Comparative Study on Antioxidant Potential of Schinus terebinthifolius Extracts Prepared by Conventional Extraction, Accelerated Solvent Extraction, and Pulsed Electric Field Method
Abstract
1. Introduction
2. Results
2.1. Yield of Extracts
2.2. Quantitative Measurement of Total Phenolic and Total Flavonoid Content
2.3. Antioxidant Capacity
2.4. Identification of Constituents by Liquid Chromatography Mass Spectrometry (LC-MS)
2.5. Cytotoxic Effect of Brazilian Pepper Extracts
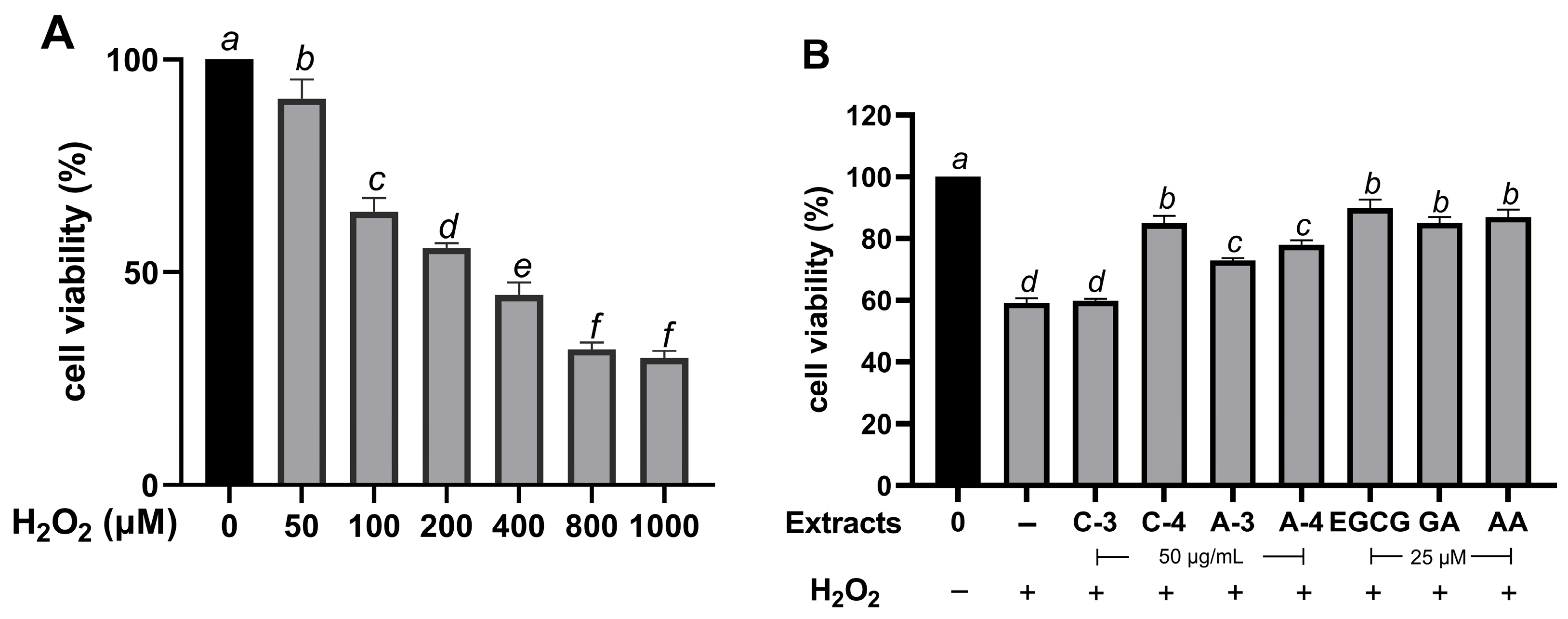
2.6. Effect of Brazilian Pepper Extracts on Cell Viability of HaCaT Cells Under Oxidative Stress
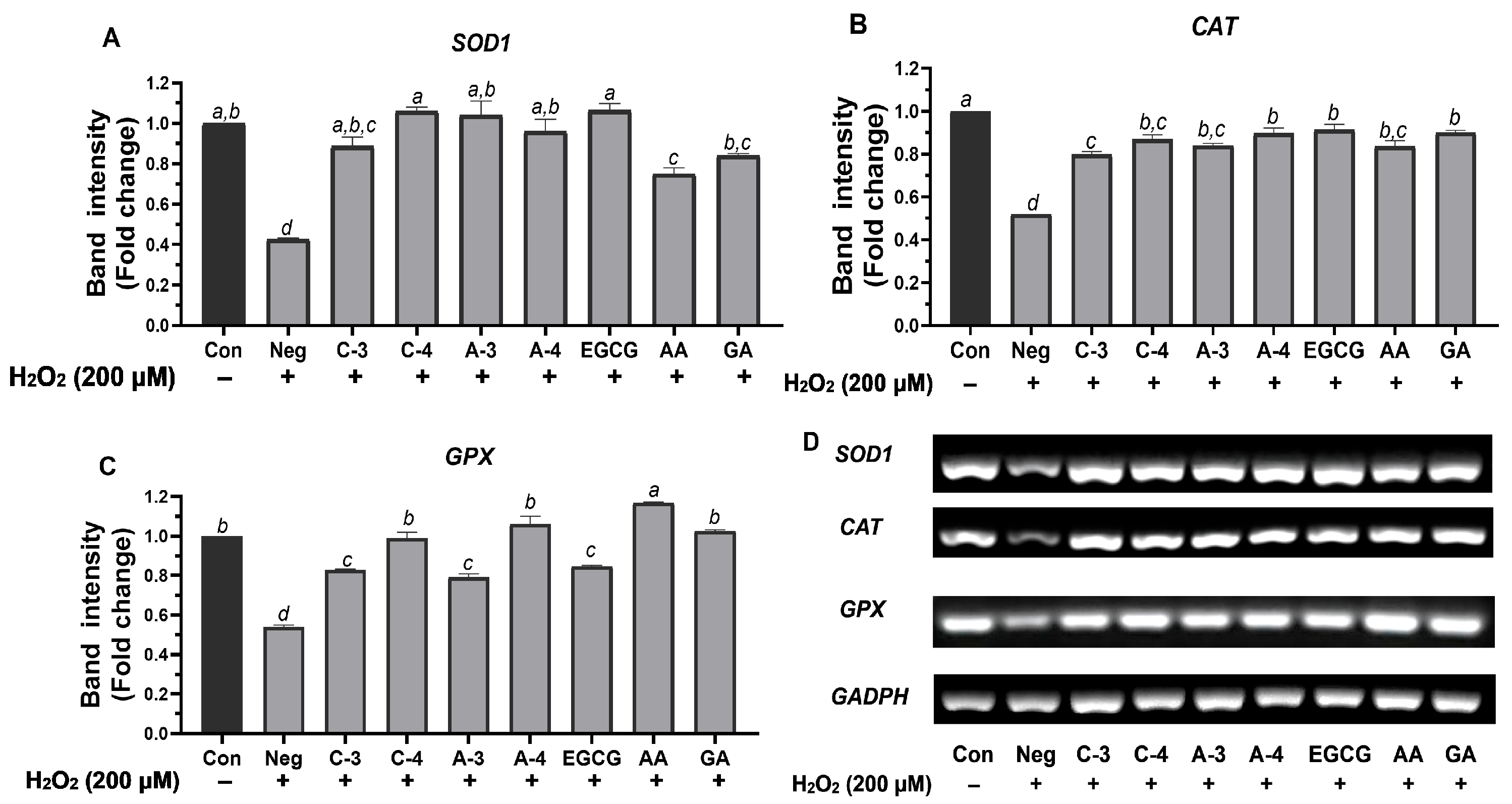
2.7. SOD1, GPX, and CAT Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. Extraction Methods
4.3.1. Conventional Extraction
4.3.2. Accelerated Solvent Extraction
4.3.3. Pulsed Electric Fields Extraction
4.4. Phytochemical Analysis
4.4.1. Total Phenolic Content
4.4.2. Total Flavonoid Content
4.4.3. Identification of Constituents by LC-MS Analysis
4.5. Antioxidant Capacity
4.5.1. ABTS Radical Scavenging Activity
4.5.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
4.6. Cell Cultures and Assessment of Cell Viability
4.6.1. Cell Culture
4.6.2. Cytotoxic Effect of Brazilian Pepper Extracts
4.6.3. Effect of H2O2 on Cell Viability of HaCaT Cells
4.6.4. Effect of Brazilian Pepper Extracts on Cell Viability of HaCaT Cells Under Oxidative Stress
4.7. RNA Extraction and Semi-Quantitative Reverse Transcriptase Polymerase Chain Reaction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery 2022, 40, 8–12. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Meyer, R. Schinus terebinthifolius. Available online: https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html (accessed on 22 August 2025).
- Silva, B.G.; Fileti, A.M.F.; Foglio, M.A.; Rosa, P.d.T.V.; Taranto, O.P. Effects of Different Drying Conditions on Key Quality Parameters of Pink Peppercorns (Schinus terebinthifolius Raddi). J. Food Qual. 2017, 2017, 3152797. [Google Scholar] [CrossRef]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Carlini, E.A.; Duarte-Almeida, J.M.; Rodrigues, E.; Tabach, R. Antiulcer Effect of the Pepper Trees Schinus terebinthifolius Raddi (aroeira-da-praia) and Myracrodruon urundeuva Allemão, Anacardiaceae (Aroeira-do-sertão). Rev. Bras. De Farmacogn. 2010, 20, 140–146. [Google Scholar] [CrossRef]
- Cavalher-Machado, S.C.; Rosas, E.C.; Brito Fde, A.; Heringe, A.P.; de Oliveira, R.R.; Kaplan, M.A.; Figueiredo, M.R.; Henriques, M. The Anti-Allergic Activity of the Acetate Fraction of Schinus terebinthifolius Leaves in IgE Induced Mice Paw Edema and Pleurisy. Int. Immunopharmacol. 2008, 8, 1552–1560. [Google Scholar] [CrossRef]
- D’Sousa’ Costa, C.O.; Ribeiro, P.R.; Loureiro, M.B.; Simões, R.C.; de Castro, R.D.; Fernandez, L.G. Phytochemical screening, antioxidant and antibacterial activities of extracts prepared from different tissues of Schinus terebinthifolius Raddi that occurs in the coast of Bahia, Brazil. Pharmacogn. Mag. 2015, 11, 607–614. [Google Scholar] [CrossRef]
- De Lima Glória, L.; de Souza Arantes, M.B.; de Faria Pereira, S.M.; De Souza Vieira, G.; Xavier Martins, C.; de Carvalho Junior, A.R.; Antunes, F.; Braz-Filho, R.; José Curcino Vieira, I.; da Cruz, L.L.; et al. Phenolic Compounds Present Schinus terebinthifolius Raddi Influence the Lowering of Blood Pressure in Rats. Molecules 2017, 22, 1792. [Google Scholar] [CrossRef]
- El-Massry, K.F.; El-Ghorab, A.H.; Shaaban, H.A.; Shibamoto, T. Chemical Compositions and Antioxidant/Antimicrobial Activities of Various Samples Prepared from Schinus terebinthifolius Leaves Cultivated in Egypt. J. Agric. Food Chem. 2009, 57, 5265–5270. [Google Scholar] [CrossRef]
- Estevão, L.R.M.; Simões, R.S.; Cassini-Vieira, P.; Canesso, M.C.C.; Barcelos, L.d.S.; Rachid, M.A.; Câmara, C.A.G.d.; Evêncio-Neto, J. Schinus terebinthifolius Raddi (Aroeira) leaves oil attenuates inflammatory responses in cutaneous wound healing in mice. Acta Cir. Bras. 2017, 32, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Feuereisen, M.M.; Hoppe, J.; Zimmermann, B.F.; Weber, F.; Schulze-Kaysers, N.; Schieber, A. Characterization of Phenolic Compounds in Brazilian Pepper (Schinus terebinthifolius Raddi) Exocarp. J. Agric. Food Chem. 2014, 62, 6219–6226. [Google Scholar] [CrossRef]
- Uliana, M.P.; Fronza, M.; da Silva, A.G.; Vargas, T.S.; de Andrade, T.U.; Scherer, R. Composition and biological activity of Brazilian rose pepper (Schinus terebinthifolius Raddi) leaves. Ind. Crops Prod. 2016, 83, 235–240. [Google Scholar] [CrossRef]
- Patocka, J.; de Almeida, J.D. Brazilian Pepper Tree: Review of Pharmacology. Mil. Med. Sci. Lett. 2017, 86, 32–41. [Google Scholar] [CrossRef]
- Estevão, L.R.M.; Mendonça, F.d.S.; Baratella-Evêncio, L.; Simões, R.S.; Barros, M.E.G.d.; Arantes, R.M.E.; Rachid, M.A.; Evêncio-Neto, J. Effects of Aroeira (Schinus terebinthifoliu Raddi) Oil on Cutaneous Wound Healing in Rats. Acta Cir. Bras. 2013, 28, 202–209. [Google Scholar] [CrossRef]
- Da Silva Nascimento, M.; Dos Santos, P.H.; De Abreu, F.F.; Shan, A.; Amaral, R.G.; Andrade, L.N.; Souto, E.B.; Santos, M.I.S.; De Souza Graça, A.; Souza, J.B.; et al. Schinus terebinthifolius Raddi (Brazilian Pepper) Leaves Extract: In Vitro and In Vivo Evidence of Anti-Inflammatory and Antioxidant Properties. Inflammopharmacology 2023, 31, 2505–2519. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Lakka, A.; Palaiogiannis, D.; Pappas, V.M.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field and Salvia officinalis L. Leaves: A Successful Combination for the Extraction of High Value Added Compounds. Foods 2021, 10, 2014. [Google Scholar] [CrossRef]
- Olech, M.; Łyko, L.; Nowak, R. Influence of Accelerated Solvent Extraction Conditions on the LC-ESI-MS/MS Polyphenolic Profile, Triterpenoid Content, and Antioxidant and Anti-Lipoxygenase Activity of Rhododendron luteum Sweet Leaves. Antioxidants 2020, 9, 822. [Google Scholar] [CrossRef]
- Azwanida, N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1000196. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical Screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Ullah, R.; Bera, G.; Gómez-Ríos, G.A.; Wang, M.; Lu, D.; Rubero, A.; Srinivasan, K.; Al-Esawi, H.; Liu, Y. Novel Fully Automated and Parallel Gas Assisted Dynamic Accelerated Solvent Extractor and Parallel Solvent Evaporator for Analysis of Solid and Semi-Solid Samples. Adv. Sample Prep. 2023, 6, 100073. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent Extraction Techniques for Natural Products: Microwave-Assisted Extraction and Pressurised Solvent Extraction. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D. Current Applications and New Opportunities for the Use of Pulsed Electric Fields in Food Science and Industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Puértolas, E.; de Marañón, I.M. Olive Oil Pilot-Production Assisted by Pulsed Electric Field: Impact on Extraction Yield, Chemical Parameters and Sensory Properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of Pulsed Electric Fields and High Voltage Electrical Discharges on Extraction of High-Added Value Compounds from Papaya Peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving Mass Transfer to Soften Tissues by Pulsed Electric Fields: Fundamentals and Applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant Activities and Protective Effects of Dendropachol, a New Bisbibenzyl Compound from Dendrobium pachyglossum, on Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef]
- Plavsa, T.; Jurinjak, N.; Antunovic, D.; Persuric, Ð.; Ganic, K.K. The Influence of Skin Maceration Time on the Phenolic Composition and Antioxidant Activity of Red Wine Teran (Vitis vinifera L.). Food Technol. Biotechnol. 2012, 50, 152. [Google Scholar]
- Rusak, G.; Komes, D.; Likić, S.; Horžić, D.; Kovač, M. Phenolic Content and Antioxidative Capacity of Green and White Tea Extracts Depending on Extraction Conditions and the Solvent Used. Food Chem. 2008, 110, 852–858. [Google Scholar] [CrossRef]
- Farooq, S.; Mir, S.A.; Shah, M.A.; Manickavasagan, A. Extraction techniques. In Plant Extracts: Applications in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–37. [Google Scholar]
- Yashashri, H.; Javalgikar, A.; Laxmi, M.; Sagar, K.; Prmod, C. Application of Magnetic Stirrer for Influencing Extraction Method on Tectona grandis as Analgesic Activity. Int. J. Pharm. Clin. Res. 2017, 9, 634–637. [Google Scholar]
- Andishmand, H.; Masoumi, B.; Torbati, M.; Homayouni-Rad, A.; Azadmard-Damirchi, S.; Hamishehkar, H. Ultrasonication/Dynamic Maceration-Assisted Extraction Method as a Novel Combined Approach for Recovery of Phenolic Compounds from Pomegranate Peel. Food Sci. Nutr. 2023, 11, 7160–7171. [Google Scholar] [CrossRef]
- Luksiene, J.; Zevzikoviene, A.; Kazlauskaite, J.A.; Marksa, M.; Majiene, D.; Zevzikovas, A. Examining the Role of Extraction Techniques and Regional Variability in the Antioxidant and Phytochemical Composition of Juglans regia L. Septa. Plants 2025, 14, 2524. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Recovery of Added-Value Compounds from Orange and Spinach Processing Residues: Green Extraction of Phenolic Compounds and Evaluation of Antioxidant Activity. Antioxidants 2021, 10, 1800. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, J.; Ngadi, M. Novel Non-Thermal Processing Technologies: Impact on Food Phenolic Compounds during Processing. In Phenolic Compounds-Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Pallarés, N.; Berrada, H.; Ferrer, E.; Rached, W.; Pinela, J.; Mandim, F.; Pires, T.C.; Finimundy, T.C.; Barba, F.J.; Barros, L. Green and Innovative Extraction: Phenolic Profiles and Biological Activities of Underutilized Plant Extracts Using Pulsed Electric Fields and Maceration. Foods 2025, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for the Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Goldstein, D.B. Effect of Alcohol on Cellular Membranes. Ann. Emerg. Med. 1986, 15, 1013–1018. [Google Scholar] [CrossRef]
- Parí, S.M.; Juárez, M.L.M.; Vilca, F.Z.; Vilca, O.M.L.; Alca, E.E.A.; Escobedo-Pacheco, E.; Huamán-Castilla, N.L. Alternative Green Extraction Techniques to Enhance Recovery of Antioxidant Compounds from Red Peel Prickly Pear (Opuntia ficus-indica L. Miller). Discov. Food 2024, 4, 58. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of Extraction Conditions on the Phenolic Composition and Antioxidant Capacity of Grape Stem Extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef]
- Mihelčič, A.; Lisjak, K.; Vanzo, A. Accelerated Solvent Extraction of Phenols from Lyophilised Ground Grape Skins and Seeds. Beverages 2023, 9, 4. [Google Scholar] [CrossRef]
- Kopjar, M.; Lončarić, A.; Mikulinjak, M.; Šrajbek, Ž.; Šrajbek, M.; Pichler, A. Evaluation of Antioxidant Interactions of Combined Model Systems of Phenolics in the Presence of Sugars. Nat. Prod. Commun. 2016, 11, 1445–1448. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.; Cuvelier, M.-E.; Berset, C. Antioxidant Activity of Phenolic Compounds in 2, 2′-Azobis (2-Amidinopropane) Dihydrochloride (AAPH)-Induced Oxidation: Synergistic and Antagonistic Effects. J. Am. Oil Chem. Soc. 2003, 80, 1007. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant activity of caffeic acid against iron-induced free radical generation—A chemical approach. PLoS ONE 2015, 10, e0129963. [Google Scholar]
- Kowalczyk, A.; Tuberoso, C.I.G.; Jerković, I. The Role of Rosmarinic Acid in Cancer Prevention and Therapy: Mechanisms of Antioxidant and Anticancer Activity. Antioxidants 2024, 13, 1313. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxidative Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress-Induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Martí-Quijal, F.J.; Pallarés, N.; Dawidowicz, K.; Ruiz, M.-J.; Barba, F.J. Enhancing Nutrient Recovery and Bioactive Compound Extraction from Spirulina Through Supercritical Fluid Extraction: Implications for SH-SY5Y Cell Viability. Foods 2023, 12, 2509. [Google Scholar] [CrossRef]
- De Aguiar Saldanha Pinheiro, A.C.; Martí-Quijal, F.J.; Barba, F.J.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Castagnini, J.M.; Tappi, S.; Rocculi, P. Pulsed Electric Fields (PEF) and Accelerated Solvent Extraction (ASE) for Valorization of Red (Aristeus antennatus) and Camarote (Melicertus kerathurus) Shrimp Side Streams: Antioxidant and HPLC Evaluation of the Carotenoid Astaxanthin Recovery. Antioxidants 2023, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Chatatikun, M.; Supjaroen, P.; Promlat, P.; Chantarangkul, C.; Waranuntakul, S.; Nawarat, J.; Tangpong, J. Antioxidant and Tyrosinase Inhibitory Properties of an Aqueous Extract of Garcinia atroviridis griff. ex. T. Anderson Fruit Pericarps. Pharmacogn. J. 2020, 12, 71–78. [Google Scholar] [CrossRef]
- Lux, P.E.; Freiling, M.; Stuetz, W.; von Tucher, S.; Carle, R.; Steingass, C.B.; Frank, J. (Poly)phenols, Carotenoids, and Tocochromanols in Corn (Zea mays L.) Kernels As Affected by Phosphate Fertilization and Sowing Time. J. Agric. Food Chem. 2020, 68, 612–622. [Google Scholar] [CrossRef]
- Ulagesan, S.; Eom, T.; Nam, T.-J.; Choi, Y.-H. Antioxidant and Chemoprotective Peptides from Simulated Gastrointestinal Digested (SGID) Protein Hydrolysate of Pyropia yezoensis Against Acetaminophen-Induced HepG2 Cells. Bioprocess. Biosyst. Eng. 2022, 45, 1645–1660. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Criado, M.N.; Belda-Galbis, C.M.; Esteve, M.J.; Rodrigo, D. Stevia rebaudiana Bertoni as a Natural Antioxidant/Antimicrobial for High Pressure Processed Fruit Extract: Processing Parameter Optimization. Food Chem. 2014, 148, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E.; et al. Phytochemical Constitution, Anti-Inflammation, Anti-Androgen, and Hair Growth-Promoting Potential of Shallot (Allium ascalonicum L.) Extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Lin, Y.-S.; Huang, Y.-W.; Fang, S.-U.; Lin, S.-Y.; Hou, W.-C. Protective Effects of Minor Components of Curcuminoids on Hydrogen Peroxide-Treated Human HaCaT Keratinocytes. J. Agric. Food Chem. 2016, 64, 3598–3608. [Google Scholar] [CrossRef]

| Code | Extraction Method | Solvent | (°C) | Time (min) | Yield (%) | TPC (mg GAE/g DM) | TFC (mg QE/100 g DM) | ABTS (µmol TE/g DM) | ORAC (µM TE/100 g DM) |
|---|---|---|---|---|---|---|---|---|---|
| C-1 | CE | Water | 25 | 15 | 16.2 ± 0.18 f | 188.45 ± 4.96 d | 180.97 ± 6.34 e,f | 2353.24 ± 112.51 f | 2924.75 ± 57.82 d |
| C-2 | 25 | 120 | 17.8 ± 0.44 e | 246.13 ± 12.31 c | 409.01 ± 10.23 c,d | 2983.94 ± 50.71 d | 2873.19 ± 59.98 d,e | ||
| C-3 | 50% EtOH | 25 | 15 | 27.4 ± 0.56 b | 542.64 ± 27.14 b | 2074.69 ± 134.12 b | 11,566.38 ± 44.25 b | 6358.00 ± 162.74 b | |
| C-4 | 25 | 120 | 29.7 ± 0.48 a | 668.56 ± 11.52 a | 2629.92 ± 112.61 a | 12,645.50 ± 60.31 a | 7180.27 ± 101.79 a | ||
| A-1 | ASE | Water | 40 | 25 | 13.5 ± 0.12 h | 52.11 ± 3.50 f | 61.17 ± 2.05 f | 639.67 ± 12.31 i | 2423.08 ± 175.37 f |
| A-2 | 120 | 25 | 14.7 ± 0.24 g,h | 106.42 ± 4.40 e | 282.37 ± 6.70 d,e | 2489.02 ± 6.64 f | 4890.34 ± 330.55 c | ||
| A-3 | 50% EtOH | 40 | 25 | 24.1 ± 0.60 d | 132.09 ± 5.54 e | 474.43 ± 12.38 c | 2699.54 ± 12.06 e | 6758.86 ± 130.26 a,b | |
| A-4 | 120 | 25 | 25.6 ± 0.28 c | 166.01 ± 1.83 d | 543.12 ± 6.13 c | 3613.60 ± 18.09 c | 6770.08 ± 43.73 a,b | ||
| P-1 | PEF | Water | 25 | 15 | 8.3 ± 0.80 k | 18.67 ± 0.61 g | ND | 1151.60 ± 6.36 h | 642.34 ± 47.76 g |
| P-2 | 25 | 120 | 10.0 ± 0.49 j | 8.81 ± 0.11 g | ND | 1062.28 ± 51.60 h | 927.87 ± 6.46 g | ||
| P-3 | 50% EtOH | 25 | 15 | 11.7 ± 0.14 i | 125.09 ± 2.81 e | 131.39 ± 3.06 e,f | 1616.93 ± 53.48 g | 2489.13 ± 211.48 e,f | |
| P-4 | 25 | 120 | 15.0 ± 0.49 f,g | 58.47 ± 3.72 f | 141.31 ± 2.59 e,f | 1543.30 ± 32.80 g | 2717.73 ± 26.60 d,e,f |
| No. | Compound | MS | Concentrations mg/g Extract | |||
|---|---|---|---|---|---|---|
| C-3 | C-4 | A-3 | A-4 | |||
| 1 | Gallic acid | 170.20 | 1.618 ± 0.032 | 1.300 ± 0.018 | 1.155 ± 0.005 | 1.542 ± 0.127 |
| 2 | p-Coumaric acid | 164.16 | ND | 0.588 ± 0.008 | 0.139 ± 0.020 | 0.167± 0.007 |
| 3 | Rutin | 610.52 | 0.031 ± 0.001 | 0.027 ± 0.002 | 0.041 ± 0.000 | 0.011± 0.001 |
| 4 | Rosmarinic acid | 360.31 | 0.254 ± 0.008 | 0.254 ± 0.001 | 0.370 ± 0.002 | 0.245 ± 0.002 |
| 5 | Caffeic acid | 180.16 | 0.309 ± 0.118 | 0.144 ± 0.033 | ND | 0.193 ± 0.006 |
| 6 | Epicatechin | 290.28 | 0.073 ± 0.004 | 0.080 ± 0.002 | ND | ND |
| Code | Extraction Method | Solvent | Temperature (°C) | Time (min) |
|---|---|---|---|---|
| C-1 | CE | Water | 25 | 15 |
| C-2 | Water | 25 | 120 | |
| C-3 | 50% (v/v) Ethanol | 25 | 15 | |
| C-4 | 50% (v/v) Ethanol | 25 | 120 | |
| A-1 | ASE | Water | 40 | 25 |
| A-2 | Water | 120 | 25 | |
| A-3 | 50% (v/v) Ethanol | 40 | 25 | |
| A-4 | 50% (v/v) Ethanol | 120 | 25 | |
| P-1 | PEF | Water | 25 | 15 |
| P-2 | Water | 25 | 120 | |
| P-3 | 50% (v/v) Ethanol | 25 | 15 | |
| P-4 | 50% (v/v) Ethanol | 25 | 120 |
| Primers | Forward Primer | Reverse Primer |
|---|---|---|
| SOD1 | AGGGCATCATCAATTTCGAG | ACATTGCCCAAGTCTCCAAC |
| CAT | CATCGCCACATGAATGGATA | CCAACTGGGATGAGAGGGTA |
| GPX | TTCCCGTGCAACCAGTTTG | GGACGTACTTGAGGGAATTCAGA |
| GADPH | GGAAGGTGAAGGTCGGAGTC | CTCAGCCTTGACGGTGCCATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaithep, T.; Muangsanguan, A.; Castagnini, J.M.; Marti-Quijal, F.J.; Sringarm, K.; Arjin, C.; Rachtanapun, P.; Barba, F.J.; Ruksiriwanich, W. Comparative Study on Antioxidant Potential of Schinus terebinthifolius Extracts Prepared by Conventional Extraction, Accelerated Solvent Extraction, and Pulsed Electric Field Method. Molecules 2025, 30, 3589. https://doi.org/10.3390/molecules30173589
Chaithep T, Muangsanguan A, Castagnini JM, Marti-Quijal FJ, Sringarm K, Arjin C, Rachtanapun P, Barba FJ, Ruksiriwanich W. Comparative Study on Antioxidant Potential of Schinus terebinthifolius Extracts Prepared by Conventional Extraction, Accelerated Solvent Extraction, and Pulsed Electric Field Method. Molecules. 2025; 30(17):3589. https://doi.org/10.3390/molecules30173589
Chicago/Turabian StyleChaithep, Tanakarn, Anurak Muangsanguan, Juan M. Castagnini, Francisco J. Marti-Quijal, Korawan Sringarm, Chaiwat Arjin, Pornchai Rachtanapun, Francisco J. Barba, and Warintorn Ruksiriwanich. 2025. "Comparative Study on Antioxidant Potential of Schinus terebinthifolius Extracts Prepared by Conventional Extraction, Accelerated Solvent Extraction, and Pulsed Electric Field Method" Molecules 30, no. 17: 3589. https://doi.org/10.3390/molecules30173589
APA StyleChaithep, T., Muangsanguan, A., Castagnini, J. M., Marti-Quijal, F. J., Sringarm, K., Arjin, C., Rachtanapun, P., Barba, F. J., & Ruksiriwanich, W. (2025). Comparative Study on Antioxidant Potential of Schinus terebinthifolius Extracts Prepared by Conventional Extraction, Accelerated Solvent Extraction, and Pulsed Electric Field Method. Molecules, 30(17), 3589. https://doi.org/10.3390/molecules30173589












