Occurrence, Properties, Applications and Analytics of Cytosine and Its Derivatives
Abstract
1. Introduction
2. Occurrence of Cytosine and Its Derivatives in Living Organisms
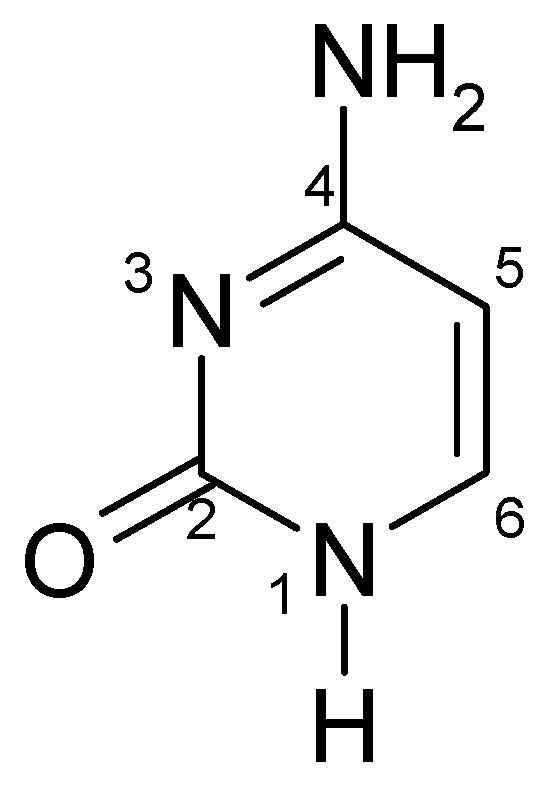
3. Chemical and Physicochemical Properties of Cytosine
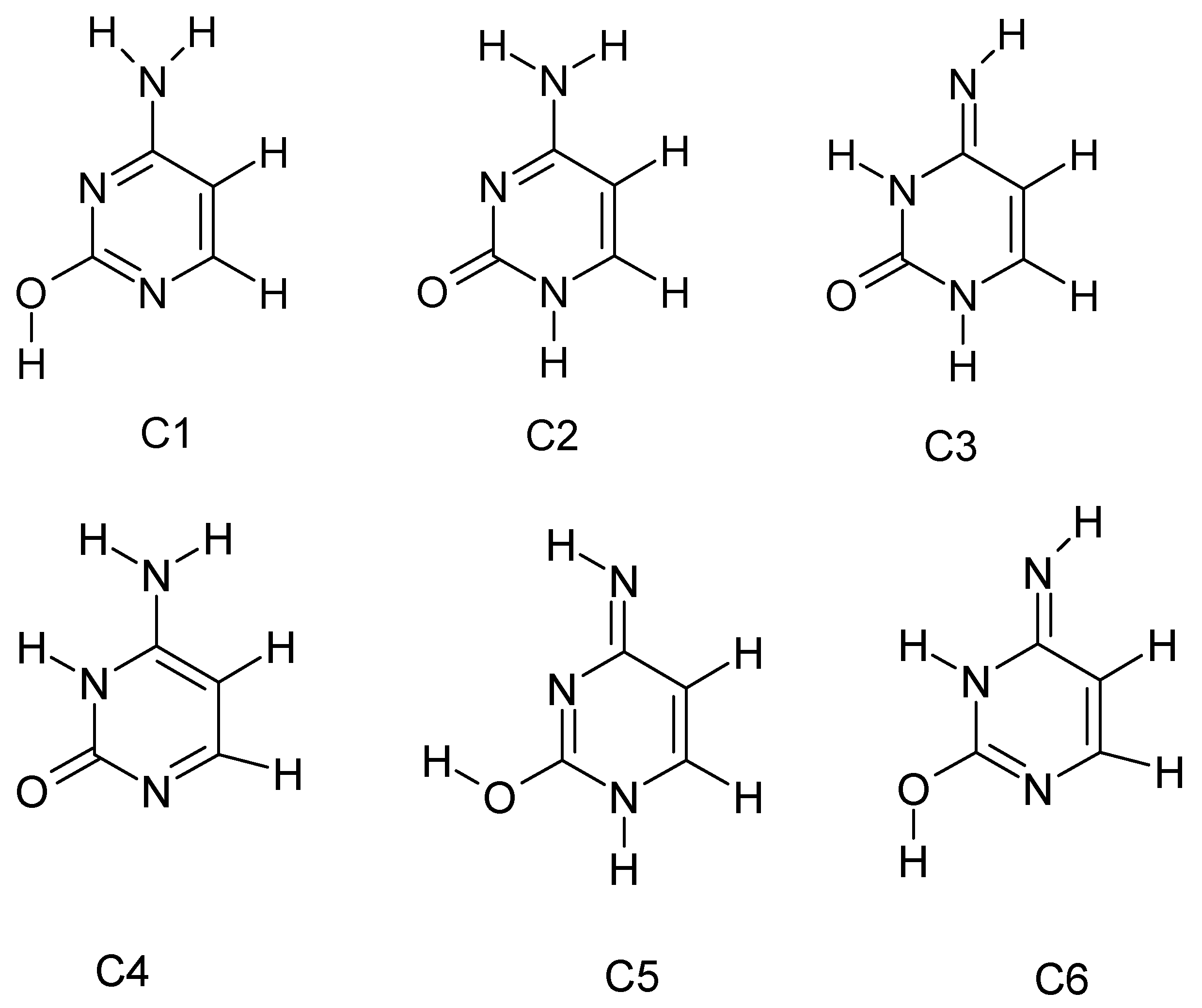
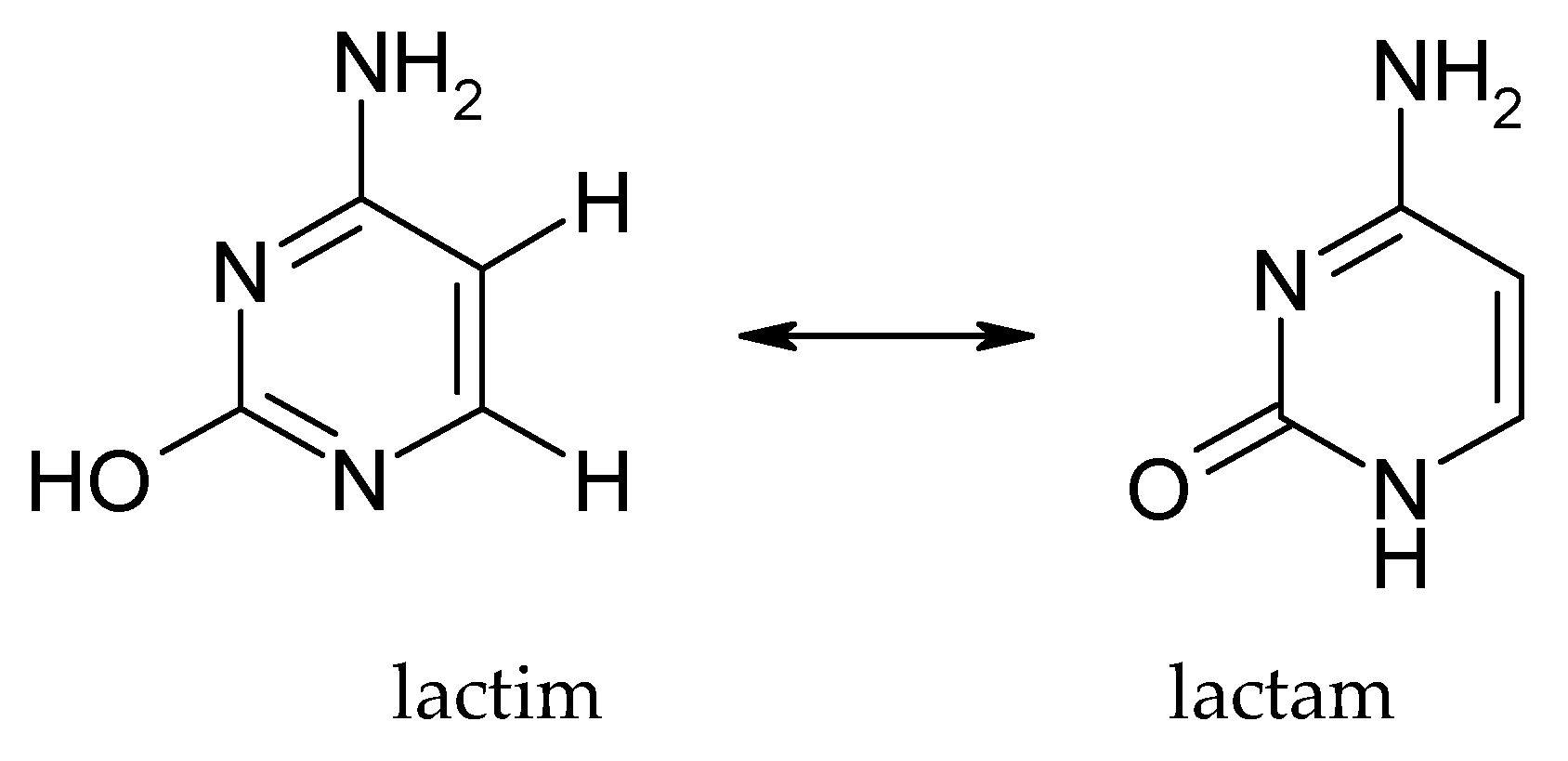
3.1. Tautomerism of Cytosine
3.2. Acid-Base Properties
3.3. Solubility and Physical Properties
3.4. Absorption Spectra and Other Properties
Properties and Applications of Selected Cytosine Derivatives
4. Biological, Medical and Industrial Applications
4.1. Biological Role of Cytosine and Its Derivatives
4.2. Medical Applications as Cytosine Drugs
- Anticancer drugs (antimetabolites): Cytarabine (Ara-C, cytosar) is 1-β-D-arabinofuranosylcytosine, a cytidine analogue containing arabinose instead of ribose [83,84]. It was introduced for therapeutic use in the 1960s (approved by the FDA in 1969), it became the primary drug for treating acute leukaemia (especially acute myeloid leukaemia). When phosphorylated to ara-CTP, cytarabine is incorporated into DNA instead of deoxycytidine and causes inhibition of DNA chain elongation and polymerase function, leading to the death of rapidly dividing cancer cells [85]. Gemcitabine (difluorodeoxycytidine, dFdC) is another cytostatic, a deoxycytidine analogue, in which the two hydrogen atoms at C2′ of the sugar have been replaced by fluorine [86,87,88]. Introduced in oncology in the 1990s (approved by the FDA in 1996 as a first-line drug for pancreatic cancer), gemcitabine has also been used in the treatment of non-small cell lung cancer, bladder cancer, breast cancer and other cancers [89,90,91]. Its mechanism involves a dual action: in the form of triphosphate, it is incorporated into DNA, causing termination of synthesis, while gemcitabine diphosphate inhibits ribonucleotide reductase, reducing the pool of deoxyribonucleotides in the cell. Other important drugs are 5-azacytosine analogues: 5-Azacytidine (AZA, Vidaza®) and 5-aza-2′-deoxycytidine (decitabine, Dacogen®). These are nucleosides in which the C5 carbon atom of pyrimidine is replaced by nitrogen (the ring becomes a triazine). They incorporate into DNA (decitabine and azacitidine) or RNA (only azacitidine) and, due to the presence of nitrogen at position 5, are not subject to methylation by DNA methyltransferases [92,93]. Furthermore, they form covalent adducts with these enzymes, leading to their degradation. As a result, these drugs cause global DNA hypomethylation and re-activation of silenced suppressor genes in cancer cells. They were approved for the treatment of myelodysplastic syndromes (MDS) and certain types of leukaemia around 2004. Although they act atypically (not so much cytotoxically as epigenetically), they improve survival of MDS patients and are being intensively studied in combination with other therapies. Other cytidine analogues are also used in chemotherapy, such as azacitidine (liposomal DepoCyt® for the treatment of CNS lymphomas) or gemcitabine (discussed above) [94,95]. New derivatives are also being developed, such as olutasydenib (FT-2102), a decitabine analogue with a modified structure, or RX-3117 (fluorocyclopentenylcytosine), a cytidine analogue active in some resistant cancers [96].
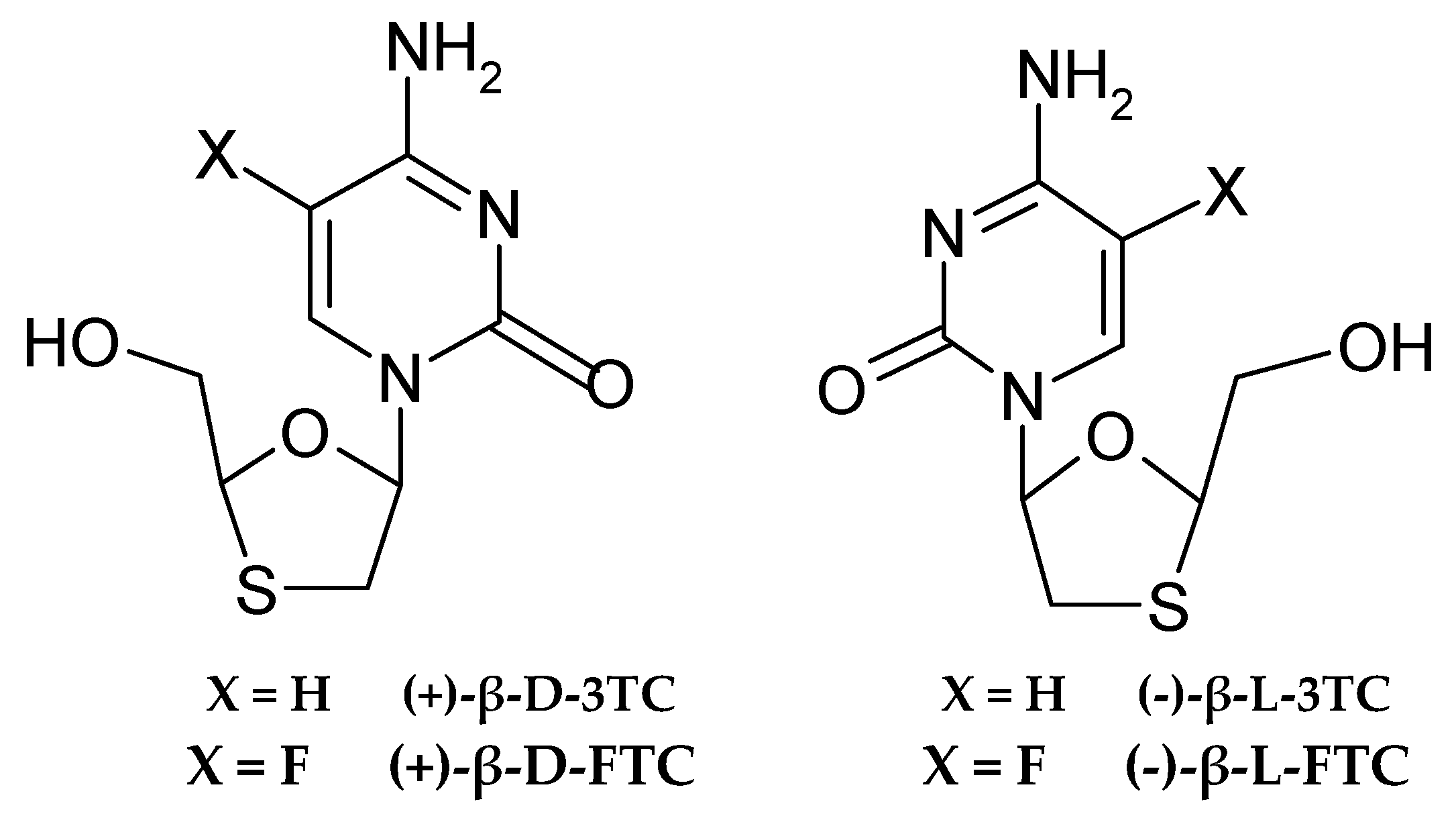
- Antiviral drugs: The structure of cytosine nucleosides provided the basis for the development of effective drugs against viruses, especially retroviruses and hepatitis B virus. Lamivudine (3TC) is a 2′,3′-dideoxy-3′-thiazolidinedione analogue of deoxycytidine, in which the ribose ring was replaced by a ring with a sulphur atom (thiazolidine), and the absence of 3′-OH groups prevents DNA chain elongation [97]. Lamivudine was approved for the treatment of HIV-1 infections in 1995 and HBV in 1998, and has become a widely used component of antiretroviral therapy (it belongs to the NRTI class of nucleoside reverse transcriptase inhibitors) [98,99]. It acts as a terminator of viral DNA synthesis after incorporation by reverse transcriptase, while competitively inhibiting the enzyme itself. Emtricitabine (FTC), a fluorinated analogue of lamivudine (Figure 4), also used in the treatment of HIV—human immunodeficiency virus (often in composite formulations), has a similar effect.
- In the context of new threats, the pro-drug molnupiravir (EIDD-2801) was emergency approved in 2021 for use against SARS-CoV-2 [102,103]. It is a modified ribonucleoside derivative of cytosine (N4-hydroxycytidine in the form of a pro-drug) which, when activated to triphosphate, incorporates into the virus RNA, causing lethal mutations in its genome. This is an example of the use of a cytosine derivative to induce viral replication errors. In the treatment of DNA viruses (such as HSV—herpes simplex virus), cytosine derivatives play a lesser role, and guanine analogues (acyclovir) are better known [104,105]. Nevertheless, work on cytidine analogues with activity against RNA and DNA viruses continues.
- Antifungal drugs: The only commonly used antifungal antimetabolite is the aforementioned 5-fluorocytosine (flucytosine, 5-FC) [106,107]. Introduced for the treatment of fungal infections in the 1970s, it is still used today (mainly in combination with amphotericin B) to treat cryptococcal meningitis and other severe fungemia [108,109]. 5-FC has no direct toxic effect on mammalian cells, as they are unable to metabolise it. In fungal cells, however, cytosine deaminase (enzyme) converts 5-FC to 5-fluorouracil (5-FU). 5-FU, in turn, is incorporated into RNA instead of uracil and inhibits thymidylate synthase (after conversion to 5-F-dUMP), which disrupts DNA synthesis. This results in a fungistatic (in higher doses fungicidal) effect of 5-FC. This drug is a valuable addition to therapy, although due to the rapid development of resistance (deaminase or pyrimidine permease mutations), it is mainly used in polytherapy. Flucytosine is an example of how a minor change in the molecule (a fluorine atom instead of hydrogen in the cytosine ring) adds a completely new pharmacological use to a compound.
- Other pharmacological uses: In addition to the examples discussed above, it is worth mentioning that cytidine and its phosphates are present in dietary supplements and products designed to enhance brain function [110]. The aforementioned citicoline (CDP-choline, which is cytidine diphosphate coupled with choline) is available as a preparation that improves cognitive function and accelerate neuronal regeneration after strokes. Its mechanism of action involves increasing the availability of cytidine (or rather uridine, which is produced from cytidine) and choline, precursors of the synthesis of important brain phospholipids. Another example is carmofur, a 5-FU derivative coupled with a carbamate residue, which also exhibits anticancer activity (although it is not a pure analogue of cytosine, but rather its metabolite) [111]. An interesting fact is the use of cytosine analogues in biotechnology, e.g., for DNA labelling. The 5-bromocytosine can be incorporated in place of cytosine and then used for specific DNA cleavage with UV light (photochemically). In medical diagnostics, the 5mC methylation profile of a patient’s genome (the so-called methylome map) is analysed using chemical conversion of cytosine to uracil by the so-called bisulfide reaction. The reagent is sodium metabisulfite, which selectively deaminates cytosine to uracil, while 5-methylcytosine remains unaffected. This technique allows the methylation pattern to be read after DNA sequencing and is routinely used in epigenetics.
4.3. Industrial and Technological Applications
5. Analytics of Cytosine and Its Derivatives
5.1. High-Performance Liquid Chromatography (HPLC)
- Columns and stationary phases: The most commonly used are strongly hydrophobic C18-type (reversed-phase) columns with high inertness, allowing the separation of polar nucleosides in the water-organic solvent system [118,119]. However, separation of very similar cytosine analogues (e.g., 5 different epigenetic derivatives: C, 5mC, 5hmC, 5fC, 5caC) can sometimes be challenging. In such cases, modification of the stationary phase can improve selectivity. For example, using a phenyl-hexyl column instead of C18 increased the differences in cytosine vs. 5-hydroxymethylcytosine retention due to π-π interactions. This allowed for the separation of all five cytosine analogues associated with DNA demethylation, which was not achieved on the classical C18 column [120]. In the analysis of highly polar derivatives, such as phosphate nucleotides, an ion-pairing reagent (e.g., tetrabutylammonium iodide) is often added to the mobile phase, or ion-exchange or HILIC (hydrophilic interaction chromatography) columns are used [121]. These approaches increase the retention of ionic or highly polar compounds on the column.
- Mobile phases: Buffered aqueous phases (e.g., ammonium or potassium phosphate buffer solutions with pH in the range of 4–7) combined with an organic solvent (methanol or acetonitrile) are typically used for the separation of cytosolic bases and nucleosides. The pH of the mobile phase is critical because it affects the degree of ionisation of analytes and interactions with the stationary phase [120,122]. Studies have shown that changes in pH induce the largest changes in the retention of cytosine and cytidine. A slightly acidic pH is usually maintained (approximately 4–6) to ensure that cytosine (pKa~4.5) is present in an ionised or partially ionised form, which reduces its retention and improves the shape of peaks. Elution gradients are often used in the analysis of mixtures of derivatives with significantly different polarities: for example, starting with a high proportion of water (or buffer) for the retention of polar nucleosides, and then gradually increasing the proportion of organic solvent to elute more non-polar analogues. This method was used to separate a mixture of cytosine and its modified derivatives, starting with 1% methanol in water with a buffer and reaching 30% methanol in several minutes, achieving excellent separation of all five analytes in <12 min [120].
- Detectors and sensitivity: Cytosine derivatives have an aromatic system, so they strongly absorb UV light in the 260–280 nm range. The most common detection technique is therefore UV detection (often using a DAD, i.e., diode array detector), set, e.g., at 270–280 nm for cytosine nucleosides. UV detection usually allows for the detection of nanomolar amounts of analyte [123,124,125]. For example, at a wavelength of 271 nm, the detection limit for cytidine in pharmaceutical analysis was about 0.15 ng and the limit of quantification was ~0.5 ng [126]. The sensitivity of HPLC–UV can be improved by increasing the injection volume (up to several dozen μL, if tolerated by the column) and by using sample extraction (analyte concentration) from the matrix. For applications requiring ultra-high sensitivity or selectivity (e.g., determination of trace modifications of bases in cellular DNA), mass spectrometry coupled with HPLC (LC-MS/MS) is used. The HPLC-ESI-MS/MS technique with selected reaction monitoring (MRM) enabled the simultaneous quantification of 5-methyl- and 5-hydroxy-2′-deoxycytidine alongside unaltered deoxycytidine with a detection limit of 0.5 femtomoles (corresponding to the analysis of 50 ng of hydrolysed genomic DNA, enabling the detection of 0.1% 5hmC content) [127]. Such high sensitivity allows for precise profiling of global DNA methylation in biological samples.
5.2. Chromatography and Electrophoresis for DNA/RNA Studies
5.3. Other Analytical Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obara, P.; Wolski, P.; Pańczyk, T. Insights into the Molecular Structure, Stability, and Biological Significance of Non-Canonical DNA Forms, with a Focus on G-Quadruplexes and i-Motifs. Molecules 2024, 29, 4683. [Google Scholar] [CrossRef]
- Wang, F.; Li, P.; Chu, H.C.; Lo, P.K. Nucleic Acids and Their Analogues for Biomedical Applications. Biosensors 2022, 12, 93. [Google Scholar] [CrossRef]
- Kossel, A.; Neumann, A. Über das Cytosin, eine neue Base aus dem Thymusnuklein. Z. Physiol. Chem. 1894, 18, 181–186. [Google Scholar]
- El-Atawy, M.A.; Alshaye, N.A.; Elrubi, N.; Hamed, E.A.; Omar, A.Z. Pyrimidines-Based Heterocyclic Compounds: Synthesis, Cytoxicity Evaluation and Molecular Docking. Molecules 2022, 27, 4912. [Google Scholar] [CrossRef] [PubMed]
- Mohi El-Deen, E.M.; Anwar, M.M.; El-Gwaad, A.A.A.; Karam, E.A.; El-Ashrey, M.K.; Kassab, R.R. Novel Pyridothienopyrimidine Derivatives: Design, Synthesis and Biological Evaluation as Antimicrobial and Anticancer Agents. Molecules 2022, 27, 803. [Google Scholar] [CrossRef]
- Sečnik, A.; Štajner, N.; Radišek, S.; Kunej, U.; Križman, M.; Jakše, J. Cytosine Methylation in Genomic DNA and Characterization of DNA Methylases and Demethylases and Their Expression Profiles in Viroid-Infected Hop Plants (Humulus lupulus Var. ‘Celeia’). Cells 2022, 11, 2592. [Google Scholar] [CrossRef] [PubMed]
- Alom, K.M.; Tukova, A.; Lyu, N.; Rodger, A.; Wang, Y. Label-Free Surface-Enhanced Raman Scattering for Genomic DNA Cytosine Methylation Reading. Molecules 2025, 30, 403. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lastra, J.M.P.D.L.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. [Google Scholar] [CrossRef]
- Békési, A.; Holub, E.; Pálinkás, H.L.; Vértessy, B.G. Detection of Genomic Uracil Patterns. Int. J. Mol. Sci. 2021, 22, 3902. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Gao, R.; Chen, Z. 5-Methylcytosine Methylation-Linked Hippo Pathway Molecular Interactions Regulate Lipid Metabolism. Int. J. Mol. Sci. 2025, 26, 2560. [Google Scholar] [CrossRef]
- Hanson, H.E.; Liebl, A.L. The Mutagenic Consequences of DNA Methylation within and across Generations. Epigenomes 2022, 6, 33. [Google Scholar] [CrossRef]
- Nikulina, A.N.; Nikulin, N.A.; Suzina, N.E.; Zimin, A.A. Treatment of E. coli Infections with T4-Related Bacteriophages Belonging to Class Caudoviricetes: Selecting Phage on the Basis of Their Generalized Transduction Capability. Viruses 2025, 17, 701. [Google Scholar] [CrossRef]
- Urbonavičius, J.; Čekytė, A.; Tauraitė, D. N4-Methylcytosine Supports the Growth of Escherichia coli Uracil Auxotrophs. Int. J. Mol. Sci. 2025, 26, 1812. [Google Scholar] [CrossRef]
- Halder, R.K.; Uddin, M.N.; Uddin, M.A.; Aryal, S.; Islam, M.A.; Hossain, F.; Jahan, N.; Khraisat, A.; Alazab, A. A Grid Search-Based Multilayer Dynamic Ensemble System to Identify DNA N4—Methylcytosine Using Deep Learning Approach. Genes 2023, 14, 582. [Google Scholar] [CrossRef]
- Bernaola-Galván, P.; Carpena, P.; Gómez-Martín, C.; Oliver, J.L. Compositional Structure of the Genome: A Review. Biology 2023, 12, 849. [Google Scholar] [CrossRef]
- Foerstner, K.U.; Mering, C.; Hooper, S.D.; Bork, P. Environments shape the nucleotide composition of genomes. Sci. Rep. 2005, 6, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, A.; Pelleri, M.C.; Antonaros, F.; Strippoli, P.; Caracausi, M.; Vitale, L. On the length, weight and GC content of the human genome. BMC Res. Notes 2019, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, J.; Liu, B.; Xu, J.; Cai, B.; Yang, H.; Straube, J.; Yu, X.; Ma, T. Biological roles of RNA m5C modification and its implications in Cancer immunotherapy. Biomark. Res. 2022, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, S.; Tsukahara, T. C-to-U RNA Editing: A Site Directed RNA Editing Tool for Restoration of Genetic Code. Genes 2022, 13, 1636. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Wang, T.; He, S. Pyrimidine Biosynthetic Enzyme CAD: Its Function, Regulation, and Diagnostic Potential. Int. J. Mol. Sci. 2021, 22, 10253. [Google Scholar] [CrossRef]
- Gorgoglione, R.; Impedovo, V.; Riley, C.L.; Fratantonio, D.; Tiziani, S.; Palmieri, L.; Dolce, V.; Fiermonte, G. Glutamine-Derived Aspartate Biosynthesis in Cancer Cells: Role of Mitochondrial Transporters and New Therapeutic Perspectives. Cancers 2022, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Bayram, S.; Fürst, S.; Forbes, M.; Kempa, S. Analysing central metabolism in ultra-high resolution: At the crossroads of carbon and nitrogen. Mol. Metab. 2019, 33, 38–47. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, R.; Krishnamurthy, R. Chemistry of Abiotic Nucleotide Synthesis. Chem. Rev. 2020, 120, 4766–4805. [Google Scholar] [CrossRef]
- Kornienko, I.V.; Aramova, O.Y.; Tishchenko, A.A.; Rudoy, D.V.; Chikindas, M.L. RNA Stability: A Review of the Role of Structural Features and Environmental Conditions. Molecules 2024, 29, 5978. [Google Scholar] [CrossRef]
- Nicu, A.-T.; Ionel, I.P.; Stoica, I.; Burlibasa, L.; Jinga, V. Recent Advancements in Research on DNA Methylation and Testicular Germ Cell Tumors: Unveiling the Intricate Relationship. Biomedicines 2024, 12, 1041. [Google Scholar] [CrossRef]
- Podolyan, Y.; Gorb, L.; Leszczynski, J. Ab Initio Study of the Prototropic Tautomerism of Cytosine and Guanine and Their Contribution to Spontaneous Point Mutations. Int. J. Mol. Sci. 2003, 4, 410–421. [Google Scholar] [CrossRef]
- Stoyanova, N.; Enchev, V. Tautomerism of cytosine, cytidine, and deoxycytidine: Proton transfer through water bridges. Int. J. Quantum Chem. 2022, 18, e26958. [Google Scholar] [CrossRef]
- Bazsó, G.; Tarczay, G.; Fogarasi, G.; Szalay, P. Tautomers of cytosine and their excited electronic states: A matrix isolation spectroscopic and quantum chemical study. Phys. Chem. Chem. Phys. 2011, 13, 6799–6807. [Google Scholar] [CrossRef]
- Mizerski, W. Tablice Chemiczne, 7th ed.; Adamantan: Warszawa, Poland, 2025; pp. 307–308. (In Polish) [Google Scholar]
- Raczyńska, E.D. On Prototropy and Bond Length Alternation in Neutral and Ionized Pyrimidine Bases and Their Model Azines in Vacuo. Molecules 2023, 28, 7282. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.; Marsden, C.G.; Rigg, B. Ionization of cysteine in aqueous solutions. Part 2.—Specific-ionization constants. Trans. Faraday Soc. 1969, 65, 3032. [Google Scholar] [CrossRef]
- Saenger, W. Physical Properties of Nucleotides: Charge Densities, pK Values, Spectra, and Tautomerism. In Principles of Nucleic Acid Structure; Springer Advanced Texts in Chemistry; Springer: New York, NY, USA, 1984; pp. 105–115. [Google Scholar] [CrossRef]
- Durant, C.; Strege, M. A Hydrophilic Interaction Chromatography Method for the Purity Analysis of Cytosine. LCGC N. Am. 2008, 26, 632–642. [Google Scholar]
- Mohajeri, A.; Nobandegani, F.F. Detection and evaluation of hydrogen bond strength in nucleic acid base pairs. J. Phys. Chem. A 2008, 112, 281–295. [Google Scholar] [CrossRef]
- Aschi, M.; Toto Brocchi, G.; Portalone, G. A Combined Experimental and Computational Study of Halogen and Hydrogen Bonding in Molecular Salts of 5-Bromocytosine. Molecules 2021, 26, 3111. [Google Scholar] [CrossRef]
- Martel, P.; Powell, B.M. Dehydration of cytosine monohydrate at physiological temperatures. Biophys. J. 1983, 41, 91–93. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; De Munno, G. Cytosine and Its Derivatives as Useful Tools to Build Unusual and Fascinating Architectures. In Modern Avenues in Metal-Nucleic Acid Chemistry, 1st ed.; Müller, B., Lipper, B., Eds.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Yousif, H.N.; Jabbar, S.A.; Al-zahawi, H.M.G. Synthesis and identification of new cytosine-derived compounds and study their binding with DNA. Mater. Today Proc. 2022, 49, 2666–2670. [Google Scholar] [CrossRef]
- Rausch, C.; Zhang, P.; Casas-Delucchi, C.S.; Daiß, J.L.; Engel, C.; Coster, G.; Hastert, F.D.; Weber, P.; Cardoso, M.C. Cytosine base modifications regulate DNA duplex stability and metabolism. Nucleic Acids Res. 2021, 49, 12870–12894. [Google Scholar] [CrossRef]
- Islam, S.M.; Ibnat, Z. New Insights into the Structure and Reactivity of Uracil Derivatives in Different Solvents—A Computational Study. ACS Omega 2020, 5, 22449–22458. [Google Scholar] [CrossRef]
- Braun, D.E.; Kahlenberg, V.; Griesser, U.J. Experimental and Computational Hydrate Screening: Cytosine, 5-Flucytosine and Their Solid Solution. Cryst. Growth Des. 2017, 17, 4347–4364. [Google Scholar] [CrossRef]
- Sigera, L.S.; Denning, D.W. Flucytosine and its clinical usage. Ther. Adv. Infect. Dis. 2023, 10. [Google Scholar] [CrossRef]
- Lewis, R.A.; Larrañaga, M.D.; Lewis, R.J. Hawley’s Condensed Chemical Dictionary, 16th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 688. [Google Scholar]
- Elangovan, N.; Arumugam, N.; Almansour, A.I.; Djearamane, S.; Wong, L.S.; Kayarohanam, S. Synthesis, solvent role, absorption and emission studies of cytosine derivative. Heliyon 2024, 10, e28623. [Google Scholar] [CrossRef]
- Stimson, M.M.; Reuter, M.A. The ultraviolet absorption spectra of cytosine and isocytosine. J. Am. Chem. Soc. 1945, 67, 2191–2193. [Google Scholar] [CrossRef]
- ChemicalBook. Available online: https://www.chemicalbook.com/ (accessed on 29 June 2025).
- National Institute of Standards and Technology. NIST—Advancing Measurement Science, Standards, and Technology. Available online: https://www.nist.gov/ (accessed on 29 June 2025).
- National Center for Biotechnology Information. PubChem Compound Summary. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 June 2025).
- Delma, F.Z.; Al-Hatmi, A.M.S.; Brüggemann, R.J.M.; Melchers, W.J.G.; de Hoog, S.; Verweij, P.E.; Buil, J.B. Molecular Mechanisms of 5-Fluorocytosine Resistance in Yeasts and Filamentous Fungi. J. Fungi 2021, 7, 909. [Google Scholar] [CrossRef]
- Bruch, A.; Lazarova, V.; Berg, M.; Krüger, T.; Schäuble, S.; Kelani, A.A.; Mertens, B.; Lehenberger, P.; Kniemeyer, O.; Kaiser, S.; et al. tRNA hypomodification facilitates 5-fluorocytosine resistance via cross-pathway control system activation in Aspergillus fumigatus. Nucleic Acids Res. 2025, 53, gkae1205. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues: Mechanisms of drug resistance and reversal strategies. Leukemia 2001, 15, 875–890. [Google Scholar] [CrossRef]
- Tsesmetzis, N.; Paulin, C.B.J.; Rudd, S.G.; Herold, N. Nucleobase and Nucleoside Analogues: Resistance and Re-Sensitisation at the Level of Pharmacokinetics, Pharmacodynamics and Metabolism. Cancers 2018, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Batasheva, S.; Fakhrullin, R. Sequence Does Not Matter: The Biomedical Applications of DNA-Based Coatings and Cores. Int. J. Mol. Sci. 2021, 22, 12884. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Muhiuddin, G.; Al-Balawi, D.A.; Samanta, S. Different DNA Sequencing Using DNA Graphs: A Study. Appl. Sci. 2022, 12, 5414. [Google Scholar] [CrossRef]
- Nie, P.; Bai, Y.; Mei, H. Synthetic Life with Alternative Nucleic Acids as Genetic Materials. Molecules 2020, 25, 3483. [Google Scholar] [CrossRef] [PubMed]
- Hajnic, M.; Alonso-Gil, S.; Polyansky, A.A.; de Ruiter, A.; Zagrovic, B. Interaction preferences between protein side chains and key epigenetic modifications 5-methylcytosine, 5-hydroxymethycytosine and N6-methyladenine. Sci. Rep. 2022, 12, 19583. [Google Scholar] [CrossRef]
- Lv, S.; Pan, Y.; Zheng, T.; Cao, Q.; Yu, B.; Zhou, F.; Wang, D. The Role of Methylation Modification in Neural Injury and Repair. Int. J. Mol. Sci. 2025, 26, 5349. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. TET enzymatic oxidation of 5-methylcytosine, 5-hydroxymethylcytosine and 5-formylcytosine. Mutat. Res. 2016, 764–765, 18–35. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Bao, D.; Chen, H.; Gong, M.; Sun, S.; Zou, G. Cross-Kingdom DNA Methylation Dynamics: Comparative Mechanisms of 5mC/6mA Regulation and Their Implications in Epigenetic Disorders. Biology 2025, 14, 461. [Google Scholar] [CrossRef]
- Nishiki, H.; Ura, H.; Togi, S.; Hatanaka, H.; Fujita, H.; Takamura, H.; Niida, Y. Integrated Analysis of Somatic DNA Variants and DNA Methylation of Tumor Suppressor Genes in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 1642. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H.; Jelinek, J.; Issa, J.-P.J. DNA Methylation, Aging, and Cancer. Epigenomes 2025, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.M.-K.; Yu, J. Promoter Hypermethylation of Tumour Suppressor Genes as Potential Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2015, 16, 2472–2496. [Google Scholar] [CrossRef]
- Meng, H.; Chen, G.; Gao, H.-M.; Song, X.; Shi, Y.; Cao, L. The Emerging Nexus of Active DNA Demethylation and Mitochondrial Oxidative Metabolism in Post-Mitotic Neurons. Int. J. Mol. Sci. 2014, 15, 22604–22625. [Google Scholar] [CrossRef]
- Zheng, K.; Lyu, Z.; Chen, J.; Chen, G. 5-Hydroxymethylcytosine: Far Beyond the Intermediate of DNA Demethylation. Int. J. Mol. Sci. 2024, 25, 11780. [Google Scholar] [CrossRef]
- Klungland, A.; Robertson, A.B. Oxidized C5-methyl cytosine bases in DNA: 5-Hydroxymethylcytosine; 5-formylcytosine; and 5-carboxycytosine. Free Radic. Biol. Med. 2017, 107, 62–68. [Google Scholar] [CrossRef]
- Stoyanova, E.; Riad, M.; Rao, A.; Heintz, N. 5-Hydroxymethylcytosine-mediated active demethylation is required for mammalian neuronal differentiation and function. Elife 2021, 10, e66973. [Google Scholar] [CrossRef]
- Besaratinia, A.; Caceres, A.; Tommasi, S. DNA Hydroxymethylation in Smoking-Associated Cancers. Int. J. Mol. Sci. 2022, 23, 2657. [Google Scholar] [CrossRef]
- Alam, W.; Tayara, H.; Chong, K.T. i4mC-Deep: An Intelligent Predictor of N4-Methylcytosine Sites Using a Deep Learning Approach with Chemical Properties. Genes 2021, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Väre, V.Y.P.; Eruysal, E.R.; Narendran, A.; Sarachan, K.L.; Agris, P.F. Chemical and Conformational Diversity of Modified Nucleosides Affects tRNA Structure and Function. Biomolecules 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, J. RNA 5-methylcytosine modification and its emerging role as an epitranscriptomic mark. RNA Biol. 2021, 18, 117–127. [Google Scholar] [CrossRef]
- Vávra, J.; Sergunin, A.; Farná, A.; Ovad, T.; Shimizu, T.; Martínková, M. Is ATP the Only Nucleoside Triphosphate among ATP, CTP, GTP, and UTP to Have a Role in Kinase Catalysis of Heme-Regulated Inhibitor toward eIF2α during Lung Cancer Development? Catalysts 2023, 13, 281. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Q.; Liu, H.; Zhou, X.; Zhang, Y.; Cai, M. Engineering substrate and energy metabolism for living cell production of cytidine-5′-diphosphocholine. Biotechnol. Bioeng. 2020, 117, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Hatcher, J.F.; Dempsey, R.J. Cytidine-5-diphosphocholine (CDP-choline) affects CTP: Phosphocholine cytidylyltransferase and lyso-phosphatidylcholine after transient brain ischemia. J. Neurosci. Res. 2004, 76, 390–396. [Google Scholar] [CrossRef]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP-Choline Cycle. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2012, 1832, 523–532. [Google Scholar] [CrossRef]
- Secades, J.J.; Trimmel, H.; Salazar, B.; González, J.A. Citicoline for the Management of Patients with Traumatic Brain Injury in the Acute Phase: A Systematic Review and Meta-Analysis. Life 2023, 13, 369. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2012, 38, 23–38. [Google Scholar] [CrossRef]
- Hangan, A.C.; Oprean, L.S.; Dican, L.; Procopciuc, L.M.; Sevastre, B.; Lucaciu, R.L. Metal-Based Drug–DNA Interactions and Analytical Determination Methods. Molecules 2024, 29, 4361. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, S.M.A.; Tabassum, R.; Munia, N.S.; Mali, S.N.; Lai, C.; Ferdous, J.; Ali, F. Potential antimicrobial properties of cytosine β-D-riboside derivatives through molecular dynamics and molecular docking exploration with bacterial and fungal proteins. Asp. Mol. Med. 2025, 5, 100077. [Google Scholar] [CrossRef]
- Matsuda, A.; Sasaki, T. Antitumor activity of sugar-modified cytosine nucleosides. Cancer Sci. 2004, 95, 105–111. [Google Scholar] [CrossRef]
- Gu, L.; Hickey, R.J.; Malkas, L.H. Therapeutic Targeting of DNA Replication Stress in Cancer. Genes 2023, 14, 1346. [Google Scholar] [CrossRef]
- Camcioglu, S.; Özyurt, B.; Oturan, N.; Trellu, C.; Oturan, M.A. Fast and Complete Destruction of the Anti-Cancer Drug Cytarabine from Water by Electrocatalytic Oxidation Using Electro-Fenton Process. Catalysts 2022, 12, 1598. [Google Scholar] [CrossRef]
- Serhan, N.; Mouchel, P.-L.; de Medina, P.; Segala, G.; Mougel, A.; Saland, E.; Rives, A.; Lamaziere, A.; Despres, G.; Sarry, J.-E.; et al. Dendrogenin A Synergizes with Cytarabine to Kill Acute Myeloid Leukemia Cells In Vitro and In Vivo. Cancers 2020, 12, 1725. [Google Scholar] [CrossRef]
- Rupavarshini, M.; Karthikeyan, S.; Anandh, S.; Ramamoorthi, A.; Ramakrishnamurthy, S.; Bharanidharan, G.; Aruna, P.; Mangaiyarkarasi, R.; Chinnathambi, S.; Pandian, G.N.; et al. A biophysical approach of cytarabine anticancer drug insights into human serum albumin and checkpoint kinase 1. Results Chem. 2023, 5, 100755. [Google Scholar] [CrossRef]
- Di Francia, R.; Crisci, S.; De Monaco, A.; Cafiero, C.; Re, A.; Iaccarino, G.; De Filippi, R.; Frigeri, F.; Corazzelli, G.; Micera, A.; et al. Response and Toxicity to Cytarabine Therapy in Leukemia and Lymphoma: From Dose Puzzle to Pharmacogenomic Biomarkers. Cancers 2021, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.; Hao, K. A Pharmacokinetic and Pharmacodynamic Evaluation of the Anti-Hepatocellular Carcinoma Compound 4-N-Carbobenzoxy-gemcitabine (Cbz-dFdC). Molecules 2020, 25, 2218. [Google Scholar] [CrossRef]
- Hawryłkiewicz, A.; Ptaszyńska, N. Gemcitabine Peptide-Based Conjugates and Their Application in Targeted Tumor Therapy. Molecules 2021, 26, 364. [Google Scholar] [CrossRef]
- Ciccolini, J.; Serdjebi, C.; Peters, G.J.; Giovannetti, E. Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: An EORTC-PAMM perspective. Cancer Chemother. Pharmacol. 2016, 78, 1–12. [Google Scholar] [CrossRef]
- Samanta, K.; Setua, S.; Kumari, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Gemcitabine Combination Nano Therapies for Pancreatic Cancer. Pharmaceutics 2019, 11, 574. [Google Scholar] [CrossRef]
- Pandit, B.; Royzen, M. Recent Development of Prodrugs of Gemcitabine. Genes 2022, 13, 466. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, P.; Liu, Z.; Xing, Y.; Xiao, Y. NXPH4 Promotes Gemcitabine Resistance in Bladder Cancer by Enhancing Reactive Oxygen Species and Glycolysis Activation through Modulating NDUFA4L2. Cancers 2022, 14, 3782. [Google Scholar] [CrossRef] [PubMed]
- Momparler, R.L. A Perspective on the Comparative Antileukemic Activity of 5-Aza-2′-deoxycytidine (Decitabine) and 5-Azacytidine (Vidaza). Pharmaceuticals 2012, 5, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Petralia, M.C.; Mazzon, E.; Basile, M.S.; Cutuli, M.; Di Marco, R.; Scandurra, F.; Saraceno, A.; Fagone, P.; Nicoletti, F.; Mangano, K. Effects of Treatment with the Hypomethylating Agent 5-aza-2′-deoxycytidine in Murine Type II Collagen-Induced Arthritis. Pharmaceuticals 2019, 12, 174. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Mileski, K.S.; Pezzani, R.; Redaelli, M.; Cho, W.C.; Kobarfard, F.; Rajabi, S.; Martorell, M.; Kumar, P.; et al. Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine. Biomolecules 2019, 9, 773. [Google Scholar] [CrossRef]
- Etrych, T.; Braunova, A.; Zogala, D.; Lambert, L.; Renesova, N.; Klener, P. Targeted Drug Delivery and Theranostic Strategies in Malignant Lymphomas. Cancers 2022, 14, 626. [Google Scholar] [CrossRef]
- Sarkisjan, D.; Julsing, J.R.; Smid, K.; de Klerk, D.; van Kuilenburg, A.B.P.; Meinsma, R.; Lee, Y.B.; Kim, D.; Peters, G.J. The Cytidine Analog Fluorocyclopentenylcy-tosine (RX-3117) Is Activated by Uridine-Cytidine Kinase 2. PLoS ONE 2016, 11, e0162901. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kim, N.D.; Seong, B.-L. Discovery and Development of Anti-HBV Agents and Their Resistance. Molecules 2010, 15, 5878–5908. [Google Scholar] [CrossRef]
- Sever, B.; Otsuka, M.; Fujita, M.; Ciftci, H. A Review of FDA-Approved Anti-HIV-1 Drugs, Anti-Gag Compounds, and Potential Strategies for HIV-1 Eradication. Int. J. Mol. Sci. 2024, 25, 3659. [Google Scholar] [CrossRef]
- Mimtsoudis, I.; Tsachouridou, O.; Akinosoglou, K.; Metallidis, S. Treatment Management Challenges in Naïve and Experienced HIV-1-Infected Individuals Carrying the M184V Mutation. Viruses 2024, 16, 1392. [Google Scholar] [CrossRef]
- Andrei, G.; Snoeck, R. Cidofovir Activity against Poxvirus Infections. Viruses 2010, 2, 2803–2830. [Google Scholar] [CrossRef] [PubMed]
- Waye, M.M.Y.; Sing, C.W. Anti-Viral Drugs for Human Adenoviruses. Pharmaceuticals 2010, 3, 3343–3354. [Google Scholar] [CrossRef]
- Teli, D.; Balar, P.; Patel, K.; Sharma, A.; Chavda, V.; Vora, L. Molnupiravir: A Versatile Prodrug against SARS-CoV-2 Variants. Metabolites 2023, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Bakos, É.; Temesszentandrási-Ambrus, C.; Özvegy-Laczka, C.; Gáborik, Z.; Sarkadi, B.; Telbisz, Á. Interactions of the Anti-SARS-CoV-2 Agents Molnupiravir and Nirmatrelvir/Paxlovid with Human Drug Transporters. Int. J. Mol. Sci. 2023, 24, 11237. [Google Scholar] [CrossRef]
- Maple, P.A.C.; Hosseini, A.A. Human Alpha Herpesviruses Infections (HSV1, HSV2, and VZV), Alzheimer’s Disease, and the Potential Benefits of Targeted Treatment or Vaccination—A Virological Perspective. Vaccines 2025, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef]
- Lenarczyk, E.; Oleksiak, D.; Janeczko, M. Antifungal Activity of 5-Fluorouridine Against Candida albicans and Candida parapsilosis Based on Virulence Reduction. Molecules 2025, 30, 2735. [Google Scholar] [CrossRef]
- Hetta, H.F.; Melhem, T.; Aljohani, H.M.; Salama, A.; Ahmed, R.; Elfadil, H.; Alanazi, F.E.; Ramadan, Y.N.; Battah, B.; Rottura, M.; et al. Beyond Conventional Antifungals: Combating Resistance Through Novel Therapeutic Pathways. Pharmaceuticals 2025, 18, 364. [Google Scholar] [CrossRef]
- Spadari, C.d.C.; Wirth, F.; Lopes, L.B.; Ishida, K. New Approaches for Cryptococcosis Treatment. Microorganisms 2020, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Melhem, M.S.C.; Leite Júnior, D.P.; Takahashi, J.P.F.; Macioni, M.B.; Oliveira, L.d.; de Araújo, L.S.; Fava, W.S.; Bonfietti, L.X.; Paniago, A.M.M.; Venturini, J.; et al. Antifungal Resistance in Cryptococcal Infections. Pathogens 2024, 13, 128. [Google Scholar] [CrossRef]
- Grieb, B. Neuroprotective Properties of Citicoline: Facts, Doubts and Unresolved Issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef]
- Lyu, W.; Zhang, Y.; Zhang, Z.; Lu, H. Carmofur Exhibits Antimicrobial Activity Against Streptococcus pneumoniae. Antibiotics 2025, 14, 231. [Google Scholar] [CrossRef]
- Manska, S.; Octaviano, R.; Rossetto, C.C. 5-Ethynyl-2′-deoxycytidine and 5-ethynyl-2′-deoxyuridine are differentially incorporated in cells infected with HSV-1, HCMV, and KSHV viruses. J. Biol. Chem. 2020, 295, 5871–5890. [Google Scholar] [CrossRef]
- Qu, D.; Wang, G.; Wang, Z.; Zhou, L.; Chi, W.; Cong, S.; Ren, X.; Liang, P.; Zhang, B. 5-Ethynyl-2′-deoxycytidine as a new agent for DNA labeling: Detection of proliferating cells. Anal. Biochem. 2011, 417, 112–121. [Google Scholar] [CrossRef]
- Chillar, K.; Awasthy, R.; Tanasova, M.; Fang, S. Synthesis of Sensitive Oligodeoxynucleotides Containing Acylated Cytosine, Adenine, and Guanine Nucleobases. DNA 2025, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cheng, T.; Pan, J. Nucleoside Analogs: A Review of Its Source and Separation Processes. Molecules 2023, 28, 7043. [Google Scholar] [CrossRef]
- Huang, Q.; Kaiser, K.; Benne, R. A simple high performance liquid chromatography method for the measurement of nucleobases and the RNA and DNA content of cellular material. Limnol. Oceanogr. Methods 2012, 10, 608–616. [Google Scholar] [CrossRef]
- Yin, R.; Mo, J.; Lu, M.; Wang, H. Detection of human urinary 5-hydroxymethylcytosine by Stable isotope dilution HPLC-MS/MS analysis. Anal. Chem. 2015, 87, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Leng, A.; Yang, Z.; Xue, Y. A Practical Method for Determination of Nine Nucleosides in Tricholoma matsutake by UPLC/MS and Quantitative Analysis of Multicomponents Using Single Marker Method. J. Anal. Methods Chem. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Andrus, A.; Kuimelis, R.G. Base Composition Analysis of Nucleosides Using HPLC. Curr. Protoc. Nucleic Acid. Chem. 2001, 10, 10.6. [Google Scholar] [CrossRef]
- Roberts, C.E.; Raner, G.M.; Isaacs, G.D. High Performance Liquid Chromatography Separation of Epigenetic Cytosine Variants. Methods Protoc. 2018, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Tuytten, R.; Lemière, F.; Van Dongen, W.; Witters, E.; Esmans, E.L.; Newton, R.P.; Dudley, E. Development of an On-Line SPE-LC–ESI-MS Method for Urinary Nucleosides: Hyphenation of Aprotic Boronic Acid Chromatography with Hydrophilic Interaction LC–ESI-MS. Anal. Chem. 2008, 80, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Pypowski, K.; Chrząścik, I.; Koval, T.; Erchak, N. Separation and Determination of Chosen λ5-Silanates by an Isotachophoresis Technique. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 896–907. [Google Scholar] [CrossRef]
- Prukała, W.; Pypowski, K.; Chrząścik, I.; Kluska, M. Separation of Biologically Active Isomers of (E)-N-Meta- and Para-Nitroazastilbenes by the HPLC Technique. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 578–585. [Google Scholar] [CrossRef]
- Prukała, W.; Prukała, D.; Pypowski, K.; Chrząścik, I.; Kluska, M. Chromatography of Biologically Active Chlorides of (E)-N-o-(m- or p-)chlorobenzyl-γ-azastilbenols-2′(3′ or 4′). J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2612–2620. [Google Scholar] [CrossRef]
- Kluska, M.; Pypowski, K.; Erchak, N. Separation of Hexabenzyldigermoxane and Hexabenzyldigermanium by HPLC. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 1777–1785. [Google Scholar] [CrossRef]
- Russell, J.J. Development of Generic Methods for the Analysis and Purification of Polar Compounds by High Performance Liquid Chromatography. Ph.D. Thesis, University of the West of England, Bristol, UK, 2016. [Google Scholar]
- Kisil, O.; Sergeev, A.; Bacheva, A.; Zvereva, M. Methods for Detection and Mapping of Methylated and Hydroxymethylated Cytosine in DNA. Biomolecules 2024, 14, 1346. [Google Scholar] [CrossRef]
- HPLC Method for Separation of Cytosine, Deoxycytidine and Cytidine on BIST B+ by SIELC Technologies. Available online: https://sielc.com/hplc-method-of-cytosine-deoxycytidine-cytidine (accessed on 29 June 2025).
- Romanova, D.; Novotny, L. Chromatographic properties of cytosine, cytidine and their synthetic analogues. J. Chromatogr. B 1996, 675, 9–15. [Google Scholar] [CrossRef]
- Magaña, A.A.; Wrobel, K.; Caudillo, A.C.; Luna, S.Z.; Wrobel, K. High-performance liquid chromatography determination of 5-methyl-2′-deoxycytidine, 2′-deoxycytidine, and other deoxynucleosides and nucleosides in DNA digests. Anal. Biochem. 2008, 374, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Turesky, R.J. Emerging Technologies in Mass Spectrometry-Based DNA Adductomics. High-Throughput 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Villalta, P.W.; Balbo, S. The Future of DNA Adductomic Analysis. Int. J. Mol. Sci. 2017, 18, 1870. [Google Scholar] [CrossRef]
- Honeywell, R.; Laan, A.C.; van Groeningen, C.J.; Strocchi, E.; Ruiter, R.; Gaccone, G.; Peters, G.J. The determination of gemcitabine and 2′-deoxycytidine in human plasma and tissue by APCI tandem mass spectrometry. J. Chromatogr. B 2007, 847, 142–152. [Google Scholar] [CrossRef]
- Prukała, D. Synthesis and physicochemical properties of new 1N o-(m- and p-) bromobenzyl substituted derivatives of 5-(aminodialkyl)methylcytosine. J. Heterocycl. Chem. 2007, 44, 1207–1212. [Google Scholar] [CrossRef]
- Kluska, M.; Prukała, D.; Prukała, W.; Kondrzycka-Dąda, A.; Komasińska, M.; Małkiewicz, K. Successful Separation And Determination of Isomers of Cytosine Derivatives for HPLC. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2172–2181. [Google Scholar] [CrossRef]
- Kluska, M.; Liepinsh, E.; Pypowski, K.; Chrząścik, I.; Michel, M.; Erchak, N. Separation of Selected Derivatives of Hoszczawa-Silanates, Taking Advantage of π–π Interactions. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2812–2820. [Google Scholar] [CrossRef]
- Kluska, M.; Pypowski, K. Separation of Tribenzylhydrogermanium Nitrile Derivatives by Means of HPLC with Participation of Π-Π Interactions. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2059–2067. [Google Scholar] [CrossRef]
- Pypowski, K.; Uszyńska, I.; Kluska, M. Chromatographic Separation of Isomers of Tribenzylgermanium Nitrile Derivatives using Chemically Bonded Aryl Stationary Phases. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 2989–2996. [Google Scholar] [CrossRef]
- Kluska, M.; Liepinsh, E.; Pypowski, K.; Erchak, N. Separation of Azepinio-Methyl Derivatives of ES-Silanates by the Use of Aryl Stationary Phases in HPLC. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 675–682. [Google Scholar] [CrossRef]
- Kluska, M. An Application of Aryl Stationary Phases for Separation of Select Organogermanium Compounds. J. Liq. Chromatogr. Relat. Technol. 2007, 31, 210–218. [Google Scholar] [CrossRef]
- Parys, W.; Dołowy, M.; Pyka-Pająk, A. Significance of Chromatographic Techniques in Pharmaceutical Analysis. Processes 2022, 10, 172. [Google Scholar] [CrossRef]
- Berdasco, M.; Fraga, M.F.; Esteller, M. Quantification of Global DNA Methylation by Capillary Electrophoresis and Mass Spectrometry. In DNA Methylation. Methods in Molecular Biology; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 2007, pp. 23–34. [Google Scholar] [CrossRef]
- Minhas-Khan, A.; Ghafar-Zadeh, M.; Shaffaf, T.; Forouhi, S.; Scime, A.; Magierowski, S.; Ghafar-Zadeh, E. UV-Vis Spectrophotometric Analysis of DNA Retrieval for DNA Storage Applications. Actuators 2021, 10, 246. [Google Scholar] [CrossRef]
- Bruijns, B.; Hoekema, T.; Oomens, L.; Tiggelaar, R.; Gardeniers, H. Performance of Spectrophotometric and Fluorometric DNA Quantification Methods. Analytica 2022, 3, 371–384. [Google Scholar] [CrossRef]
- Shen, C. Diagnostic Molecular Biology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]

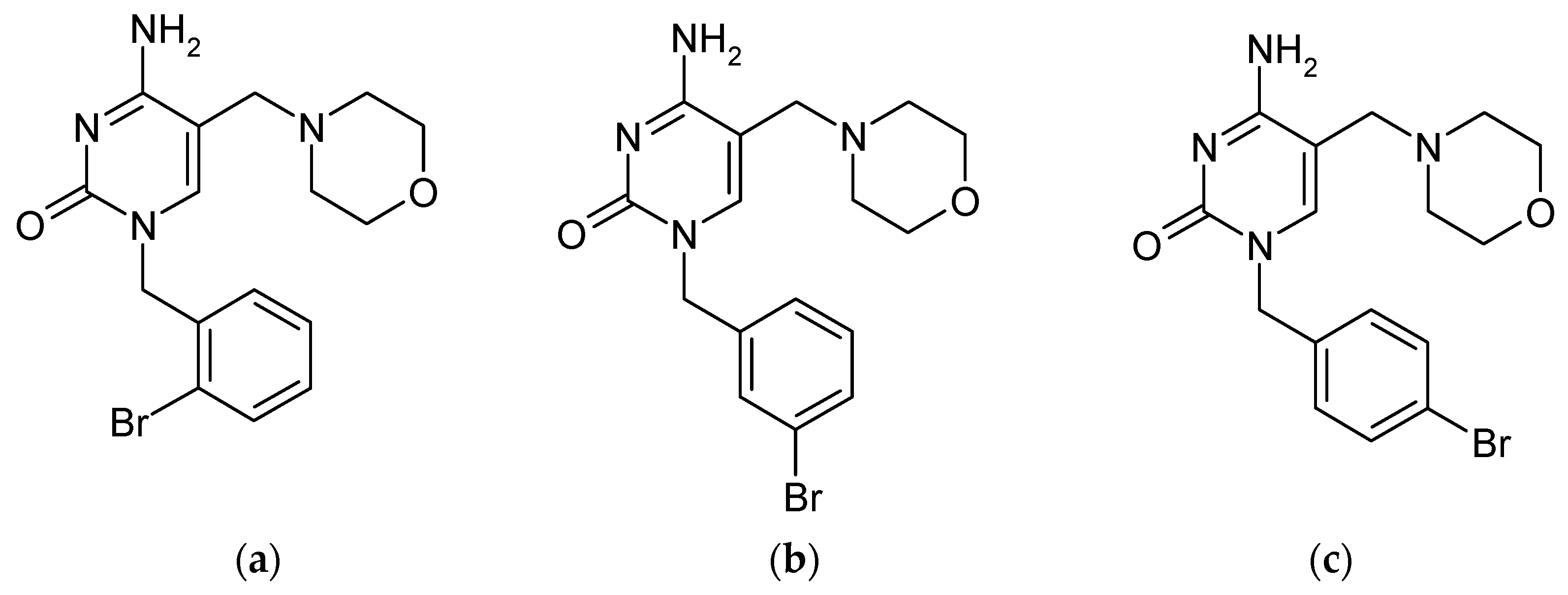
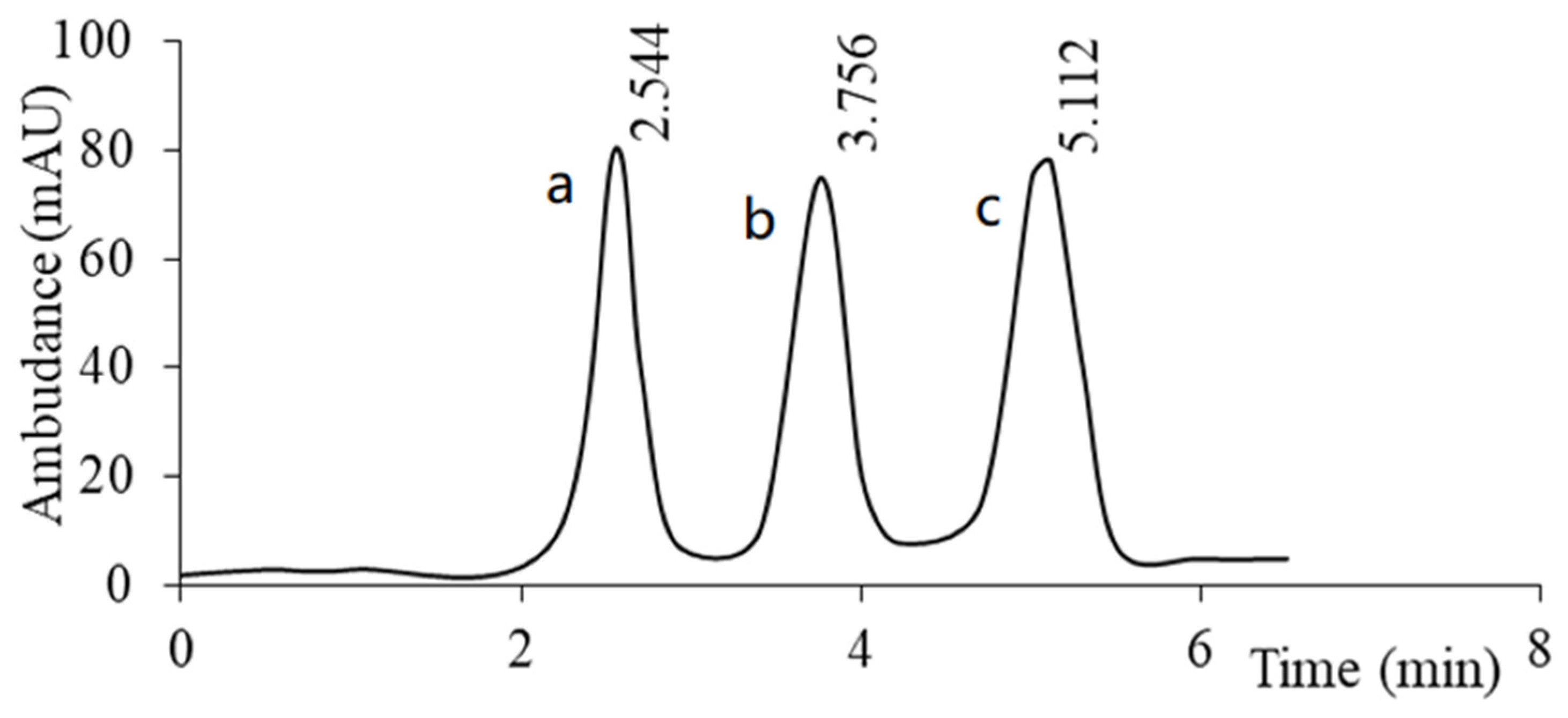
| Compound (Abbreviation) | Structure (Modification) | pKa (≈) | Melting Point | Solubility in H2O (25 °C) | Applications/Remarks |
|---|---|---|---|---|---|
| Cytosine (C) | 4-aminopyrimidin-2(1H)-one | ~4.6; 12.2 | 320–325 °C # | ~7–8 mg/mL | DNA/RNA principle; undergoes methylation to 5-mC |
| 5-Methylcytosine (5-mC) | cytosine with –CH3 in position 5 | 4.6; 12.4 (estimate) | 270 °C # | poorly soluble < 5 mg/mL (estimate) | Epigenetic modification of DNA (gene regulation) |
| 5-Fluorocytosine (5-FC) | cytosine with –F in position 5 | ~3.3; ~11 | 295–297 °C # | 15 mg/mL | Antifungal drug (5-FU prodrug) |
| 5-Azacytosine (in 5-aza-Cyd) | pyrimidine with N instead of C5 | ~1.1; 9.2 (for 5-azaC) | >300 °C | highly soluble (in nucleoside form) | Component of azacitidine and decitabine drugs (DNA hypomethylation) |
| Cytidine (Cyd) | nucleoside: cytosine + ribose β | 4.2; ~12.5 (principle) | 230.5 °C # | ≥60 mg/mL | RNA component; supplement (citicoline) |
| Deoxycytidine (dCyd) | nucleoside: cytosine + 2′-deoxyribose | 4.3; ~12 (principle) | 207–210 °C # | highly soluble (≥50 mg/mL) | DNA component |
| 5-Azacytidine (5-aza-Cyd) | cytidine analogue with C5 → N (triazine) | - | ~229 °C # | ≥50 mg/mL (water, unstable) | Anti-cancer drug (Vidaza®; MDS, leukaemia) |
| Decitabine (5-aza-dCyd) | deoxycytidine analogue (C5 → N) | - | 209 °C # | good solubility in water | Anti-cancer drug (Dacogen®; MDS) |
| Cytarabine (Ara-C) | 1-β-D- arabinofuranosylcytosine | 4.2; 12 (principle) | 212–213 °C # | ~10 mg/mL (water) | Cytostatic drug (Ara-C; leukaemia) |
| Gemcitabine (dFdC) | 2′,2′-difluorodeoxycytidine | 3.6; 12 (principle) | ~168 °C # | ~15 mg/mL (water) | Cytostatic drug (Gemzar®; pancreatic cancer, others) |
| Lamivudine (3TC) | 2′,3′-dideoxy-3′-thiacytidine (S in the ring) | ~4; 12 (principle) | 160–162 °C | ≥20 mg/mL (water) | Antiretroviral drug (NRTI; HIV, HBV) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kluska, M.; Jabłońska, J.; Prukała, D.; Prukała, W. Occurrence, Properties, Applications and Analytics of Cytosine and Its Derivatives. Molecules 2025, 30, 3598. https://doi.org/10.3390/molecules30173598
Kluska M, Jabłońska J, Prukała D, Prukała W. Occurrence, Properties, Applications and Analytics of Cytosine and Its Derivatives. Molecules. 2025; 30(17):3598. https://doi.org/10.3390/molecules30173598
Chicago/Turabian StyleKluska, Mariusz, Joanna Jabłońska, Dorota Prukała, and Wiesław Prukała. 2025. "Occurrence, Properties, Applications and Analytics of Cytosine and Its Derivatives" Molecules 30, no. 17: 3598. https://doi.org/10.3390/molecules30173598
APA StyleKluska, M., Jabłońska, J., Prukała, D., & Prukała, W. (2025). Occurrence, Properties, Applications and Analytics of Cytosine and Its Derivatives. Molecules, 30(17), 3598. https://doi.org/10.3390/molecules30173598







