Microstructure and First Hydrogenation Properties of Zr1−xTixCr2 Alloys Where x = 0, 0.25, 0.5, 0.75, and 1
Abstract
1. Introduction
2. Results and Discussion
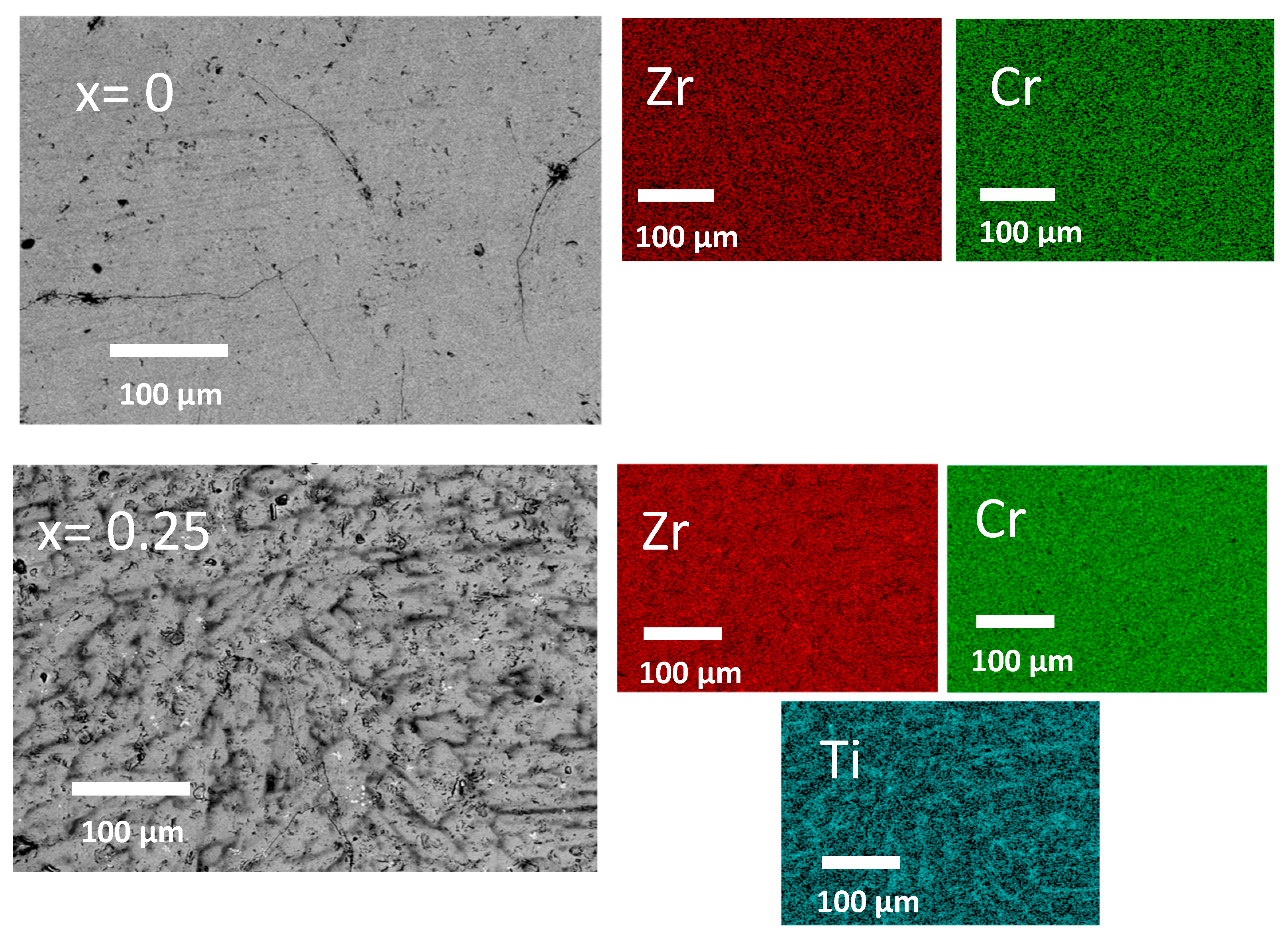
2.1. Microstructural Study
2.2. Crystal Structure
2.3. First Hydrogenation
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Rampai, M.M.; Mtshali, C.B.; Seroka, N.S.; Khotseng, L. Hydrogen production, storage, and transportation: Recent advances. RSC Adv. 2024, 14, 6699–6718. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Sakaki, K.; Akiba, E.; Allendorf, M.D.; Alvares, E.; Ares, J.R.; Babai, D.; Baricco, M.; Von Colbe, J.B.; Bereznitsky, M. Magnesium-and intermetallic alloys-based hydrides for energy storage: Modelling, synthesis and properties. Prog. Energy 2022, 4, 032007. [Google Scholar] [CrossRef]
- Van Mal, H.H.; Buschow, K.H.J.; Miedema, A.R. Hydrogen absorption in LaNi5 and related compounds: Experimental observations and their explanation. J. Less Common Met. 1974, 35, 65–76. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Li, C.; Yuan, Z.-M.; Qi, Y.; Guo, S.-H.; Zhao, D.-L. Research progress of TiFe-based hydrogen storage alloys. J. Iron Steel Res. Int. 2022, 29, 537–551. [Google Scholar] [CrossRef]
- Gesari, S.B.; Pronsato, M.E.; Visintin, A.; Juan, A. Hydrogen Storage in AB2 Laves Phase (A = Zr, Ti; B = Ni, Mn, Cr, V): Binding Energy and Electronic Structure. J. Phys. Chem. C 2010, 114, 16832–16836. [Google Scholar] [CrossRef]
- Stein, F.; Leineweber, A. Laves phases: A review of their functional and structural applications and an improved fundamental understanding of stability and properties. J. Mater. Sci. 2021, 56, 5321–5427. [Google Scholar] [CrossRef]
- Cao, Z.; Habermann, F.; Burkmann, K.; Felderhoff, M.; Mertens, F. Unstable Metal Hydrides for Possible on-Board Hydrogen Storage. Hydrogen 2024, 5, 241–279. [Google Scholar] [CrossRef]
- Ivey, D.G.; Northwood, D.O. Storing Hydrogen in AB2 Laves-Type Compounds. Z. Für Phys. Chem. 1986, 147, 191–209. [Google Scholar] [CrossRef]
- Pebler, A.; Gulbransen, E. Equilibrium studies on the systems ZrCr2-H2, ZrV2-H2, and ZrMo2-H2 between 0 and 900 °C. AIME Met. Soc. Trans. 1967, 239, 1597. [Google Scholar]
- Aufrecht, J.; Baumann, W.; Leineweber, A.; Duppel, V.; Mittemeijer, E.J. Layer-stacking irregularities in C36-type Nb–Cr and Ti–Cr Laves phases and their relation with polytypic phase transformations. Philos. Mag. 2010, 90, 3149–3175. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Yuan, F.; Han, F.; Zhang, Y.; Gu, H. Stacking faults in Zr(Fe, Cr)2 Laves structured secondary phase particle in Zircaloy-4 alloy. Nanoscale 2018, 10, 2249–2254. [Google Scholar] [CrossRef]
- Yang, T.; Lu, J.; Li, K.; Kong, Y.; Zhang, Z.; Long, Q.; Lan, X.; Lu, Q.; Du, Y. Discovery of a bulk C36-type MgZn2 structure step by step transformed from the C14 prototype laves phase structure. J. Mater. Sci. 2022, 57, 2999–3009. [Google Scholar] [CrossRef]
- Arias, D.; Abriata, J. The Cr−Zr (chromium-zirconium) system. Bull. Alloy Phase Diagr. 1986, 7, 237–244. [Google Scholar] [CrossRef]
- Drašner, A.; Blaẑina, Ẑ. The influence of Si and Ge on the hydrogen sorption properties of the intermetallic compound ZrCr2. J. Alloys Compd. 1993, 199, 101–104. [Google Scholar] [CrossRef]
- Bodega, J.; Fernández, J.F.; Leardini, F.; Ares, J.R.; Sánchez, C. Synthesis of hexagonal C14/C36 and cubic C15 ZrCr2 Laves phases and thermodynamic stability of their hydrides. J. Phys. Chem. Solids 2011, 72, 1334–1342. [Google Scholar] [CrossRef]
- Murashkina, T.L.; Syrtanov, M.S.; Laptev, R.S.; Stepanova, E.N.; Lider, A.M. Structure and defects evolution at temperature and activation treatments of the TiCr2 intermetallic compound of Laves phase C36-type. Int. J. Hydrogen Energy 2019, 44, 10732–10743. [Google Scholar] [CrossRef]
- Beeri, O.; Cohen, D.; Gavra, Z.; Mintz, M.H. Sites occupation and thermodynamic properties of the TiCr2−xMnx–H2 (0 ≤ x ≤ 1) system: Statistical thermodynamics analysis. J. Alloys Compd. 2003, 352, 111–122. [Google Scholar] [CrossRef]
- Bulyk, I.; Basaraba, Y.B.; Dovhyj, Y.O. Influence of Ti on the hydrogen-induced phase-structure transformations in the ZrCr2 intermetallic compound. Intermetallics 2006, 14, 735–741. [Google Scholar] [CrossRef]
- Dovhyi, Y.O. Energy conditions of stability of the phases of intermetallic compounds with variable compositions of the form Zr1−xTixCr2. Mater. Sci. 2008, 44, 556–560. [Google Scholar] [CrossRef]
- Klyamkin, S.; Kovriga, A.Y.; Verbetsky, V. Effect of substitution on FCC and BCC hydridephase formation in the TiCr2–H2 system. Int. J. Hydrogen Energy 1999, 24, 149–152. [Google Scholar] [CrossRef]
- Khajavi, S.; Rajabi, M.; Huot, J. Crystal structure of as-cast and heat-treated Ti0.5Zr0.5 (Mn1−xFex)Cr1, x = 0, 0.2, 0.4. J. Alloys Compd. 2018, 767, 432–438. [Google Scholar] [CrossRef]
- Rudy, E. Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems: Part V. Compendium of Phase Diagram Data; Air Force Materials Laboratory: Arlington, VA, USA, 1969. [Google Scholar]
- Johnson, J.R. Reaction of hydrogen with the high temperature (C14) form of TiCr2. J. Less Common Met. 1980, 73, 345–354. [Google Scholar] [CrossRef]
- Peisl, H. Lattice strains due to hydrogen in metals. In Hydrogen in Metals I; Springer: Berlin/Heidelberg, Germany, 1978; pp. 53–74. [Google Scholar]
- Broom, D.P. Hydrogen Storage Materials: The Characterisation of Their Storage Properties; Springer: Berlin/Heidelberg, Germany, 2011; Volume 1. [Google Scholar]
- Bruker, A. Topas V3: General Profile and Structure Analysis Software for Powder Diffraction Data–User’s Manual; Bruker AXS: Karlsruhe, Germany; Coehlo AA (2007) TOPAS Academic, Coelho Software: Brisbane, Australia, 2005. [Google Scholar]






| Sample | Zr (at.%) | Ti (at.%) | Cr (at.%) | |
|---|---|---|---|---|
| x = 0 | Nominal | 33 | -- | 67 |
| Measurement | 32 | -- | 68 | |
| x = 0.25 | Nominal | 25 | 8 | 67 |
| Measurement | 24 | 9 | 67 | |
| x = 0.5 | Nominal | 16 | 17 | 67 |
| Measurement | 15 | 18 | 67 | |
| x = 0.75 | Nominal | 8 | 25 | 67 |
| Measurement | 8 | 26 | 66 | |
| x = 0.25 | Nominal | -- | 33 | 67 |
| Measurement | -- | 33 | 67 |
| Sample | Zr (at.%) | Ti (at.%) | Cr (at.%) | |
|---|---|---|---|---|
| x = 0 | Bulk | 32 | -- | 68 |
| x = 0.25 | Light grey | 26 | 6 | 68 |
| Dark grey | 14 | 18 | 68 | |
| x = 0.5 | Light grey | 20 | 12 | 68 |
| Dark grey | 10 | 23 | 67 | |
| x = 0.75 | Light grey | 9 | 25 | 66 |
| Dark grey | 5 | 29 | 66 | |
| x = 0.25 | Light grey | -- | 30 | 70 |
| Dark grey | -- | 35 | 65 |
| Sample | Lattice Parameter (Å) | Unit Cell Volume (Å3) | Crystallite Size (nm) | Microstrain (%) |
|---|---|---|---|---|
| x = 0 | a = 5.1137 (3) | 187.74 (3) | 113 (9) | 0.034 (2) |
| c = 8.2897 (7) | ||||
| x = 0.25 | a = 5.085 (1) | 184.4 (1) | 38 (3) | 0.112 (7) |
| c = 8.224 (2) | ||||
| x = 0.5 | a = 5.027 (3) | 178.1 (3) | -- | 0.328 (5) |
| c = 8.141 (5) | ||||
| x = 0.75 | a = 4.971 (1) | 172.29 (9) | -- | 0.153 (3) |
| c = 8.051 (2) | ||||
| x = 1 | a = 4.942 (2) | 169.4 (1) | -- | 0.205 (3) |
| c = 7.992 (3) |
| Sample | Phase | Abundance (wt.%) | Lattice Parameter (Å) | Unit Cell Volume (Å3) | Crystallite Size (nm) | Microstrain (%) |
|---|---|---|---|---|---|---|
| x = 0 | C14 | 100 | a = 5.4208 (6) | 225.23 (6) | 52 (4) | 0.082 (5) |
| c = 8.850 (1) | ||||||
| x = 0.25 | C14 | 100 | a = 5.289 (2) | 219.1 (1) | 19 (1) | 0.24 (1) |
| c = 8.784 (3) | ||||||
| x = 0.5 | C14 | 29 (1) | a = 5.289 (2) | 210.3 (2) | -- | 0.319 (5) |
| c = 8.680 (5) | ||||||
| C14_2 | 66 (2) | a = 5.172 (3) | 197.0 (3) | -- | 0.79 (2) | |
| c = 8.503 (7) | ||||||
| Cr0.8Ti0.2 | 4.7 (4) | a = 2.937 (1) | 25.33 (3) | 30 (4) | -- |
| Sample | ΔV (Å3) | H/M | Estimated Capacity (wt.%) |
|---|---|---|---|
| x = 0 | 37.5 | 1.3 | 2 |
| x = 0.25 | 34.89 | 1.2 | 1.97 |
| x = 0.5 | C14_1: 31.7 | 1.1 | 1.84 |
| C14_2: 31.7 | 0.6 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtiari, T.; Sleiman, S.; Huot, J. Microstructure and First Hydrogenation Properties of Zr1−xTixCr2 Alloys Where x = 0, 0.25, 0.5, 0.75, and 1. Molecules 2025, 30, 3611. https://doi.org/10.3390/molecules30173611
Bakhtiari T, Sleiman S, Huot J. Microstructure and First Hydrogenation Properties of Zr1−xTixCr2 Alloys Where x = 0, 0.25, 0.5, 0.75, and 1. Molecules. 2025; 30(17):3611. https://doi.org/10.3390/molecules30173611
Chicago/Turabian StyleBakhtiari, Tanin, Salma Sleiman, and Jacques Huot. 2025. "Microstructure and First Hydrogenation Properties of Zr1−xTixCr2 Alloys Where x = 0, 0.25, 0.5, 0.75, and 1" Molecules 30, no. 17: 3611. https://doi.org/10.3390/molecules30173611
APA StyleBakhtiari, T., Sleiman, S., & Huot, J. (2025). Microstructure and First Hydrogenation Properties of Zr1−xTixCr2 Alloys Where x = 0, 0.25, 0.5, 0.75, and 1. Molecules, 30(17), 3611. https://doi.org/10.3390/molecules30173611







