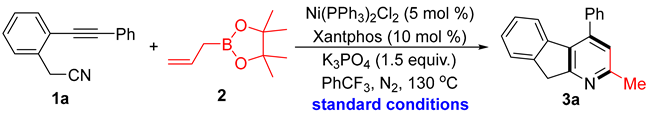
Abstract
An unprecedented nickel-catalyzed [2 + 2 + 2] cycloaddition that enables efficient construction of fused pyridine frameworks with allyl boronate was reported. This transformation is proposed to occur through a mechanism involving aza-nickelacyclopentadiene intermediates, wherein the boryl group of the allyl boronate plays a critical role in enabling the following cyclization via the control experiments. This work not only expands the structural diversity accessible via transition-metal-catalyzed [2 + 2 + 2] cycloadditions but also showcases the untapped potential of unsaturated substrates in cycloaddition reactions.
1. Introduction
Fused pyridine skeleton represents a privileged scaffold prevalent in numerous heterocyclic compounds and natural products (Figure 1), serving as fundamental structural units in pharmaceuticals, functional materials, and as versatile ligands or catalysts [1,2,3,4,5,6,7]. Although significant progress has been made in developing synthetic strategies for pyridine derivatives [8,9,10,11,12,13], the catalytic construction of densely substituted pyridines remains a formidable challenge in synthetic chemistry.
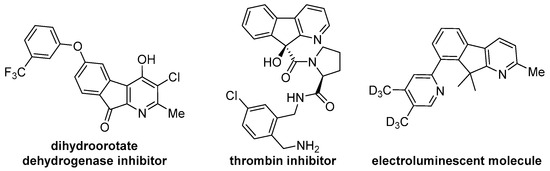
Figure 1.
Pyridine-based functional molecules.
De novo synthetic approaches offer distinct advantages for assembling pyridyl cores with diverse substitution patterns from readily accessible building blocks. In this context, transition-metal-catalyzed [2 + 2 + 2] cycloaddition of two alkyne units with a nitrile component has emerged as an atom-economical and versatile strategy for synthesizing six-membered cyclic and aromatic compounds [14,15,16].
A key intermediate in these transformations is the azametallacyclopentadiene, featuring one M-C(sp2) and one M-N(sp2) bond, which forms via metal-mediated oxidative cyclometalation of two triple-bond units [17,18,19]. Typically, azametallacyclopentadienes subsequently undergo alkyne insertion to afford the final cyclic/aromatic products (Scheme 1I) [20,21,22,23]. Considerable efforts have explored diverse catalytic systems based on (aza)metallacyclopentadienes, with successful implementations reported using ruthenium [24], rhodium [25], iridium [26], cobalt [27], iron [28], nickel [29,30], and niobium [31] catalysts [14,15]. For instance, the Liu group reported a Ni/BPh3 co-catalyzed [2 + 2 + 2] cycloaddition of alkyne-nitriles with internal alkynes, providing an efficient route to fused pyridines (Scheme 1(IIa)) [32]. Recently, Liu and coauthors developed a palladium/copper dual-catalyzed [2 + 2 + 2] cycloaddition of alkyne-tethered malononitriles and alkynes (Scheme 1(IIb)) [33].
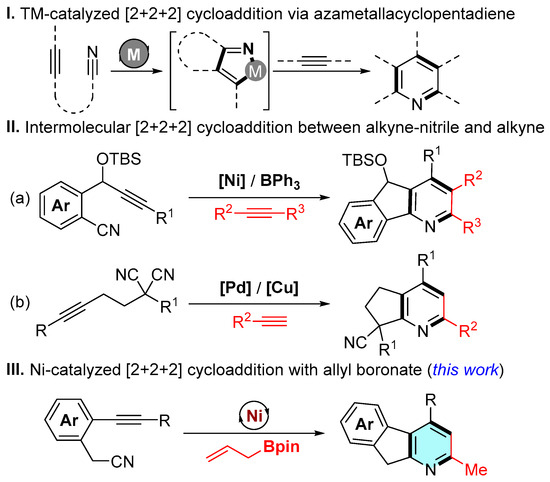
Scheme 1.
Background and project synopsis.
Notably, despite the diverse transformations of azametallacyclopentadienes in pyridine synthesis, alkene derivatives remained insurmountable substrates in this process due to subtle electrical property differences with the triple bond. Motivated by our interest in transition-metal-catalyzed transformations of unsaturated hydrocarbons for heterocycle assembly [34,35,36], we herein report a nickel-catalyzed [2 + 2 + 2] cycloaddition for constructing pyridine scaffolds using allyl boronate as cycloaddition partner (Scheme 1III).
2. Results and Discussion
The [2 + 2 + 2] cycloaddition of an alkyne-nitrile substrate with allyl boronate was investigated using a nickel catalytic system, affording 3a in 80% isolated yield under optimal conditions (Table 1, entry 1). Firstly, control experiments established that the nickel catalyst, diphosphine ligand, and base were all essential for the conversion (entries 2–4). Replacing Ni(Ph3P)2Cl2 with Ni(acac)2 as the catalyst significantly decreased reaction efficiency (entry 5), while other ligands, such as DPEphos, dppp, dppf, and Cy3P, failed to produce the desired cycloaddition product (entries 6–9). Additionally, performing the transformation with Na3PO4 as a base, a very low yield is obtained (entry 10). Reactions in other solvents, particularly polar solvents, resulted in a complex mixture (entries 11–12). On the other hand, performing the reaction under air did not improve the outcome (entry 13), and lower reaction temperatures were detrimental to the [2 + 2 + 2] cycloaddition (entry 14). Therefore, the optimal reaction conditions were identified employing Ni(PPh3)2Cl2 as the catalyst, XantPhos as the ligand, and K3PO4 as the base in trifluorotoluene.

Table 1.
Optimization of the reaction conditions.
After establishing optimal conditions, the scope of the nickel-catalyzed [2 + 2 + 2] cycloaddition was explored (Scheme 2). Generally, para-substituted internal alkynes bearing both electron-donating (Me, OMe, Ph, tBu) and electron-withdrawing groups (F, Cl, CF3, CO2Me) proved compatible, affording tricyclic derivatives in moderate yields (3b–3i). The constitution of product 3h was confirmed by X-ray crystallographic analysis (CCDC: 2474292). meta-Substituted substrates (tBu, OMe, F) also exhibited good reactivity under standard conditions (3j–3l). Notably, ortho-substituted substrates afforded the desired products in lower yields (3m–3o), indicating steric hindrance influenced reaction efficiency. Significantly, fused pyridine tricyclic derivatives were synthesized from biaryl alkynes bearing diverse substituents (silyl ether, 2-naphthyl, triphenylenyl, thienyl, benzothienyl, etc.), yielding products 3p–3v in moderate yields. Finally, variations in substituents on the acetonitrile-derived phenyl ring were well-tolerated with negligible impact on yields (3w–3y).
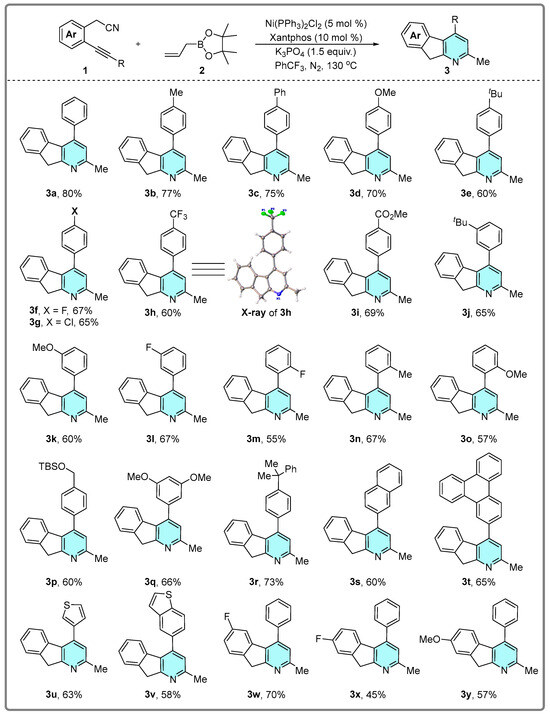
Scheme 2.
Substrate scope of the Ni-catalyzed [2 + 2 + 2] cycloaddition. The synthetic utility of this reaction was demonstrated through a gram-scale synthesis and product derivatizations. Compound 3a was successfully synthesized on a gram scale under the optimized conditions, affording the product in good yield (Scheme 3I). Subsequently, compounds 3a and 3i were subjected to a copper-mediated oxidation system, yielding the corresponding oxidative products 4a and 4i in moderate to good yields (Scheme 3II).
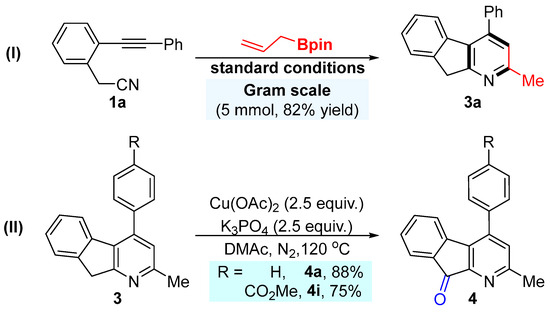
Scheme 3.
The gram-scale experiment and synthetic utility.
To gain insight into the reaction mechanism, several control experiments were conducted (Scheme 4). First, various allyl derivatives, such as trifluoroborate, trimethylsilyl, halide, and acetate groups, were used in place of allyl boronate under the standard conditions (Scheme 4I). However, these failed to afford the desired product due to the low conversion of 1a. Furthermore, crotyl boronate also failed to produce the fused pyridine products 3z or 3aa under the optimal conditions (Scheme 4II). These results indicate that both the boronate group and a terminal double bond are essential for the [2 + 2 + 2] cycloaddition.
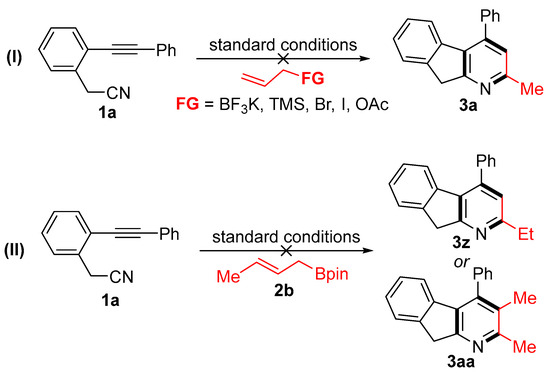
Scheme 4.
Control experiments.
Based on experimental results and the previous literature [32,33,37,38], a plausible mechanism is proposed in Scheme 5. Initially, coordination of the substrate’s alkyne group to the nickel(0) catalyst forms intermediate Int-1. Subsequent oxidative hetero-cyclometallation then generates azanickelcyclopentadiene intermediate Int-2. This species undergoes migration insertion of allyl boronate’s double bond, yielding seven-membered aza-nickelacycle Int-3. Reductive elimination of Int-3 releases both the active nickel(0) species and the six-membered framework complex Int-4. Finally, Int-4 undergoes base-facilitated olefin isomerization to afford the target product 3a.
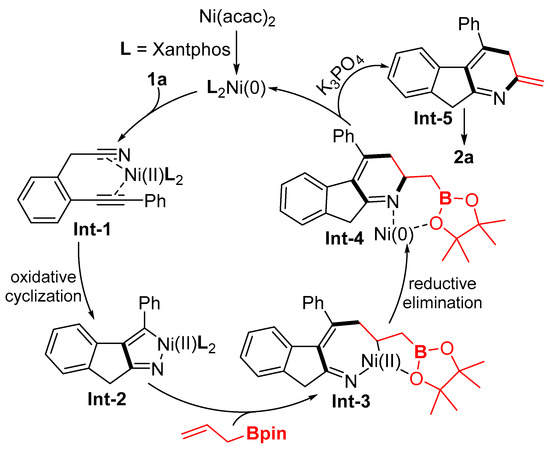
Scheme 5.
Plausible mechanistic pathways.
3. Materials and Methods
3.1. Materials
All the reagents were obtained from commercial sources and used directly without further purification, unless otherwise noted. Synthetic methods and spectral data for the start substrates were consistent with the methods and data reported in the studies. 1H NMR, 19F NMR, and 13C NMR spectra were recorded on a Bruker AVANCE III 400 MHz. 1H NMR, 19F NMR, and 13C NMR chemical shifts were determined relative to the internal standard TMS at δ 0.0. Chemical shifts (δ) are reported in ppm, and coupling constants (J) are reported in Hertz (Hz).
3.2. General Methods for the Preparation of Fused Pyridine Derivatives
To a 25 mL oven-dried Schlenk-tube with magnetic stirrer bar, substrate (1, 0.2 mmol), Ni(PPh3)Cl2 (5 mol%), Xantphos (10 mol%), K3PO4 (1.5 equiv), allyl boronate (2, 1.5 equiv, 0.3 mmol), and PhCF3 (2.0 mL) were successively added and vigorously stirred together in 130 °C oil bath under N2 atmosphere for 12 h. After the reaction was finished, the mixture was cooled to room temperature. The reaction was quenched with saturated NH4Cl aq. and extracted with EtOAc (3 × 25 mL). The combined ethyl acetate layer was washed with brine (25 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuum. The crude product was purified by flash column chromatography (eluting with petroleum ether/ethyl acetate) on silica gel to afford the product 3.
2-methyl-4-phenyl-9H-indeno [2,1-b]pyridine (3a): Compound 3a was prepared according to the general procedure using 1a with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 8/1, v/v) afforded 3a as a brown solid, m.p. = 127.4—129.6 °C, 41.1 mg, 80% yield. 1H NMR (400 MHz, CDCl3) δ 7.56—7.46 (m, 6H), 7.26—7.22 (m, 1H), 7.12—7.07 (m, 2H), 7.01 (s, 1H), 4.02 (s, 2H), 2.65 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.77, 155.93, 144.92, 141.38, 139.44, 138.83, 129.56, 128.71, 128.43, 128.41, 126.92, 126.55, 125.04, 122.84, 122.69, 38.85, 24.24. HRMS-ESI (m/z): calcd for C19H15N, [M+H]+: 258.1283, found, 258.1302.
2-methyl-4-(p-tolyl)-9H-indeno [2,1-b]pyridine (3b): Compound 3b was prepared according to the general procedure using 1b with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 8/1, v/v) afforded 3b as a brown solid, m.p. = 99.2—103.3 °C, 41.7 mg, 77% yield. 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.5 Hz, 1H), 7.38 (d, J = 7.9 Hz, 2H), 7.32 (d, J = 7.8 Hz, 2H), 7.26—7.23 (m, 1H), 7.17 (d, J = 7.6 Hz, 1H), 7.11 (t, J = 7.5 Hz, 1H), 6.99 (s, 1H), 4.02 (s, 2H), 2.65 (s, 3H), 2.48 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.70, 155.83, 145.09, 141.35, 139.55, 138.27, 135.85, 129.64, 129.38, 128.32, 126.86, 126.51, 125.01, 122.90, 122.83, 38.82, 24.19, 21.40. HRMS-ESI (m/z): calcd for C20H17N, [M+H]+: 272.1439, found, 272.1432.
4-([1,1’-biphenyl]-4-yl)-2-methyl-9H-indeno [2,1-b]pyridine (3c): Compound 3c was prepared according to the general procedure using 1c with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 6/1, v/v) afforded 3c as a brown solid, m.p. = 180.1—185.4 °C, 50 mg, 75% yield. 1H NMR (400 MHz, CDCl3) δ 7.79—7.70 (m, 4H), 7.60—7.55 (m, 3H), 7.50 (dd, J = 8.4, 6.9 Hz, 2H), 7.43—7.38 (m, 1H), 7.28 (dd, J = 7.5, 1.2 Hz, 1H), 7.25—7.22 (m, 1H), 7.15—7.10 (m, 1H), 7.06 (s, 1H), 4.04 (s, 2H), 2.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.81, 155.92, 144.64, 141.40, 141.25, 140.44, 139.41, 137.70, 129.61, 128.97, 128.95, 127.70, 127.37, 127.16, 127.02, 126.61, 125.10, 122.93, 122.77, 38.84, 24.23. HRMS-ESI (m/z): calcd for C25H19N, [M+H]+: 334.1596, found, 334.1609.
4-(4-methoxyphenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3d): Compound 3d was prepared according to the general procedure using 1d with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 3/1, v/v) afforded 3d as brown solid, m.p. = 133.2—135.4 °C, 40.2 mg, 70% yield. 1H NMR (400 MHz, CDCl3) δ 7.57—7.52 (m, 1H), 7.45—7.39 (m, 2H), 7.27 (d, J = 1.2 Hz, 1H), 7.24—7.18 (m, 1H), 7.12 (t, J = 7.6 Hz, 1H), 7.07—7.03 (m, 2H), 6.99 (s, 1H), 4.02 (s, 2H), 3.92 (s, 3H), 2.65 (s, 3H). 3C NMR (101 MHz, CDCl3) δ 164.72, 159.81, 155.82, 144.81, 141.35, 139.57, 131.07, 129.73, 129.71, 126.86, 126.52, 125.03, 122.90, 122.84, 114.10, 55.40, 38.83, 24.17. HRMS-ESI (m/z): calcd for C25H19N, [M+H]+: 288.1388, found, 288.1388.
4-(4-(tert-butyl)phenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3e): Compound 3e was prepared according to the general procedure using 1e with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 9/1, v/v) afforded 3e as a brown solid, m.p. = 120.2-125.4 °C, 37.6 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ 7.57—7.50 (m, 3H), 7.46—7.40 (m, 2H), 7.26—7.23 (m, 1H), 7.20 (dt, J = 7.8, 1.0 Hz, 1H), 7.14—7.10 (m, 1H), 7.01 (s, 1H), 4.02 (s, 2H), 2.64 (s, 3H), 1.42 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 164.72, 155.81, 151.58, 145.05, 141.35, 139.57, 135.77, 129.61, 128.12, 126.86, 126.52, 125.57, 125.00, 122.93, 122.91, 38.84, 34.80, 31.43, 24.17. HRMS-ESI (m/z): calcd for C23H23N, [M+H]+: 314.1909, found, 314.1928.
4-(4-fluorophenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3f): Compound 3f was prepared according to the general procedure using 1f with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1, v/v) afforded 3f as a brown solid, m.p. = 159.4—162.2 °C, 36.8 mg, 67% yield. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 7.5 Hz, 1H), 7.49—7.44 (m, 2H), 7.29—7.19 (m, 4H), 7.15—7.06 (m, 2H), 6.98 (s, 1H), 4.02 (s, 2H), 2.66 (s, 3H).13C NMR (101 MHz, CDCl3) δ 164.85, 162.88 (d, J = 247.8 Hz), 156.01, 143.85, 141.41, 139.25, 134.78, 130.24 (d, J = 8.3 Hz), 129.64, 126.84 (d, J = 45.5 Hz), 125.16, 122.74, 122.67, 115.90, 115.69, 38.83, 24.22. 19F NMR (377 MHz, CDCl3) δ -113.29. HRMS-ESI (m/z): calcd for C19H14FN, [M+H]+: 276.1189, found, 276.1188.
4-(4-chlorophenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3g): Compound 3g was prepared according to the general procedure using 1g with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1, v/v) afforded 3g as a brown solid, m.p. = 133.2—135.4 °C, 37.8 mg, 65% yield. 1H NMR (400 MHz, CDCl3) δ 7.58—7.49 (m, 3H), 7.46—7.42 (m, 2H), 7.29 (dd, J = 7.3, 1.5 Hz, 1H), 7.16—7.09 (m, 2H), 6.98 (s, 1H), 4.03 (s, 2H), 2.66 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.87, 156.02, 141.41, 139.11, 137.21, 129.88, 129.52, 129.02, 127.16, 126.67, 125.19, 122.72, 122.57, 38.81, 24.21.HRMS-ESI (m/z): calcd for C19H14ClN, [M+H]+: 292.0893, found, 292.0893.
2-methyl-4-(4-(trifluoromethyl)phenyl)-9H-indeno [2,1-b]pyridine (3h): Compound 3h was prepared according to the general procedure using 1h with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1, v/v) afforded 3h as a brown solid, m.p. = 139—142 °C, 39 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.0 Hz, 2H), 7.60 (dd, J = 20.3, 7.8 Hz, 3H), 7.31—7.27 (m, 1H), 7.13 (t, J = 7.6 Hz, 1H), 7.05—6.97 (m, 2H), 4.05 (s, 2H), 2.68 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.99, 156.14, 143.24, 142.50, 141.46, 138.90, 130.81, 129.39, 128.95, 127.31, 126.74, 125.77 (q, J = 3.7 Hz), 125.26, 122.62, 122.41, 38.82, 24.22. 19F NMR (377 MHz, CDCl3) δ -62.45. HRMS-ESI (m/z): calcd for C20H14F3N, [M+H]+: 326.1157, found, 326.1154.
methyl 4-(2-methyl-9H-indeno [2,1-b]pyridin-4-yl)benzoate (3i): Compound 3i was prepared according to the general procedure using 1i with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 2/1, v/v) afforded 3i as a brown solid, m.p. = 164.8—168.8 °C, 43.5 mg, 69% yield. 1H NMR (400 MHz, CDCl3) δ 8.23—8.18 (m, 2H), 7.59—7.55 (m, 3H), 7.27 (td, J = 7.4, 1.2 Hz, 2H), 7.10 (td, J = 7.6, 1.1 Hz, 1H), 7.05—6.99 (m, 2H), 4.04 (s, 2H), 3.99 (s, 3H), 2.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 166.84, 164.89, 156.03, 143.76, 143.45, 141.43, 138.99, 130.17, 130.05, 129.39, 128.63, 127.22, 126.69, 125.19, 122.74, 122.30, 52.39, 38.81, 24.21. HRMS-ESI (m/z): calcd for C21H17NO2, [M+H]+: 316.1338, found, 316.1317.
4-(3-(tert-butyl)phenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3j): Compound 3i was prepared according to the general procedure using 1j with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 8/1, v/v) afforded 3j as a brown solid, m.p. = 115.3—118.4 °C, 40.7 mg, 65% yield. 1H NMR (400 MHz, CDCl3) δ 7.57—7.44 (m, 4H), 7.32—7.27 (m, 1H), 7.25 (dd, J = 7.3, 1.5 Hz, 1H), 7.15—7.07 (m, 2H), 7.04 (s, 1H), 4.03 (s, 2H), 2.67 (s, 3H), 1.37 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 164.71, 155.85, 151.46, 145.56, 141.37, 139.50, 138.31, 129.63, 128.56, 126.91, 126.45, 125.83, 125.44, 125.27, 125.03, 122.96, 122.78, 38.82, 34.92, 31.36, 24.21. HRMS-ESI (m/z): calcd for C23H23N, [M+H]+: 314.1909, found, 314.1891.
4-(3-methoxyphenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3k): Compound 3k was prepared according to the general procedure using 1k with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 3/1, v/v) afforded 3k as a brown solid, m.p. = 119.4—120.2 °C, 34.4 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 7.6 Hz, 1H), 7.44 (td, J = 7.9, 2.2 Hz, 1H), 7.31—7.24 (m, 2H), 7.17—7.11 (m, 2H), 7.09—7.00 (m, 4H), 4.04 (s, 2H), 3.85 (d, J = 2.2 Hz, 3H), 2.67 (d, J = 2.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 164.60, 159.76, 155.74, 144.93, 141.34, 140.06, 139.28, 129.86, 129.65, 127.02, 126.62, 125.05, 122.99, 122.65, 120.73, 114.28, 113.61, 55.40, 38.76, 24.12. HRMS-ESI (m/z): calcd for C20H17NO, [M+H]+: 288.1388, found, 288.1388.
4-(3-fluorophenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3l): Compound 3l was prepared according to the general procedure using 1l with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1, v/v) afforded 3l as a brown solid, m.p. = 130.2—135.2 °C, 36.9 mg, 67% yield. 1H NMR (400 MHz, CDCl3) δ 7.57 (dt, J = 7.5, 1.0 Hz, 1H), 7.53—7.47 (m, 1H), 7.31—7.27 (m, 2H), 7.24—7.18 (m, 2H), 7.16—7.07 (m, 2H), 7.00 (s, 1H), 4.04 (s, 2H), 2.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.80, 162.85 (d, J = 248.0 Hz), 155.94, 143.55, 141.40, 140.84, 138.99, 130.42 (d, J = 8.2 Hz), 129.53, 126.95 (d, J = 50.2 Hz), 125.17, 124.26 (d, J = 2.9 Hz), 122.75, 122.50, 115.68, 115.49 (d, J = 5.2 Hz), 115.31, 38.77, 24.14. 19F NMR (377 MHz, CDCl3) δ -112.17. HRMS-ESI (m/z): calcd for C19H14FN, [M+H]+: 276.1189, found, 276.1188.
4-(2-fluorophenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3m): Compound 3m was prepared according to the general procedure using 1m with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1, v/v) afforded 3m as a brown solid, m.p. = 158.9—162.4 °C, 30.2 mg, 55% yield. 1H NMR (400 MHz, CDCl3) δ 7.58—7.47 (m, 2H), 7.40 (td, J = 7.4, 1.9 Hz, 1H), 7.34—7.26 (m, 2H), 7.24 (dd, J = 6.0, 1.1 Hz, 1H), 7.13 (td, J = 7.6, 1.1 Hz, 1H), 7.04 (s, 1H), 6.95 (d, J = 7.8 Hz, 1H), 4.04 (d, J = 3.5 Hz, 2H), 2.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.62, 159.47 (d, J = 247.9 Hz), 155.89, 141.35, 139.27, 138.13, 130.82 (d, J = 3.3 Hz), 130.58, 130.50, 130.47, 126.99 (d, J = 33.3 Hz), 126.25 (d, J = 16.3 Hz), 125.06, 124.57 (d, J = 3.7 Hz), 123.01, 122.28, 116.10 (d, J = 21.5 Hz), 38.79, 24.23. 19F NMR (377 MHz, CDCl3) δ -114.66. HRMS-ESI (m/z): calcd for C19H14FN, [M+H]+: 276.1189, found, 276.1188.
2-methyl-4-(o-tolyl)-9H-indeno [2,1-b]pyridine (3n): Compound 3n was prepared according to the general procedure using 1n with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1, v/v) afforded 3n as a brown solid, m.p. = 138.9—142.4 °C, 36.3 mg, 67% yield. 1H NMR (400 MHz, CDCl3) δ 7.55 (dt, J = 7.5, 1.0 Hz, 1H), 7.44—7.31 (m, 3H), 7.22 (ddd, J = 10.7, 7.5, 1.3 Hz, 3H), 7.08 (td, J = 7.6, 1.1 Hz, 1H), 6.97 (s, 1H), 6.63 (dt, J = 7.8, 0.9 Hz, 1H), 4.04 (d, J = 3.3 Hz, 2H), 2.67 (s, 3H), 2.09 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.31, 155.98, 144.41, 141.20, 139.58, 138.23, 135.54, 130.28, 130.22, 128.45, 128.43, 126.94, 126.25, 124.94, 122.42, 122.23, 38.80, 24.27, 19.78. HRMS-ESI (m/z): calcd for C20H17N, [M+H]+: 272.1439, found, 272.1432.
4-(2-methoxyphenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3o): Compound 3o was prepared according to the general procedure using 1o with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 3/1, v/v) afforded 3o as a brown solid, m.p. = 152.1—153.3 °C, 32.8 mg, 57% yield. 1H NMR (400 MHz, CDCl3) δ 7.55—7.46 (m, 2H), 7.29—7.26 (m, 1H), 7.23 (dd, J = 7.5, 1.2 Hz, 1H), 7.13—7.05 (m, 3H), 7.02 (s, 1H), 6.86 (d, J = 7.8 Hz, 1H), 4.03 (d, J = 6.9 Hz, 2H), 3.69 (s, 3H), 2.66 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.02, 156.53, 141.20, 139.88, 130.26, 130.04, 127.49, 126.77, 126.62, 124.82, 123.23, 122.43, 120.97, 110.94, 55.56, 38.72, 24.17. HRMS-ESI (m/z): calcd for C20H17NO, [M+H]+: 288.1388, found, 288.1388.
4-(4-(((tert-butyldimethylsilyl)oxy)methyl)phenyl)-2-methyl-9H-indeno.[2,1-b]pyridine (3p): Compound 3p was prepared according to the general procedure using 1p with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1, v/v) afforded 3p as Brown oil, 48.1 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ 7.38 (dt, J = 7.5, 1.0 Hz, 1H), 7.33—7.27 (m, 4H), 7.11—7.06 (m, 2H), 6.99—6.89 (m, 2H), 6.84 (s, 1H), 4.71 (s, 2H), 3.86 (s, 2H), 2.49 (s, 3H), 0.82 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 164.69, 155.82, 144.99, 141.82, 141.35, 139.45, 137.36, 129.67, 128.32, 126.91, 126.52, 126.32, 125.01, 122.93, 122.79, 64.82, 38.80, 26.00, 24.17, 18.49, -5.16. HRMS-ESI (m/z): calcd for C26H31NOSi, [M+H]+: 402.2253, found, 402.2273.
4-(3,5-dimethoxyphenyl)-2-methyl-9H-indeno [2,1-b]pyridine (3q): Compound 3q was prepared according to the general procedure using 1q with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 2/1, v/v) afforded 3q as a brown solid, m.p. = 133.4—135.5 °C, 41.9 mg, 66% yield. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 7.5 Hz, 1H), 7.30—7.27 (m, 1H), 7.26—7.21 (m, 1H), 7.15 (t, J = 7.5 Hz, 1H), 7.03 (s, 1H), 6.63—6.58 (m, 3H), 4.03 (s, 2H), 3.83 (s, 6H), 2.66 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.68, 160.98, 155.84, 144.86, 141.34, 140.71, 139.28, 129.49, 126.98, 126.66, 125.03, 123.12, 122.40, 106.24, 100.62, 55.54, 38.80, 24.20. HRMS-ESI (m/z): calcd for C21H19NO2, [M+H]+: 318.1494, found, 318.1496.
2-methyl-4-(4-(2-phenylpropan-2-yl)phenyl)-9H-indeno [2,1-b]pyridine (3r): Compound 3r was prepared according to the general procedure using 1r with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 5/1, v/v) afforded 3r as a brown solid, m.p. = 130.8—131.8 °C, 54.7 mg, 73% yield. 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 7.5 Hz, 1H), 7.44—7.35 (m, 4H), 7.33 (d, J = 5.2 Hz, 4H), 7.24—7.20 (m, 1H), 7.19—7.09 (m, 2H), 7.02 (s, 1H), 4.02 (s, 2H), 2.65 (s, 3H), 1.78 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 164.68, 155.79, 151.16, 150.46, 144.98, 141.36, 139.50, 135.98, 129.65, 128.14, 128.11, 127.15, 126.91, 126.86, 126.52, 125.84, 125.04, 122.88, 43.06, 38.82, 30.81, 24.18. HRMS-ESI (m/z): calcd for C28H25N, [M+H]+: 376.2065, found, 376.2085.
2-methyl-4-(naphthalen-2-yl)-9H-indeno [2,1-b]pyridine (3s): Compound 3s was prepared according to the general procedure using 1s with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1, v/v) afforded 3s as a brown solid, m.p. = 138.0—142.8 °C, 36.8 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ 8.02—7.95 (m, 3H), 7.93—7.89 (m, 1H), 7.63—7.56 (m, 4H), 7.26 (dd, J = 14.7, 1.4 Hz, 2H), 7.14—7.02 (m, 3H), 4.09 (s, 2H), 2.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.74, 155.86, 141.39, 139.35, 136.24, 133.37, 133.10, 128.40, 128.28, 127.92, 127.43, 127.04, 126.66, 126.64, 126.51, 125.08, 123.02, 122.96, 38.84, 24.20. HRMS-ESI (m/z): calcd for C25H17N, [M+H]+: 308.1439, found, 308.1460.
2-methyl-4-(triphenylen-2-yl)-9H-indeno [2,1-b]pyridine (3t): Compound 3t was prepared according to the general procedure using 1t with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 5/1, v/v) afforded 3t as a brown solid, m.p. = 143—147 °C, 52.9 mg, 65% yield. 1H NMR (400 MHz, CDCl3) δ 8.90—8.53 (m, 7H), 7.83—7.55 (m, 7H), 7.27—7.15 (m, 4H), 7.02 (t, J = 7.7 Hz, 1H), 4.11 (s, 2H), 2.76—2.70 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 164.94, 156.06, 144.90, 141.45, 139.44, 137.48, 130.08, 130.05, 130.03, 129.81, 129.78, 129.55, 129.45, 127.68, 127.66, 127.50, 127.46, 127.43, 127.06, 126.74, 125.14, 123.88, 123.51, 123.48, 123.45, 123.36, 122.88, 122.85, 38.91, 24.30. HRMS-ESI (m/z): calcd for C31H21N, [M+H]+: 408.1751, found, 408.1765.
2-methyl-4-(thiophen-3-yl)-9H-indeno [2,1-b]pyridine (3u): Compound 3u was prepared according to the general procedure using 1u with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 4/1, v/v) afforded 3u as a brown solid, m.p. = 138.9—142.2 °C, 33.2 mg, 63% yield. 1H NMR (400 MHz, CDCl3) δ 7.58—7.54 (m, 1H), 7.51 (dd, J = 4.9, 3.0 Hz, 1H), 7.45 (dd, J = 3.0, 1.3 Hz, 1H), 7.30—7.27 (m, 2H), 7.20—7.15 (m, 1H), 7.05 (s, 1H), 4.02 (s, 2H), 2.65 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 141.29, 139.32, 139.10, 128.17, 127.11, 126.73, 126.38, 125.09, 123.81, 122.91, 122.76, 38.74, 24.01. HRMS-ESI (m/z): calcd for C17H13NS, [M+H]+: 264.0847, found, 264.0823.
4-(benzo[b]thiophen-5-yl)-2-methyl-9H-indeno [2,1-b]pyridine (3v): Compound 3v was prepared according to the general procedure using 1v with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 5/1, v/v) afforded 3v as a brown solid, m.p. = 147.9—150.2 °C, 36.3 mg, 58% yield. 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 8.1 Hz, 1H), 7.94 (d, J = 1.7 Hz, 1H), 7.58—7.54 (m, 2H), 7.47 (dt, J = 8.4, 1.6 Hz, 1H), 7.41 (d, J = 5.4 Hz, 1H), 7.25 (dd, J = 7.4, 1.8 Hz, 1H), 7.08 (dt, J = 7.9, 5.4 Hz, 3H), 4.05 (s, 2H), 2.68 (d, J = 1.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 164.77, 155.89, 145.02, 141.39, 139.91, 139.77, 139.43, 134.96, 129.80, 127.53, 126.99, 126.59, 125.08, 124.79, 124.09, 123.36, 123.11, 122.90, 122.78, 38.85, 24.24. HRMS-ESI (m/z): calcd for C21H15NS, [M+H]+: 314.1003, found, 314.1021.
6-fluoro-2-methyl-4-phenyl-9H-indeno [2,1-b]pyridine (3w): Compound 3w was prepared according to the general procedure using 1w with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1, v/v) afforded 3w as a brown solid, m.p. = 120.3—124.5 °C, 38.5 mg, 70% yield. 1H NMR (400 MHz, CDCl3) δ 7.53 (pd, J = 4.7, 1.7 Hz, 3H), 7.50—7.44 (m, 3H), 7.03 (s, 1H), 6.97—6.91 (m, 1H), 6.76 (dd, J = 9.9, 2.5 Hz, 1H), 3.98 (s, 2H), 2.66 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 165.45, 161.79 (d, J = 242.5 Hz), 156.58, 145.29, 141.09 (d, J = 9.4 Hz), 138.17, 136.65, 136.62, 129.03, 129.00, 128.88, 128.73, 128.29, 125.92 (d, J = 8.9 Hz), 122.80, 113.83 (d, J = 23.0 Hz), 109.90 (d, J = 24.3 Hz), 38.24, 24.28. 19F NMR (377 MHz, CDCl3) δ -115.19. HRMS-ESI (m/z): calcd for C19H14FN, [M+H]+: 276.1189, found, 276.1188.
7-fluoro-2-methyl-4-phenyl-9H-indeno [2,1-b]pyridine (3x): Compound 3x was prepared according to the general procedure using 1x with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 6/1, v/v) afforded 3x as a brown solid, m.p. = 123.4—125.5 °C, 24.7 mg, 45% yield. 1H NMR (400 MHz, CDCl3) δ 7.58—7.45 (m, 6H), 7.04 (s, 1H), 6.95 (td, J = 8.7, 2.5 Hz, 1H), 6.76 (dd, J = 9.9, 2.5 Hz, 1H), 3.99 (s, 2H), 2.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 165.39, 161.79 (d, J = 242.4 Hz), 156.52, 145.36, 141.06 (d, J = 9.2 Hz), 138.14, 136.61, 128.89, 128.75, 128.28, 125.93 (d, J = 9.1 Hz), 122.86, 113.86 (d, J = 22.9 Hz), 109.91 (d, J = 24.4 Hz), 38.20, 24.23.19F NMR (377 MHz, CDCl3) δ -115.19. HRMS-ESI (m/z): calcd for C19H14FN, [M+H]+: 276.1189, found, 276.1188.
7-methoxy-2-methyl-4-phenyl-9H-indeno [2,1-b]pyridine (3y): Compound 3y was prepared according to the general procedure using 1y with allyl borate 2. Purification by flash column chromatography (petroleum ether/ethyl acetate = 3/1, v/v) afforded 3y as a brown solid, m.p. = 117.3—120.5 °C, 32.7 mg, 57% yield. 1H NMR (400 MHz, CDCl3) δ 7.57—7.48 (m, 2H), 7.40 (td, J = 7.4, 1.9 Hz, 1H), 7.33—7.27 (m, 2H), 7.26—7.23 (m, 1H), 7.13 (td, J = 7.6, 1.1 Hz, 1H), 7.04 (s, 1H), 6.95 (d, J = 7.8 Hz, 1H), 4.04 (d, J = 3.5 Hz, 2H), 2.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 192.69, 164.06, 159.19, 143.31, 138.81, 132.15, 129.83, 128.73, 128.42, 128.40, 123.64, 122.68, 112.81, 110.44, 55.46, 38.88, 23.94. HRMS-ESI (m/z): calcd for C20H17NO, [M+H]+: 288.1388, found, 288.1388.
3.3. General Procedure for the Derivatization of Pyridine Products
To a 25 mL Schlenk tube with a magnetic stirrer bar, substrates (3, 0.2 mmol), Cu(OAc)2 (1.5 equiv, 0.3 mmol), K3PO4 (2.5 equiv), and DMAc (2.0 mL) were successively added and vigorously stirred together in a 120 °C oil bath under N2 atmosphere for 8 h. After the reaction was finished, the mixture was cooled to room temperature. The reaction was quenched with saturated NH4Cl aq. and extracted with EtOAc (3 × 20 mL). The combined ethyl acetate layer was washed with brine (15 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuum. The crude product was purified by flash column chromatography (eluting with petroleum ether/ethyl acetate) on silica gel to afford the product 4.
2-methyl-4-phenyl-9H-indeno [2,1-b]pyridin-9-one (4a): Compound 4a was prepared according to the general procedure using 3a. Purification by flash column chromatography (petroleum ether/ethyl acetate = 3/1, v/v) afforded 4a as a light yellow solid, m.p. = 116.5—119.5 °C, 47.7 mg, 88% yield. 1H NMR (400 MHz, Chloroform-d) δ 7.71 (dd, J = 5.7, 3.0 Hz, 1H), 7.57—7.46 (m, 5H), 7.26—7.23 (m, 2H), 7.07 (s, 1H), 6.90 (dd, J = 6.4, 2.6 Hz, 1H), 2.66 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 193.02, 159.49, 153.24, 145.59, 142.21, 137.26, 135.11, 134.70, 132.45, 129.20, 129.11, 128.99, 128.17, 128.05, 124.69, 123.14, 24.12. HRMS-ESI (m/z): calcd for C19H13NO, [M+H]+: 272.1070, found, 272.1061.
methyl 4-(2-methyl-9-oxo-9H-indeno [2,1-b]pyridin-4-yl)benzoate (4i): Compound 4i was prepared according to the general procedure using 3i. Purification by flash column chromatography (petroleum ether/ethyl acetate = 1/1, v/v) afforded 4i as a light yellow solid, m.p. = 112.5—114.5 °C, 49.3 mg, 75% yield. 1H NMR (400 MHz, Chloroform-d) δ 8.28—8.17 (m, 2H), 7.73 (d, J = 6.6 Hz, 1H), 7.61—7.54 (m, 2H), 7.28 (d, J = 9.0 Hz, 2H), 7.08 (s, 1H), 6.84 (d, J = 6.8 Hz, 1H), 4.00 (d, J = 1.5 Hz, 3H), 2.68 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 192.69, 166.54, 159.72, 153.34, 144.38, 141.82, 141.80, 135.21, 134.48, 132.44, 130.84, 130.26, 129.47, 128.40, 127.54, 124.87, 123.04, 52.48, 24.15. HRMS-ESI (m/z): calcd for C21H15NO3, [M+H]+: 330.1125, found, 330 1112.
4. Conclusions
In summary, we have developed a nickel-catalyzed [2 + 2 + 2] cycloaddition of alkynes, nitriles, and allyl boronates using a concise and stable catalytic system. This protocol employs readily available starting materials, enabling the efficient synthesis of fused pyridine derivatives with good functional group compatibility and excellent regioselectivity. Mechanistic studies revealed the essential roles of the terminal double bond and the Bpin group. Collectively, these findings provide fundamental insights into the [2 + 2 + 2] cycloaddition mechanism and the reactivity of azametallacyclopentadienes, thereby opening new avenues for metal-catalyzed cycloadditions of unsaturated compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30173629/s1, the X-ray of 3h, NMR data and spectra of the catalytic products. References [36,37,39,40,41,42,43] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, K.D. and J.H.; methodology, K.D., T.Z. and G.L.; investigation, K.D., T.Z., T.S., S.H. and R.G.; writing—original draft preparation, J.H. and C.L.; writing—review and editing, Z.-Y.W. and C.L.; supervision, Z.-Y.W. and J.H.; project administration, J.H.; funding acquisition, Z.-Y.W., C.L. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22361003), Guizhou Province Science and Technology Support Program (QKHZC-[2025]YB133), the Jiangxi Provincial Natural Science Foundation (20242BAB25591), Zhaoqing University Innovative Research Team Funding Program (TD202413).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef]
- Guan, A.-Y.; Liu, C.-L.; Sun, X.-F.; Xie, Y.; Wang, M.-A. Discovery of Pyridine-based Agrochemicals by Using Intermediate Derivatization Methods. Bioorg. Med. Chem. 2016, 24, 342–353. [Google Scholar] [CrossRef]
- Desimoni, G.; Faita, G.; Quadrelli, P. Pyridine-2,6-bis-(oxazolines), Helpful Ligands for Asymmetric Catalysts. Chem. Rev. 2003, 103, 3119–3154. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly Reactive Ligands and a Gateway to New Families of Catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef] [PubMed]
- Wurz, R.P. Chiral Dialkylaminopyridine Catalysts in Asymmetric Synthesis. Chem. Rev. 2007, 107, 5570–5595. [Google Scholar] [CrossRef] [PubMed]
- Kwong, H.-L.; Yeung, H.-L.; Yeung, C.-T.; Lee, W.-S.; Lee, C.-S.; Wong, W.-L. Chiral Pyridine-containing Ligands in Asymmetric Catalysis. Coord. Chem. Rev. 2007, 251, 2188–2222. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, W. Renaissance of Pyridine-oxazolines as Chiral Ligands for Asymmetric Catalysis. Chem. Soc. Rev. 2018, 47, 1783–1810. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.D. Recent Strategies for the Synthesis of Pyridine Derivatives. Chem. Eur. J. 2010, 16, 12052–12062. [Google Scholar] [CrossRef]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition Metal-mediated Synthesis of Monocyclic Aromatic Heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef]
- Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constantieux, T. Metal-free Multicomponent Syntheses of Pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Li, Z.-H.; Cao, Z.-H.; Li, Z.; Pang, C.-M.; Zhang, Z.-Q.; Wang, Z.-Y. Multifunctional 3-Cyanopyridine Compounds: Synthesis Based on A Tandem Reaction with 100% Atom Economy and Their Applications. Green Chem. 2025, 27, 7300–7306. [Google Scholar] [CrossRef]
- Zeng, J.; Zhou, T.; Liu, J.; Wan, J.-P. Photocatalytic Pyridine Synthesis with Enaminones and TMEDA under Metal-Free Conditions. J. Org. Chem. 2024, 89, 11060–11066. [Google Scholar] [CrossRef]
- Zhan, J.-L.; Wu, M.-W.; Wei, D.; Wei, B.-Y.; Jiang, Y.; Yu, W.; Han, B. 4-HO-TEMPO-Catalyzed Redox Annulation of Cyclopropanols with Oxime Acetates toward Pyridine Derivatives. ACS Catal. 2019, 9, 4179–4188. [Google Scholar] [CrossRef]
- Roglans, A.; Pla-Quintana, A.; Solà, M. Mechanistic Studies of Transition-Metal-Catalyzed [2 + 2 + 2] Cycloaddition Reactions. Chem. Rev. 2021, 121, 1894–1979. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Cen, K.; Shen, W.; Bai, L.-G.; Liu, W.-B. [2 + 2 + 2] Cycloaddition of Nitriles to Enantioenriched and Highly Substituted Pyridines. Chem. Catal. 2022, 2, 2889–2897. [Google Scholar] [CrossRef]
- Domínguez, G.; Pérez-Castells, J. Alkenes in [2 + 2 + 2] Cycloadditions. Chem. Eur. J. 2016, 22, 6720–6739. [Google Scholar] [CrossRef]
- Lv, Z.-J.; Liu, W.; Zhang, W.-X. Progress of Azametallacyclopentadienes in the New Century. Chem. Eur. J. 2023, 29, e202204079. [Google Scholar] [CrossRef]
- Whitehurst, W.G.; Kim, J.; Koenig, S.G.; Chirik, P.J. Three-Component Coupling of Arenes, Ethylene, and Alkynes Catalyzed by a Cationic Bis(phosphine) Cobalt Complex: Intercepting Metallacyclopentenes for C-H Functionalization. J. Am. Chem. Soc. 2022, 144, 4530–4540. [Google Scholar] [CrossRef]
- Ning, C.; Rui, K.-H.; Wei, Y.; Shi, M. Rh(I)-Catalyzed Dimerization of Ene-Vinylidenecyclopropanes for the Construction of Spiro[4,5]decanes and Mechanistic Studies. Chem. Sci. 2022, 13, 7310–7317. [Google Scholar] [CrossRef]
- Peng, J.-H.; Zheng, Y.-Q.; Bai, L.-G.; Liu, W.-B. Chiral Discrimination of Small Substituents in Biaryl Atropisomer Construction: Enantioselective Synthesis of Axially Chiral 1-Azafluorene via Ni-catalyzed [2 + 2 + 2] Cycloaddition. Sci. China Chem. 2023, 66, 3148–3153. [Google Scholar] [CrossRef]
- Huh, D.N.; Cheng, Y.; Frye, C.W.; Egger, D.T.; Tonks, I.A. Multicomponent Syntheses of 5- and 6-Membered Aromatic Heterocycles using Group 4-8 Transition Metal Catalysts. Chem. Sci. 2021, 12, 9574–9590. [Google Scholar] [CrossRef]
- Sheng, J.; Wang, Y.; Su, X.; He, R.; Chen, C. Copper-catalyzed [2 + 2 + 2] Modular Synthesis of Multisubstituted Pyridines: Alkenylation of Nitriles with Vinyliodonium Salts. Angew. Chem. Int. Ed. 2017, 56, 4824–4828. [Google Scholar] [CrossRef]
- Kumar, P.; Prescher, S.; Louie, J. A Serendipitous Discovery: Nickel Catalyst for the Cycloaddition of Diynes with Unactivated Nitriles. Angew. Chem. Int. Ed. 2011, 50, 10694–10698. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ogawa, R.; Itoh, K. Significant Chemo- and Regioselectivies in the Ru(II)-Catalyzed [2 + 2 + 2] Cycloaddition of 1,6-Diynes with Dicyanides. J. Am. Chem. Soc. 2001, 123, 6189–6190. [Google Scholar] [CrossRef] [PubMed]
- Cioni, P.; Diversi, P.; Ingrosso, G.; Lucherini, A.; Ronca, P. Rhodium-catalyzed Synthesis of Pyridines from Alkynes and Nitriles. J. Mol. Catal. 1987, 40, 337–357. [Google Scholar] [CrossRef]
- Onodera, G.; Shimizu, Y.; Kimura, J.; Kobayashi, J.; Ebihara, Y.; Kondo, K.; Sakata, K.; Takeuchi, R. Iridium-Catalyzed [2 + 2 + 2] Cycloaddition of α,ω-Diynes with Nitriles. J. Am. Chem. Soc. 2012, 134, 10515–10531. [Google Scholar] [CrossRef]
- Wakatsuki, Y.; Yamazaki, H. Cobalt-catalyzed Synthesis of Pyridines from Acetylenes and Nitriles. Tetrahedron Lett. 1973, 14, 3383–3384. [Google Scholar] [CrossRef]
- Schmidt, U.; Zenneck, U. Katalytische Cocyclisierungen von Ethin Mit Nitrilen an Bis(η2-ethen)(η6-toluol)eisen als Katalysator. J. Organomet. Chem. 1992, 440, 187–190. [Google Scholar] [CrossRef]
- McCormick, M.M.; Duong, H.A.; Zuo, G.; Louie, J. A Nickel Catalyzed Route to Pyridines. J. Am. Chem. Soc. 2005, 127, 5030–5031. [Google Scholar] [CrossRef]
- Stolley, R.M.; Duong, H.A.; Thomas, D.R.; Louie, J. The Discovery of [Ni(NHC)RCN]2 Species and Their Role as Cycloaddition Catalysts for the Formation of Pyridines. J. Am. Chem. Soc. 2012, 134, 15154–15162. [Google Scholar] [CrossRef]
- Satoh, Y.; Obora, Y. Low-Valent Niobium-Catalyzed Intermolecular [2 + 2 + 2] Cycloaddition of tert-Butylacetylene and Arylnitriles to Form 2,3,6-Trisubstituted Pyridine Derivatives. J. Org. Chem. 2013, 78, 7771–7776. [Google Scholar] [CrossRef]
- Mulcahy, S.P.; Varelas, J.G. Three-step Synthesis of an Annulated β-Carboline via Palladium Catalysis. Tetrahedron Lett. 2013, 54, 6599–6601. [Google Scholar] [CrossRef] [PubMed]
- Saliba, B.M.; Khanal, S.; O’Donnell, M.A.; Queenan, K.E.; Song, J.; Gentile, M.R.; Mulcahy, S.P. Parallel Strategies for the Synthesis of Annulated Pyrido[3,4-b]indoles via Rh(I)-and Pd(0)-Catalyzed Cyclotrimerization. Tetrahedron Lett. 2018, 59, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Nie, B.; Li, X.; Liu, T.; Li, C.; Huang, J. Ligand-controlled Regiodivergent Ni-Catalyzed trans-Hydroboration/Carboboration of Internal Alkynes with B2pin2. Chem. Sci. 2024, 15, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, T.; Li, S.; Li, G.; Wu, G.; Gao, Y.; Xu, Z.; Wu, Y.; Peng, X.; Huang, J. Redox-Neutral Nickel-Catalyzed Selective Hydroalkynylation of Internal Alkyne and Its Application in Anticancer Agent Discovery. Chin. J. Chem. 2024, 42, 3317–3323. [Google Scholar] [CrossRef]
- Huang, J.; Yan, W.; Tan, C.; Wu, W.; Jiang, H. Palladium(II)-catalyzed Hydroboration of Alkene with B2pin2. Chem. Comm. 2018, 54, 1770–1773. [Google Scholar] [CrossRef]
- Ma, W.; Yu, C.; Chen, T.; Xu, L.; Zhang, W.-X.; Xi, Z. Metallacyclopentadienes: Synthesis, Structure and Reactivity. Chem. Soc. Rev. 2017, 46, 1160–1192. [Google Scholar] [CrossRef]
- Li, M.; Wu, W.; Jiang, H. Recent Advances in Silver-Catalyzed Transformations of Electronically Unbiased Alkenes and Alkyne. Chem. Cat. Chem. 2020, 12, 5034–5050. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, C.; Hu, X.; Xie, X.; Liu, Y. Nickel-Catalyzed C(sp3)-H Functionalization of Benzyl Nitriles: Direct Michael Addition to Terminal Vinyl Ketones. Org. Lett. 2021, 23, 6004–6009. [Google Scholar] [CrossRef]
- Chen, X.; He, Q.; Xie, Y.; Yang, C. Palladium(II)-Catalyzed Synthesis of Functionalized Indenones via Oxidation and Cyclization of 2-(2-Arylethynylphenyl)acetonitriles. Org. Biomol. Chem. 2013, 11, 2582–2585. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, J.W.; Yang, W.W.; Fu, J.Y.; Zhu, J.Y.; Wang, Y.B. Synthesis of 1-Cyano-3-acylnaphthalenes via Formal [4+2] Benzannulation of 2-(2-Alkynylphenyl)acetonitriles and Alkynones. J. Org. Chem. 2019, 84, 8090–8099. [Google Scholar] [CrossRef]
- Lin, H.S.; Pan, Y.Z.; Tian, Y.H.; Pan, Y.M.; Wang, X. Palladium-Catalyzed Tandem Cyclization of 2-(2 Ethynylphenyl)acetonitriles and Isocyanides: Access to Indeno[2,1-b]pyrroles. Adv. Synth. Catal. 2022, 364, 1117–1121. [Google Scholar] [CrossRef]
- Sedelmeier, J.; Ley, S.V.; Lange, H.; Baxendale, I.R. Pd-EnCatTM TPP30 as a Catalyst for the Generation of Highly Functionalized Aryl- and Alkenyl-Substituted Acetylenes via Microwave-Assisted Sonogashira Type Reactions. Eur. J. Org. Chem. 2009, 26, 4412–4420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).