Seasonal Variation in Essential Oil Composition and Bioactivity of Three Ocimum Species from Nepal
Abstract
1. Introduction
2. Results and Discussion
2.1. Variation in the Yield of Essential Oils
2.2. Essential Oil Composition
2.3. Enantiomeric Distributions of Essential Oils
2.4. Hierarchical Cluster Analysis of Ocimum Essential Oils
2.5. Antimicrobial Efficacy
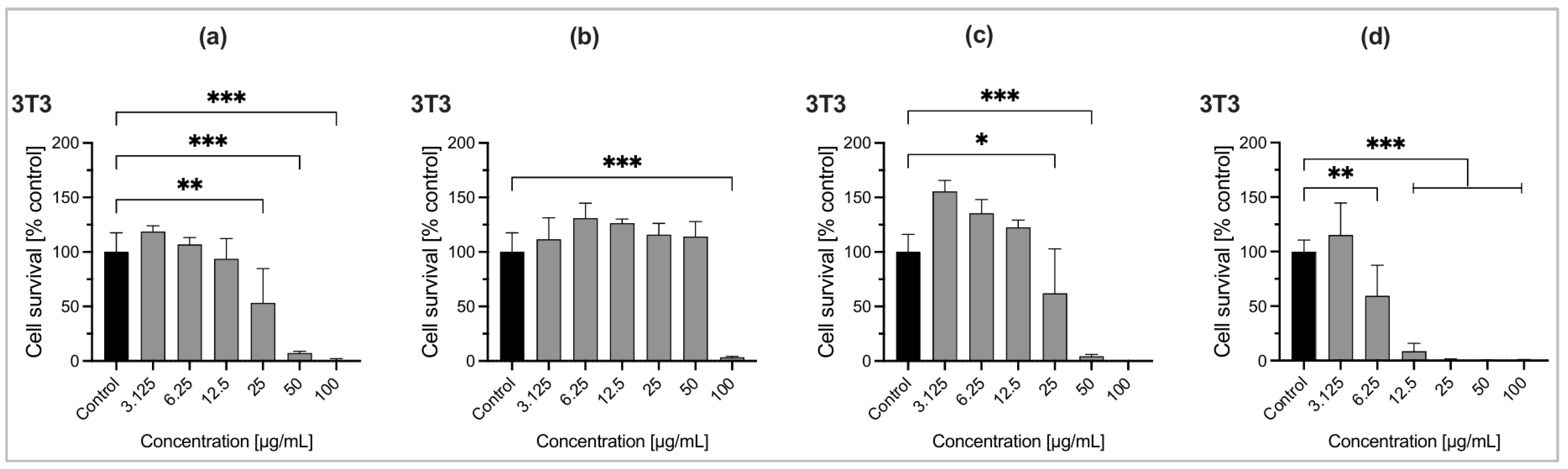
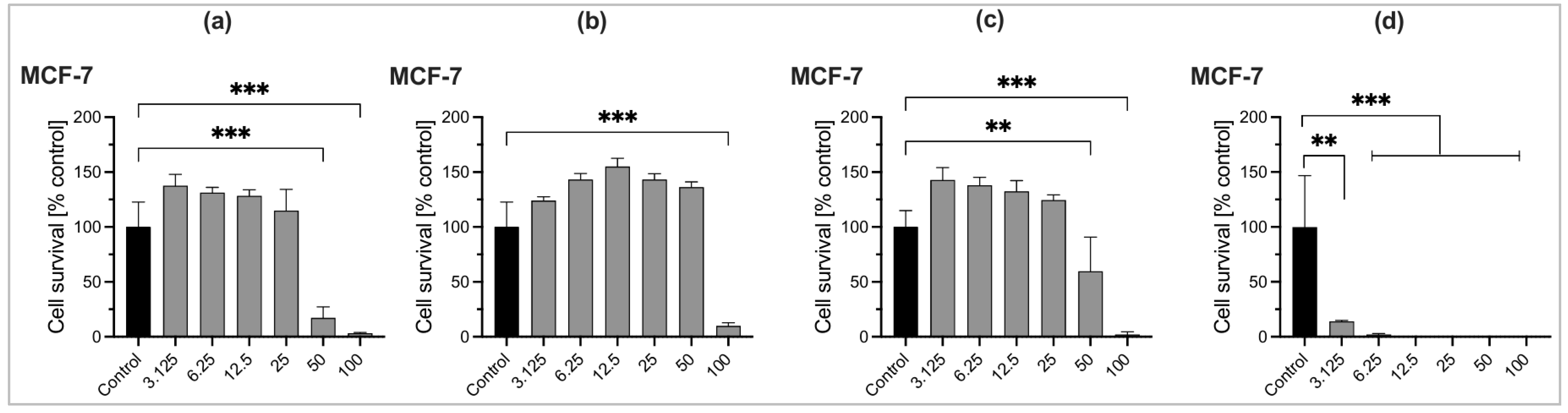
2.6. Cytotoxic Activity of Essential Oils
2.7. Antioxidant Activity (DPPH and ABTS Assays)
3. Materials and Methods
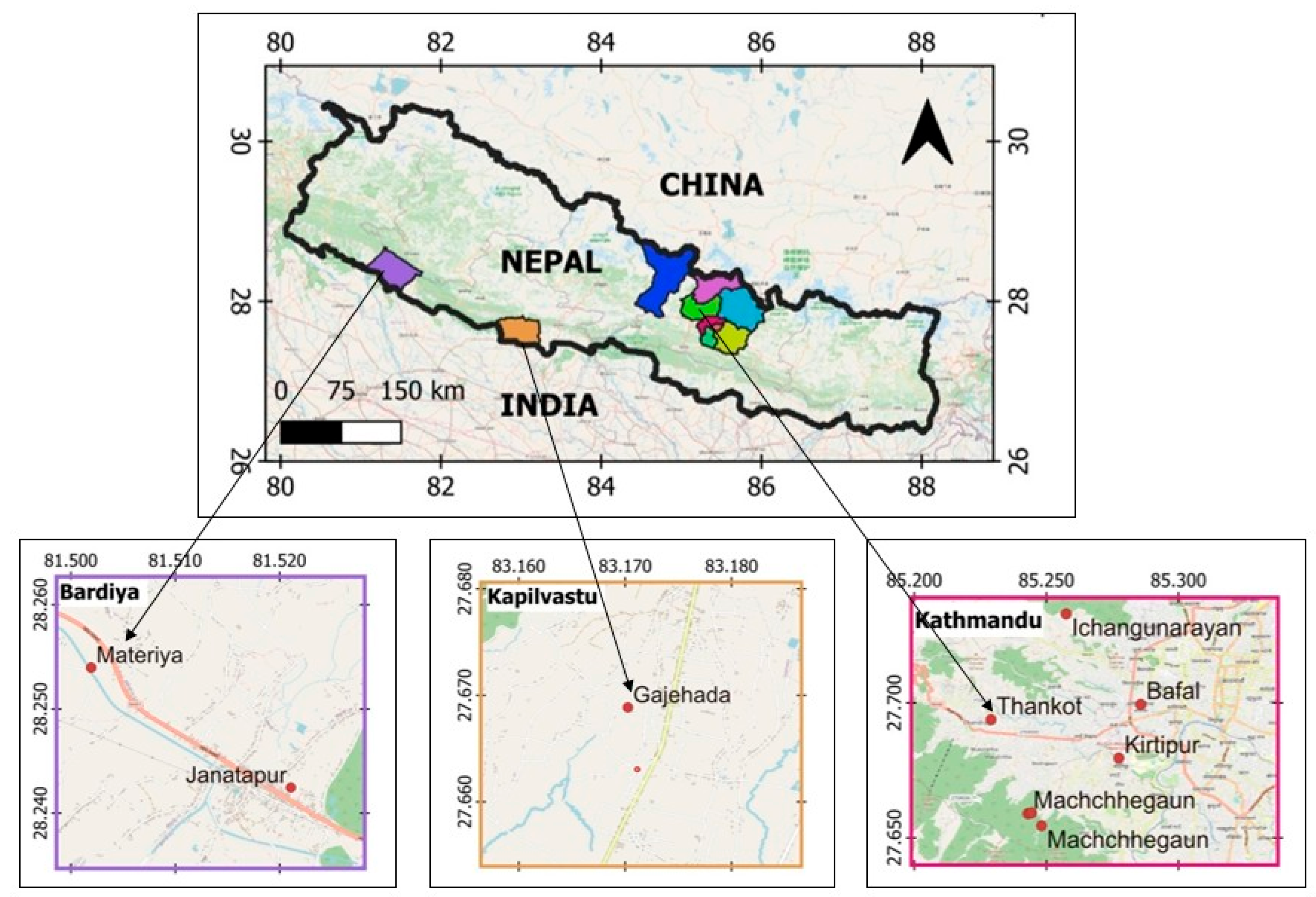
3.1. Collection of Plant Materials
3.2. Essential Oil Extraction
3.3. Gas Chromatography–Mass Spectrometry Analysis
3.4. Hierarchical Cluster Analysis
3.5. Antimicrobial Analysis
3.6. Cytotoxicity Analysis
3.7. Antioxidant Potential
3.7.1. DPPH Radical-Scavenging Assay
3.7.2. ABTS Radical-Scavenging Assay
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC-MS | Gas Chromatography–Mass Spectrometry |
| EOs | Essential Oils |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid |
| IC50 | Inhibitory Concentration at 50% |
| RI | Retention Index |
| RSA | Radical Scavenging Activity |
| ATCC | American Type Culture Collection |
| DMSO | Dimethyl Sulphoxide |
| SM | Supplementary Materials |
| BHT | Butylated Hydroxytoluene |
| HCA | Hierarchial Cluster Analysis |
| MIC | Minimum Inhibitory Concentration |
| DMEM | Dulbecco’s Modified Eagle Medium |
| CAMHB | Cation-adjusted Muller Hinton Broth |
| PBS | Phosphate-Buffered Saline |
References
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Stefan, M.; Zamfirache, M.M.; Padurariu, C.; Trutǎ, E.; Gostin, I. The composition and antibacterial activity of essential oils in three Ocimum species growing in Romania. Cent. Eur. J. Biol. 2013, 8, 600–608. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Tekwani, B.; Khan, S.I. Content, composition, and bioactivity of the essential oils of three basil genotypes as a function of harvesting. J. Agric. Food Chem. 2008, 56, 380–385. [Google Scholar] [CrossRef]
- Pérez-González, C.; Pérez-Ramos, J.; Alberto Méndez-Cuesta, C.; Serrano-Vega, R.; Martell-Mendoza, M.; Pérez-Gutiérrez, S. Cytotoxic activity of essential oils of some species from Lamiaceae family. Cytotox.—Defin. Identif. Cytotoxic Compd. 2019, 1–15. [Google Scholar] [CrossRef]
- Sajjadi, S.E. Analysis of the essential oil of two cultivated Basil (Ocimum basilicum L.) from Iran. Department of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Sciences. Daru 2006, 14, 128–130. [Google Scholar]
- Ahmed, A.F.; Attia, F.A.K.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Jahanger, M.A.; Patra, K.K.; Kumari, S.; Singh, A.; Manika, N.; Srivastava, R.P.; Saxena, G.; Singh, L. A glance at the phytochemical and ethno-pharmacological understanding of four Ocimum species. Curr. Pharm. Biotechnol. 2023, 24, 1094–1107. [Google Scholar] [CrossRef]
- ACFPN. Annotated Checklist of the Flowering Plants of Nepal. 2023. Available online: http://www.efloras.org/florataxon.aspx?flora_id=110&taxon_id=122604 (accessed on 16 May 2023).
- Aissi, O.; Boussaid, M.; Messaoud, C. Essential oil composition in natural populations of Pistacia lentiscus L. from Tunisia: Effect of ecological factors and incidence on antioxidant and antiacetylcholinesterase activities. Ind. Crops Prod. 2016, 91, 56–65. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Van Zyl, R.L.; Van Vuuren, S.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Viljoen, A.M. Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. South Afr. J. Bot. 2008, 74, 230–237. [Google Scholar] [CrossRef]
- Tran, T.H.; Cam Quyen, N.T.; Kieu Linh, H.T.; Le Ngoc, T.T.; Quan, P.M.; Toan, T.Q. Essential oil from vietnamese mandarin (Citrus reticulata blanco) using hydrodistillation extraction process and identification of it’s components. Solid State Phenom. 2019, 298, 100–105. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, H.; Tufail, S.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Smitha, G.R.; Tripathy, V. Seasonal variation in the essential oils extracted from leaves and inflorescence of different Ocimum species grown in Western plains of India. Ind. Crops Prod. 2016, 94, 52–64. [Google Scholar]
- Dangol, S.; Poudel, D.K.; Ojha, P.K.; Maharjan, S.; Poudel, A.; Satyal, R.; Rokaya, A.; Timsina, S.; Dosoky, N.S.; Satyal, P.; et al. Essential oil composition analysis of cymbopogon species from eastern Nepal by GC-MS and chiral GC-MS, and antimicrobial activity of some major compounds. Molecules 2023, 28, 543. [Google Scholar] [CrossRef]
- Ojha, P.K.; Poudel, D.K.; Dangol, S.; Rokaya, A.; Timsina, S.; Satyal, P.; Setzer, W.N. Volatile constituent analysis of wintergreen essential oil and comparison with synthetic methyl salicylate for authentication. Plants 2022, 11, 1090. [Google Scholar] [CrossRef]
- Ojha, P.K.; Poudel, D.K.; Rokaya, A.; Satyal, R.; Setzer, W.N.; Satyal, P. Comparison of volatile constituents present in commercial and lab-distilled frankincense (Boswellia carteri) essential oils for authentication. Plants 2022, 11, 2134. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Camphor and eucalyptol—Anticandidal spectrum, antivirulence effect, efflux pumps interference and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Jianhua, W.; Hai, W. Antifungal susceptibility analysis of berberine, baicalin, eugenol and curcumin on Candida albicans. J. Med. Coll. PLA 2009, 24, 142–147. [Google Scholar] [CrossRef]
- Adiguzel, A.; Gulluce, M.; Sengul, M.; Ogutcu, H.; Sahin, F.; Karaman, I. Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turk. J. Biol. 2005, 29, 155–160. [Google Scholar]
- Yamani, H.A.; Pang, E.C.; Mantri, N.; Deighton, M.A. Antimicrobial activity of Tulsi (Ocimum tenuiflorum) essential oil and their major constituents against three species of bacteria. Front. Microbiol. 2016, 7, 681. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Sethi, V. Antimicrobial activity of essential oils from spices. J. Food Sci. Technol. 1994, 31, 68–70. [Google Scholar]
- Phan, N.D.; Omar, A.M.; Takahashi, I.; Baba, H.; Okumura, T.; Imura, J.; Okada, T.; Toyooka, N.; Fujii, T.; Awale, S. Nicolaioidesin C: An antiausterity agent shows promising antitumor activity in a pancreatic cancer xenograft mouse model. J. Nat. Prod. 2023, 86, 1402–1410. [Google Scholar] [CrossRef]
- Gad, S.C. Alternatives to in vivo studies in toxicology. Gen. Appl. Toxicol. 2009, 6. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry 2004, 65, 881–884. [Google Scholar] [CrossRef]
- Sylvestre, M.; Legault, J.; Dufour, D.; Pichette, A. Chemical composition and anticancer activity of leaf essential oil of Myrica gale L. Phytomedicine 2005, 12, 299–304. Phytomedicine 2005, 12, 299–304. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components? An experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef]
- Badr, M.M.; Taktak, N.E.M.; Badawy, M.E.I. Comparison of the antimicrobial and antioxidant activities of tea tree (Melaleuca alternifolia) oil and its main component terpinen-4-ol with their nanoemulsions. Egypt. J. Chem. 2023, 66, 111–120. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.D.D.S.; De Oliveira E Silva, A.M.; Blank, A.F.; Nogueira, P.C.D.L.; Nizio, D.A.D.C.; Almeida-Pereira, C.S.; Pereira, R.O.; Menezes-Sá, T.S.A.; Santana, M.H.D.S.; Arrigoni-Blank, M.D.F. Radical scavenging activity of the essential oils from Croton grewioides Baill accessions and the major compounds eugenol, methyl eugenol and methyl chavicol. J. Essent. Oil Res. 2021, 33, 94–103. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Guerra-Boone, L.; Alvarez-Román, R.; Salazar-Aranda, R.; Torres-Cirio, A.; Rivas-Galindo, V.M.; de-Torres, N.W.; González, G.; Pérez-López, L.A. Antimicrobial and antioxidant activities and chemical characterization of essential oils of Thymus vulgaris, Rosmarinus officinalis, and Origanum majorana from northeastern México. Pak. J. Pharm. Sci. 2015, 28, 363–369. [Google Scholar]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Joshi, R. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J. Pharm. Sci. 2013, 75, 457–462. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, Y.R. Variations in compositions and antioxidant activities of essential oils from leaves of Luodian Blumea balsamifera from different harvest times in China. PLoS ONE 2020, 15, e0234661. [Google Scholar] [CrossRef]
- Chu, S.S.; Jiang, G.H.; Liu, Z.L. Insecticidal compounds from the essential oil of Chinese medicinal herb Atractylodes chinensis. Pest Manag. Sci. 2011, 67, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.N.; Satyal, P.; Satyal, R.; Setzer, W.N.; Gyawali, R. Chemical composition, enantiomeric distribution, antimicrobial and antioxidant activities of Origanum majorana L. essential oil from Nepal. Molecules 2022, 27, 6136. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, A.; Johnson, S.; Poudel, A.; Satyal, P.; Bangerter, L.; Setzer, W.N. Chemical variation in essential oils from the oleo-gum resin of Boswellia carteri: A preliminary investigation. Chem. Biodivers. 2018, 15, e1800047. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2015; p. 63. [Google Scholar]
- Poudel, D.K.; Ojha, P.K.; Rokaya, A.; Satyal, R.; Satyal, P.; Setzer, W.N. Analysis of volatile constituents in Curcuma species, viz. C. aeruginosa, C. zedoaria, and C. longa, from Nepal. Plants 2022, 11, 1932. [Google Scholar]
- Decarlo, A.; Zeng, T.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The essential oil composition and antimicrobial activity of liquidambar Formosana oleoresin. Plants 2020, 9, 822. [Google Scholar] [CrossRef]
- Sahm, D.H.; Washington, J.A. Antibacterial susceptibility tests: Dilution methods. In Manual of Clinical Microbiology, 5th ed.; Balows, A., Hausler, W.J., Herrmann, K.L., Isenberg, H.D., Shamody, H.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1991; pp. 1105–1116. [Google Scholar]
- Kim, M.K.; Lee, H.S.; Kim, E.J.; Won, N.H.; Chi, Y.M.; Kim, B.C.; Lee, K.W. Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem. Toxicol. 2007, 45, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Modi, H.A. Comparative study of DPPH, ABTS and FRAP sssays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. (IJRASET) 2015, 3, 636–641. [Google Scholar]



| Lit. RI | Exp. RI | Components | O. tenuiflorum (Bardiya-Winter) (%) | O. tenuiflorum (Bardiya-Autumn) (%) |
|---|---|---|---|---|
| Monoterpene hydrocarbons | 0.20% | 0.31% | ||
| 932 | 933 | α-Pinene | 0.02 | 0.12 |
| 946 | 950 | Camphene | - | 0.06 |
| 969 | 972 | Sabinene | 0.03 | 0.02 |
| 974 | 978 | β-Pinene | 0.08 | 0.05 |
| 988 | 989 | Myrcene | 0.01 | - |
| 1024 | 1029 | Limonene | 0.04 | 0.06 |
| 1032 | 1035 | (Z)-β-Ocimene | 0.01 | - |
| 1044 | 1046 | (E)-β-Ocimene | 0.01 | - |
| Oxygenated monoterpenoids | 1.15% | 0.99% | ||
| 1026 | 1032 | 1,8-Cineol | 0.01 | 0.06 |
| - | 1044 | Dihydrotagetone | 1.02 | - |
| 1095 | 1099 | Linalool | 0.02 | 0.52 |
| 1101 | 1105 | cis-Thujone | 0.03 | - |
| 1160 | 1163 | Pinocarvone | 0.04 | - |
| 1165 | 1173 | Borneol | - | 0.41 |
| Sesquiterpene hydrocarbons | 57.11% | 62.54% | ||
| - | 1381 | cis-β-Elemene | 1.72 | 1.92 |
| - | 1391 | (E)-β-Elemene | 29.08 | 32.85 |
| 1403 | 1404 | Methyl eugenol | 1.21 | 1.43 |
| 1400 | 1400 | β-Longipinene | 0.04 | - |
| 1408 | 1405 | cis-Caryophyllene | - | 0.02 |
| 1407 | 1415 | α-Barbatene | 0.19 | 0.2 |
| 1417 | 1418 | β-Caryophyllene | 19.85 | 21.64 |
| - | 1394 | epi-Cubebene isomer | - | 0.02 |
| 1429 | 1423 | cis-Thujopsene | - | 0.03 |
| 1436 | 1437 | Isobazzanene | - | 0.08 |
| 1440 | 1447 | β-Barbatene | 0.22 | 0.23 |
| 1454 | 1452 | (E)-β-Farnesene | - | 0.06 |
| 1452 | 1454 | α-Humulene | 1.12 | 1.18 |
| 1476 | 1483 | β-Chamigrene | 0.24 | 0.2 |
| 1475 | 1480 | γ-Gurjunene | - | 0.03 |
| 1489 | 1489 | β-Selinene | 1.06 | 0.81 |
| 1496 | 1499 | Valencene | 0.04 | 0.04 |
| 1498 | 1498 | α-Selinene | 1.01 | 0.92 |
| 1505 | 1505 | α-Cuprenene | - | 0.03 |
| 1508 | 1505 | Germacrene A | 1.19 | 0.69 |
| 1513 | 1514 | γ-Cadinene | 0.07 | - |
| 1522 | 1519 | δ-Cadinene | - | 0.01 |
| 1520 | 1522 | 7-epi-a-Selinene | - | 0.04 |
| - | 1523 | 1,4-Dihydro cuparene | - | 0.03 |
| 1529 | 1528 | (E)-γ-Bisabolene | 0.04 | 0.07 |
| - | 1599 | Bisabolenol isomer | 0.03 | - |
| 1532 | 1534 | γ-Cuprenene | - | 0.03 |
| Oxygenated sesquiterpenoids | 7.28% | 1.15% | ||
| - | 1457 | Dehydrosesquicineole | 0.02 | - |
| 1471 | 1468 | 4,5-Di-epi-aristolochene | - | 0.02 |
| - | 1533 | 10-epi-Cubenol | 0.07 | 0.02 |
| - | 1549 | α-Elemol | - | 0.04 |
| 1582 | 1583 | Caryophyllene oxide | 3.37 | 0.75 |
| 1608 | 1612 | Humulene epoxide II | 0.35 | 0.03 |
| 1608 | 1606 | β-Atlantol | 0.04 | - |
| - | 1617 | Intermedeol isomer | 0.15 | 0.04 |
| 1642 | 1644 | Selina-3,11-dien-6-a-ol | - | 0.02 |
| 1658 | 1656 | neo-Intermedeol | - | 0.19 |
| 1639 | 1634 | Caryophylla-4(12),8(13)-dien-5-β-ol | 0.14 | - |
| 1639 | 1636 | allo-Aromadendrene epoxide | 0.05 | - |
| 1658 | 1659 | Selin-11-en-4-a-ol | 1.45 | - |
| - | 1668 | Isospathulenol | 0.97 | 0.06 |
| 1683 | 1683 | epi-a-Bisabolol | 0.04 | - |
| - | 1644 | Sesquiterpineol | 0.63 | - |
| Others | 33.53% | 35.49% | ||
| 850 | 860 | (3Z)-Hexenol | 0.07 | 0.07 |
| - | 896 | 2,5-Diethyl tetrahydro furan | - | 0.01 |
| 974 | 982 | 1-Octen-3-ol | 0.02 | 0.02 |
| 988 | 996 | 3-Octanol | - | 0.01 |
| 1195 | 1198 | Methyl chavicol | 0.01 | - |
| - | 1294 | Dihydroedulan | 0.03 | - |
| 1306 | 1357 | Eugenol | 32.15 | 34.95 |
| 1100 | 1105 | n-Nonanal | 0.02 | - |
| 1436 | 1437 | Isobazzanene | 0.08 | - |
| - | 1726 | Sesquiterpinyl alcohol | 1.16 | - |
| - | 1846 | Phytone | 0.03 | 0.1 |
| - | 1879 | Phytadiene isomer | - | 0.03 |
| 2300 | 2300 | Tricosane | - | 0.03 |
| 2400 | 2400 | Tetracosane | - | 0.04 |
| 2500 | 2498 | Pentacosane | - | 0.05 |
| 2600 | 2600 | Hexacosane | - | 0.04 |
| 2700 | 2698 | Heptacosane | - | 0.03 |
| Total | 98.28 | 99.71 | ||
| EO yield (%) | 0.50 ± 0.05 | 1.67 ± 0.13 | ||
| Lit. RI | Exp. RI | Components | O. americanum (Thankot-Winter) (%) | O. americanum (Thankot-Summer) (%) |
|---|---|---|---|---|
| Monoterpene hydrocarbons | 11.35% | 6.64% | ||
| 924 | 925 | α-Thujene | 0.03 | - |
| 932 | 933 | α-Pinene | 0.35 | 0.19 |
| 946 | 950 | Camphene | 1.44 | 0.72 |
| 969 | 972 | Sabinene | 0.09 | 0.14 |
| 974 | 978 | β-Pinene | 0.38 | 0.24 |
| 988 | 989 | Myrcene | 0.58 | 0.37 |
| 1002 | 1008 | α-Phellandrene | 0.12 | - |
| 1014 | 1017 | α-Terpinene | 0.06 | - |
| 1020 | 1025 | p-Cymene | 0.18 | 0.69 |
| 1024 | 1029 | Limonene | 4.4 | 3.96 |
| 1025 | 1032 | β-Phellandrene | 0.04 | 0.02 |
| 1032 | 1035 | (Z)-β-Ocimene | 0.12 | - |
| 1044 | 1046 | (E)-β-Ocimene | 2.43 | 0.28 |
| 1054 | 1058 | γ-Terpinene | 0.25 | - |
| 1086 | 1086 | Terpinolene | 0.88 | 0.03 |
| Oxygenated monoterpenoids | 68.57% | 82.38% | ||
| 1026 | 1032 | 1,8-Cineol | 0.31 | 0.31 |
| - | 1044 | Dihydrotagetone | 0.15 | 0.09 |
| 1065 | 1071 | cis-Sabinene hydrate | 1.2 | 1.3 |
| 1095 | 1099 | Linalool | 9.91 | 9.72 |
| 1122 | 1127 | α-Campholenal | 0.18 | - |
| 1141 | 1148 | Camphor | 51.3 | 65.88 |
| 1159 | 1155 | (E)-β-Terpineol | - | 0.41 |
| 1145 | 1156 | Camphene hydrate | 0.3 | - |
| 1148 | 1157 | Menthone | 0.04 | - |
| 1160 | 1163 | Pinocarvone | 0.16 | - |
| - | 1167 | exo-Acetoxy camphene | 0.06 | - |
| 1165 | 1173 | Borneol | 0.46 | 0.26 |
| 1174 | 1181 | Terpinen-4-ol | 1.5 | 1.64 |
| 1179 | 1187 | p-Cymen-8-ol | - | 0.28 |
| 1186 | 1195 | α-Terpineol | 2.54 | 1.6 |
| 1194 | 1194 | Myrtenol | 0.73 | |
| - | 1202 | epi-Borneol | 0.18 | 0.08 |
| - | 1179 | p-1,8-Menthadien-4-ol | 0.1 | 0.13 |
| 1239 | 1242 | Carvone | - | 0.08 |
| - | 1388 | Terpenediol | - | 0.04 |
| Sesquiterpene hydrocarbons | 18.54% | 6.75% | ||
| 1374 | 1376 | α-Copaene | 0.56 | 0.29 |
| 1387 | 1391 | β-Bourbonene | 0.2 | 0.13 |
| 1387 | 1388 | β-Cubebene | 0.16 | 0.1 |
| - | 1391 | (E)-β-Elemene | 0.38 | 0.19 |
| 1417 | 1418 | β-Caryophyllene | 6.35 | 3.97 |
| 1430 | 1431 | β-Copaene | 0.11 | 0.03 |
| 1452 | 1454 | α-Humulene | 0.37 | 0.27 |
| 1484 | 1484 | Germacrene D | 7.75 | 1.38 |
| - | 1482 | iso-bicyclogeramcrene | 1.6 | 0.3 |
| 1500 | 1496 | Bicyclogermacrene | 0.59 | 0.04 |
| 1500 | 1500 | α-Muurolene | 0.08 | - |
| 1513 | 1514 | γ-Cadinene | 0.05 | - |
| 1522 | 1519 | δ-Cadinene | 0.34 | 0.05 |
| Oxygenated sesquiterpenoids | 0.57% | 2.58% | ||
| - | 1549 | Isocaryphyllene oxide | - | 0.09 |
| 1577 | 1579 | Spathulenol | - | 0.29 |
| 1582 | 1583 | Caryophyllene oxide | 0.24 | 1.91 |
| 1608 | 1612 | Humulene epoxide II | - | 0.07 |
| 1639 | 1636 | allo-Aromadendrene epoxide | - | 0.16 |
| 1601 | 1594 | (E)-β-Elemenone | 0.04 | - |
| 1638 | 1643 | epi-α-Cadinol | 0.11 | - |
| 1640 | 1642 | epi-α-Muurolol | 0.08 | - |
| 1514 | 1513 | γ-Cadinene | 0.05 | |
| Others | 0.96% | 1.49% | ||
| 850 | 860 | (3Z)-Hexenol | 0.11 | 0.27 |
| - | 880 | 2-Butyl furan | - | 0.04 |
| - | 960 | Benzaldehyde | - | 0.03 |
| 974 | 982 | 1-Octen-3-ol | 0.1 | 0.07 |
| 988 | 996 | 3-Octanol | 0.15 | 0.06 |
| - | 1005 | Hexenyl acetate | 0.1 | - |
| 1100 | 1105 | n-Nonanal | 0.06 | - |
| 1195 | 1198 | Methyl chavicol | 0.13 | - |
| - | 1183 | 4-Methyl acetophenone | - | 0.06 |
| 1179 | 1187 | p-Cymen-8-ol | 0.06 | - |
| 1190 | 1199 | Methyl salicylate | 0.07 | 0.06 |
| 1229 | 1230 | (3Z)-Hexenyl 2-methyl butanoate | 0.04 | - |
| 1306 | 1357 | Eugenol | 0.21 | - |
| - | 1345 | 2-Methyl-2-(para-tolyl) propionaldehyde | - | 0.18 |
| - | 1668 | Dihydrogermacrene D | - | 0.12 |
| 1863 | 1827 | cis-Thujopsenic acid | - | 0.1 |
| 1475 | 1473 | trans-Cadina-1(6),4-diene | 0.06 | - |
| 1565 | 1571 | 3-cis-Hexenyl benzoate | 0.05 | - |
| 2300 | 2301 | n-Tricosane | - | 0.05 |
| 2400 | 2401 | n-Tetracosane | - | 0.07 |
| 2500 | 2499 | n-Pentacosane | - | 0.08 |
| 2600 | 2600 | n-Hexacosane | - | 0.07 |
| 2700 | 2698 | n-Heptacosane | - | 0.06 |
| Total | 99.89 | 99.84 | ||
| EO yield (%) | 0.35 ± 0.02 | 0.42 ± 0.03 | ||
| Lit. RT | Exp. RI | Components | O. basilicum (Kapilvastu-Summer) (%) | O. basilicum (Kapilvastu-Summer) (%) |
|---|---|---|---|---|
| Monoterpene hydrocarbons | 0.71% | 0.3% | ||
| 924 | 925 | α-Thujene | 0.02 | 0.02 |
| 932 | 933 | α-Pinene | 0.19 | 0.06 |
| 969 | 972 | Sabinene | 0.03 | 0.03 |
| 974 | 978 | β-Pinene | 0.2 | 0.04 |
| 988 | 989 | Myrcene | 0.04 | 0.04 |
| 1024 | 1029 | Limonene | 0.02 | 0.05 |
| 1032 | 1035 | (Z)-β-Ocimene | - | 0.02 |
| 1044 | 1046 | (E)-β-Ocimene | 0.21 | 0.04 |
| Oxygenated monoterpenoids | 28.09% | 28.65% | ||
| 1026 | 1032 | 1,8-Cineol | 0.05 | 0.6 |
| - | 1044 | Dihydrotagetone | 0.12 | 0.03 |
| 1067 | 1071 | cis-Linalool oxide (furanoid) | 0.13 | 0.14 |
| 1084 | 1087 | trans-Linalool oxide (furanoid) | 0.13 | 0.18 |
| 1083 | 1092 | Fenchone | - | 0.02 |
| 1095 | 1099 | Linalool | 27.05 | 26.92 |
| - | 1131 | Limona ketone | 0.02 | 0.03 |
| 1186 | 1195 | α-Terpineol | 0.01 | - |
| 1235 | 1238 | Neral | 0.24 | 0.28 |
| 1247 | 1258 | Chavicol | 0.02 | 0.01 |
| 1247 | 1252 | p-Anis aldehyde | - | 0.02 |
| 1264 | 1268 | Geranial | 0.31 | 0.11 |
| 1282 | 1291 | (E)-Anethole | 0.12 | 0.16 |
| 1306 | 1357 | Eugenol | 0.02 | 0.02 |
| Sesquiternepe hydrocarbons | 5.6% | 4.09% | ||
| 1374 | 1376 | α-Copaene | 0.08 | 0.08 |
| 1387 | 1388 | β-Cubebene | 0.05 | 0.09 |
| - | 1391 | (E)-β-Elemene | 0.07 | 0.07 |
| 1403 | 1404 | Methyl Eugenol | 0.02 | 0.11 |
| 1409 | 1416 | α-Gurjunene | 0.02 | 0.02 |
| 1417 | 1418 | β-Caryophyllene | 0.79 | 0.24 |
| 1432 | 1434 | trans-a-Bergamotene | 0.58 | 0.68 |
| 1440 | 1440 | (Z)-β-Farnesene | 0.08 | 0.12 |
| 1454 | 1452 | (E)-β-Farnesene | 0.25 | 0.14 |
| 1452 | 1454 | α-Humulene | 0.13 | 0.44 |
| - | 1452 | epi-Caryophyllene | 0.03 | 0.03 |
| 1484 | 1484 | Germacrene D | 0.79 | 0.14 |
| - | 1485 | trans-β-Bergamotene | 0.1 | - |
| 1500 | 1496 | Bicyclogermacrene | 0.09 | 0.03 |
| 1505 | 1507 | β-Bisabolene | 0.1 | 0.02 |
| 1522 | 1519 | δ-Cadinene | 0.04 | 0.04 |
| 1529 | 1528 | (E)-γ-Bisabolene | 2.38 | 1.84 |
| Oxygenated sesquiterpenes | 0.44% | 1.06% | ||
| 1515 | 1515 | Sesquicineole | 0.01 | - |
| 1561 | 1561 | (E)-Nerolidol | 0.07 | 0.07 |
| 1574 | 1577 | Germacrene D-4-ol | 0.04 | 0.02 |
| 1582 | 1583 | Caryophyllene oxide | 0.22 | 0.64 |
| 1608 | 1612 | Humulene epoxide II | 0.02 | - |
| 1649 | 1655 | β-Eudesmol | 0.02 | 0.21 |
| 1685 | 1685 | α-Bisabolol | 0.06 | 0.12 |
| Others | 64.9% | 62.49% | ||
| 846 | 856 | (2E)-Hexenal | 0.01 | - |
| - | 960 | Benzaldehyde | 0.01 | 0.05 |
| 981 | 986 | 6-Methyl-5-hepten-2-one | 0.04 | 0.01 |
| 998 | 1005 | n-Octanal | 0.11 | 0.16 |
| - | 1005 | Hexenyl acetate | 0.03 | 0.03 |
| - | 1101 | 6-Methyl-3,5-heptadien-2-one | 0.01 | 0.01 |
| 1110 | 1107 | 1-Octen-3-yl acetate | 0.01 | - |
| 1195 | 1198 | Methyl chavicol | 64.42 | 62.16 |
| 1211 | 1211 | Octyl acetate | 0.06 | 0.01 |
| 1384 | 1375 | (3Z)-Hexenyl-(3Z)-hexenoate | 0.15 | 0.04 |
| - | 1559 | (E)-p-Methoxycinnamaldehyde | 0.05 | 0.02 |
| Total | 99.74 | 96.59 | ||
| EO yield (%) | 0.66 ± 0.09 | 0.88 ± 0.09 | ||
| Compounds | RT | RT | O. tenuiflorum (Bardiya-Winter) (%) | O. tenuiflorum (Bardiya-Autumn) (%) | ||
|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | (+) | (−) | |
| α-Pinene | 16.40 | 15.92 | 64.32 | 35.68 | 53.62 | 46.38 |
| Sabinene | 19.74 | 20.60 | 20.8 | 79.2 | - | - |
| Camphene | 18.30 | 17.73 | - | - | 2.72 | 97.28 |
| β-Pinene | 20.27 | 20.62 | 33.56 | 66.44 | 19.66 | 80.34 |
| Limonene | 25.99 | 25.06 | 17.14 | 82.86 | 38.12 | 61.88 |
| Borneol | 59.11 | 58.59 | - | - | 0 | 100 |
| β-Caryophyllene | NA | 69.33 | 0 | 100 | 0 | 100 |
| Compounds | RT | RT | O. americanum (Thankot-Winter) (%) | O. americanum (Thankot-Summer) (%) | ||
|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | (+) | (−) | |
| α-Pinene | 16.40 | 15.92 | 71.59 | 28.41 | 67.83 | 32.17 |
| Camphene | 18.30 | 17.73 | 91.39 | 8.61 | 93.19 | 8.81 |
| β-Pinene | 20.27 | 20.62 | 64.97 | 35.03 | 55.41 | 44.59 |
| Limonene | 25.99 | 25.06 | 82.63 | 17.37 | 75.56 | 23.44 |
| cis-Sabinene hydrate | 40.70 | 41.25 | - | - | 91.23 | 8.77 |
| Linalool | 44.69 | 45.30 | 10.27 | 89.73 | 1.36 | 98.64 |
| Camphor | 50.12 | 49.31 | 99.33 | 0.67 | 100 | 0 |
| Terpinen-4-ol | 54.64 | 54.93 | 79.85 | 20.15 | 76.04 | 23.96 |
| Borneol | 59.11 | 58.59 | - | - | 7.4 | 92.6 |
| α-Terpineol | 60.58 | 59.73 | 34.59 | 65.41 | 44.27 | 55.73 |
| β-Caryophyllene | NA | 69.33 | 0 | 100 | 0 | 100 |
| Germacrene D | 73.48 | 73.73 | 0 | 100 | 0 | 100 |
| Compounds | RT | RT | O. basilicum (Kapilvastu-Summer) (%) | O. basilicum (Kapilvastu-Winter) (%) | ||
|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | (+) | (−) | |
| α-Pinene | 16.40 | 15.92 | 55.74 | 44.26 | 56.08 | 43.92 |
| β-Pinene | 20.27 | 20.62 | 78.89 | 21.11 | 57.35 | 42.65 |
| Limonene | 25.99 | 25.06 | 49.05 | 50.95 | 49.45 | 50.55 |
| Linalool | 44.69 | 45.30 | 0.14 | 99.86 | 2.12 | 97.88 |
| α-Terpineol | 60.58 | 59.73 | 21.75 | 78.25 | 25.32 | 74.68 |
| β-Caryophyllene | 69.33 | NA | 0 | 100 | 0 | 100 |
| Germacrene D | 73.48 | 73.73 | 0 | 100 | 0 | 100 |
| β-Bisabolene | 75.55 | 75.73 | 91.71 | 8.29 | 92.20 | 7.80 |
| Name of Micro-Organism | O. tenuiflorum | O. americanum | O. basilicum | |||
|---|---|---|---|---|---|---|
| MICs (µg/mL) | ||||||
| (Bardiya- Winter) | (Bardiya-Autumn) | (Thankot-Summer) | (Thankot-Winter) | (Kapilvastu-Summer) | (Kapilvastu-Winter) | |
| Bacillus cereus (ATCC 11778) | 650 | 1300 | 650 | 1300 | 1579 | 1314 |
| Staphylococcus aureus (ATCC 6538) | 1300 | 650 | 650 | 1300 | 1257 | 1579 |
| Pseudomonas aeruginosa (ATCC 9027) | 1300 | 650 | 650 | 650 | 1300 | 650 |
| Escherichia coli (ATCC 8739) | 650 | 1300 | 1300 | 2600 | 2628 | 1530 |
| Candida albicans (ATCC 10231) | 162.5 | 325 | 350 | 650 | 1314 | 1579 |
| Aspergillusniger (ATCC 16888) | 650 | 1300 | 650 | 1300 | 2600 | 2557 |
| EO Samples and Standard | IC50 (µg/mL) | IC50 (µg/mL) |
|---|---|---|
| NIH-3T3 Cell Line | MCF-7 Cell Line | |
| O. tenuiflorum (Bardiya-Autumn) | 34.62 | 23.43 |
| O. basilicum (Kapilvastu-Winter) | 90.56 | 92.88 |
| O. americanum (Thankot-Summer) | 49.45 | 57.42 |
| Gemcitabine | 0.5175 | 0.4977 |
| EO Samples and Standard | DPPH Radical-Scavenging IC50 Value (µg/mL) | ABTS Radical-Scavenging IC50 Value (µg/mL) |
|---|---|---|
| O. tenuiflorum (Bardiya-Winter) | 78.96 ± 0.1 | 5.88 ± 0.80 |
| O. tenuiflorum (Bardiya-Autumn) | 69.23 ± 0.10 | 9.05 ± 0.24 |
| O. americanum (Thankot-Winter) | 359.17 ± 0.10 | 129.51 ± 1.21 |
| O. americanum (Thankot-Summer) | 452.79 ± 0.90 | 145.67 ± 0.20 |
| O. basilicum (Kapilvastu-Summer) | 448.21 ± 0.09 | 61.40 ± 0.26 |
| O. basilicum (Kapilvastu-Winter) | 236.14 ± 0.09 | 44.38 ± 0.81 |
| Ascorbic acid | 6.37 ± 0.34 | 1.98 ± 1.20 |
| BHT | 12.46 ± 0.09 | - |
| Quercetin | - | 7.79 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, P.N.; Satyal, P.; Setzer, W.N.; Awale, S.; Watanabe, S.; Maneenet, J.; Satyal, R.; Acharya, A.; Shrestha, A.; Gyawali, R. Seasonal Variation in Essential Oil Composition and Bioactivity of Three Ocimum Species from Nepal. Molecules 2025, 30, 3581. https://doi.org/10.3390/molecules30173581
Paudel PN, Satyal P, Setzer WN, Awale S, Watanabe S, Maneenet J, Satyal R, Acharya A, Shrestha A, Gyawali R. Seasonal Variation in Essential Oil Composition and Bioactivity of Three Ocimum Species from Nepal. Molecules. 2025; 30(17):3581. https://doi.org/10.3390/molecules30173581
Chicago/Turabian StylePaudel, Prem Narayan, Prabodh Satyal, William N. Setzer, Suresh Awale, Shiro Watanabe, Juthamart Maneenet, Rakesh Satyal, Ajaya Acharya, Anjila Shrestha, and Rajendra Gyawali. 2025. "Seasonal Variation in Essential Oil Composition and Bioactivity of Three Ocimum Species from Nepal" Molecules 30, no. 17: 3581. https://doi.org/10.3390/molecules30173581
APA StylePaudel, P. N., Satyal, P., Setzer, W. N., Awale, S., Watanabe, S., Maneenet, J., Satyal, R., Acharya, A., Shrestha, A., & Gyawali, R. (2025). Seasonal Variation in Essential Oil Composition and Bioactivity of Three Ocimum Species from Nepal. Molecules, 30(17), 3581. https://doi.org/10.3390/molecules30173581










