Multiple Functions of Carbon Additives in NASICON-Type Electrodes for Stabilizing the Sodium Storage Performance
Abstract
1. Introduction
2. Results
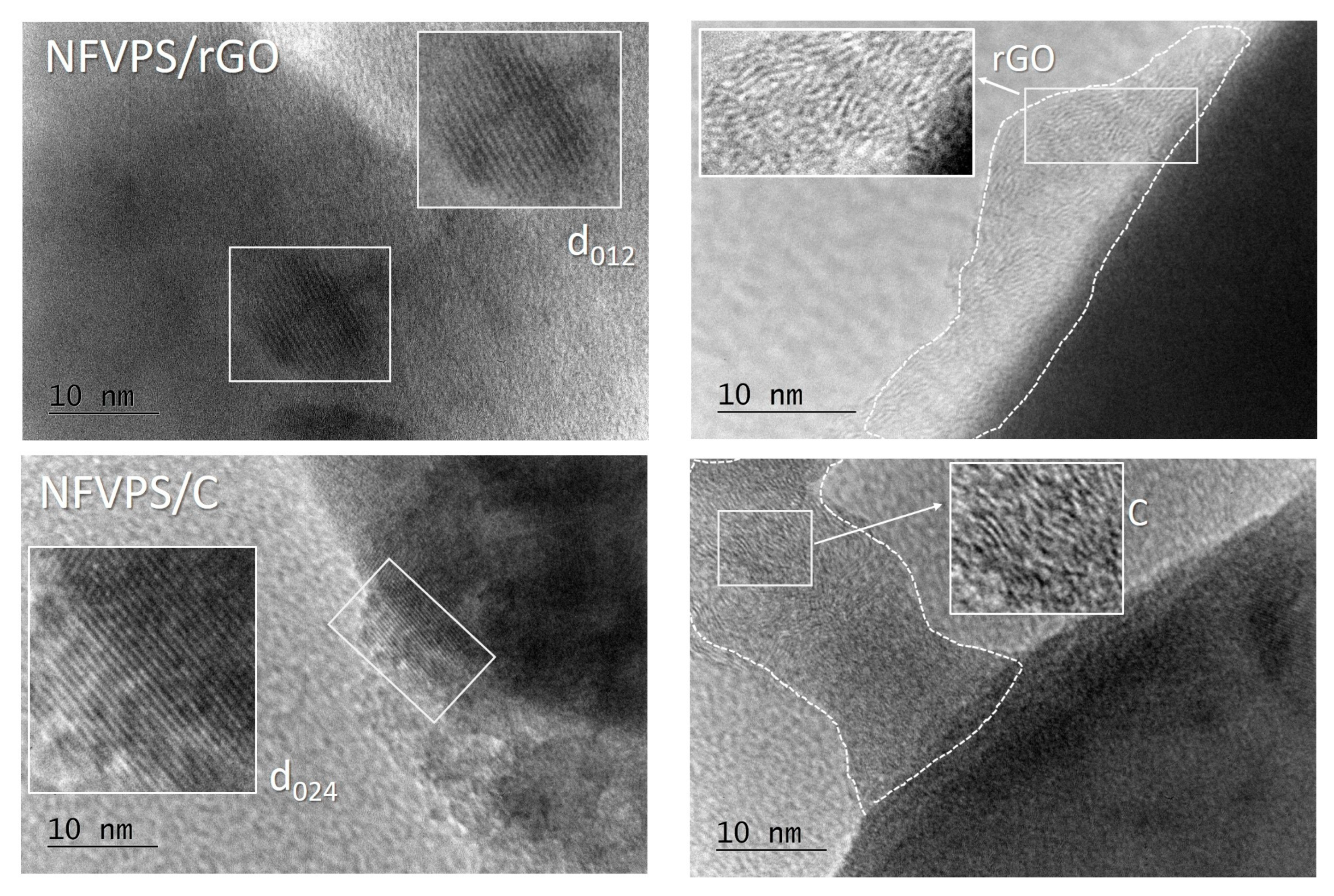
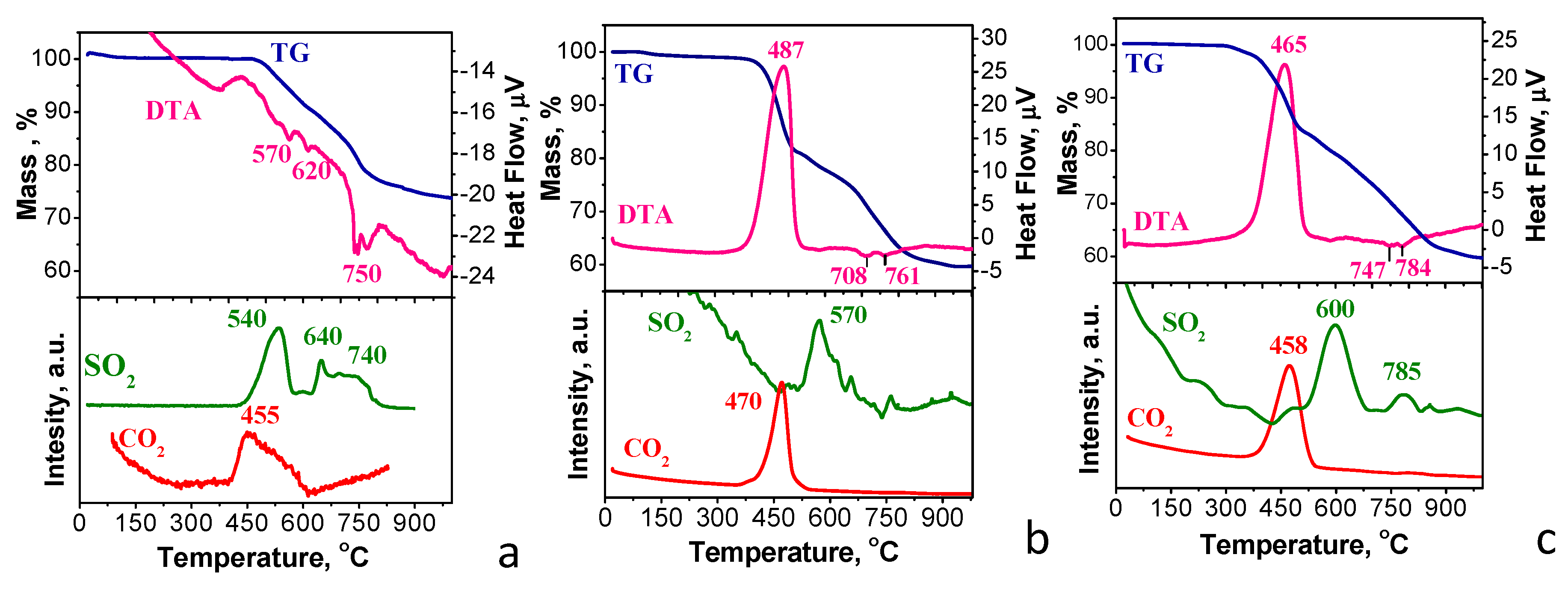
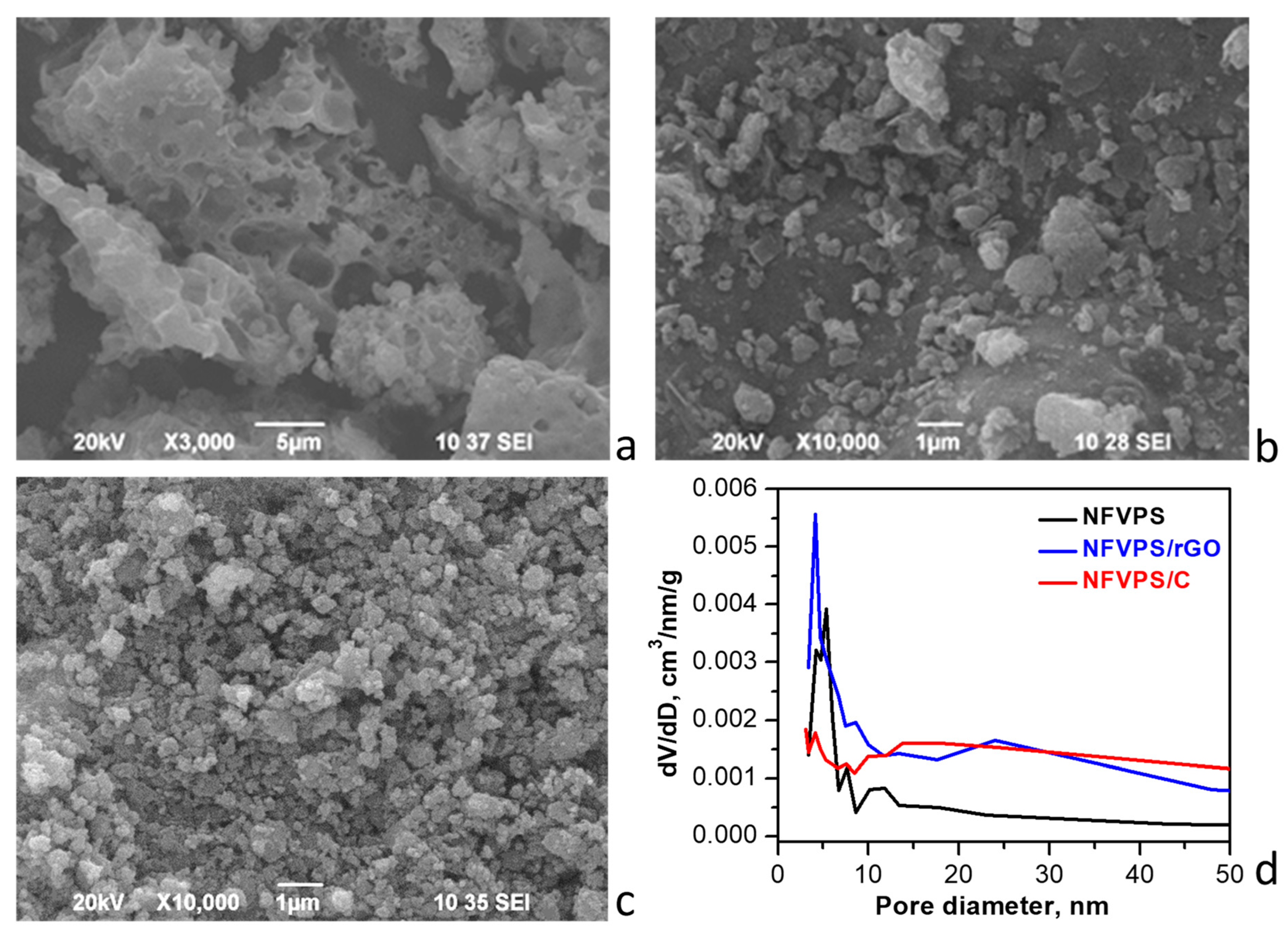
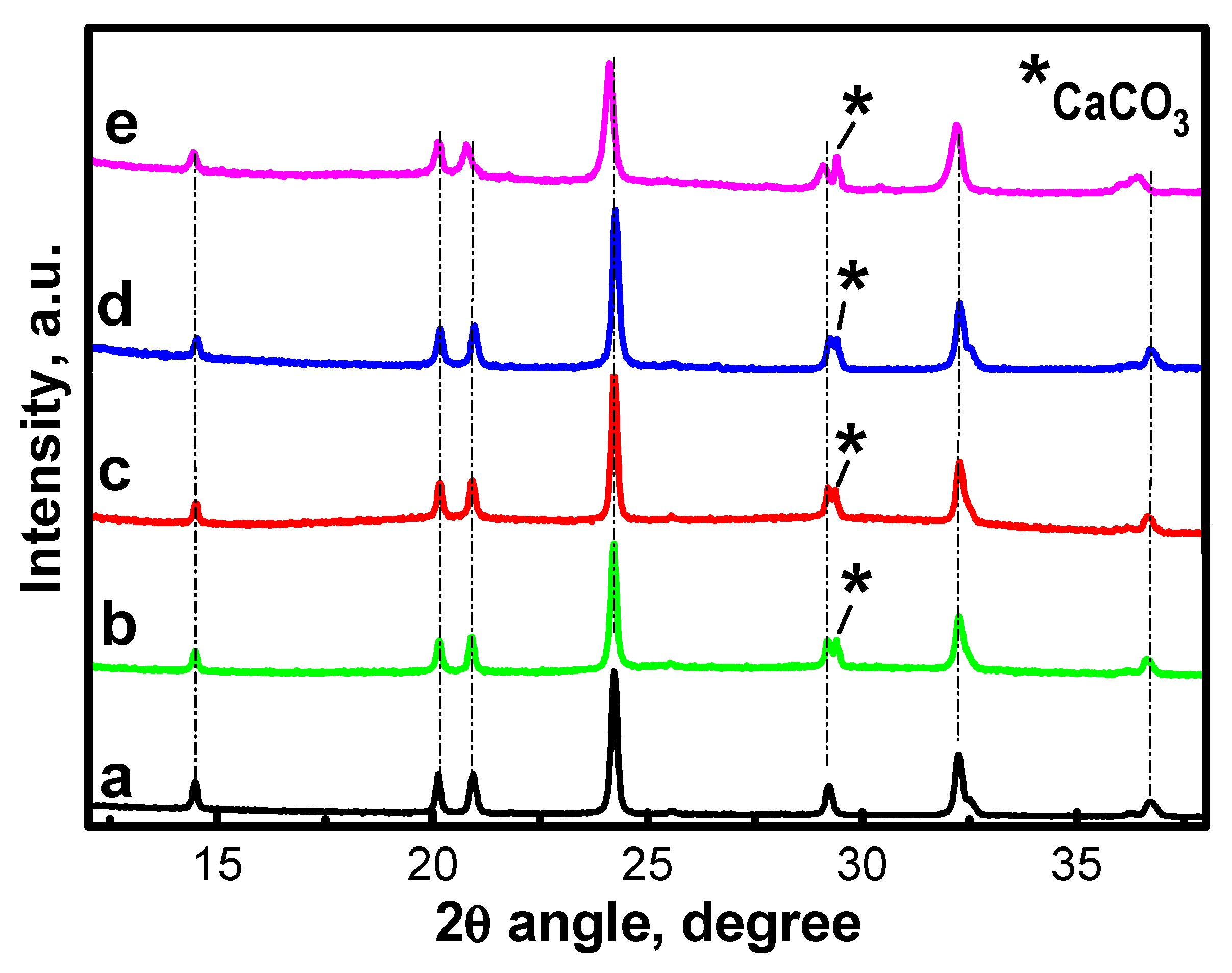
2.1. Characterization of NFVPS/rGO and NFVPS/C Composites
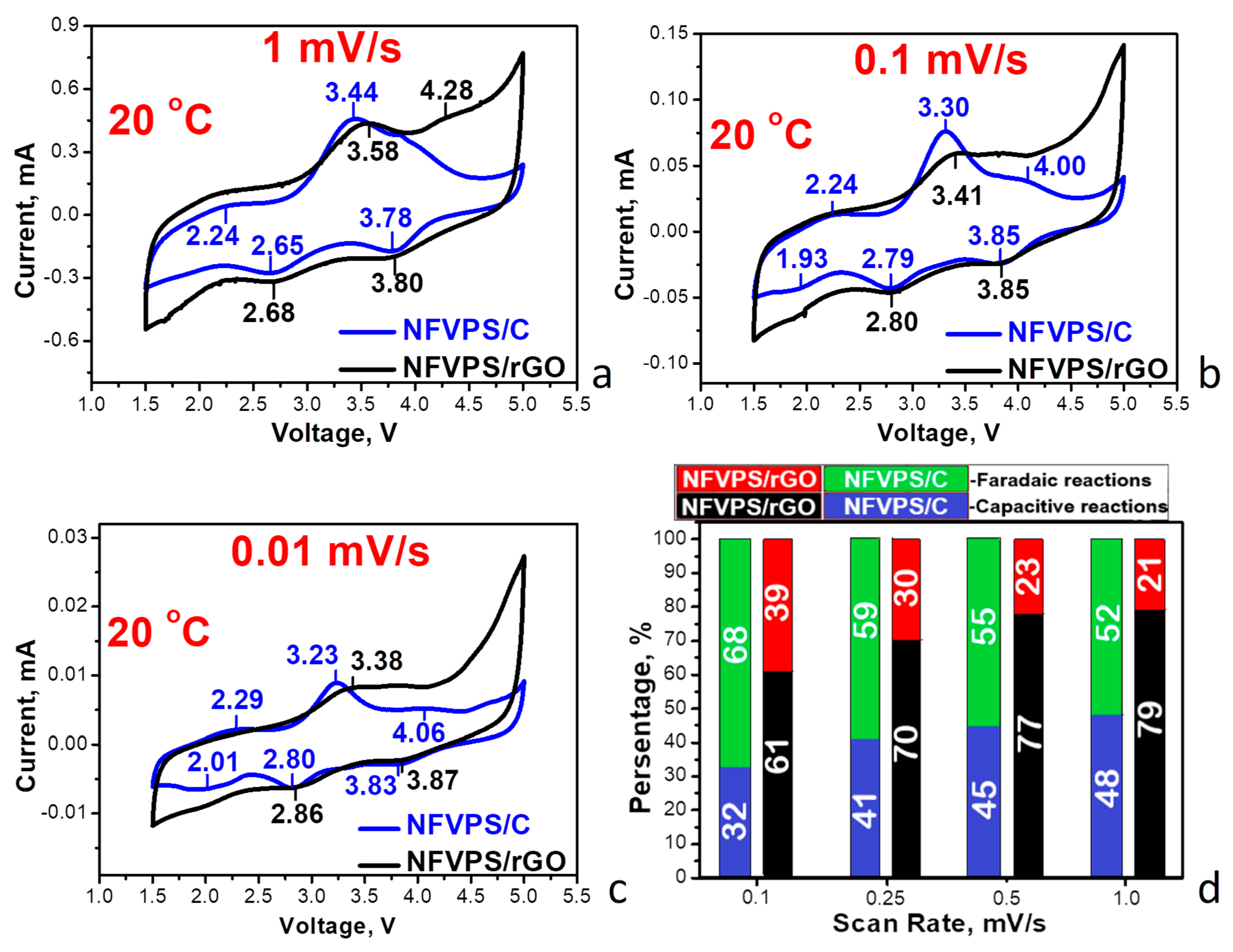
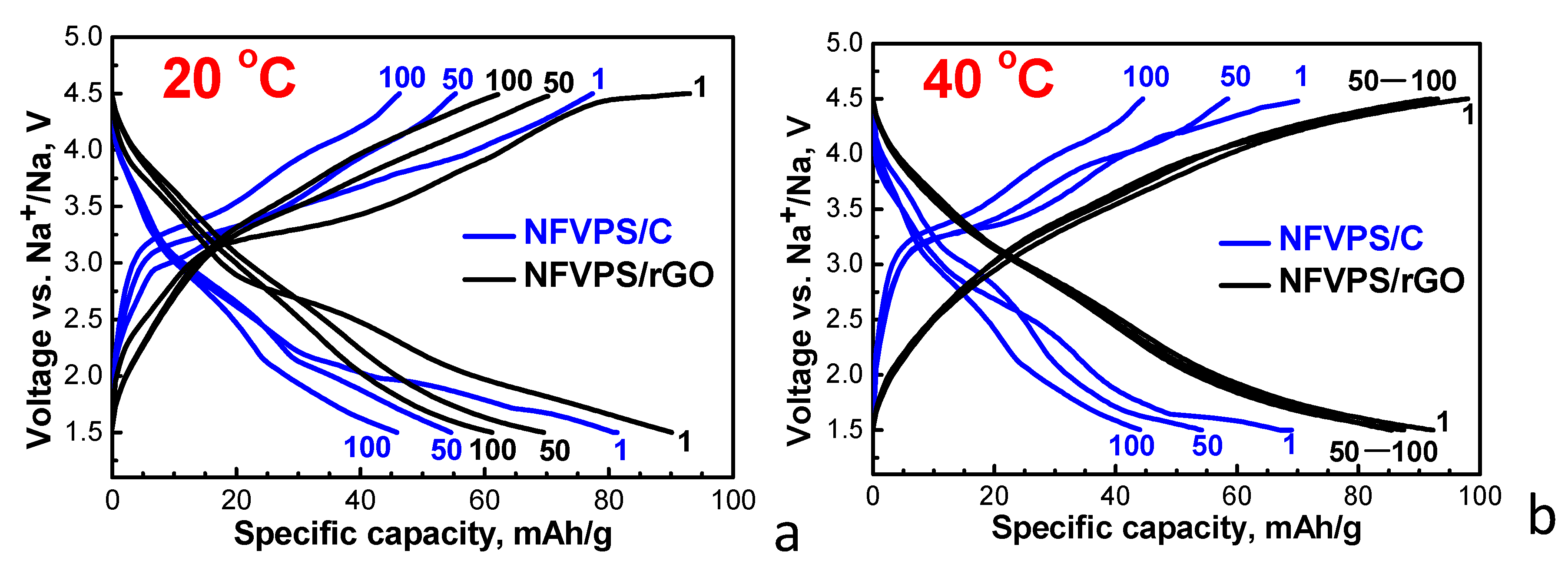
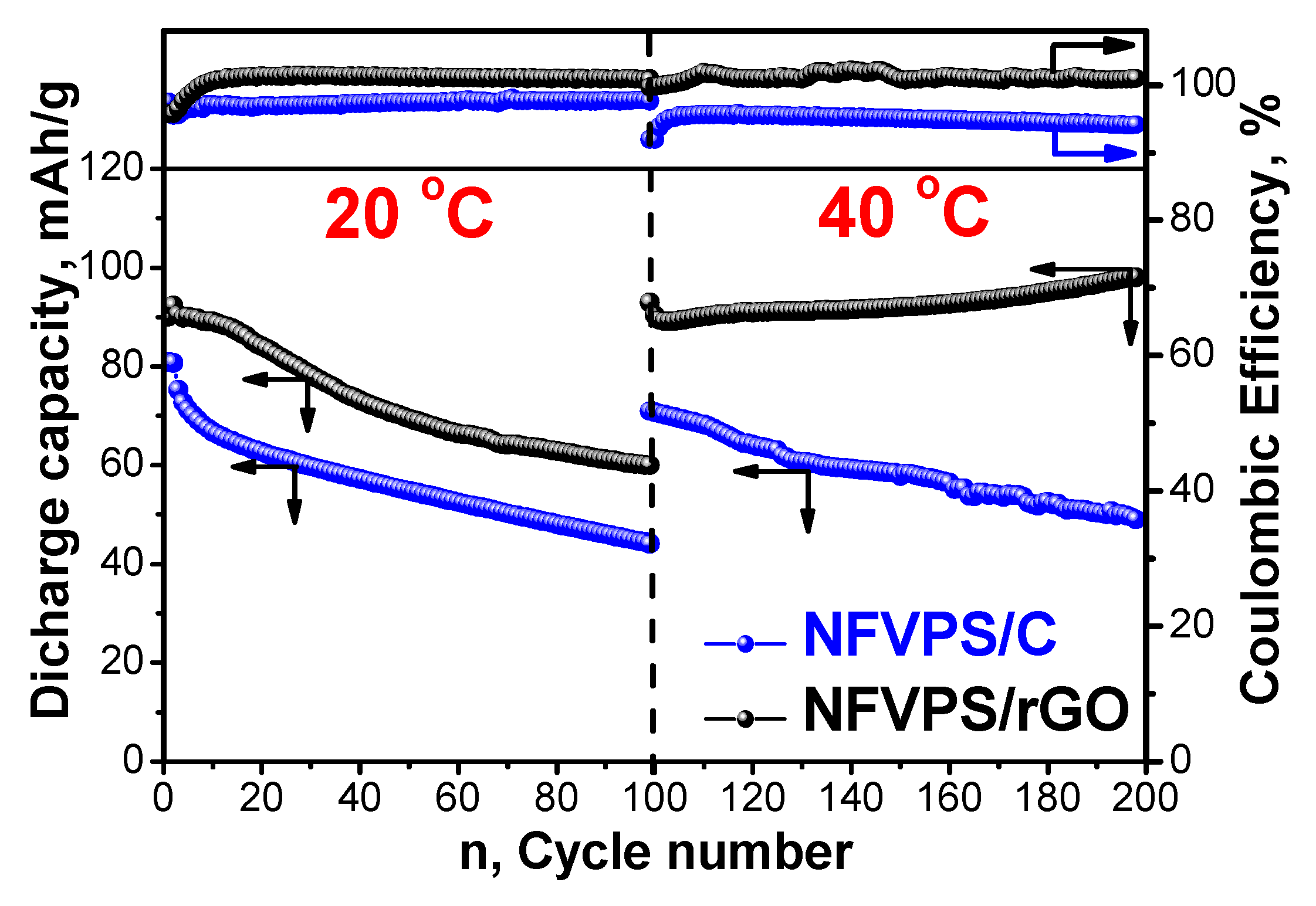
2.2. Electrochemical Studies
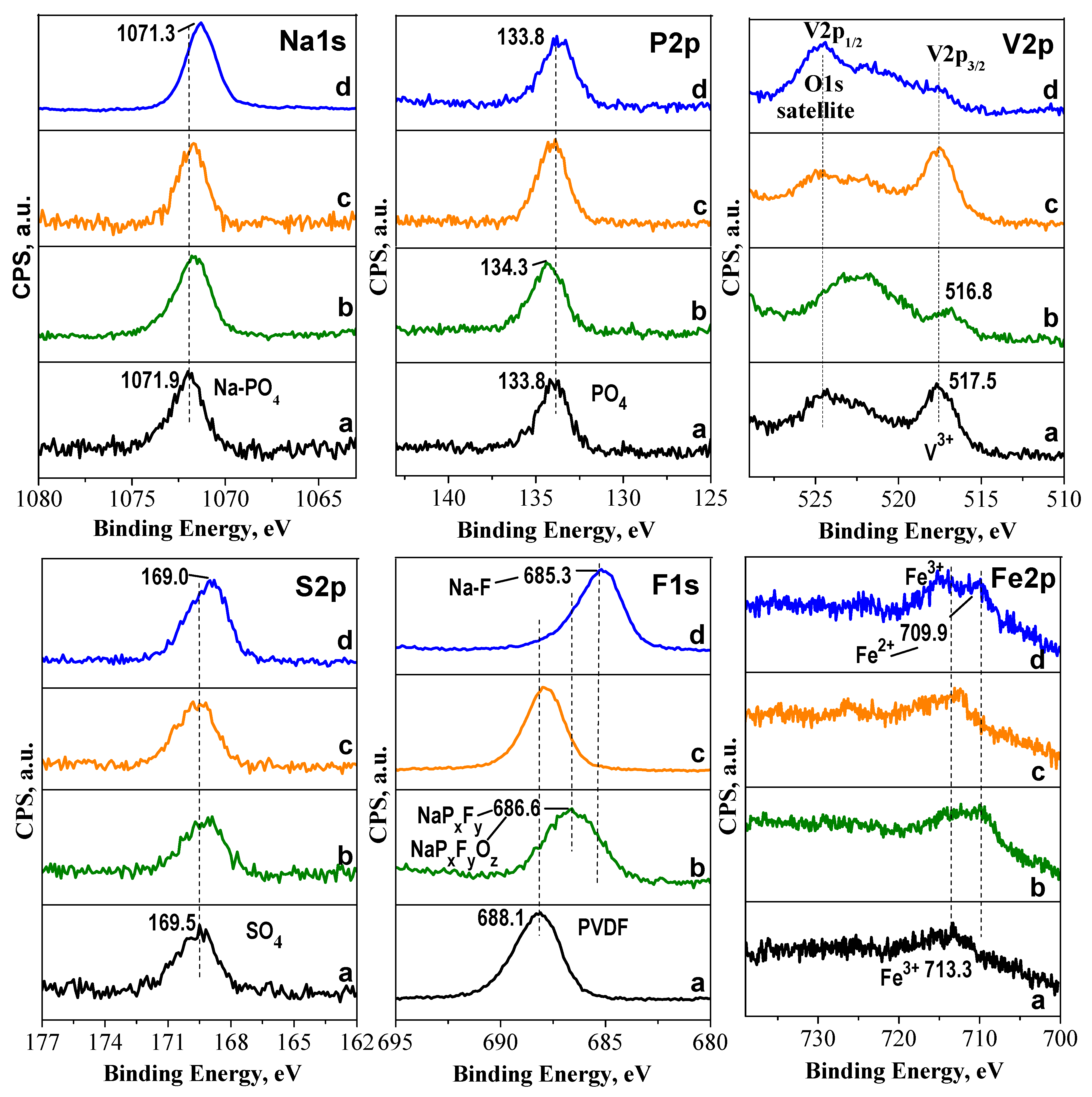
2.3. Ex-Situ Studies
3. Materials and Methods
3.1. Synthesis
3.2. Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of sodium-ion batteries: Opportunities and challenges. Engineering 2023, 24, 172–183. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Xiao, L.; Ai, X.; Cao, Y. Phosphate Framework Electrode Materials for Sodium Ion Batteries. Adv. Sci. 2017, 4, 1600392–1600413. [Google Scholar] [CrossRef]
- Mao, J.; Luo, C.; Gao, T.; Fan, X.; Wang, C. Scalable synthesis of Na3V2(PO4)3/C porous hollow spheres as a cathode for Na-ion batteries. J. Mater. Chem. A 2015, 3, 10378–10385. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, Q.; Yang, S.; Zhang, X.; Jia, M.; Yuan, J.; Yan, X. Towards high-performance phosphate-based polyanion-type materials for sodium-ion batteries. Energy Storage Mater. 2022, 50, 760–782. [Google Scholar] [CrossRef]
- Song, Z.; Liu, R.; Liu, W.-D.; Chen, Y.; Hu, W. Low-cost polyanion-type cathode materials for sodium-ion battery. Adv. Energy Sustain. Res. 2023, 4, 2300102–2300124. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Z.; Zheng, Y.; Gao, Y. NaFePO4 for sodium-ion batteries: Mechanism, synthesis and optimization strategies toward commercialization. Energy Storage Mater. 2024, 65, 103157–103177. [Google Scholar] [CrossRef]
- Chen, G.; Huang, Q.; Wu, T.; Lu, L. Polyanion sodium vanadium phosphate for next generation of sodium-ion batteries—A review. Adv. Funct. Mater. 2020, 30, 2001289–2001312. [Google Scholar] [CrossRef]
- Singh, B.; Wang, Z.; Park, S.; Sai Gautam, G.; Chotard, J.-N.; Croguennec, L.; Carlier, D.; Cheetham, A.K.; Masquelier, C.; Canepa, P. A chemical map of NASICON electrode materials for Sodium-ion batteries. J. Mater. Chem. A 2021, 9, 281–292. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Chen, H.; Dong, H.; Wang, H.; Wang, Y.; Xiao, Y.; Wang, J.; Chen, S. Fast Screening Suitable Doping Transition Metals to Na3V2(PO4)2F3 for Sodium-Ion Batteries with High Energy Density in Wide-Temperature Range. Adv. Mater. 2025, 37, 2505093. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, H.; Dou, S.; Tian, H.; Gan, W.; Yuan, Q. Dynamic template directed construction of three-dimensional porous bismuth aerogels for high-rate Na-ion storage. J. Mater. Chem. A 2023, 11, 5945–5955. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Y.; Bai, Q.; Tian, Z.; Huang, Q.; Liu, C.; He, S.; Yang, Y.; Wang, Y.; Guo, L. Unravelling the regulation mechanism of nanoflower shaped Na3V2(PO4)3 in methanol–water system for high performance sodium ion batteries. Chem. Eng. J. 2023, 451, 138780–138791. [Google Scholar] [CrossRef]
- Kate, R.S.; Jadhav, H.S.; Chothe, U.P.; Bhattacharjee, K.; Kulkarni, M.V.; Deokate, R.J.; Kale, B.B.; Kalubarme, R.S. Critical review of the recent progress and challenges of polyanion Na3V2(PO4)3 cathode materials in rechargeable sodium-ion batteries. J. Mater. Chem. A 2024, 12, 7418–7451. [Google Scholar] [CrossRef]
- Thirupathi, R.; Kumari, V.; Chakrabarty, S.; Omar, S. Recent progress and prospects of NASICON framework electrodes for Na-ion batteries. Prog. Mater. Sci. 2023, 137, 101128–101181. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Singh, R.K. Nanostructured coating strategies of cathode for improved sodium ion battery performance. Chem. Eng. J. 2023, 471, 144592–144615. [Google Scholar] [CrossRef]
- Li, W.; Yao, Z.; Zhou, C.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Boosting High-Rate Sodium Storage Performance of N-Doped Carbon-Encapsulated Na3V2(PO4)3 Nanoparticles Anchoring on Carbon Cloth. Small 2019, 15, 1902432–1902442. [Google Scholar] [CrossRef]
- A Jiang, Y.; Zeng, L.; Wang, J.; Li, W.; Pan, F.; Yu, Y. A carbon coated NASICON structure material embedded in porous carbon enabling superior sodium storage performance: NaTi2(PO4)3 as an example. Nanoscale 2015, 7, 14723–14729. [Google Scholar] [CrossRef]
- Zhan, L.; Zhang, L.; Li, Y.; Cai, H.; Wu, Y. Performance and Stability Enhancement of Hole-Transporting Materials in Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2025, 8, 4355–4361. [Google Scholar] [CrossRef]
- Duan, W.; Zhu, Z.; Li, H.; Hu, Z.; Zhang, K.; Cheng, F.; Chen, J. Na3V2(PO4)3@C core–shell nanocomposites for rechargeable sodium-ion batteries. J. Mater. Chem. A 2014, 2, 8668–8675. [Google Scholar] [CrossRef]
- Li, H.; Bai, Y.; Wu, F.; Ni, Q.; Wu, C. Na3V2(PO4)3/C nanorods as advanced cathode material for sodium ion batteries. Solid State Ion. 2015, 278, 281–286. [Google Scholar] [CrossRef]
- Liu, R.; Li, S.; Wang, Z.; Xu, H.; Wang, W.; Jia, Y.; Zhang, L.; Xie, Z.; Wang, L. Carbon decorated Na3V2(PO4)3 nanoparticles as a high-rate-capability cathode for fast chargeable sodium-ion batteries. Electrochim. Acta 2025, 552, 145945–145953. [Google Scholar] [CrossRef]
- Li, G.; Jiang, D.; Wang, H.; Lan, X.; Zhong, H.; Jiang, Y. Glucose-assisted synthesis of Na3V2(PO4)3/C composite as an electrode material for high-performance sodium-ion batteries. J. Power Sources 2014, 265, 325–334. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Wang, C.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y. Improved electrochemical performance of the Na3V2(PO4)3 cathode by B-doping of the carbon coating layer for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 15190–15201. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Q.; Xu, C.; Li, Q.; An, Q.; Zhang, P.; Sheng, J.; Zhou, L.; Mai, L. Layer-by-Layer Na3V2(PO4)3 Embedded in Reduced Graphene Oxide as Superior Rate and Ultralong-Life Sodium-Ion Battery Cathode. Adv. Energy Mater. 2016, 6, 1600389–1600395. [Google Scholar] [CrossRef]
- Zhu, C.; Song, K.; van Aken, P.A.; Maier, J.; Yu, Y. Carbon-Coated Na3V2(PO4)3 Embedded in Porous Carbon Matrix: An Ultrafast Na-Storage Cathode with the Potential of Outperforming Li Cathodes. Nano Lett. 2014, 14, 2175–2180. [Google Scholar] [CrossRef]
- Ma, X.; Xia, J.; Wu, X.; Pan, Z.; Shen, P.K. Remarkable enhancement in the electrochemical activity of maricite NaFePO4 on high-surface-area carbon cloth for sodium-ion batteries. Carbon 2019, 146, 78–87. [Google Scholar] [CrossRef]
- Hwang, J.; Matsumoto, K.; Hagiwara, R. Na3V2(PO4)3 @Carbon Nanofibers: High Mass Loading Electrode Approaching Practical Sodium Secondary Batteries Utilizing Ionic Liquid Electrolytes. ACS Appl. Energy Mater. 2019, 2, 2818–2827. [Google Scholar] [CrossRef]
- Tang, K.; Tian, H.; Zhang, Y.; Cai, Y.; Du, H.; Zhu, M.; Yao, X.; Su, Z. Multilevel carbon composite construction of NASICON-type NaVPO4F/C/CNT cathode material for enhanced-performance sodium-ion batteries. J. Mater. Chem. C 2025, 13, 6605–6613. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, D.; Chen, Y.; Xia, X.; Liu, H.; He, Y.; Ma, Q. A facile surfactant-assisted self-assembly of LiFePO4/graphene composites with improved rate performance for lithium ion batteries. J. Electroanal. Chem. 2018, 818, 68–75. [Google Scholar] [CrossRef]
- Samigullin, R.R.; Zakharkin, M.V.; Drozhzhin, O.A.; Antipov, E.V. Thermal Stability of NASICON-Type Na3V2(PO4)3 and Na4VMn(PO4)3 as Cathode Materials for Sodium-ion Batteries. Energies 2023, 16, 3051. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, H.; Shakoor, R.A.; Jung, Y.; Choi, J.W. Electrochemical and Thermal Properties of NASICON Structured Na3V2(PO4)3 as a Sodium Rechargeable Battery Cathode: A Combined Experimental and Theoretical Study. J. Electrochem. Soc. 2012, 159, A1393–A1397. [Google Scholar] [CrossRef]
- Koleva, V.; Tushev, T.; Harizanova, S.; Kukeva, R.; Shipochka, M.; Markov, P.; Stoyanova, R. Multi-electron redox reactions with iron and vanadium ions at a mixed phosphate–sulfate electrode during sodium intercalation. Mater. Adv. 2024, 5, 8599–8614. [Google Scholar] [CrossRef]
- Essehli, R.; Alkhateeb, A.; Mahmoud, A.; Boschini, F.; Yahia, H.B.; Amin, R.; Belharouak, I. Optimization of the compositions of polyanionic sodium-ion battery cathode NaFe2-xVx(PO4)(SO4)2. J. Power Sources 2020, 469, 228417–228426. [Google Scholar] [CrossRef]
- Li, S.-F.; Hou, X.-K.; Gu, Z.-Y.; Meng, Y.-F.; Zhao, C.-D.; Zhang, H.-X.; Wu, X.-L. Sponge-like NaFe2PO4(SO4)2@rGO as a high-performance cathode material for sodium-ion batteries. New J. Chem. 2021, 45, 4854–4860. [Google Scholar] [CrossRef]
- Pati, J.; Dhaka, R.S. Mixed polyanionic NaFe1.6V0.4(PO4)(SO4)2@CNT cathode for sodium-ion batteries: Electrochemical diffusion kinetics and distribution of relaxation time analysis at different temperatures. J. Power Sources 2024, 609, 234646–234659. [Google Scholar] [CrossRef]
- Zboril, R.; Mashlan, M.; Papaefthymiou, V.; Hadjipanayis, G. Thermal decomposition of Fe2(SO4)3: Demonstration of Fe2O3 polymorphism. J. Radioanal. Nucl. Chem. 2003, 255, 413–417. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Li, W.; Zeng, L.; Pan, F.; Wang, M.; Wei, X.; Hu, G.; Gu, L.; Yu, Y. Nanoconfined carbon-coated Na3V2(PO4)3 particles in mesoporous carbon enabling ultralong cycle life for sodium-ion batteries. Adv. Energy Mater. 2015, 5, 1402104. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.; Zhu, Y.; Chen, G.; Wu, S.; Qin, Y. Adsorption of SO2 by typical carbonaceous material: A comparative study of carbon nanotubes and activated carbons. Adsorption 2013, 19, 959–966. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.M.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative highresolution XPS and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Clark, D.T.; Dilks, A. ESCA applied to polymers. 23. RF glow discharge modification of polymers in pure oxygen and helium oxygen mixtures. J. Polym. Sci. Polym. Chem. 1979, 17, 957–976. [Google Scholar] [CrossRef]
- Harizanova, S.; Tushev, T.; Koleva, V.; Stoyanova, R. Carbon-based composites with mixed phosphate-pyrophosphates with improved electrochemical performance at elevated temperature. Materials 2023, 16, 6546. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Veleva, S.; Marinova, D.; Harizanova, S.; Koleva, V.; Lefterova, E.; Shipochka, M.; Dimitrov, O.; Stoyanova, A.; Stoyanova, R. Mixing approaches in enhancing the capacitive performance of rGO-based hybrid electrodes. Materials 2025, 18, 2460. [Google Scholar] [CrossRef]
- Affi, J.; Handayani, M.; Anggoro, M.A.; Esmawan, A.; Sabarman, H.; Satriawan, A.; Shalannanda, W.; Siburian, R.; Anshori, I. Electrochemical and capacitive behavior of reduced graphene oxide from green reduction of graphene oxide by urea for supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2023, 34, 1638. [Google Scholar] [CrossRef]
- Kachmar, A.; Tobis, M.; Frąckowiak, E. Electrochemical capacitor based on reduced graphene oxide/NiS2 composite. ChemElectroChem 2022, 9, e202200834. [Google Scholar] [CrossRef]
- Schulz, N.; Hausbrand, R.; Wittich, C.; Dimesso, L.; Jaegermann, W. XPS-surface analysis of SEI layers on Li-ion cathodes: Part II. SEI-composition and formation inside composite electrodes. J. Electrochem. Soc. 2018, 165, A833–A846. [Google Scholar] [CrossRef]
- Kalapsazova, M.; Kostov, K.; Zhecheva, E.; Stoyanova, R. Hybrid Li/Na ion batteries: Temperature-induced reactivity of three-layered oxide (P3-Na2/3 Ni1/3Mg1/6Mn1/2O2) toward lithium ionic liquid electrolytes. Front. Chem. 2020, 8, 600140–600151. [Google Scholar] [CrossRef]
- Shipitsyn, V.; Antrasian, N.; Soni, V.; Mu, L.; Ma, L. Fundamentals and perspectives of electrolyte additives for non-aqueous Na-ion batteries. Energy Mater. 2023, 3, 300038–300075. [Google Scholar] [CrossRef]
- Jin, Y.; Le, P.M.L.; Gao, P.; Xu, Y.; Xiao, B.; Engelhard, M.H.; Cao, X.; Vo, T.D.; Hu, J.; Zhong, L.; et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat Energy 2022, 7, 718–725. [Google Scholar] [CrossRef]
- Kukeva, R.; Vassilev, G.; Kalapsazova, M.; Rasheev, H.; Tadjer, A.; Simova, S.; Stoyanova, R. Electrochemical oxidation and reduction of sodium electrolytes tracked by in-situ/ex-situ magnetic resonance spectroscopy and computational modelling. Electrochim. Acta 2025, 526, 146191. [Google Scholar] [CrossRef]
- Kim, T.; Choi, W.; Shin, H.-C.; Choi, J.-Y.; Kim, J.M.; Park, M.S.; Yoon, W.S. Applications of voltammetry in lithium ion battery research. J. Electrochem. Sci. Technol. 2020, 11, 14–25. [Google Scholar] [CrossRef]


| № | Electrodes | ||
|---|---|---|---|
| 1 | pristine NFVPS/rGO electrode | 0.49 | 0.29 |
| 2 | NFVPS/rGO electrode stopped at 1.5 V after 200 cycles: 100 cycles at 20 °C and next 100 cycles at 40 °C | 0.61 | 0.25 |
| 3 | pristine NFVPS/C electrode | 0.33 | 0.08 |
| 4 | NFVPS/C electrode stopped at 1.5 V after 200 cycles: 100 cycles at 20 °C and next 100 cycles at 40 °C | 0.83 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tushev, T.; Harizanova, S.; Shipochka, M.; Stoyanova, R.; Koleva, V. Multiple Functions of Carbon Additives in NASICON-Type Electrodes for Stabilizing the Sodium Storage Performance. Molecules 2025, 30, 3547. https://doi.org/10.3390/molecules30173547
Tushev T, Harizanova S, Shipochka M, Stoyanova R, Koleva V. Multiple Functions of Carbon Additives in NASICON-Type Electrodes for Stabilizing the Sodium Storage Performance. Molecules. 2025; 30(17):3547. https://doi.org/10.3390/molecules30173547
Chicago/Turabian StyleTushev, Trajche, Sonya Harizanova, Maria Shipochka, Radostina Stoyanova, and Violeta Koleva. 2025. "Multiple Functions of Carbon Additives in NASICON-Type Electrodes for Stabilizing the Sodium Storage Performance" Molecules 30, no. 17: 3547. https://doi.org/10.3390/molecules30173547
APA StyleTushev, T., Harizanova, S., Shipochka, M., Stoyanova, R., & Koleva, V. (2025). Multiple Functions of Carbon Additives in NASICON-Type Electrodes for Stabilizing the Sodium Storage Performance. Molecules, 30(17), 3547. https://doi.org/10.3390/molecules30173547









