Pharmacognosy and Antioxidant Activity of Pruned Leaves from the Unexplored Olea europaea L. ‘Lavagnina’ (Liguria, Italy)
Abstract
1. Introduction
2. Results
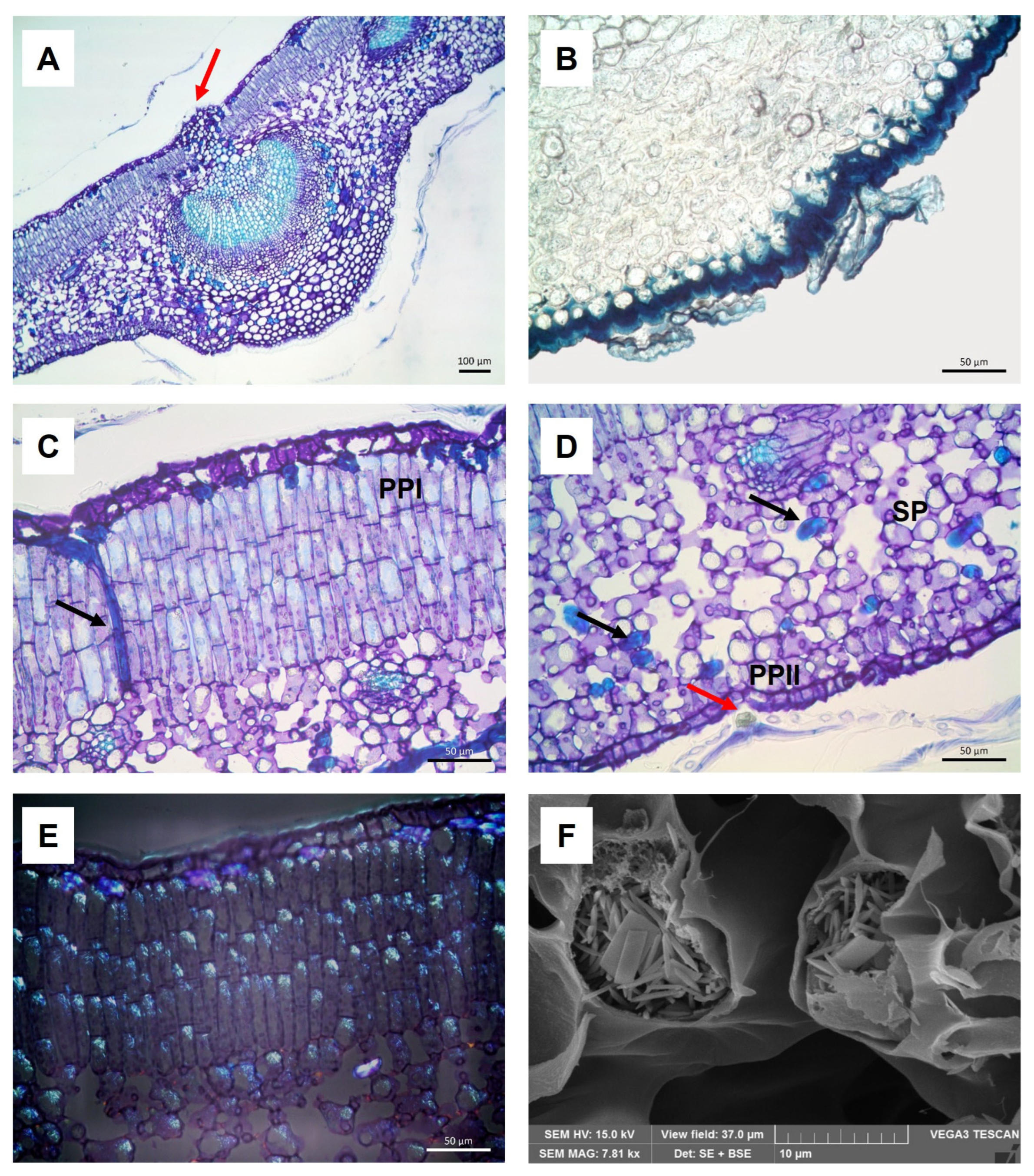
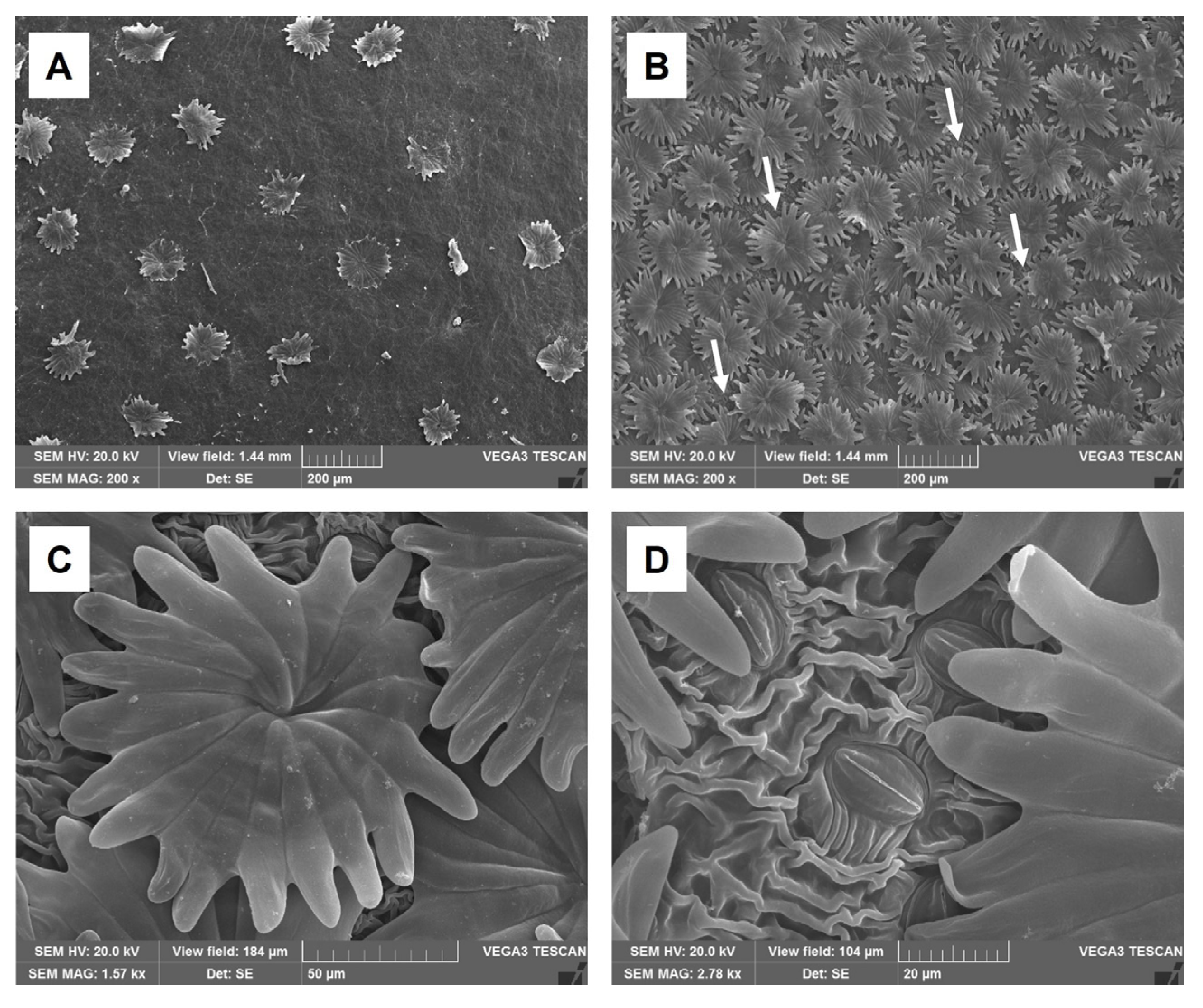
2.1. Micro-Morphological and Anatomical Analysis
2.2. Extraction
2.3. Antioxidant Activity
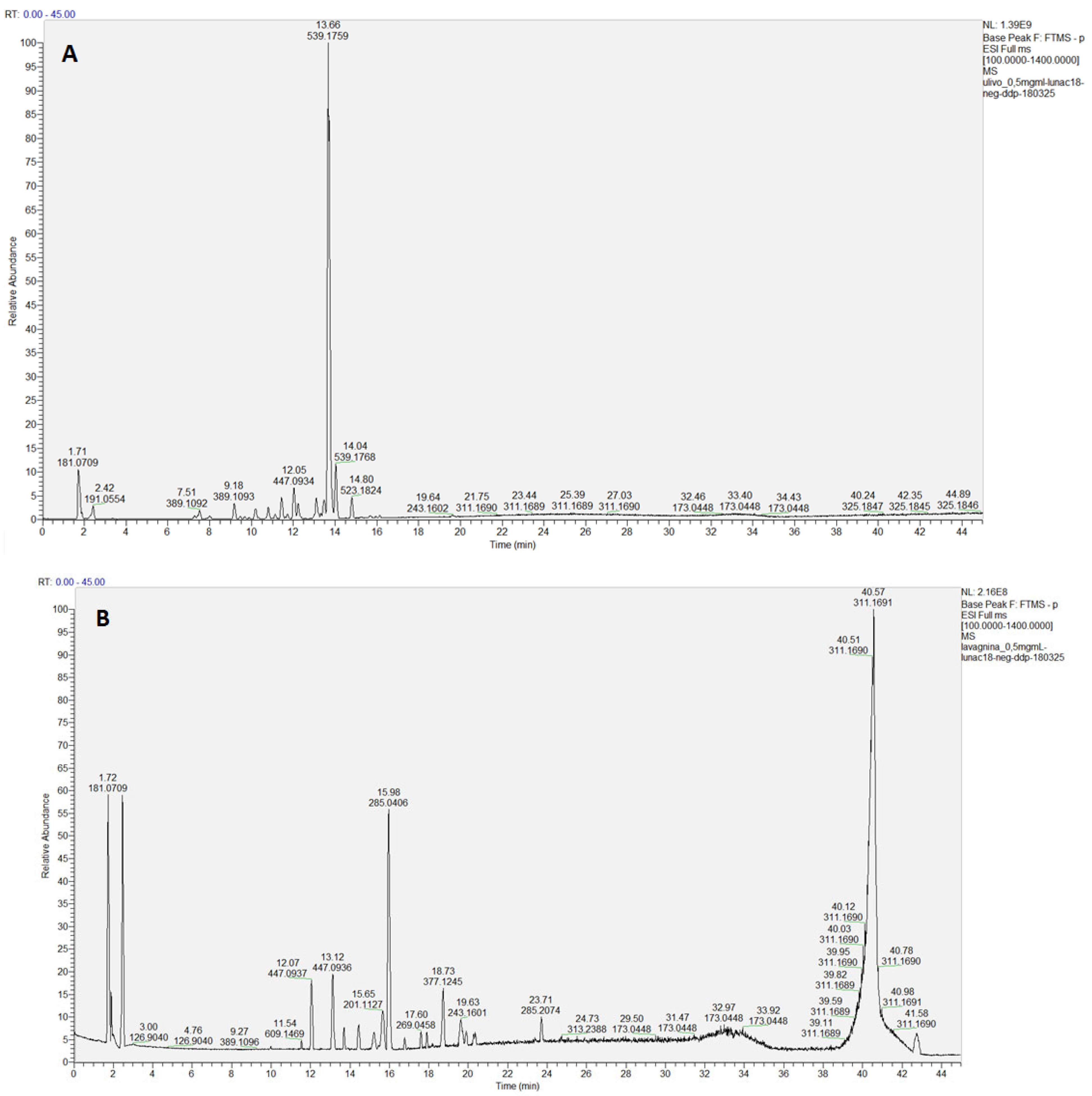
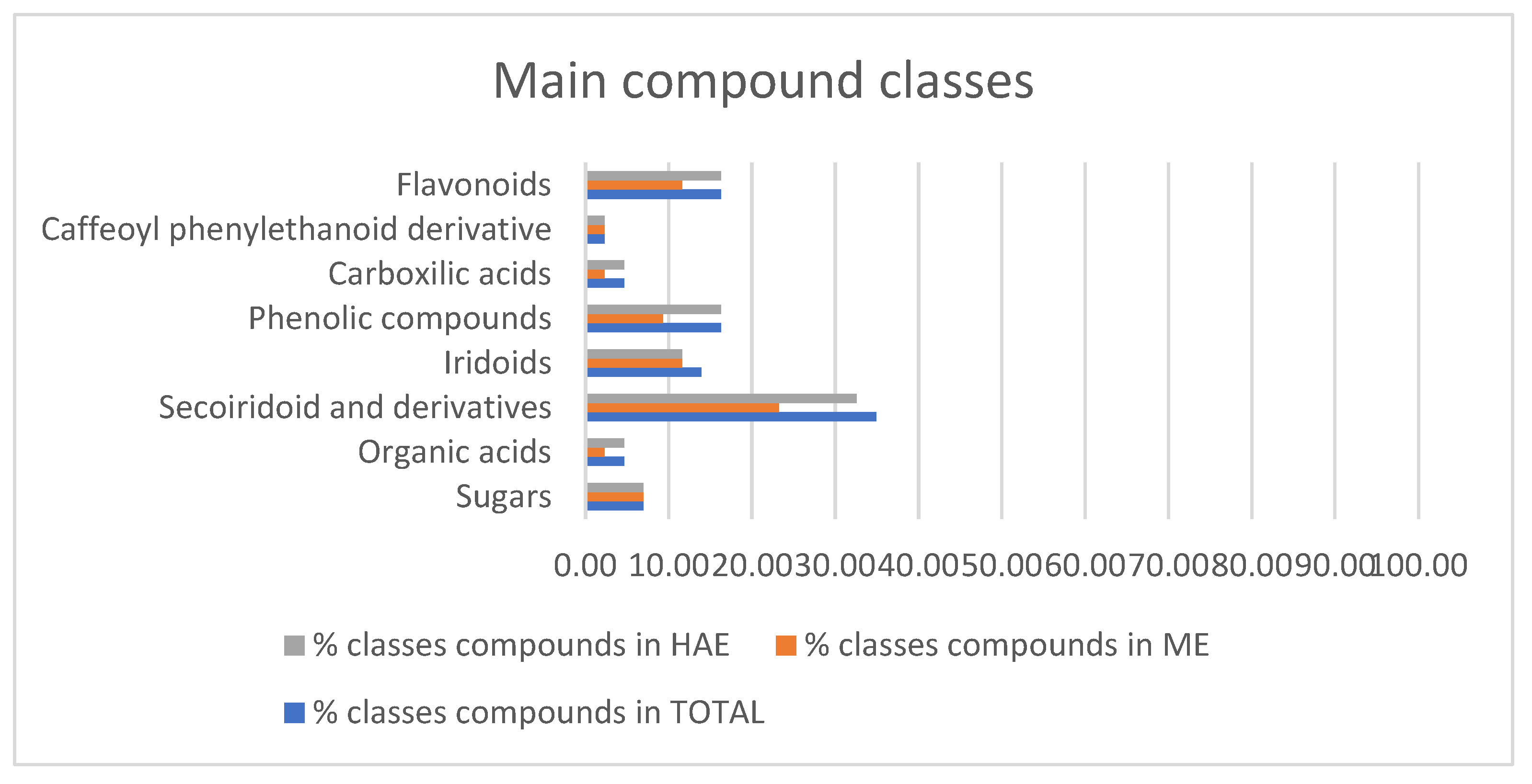
2.4. Chemical Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Micro-Morphological and Anatomical Analyses
4.3. Extraction
4.4. Antioxidant Activity
4.4.1. DPPH Assay
4.4.2. ABTS Assay
4.4.3. FRAP Assay
4.5. Chemical Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Lo Giudice, V.; Faraone, I.; Bruno, M.R.; Ponticelli, M.; Labanca, F.; Bisaccia, D.; Massarelli, C.; Milella, L.; Todaro, L. Olive Trees By-Products as Sources of Bioactive and Other Industrially Useful Compounds: A Systematic Review. Molecules 2021, 26, 5081. [Google Scholar] [CrossRef]
- Consorzio per la Tutela dell’Olio Extra Vergine di Oliva DOP Riviera Ligure. Available online: https://www.oliorivieraligure.it/ (accessed on 21 February 2025).
- Rellini, I.; Demasi, M.; Scopesi, C.; Ghislandi, S.; Salvidio, S.; Pini, S.; Stagno, A. Evaluation of the environmental components of the Taggiasca “terroir” olive (Imperia, Italy). Bull. Environ. Life Sci. 2022, 4, 24–42. [Google Scholar] [CrossRef]
- Official Gazette of the Italian Republic 193/1998. Available online: https://www.gazzettaufficiale.it/home (accessed on 21 February 2025).
- Quaini, M. Per la Storia del Paesaggio Agrario in Liguria: Note di Geografia Storica Sulle Strutture Agrarie Della Liguria Medievale e Moderna; Camera di Commercio Industria Artigianato e Agricoltura: Savona, Italy, 1973; pp. 75–80. [Google Scholar]
- Barichello, R.; Monticelli, A.; Cafici, E.; Guardavilla, A.; Martoni, S.; Ghironi, M.; Rossi, N.; Pini, S.; Spagnoli, F.; Bertucci, A.; et al. Le Varietà di Olivo Liguri; Regione Liguria: Liguria, Italy, 2006. [Google Scholar]
- Lombardo, N.; Muzzalupo, I.; Perri, E.; Sajiad, A. Microsatellite markers for olive (Olea europaea L.). In Proceedings of the 12th Simposium Científico-Técnico Expoliva, Jaén, Spain, 12–13 May 2005; Fundación para la promoción y el desarrollo del olivar y el aceite de oliva (ed). pp. 1–8. [Google Scholar]
- Bracci, T.; Sebastiani, L.; Busconi, M.; Fogher, C.; Belaj, A.; Trujillo, I. SSR markers reveal the uniqueness of olive cultivars from the Italian region of Liguria. Sci. Hortic. 2009, 122, 209–215. [Google Scholar] [CrossRef]
- Carciochi, R.A.; D’Alessandro, L.G.; Vauchel, P.; Rodriguez, M.M.; Nolasco, S.M.; Dimitrov, K. Valorization of Agrifood By-Products by Extracting Valuable Bioactive Compounds Using Green Processes. In Ingredients Extraction by Physicochemical Methods in Food—Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 4, pp. 191–228. [Google Scholar]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Magyari-Pavel, I.Z.; Moacă, E.-A.; Avram, Ș.; Diaconeasa, Z.; Haidu, D.; Ștefănuț, M.N.; Rostas, A.M.; Muntean, D.; Bora, L.; Badescu, B.; et al. Antioxidant Extracts from Greek and Spanish Olive Leaves: Antimicrobial, Anticancer and Antiangiogenic Effects. Antioxidants 2024, 13, 774. [Google Scholar] [CrossRef]
- Ghanem, M.T.M.; Tawfik, W.A.; Mahdy, E.M.; Abdelgawa, M.E.; Abdel-Azim, N.S.; El-Missiry, M.M. Chemical and biological evaluation of olive leaves as a waste by-product of olive oil industry. Egypt. Pharm. J. 2019, 18, 172–177. [Google Scholar]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M. Usi e Tradizioni Della Flora Italiana—Medicina Popolare ed Etnobotanica; Aracne: Rome, Italy, 2006. [Google Scholar]
- González, J.A.; García-Barriuso, M.; Amich, F. Ethnobotanical study of medicinal plants traditionally used in the Arribes del Duero, western Spain. J. Ethnopharmacol. 2010, 131, 343–355. [Google Scholar] [CrossRef] [PubMed]
- El Khomsi, M.; Dandani, Y.; Chaachouay, N.; Hmouni, D. Ethnobotanical study of plants used for medicinal, cosmetic, and food purposes in the region of Moulay Yacoub, Northeast of Morocco. J. Pharm. Pharmacogn. Res. 2022, 10, 13–29. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Palmeri, R.; Siracusa, L.; Carrubba, M.; Parafati, L.; Proetto, I.; Pesce, F.; Fallico, B. Olive Leaves, a Promising Byproduct of Olive Oil Industry: Assessment of Metabolic Profiles and Antioxidant Capacity as a Function of Cultivar and Seasonal Change. Agronomy 2022, 12, 2007. [Google Scholar] [CrossRef]
- Khelouf, I.; Karoui, I.J.; Lakoud, A.; Hammami, M.; Abderrabba, M. Comparative chemical composition and antioxidant activity of olive leaves Olea europaea L. of Tunisian and Algerian varieties. Heliyon 2023, 9, e22217. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z. Physicochemical Characteristics and Antioxidant Potential of the Greek PDO and PGI Virgin Olive Oils (VOOs). Eur. J. Lipid Sci. Technol. 2018, 121, 1800172. [Google Scholar] [CrossRef]
- Toumi, K.; S’wiatek, Ł.; Boguszewska, A.; Skalicka-Wo’zniak, K.; Bouaziz, M. Comprehensive Metabolite Profiling of Chemlali Olive Tree Root Extracts Using LC-ESI-QTOF-MS/MS, Their Cytotoxicity, and Antiviral Assessment. Molecules 2023, 28, 4829. [Google Scholar] [CrossRef]
- Serrano-García, I.; Martakos, I.C.; Olmo-García, L.; León, L.; de la Rosa, R.; Gómez-Caravaca, A.M.; Belaj, A.; Serrano, A.; Dasenaki, M.E.; Thomaidis, N.S.; et al. Application of Liquid Chromatography-Ion Mobility Spectrometry-Mass Spectrometry-Based Metabolomics to Investigate the Basal Chemical Profile of Olive Cultivars Differing in Verticillium dahliae Resistance. J. Agric. Food Chem. 2024, 72, 27561–27574. [Google Scholar] [CrossRef] [PubMed]
- Campeol, E.; Flamini, G.; Cioni, P.L.; Morelli, I.; D’Andrea, F.; Cremonini, R. 1,5-Anhydroxylitol from leaves of Olea europaea. Carbohydr. Res. 2004, 339, 2731–2732. [Google Scholar] [CrossRef]
- Villegas-Aguilar, M.D.C.; Cádiz-Gurrea, M.L.; Sánchez-Marzo, N.; Barrajón-Catalán, E.; Arráez-Román, D.; Fernández-Ochoa, Á.; Segura-Carretero, A. The Application of Untargeted Metabolomic Approaches for the Search of Common Bioavailable Metabolites in Human Plasma Samples from Lippia citriodora and Olea europaea Extracts. J. Agric. Food Chem. 2024, 72, 24879–24893. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.D.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS based metabolic profiling of the potential Olea europaea by-product “wood” and its comparison with leaf counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Analysis. 2013, 24, 213–223. [Google Scholar] [CrossRef]
- Beteinakis, S.; Papachristodoulou, A.; Stathopoulos, P.; Mikros, E.; Halabalaki, M. A multilevel LC-HRMS and NMR correlation workflow towards foodomics advancement: Application in table olives. Talanta 2024, 280, 126641. [Google Scholar] [CrossRef]
- Divya Priya, A.; Martin, A. UHPLC-MS/MS based comprehensive phenolic profiling, antimicrobial and antioxidant activities of Indian Rhodomyrtus tomentosa fruits. Sci. Rep. 2025, 15, 945. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; Rodriguez-Gutierrez, G.; Lama-Munoz, A.; Fernandez-Bolanos, J. New phenolic compounds hydrothermally extracted from the olive oil byproduct alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.M. Comparative metabolic profiling of olive leaf extracts from twelve different cultivars collected in both fruiting and flowering seasons. Sci. Rep. 2023, 13, 612. [Google Scholar] [CrossRef]
- Lv, X.; Sun, J.-Z.; Xu, S.-Z.; Cai, Q.; Li, Y.-Q. Rapid Characterization and Identification of Chemical Constituents in Gentiana radix before and after Wine-Processed by UHPLC-LTQ-Orbitrap MSn. Molecules 2018, 23, 3222. [Google Scholar] [CrossRef]
- Fayek, N.M.; Zayed, A.; Zahran, H.A.; Ramadan, N.S.; Capanoglu, E.; Li, Z.; Fang, Y.; Khalifa, I.; Farag, M.A. Effect of cultivar type, ontogeny and extraction methods as determinant factors of olive leaf metabolome: A case study in 8 Egyptian cultivars as analyzed using LC/MS-based metabolomics. Ind. Crops Prod. 2024, 222, 120085. [Google Scholar] [CrossRef]
- Ventura, G.; Calvano, C.D.; Abbattista, R.; Bianco, M.; De Ceglie, C.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Characterization of bioactive and nutraceutical compounds occurring in olive oil processing wastes. Rapid Commun. Mass Spectrom. 2019, 33, 1670–1681. [Google Scholar] [CrossRef]
- Fu, S.; Arráez-Roman, D.; Segura-Carretero, A.; Menéndez, J.A.; Menéndez-Gutiérrez, M.P.; Micol, V.; Fernández-Gutiérrez, A. Qualitative screening of phenolic compounds in olive leaf extracts by hyphenated liquid chromatography and preliminary evaluation of cytotoxic activity against human breast cancer cells. Anal. Bioanal. Chem. 2010, 397, 643–654. [Google Scholar] [CrossRef]
- Tarchi, I.; Olewnik-Kruszkowska, E.; Aït-Kaddour, A.; Bouaziz, M. Innovative Process for the Recovery of Oleuropein-Rich Extract from Olive Leaves and Its Biological Activities: Encapsulation for Activity Preservation with Concentration Assessment Pre and Post Encapsulation. ACS Omega 2025, 10, 6135–6146. [Google Scholar] [CrossRef]
- Mushtaq, H.; Piccolella, S.; Cimmino, G.; Ferrara, E.; Brahmi-Chendouh, N.; Petriccione, M.; Pacifico, S. Polyphenols from Olea europaea L. cv. Caiazzana leaf pruning waste for antioxidant and UV-blocking starch-based plastic films. Food Packag. Shelf Life 2025, 47, 101447. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Granados, M.; Saurina, J.; Sentellas, S. Olive tree leaves as a great source of phenolic compounds: Comprehensive profiling of NaDES extracts. Food Chem. 2024, 456, 140042. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS(2). J. Agric. Food Chem. 2012, 60, 791–798. [Google Scholar] [CrossRef]
- Michel, T.; Khlif, I.; Kanakis, P.; Termentzi, A.; Allouche, N.; Halabalaki, M.; Skaltsounis, A.-L. UHPLC-DAD-FLD and UHPLC-HRMS/MS based metabolic profiling and characterization of different Olea europaea organs of Koroneiki and Chetoui varieties. Phytochem. Lett. 2015, 11, 424–439. [Google Scholar] [CrossRef]
- Fayek, N.M.; Farag, M.A.; Saber, F.R. Metabolome classification via GC/MS and UHPLC/MS of olive fruit varieties grown in Egypt reveal pickling process impact on their composition. Food Chem. 2021, 339, 127861. [Google Scholar] [CrossRef]
- López-Salas, L.; Díaz-Moreno, J.; Ciulu, M.; Borrás-Linares, I.; Quirantes-Piné, R.; Lozano-Sánchez, J. Monitoring the Phenolic and Terpenic Profile of Olives, Olive Oils and By-Products throughout the Production Process. Foods 2024, 13, 1555. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Giambanelli, E.; Quirantes-Piné, R.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Wastes generated during the storage of extra virgin olive oil as a natural source of phenolic compounds. J. Agric. Food Chem. 2011, 59, 11491–11500. [Google Scholar] [CrossRef]
- Grigoletto, I.; García Salas, P.; Valli, E.; Bendini, A.; Ferioli, F.; Pasini, F.; Sánchez Villasclaras, S.; García-Ruiz, R.; Gallina Toschi, T. HPLC-MS/MS Phenolic Characterization of Olive Pomace Extracts Obtained Using an Innovative Mechanical Approach. Foods 2024, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Do, T.K.T.; De Vaumas, R.; Reich, E. Phytochemical profiling of iridoids by high-performance thin-layer chromatography. J. Planar Chrom. Modern TLC 2021, 34, 361–366. [Google Scholar] [CrossRef]
- Zou, W.; Wang, B.; Feng, X.; Lai, C.; Tan, Q. Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Combined With Network Pharmacology to Elucidate the Bioactive Ingredients and Potential Mechanism of Wu-Teng Decoction for Treatment of Rheumatoid Arthritis. Chem. Biodivers. 2025, 22, e202403454. [Google Scholar] [CrossRef]
- Lozano-Sanchez, J.; Bendini, A.; Quirantes-Piné, R.; Cerretani, L.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Monitoring the bioactive compounds status of extravirgin olive oil and storage by-products over the shelf life. Food Control 2013, 30, 606–615. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Costa Célia, M.; Bonifácio–Lopes, T.; Silva, S.; Veiga, M.; Monforte, A.R.; Nunes, J.; Vicente, A.; Pintado, M. Prebiotic effects of olive pomace powders in the gut: In vitro evaluation of the inhibition of adhesion of pathogens, prebiotic and antioxidant effects. Food Hydrocoll. 2021, 112, 106312. [Google Scholar] [CrossRef]
- Expósito-Díaz, A.; Miho, H.; Ledesma-Escobar, C.A.; Moral, J.; Díez, C.M.; Priego-Capote, F. Influence of genetic and interannual factors on bioactive compounds of olive pomace determined through a germplasm survey. Food Chem. 2022, 378, 132107. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Sánchez-González, C.; Sumalla-Cano, S.; Battino, M.; García-Ruiz, R.; Sánchez, S.; et al. Effect of olive leaf phytochemicals on the anti-acetylcholinesterase, anti-cyclooxygenase-2 and ferric reducing antioxidant capacity. Food Chem. 2024, 444, 138516. [Google Scholar] [CrossRef]
- Johnson, R.; Melliou, E.; Zweigenbaum, J.; Mitchell, A.E. Quantitation of Oleuropein and Related Phenolics in Cured Spanish-Style Green, California-Style Black Ripe, and Greek-Style Natural Fermentation Olives. J. Agric. Food Chem. 2018, 66, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Peralbo-Molina, A.; Priego-Capote, F.; Luque de Castro, M.D. Tentative identification of phenolic compounds in olive pomace extracts using liquid chromatography-tandem mass spectrometry with a quadrupole-quadrupole-time-of-flight mass detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar] [CrossRef] [PubMed]
- Moudache, M.; Silva, F.; Nerín, C.; Zaidi, F. Olive cake and leaf extracts as valuable sources of antioxidant and antimicrobial compounds: A comparative study. Waste Biomass Valorization 2021, 12, 1431–1445. [Google Scholar] [CrossRef]
- Zhao, H.; Kim, Y.; Avena-Bustillos, R.J.; Nitin, N.; Wang, S.C. Characterization of California olive pomace fractions and their in vitro antioxidant and antimicrobial activities. LWT—Food Sci. Technol. 2023, 180, 114677. [Google Scholar] [CrossRef]
- Beer, F.; Weinert, C.H.; Wellmann, J.; Hillebrand, S.; Ley, J.P.; Soukup, S.T.; Kulling, S.E. Comprehensive metabolome characterization of leaves, internodes, and aerial roots of Vanilla planifolia by untargeted LC-MS and GC × GC-MS. Phytochem. Anal. 2025, 36, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; d’Errico, G.; Crasto, A.; Mazzei, P.; Piccolo, A.; Incerti, G.; Woo, S.L.; et al. Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes. PLoS ONE 2018, 13, e0198728. [Google Scholar] [CrossRef]
- Xue, Y.; Guan, T.; Liu, J.; Wang, J.; Yang, Z.; Guan, F.; Li, W.; Wang, Y. Chemical Composition Analysis and Multi-index Component Content Determination of Compounds in Goupi Plaster Based on UPLC–Q-Exactive-MS and UPLC–MS/MS. Chromatographia 2024, 87, 45–57. [Google Scholar] [CrossRef]
- Ali, K.; Ali, A.; Khan, M.N.; Rahman, S.; Faizi, S.; Ali, M.S.; Khalifa, S.A.M.; El-Seedi, H.R.; Musharraf, S.G. Rapid Identification of Common Secondary Metabolites of Medicinal Herbs Using High-Performance Liquid Chromatography with Evaporative Light Scattering Detector in Extracts. Metabolites 2021, 11, 489. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, M.; Zhou, P.; Tian, M.; Zhou, J.; Zhang, L. Analysis of chemical composition in Chinese olive leaf tea by UHPLC-DAD-Q-TOF-MS/MS and GC-MS and its lipid-lowering effects on the obese mice induced by high-fat diet. Food Res. Int. 2020, 128, 108785. [Google Scholar] [CrossRef]
- Luján, R.J.; Capote, F.P.; Marinas, A.; de Castro, M.D. Liquid chromatography/triple quadrupole tandem mass spectrometry with multiple reaction monitoring for optimal selection of transitions to evaluate nutraceuticals from olive-tree materials. Rapid Commun. Mass Spectrom. 2008, 22, 855–864. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; de Jesús Avena-Bustillos, R.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 660582. [Google Scholar] [CrossRef]
- WHO. Quality Control Methods for Herbal Materials; WHO Press: Valletta, Malta, 2011; Available online: https://apps.who.int/iris/handle/10665/44479 (accessed on 26 March 2025).
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef]
- Moreno-Alías, I.; León, L.; de la Rosa, R.; Rapoport, H.F. Morphological and anatomical evaluation of adult and juvenile leaves of olive plants. Trees 2009, 23, 181–187. [Google Scholar] [CrossRef]
- Rahfeld, B. Mikroskopischer Farbatlas Pflanzlicher Drogen, 2nd ed.; Spektrum Akademischer Verlag Gmbh: Heidelberg, Germany, 2011. [Google Scholar]
- de Menezes, T.P.; de Oliveira, A.F.; Pasqual, M.; de Souza, A.R.; de Castro, E.M. Conteúdo de DNA e caracterização anatômica de cultivares de oliveiras (Olea europea L.). Agrarian 2018, 11, 196–202. [Google Scholar] [CrossRef][Green Version]
- Marchioni, I.; Rodolfi, M.; Massa, D.; Cacini, S.; Ughini, V.; Bartolini, G.; Fabbri, A.; Petruccelli, R.; Ganino, T. Comparative effects of drought stress on three olive cultivars focusing on older leaves. Sci. Hortic. 2024, 332, 113234. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Rombaut, N.; Chave, T.; Nikitenko, S.I.; Maâtaoui, M.E.; Fabiano-Tixier, A.S.; Chemat, F. Modification of Olive Leaves’ Surface by Ultrasound Cavitation. Correlation with Polyphenol Extraction Enhancement. Appl. Sci. 2021, 11, 232. [Google Scholar] [CrossRef]
- Žuna Pfeiffer, T.; Štolfa, I.; Hoško, M.; Žanić, M.; Pavičić, N.; Cesar, V.; Lepeduš, H. Comparative Study of Leaf Anatomy and Certain Biochemical Traits in Two Olive Cultivars. Agric. Conspec. Sci. 2010, 75, 91–97. [Google Scholar]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Stavrianakou, S.; Karabourniotis, G. Trichome layers versus dehaired lamina of Olea europaea leaves: Differences in flavonoid distribution, UV-absorbing capacity, and wax yield. Environ. Exp. Bot. 2006, 55, 294–304. [Google Scholar] [CrossRef]
- Filardo, S.; Roberto, M.; Di Risola, D.; Mosca, L.; Di Pietro, M.; Sessa, R. Olea europaea L-derived secoiridoids: Beneficial health effects and potential therapeutic approaches. Pharmacol. Ther. 2024, 254, 108595. [Google Scholar] [CrossRef]
- Marchetti, C.; Clericuzio, M.; Borghesi, B.; Cornara, L.; Ribulla, S.; Gosetti, F.; Marengo, E.; Burlando, B. Oleuropein-Enriched Olive Leaf Extract Affects Calcium Dynamics and Impairs Viability of Malignant Mesothelioma Cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 908493. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. Potential Protective Role Exerted by Secoiridoids from Olea europaea L. in Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Immunoinflammatory Diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micromorphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- Yeung, E. A beginner’s guide to the study of plant structure. In Tested Studies for Laboratory Teaching; Karcher, S.J., Ed.; Purdue University: Lafayatte, IN, USA, 1998; Volume 19, pp. 125–142. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Badria, F.A.; Aboelmaaty, W.S. Plant Histochemistry: A Versatile and Indispensible Tool in Localization of Gene Expression, Enzymes, Cytokines, Secondary Metabolites and Detection of Plants Infection and Pollution. Acta Sci. Pharm. Sci. 2019, 3, 88–100. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; Cerulli, A.; Montoro, P.; Piacente, S. Metabolite Profiling for Typization of “Rucola della Piana del Sele” (PGI), Eruca sativa, through UHPLC-Q-Exactive-Orbitrap-MS/MS Analysis. Foods 2023, 12, 3384. [Google Scholar] [CrossRef]

| Extract | DPPH IC50 1 (μg/mL) | FRAP mmol Fe2+ Equivalents/g Extract | ABTS mmol TEAC 2/g |
|---|---|---|---|
| PEE | >1000 | - | >1000 |
| CHE | 79.4 ± 0.7 a | 1.4 ± 0.5 b | 0.9 ± 0.03 b |
| ME | 29.7 ± 0.6 b | 2.6 ± 0.3 b | 0.7 ± 0.05 b |
| HAE | 17.3 ± 0.6 c | 2.8 ± 0.7 b | 1.3 ± 0.04 b |
| Trolox | 3.21 ± 0.2 d | 84.2 ± 0.9 a | - |
| Ascorbic acid | - | - | 39.3 ± 0.8 a |
| No. | Family | Retention Time | Measured (m/z) [M-H]− | Molecular Formula | Δppm | Fragment | Fragment Formula | Fragment ion (m/z) | Δppm | Identification | ME | HAE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alcohol sugar | 1.72 | 181.0709 | C6H14O6 | 1.190 | [M-H2O-H]− | C6H11O5 | 163.0604 | 1.963 | Mannitol | x | x |
| [M-CH6O2-H]− | C5H7O4 | 131.0338 | −0.65 | |||||||||
| [M-C2H6O2-H]− | C4H7O4 | 119.0339 | 0.04 | |||||||||
| [M-C2H8O3-H]− | C4H5O3 | 101.0232 | −0.797 | |||||||||
| 2 | Organic acid | 1.87 | 195.0503 | C6H12O7 | 0.411 | [M-H2O-H]− | C6H9O6 | 177.0398 | 2.347 | Gluconic acid | x | |
| [M-2H2O-H]− | C6H7O5 | 159.0289 | 0.756 | |||||||||
| [M-CH6O3-H]− | C5H5O4 | 129.0183 | 0.348 | |||||||||
| [M-C2H8O4-H]− | C4H3O3 | 99.0075 | −1.217 | |||||||||
| [M-C4H8O4-H]− | C2H3O3 | 75.0075 | −1.606 | |||||||||
| 3 | Organic acid | 2.48 | 191.0553 | C7H12O6 | 1.547 | [M-H2O-H]− | C7H9O5 | 173.0445 | 0.174 | Quinic acid | x | x |
| [M-2H2O-CO-H]− | C6H7O3 | 127.0388 | −0.949 | |||||||||
| [M-2H2O-CO2-H]− | C6H7O2 | 111.0439 | −0.955 | |||||||||
| [M-3H2O-CO2-H]− | C6H5O | 93.0334 | −1 412 | |||||||||
| [M-C3H6O4-H]− | C4H5O2 | 85.0283 | −1 599 | |||||||||
| 4 | Sugar | 2.59 | 237.0616 | C8H14O8 | 4.582 | [M-C3H8O4-H]− | C5H5O4 | 129.0183 | 0.193 | unknown (3-deoxy-D-manno-octulosonate) | x | x |
| [M-C5H10O5-H]− | C3H3O3 | 87.0075 | −1.385 | |||||||||
| 5 | Secoiridoid | 7.29 | 407.15652 | C17H28O11 | 4.254 | [M-C8H16O9-H]− | C9H11O2 | 151.07536 | 0.026 | 1-b-D-glucopyranosyl acyclodihydroelenolic acid | x | |
| [M-C9H16O9-H]− | C8H11O2 | 139.07545 | 0.675 | |||||||||
| [M-C13H20O7-H]− | C4H7O4 | 119.03346 | −3.572 | |||||||||
| 6 | Iridoid | 7.38 | 199.0608 | C9H12O5 | 3.617 | [M-CO2-H]− | C8H11O3 | 155.0705 | 1.285 | Hydroxylated product of the dialdehydic form of decarboxymethyl-elenolic acid | x | |
| [M-2CO2-H]− | C7H11O | 111.0804 | 0.076 | |||||||||
| [M-C7H8O3-H]− | C2H3O2 | 59.0127 | −1.286 | |||||||||
| 7 | Phenolic group | 7.48 | 315.1091 | C14H20O8 | 5.160 | [M-C6H10O5-H]− | C8H9O3 | 153.0549 | 1.564 | Hydroxytyrosol glucoside | x | x |
| [M-C6H10O5-H2O-H]− | C8H7O2 | 135.0440 | −0.489 | |||||||||
| [M-C7H12O6-H]− | C7H7O2 | 123.0441 | 0.195 | |||||||||
| [M-C11H14O5-H]− | C3H5O3 | 89.0232 | −0.792 | |||||||||
| 8 | Secoiridoid | 7.53 | 389.10925 | C16H22O11 | 3.629 | [M-C6H10O5-H]− | C10H11O6 | 227.0558 | 3.327 | Oleoside (isomers) | x | x |
| [M-C6H10O5-CO2-H]− | C9H11O4 | 183.0657 | 3.085 | |||||||||
| [M-C8H10O9-H]− | C8H11O2 | 139.0755 | 1.034 | |||||||||
| [M-C8H12O10-H]− | C8H9O | 121.0648 | 0.318 | |||||||||
| [M-C12H16O8-H]− | C4H5O3 | 101.0233 | −0.401 | |||||||||
| [M-C13H16O8-H]− | C3H5O3 | 89.0232 | −1.129 | |||||||||
| [M-C14H18O9-H]− | C2H3O2 | 59.0127 | −1.286 | |||||||||
| 9 | Phenolic group | 7.92 | 477.1620 | C20H30O13 | 3.610 | [M-C14H20O8-H]− | C6H9O5 | 161.0447 | 1.305 | Hydroxytyrosol-diglucoside | x | x |
| [M-C12H20O10-H]− | C8H9O3 | 153.0549 | 1.629 | |||||||||
| [M-C12H20O10-CH2O-H]- | C7H7O2 | 123.0440 | −0.455 | |||||||||
| 10 | Phenolic group | 8.01 | 153.0549 | C8H10O3 | 1.433 | [M-CH2O-H]− | C7H7O2 | 123.0441 | 0.276 | Hydroxytyrosol | x | x |
| [M-C2H2O2-H]− | C6H7O | 95.0492 | 0.722 | |||||||||
| [M-C3H4O2-H]− | C5H5O | 81.0333 | −2.732 | |||||||||
| 11 | Secoiridoid | 8.13 | 375.1301 | C16H24O10 | 3.803 | [M-C6H10O5-H2O-CO2-H]− | C9H11O2 | 151.0760 | 4.063 | Loganic acid | x | x |
| [M-C8H10O9-H]− | C8H13O | 125.0958 | −2.411 | |||||||||
| [M-C12H16O6-H]− | C4H7O4 | 119.0334 | −3.824 | |||||||||
| [M-C11H18O7-H]− | C5H5O3 | 113.0232 | −1.244 | |||||||||
| [M-C12H18O7-H]− | C4H5O3 | 101.0223 | −3.866 | |||||||||
| [M-C13H18O7-H]− | C3H5O3 | 89.0230 | −4.275 | |||||||||
| [M-C13H20O8-H]− | C3H3O2 | 71.0125 | −3.040 | |||||||||
| 12 | Iridoid | 8.50 | 345.1195 | C15H22O9 | 4.263 | [M-C6H10O5-H]− | C9H11O4 | 183.0656 | 4.232 | Aucubin | x | |
| [M-C6H10O5-H2O-H]− | C9H9O3 | 165.0552 | 3.449 | |||||||||
| [M-C11H14O5-H]− | C4H7O4 | 119.0338 | −0.716 | |||||||||
| [M-C12H16O6-H]− | C3H5O3 | 89.0233 | −0.568 | |||||||||
| 13 | Iridoid | 8.93 | 215.0922 | C10H16O5 | 3.766 | [M-CO2-H]− | C9H15O3 | 171.1020 | 2.274 | Aldehydic form of decarboxymethyl elenolic acid | x | x |
| [M-CO2-H2O-H]− | C9H13O2 | 153.0912 | 1.266 | |||||||||
| [M-CO2-H2O-CO-H]− | C8H13O | 125.0961 | 0.227 | |||||||||
| 14 | Phenolic aldehyde derivative | 8.96 | 313.0933 | C14H18O8 | 4.683 | [M-C6H10O5-H]− | C8 H7 O3 | 151.0393 | 2.048 | Vanilloside | x | |
| [M-C6H10O5-CO-H]− | C7H7O2 | 123.0441 | 0.276 | |||||||||
| [M-C11H14O5-H]− | C3H3O3 | 87.0075 | −1.615 | |||||||||
| 15 | Iridoid | 8.98 | 359.1353 | C16H24O9 | 4.514 | [M-C6H10O5-H]− | C10H13O4 | 197.0814 | 2.662 | 7-Deoxyloganic acid | x | x |
| [M-C6H10O5- C4H6O-H]− | C6H7O3 | 127.0389 | −0.713 | |||||||||
| 16 | Secoiridoid | 9.00 | 421.1724 | C18H30O11 | 4.635 | [M-C9H18O9-H]− | C9H11O2 | 151.075 | −2.093 | Oleoside methyl ester derivative | x | |
| [M-C13H22O8-H]− | C5 H7 O3 | 115.0388 | −1.309 | |||||||||
| 17 | Secoiridoid glycoside | 9.04 | 257.1032 | C12H18O6 | 4.960 | [M-CO2-H]− | C11H17O4 | 213.1130 | 4.103 | 3-Hydroxymethyl-2.3-dihydro-5-(methoxycarbonyl)-2-methyl-2H-pyran-4-acetic methyl ester | x | |
| [M-C3H6O4-H]− | C9H11O2 | 151.0758 | 2.806 | |||||||||
| [M-C9H14O3-H]− | C3H3O3 | 87.0075 | −1.730 | |||||||||
| 18 | Secoiridoid | 9.10 | 565.1779 | C23H34O16 | 2.847 | / | / | / | / | Elenolic acid dihexoside isomer | x | x |
| 19 | 9.27 | 345.1558 | C16H26O8 | 4.131 | / | / | / | / | 1,1,12,12-Dodecanetetracarboxylic acid | x | ||
| 20 | Secoiridoid | 9.43 | 245.1031 | C11H18O6 | 4.632 | [M-H2O-CO2-H]− | C10H15O3 | 183.1020 | 2.289 | Acyclodihydroelenolic acid | x | x |
| [M-C3H7O4-H]− | C8H11O2 | 139.0752 | −1.123 | |||||||||
| 21 | Iridoid | 9.57 | 389.1459 | C17H26O10 | 3.846 | [M-CO2-H]− | C16H25O8 | 345.1531 | −3.547 | Loganin | x | x |
| [M-2CO2-H]− | C15H25O6 | 301.1654 | 2.673 | |||||||||
| [M-C13H18O6-H]− | C4H7O4 | 119.0338 | −0.296 | |||||||||
| [M-C13H20O7-H]− | C4H5O3 | 101.0233 | −0.500 | |||||||||
| 22 | Phenolic aldehyde | 9.99 | 151.0392 | C8 H8O3 | 1.717 | [M-CH2-H]− | C7H5O3 | 137.0237 | 2.623 | Vanillin | x | |
| [M-CO-H]− | C7H7O2 | 123.0441 | 0.764 | |||||||||
| [M-CHO-H]− | C7H6O2 | 122.0363 | 0.729 | |||||||||
| 23 | Iridoid | 10.36 | 377.1459 | C16H26O10 | 4.048 | [M-C6H12O6-H]− | C10H13O4 | 197.0815 | 3.271 | Lamiol | x | x |
| [M-C6H12O6-CO2-H]− | C9H13O2 | 153.0913 | 1.854 | |||||||||
| [M-C6H12O6-C6H6-H]− | C4H7O4 | 119.034 | 0.964 | |||||||||
| 24 | Secoiridoid | 10.77 | 403.1250 | C17H24O11 | 3.453 | [M-C6H12O6-H]− | C11H11O5 | 223.0613 | 5.335 | Elenolic acid glucoside (oleoside methyl ester) | x | x |
| [M-C8H14O7-H]− | C9H9O4 | 181.0501 | 2.898 | |||||||||
| [M-C6H12O6-CO2-H]− | C10H11O3 | 179.0702 | −0.283 | |||||||||
| [M-C13H18O8-H]− | C4H5O3 | 101.0233 | −0.203 | |||||||||
| [M-C14H18O8 -H]− | C3H5O3 | 89.0232 | −1.129 | |||||||||
| 25 | Caffeoyl phenylethanoid derivatives | 11.20 | 461.1672 | C20H30O12 | 3.962 | [M-C13H18O6-H]− | C7H11O6 | 191.0556 | 3.169 | Decaffeoylverbascoside | x | x |
| [M-C15H20O7-H]− | C5H9O5 | 149.0444 | 0.001 | |||||||||
| [M-C15H24O9-H]− | C5H5O3 | 113.0233 | 0.172 | |||||||||
| 26 | Secoiridoid | 11.47 | 525.1621 | C24H30O13 | 2119 | [M-C8H8O2-H]− | C16H21O11 | 389.1088 | 2.473 | Demethyl oleuropein | x | x |
| [M-C14H20O8-H]− | C10H9O5 | 209.0452 | 3.588 | |||||||||
| [M-C14H18O9-H]− | C10H11O4 | 195.0656 | 2.331 | |||||||||
| [M-C14H20O8-CO2-H]− | C9H9O3 | 165.0550 | 2.177 | |||||||||
| [M-C14H20O8-2CO2-H]− | C8H9O | 121.0649 | 0.566 | |||||||||
| 27 | Flavonoid | 11.55 | 609.1468 | C27H30O16 | 2.920 | [M-C6H18O2-H]− | C21H11O14 | 487.0153 | 2.030 | Rutin | x | x |
| [M-C11H20O9-H]− | C16H9O7 | 313.0347 | 1.249 | |||||||||
| [M-C12H20O9-H]− | C15H9O7 | 301.0351 | 2.727 | |||||||||
| [M-C12H21O9-H]− | C15H8O7 | 300.0279 | 4.786 | |||||||||
| [M-C13H22O11-H]− | C14H7O5 | 255.0296 | 3.255 | |||||||||
| [M-C19H26O11-H]- | C8H3O5 | 178.9981 | 3.298 | |||||||||
| [M-C20H26O12-H]- | C7H3O4 | 151.0028 | 1.357 | |||||||||
| 28 | Secoiridoid | 11.65 | 555.1727 | C25H32O14 | 3.203 | [M-C10H16O6-H]− | C15H15O8 | 323.0771 | 3.021 | 10-Hydroxyoleuropein | x | x |
| [M-C14H20O9-H]− | C11H11O5 | 223.0609 | 3.766 | |||||||||
| [M-C15H24O10-H]− | C10H7O4 | 191.0342 | 1.805 | |||||||||
| [M-C17H24O11-H]− | C8H7O3 | 151.0392 | 1.717 | |||||||||
| 29 | Flavonoid | 11.76 | 593.152 | C27H30O15 | 3.175 | [M-C6H10O4-H]− | C21H19O11 | 447.0936 | 3.092 | Luteolin-7-O-rutinoside | x | x |
| [M-C12H20O9-H]− | C15H9O6 | 285.0407 | 4.826 | |||||||||
| 30 | Flavonoid | 12.05 | 447.0936 | C21H20O11 | 3.226 | [M-C6H10O5-H]− | C15H9O6 | 285.0407 | 4.931 | Luteolin-O-hexoside isomer | x | x |
| 31 | Secoiridoid | 12.23 | 569.1882 | C26H34O14 | 3.071 | [M-C9H10O3-H]− | C17H23O11 | 403.1241 | 1.543 | 2′′-Methoxyoleuropein | x | |
| [M-C9H10O3-C6H12O6-H]− | C11H11O5 | 223.061 | 3.901 | |||||||||
| 32 | Secoiridoid | 13.66 | 539.1759 | C25H32O13 | 0.543 | [M-C6H10O5-H]− | C19H21O8 | 377.1253 | 5.876 | Oleuropein | x | x |
| [M-C7H16O7-H]− | C18H15O6 | 327.0865 | 0.628 | |||||||||
| [M-C6H10O5-C4H6O-H]− | C15H15O7 | 307.0826 | 4.594 | |||||||||
| [M-C6H10O5-C4H6O-CH3OH-H]− | C14H11O6 | 275.0561 | 4.128 | |||||||||
| [M-C14H20O8-H]− | C11H11O5 | 223.0610 | 3.856 | |||||||||
| [M-C19H20O7-H]− | C6H11O6 | 179.0550 | −0.193 | |||||||||
| 33 | Secoiridoid | 14.40 | 583.2039 | C27H36O14 | 3.066 | [M-C10H12O3-H]− | C17H23O11 | 403.1261 | 6.480 | Lucidumoside C | x | x |
| [M-C10H12O3-C6H12O6-H]− | C11H11O5 | 223.0610 | 3.632 | |||||||||
| [M-C17H24O11-H]− | C10H11O3 | 179.0707 | 2.621 | |||||||||
| [M-C19H29O11-H]− | C8H7O3 | 151.0392 | 1.320 | |||||||||
| 34 | Secoiridoid | 14.83 | 523.1826 | C25H32O12 | 2.977 | [M-C6H10O5-H]− | C19H21O7 | 361.1298 | 4.626 | Ligstroside (deoxy Oleuropein) | x | |
| [M-C6H10O5-C4H6O-H]− | C15H15O6 | 291.0877 | 4.897 | |||||||||
| [M-C6H10O5-C4H6O3-H]− | C15H15O4 | 259.0979 | 5.344 | |||||||||
| 35 | Simple phenol | 15.64 | 201.1127 | C10H18O4 | 2.757 | [M-H2O-H]− | C10H15O3 | 183.1020 | 2.398 | Sebacic acid | x | |
| [M-CO2-H]− | C9H17O2 | 157.1226 | 1.996 | |||||||||
| [M-H2O-CO2-H]− | C9H15O | 139.1119 | 0.851 | |||||||||
| 36 | Flavonoid | 15.98 | 285.2074 | C15H10O6 | 4.510 | [M-C3H2O3-H]− | C12H7O3 | 199.0395 | 2.911 | Luteolin | x | x |
| [M-C3O2-C2H2O-H]− | C10H7O3 | 175.0392 | 1.425 | |||||||||
| [M-C8H6O2-H]− | C7H3O4 | 151.0029 | 2.284 | |||||||||
| [M-C7H4O4-H]− | C8H5O2 | 133.0283 | −0.646 | |||||||||
| [M-C13H6O4-H]− | C2H3O2 | 59.0126 | −3.150 | |||||||||
| 37 | Flavonoid | 16.14 | 301.0358 | C15H10O7 | 4.953 | [M-CO-H]− | C14H9O6 | 273.0406 | 4.598 | Quercetin | x | x |
| [M-C7H6O2-H]− | C8H3O5 | 178.9980 | 2.907 | |||||||||
| [M-C8H6O3-H]− | C7H3O4 | 151.0029 | 1.887 | |||||||||
| 38 | Flavonoid | 17.60 | 269.0458 | C15H10O5 | 5.055 | [M-C8H6O-H]− | C7H3O4 | 151.0027 | 0.695 | Apigenin | x | |
| [M-C7H4O2-H]− | C8H5O3 | 149.0234 | 0.802 | |||||||||
| [M-C7H4O4-H]− | C8H5O | 117.0332 | −2.233 | |||||||||
| [M-C8H6O-CO2-H]− | C6H3O2 | 107.0127 | −0.148 | |||||||||
| 39 | Flavonoid | 17.90 | 299.0562 | C16H12O6 | 4.098 | [M-CH3-H]− | C15H8O6 | 284.0329 | 4.685 | Diosmetin | x | |
| [M-C2H4-H]− | C14H7O6 | 271.0450 | 4.596 | |||||||||
| [M-CH3-CO-H]− | C14H8O5 | 256.0369 | 0.958 | |||||||||
| 40 | Secoiridoid | 18.73 | 377.1245 | C19H22O8 | 3.834 | [M-CH3OH-H2O-H]− | C18H15O6 | 327.0869 | 1.912 | Oleuropein aglycone | x | |
| [M-C4H6O-H]− | C15H15O7 | 307.0825 | 4.106 | |||||||||
| [M-C4H6O-CH3OH-H]− | C14H11O6 | 275.0571 | 7.582 | |||||||||
| [M-C11H16O5-H]− | C8H5O3 | 149.0236 | 1.674 | |||||||||
| [M-C12H14O5-H]− | C7H7O3 | 139.0391 | 0.787 | |||||||||
| [M-C13H14O7-H]− | C6H7O | 95.0491 | −0.857 | |||||||||
| 41 | Phenolic acid | 22.34 | 173.0448 | C7H10O5 | 1.850 | [M-H2O-H]− | C7H7O4 | 155.0342 | 1.772 | Shikimic acid | x | x |
| [M-CO-H]− | C6H9O4 | 145.0496 | 0.515 | |||||||||
| [M-CO2-H]− | C6H9O3 | 129.0547 | 0.77 | |||||||||
| 42 | Dicarboxylic acid | 23.71 | 285.2074 | C16H30O4 | 4.888 | [M-H2O-H]− | C16H27O3 | 267.1969 | 5.235 | Hexadecanedioic acid | x | x |
| [M-H2O-CO2-H]− | C15H27O | 223.2066 | 4.158 | |||||||||
| [M-C6H14O3-H]− | C10H15O | 151.1112 | −3.651 | |||||||||
| 43 | Sugar | 28.66 | 259.0825 | C11H16O7 | 4.982 | [M-C2H2O-H]− | C9H13O6 | 217.0701 | −2.739 | 1.5-Anhydroxylitol | x | x |
| [M-C5H6O3-H]− | C6H9O4 | 145.0494 | −0.588 | |||||||||
| [M-C6H8O4-H]− | C5H7O3 | 115.039 | 0.603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betuzzi, F.; Malaspina, P.; Polito, F.; Bottino, G.; De Feo, V.; De Martino, L.; Cornara, L. Pharmacognosy and Antioxidant Activity of Pruned Leaves from the Unexplored Olea europaea L. ‘Lavagnina’ (Liguria, Italy). Molecules 2025, 30, 3605. https://doi.org/10.3390/molecules30173605
Betuzzi F, Malaspina P, Polito F, Bottino G, De Feo V, De Martino L, Cornara L. Pharmacognosy and Antioxidant Activity of Pruned Leaves from the Unexplored Olea europaea L. ‘Lavagnina’ (Liguria, Italy). Molecules. 2025; 30(17):3605. https://doi.org/10.3390/molecules30173605
Chicago/Turabian StyleBetuzzi, Federica, Paola Malaspina, Flavio Polito, Giovanni Bottino, Vincenzo De Feo, Laura De Martino, and Laura Cornara. 2025. "Pharmacognosy and Antioxidant Activity of Pruned Leaves from the Unexplored Olea europaea L. ‘Lavagnina’ (Liguria, Italy)" Molecules 30, no. 17: 3605. https://doi.org/10.3390/molecules30173605
APA StyleBetuzzi, F., Malaspina, P., Polito, F., Bottino, G., De Feo, V., De Martino, L., & Cornara, L. (2025). Pharmacognosy and Antioxidant Activity of Pruned Leaves from the Unexplored Olea europaea L. ‘Lavagnina’ (Liguria, Italy). Molecules, 30(17), 3605. https://doi.org/10.3390/molecules30173605









