Comparison of Composite Materials Designed to Optimize Heterogeneous Decatungstate Oxidative Photocatalysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Materials
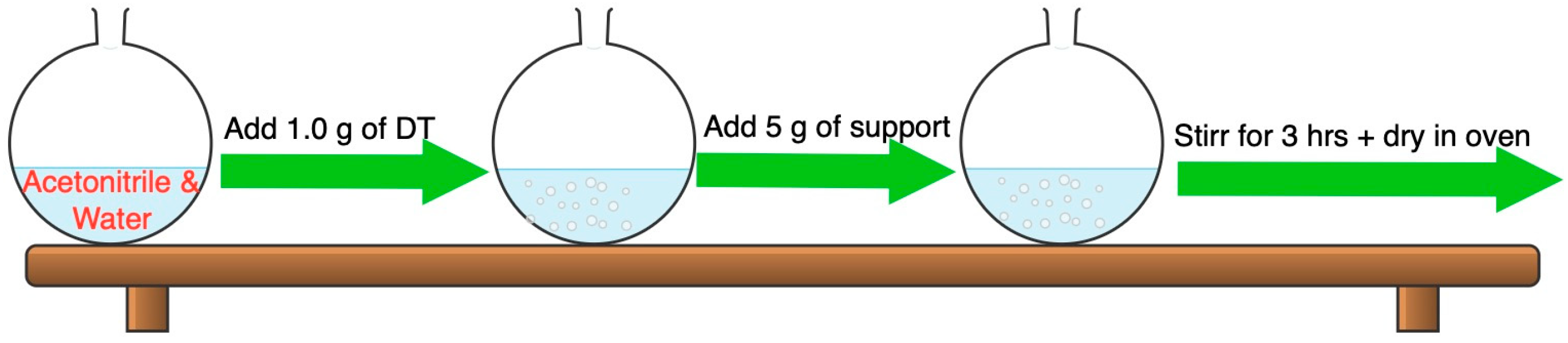
2.1.1. Catalyst Preparation
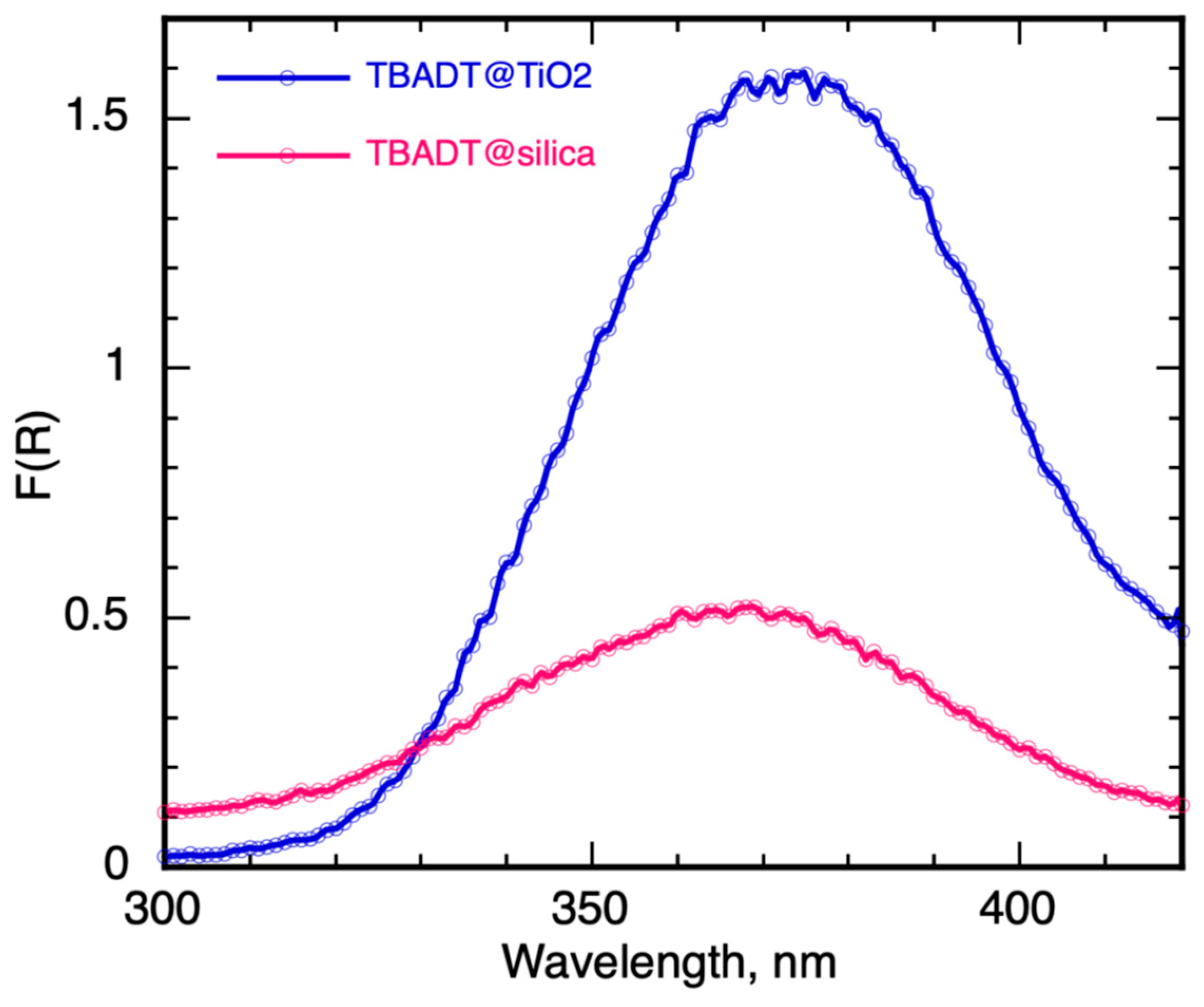
2.1.2. Catalyst Diffuse Reflectance and BET Characterization
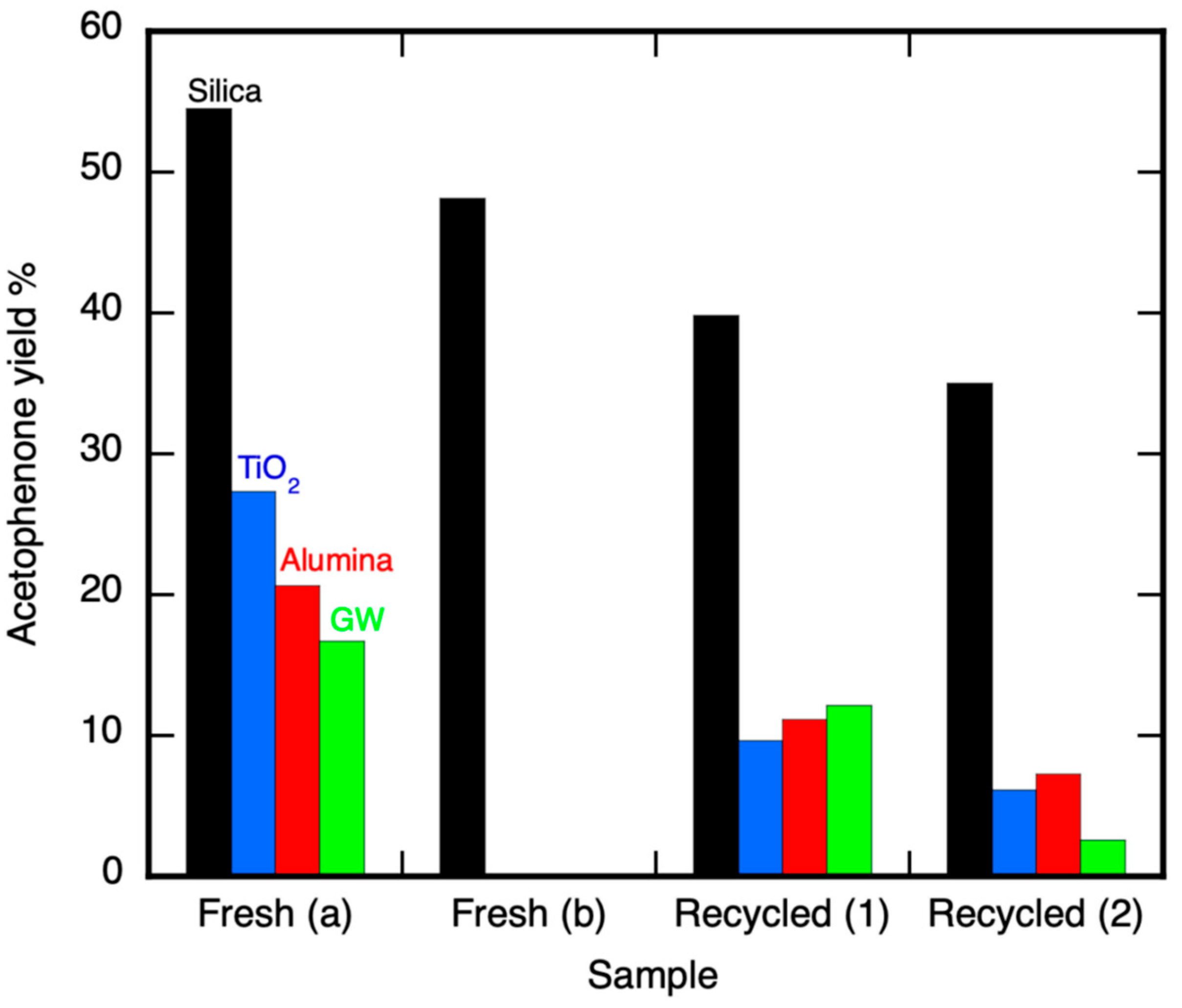
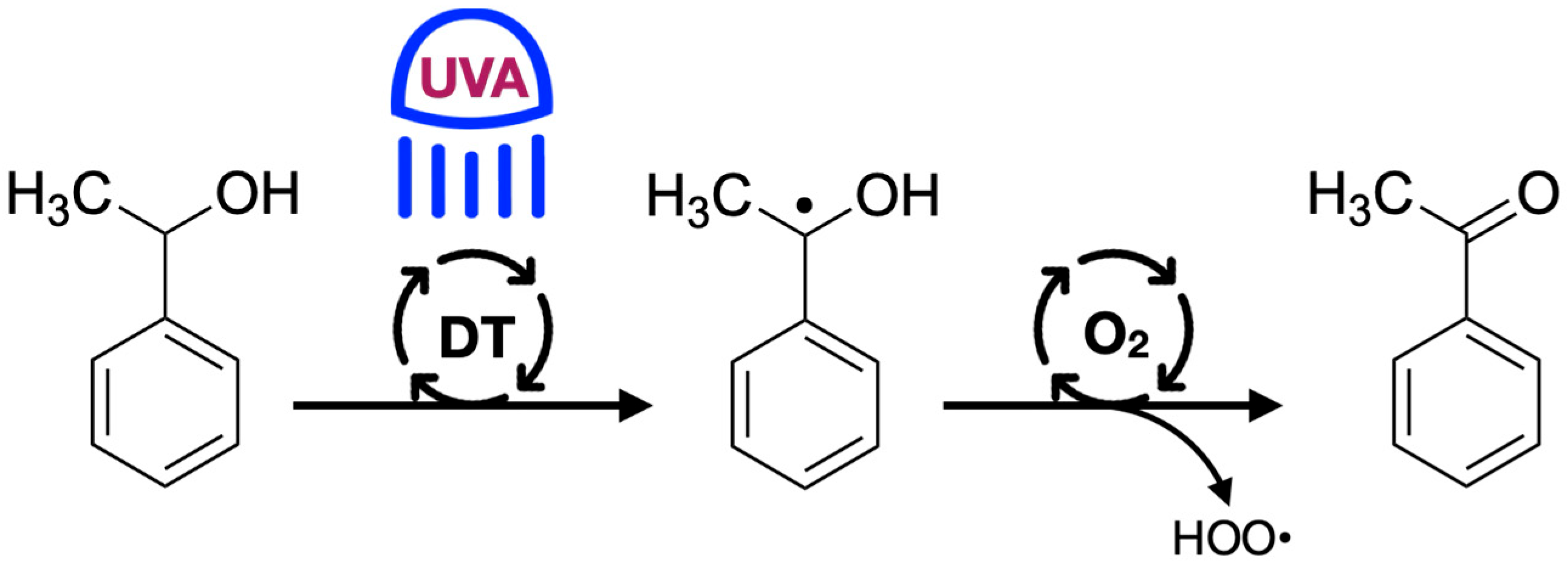
2.2. 1-Phenylethanol Sample Irradiation Using TBADT on Various Supports
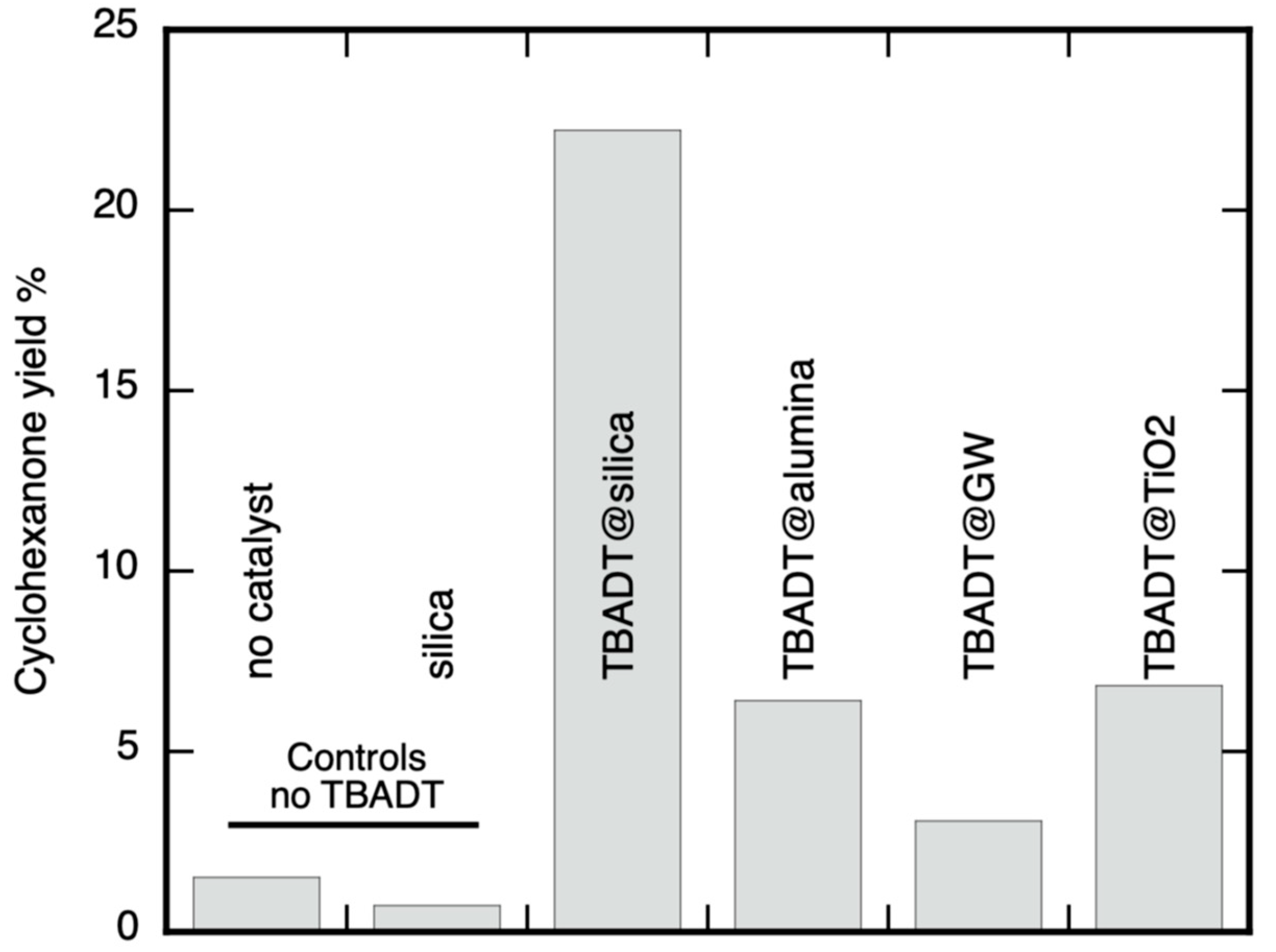
2.2.1. Cyclohexanol Sample UVA Irradiation Using TBADT on Different Supports
2.2.2. Cyclohexanol Irradiations at 280 nm
2.2.3. Exploratory Studies Using NaDT
2.2.4. Exploratory Studies Using Black-TiO2 as Support
3. Materials and Methods
3.1. Sources of Materials
3.1.1. Commercial Materials
3.1.2. Preparation of NaDT and TBADT
3.2. Modification of Supports
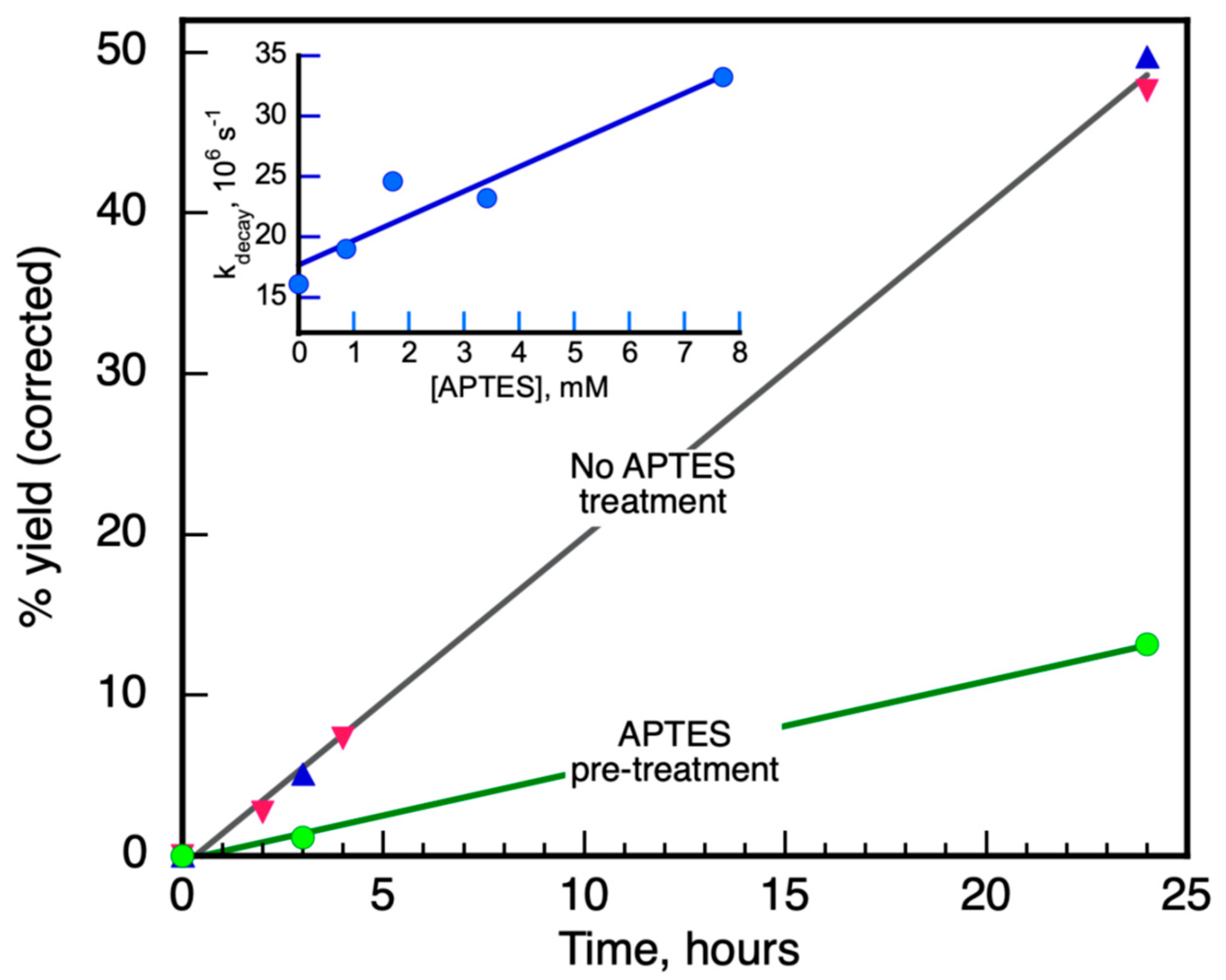
3.2.1. APTES@Glass Wool
3.2.2. Synthesis of Black TiO2
3.3. Preparation of DT@support Catalysts
3.4. Irradiation of the Samples
3.5. Leaching Tests for TBADT@silica
3.6. Tools and Instruments Used for Analysis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claros, M.; Quévarec, J.; Fernández-García, S.; Noël, T. Design and application of a decatungstate-based ionic liquid photocatalyst for sustainable hydrogen atom transfer reactions. Green Chem. 2025, 27, 7660–7666. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Capaldo, L.; Laudadio, G.; Nyuchev, A.V.; Rincón, J.A.; García-Losada, P.; Mateos, C.; Frederick, M.O.; Nuño, M.; Noël, T. Decatungstate-Mediated C(sp3)–H Heteroarylation via Radical-Polar Crossover in Batch and Flow. Angew. Chem. Int. Ed. 2021, 60, 17893–17897. [Google Scholar] [CrossRef]
- Wen, Z.; Maheshwari, A.; Sambiagio, C.; Deng, Y.; Laudadio, G.; Van Aken, K.; Sun, Y.; Gemoets, H.P.L.; Noël, T. Optimization of a Decatungstate-Catalyzed C(sp3)–H Alkylation Using a Continuous Oscillatory Millistructured Photoreactor. Org. Proc. Res. Dev. 2020, 24, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, G.; Deng, Y.; van der Wal, K.; Ravelli, D.; Nuño, M.; Fagnoni, M.; Guthrie, D.; Sun, Y.; Noël, T. C(sp3)–H functionalizations of light hydrocarbons using decatungstate photocatalysis in flow. Science 2020, 369, 92–96. [Google Scholar] [CrossRef]
- Laudadio, G.; Govaerts, S.; Wang, Y.; Ravelli, D.; Koolman, H.F.; Fagnoni, M.; Djuric, S.W.; Noël, T. Selective C(sp3)−H Aerobic Oxidation Enabled by Decatungstate Photocatalysis in Flow. Angew. Chem. Int. Ed. 2018, 57, 4078–4082. [Google Scholar] [CrossRef]
- Didarataee, S.; Ong, J.; Suprun, A.; Joshi, N.; Scaiano, J.C. Kinetics, quantum yield and mechanism of the decatungstate-catalyzed photooxidation of C–H hydrogen donors: Role of the persistent radical effect. Catal. Sci. Technol. 2025, 15, 1149–1156. [Google Scholar] [CrossRef]
- Foote, C.S. Type I and Type II Mechanisms of Photodynamic Action. ACS Symp. Ser. 1987, 339, 22–38. [Google Scholar]
- Pasti, L.; Sarti, E.; Martucci, A.; Marchetti, N.; Stevanin, C.; Molinari, A. An advanced oxidation process by photoexcited heterogeneous sodium decatungstate for the degradation of drugs present in aqueous environment. Appl. Catal. B Environ. 2018, 239, 345–351. [Google Scholar] [CrossRef]
- Su, A.; Jiang, D.; Hu, W.; Liang, S.; Yu, K.; Zhou, W.; Wang, J.; Fu, Z.; Liu, Y.; Liu, J. Hybridizing engineering strategy of decatungstate III: Transition metal modified carbon quantum dot-regulated photo-catalytic oxidation performance of decatungstate. J. Organomet. Chem. 2025, 1023, 123432. [Google Scholar] [CrossRef]
- Fornal, E.; Giannotti, C. Photocatalyzed oxidation of cyclohexane with heterogenized decatungstate. J. Photochem. Photobiol. A Chem. 2007, 188, 279–286. [Google Scholar] [CrossRef]
- Molinari, A.; Amadelli, R.; Mazzacani, A.; Sartori, G.; Maldotti, A. Tetralkylammonium and Sodium Decatungstate Heterogenized on Silica: Effects of the Nature of Cations on the Photocatalytic Oxidation of Organic Substrates. Langmuir 2002, 18, 5400–5405. [Google Scholar] [CrossRef]
- Tzirakis, M.D.; Lykakis, I.N.; Panagiotou, G.D.; Bourikas, K.; Lycourghiotis, A.; Kordulis, C.; Orfanopoulos, M. Decatungstate catalyst supported on silica and γ-alumina: Efficient photocatalytic oxidation of benzyl alcohols. J. Catal. 2007, 252, 178–189. [Google Scholar] [CrossRef]
- Bigi, F.; Corradini, A.; Quarantelli, C.; Sartori, G. Silica-bound decatungstates as heterogeneous catalysts for H2O2 activation in selective sulfide oxidation. J. Catal. 2007, 250, 222–230. [Google Scholar] [CrossRef]
- Wang, M.; Jia, C.; Hui, H.; Xu, Q.; Li, X.; Ren, Y.; Yue, B.; He, H. Engineering the heterogeneous photocatalytic activity of crystalline decatungstate-based coordination polymers. CrystEngComm 2024, 26, 3303–3310. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, J.; Hu, S.; Deng, Y.; Luo, M.; She, J.; Liu, Y.; Zhang, C.; Tang, S.; Fu, Z. Hybridizing strategy of decatungstate by Au nanoparticles for enhanced photo-catalytic oxidation of hydrocarbons by dioxygens. Appl. Catal. A Gen. 2022, 630, 118473. [Google Scholar] [CrossRef]
- Kulikov, V.; Meyer, G. Organoamine silver(i) decatungstate structures: Remarkable chemoselectivity and the exploration of the intramolecular redox reaction upon thermolysis. New J. Chem. 2014, 38, 3408–3412. [Google Scholar] [CrossRef]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Modern Molecular Photochemistry of Organic Molecules; University Science Publishers: New York, NY, USA, 2010; p. 1100. [Google Scholar]
- Didarataee, S.; Suprun, A.; Joshi, N.; Scaiano, J.C. NIR phosphorescence from decatungstate anions allows the conclusive characterization of its elusive excited triplet behaviour and kinetics. Chem. Commun. 2024, 60, 1896–1899. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. J. Opt. Soc. Am. 1948, 38, 448. [Google Scholar] [CrossRef]
- Morsella, M.; d’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 1688. [Google Scholar]
- Sarver, P.J.; Bacauanu, V.; Schultz, D.M.; DiRocco, D.A.; Lam, Y.-h.; Sherer, E.C.; MacMillan, D.W.C. The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nat. Chem. 2020, 12, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Yaghmaei, M.; da Silva, D.R.C.; Rutajoga, N.; Currie, S.; Li, Y.; Vallieres, M.; Silvero, M.J.; Joshi, N.; Wang, B.; Scaiano, J.C. Innovative Black TiO2 Photocatalyst for Effective Water Remediation Under Visible Light Illumination Using Flow Systems. Catalysts 2024, 14, 775. [Google Scholar] [CrossRef]
- Alcaraz de la Osa, R.; Iparragirre, I.; Ortiz, D.; Saiz, J.M. The extended Kubelka–Munk theory and its application to spectroscopy. ChemTexts 2019, 6, 1. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, J.; Cajka, B.; Scaiano, J.C. Comparison of Composite Materials Designed to Optimize Heterogeneous Decatungstate Oxidative Photocatalysis. Molecules 2025, 30, 3597. https://doi.org/10.3390/molecules30173597
Ong J, Cajka B, Scaiano JC. Comparison of Composite Materials Designed to Optimize Heterogeneous Decatungstate Oxidative Photocatalysis. Molecules. 2025; 30(17):3597. https://doi.org/10.3390/molecules30173597
Chicago/Turabian StyleOng, Julia, Benjamin Cajka, and Juan C. Scaiano. 2025. "Comparison of Composite Materials Designed to Optimize Heterogeneous Decatungstate Oxidative Photocatalysis" Molecules 30, no. 17: 3597. https://doi.org/10.3390/molecules30173597
APA StyleOng, J., Cajka, B., & Scaiano, J. C. (2025). Comparison of Composite Materials Designed to Optimize Heterogeneous Decatungstate Oxidative Photocatalysis. Molecules, 30(17), 3597. https://doi.org/10.3390/molecules30173597




