Comparative Analysis of Biochemical and Cellular Assay Conditions and the Need for a Buffer That Mimics Cytoplasmic Environments
Abstract
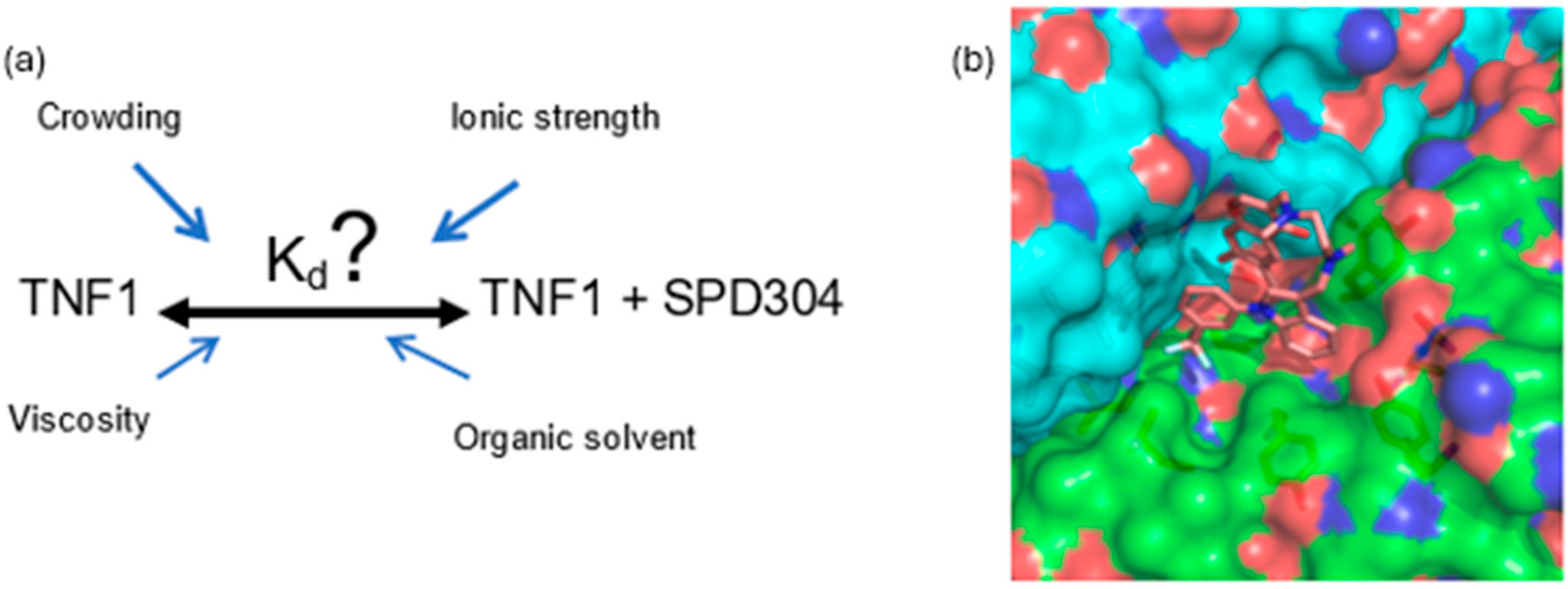
1. Introduction
2. Cytoplasmic Versus Solution Environment
3. Intracellular pH and Differences with Extracellular pH
4. Molecular Crowding
5. Intracellular Salt Differences with Respect to Extracellular pH and Common Buffers
5.1. Cations
5.2. Anions
6. Intracellular Lipophilicity (Hydrophobic Effect) and Differences from Common Biochemical Buffers (BPS Buffer)
7. Proof of Concept: Alignment Between In Vitro and Cellular Assays
8. Key Thoughts
Applications and Implementations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| Bc | Biochemical |
| BcA | Biochemical assay |
| BPS | Biochemical buffers |
| BS | Buffer solution |
| Ca | Calcium |
| CaCl2·2H2O | Calcium chloride dihydrate |
| Cb | Cell-based |
| CBA | Cell-based assays |
| CETSA | Cellular Thermal Shift Assay |
| Cl | Chloride |
| Co | Cobalt |
| CO2 | Carbon dioxide |
| Cu | Copper |
| D(t) | Time-dependent diffusion coefficient |
| DTT | Dithiothreitol |
| dH2O | Distilled water |
| DMSO | Dimethyl sulfoxide |
| Fe | Ferrum |
| H2PO4− | Dihydrogen phosphate |
| HCO3− | Bicarbonate |
| HEPES | (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) |
| HHBS | Hank’s Buffer with HEPES |
| HPO42− | Hydrogen phosphate |
| IC50 | Half-maximal inhibitory concentration |
| IDP | Intrinsically disordered protein |
| K | Potassium |
| Ka | equilibrium association constant |
| KCl | Potassium chloride |
| Kd | equilibrium dissociation constant |
| KH2PO4 | Potassium dihydrogen phosphate |
| Ki | Inhibition constant |
| Km | Michaelis-Menten constant |
| logD | Distribution coefficient |
| logP | Partition coefficient |
| MAPK | Mitogen-activated protein kinase |
| Mg | Magnesium |
| MgCl2·6H2O | Magnesium chloride hexahydrate |
| MgSO4 | Magnesium sulfate |
| MgSO4·7H2O | Magnesium sulfate heptahydrate |
| Mn | Manganese |
| Mo | Molybdenum |
| M-PER | Mammalian protein extraction reagent |
| MSD | Mean Square Displacement |
| Na | Sodium |
| Na2HPO4 | Disodium phosphate |
| NaCl | Sodium chloride |
| NaH2PO4 | Sodium dihydrogen phosphate |
| NaHCO3 | Sodium bicarbonate |
| NaOH | Sodium hydroxide |
| NHE | Na+/H+ exchangers |
| Ni | Nickel |
| NMR | Nuclear magnetic resonance |
| PBS | Phosphate-Buffered Saline |
| PCh | Physicochemical |
| PDB | Protein Data Bank |
| PEG | Polyethylene glycol |
| PGK | Phosphoglycerate kinase |
| pHe | Extracellular pH |
| pHi | Intracellular pH |
| Pi | Inorganic phosphate |
| S | Entropy |
| SAR | Structure Activity Relationship |
| SL2 | Stem loop 2 |
| SO4−2 | Sulfate |
| T | Temperature |
| TBS | Tris-buffered saline |
| TNF | Tumor Necrosis Factor |
| TPSA | Topological polar surface area |
| V | Vanadium |
| VlsE | variable major protein-like sequence expressed |
| W | Tungsten |
| Zn | Zinc |
| ΔG | Change in Gibbs free energy |
| ΔH | Change in enthalpy |
| ΔS | Change in entropy |
References
- Pinne, M.; Raucy, J.L. Advantages of cell-based high-volume screening assays to assess nuclear receptor activation during drug discovery. Expert Opin. Drug Discov. 2014, 9, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Guin, D.; Gruebele, M. Chaperones Hsc70 and Hsp70 Bind to the Protein PGK Differently inside Living Cells. J. Phys. Chem. B 2020, 124, 3629–3635. [Google Scholar] [CrossRef]
- Wang, S.; Midgley, C.A.; Scaërou, F.; Grabarek, J.B.; Griffiths, G.; Jackson, W.; Kontopidis, G.; McClue, S.J.; McInnes, C.; Meades, C.; et al. Discovery of N-phenyl-4-(thiazol-5-yl)pyrimidin-2-amine aurora kinase inhibitors. J. Med. Chem. 2010, 53, 4367–4378. [Google Scholar] [CrossRef]
- Alexiou, P.; Papakyriakou, A.; Ntougkos, E.; Papaneophytou, C.P.; Liepouri, F.; Mettou, A.; Katsoulis, I.; Maranti, A.; Tsiliouka, K.; Strongilos, A.; et al. Rationally designed less toxic SPD-304 analogs and preliminary evaluation of their TNF inhibitory effects. Arch. Pharm. 2014, 347, 798–805. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Chiosis, G.; Huezo, H.; Rosen, N.; Mimnaugh, E.; Whitesell, L.; Neckers, L. 17AAG: Low Target Binding Affinity and Potent Cell Activity—Finding an Explanation1. Mol. Cancer Ther. 2003, 2, 123–129. [Google Scholar] [PubMed]
- Knapinska, A.M.; Singh, C.; Drotleff, G.; Blanco, D.; Chai, C.; Schwab, J.; Herd, A.; Fields, G.B. Matrix Metalloproteinase 13 Inhibitors for Modulation of Osteoclastogenesis: Enhancement of Solubility and Stability. ChemMedChem 2021, 16, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Price, I.R.; Bai, J.J.; Lin, H. A Glycoconjugated SIRT2 Inhibitor with Aqueous Solubility Allows Structure-Based Design of SIRT2 Inhibitors. ACS Chem. Biol. 2019, 14, 1802–1810. [Google Scholar] [CrossRef]
- Mateus, A.; Gordon, L.J.; Wayne, G.J.; Almqvist, H.; Axelsson, H.; Seashore-Ludlow, B.; Treyer, A.; Matsson, P.; Lundbäck, T.; West, A. Prediction of intracellular exposure bridges the gap between target-and cell-based drug discovery. Proc. Natl. Acad. Sci. USA 2017, 114, E6231–E6239. [Google Scholar] [CrossRef]
- Lee, E.J.; Duggirala, K.B.; Lee, Y.; Yun, M.R.; Jang, J.; Cyriac, R.; Jung, M.E.; Choi, G.; Chae, C.H.; Cho, B.C. Novel allosteric glutaminase 1 inhibitors with macrocyclic structure activity relationship analysis. Bioorg. Med. Chem. Lett. 2022, 75, 128956. [Google Scholar] [CrossRef]
- Luchinat, E.; Barbieri, L.; Cremonini, M.; Nocentini, A.; Supuran, C.T.; Banci, L. Drug Screening in Human Cells by NMR Spectroscopy Allows the Early Assessment of Drug Potency. Angew. Chem. Int. Ed. 2020, 59, 6535–6539. [Google Scholar] [CrossRef]
- Luzzio, M.J.; Besterman, J.M.; Emerson, D.L.; Evans, M.G.; Lackey, K.; Leitner, P.L.; McIntyre, G.; Morton, B.; Myers, P.L.; Peel, M. Synthesis and antitumor activity of novel water soluble derivatives of camptothecin as specific inhibitors of topoisomerase I. J. Med. Chem. 1995, 38, 395–401. [Google Scholar] [CrossRef]
- Kontopidis, G.; Andrews, M.J.I.; McInnes, C.; Cowan, A.; Powers, H.; Innes, L.; Plater, A.; Griffiths, G.; Paterson, D.; Zheleva, D.I.; et al. Insights into Cyclin Groove Recognition: Complex Crystal Structures and Inhibitor Design through Ligand Exchange. Structure 2003, 11, 1537–1546. [Google Scholar] [CrossRef]
- Wu, S.Y.; McNae, I.; Kontopidis, G.; McClue, S.J.; McInnes, C.; Stewart, K.J.; Wang, S.; Zheleva, D.I.; Marriage, H.; Lane, D.P.; et al. Discovery of a Novel Family of CDK Inhibitors with the Program LIDAEUS: Structural Basis for Ligand-Induced Disordering of the Activation Loop. Structure 2003, 11, 399–410. [Google Scholar] [CrossRef]
- Rinotas, V.; Liepouri, F.; Ouzouni, M.-D.; Chalkidi, N.; Papaneophytou, C.; Lampropoulou, M.; Vidali, V.P.; Kontopidis, G.; Couladouros, E.; Eliopoulos, E. Structure-Based Discovery of Receptor Activator of Nuclear Factor-κB Ligand (RANKL)-Induced Osteoclastogenesis Inhibitors. Int. J. Mol. Sci. 2023, 24, 11290. [Google Scholar] [CrossRef]
- Camara, R.; Ogbeni, D.; Gerstmann, L.; Ostovar, M.; Hurer, E.; Scott, M.; Mahmoud, N.G.; Radon, T.; Crnogorac-Jurcevic, T.; Patel, P.; et al. Discovery of novel small molecule inhibitors of S100P with in vitro anti-metastatic effects on pancreatic cancer cells. Eur. J. Med. Chem. 2020, 203, 112621. [Google Scholar] [CrossRef]
- Jung, F.H.; Pasquet, G.; Lambert-van der Brempt, C.; Lohmann, J.-J.M.; Warin, N.; Renaud, F.; Germain, H.; De Savi, C.; Roberts, N.; Johnson, T.; et al. Discovery of Novel and Potent Thiazoloquinazolines as Selective Aurora A and B Kinase Inhibitors. J. Med. Chem. 2006, 49, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Suzuki, T.; Mino, K.; Koseki, K.; Oehme, F.; Flamme, I.; Ozasa, H.; Itoh, Y.; Ogasawara, D.; Komaarashi, H.; et al. Design, Synthesis, Enzyme-Inhibitory Activity, and Effect on Human Cancer Cells of a Novel Series of Jumonji Domain-Containing Protein 2 Histone Demethylase Inhibitors. J. Med. Chem. 2010, 53, 5629–5638. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nagano, Y.; Kouketsu, A.; Matsuura, A.; Maruyama, S.; Kurotaki, M.; Nakagawa, H.; Miyata, N. Novel inhibitors of human histone deacetylases: Design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxamates. J. Med. Chem. 2005, 48, 1019–1032. [Google Scholar] [CrossRef]
- Luby-Phelps, K. Cytoarchitecture and physical properties of cytoplasm: Volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 1999, 192, 189–221. [Google Scholar]
- Luby-Phelps, K. Physical properties of cytoplasm. Curr. Opin. Cell Biol. 1994, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, O.S.E.; Tsika, A.C.; Rovoli, M.; Papadopoulos, G.E.; Kontopidis, G.; Spyroulias, G.A.; Leonidas, D.D. Structural and Biochemical Characterization of the Human Angiogenin–Proliferating Cell Nuclear Antigen Interaction. Biochemistry 2023, 62, 1706–1715. [Google Scholar] [CrossRef]
- Lázár, L.; Tsagkarakou, A.S.; Stravodimos, G.; Kontopidis, G.; Leffler, H.; Nilsson, U.J.; Somsák, L.; Leonidas, D.D. Strong Binding of C-Glycosylic1, 2-Thiodisaccharides to Galectin-3—Enthalpy-Driven Affinity Enhancement by Water-Mediated Hydrogen Bonds. J. Med. Chem. 2023, 66, 12420–12431. [Google Scholar] [CrossRef]
- Zhang, A.; Guo, Z.; Ge, G.; Liu, Z. Insights into In Vivo Environmental Effects on Quantitative Biochemistry in Single Cells. Anal. Chem. 2023, 95, 17246–17255. [Google Scholar] [CrossRef]
- Clegg, J.S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am. J. Physiol. Integr. Comp. Physiol. 1984, 246, R133–R151. [Google Scholar] [CrossRef] [PubMed]
- Schnell, S.; Turner, T.E. Reaction kinetics in intracellular environments with macromolecular crowding: Simulations and rate laws. Prog. Biophys. Mol. Biol. 2004, 85, 235–260. [Google Scholar] [CrossRef]
- Stadmiller, S.S.; Aguilar, J.S.; Parnham, S.; Pielak, G.J. Protein–Peptide Binding Energetics under Crowded Conditions. J. Phys. Chem. B 2020, 124, 9297–9309. [Google Scholar] [CrossRef]
- Papaneophytou, C.P.; Grigoroudis, A.I.; McInnes, C.; Kontopidis, G. Quantification of the effects of ionic strength, viscosity, and hydrophobicity on protein-ligand binding affinity. ACS Med. Chem. Lett. 2014, 5, 931–936. [Google Scholar] [CrossRef]
- Luh, L.M.; Hänsel, R.; Löhr, F.; Kirchner, D.K.; Krauskopf, K.; Pitzius, S.; Schäfer, B.; Tufar, P.; Corbeski, I.; Güntert, P.; et al. Molecular crowding drives active Pin1 into nonspecific complexes with endogenous proteins prior to substrate recognition. J. Am. Chem. Soc. 2013, 135, 13796–13803. [Google Scholar] [CrossRef] [PubMed]
- Seashore-Ludlow, B.; Axelsson, H.; Almqvist, H.; Dahlgren, B.; Jonsson, M.; Lundback, T. Quantitative interpretation of intracellular drug binding and kinetics using the cellular thermal shift assay. Biochemistry 2018, 57, 6715–6725. [Google Scholar] [CrossRef]
- Chen, Q.; Schönherr, H.; Vancso, G.J. Block-Copolymer Vesicles as Nanoreactors for Enzymatic Reactions. Small 2009, 5, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.G.S.; Malys, N. What is the true enzyme kinetics in the biological system? An investigation of macromolecular crowding effect upon enzyme kinetics of glucose-6-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 2011, 405, 388–392. [Google Scholar] [CrossRef]
- Fulton, A.B. How crowded is the cytoplasm? Cell 1982, 30, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Golding, I.; Cox, E.C. Physical Nature of Bacterial Cytoplasm. Phys. Rev. Lett. 2006, 96, 98102. [Google Scholar] [CrossRef]
- McGuffee, S.R.; Elcock, A.H. Diffusion, crowding & protein stability in a dynamic molecular model of the bacterial cytoplasm. PLoS Comput. Biol. 2010, 6, e1000694. [Google Scholar] [CrossRef]
- Rivas, G.; Minton, A.P. Toward an understanding of biochemical equilibria within living cells. Biophys. Rev. 2018, 10, 241–253. [Google Scholar] [CrossRef]
- Rivas, G.; Minton, A.P. Editorial: Biochemical Reactions in Cytomimetic Media. Front. Mol. Biosci. 2020, 6, 145. [Google Scholar] [CrossRef]
- Hann, M.M.; Simpson, G.L. Intracellular drug concentration and disposition—The missing link? Methods 2014, 68, 283–285. [Google Scholar] [CrossRef]
- Wu, S.Y.; Dornan, J.; Kontopidis, G.; Taylor, P.; Walkinshaw, M.D. The First Direct Determination of a Ligand Binding Constant in Protein Crystals. Angew. Chem. Int. Ed. Engl. 2001, 40, 582–586. [Google Scholar] [CrossRef]
- Mettou, A.; Papaneophytou, C.; Melagraki, G.; Maranti, A.; Liepouri, F.; Alexiou, P.; Papakyriakou, A.; Couladouros, E.; Eliopoulos, E.; Afantitis, A.; et al. Aqueous Solubility Enhancement for Bioassays of Insoluble Inhibitors and QSPR Analysis: A TNF-α Study. SLAS Discov. Adv. Life Sci. RD 2018, 23, 84–93. [Google Scholar] [CrossRef]
- Papaneophytou, C.P.; Mettou, A.K.; Rinotas, V.; Douni, E.; Kontopidis, G.A. Solvent Selection for Insoluble Ligands, a Challenge for Biological Assay Development: A TNF-α/SPD304 Study. ACS Med. Chem. Lett. 2013, 4, 137–141. [Google Scholar] [CrossRef]
- Busa, W.B.; Nuccitelli, R. Metabolic regulation via intracellular pH. Am. J. Physiol. Integr. Comp. Physiol. 1984, 246, R409–R438. [Google Scholar] [CrossRef]
- Marakhova, I.; Yurinskaya, V.; Aksenov, N.; Zenin, V.; Shatrova, A.; Vereninov, A. Intracellular K+ and water content in human blood lymphocytes during transition from quiescence to proliferation. Sci. Rep. 2019, 9, 16253. [Google Scholar] [CrossRef]
- Morse, P.D., 2nd. Determining Intracellular Viscosity from the Rotational Motion of Spin Labels. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1986; Volume 127, pp. 239–249. [Google Scholar] [CrossRef]
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434. [Google Scholar] [CrossRef]
- Tverskoi, A.M.; Sidorenko, S.V.; Klimanova, E.A.; Akimova, O.A.; Smolyaninova, L.V.; Lopina, O.D.; Orlov, S.N. Effects of ouabain on proliferation of human endothelial cells correlate with Na+,K+-ATPase activity and intracellular ratio of Na+ and K+. Biochemistry 2016, 81, 876–883. [Google Scholar] [CrossRef]
- Banerjee, R. Redox outside the box: Linking extracellular redox remodeling with intracellular redox metabolism. J. Biol. Chem. 2012, 287, 4397–4402. [Google Scholar] [CrossRef]
- Kaludercic, N.; Deshwal, S.; Di Lisa, F. Reactive oxygen species and redox compartmentalization. Front. Physiol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta-Gen. Subj. 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Jiménez-Moreno, N.; Dias, I.H.K.; Debelec, B.; Vucetic, M.; Fladmark, K.E.; Basaga, H.; Ribaric, S.; Milisav, I.; Cuadrado, A. Redox control of protein degradation. Redox Biol. 2015, 6, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Torres, J.; Truong, L.; Chaudhuri, R.; Mittal, A.; Johnson, M.E. Reducing agents affect inhibitory activities of compounds: Results from multiple drug targets. Anal. Biochem. 2012, 423, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Gusenbauer, M. Google Scholar to overshadow them all? Comparing the sizes of 12 academic search engines and bibliographic databases. Scientometrics 2019, 118, 177–214. [Google Scholar] [CrossRef]
- Luby-Phelps, K. The physical chemistry of cytoplasm and its influence on cell function: An update. Mol. Biol. Cell 2013, 24, 2593–2596. [Google Scholar] [CrossRef] [PubMed]
- van der Vegt, N.F.A.; Nayar, D. The Hydrophobic Effect and the Role of Cosolvents. J. Phys. Chem. B 2017, 121, 9986–9998. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Kneen, M.; Farinas, J.; Li, Y.; Verkman, A.S. Green Fluorescent Protein as a Noninvasive Intracellular pH Indicator. Biophys. J. 1998, 74, 1591–1599. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef]
- Onufriev, A.V.; Alexov, E. Protonation and pK changes in protein-ligand binding. Q. Rev. Biophys. 2013, 46, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Raghunand, N.; Garcia-Martin, M.L.; Gatenby, R.A. pH imaging. IEEE Eng. Med. Biol. Mag. 2004, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Rolver, M.G.; Pedersen, S.F. Putting Warburg to work: How imaging of tumour acidosis could help predict metastatic potential in breast cancer. Br. J. Cancer 2021, 124, 1–2. [Google Scholar] [CrossRef]
- Doyen, D.; Poet, M.; Jarretou, G.; Pisani, D.F.; Tauc, M.; Cougnon, M.; Argentina, M.; Bouret, Y.; Counillon, L. Intracellular pH Control by Membrane Transport in Mammalian Cells. Insights Into the Selective Advantages of Functional Redundancy. Front. Mol. Biosci. 2022, 9, 825028. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Reshkin, S.J.; Greco, M.R.; Cardone, R.A. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos. Trans. R. Soc. Biol. Sci. 2014, 369, 20130100. [Google Scholar] [CrossRef]
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell Sci. 2017, 130, 663–669. [Google Scholar] [CrossRef]
- Segeritz, C.-P.; Vallier, L. Cell Culture. In Basic Science Methods for Clinical Researchers; Academic Press: Cambridge, MA, USA, 2017; pp. 151–172. ISBN 9780128030776. [Google Scholar]
- Sherman, T.A.; Rongali, S.C.; Matthews, T.A.; Pfeiffer, J.; Nehrke, K. Identification of a nuclear carbonic anhydrase in Caenorhabditis elegans. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2012, 1823, 808–817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yong, M.J.; Kang, B.; Yang, U.; Oh, S.S.; Je, J.H. Live Streaming of a Single Cell’s Life over a Local pH-Monitoring Nanowire Waveguide. Nano Lett. 2022, 22, 6375–6382. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, Y.; Li, C.; Wang, M.; Xu, X.; Jin, Y. Single-cell pH imaging and detection for pH profiling and label-free rapid identification of cancer-cells. Sci. Rep. 2017, 7, 1759. [Google Scholar] [CrossRef]
- Ganji, M.; Docter, M.; Le Grice, S.F.J.; Abbondanzieri, E.A. DNA binding proteins explore multiple local configurations during docking via rapid rebinding. Nucleic Acids Res. 2016, 44, 8376–8384. [Google Scholar] [CrossRef]
- Feig, M. Virtual Issue on Protein Crowding and Stability. J. Phys. Chem. B 2021, 125, 10649–10651. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Amon, A. Relevance and Regulation of Cell Density. Trends Cell Biol. 2020, 30, 213–225. [Google Scholar] [CrossRef]
- Molines, A.T.; Lemiere, J.; Gazzola, M.; Steinmark, I.E.; Edrington, C.H.; Hsu, C.T.; Real-Calderon, P.; Suhling, K.; Goshima, G.; Holt, L.J.; et al. Physical properties of the cytoplasm modulate the rates of microtubule polymerization and depolymerization. Dev. Cell 2022, 57, 466–479 e6. [Google Scholar] [CrossRef] [PubMed]
- Nenninger, A.; Mastroianni, G.; Mullineaux, C.W. Size dependence of protein diffusion in the cytoplasm of Escherichia coli. J. Bacteriol. 2010, 192, 4535–4540. [Google Scholar] [CrossRef] [PubMed]
- Haspinger, D.C.; Klinge, S.; Holzapfel, G.A. Numerical analysis of the impact of cytoskeletal actin filament density alterations onto the diffusive vesicle-mediated cell transport. PLoS Comput. Biol. 2021, 17, e1008784. [Google Scholar] [CrossRef] [PubMed]
- Potma, E.O.; De Boeij, W.P.; Bosgraaf, L.; Roelofs, J.; Van Haastert, P.J.M.; Wiersma, D.A. Reduced protein diffusion rate by cytoskeleton in vegetative and polarized Dictyostelium cells. Biophys. J. 2001, 81, 2010–2019. [Google Scholar] [CrossRef]
- Pei, D.; Buyanova, M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjugate Chem. 2017, 30, 273–283. [Google Scholar] [CrossRef]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomal accumulation of anticancer drugs triggers lysosomal exocytosis. Oncotarget 2017, 8, 45117–45132. [Google Scholar] [CrossRef]
- Lismont, C.; Nordgren, M.; Van Veldhoven, P.P.; Fransen, M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015, 3, 35. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Nowotny-Różańska, M. Viscosity of aqueous solutions of saccharides. Polish J. Food Nutr. Sci. 1998, 48, 171–180. [Google Scholar]
- Lavrenko, P.N.; Mikryukova, O.I.; Didenko, S.A. Hydrodynamic properties and the shape of the molecules of the polysaccharide ficoll-400 in solution. Polym. Sci. USSR 1986, 28, 576–584. [Google Scholar] [CrossRef]
- Ernst, D.; Hellmann, M.; Köhler, J.; Weiss, M. Fractional Brownian motion in crowded fluids. Soft Matter 2012, 8, 4886–4889. [Google Scholar] [CrossRef]
- El-Morched, M.; Barraco, C.; Simionescu, R.; Harroun, T.; Yan, H. Characterization of Simulated Cytoplasmic Fluids. ChemistrySelect 2024, 9, e202402861. [Google Scholar] [CrossRef]
- Chalikian, T.V. Excluded volume contribution to cosolvent-mediated modulation of macromolecular folding and binding reactions. Biophys. Chem. 2016, 209, 1–8. [Google Scholar] [CrossRef]
- Dupuis, N.F.; Holmstrom, E.D.; Nesbitt, D.J. Molecular-crowding effects on single-molecule RNA folding/unfolding thermodynamics and kinetics. Proc. Natl. Acad. Sci. USA 2014, 111, 8464–8469. [Google Scholar] [CrossRef]
- Rajendran, D.; Mitra, S.; Oikawa, H.; Madhurima, K.; Sekhar, A.; Takahashi, S.; Naganathan, A.N. Quantification of Entropic Excluded Volume Effects Driving Crowding-Induced Collapse and Folding of a Disordered Protein. J. Phys. Chem. Lett. 2022, 13, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Ponte, I.; Suau, P. Macromolecular crowding induces a molten globule state in the C-terminal domain of histone H1. Biophys. J. 2007, 93, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Altamash, T.; Ahmed, W.; Rasool, S.; Biswas, K.H. Intracellular Ionic Strength Sensing Using NanoLuc. Int. J. Mol. Sci. 2021, 22, 677. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Peters, K.; Staehlke, S.; Rebl, H.; Jonitz-Heincke, A.; Hahn, O. Impact of Metal Ions on Cellular Functions: A Focus on Mesenchymal Stem/Stromal Cell Differentiation. Int. J. Mol. Sci. 2024, 25, 10127. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F. Nickel. Adv. Nutr. 2021, 12, 281–282. [Google Scholar] [CrossRef]
- Brooke, D.; Movahed, N.; Bothner, B. Universal buffers for use in biochemistry and biophysical experiments. AIMS Biophys. 2015, 2, 336–342. [Google Scholar] [CrossRef]
- Patterson, C.J.; Pernil, R.; Foster, A.W.; Robinson, N.J. Cyanobacterial Models that Address Cross-Talk in Metal Homeostasis. In Metals in Cells; Culotta, V., Scott, R.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Permyakov, E.A. Metal Binding Proteins. Encyclopedia 2021, 1, 261–292. [Google Scholar] [CrossRef]
- Lodish, H.; Kaiser, C.A.; Krieger, M.; Bretscher, A.; Ploegh, H.; Martin, K.C.; Yaffe, M.; Amon, A. Transmembrane Transport of Ions and Small Molecules. In Molecular Cell Biology, 9th ed.; Macmillan International Higher Education: New York, NY, USA, 2021; pp. 1422–1443. [Google Scholar]
- Strazzullo, P.; Leclercq, C. Sodium. Adv. Nutr. 2014, 5, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Burkitt Creedon, J.M. Sodium Disorders. In Small Animal Critical Care Medicine, 2nd ed.; Elsevier Saunders: St. Louis, MO, USA, 2015; pp. 263–268. ISBN 9781455703067. [Google Scholar]
- Zacchia, M.; Abategiovanni, M.L.; Stratigis, S.; Capasso, G. Potassium: From Physiology to Clinical Implications. Kidney Dis. 2016, 2, 72–79. [Google Scholar] [CrossRef]
- Romani, A.M.P. Intracellular*magnesium*homeostasis. In Magnesium in the Central Nervous System; Vink R, N.M., Ed.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Ramappa, V.K.; Srivastava, D.; Singh, P.; Kumar, U.; Kumar, D.; Gosipatala, S.B.; Saha, S.; Kumar, D.; Raj, R. Mulberries: A Promising Fruit for Phytochemicals, Nutraceuticals, and Biological Activities. Int. J. Fruit Sci. 2020, 20, S1254–S1279. [Google Scholar] [CrossRef]
- Das, S.; Khatua, K.; Rakshit, A.; Carmona, A.; Sarkar, A.; Bakthavatsalam, S.; Ortega, R.; Datta, A. Emerging chemical tools and techniques for tracking biological manganese. Dalt. Trans. 2019, 48, 7047–7061. [Google Scholar] [CrossRef]
- Philpott, C.C.; Jadhav, S. The ins and outs of iron: Escorting iron through the mammalian cytosol. Free. Radic. Biol. Med. 2019, 133, 112–117. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Molybdenum. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; p. 421. [Google Scholar]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Bretscher, A.; Ploegh, H.; Martin, K.C.; Yaffe, M.; Amon, A. Culturing and Visualizing Cells. In Molecular Cell Biology, 9th ed.; Macmillan International Higher Education: New York, NY, USA, 2021; pp. 586–597. [Google Scholar]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Gamaj, M.I. Estimation of the Diffusion Coefficient and Hydrodynamic Radius (stokes Radius) for Inorganic Ions in Solution Depending on Molar Conductivity as Electro-Analytical Technique—A Review. J. Chem. Rev. 2020, 2, 182–188. [Google Scholar] [CrossRef]
- Kritmetapak, K.; Kumar, R. Phosphate as a Signaling Molecule. Calcif. Tissue Int. 2021, 108, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Shcheynikov, N.; Son, A.; Hong, J.H.; Yamazaki, O.; Ohana, E.; Kurtz, I.; Shin, D.M.; Muallem, S. Intracellular Cl- as a signaling ion that potently regulates Na+/HCO3− transporters. Proc. Natl. Acad. Sci. USA 2015, 112, E329–E337. [Google Scholar] [CrossRef]
- Koulouridis, I.; Koulouridis, E. The Integral Role of Chloride & With-No-Lysine Kinases in Cell Volume Regulation & Hypertension. Int. J. Nephrol. Renov. Dis. 2023, 16, 183–196. [Google Scholar] [CrossRef]
- Markovich, D. Physiological Roles and Regulation of Mammalian Sulfate Transporters. Physiol. Rev. 2001, 81, 1499–1533. [Google Scholar] [CrossRef] [PubMed]
- Kucernak, A.R.; Wang, H.; Lin, X. Avoid Using Phosphate Buffered Saline (PBS) as an Electrolyte for Accurate OER Studies. ACS Energy Lett. 2024, 9, 3939–3946. [Google Scholar] [CrossRef]
- AAT Bioquest Inc. Phosphate Buffer (pH 5.8 to 7.4) Preparation and Recipe. 2025. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/phosphate-buffer-ph-5-8-to-7-4 (accessed on 24 March 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 23831, Hepes. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hepes (accessed on 29 March 2025).
- AAT Bioquest Inc. HHBS (Hank’s Buffer with Hepes) Preparation and Recipe. 2025. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/hhbs-hanks-buffer-with-hepes (accessed on 29 March 2025).
- AAT Bioquest Inc. HEPES Buffer (1 M, 7.5 pH) Preparation and Recipe. 2025. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/hepes-buffer-ph-7-5 (accessed on 29 March 2025).
- Green, M.R.; Sambrook, J. Buffers. Cold Spring Harb Protoc. 2018, 2018, pdb-top098210. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6503, Tris(hydroxymethyl)aminomethane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tris_hydroxymethyl_aminomethane (accessed on 22 August 2025).
- AAT Bioquest Inc. Tris Buffer (1 M, pH 7.2) Preparation and Recipe. 2025. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/tris-buffer (accessed on 29 March 2025).
- AAT Bioquest Inc. Tris-Buffered Saline (TBS, 0.1 M) Preparation and Recipe. 2025. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/tris-buffered-saline-tbs-0-1-m (accessed on 29 March 2025).
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Reckitt Benckiser. Partition and distribution coefficients. RSC Adv. Chem. Sci. 2012, 1–2. [Google Scholar]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Asokan, A.; Cho, M.J. Exploitation of Intracellular pH Gradients in the Cellular Delivery of Macromolecules. J. Pharm. Sci. 2002, 91, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; Gruebele, M. Cellular Sticking Can Strongly Reduce Complex Binding by Speeding Dissociation. J. Phys. Chem. B 2021, 125, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; Deutsch, J.; Gruebele, M. An in vitro mimic of in-cell solvation for protein folding studies. Protein Sci. 2020, 29, 1060–1068. [Google Scholar] [CrossRef]
- Knab, E.; Davis, C.M. Chemical interactions modulate λ6-85 stability in cells. Protein Sci. 2023, 32, e4698. [Google Scholar] [CrossRef]
- Yoo, H.; Davis, C.M. An in Vitro Cytomimetic of In-Cell RNA Folding. ChemBioChem 2022, 23, e202200406. [Google Scholar] [CrossRef]
- Luchinat, E.; Banci, L. In-cell NMR: From target structure and dynamics to drug screening. Curr. Opin. Struct. Biol. 2022, 74, 102374. [Google Scholar] [CrossRef]
- Molina, D.M.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.; Nordlund, P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Almqvist, H.; Axelsson, H.; Ignatushchenko, M.; Lundbäck, T.; Nordlund, P.; Molina, D.M. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 2014, 9, 2100–2122. [Google Scholar] [CrossRef] [PubMed]
- Mansouritorghabeh, H.; Jabbari-Azad, F.; Varasteh, A.; Sankian, M.; Farid-Hosseini, R. Common solvents for making extraction of allergenic proteins from plants’ pollens for prick tests and related factors: A technical review. Electron. Physician 2017, 9, 4440–4446. [Google Scholar] [CrossRef][Green Version]
- Yu, B.; Pettitt, B.M.; Iwahara, J. Dynamics of Ionic Interactions at Protein-Nucleic Acid Interfaces. Acc. Chem. Res. 2020, 53, 1802–1810. [Google Scholar] [CrossRef]
- Good, N.E.; Winget, G.D.; Winter, W.; Connolly, T.N.; Izawa, S.; Singh, R.M.M. Hydrogen Ion Buffers for Biological Research. Biochemistry 1966, 5, 467–477. [Google Scholar] [CrossRef]
- Michl, J.; Park, K.C.; Swietach, P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun. Biol. 2019, 2, 144. [Google Scholar] [CrossRef] [PubMed]
- Delmouly, K.; Belondrade, M.; Casanova, D.; Milhavet, O.; Lehmann, S. HEPES inhibits the conversion of prion protein in cell culture. J. Gen. Virol. 2011, 92, 1244–1250. [Google Scholar] [CrossRef]
- Padan, E.; Schuldiner, S. Intracellular pH regulation in bacterial cells. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1986; Volume 125, pp. 337–352. ISBN 0076-6879. [Google Scholar]
- Tritsch, G.L.; Niswander, P.R.; Rosenfeld, J.; Nechaev, A.; Mittelman, A. Cosolvent-buffer mixtures as models for the cytoplasmic milieu: The enzymology of adenosine aminohydrolase. Mol. Cell. Biochem. 1976, 12, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wallerstein, J.; Akke, M. Minute Additions of DMSO Affect Protein Dynamics Measurements by NMR Relaxation Experiments through Significant Changes in Solvent Viscosity. ChemPhysChem 2019, 20, 326–332. [Google Scholar] [CrossRef]
- Chan, D.S.H.; Kavanagh, M.E.; McLean, K.J.; Munro, A.W.; Matak-Vinković, D.; Coyne, A.G.; Abell, C. Effect of DMSO on Protein Structure and Interactions Assessed by Collision-Induced Dissociation and Unfolding. Anal. Chem. 2017, 89, 9976–9983. [Google Scholar] [CrossRef]
- McNae, I.W.; Kan, D.; Kontopidis, G.; Patterson, A.; Taylor, P.; Worrall, L.; Walkinshaw, M.D. Studying protein–ligand interactions using protein crystallography. Crystallogr. Rev. 2005, 11, 61–71. [Google Scholar] [CrossRef]
- Lowe, E.D.; Tews, I.; Cheng, K.Y.; Brown, N.R.; Gul, S.; Noble, M.E.M.; Gamblin, S.J.; Johnson, L.N. Specificity Determinants of Recruitment Peptides Bound to Phospho-CDK2/Cyclin A. Biochemistry 2002, 41, 15625–15634. [Google Scholar] [CrossRef] [PubMed]
- Keleti, T. Two rules of enzyme kinetics for reversible Michaelis-Menten mechanisms. FEBS Lett. 1986, 208, 109–112. [Google Scholar] [CrossRef]
- Ohno, A.; Inomata, K.; Tochio, H.; Shirakawa, M. In-Cell NMR Spectroscopy in Protein Chemistry and Drug Discovery. Curr. Top. Med. Chem. 2011, 11, 68–73. [Google Scholar] [CrossRef]
- Peters, J.; Oliva, R.; Caliò, A.; Oger, P.; Winter, R. Effects of Crowding and Cosolutes on Biomolecular Function at Extreme Environmental Conditions. Chem. Rev. 2023, 123, 13441–13488. [Google Scholar] [CrossRef]
- Bull, S.C.; Doig, A.J. Properties of protein drug target classes. PLoS ONE 2015, 10, e0117955. [Google Scholar] [CrossRef] [PubMed]
| Essential Cations | Approximate Intracellular Concentration | Source |
|---|---|---|
| Calcium (Ca) | 10−1–10−4 mM | [105] |
| Cobalt (Co) | Low Concentration, dependent on cell exposure | [103] |
| Copper (Cu) | Zeptomolar to femtomolar | [93] |
| Iron (Fe) | 1–7 μM | [102] |
| Magnesium (Mg) | 17–20 mM total, (0.5–1 mM free) | [99] |
| Manganese (Mn) | low μΜ to sub-mM | [101] |
| Molybdenum (Mo) | 5 nm, can vary | [104] |
| Nickel (Ni) | Very low: not defined concentration in mammals | [93] |
| Potassium (K) | 140–150 mM | [98] |
| Sodium (Na) | 10 mM | [95,96] |
| Zinc (Zn) | 200–300 μΜ | [106] |
| Ion | Concentration in solution with pH 7.4, without CaCl2·2H2O and MgCl2·6H2O |
|---|---|
| Na+ | 157 mM |
| K+ | 4.50 mM |
| Cl− | 139.70 mM |
| HPO42− | 7.23 mM |
| H2PO4− | 4.57 mM |
| Ion | Concentration in Extracellular Saline Buffer | Concentration in HHBS (pH 7.3, 25 °C) |
|---|---|---|
| Na+ | 140 mM | 142.85 mM |
| K+ | 5.40 mM | 5.77 mM |
| Cl− | 149.40 mM | 146.83 mM |
| Ca2+ | 2 mM | 1.26 mM |
| Mg2+ | 10 mM | 0.90 mM |
| SO42− | 10 mM | 0.41 mM |
| HEPES | 5 mM | 20 mM |
| H2PO4− | - | 0.44 mM |
| HPO42− | - | 0.34 mM |
| HCO3− | - | 4.17 mM |
| Parameter | Suggested Value/Strategy | Notes |
|---|---|---|
| Temperature | 37 °C | Must match physiological conditions for accurate thermodynamic constants |
| pH | ~7.2–7.4 typical cytoplasmic 7.2 (nuclear studies); 7.1–7.7 in tumor cells | Should be tailored to target cell type |
| Main Cations | 140–150 mM K+ 10 mM Na+ 1 mM Mg2+ | K+ predominant over Na+ and Mg2+ |
| Main Anions | 95–120 mM Cl− 12 mM HCO3− or | NaHCO3− may be used as a weak buffer |
| Cosolvent for lipophilicity | <3% | Although there is no literature to support certain values, some relevant studies include [28,41,140,141]. Certain proteins may exhibit sensitivity even at concentrations as low as 3% [141]. |
| Other Metal ions | Zn2+, Ca2+, etc. | May be used if enzyme cofactors or structural ions are required |
| Crowding Agents | Peg 8000/Ficoll 70/Dextran (10–35%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontopidis, G.; Patergiannakis, I.-S. Comparative Analysis of Biochemical and Cellular Assay Conditions and the Need for a Buffer That Mimics Cytoplasmic Environments. Molecules 2025, 30, 3630. https://doi.org/10.3390/molecules30173630
Kontopidis G, Patergiannakis I-S. Comparative Analysis of Biochemical and Cellular Assay Conditions and the Need for a Buffer That Mimics Cytoplasmic Environments. Molecules. 2025; 30(17):3630. https://doi.org/10.3390/molecules30173630
Chicago/Turabian StyleKontopidis, George, and Iason-Spyridon Patergiannakis. 2025. "Comparative Analysis of Biochemical and Cellular Assay Conditions and the Need for a Buffer That Mimics Cytoplasmic Environments" Molecules 30, no. 17: 3630. https://doi.org/10.3390/molecules30173630
APA StyleKontopidis, G., & Patergiannakis, I.-S. (2025). Comparative Analysis of Biochemical and Cellular Assay Conditions and the Need for a Buffer That Mimics Cytoplasmic Environments. Molecules, 30(17), 3630. https://doi.org/10.3390/molecules30173630







