Grape stems as second product in the wine industry are an inexpensive and rich source of natural antioxidants with proven health-promoting potential, making them valuable for use in dietary supplements and phytochemical applications. Our screening results identified Italian Riesling, Cabernet Sauvignon, and Merlot stems as the most promising cultivars, with combined contents of (+)-catechin (CA), quercetin-3-glucuronide (Q-gluc), and quercetin-3-glucoside (Q-glc) accounting for 59%, 68%, and 78% of total phenolics, respectively [
13]. To enable their use in food and pharmaceutical formulations, further screening and targeted optimization of the extraction process using safe, non-toxic solvents is essential for maximizing recovery of these valuable compounds.
2.1. Individual Phenolic Composition of Grape Stems
As part of the initial screening, the phenolic profiles of seven grape stem varieties were analyzed to quantify the content of key bioactive compounds (as described in
Section 2.2). The concentrations of individual phenolic compounds are presented in
Table 1. The total identified phenolic content, calculated as the sum of all quantified compounds, ranged from 1.465 to 4.783 mg/g of dry matter. Among the identified compounds, (+)-catechin (CA) was the most abundant, followed by quercetin-3-glucuronide (Q-gluc).
The highest levels of CA, Q-gluc, Caf, and Cm were observed in Merlot (Me), Cabernet Sauvignon (Cs), and Italian Riesling (IR). Merlot stems also showed the highest content of Q-glc, while L-glc was most concentrated in both Merlot and Italian Riesling samples.
When considering the sum of the three target compounds—CA, Q-gluc, and Q-glc—these accounted for 79.15% of the total phenolics in Merlot, 74.88% in Cabernet Sauvignon, and 70.44% in Italian Riesling. Based on this predominance, these three phenolic compounds were selected as the focus for optimization, and the corresponding grape stem varieties (Me, Cs, IR) were chosen for further study.
The extraction process was optimized using a 23 full factorial design, with ethanol concentration (x1), extraction time (x2), and extraction temperature (x3) as independent variables. The dependent variables were the yields of Q-gluc, Q-glc, and CA in the selected grape stem samples. Process levels for each factor were selected based on preliminary screening experiments that indicated their influence on phenolic recovery.
2.2. Quercetin-3-Glucuronide (Q-Gluc)
The experimentally determined concentrations of Q-gluc under various extraction conditions are presented in
Table 2. Values ranged from 0.217 to 0.984 mg/g, depending on the grape stem variety and extraction parameters. The highest Q-gluc yield was obtained from Merlot stems using 60% ethanol at 65 °C for 80 min. In contrast, the lowest yield was recorded for Italian Riesling stems under conditions of 30% ethanol, 25 °C, and 30 min of extraction.
The ANOVA results are summarized in
Table 3. The significance of the main factors, as well as their two-way and three-way interactions, was evaluated based on the corresponding F- and
p-values. Higher F-values indicate a stronger effect on the model, while
p-values less than 0.05 denote statistical significance.
To simplify the regression model, all terms with
p-values ≥ 0.05 were considered statistically insignificant and excluded. The resulting reduced regression equations are as follows:
The predicted values generated by the polynomial regression model showed strong agreement with the experimentally observed data (
Table 2), confirming the model’s accuracy in predicting Q-gluc yields from grape stems. This high level of agreement demonstrates that the developed regression model is well-suited for describing the extraction process.
The coefficient of determination (R2) exceeded 99%, indicating excellent model fit, while adjusted R2 values remained above 98% for all responses. Additionally, the coefficient of variation (CV) was below 2%, further supporting the model’s precision, reliability, and robustness.
ANOVA results revealed that all three linear factors—ethanol concentration (x1), extraction time (x2), and temperature (x3)—were statistically significant, with F-values ranging from 257.77 to 645.27. These findings confirm their strong influence on Q-gluc content.
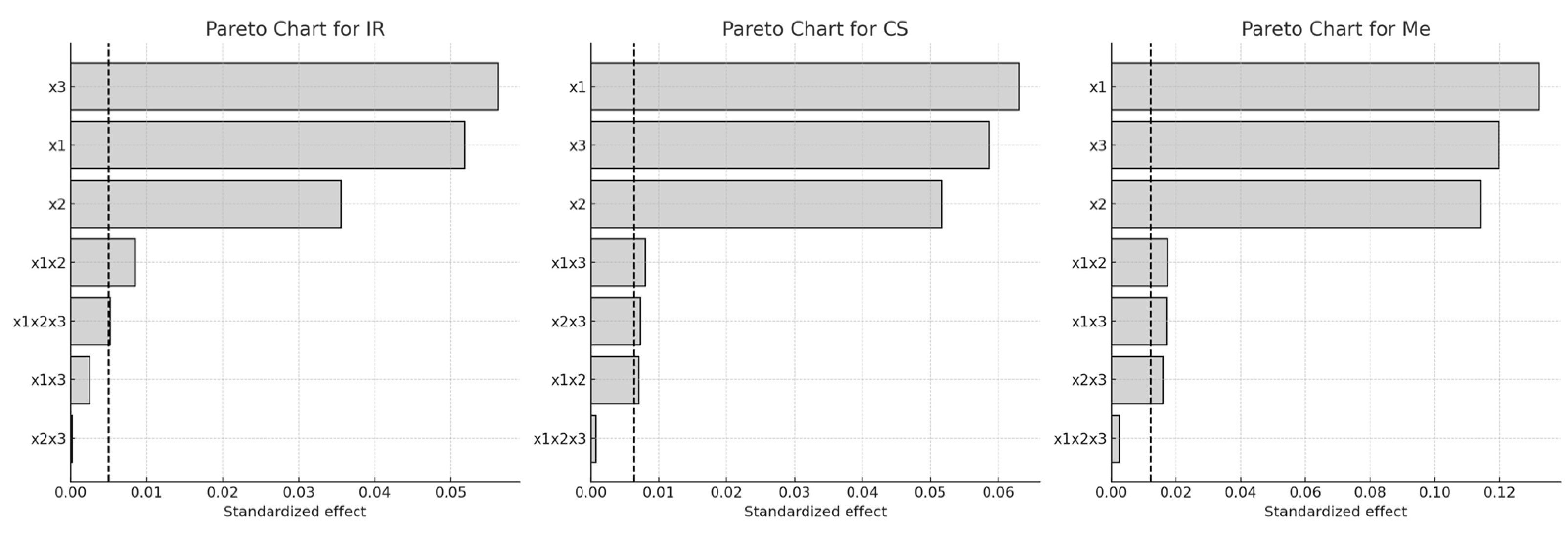
The Pareto chart (
Figure 1) further illustrates the relative impact of each factor. The length of each horizontal bar represents the magnitude of the standardized effect, while the dashed line marks the 95% confidence threshold for statistical significance.
Among the factors examined, extraction temperature (x3) had the most pronounced impact on Q-gluc yield, followed by ethanol concentration (x1), particularly in the IR and CS samples. For Merlot (Me), the order of influence was x1 > x3 > x2. All two-way interactions were found to significantly affect Q-gluc extraction in CS and Me, while the three-way interaction (x1·x2·x3) showed no statistical relevance. In contrast, this three-way interaction was statistically significant in the case of IR (p < 0.05).
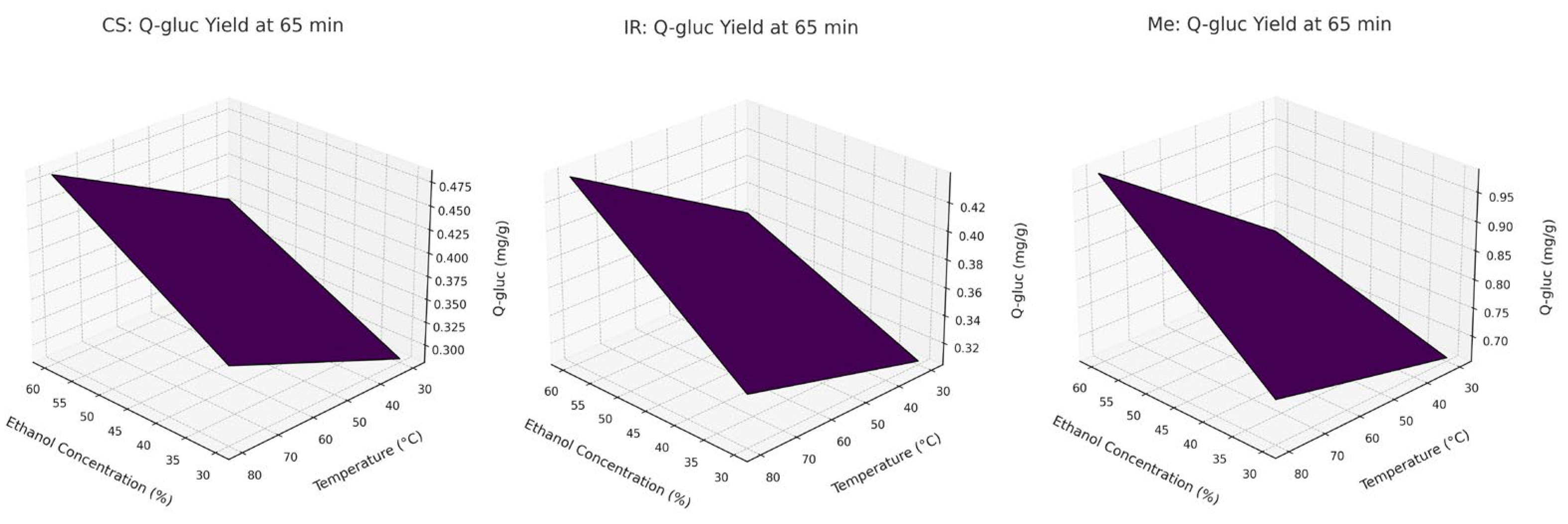
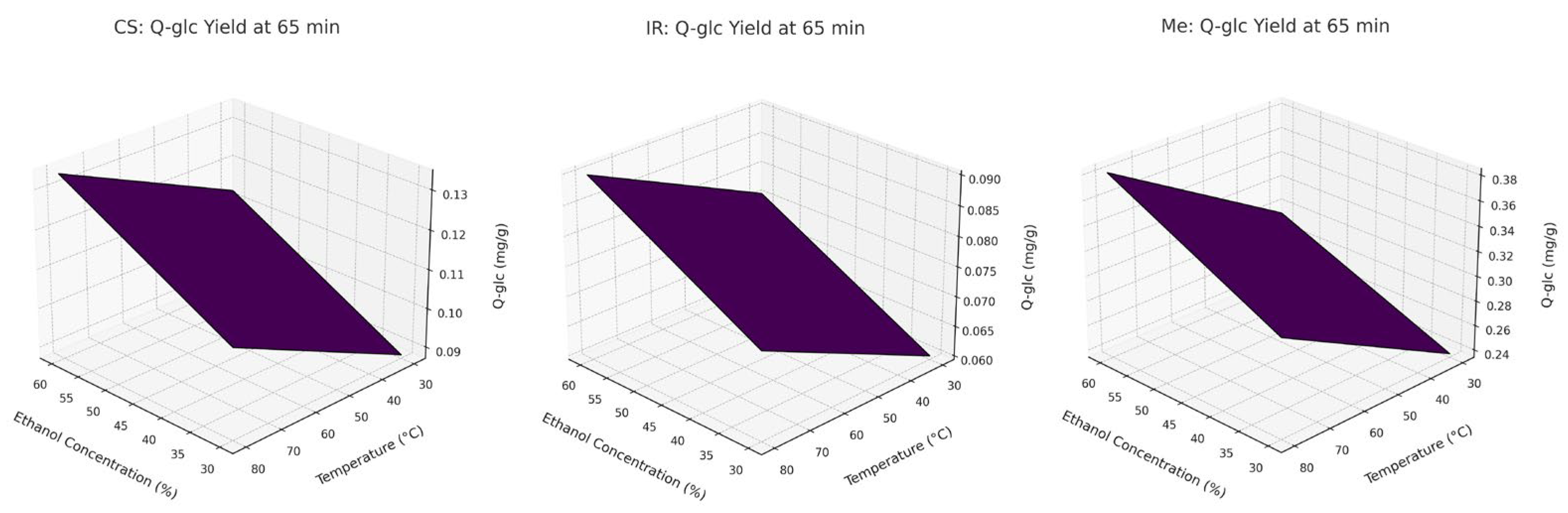
To further visualize these interaction effects, response surface plots are presented in
Figure 2. These 3D surfaces clearly demonstrate that temperature and ethanol concentration have the strongest combined influence on Q-gluc extraction, whereas extraction time has a relatively minor effect within the tested range. The plots were derived from the full factorial design and align with the trends observed experimentally for Q-gluc.
In the next phase of the study, the kinetic and thermodynamic parameters of Q-gluc extraction were evaluated for stem samples from three grape varieties.
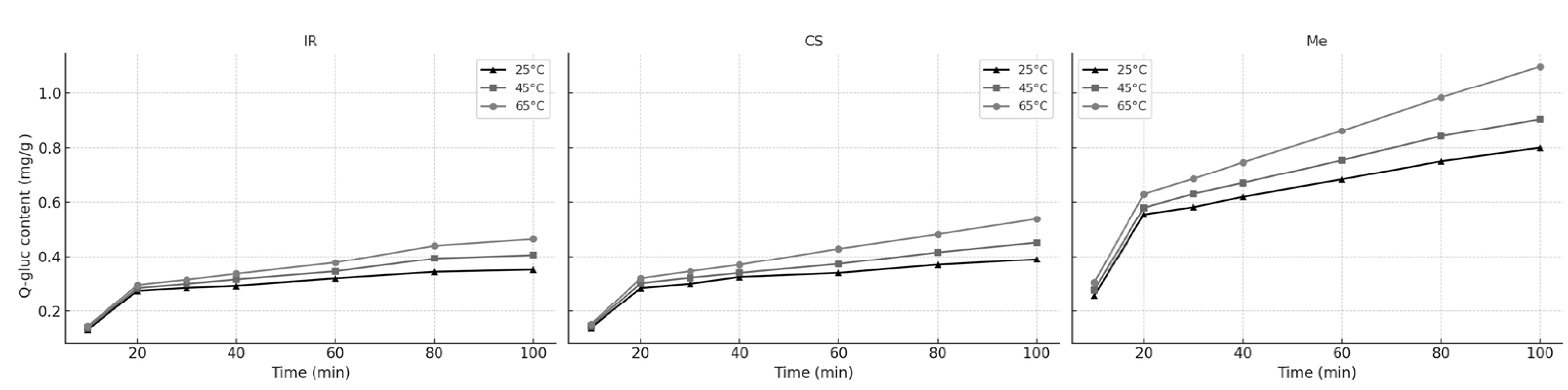
Figure 3 presents the variation in Q-gluc concentration over time for Italian Riesling (IR), Cabernet Sauvignon (CS), and Merlot (Me) stems, extracted at different temperatures.
During the initial extraction phase, which lasts approximately 20 min, the process is primarily governed by a leaching mechanism. In this stage—often referred to as the fast extraction phase—the solvent quickly dissolves Q-gluc that is readily accessible on the surface of the plant particles, resulting in a rapid increase in extract concentration over time.
Following this initial period, the extraction rate declines markedly as the process becomes controlled by internal diffusion. In this slower phase, Q-gluc diffuses from the interior of the plant matrix into the solvent, leading to a more gradual rise in concentration. This phase is known as the slow extraction or diffusion phase and is significantly slower than surface leaching.
To describe the observed extraction kinetics of Q-gluc, the experimental data were fitted to an unsteady-state diffusion model (Equation (6)). Kinetic parameters were estimated through linear regression based on the linearized form of the model equation.
The results revealed that temperature plays a key role in the slow extraction phase. An increase in extraction temperature led to a higher diffusion rate constant (k), indicating enhanced mass transfer from the plant matrix. The model’s fit quality was assessed using the coefficient of determination (R
2) and the root mean square (RMS) error. As shown in
Table 4, all R
2 values exceeded 0.80, and RMS values remained below ±10%, confirming that the model adequately describes the experimental data across all grape stem varieties.
Figure 3 and
Table 4 illustrate how Q-gluc concentrations change over time for different grape stem types. These variations resulted in distinct leaching and diffusion rate constants, reflecting the diverse physical and chemical characteristics of raw materials. Differences in cell wall thickness, pore structure, and initial phenolic content are likely contributors to the observed variation in extraction behavior.
The activation energy (Ea) for Q-gluc extraction from grape stems ranged between 8.47 and 10.96 kJ/mol, which aligns well with previously reported values. For comparison, Bucić-Kojić et al. (2007) [
27] reported activation energies between 1.10 and 7.70 kJ/mol for polyphenol extraction from grape seeds using 50% aqueous ethanol. In general, lower activation energy indicates a faster extraction rate, as more molecules possess sufficient energy to overcome the energy barrier under given conditions.
The positive values of enthalpy (ΔH°) and entropy (ΔS°) across all tested temperatures confirmed that Q-gluc extraction using 60% ethanol is an endothermic and irreversible process. The Gibbs free energy change (ΔG°) ranged from −1.12 to −4.88 kJ/mol, indicating that the extraction was spontaneous and thermodynamically feasible under all conditions. Moreover, the increasing negativity of ΔG° with temperature suggests that spontaneity improves at higher extraction temperatures.
Among the tested conditions, extraction from Merlot (Me) stems at 65 °C proved most favorable, characterized by the lowest activation energy, the highest diffusion and equilibrium constants (k and K), and the most negative ΔG°, confirming both efficiency and spontaneity of the process.
2.3. Quercetin-Glucoside (Q-Glc)
The experimental results obtained for Q-glc during the optimization process are presented in
Table 5. The highest Q-glc yield (0.386 mg/g) was achieved using 60% ethanol at 65 °C for 80 min in Merlot (Me) grape stems. Conversely, the lowest yield (0.045 mg/g) was recorded under the mildest conditions—30% ethanol, 25 °C, and 30 min—in Italian Riesling (IR) stems.
The statistical evaluation of the extraction process using ANOVA is summarized in
Table 6.
The resulting simplified regression equations are:
The predicted values of Q-glc content, calculated using regression Equations (4)–(6), are presented in
Table 5. A comparison between predicted and experimental values shows strong agreement, confirming that the polynomial regression models accurately describe Q-glc extraction behavior under the tested conditions.
This strong correlation is further supported by the high coefficient of determination (R
2), which exceeded 0.96 for all models (
Table 6), indicating that over 96% of the variability in Q-glc yield can be explained by the selected factors.
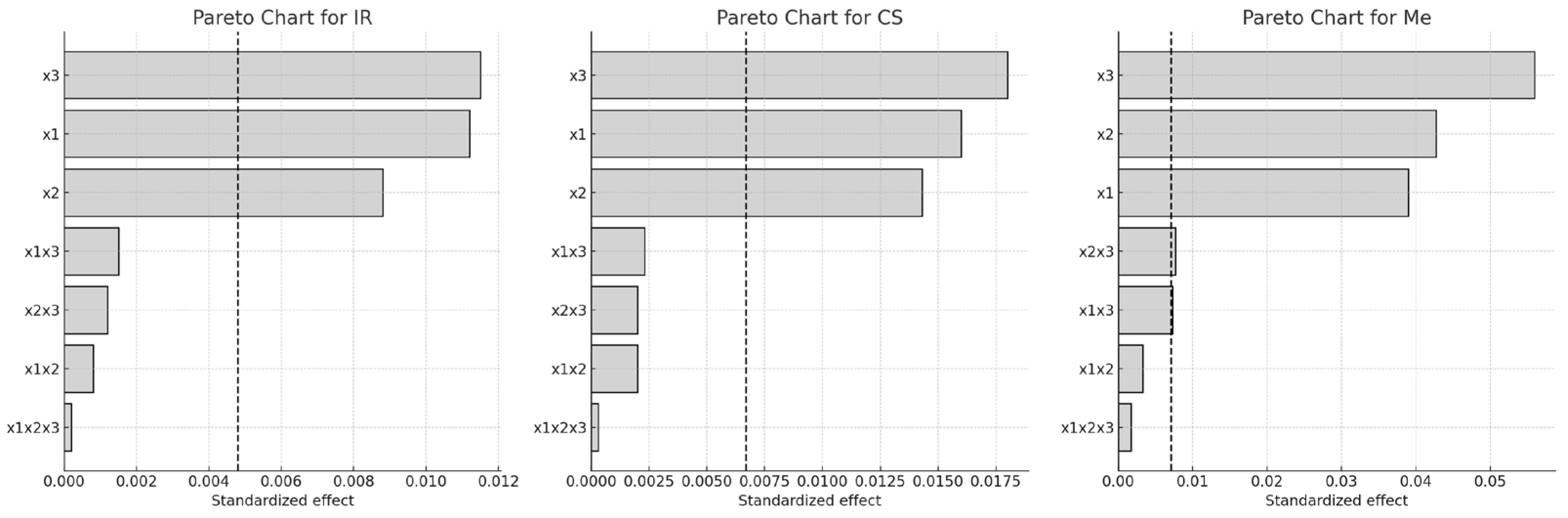
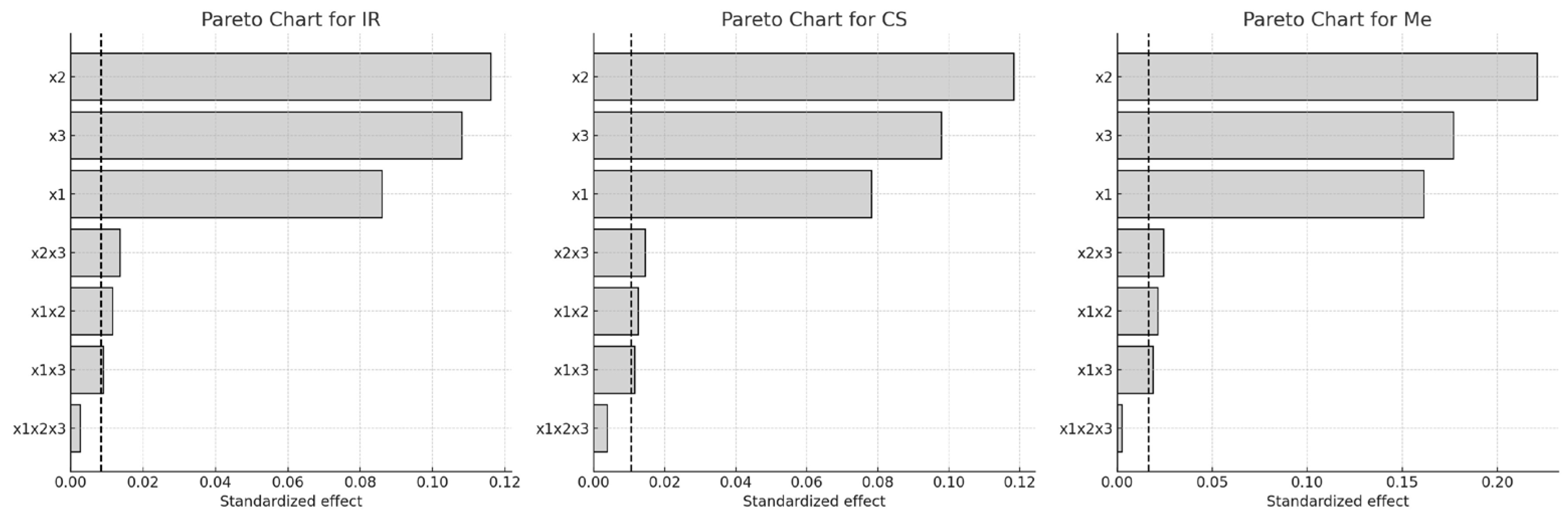
For Italian Riesling (IR) and Cabernet Sauvignon (CS), extraction temperature (x3) had the greatest influence on Q-glc content, followed by ethanol concentration (x1) and extraction time (x2). None of the two- or three-way interactions were statistically significant for these two varieties. In contrast, for Merlot (Me), the factor influence followed the order x3 > x2 > x1, with two significant two-factor interactions: x1·x3 and x2·x3.
These findings are further illustrated in
Figure 4 (Pareto chart) and
Figure 5 (3D response surface plots), which visualize the relative importance and combined effects of the extraction parameters on Q-glc yield.
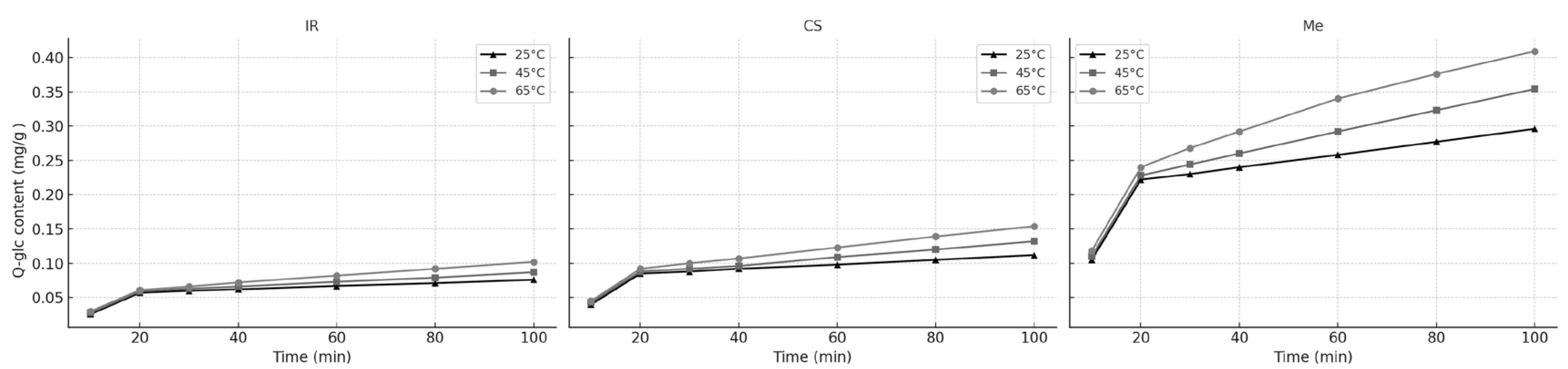
The effect of different extraction times (10, 20, 30, 40, 60, 80, and 100 min) on Q-glc extraction from different stems are presented in
Figure 6. All extraction was carried out at 25, 45, and 65 °C with 60% ethanol.
As with Q-gluc, the extraction of Q-glc followed a two-phase pattern: an initial washing phase during the first ~20 min, followed by a slower diffusion-controlled phase. The kinetic parameters calculated using the unsteady-state diffusion model at different temperatures are presented in
Table 7.
Activation energy (Ea) is a key parameter for understanding the dynamics of the extraction process [
28]. Its value can be influenced by several factors, including the structural properties of the plant matrix and the target compound, pre-treatment of the sample, and the nature of the extraction solvent [
29].
The activation energies (Ea) calculated for the slow extraction phase using the unsteady-state diffusion model are presented in
Table 7. The values were 12.49 kJ/mol for Italian Riesling (IR), 11.39 kJ/mol for Cabernet Sauvignon (CS), and 12.68 kJ/mol for Merlot (Me). These results suggest that Q-glc extraction from Merlot stems was more temperature-dependent, while the extraction from CS was less affected by temperature changes. Although specific Ea values for Q-glc extraction from grape stems have not been previously reported, the obtained results are consistent with values observed for other phenolic compounds in plant matrices.
Thermodynamic analysis further supports these findings. The extraction process was characterized by positive entropy changes (ΔS°: 84.47–129.18 J/mol·K) and positive enthalpy values (ΔH°: 26.53–36.58 kJ/mol), confirming the endothermic and irreversible nature of the process. The negative Gibbs free energy values (ΔG°: –1.40 to –7.08 kJ/mol) indicate that the extraction is spontaneous under all tested conditions (
Table 7). Among the tested samples, the most favorable conditions for Q-glc extraction were observed for Cabernet Sauvignon at 65 °C, which exhibited the lowest Ea, highest kinetic and equilibrium constants (k and K), and the most negative ΔG°, indicating a more efficient and spontaneous process.
2.4. Catechin (CA)
The results obtained using a 2
3 experimental design for CA extractions from the stems of three different grape varieties are shown in
Table 8. The ANOVA results are presented in
Table 9.
The resulting simplified regression equations are:
Among the model terms, extraction time (x2) had the most significant effect on CA yield, with extremely high F-values ranging from 623.00 to 931.69 (p < 0.00001), indicating it was the dominant factor influencing extraction efficiency. This was followed by extraction temperature (x3), which also showed a strong and significant impact (F = 422.26–807.50, p < 0.00001). Ethanol concentration (x1) was also statistically significant, with F-values between 269.55 and 512.03 (p < 0.00001).
Notably, the interaction between extraction time and temperature (x2·x3) had a meaningful influence on CA content, with F-values between 9.27 and 12.94 (p < 0.01593), suggesting a synergistic effect. In contrast, the three-way interaction (x1·x2·x3) was not statistically significant (F = 0.1052–0.6470, p > 0.05), indicating it had little to no effect on the extraction process.
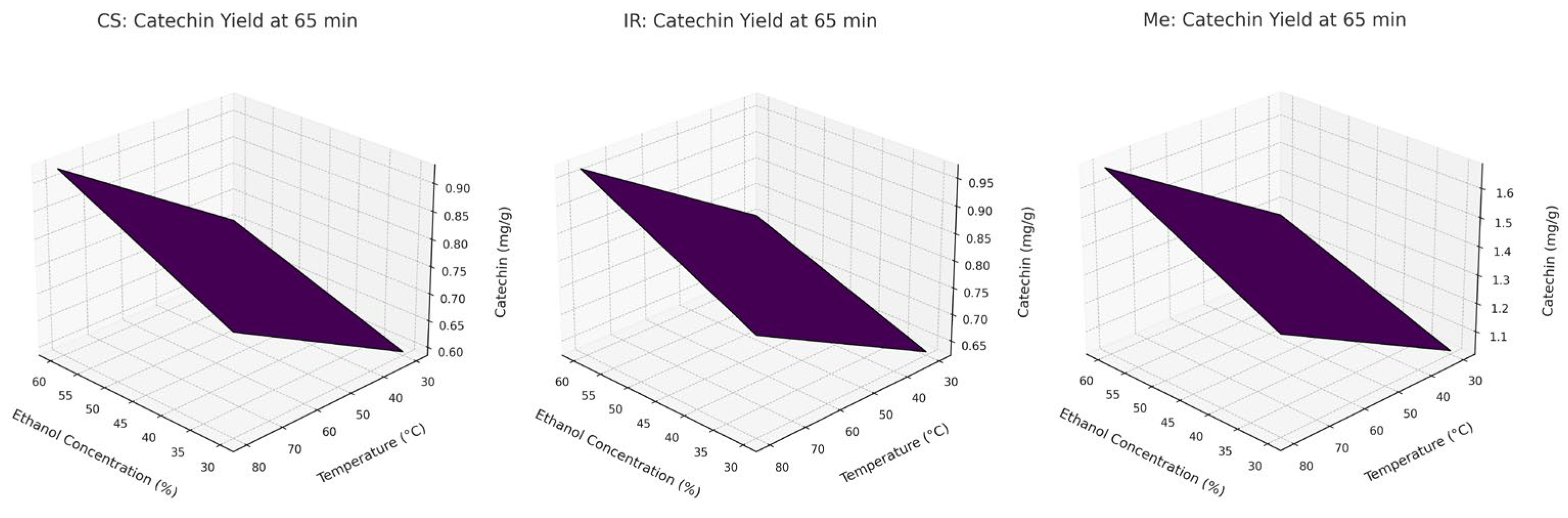
These effects of factors on the CA extraction process are shown in
Figure 7 using a Pareto diagram and
Figure 8 using a 3D graph.
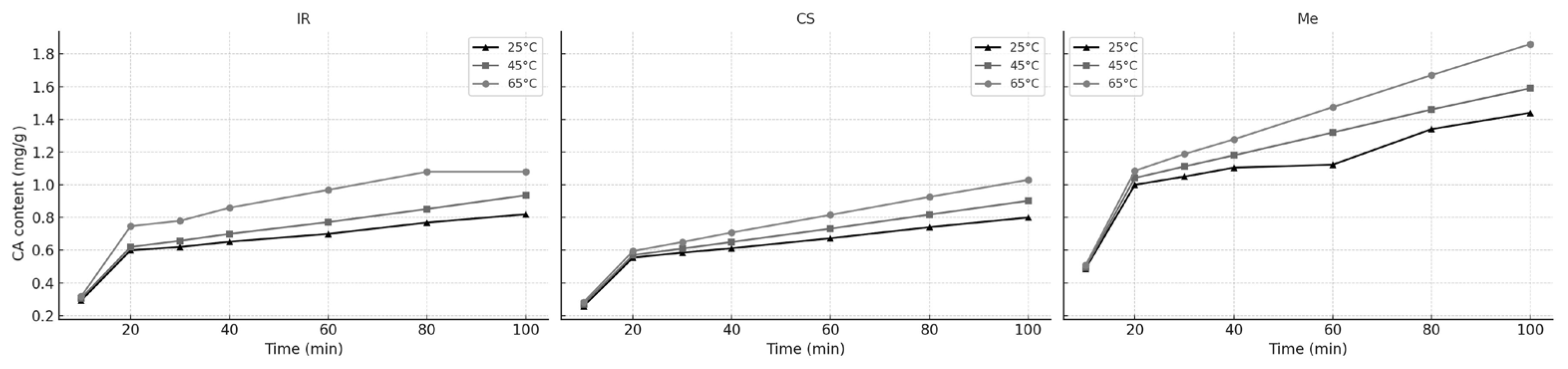
Figure 9 illustrates the increase in CA content over time during extraction with 60% ethanol from different grape stem varieties at varying temperatures. The resulting curves display a typical extraction profile for plant-derived compounds, consistent with trends observed in our previous experiments.
Across all grape stem varieties and extraction temperatures (25 °C, 45 °C, and 65 °C), the unsteady-state diffusion model provided an excellent fit to the experimental data. The coefficients of determination (R
2) ranged from 0.9852 to 0.9992, while the root mean square (RMS) errors remained low, between 0.62 and 2.87 (
Table 10). These results confirm that the derived empirical equations are suitable for predicting system responses within the studied parameter ranges.
The calculated activation energies for catechin extraction were all positive, indicating that the process is endothermic. The Ea values were 8.54 kJ/mol for Italian Riesling (IR), 8.39 kJ/mol for Cabernet Sauvignon (CS), and 7.85 kJ/mol for Merlot (Me). Regardless of grape stem variety, the extraction rate constant increased with temperature, confirming the temperature dependence of the process.
The thermodynamic parameters for CA extraction from grape stems are summarized in
Table 10. The enthalpy change (ΔH°) values ranged from 26.29 to 35.25 kJ/mol across the different grape varieties, confirming the endothermic nature of the process. Similarly, the entropy change (ΔS°) was positive for all samples, with values ranging from 95.15 to 131.57 J/mol·K. Merlot (Me) stems exhibited the highest ΔS°, indicating a greater degree of disorder and further supporting the irreversibility of the process.
The Gibbs free energy change (ΔG°) values were negative in all cases, ranging from –2.04 to –9.22 kJ/mol, demonstrating that the extraction of CA is thermodynamically feasible and spontaneous. The most negative ΔG° values were observed in extracts from Merlot stems, indicating the highest spontaneity.
Overall, the most favorable extraction conditions for CA were observed at 65 °C from Merlot stems, which exhibited the lowest activation energy, highest kinetic and equilibrium constants (k and K), and the most negative ΔG°, confirming the efficiency and thermodynamic favorability of the process.
2.5. Characterization of Extracts Obtained Under Optimal Conditions
The composition and antioxidant activity of grape stem extracts obtained under optimal conditions (60% ethanol, 65 °C, and 80 min) are presented in
Table 11. Among the varieties tested, Merlot (Me) showed the highest total phenolic (72.22 mg GAE/g) and flavonoid content (62.83 mg CAE/g), along with the highest levels of Q-gluc (1.098 mg/g), Q-glc (0.409 mg/g), and catechin (1.860 mg/g).
Merlot also exhibited the strongest antioxidant activity across all assays (DPPH, ABTS, FRAP, CUPRAC, TRP), confirming a strong correlation between phenolic content and antioxidant potential. These results are consistent with literature data, where Merlot stems have been reported as rich in bioactive flavonoids [
30,
31].
Cabernet Sauvignon (CS) showed intermediate values, while Italian Riesling (IR) had the lowest, supporting the conclusion that Merlot stems are the most promising source of natural antioxidants for potential food, pharmaceutical, or cosmetic applications [
32,
33].
From an applied perspective, these optimized extractions could be scaled for industrial use, where grape stems represent a low-cost raw material generated in large volumes by wineries. Developing stable antioxidant extracts from this by-product may offer the food, nutraceutical, and cosmetic industries a natural alternative to synthetic antioxidants, while simultaneously reducing waste management challenges for the wine sector. When compared to other well-known plant sources such as tea leaves [
34], grape stems generally show lower absolute levels of catechins and quercetin derivatives. However, their abundance as a winery by-product makes them a sustainable and attractive alternative source for these bioactive compounds.