C.; Tran, Q.T.; Onda, S.; Usuki, T. Extraction and isolation of ganoderic acid Σ from Ganoderma lucidum. Tetrahedron Lett. 2016, 57, 5368–5371. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, B.-J.; Deng, S.; Zhang, H.-L.; Huang, S.-S.; Huo, X.-K.; Wang, C.; Liu, F.; Ma, X.-C. Isolation and identification of oxygenated lanostane-type triterpenoids from the fungus Ganoderma lucidum. Phytochem. Lett. 2016, 16, 87–91. [Google Scholar] [CrossRef]
- Shen, B. A new golden age of natural products drug discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef]
- Abdel-Razek, A.S.; El-Naggar, M.E.; Allam, A.; Morsy, O.M.; Othman, S.I. Microbial natural products in drug discovery. Processes 2020, 8, 470. [Google Scholar] [CrossRef]
- Singh, G.; Armaleo, D.; Dal Grande, F.; Schmitt, I. Depside and Depsidone Synthesis in Lichenized Fungi Comes into Focus through a Genome-Wide Comparison of the Olivetoric Acid and Physodic Acid Chemotypes of Pseudevernia furfuracea. Biomolecules 2021, 11, 1445. [Google Scholar] [CrossRef]
- Kulinowska, M.; Dresler, S.; Skalska-Kamińska, A.; Hanaka, A.; Strzemski, M. Methodological Aspects of Green Extraction of Usnic Acid Using Natural Deep Eutectic Solvents. Molecules 2023, 28, 5321. [Google Scholar] [CrossRef]
- Baczewska, I.; Strzemski, M.; Feldo, M.; Hanaka, A.; Dresler, S. Green Extraction of Depsidones and Depsides from Hypogymnia physodes (L.) Nyl. Using Natural Deep Eutectic Solvents. Int. J. Mol. Sci. 2024, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen depsidones with biological interest. Planta Medica 2022, 88, 855–880. [Google Scholar] [CrossRef]
- Du, Y.; Tian, L.; Wang, Y.; Li, Z.; Xu, Z. Chemodiversity, pharmacological activity, and biosynthesis of specialized metabolites from medicinal model fungi Ganoderma lucidum. Chin. Med. 2024, 19, 51. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Omidifar, N.; Chiang, W.-H.; Neralla, V.R.; Yousefi, K.; Shokripour, M. Ganoderma lucidum methanolic extract as a potent phytoconstituent: Characterization, in-vitro antimicrobial and cytotoxic activity. Sci. Rep. 2023, 13, 17326. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.-S.; Tomescu, C.L.; Ionescu, A.-M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Younis, A.; Stewart, J.; Wu, F.S.; El Shikh, H.; Hassan, F.; Elaasser, M. Effectiveness of different solvents extracts from edible mushrooms in inhibiting the growth of tumor cells. Cancer Biol. 2014, 4, 1–15. [Google Scholar]
- Mondal, T.; Some, R.; Dutta, S. Studies on antioxidant and antimicrobial properties of some common mushrooms. J. Today’s Biol. Sci. Res. Rev. 2013, 2, 60–67. [Google Scholar]
- Dong, Q.; He, D.; Ni, X. Comparative study on phenolic compounds, triterpenoids, and antioxidant activity of Ganoderma lucidum affected by different drying methods. J. Food Meas. Charact. 2019, 13, 3198–3205. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmianski, J.; Szyjka, A.; Moreira, H.; Barg, E. Anticancer and Antioxidant Activities in Ganoderma lucidum Wild Mushrooms in Poland, as Well as Their Phenolic and Triterpenoid Compounds. Int. J. Mol. Sci. 2022, 23, 9359. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Al Masud, A.; Lira, N.Y.; Shakil, S. Proximate analysis, phytochemical screening and antioxidant activity of different strains of Ganoderma lucidum (Reishi Mushroom). Open J. Biol. Sci. 2020, 5, 24–27. [Google Scholar]
- Lin, M.S.; Yu, Z.R.; Wang, B.J.; Wang, C.C.; Weng, Y.M.; Koo, M. Bioactive constituent characterization and antioxidant activity of Ganoderma lucidum extract fractionated by supercritical carbon dioxide. Sains Mal. 2015, 44, 1685–1691. [Google Scholar]
- Song, S.; Liu, Y.; Kong, X.; Chang, W.; Song, G. Progress on understanding the anticancer mechanisms of medicinal mushroom: Inonotus obliquus. Asian Pac. J. Cancer Prev. 2013, 14, 1571–1578. [Google Scholar] [CrossRef]
- Chen, P.; Qin, H.J.; Li, Y.W.; Ma, G.; Yang, J.S.; Wang, Q. Study on chemical constituents of an edible mushroom Volvariella volvacea and their antitumor activity in vitro. Nat. Prod. Res. 2018, 34, 1417–1422. [Google Scholar] [CrossRef]
- Panda, S.K.; Sahoo, G.; Swain, S.S.; Luyten, W. Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery. Pharmaceuticals 2022, 15, 176. [Google Scholar] [CrossRef]
- Park, H.J. Current Uses of Mushrooms in Cancer Treatment and Their Anticancer Mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef]
- Cör Andrejč, D.; Knez, Ž.; Knez Marevci, M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Mao, Z.; Guo, H.; Xie, Y.; Cui, Z.; Sun, J.; Wu, H.; Wen, X.; Wang, J.; Shan, T. Mollicellins O–R, Four New Depsidones Isolated from the Endophytic Fungus Chaetomium sp. Eef-10. Molecules 2018, 23, 3218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Li, S.; Zhang, X.; Zhao, C. Biological Activities of Some New Secondary Metabolites Isolated from Endophytic Fungi: A Review Study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef]
- Liu, R.M.; Zhong, J.J. Ganoderic acid Mf and S induce mitochondria mediated apoptosis in human cervical carcinoma HeLa cells. Phytomedicine 2011, 18, 349–355. [Google Scholar] [CrossRef]
- Gurovic, M.S.V.; Viceconte, F.R.; Pereyra, M.T.; Bidegain, M.A.; Cubitto, M.A. DNA damaging potential of Ganoderma lucidum extracts. J. Ethnopharmacol. 2018, 217, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, S.; Sang, Q.; Cheng, X.; Bi, Y. Potential Active Compounds of Ganoderma lucidum and Their Anticancer Effects: A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70741. [Google Scholar] [CrossRef]
- Khumkomkhet, P.; Kanokmedhakul, S.; Kanokmedhakul, K.; Hahnvajanawong, C.; Soytong, K. Antimalarial and cytotoxic depsidones from the fungus Chaetomium brasiliense. J. Nat. Prod. 2009, 72, 1487–1491. [Google Scholar] [CrossRef]
- Zeng, Z.; Cai, J.; Chen, Y.; Li, X.; Chen, C.; Liu, Y.; Jayasinghe, L.; Zhou, X. Three New Dipeptide and Two New Polyketide Derivatives from the Mangrove-Derived Fungus Talaromyces sp.: Antioxidant Activity of Two Isolated Substances. Mar. Drugs 2024, 22, 559. [Google Scholar] [CrossRef]
- Saetang, P.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J.; Borwornpinyo, S.; Seemakhan, S.; Muanprasat, C. Depsidones and an α-pyrone derivative from Simplicillium sp. PSU-H41, an endophytic fungus from Hevea brasiliensis leaf [corrected]. Phytochemistry 2017, 143, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Tomoda, H.; Cao, J.; Okuda, S.; Omura, S. Purpactins, new inhibitors of acyl-CoA: Cholesterol acyltransferase produced by Penicillium purpurogenum. II. Structure elucidation of purpactins A, B and C. J. Antibiot. 1991, 44, 144–151. [Google Scholar] [CrossRef]
- Domingos, L.T.S.; Martins, R.d.S.; Lima LMd Ghizelini, A.M.; Ferreira-Pereira, A.; Cotinguiba, F. Secondary Metabolites Diversity of Aspergillus unguis and Their Bioactivities: A Potential Target to Be Explored. Biomolecules 2022, 12, 1820. [Google Scholar] [CrossRef]
- Khayat, M.T.; Ghazawi, K.F.; Samman, W.A.; Alhaddad, A.A.; Mohamed, G.A.; Ibrahim, S.R. Recent advances on natural depsidones: Sources, biosynthesis, structure-activity relationship, and bioactivities. PeerJ 2023, 11, e15394. [Google Scholar] [CrossRef]
- Arce-Ramos, L.; Castillo, J.-C.; Becerra, D. Synthesis and Biological Studies of Benzo[b]furan Derivatives: A Review from 2011 to 2022. Pharmaceuticals 2023, 16, 1265. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; An, F.; Zhu, X.; Yu, H.; Hao, L.; Lu, Y. Curdepsidones B–G, Six Depsidones with Anti-Inflammatory Activities from the Marine-Derived Fungus Curvularia sp. IFB-Z10. Mar. Drugs 2019, 17, 266. [Google Scholar] [CrossRef]
- Blake, J.F.; Kallan, N.C.; Xiao, D.; Xu, R.; Bencsik, J.R.; Skelton, N.J.; Spencer, K.L.; Mitchell, I.S.; Woessner, R.D.; Gloor, S.L.; et al. Discovery of pyrrolopyrimidine inhibitors of Akt. Bioorg. Med. Chem. Lett. 2010, 20, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.J.; Korolchuk, S.; Tatum, N.J.; Wang, L.Z.; Endicott, J.A.; Noble, M.E.M.; Martin, M.P. Differences in the Conformational Energy Landscape of CDK1 and CDK2 Suggest a Mechanism for Achieving Selective CDK Inhibition. Cell Chem. Biol. 2019, 26, 121–130.e5. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, Y. Molecular Modeling and Design Studies of Purine Derivatives as Novel CDK2 Inhibitors. Molecules 2018, 23, 2924. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yoshida, I.; Nakae, S.; Okita, K.; Gouda, M.; Matsubara, M.; Yokota, K.; Ishiguro, H.; Tada, T. Crystal structure of human mono-phosphorylated ERK1 at Tyr204. Biochem. Biophys. Res. Commun. 2008, 377, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C.; et al. Small-molecule inhibition of TNF-alpha. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Loyd, A.L.; Richter, B.S.; Jusino, M.A.; Truong, C.; Smith, M.E.; Blanchette, R.A.; Smith, J.A. Identifying the “Mushroom of Immortality”: Assessing the Ganoderma Species Composition in Commercial Reishi Products. Front. Microbiol. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Li, G.; Yu, K.; Li, F.; Xu, K.; Li, J.; He, S.; Cao, S.; Tan, G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef]
- Khunoana, E.T.; Nkadimeng, S.M. Current Advances in the Use of Mushrooms as Therapeutics for Lung Cancer: A Review. Molecules 2025, 30, 1322. [Google Scholar] [CrossRef] [PubMed]
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants 2022, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolic and total flavonoids in ulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Calvo, M.M.; Martín-Diana, A.B.; Rico, D.; López-Caballero, M.E.; Martínez-Álvarez, O. Antioxidant, Antihypertensive, Hypoglycaemic and Nootropic Activity of a Polyphenolic Extract from the Halophyte Ice Plant (Mesembryanthemum crystallinum). Foods 2022, 11, 1581. [Google Scholar] [CrossRef]
- Zulkipli, N.N.; Rahman, S.A.; Taib, W.R.W.; Razali, R.M.; Ismail, I.; Ahmad, W.A.N.W.; Daud, C.K.D.C.K. The cytotoxicity effect and identification of bioactive compounds of Prismatomeris glabra crude leaf extracts against breast cancer cells. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 33. [Google Scholar] [CrossRef]
- Alam, M.M.; Emon, N.U.; Alam, S.; Rudra, S.; Akhter, N.; Mamun, M.M.; Ganguly, A. Assessment of pharmacological activities of Lygodium microphyllum Cav. leaves in the management of pain, inflammation, pyrexia, diarrhea, and helminths: In vivo, in vitro and in silico approaches. Biomed. Pharmacother. 2021, 139, 111644. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE), 2024.0601 Chemical Computing Group ULC, 910-1010 Sher-brooke St. W., Montreal, QC H3A 2R7, 2025. Available online: http://www.chemcomp.com (accessed on 2 July 2025).

| Sample Code | (1) Petroleum Ether Extract | (2) Ethyl Acetate Extract | (3) N-Butanol Extract | (4) Methanol Extract |
|---|---|---|---|---|
| Total Phenolics (mg/g) | 0.21 ± 0.12 | 9.87 ± 0.71 * | 0.11 ± 0.01 | 4.27 ± 0.11 |
| No | Name of the Depsidone Compound | Rt (Min) | Mol. Weight [M-H]- | Error (ppm) | Characteristic Frag. | References |
|---|---|---|---|---|---|---|
| 1 | Mollicellin G C22H23O7 | 1.11235 | 398.1352 | 3.5 | 202.037, 238.0756, 396.855, 98.128450, 188.0453.195.0500, 200.0599, 218.0507, 218.105, 278.0984, 308.097, 330.107, 362.144, 398.10425 | [37] |
| 2 | Simplicildone I C31H28O8 | 1.21 | 527.1707 | 1 | 527.1746, 376.212, 348.402, 177.542 | [38] |
| 3 | Mollicellin B C21H18O7 | 1.244983 | 381.0977 | 0.8 | 381.097, 366.371, 322.804, 179.374, 167.529, 217.1236, 219.04, 219.096, 243.076, 261.0647, 363.1362, 381.102 | [42] |
| 4 | Talaromyone B C23H26O7 | 1.31 | 413.4565 | −8.4 | 413.1656, 353.221, 339.391, 283.024 | [38] |
| 5 | Simplicildone A C18H18O6 | 1.3228 | 329.103 | 1.5 | 329.103, 314.453, 314.409, 85.1782, 285.324, 155.164, 154. 328, 91.443, 76.221 | [44] |
| 6 | Purpactin C C23H21O7 | 1.43 | 408.1255 | 4.6 | 408.125, 393.851, 365.344 | [45] |
| 7 | Emeguisin B C24H25ClO5 | 1.72 | 427.8752 | −13.1 | 112.986, 247.071, 256.93, 265.130, 293.103, 337.1281, 427.167, 381.2082, 71.427,1349, 247.0716, 398.021, 427.1356 | [46] |
| 8 | Mollicellin E C22H19ClO8 | 3.390283 | 445.0675 | −3.4 | 445.0675, 312.865, 220.674, 196.034, 191.350, 225.0928, 237.0142, 250.097, 264.9148, 265.108, 279.008, 279.034, 293.046, 309.069, 313.110 361.118, 377.101, 386.1417, 445.069 | [37] |
| 9 | Simplicildone D C25H24O7 | 7.60215 | 435.1465 | 4.8 | 435.1465, 429.432, 381.246, 201.176, 154.369, 91.66, 76.33 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, S.H.A.; Mahmoud, Y.A.-G.; Bediway, M.Y.; Elsilk, S.E.; Yosri, M.; Metwally, K.; Abo-Dya, N.E.; Yahya, G.; Almostafa, M.; El-Hela, A.A. From Mushrooms to Molecules: Exploring Depsidones in Ganoderma lucidum for Antioxidant and Anticancer Applications. Molecules 2025, 30, 3650. https://doi.org/10.3390/molecules30173650
Mohamed SHA, Mahmoud YA-G, Bediway MY, Elsilk SE, Yosri M, Metwally K, Abo-Dya NE, Yahya G, Almostafa M, El-Hela AA. From Mushrooms to Molecules: Exploring Depsidones in Ganoderma lucidum for Antioxidant and Anticancer Applications. Molecules. 2025; 30(17):3650. https://doi.org/10.3390/molecules30173650
Chicago/Turabian StyleMohamed, Sayed H. A., Yehia A.-G. Mahmoud, Mohamed Y. Bediway, Sobhy E. Elsilk, Mohammed Yosri, Kamel Metwally, Nader E. Abo-Dya, Galal Yahya, Mervt Almostafa, and Atef A. El-Hela. 2025. "From Mushrooms to Molecules: Exploring Depsidones in Ganoderma lucidum for Antioxidant and Anticancer Applications" Molecules 30, no. 17: 3650. https://doi.org/10.3390/molecules30173650
APA StyleMohamed, S. H. A., Mahmoud, Y. A.-G., Bediway, M. Y., Elsilk, S. E., Yosri, M., Metwally, K., Abo-Dya, N. E., Yahya, G., Almostafa, M., & El-Hela, A. A. (2025). From Mushrooms to Molecules: Exploring Depsidones in Ganoderma lucidum for Antioxidant and Anticancer Applications. Molecules, 30(17), 3650. https://doi.org/10.3390/molecules30173650








