Nano-Based Technology in Glioblastoma
Abstract
1. Introduction to Glioblastoma
1.1. Overview of Nanotechnology
1.2. Aim of the Review
2. GBM: Characteristics and Therapeutic Challenges
2.1. Biology of GBM
2.1.1. Pathophysiology of GBM: Neoplastic Cells, Angiogenesis, and Tumor Heterogeneity
2.1.2. IDH Mutation
2.1.3. The Notch Signaling Pathway
2.1.4. Platelet-Derived Growth Factor (PDGF)
2.1.5. Epidermal Growth Factor Receptor (EGFR)
2.1.6. Ceramide Signaling
2.2. Mechanisms of Drug Resistance in GBM, Including the Blood–Brain Barrier
2.3. Conventional Treatment Modalities
Description of Surgery, Radiotherapy, and Chemotherapy and Their Limitations
3. Nanotechnology in the Treatment of GBM
3.1. Mechanisms of Nanoparticle Action in Cancer Therapy
3.2. Types of Nanomaterials in GBM Therapy
3.2.1. Lipid Nanoparticles: Liposomes, Micelles, and Their Application in Drug Delivery to Brain Tumors
3.2.2. Metallic and Carbon Nanoparticles: Gold, Iron, Carbon Nanotubes–Their Properties and Therapeutic Potential
3.2.3. Polymeric Nanoparticles: Applications in Controlled Drug Release and Precision Therapy
4. Examples of Studies and Clinical Trials
4.1. Preclinical Studies
4.1.1. In Vitro Studies
PLGA-PEG Nanoparticles with antagomiR-21 and antagomiR-10b
PTX–Oligo(p-phenylenevinylene) Nanoformulation
Chitosan-PLGA for Intranasal Application
Hemoglobin + Glucose Oxidase Nanoparticles (RBC-Coated)
Lipid–Amphiphilic Nanoformulations with PTX and PDL1-siRNA
Transferrin-Targeted Lipid Nanoparticles (Tf-PTX-LNPs)–Intranasal Administration
4.1.2. In Vivo Studies
In Vivo Tests with PLGA-PEG Nanoparticles + antagomiRs
Gold Nanoparticles (AuNCs, AuNTs) as Radiosensitizers
Doxorubicin-Loaded Nanoparticles Encapsulated in Exosomes (ENP_DOX)
Fe3O4 Magnetic Nanoparticles with Antisense miR-10b (MN-Anti-miR10b)
PLGA-Chlorotoxin (CTX) + Ionizing Radiation (IR)
CRLX101 (Camptothecin Conjugate)
4.1.3. Summary of Efficacy and Safety
Efficacy
Safety
4.2. Examples of Clinical Trials
- Gold Nanoparticles with RNAi: NU-0129 (Spherical Nucleic Acids)
- ○
- Mechanism: Spherical gold-core nanoparticles coated with siRNA targeting the oncogene Bcl2L12.
- ○
- Trial: Phase 0; eight patients with recurrent GBM received a very low intravenous dose of NU-0129 prior to tumor resection.
- ○
- Results: Particles penetrated the tumor and reduced Bcl2L12 levels without significant toxicity (no grade 4/5 adverse events) [289].
- Liposomes and Lipid-RNA Structures (LNPs): Vaccines and p53 Recycling
- SGT-53: Liposomal pDNA encoding p53 with anti-TfR targeting, combined with temozolomide or radiotherapy. Phase II trial terminated early due to low recruitment (NCT02340156).
- RNA–lipid NPs: RNA vaccine for newly diagnosed MGMT-unmethylated GBM patients (Phase I, NCT04573140) aimed at “reprogramming” the immune microenvironment [278].
- 2.
- Nanoparticles Enhancing Radiotherapy (Radiosensitizers)
- 3.
- Photodynamic/Photothermal Therapy + Nanoparticles
- Preclinical: Hybrid particles with angiopep and IR-780/mTHPC promoting PDT/PTT and selective apoptosis [291].
- Other approach: Iridium(III) cores combined with gold nanoparticles, inducing devascularization and tumor elimination.
- 4.
- HDL-like Lipid Nanodiscs with LXR Agonists
- Injection after tumor removal combined with radiotherapy in mouse models.
- Results: >60% survival at 60 days, with immunological memory and rejection of subsequent tumors in 68% of mice. Preparations are underway for clinical trials.
- 5.
- Immunotherapeutic Approaches Using Nano-Elements
- HSP-gp96: Peptide adjuvant in nanostructured vaccines (HSPPC), Phase I/II: immune response induced in 11/12 patients, progression delay in 41 individuals.
- mRNA-LNP vaccines (similar to COVID-19 vaccines), currently in Phase I (NCT04573140) and further developed due to technological success [292].
4.2.1. Experimental Therapies Examples, Including Nanoparticles for Chemotherapeutic Drug Delivery
- Nanoparticles for Chemotherapy
- Lipid-based Nanostructures—Temozolomide (TMZ):A comparative study of polymeric nanoparticles (PNP), solid lipid nanoparticles (SLN), and nanostructured lipid carriers (NLC) showed that TMZ-loaded NLCs (T-NLCs) exhibited superior anti-glioma efficacy—demonstrating better in vitro and in vivo outcomes, with stronger tumor growth inhibition and minimal side effects [293].
- Surface-functionalized Liposomes:Liposomes loaded with TMZ, modified with anti-CD133 antibodies and angiopep-2, increased median survival from ~23 to ~49 days in mouse models. Co-loading TMZ with the BET inhibitor JQ1 and transferrin further enhanced therapeutic efficacy and reduced adverse effects [294].
- Albumin and Metal Nanoparticles:Albumin nanoparticles (ABI-009) and polysiloxane gadolinium chelates (AGuIX) are being studied in combination with radiochemotherapy in ongoing phase I/II trials [291].
- Platinum Conjugates and PEGylated Micelles:PEG-Glu micelles bearing cyclic RGD peptides facilitated oxaliplatin transport across the blood–brain barrier (BBB), significantly inhibiting tumor growth in animal models compared to standard drug formulations [295].
- Chemodynamic Nanoreactors:DOX@MTP/HA-EGCG nanoparticles act as ‘cascade nanoreactors’ combining chemodynamic therapy (CDT) with chemotherapy. They efficiently cross the BBB, accumulate in tumors, and generate reactive oxygen species (ROS), resulting in enhanced cytotoxicity [296].
- Technologies Supporting Drug Penetration
- Focused Ultrasound (FUS)/Sonodynamic Therapy:FUS increases BBB permeability and, combined with microbubbles and agents such as doxorubicin or anti-PD-L1 antibodies, enhances drug accumulation in tumors. Sonodynamic therapy using fluorescent dyes and ultrasound showed clinical benefits, with documented neurological improvement in a patient case.
- Convection-Enhanced Delivery (CED):Direct intracerebral delivery of drugs, such as radiolabeled liposomes (e.g., Re-186-containing 186RNL), improves dosing precision and reduces exposure of healthy tissue [297].
- Gene Therapies and Biological Carriers
- Liposomal Probes–p53 (SGT-53):Liposomal plasmid p53 combined with transferrin targeting. Despite promising preclinical data, the phase II trial was terminated due to low recruitment [3].
- RNA/Lipid Nanoparticles (LNP-mRNA):mRNA carriers encoding, for example, CMV pp65, combined with dendritic cell immunotherapy, activated immune responses and are under phase I investigation (NCT04573140).
- Oncolytic Viruses and Neural Stem Cell Carriers:Teserpaturev (Delytact, G47Δ)—an oncolytic HSV-1 virus approved in Japan after phase II, showing a ~84% one-year survival rate and median survival of ~20 months.NSC-CRAd-S-pk7—a replicating adenovirus (~70–90 nm) delivered via stem cells, shown to be safe in phase I.Toca 511 (vocimagene amiretrorepvec) + flucytosine: Viral gene therapy combining cytosine deaminase with a prodrug, in phase II/III for recurrent high-grade gliomas, with FDA/EMA priority designations.
- Immunotherapy Supported by Nanotechnology
- HSP-gp96 Biotherapeutic Vaccines:Protein complexes with tumor peptides in phase I/II trials showed activation of immune response, tumor immune infiltration, and a favorable safety profile [292].
4.2.2. Conclusions and Future Research Directions
Personalized Targeted Therapy
Safe Brain Access
Breakthroughs in Oncolysis and Immunoactivation
4.3. Challenges in Clinical Application of Nanotechnology
5. Summary and Conclusions
5.1. Future of Nanotechnology in GBM Treatment
5.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the management of glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Kanderi, T.; Munakomi, S.; Gupta, V. Glioblastoma Multiforme. StatPearls. 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558954/ (accessed on 16 May 2025).
- Pouyan, A.; Ghorbanlo, M.; Eslami, M.; Jahanshahi, M.; Ziaei, E.; Salami, A.; Mokhtari, K.; Shahpasand, K.; Farahani, N.; Meybodi, T.E.; et al. Glioblastoma multiforme: Insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol. Cancer 2025, 24, 58. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20 (Suppl. S5), S2–S8. [Google Scholar] [CrossRef]
- Gilard, V.; Tebani, A.; Dabaj, I.; Laquerrière, A.; Fontanilles, M.; Derrey, S.; Marret, S.; Bekri, S. Diagnosis and management of Glioblastoma: A comprehensive perspective. J. Pers. Med. 2021, 11, 258. [Google Scholar] [CrossRef]
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovács, Á. GBM: Clinical presentation, multidisciplinary management, and long-term outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef]
- Seyhan, A.A. Circulating liquid biopsy biomarkers in Glioblastoma: Advances and challenges. Int. J. Mol. Sci. 2024, 25, 7974. [Google Scholar] [CrossRef]
- Young, R.M.; Jamshidi, A.; Davis, G.; Sherman, J.H. Current trends in the surgical management and treatment of adult Glioblastoma. Ann. Transl. Med. 2015, 3, 121. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and other primary brain malignancies in adults: A review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Sizoo, E.M.; Braam, L.; Postma, T.J.; Pasman, H.R.; Heimans, J.J.; Klein, M.; Reijneveld, J.C.; Taphoorn, M.J. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro-oncology 2010, 12, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Breen, W.G.; Aryal, M.P.; Cao, Y.; Kim, M.M. Integrating multi-modal imaging in radiation treatments for GBM. Neuro-oncology 2024, 26 (Suppl. S1), S17–S25. [Google Scholar] [CrossRef]
- Sipos, D.; Debreczeni-Máté, Z.; Ritter, Z.; Freihat, O.; Simon, M.; Kovács, Á. Complex diagnostic challenges in GBM: The role of 18F-FDOPA PET imaging. Pharmaceuticals 2024, 17, 1215. [Google Scholar] [CrossRef]
- Sadowski, K.; Jażdżewska, A.; Kozłowski, J.; Zacny, A.; Lorenc, T.; Olejarz, W. Revolutionizing GBM treatment: A comprehensive overview of modern therapeutic approaches. Int. J. Mol. Sci. 2024, 25, 5774. [Google Scholar] [CrossRef] [PubMed]
- Taal, W.; Bromberg, J.E.; van den Bent, M.J. Chemotherapy in glioma. CNS Oncol. 2015, 4, 179–192. [Google Scholar] [CrossRef]
- Tosoni, A.; Franceschi, E.; Poggi, R.; Brandes, A.A. Relapsed Glioblastoma Treatment strategies for initial and subsequent recurrences. Curr. Treat. Options Oncol. 2016, 17, 49. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Mosleh-Shirazi, S.; Abbasi, M.; Moaddeli, M.R.; Vaez, A.; Shafiee, M.; Kasaee, S.R.; Amani, A.M.; Hatam, S. Nanotechnology advances in the detection and treatment of cancer: An overview. Nanotheranostics 2022, 6, 400–423. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Talaie, Z.; Syahir, A. Nanotechnology-based combinatorial anti-Glioblastoma therapies: Moving from terminal to treatable. Pharmaceutics 2022, 14, 1697. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in healthcare, and its safety and environmental risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; SAbdalla, M.; Bechelany, M. Review on natural, incidental, bioinspired, and engineered nanomaterials. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Wang B, Hu S, Teng Y, Chen J, Wang H, Xu Y, Wang K, Xu J, Cheng Y, Gao X Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [CrossRef]
- Forjaz, G.; Barnholtz-Sloan, J.S.; Kruchko, C.; Siegel, R.; Negoita, S.; Ostrom, Q.T.; Dickie, L.; Ruhl, J.; Van Dyke, A.; Patil, N.; et al. An updated histology recode for the analysis of brain and CNS tumors. Neuro-Oncol. Adv. 2021, 3, vdaa175. [Google Scholar] [CrossRef]
- Stoyanov, G.S.; Dzhenkov, D.L. On the concepts and history of Glioblastoma multiforme—Morphology, genetics and epigenetics. Folia Med. 2018, 60, 48–66. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.J.; Moon, B.H.; Song, S.H.; Lee, M.O.; Shim, S.H.; Kim, H.S.; Lee, M.C.; Kwon, J.T.; Fornace, A.J., Jr.; et al. Generation of cancerous neural stem cells forming glial tumor by oncogenic stimulation. Stem Cell Rev. Rep. 2012, 8, 532–545. [Google Scholar] [CrossRef]
- Waugh, M.G. Chromosomal instability and phosphoinositide pathway gene signatures in Glioblastoma multiforme. Mol. Neurobiol. 2016, 53, 621–630. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human GBM genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- von Deimling, A.; Louis, D.N.; von Ammon, K.; Petersen, I.; Hoell, T.; Chung, R.Y.; Martuza, R.L.; Schoenfeld, D.A.; Yaşargil, M.G.; Wiestler, O.D. Association of epidermal growth factor receptor gene amplification with loss of chromosome 10 in human GBM multiforme. J. Neurosurg. 1992, 77, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Fedoy, A.E.; Yang, N.; Martinez, A.; Leiros, H.K.; Steen, I.H. Structural and functional properties of isocitrate dehydrogenase from the psychrophilic bacterium Desulfotalea psychrophila reveal a cold-active enzyme with an unusual high thermal stability. J. Mol. Biol. 2007, 372, 130–149. [Google Scholar] [CrossRef]
- Kaminska, B.; Czapski, B.; Guzik, R.; Krol, S.K.; Gielniewski, B. Consequences of IDH1/2 mutations in gliomas and an assessment of inhibitors targeting mutated IDH proteins. Molecules 2019, 24, 968. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zeng, Y.; Zhang, D.F.; Zou, S.H.; Cheng, Y.F.; Yao, Y.G. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef]
- Ward, P.S.; Cross, J.R.; Lu, C.; Weigert, O.; Abel-Wahab, O.; Levine, R.L.; Weinstock, D.M.; Sharp, K.A.; Thompson, C.B. Identification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate production. Oncogene 2012, 31, 2491–2498. [Google Scholar] [CrossRef]
- Turkalp, Z.; Karamchandani, J.; Das, S. IDH mutation in glioma: New insights and promises for the future. JAMA Neurol. 2014, 71, 1319–1325. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Grassian, A.R.; Parker, S.J.; Davidson, S.M.; Divakaruni, A.S.; Green, C.R.; Zhang, X.; Slocum, K.L.; Pu, M.; Lin, F.; Vickers, C.; et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 2014, 74, 3317–3331. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef]
- Chesnelong, C.; Chaumeil, M.M.; Blough, M.D.; Al-Najjar, M.; Stechishin, O.D.; Chan, J.A.; Pieper, R.O.; Ronen, S.M.; Weiss, S.; Luchman, H.A.; et al. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro-oncology 2014, 16, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.X.; Liang, J.Y.; Zhang, C.; Xiong, Y.; Guan, K.L.; Yuan, H.X. The oncometabolite 2-hydroxyglutarate produced by mutant IDH1 sensitizes cells to ferroptosis. Cell Death Dis. 2019, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- YLai, K.; Chen, Z.; Lin, S.; Ye, K.; Yuan, Y.; Li, G.; Song, Y.; Ma, H.; Mak, T.W.; Xu, Y. The IDH1-R132H mutation aggravates cisplatin-induced acute kidney injury by promoting ferroptosis through disrupting NDUFA1 and FSP1 interaction. Cell Death Differ. 2025, 32, 242–255. [Google Scholar] [CrossRef]
- Lino, M.M.; Merlo, A.; Boulay, J.L. Notch signaling in Glioblastoma: A developmental drug target? BMC Med. 2010, 8, 72. [Google Scholar] [CrossRef]
- Bazzoni, R.; Bentivegna, A. Role of Notch signaling pathway in Glioblastoma pathogenesis. Cancers 2019, 11, 292. [Google Scholar] [CrossRef]
- Yan, D.; Hao, C.; Xiao-Feng, L.; Yu-Chen, L.; Yu-Bin, F.; Lei, Z. Molecular mechanism of Notch signaling with special emphasis on microRNAs: Implications for glioma. J. Cell. Physiol. 2018, 234, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef]
- Wang, S.; Gu, S.; Chen, J.; Yuan, Z.; Liang, P.; Cui, H. Mechanism of Notch signaling pathway in malignant progression of GBM and targeted therapy. Biomolecules 2024, 14, 480. [Google Scholar] [CrossRef]
- Moutal, A.; Honnorat, J.; Massoma, P.; Désormeaux, P.; Bertrand, C.; Malleval, C.; Watrin, C.; Chounlamountri, N.; Mayeur, M.E.; Besançon, R.; et al. CRMP5 controls Glioblastoma cell proliferation and survival through Notch-dependent signaling. Cancer Res. 2015, 75, 3519–3528. [Google Scholar] [CrossRef]
- Li, Y.; Guessous, F.; Zhang, Y.; Dipierro, C.; Kefas, B.; Johnson, E.; Marcinkiewicz, L.; Jiang, J.; Yang, Y.; Schmittgen, T.D.; et al. MicroRNA-34a inhibits Glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009, 69, 7569–7576. [Google Scholar] [CrossRef]
- Liu, B.; Lin, X.; Yang, X.; Dong, H.; Yue, X.; Andrade, K.C.; Guo, Z.; Yang, J.; Wu, L.; Zhu, X.; et al. Downregulation of RND3/RhoE in Glioblastoma patients promotes tumorigenesis through augmentation of notch transcriptional complex activity. Cancer Med. 2015, 4, 1404–1416. [Google Scholar] [CrossRef]
- Panza, S.; Russo, U.; Giordano, F.; Leggio, A.; Barone, I.; Bonofiglio, D.; Gelsomino, L.; Malivindi, R.; Conforti, F.L.; Naimo, G.D.; et al. Leptin and Notch signaling cooperate in sustaining Glioblastoma multiforme progression. Biomolecules 2020, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Q.; Geng, R.; Liu, H.; Yuan, F.; Xu, Y.; Qi, Y.; Jiang, H.; Chen, Q.; Liu, B. Notch intracellular domain regulates Glioblastomaproliferation through the Notch1 signaling pathway. Oncol. Lett. 2021, 21, 303. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, N.; Yang, Y.; Jiang, G.; Zhan, H.; Li, F. LINC01152 upregulates MAML2 expression to modulate the progression of Glioblastoma multiforme via Notch signaling pathway. Cell Death Dis. 2021, 12, 115. [Google Scholar] [CrossRef]
- Cenciarelli, C.; Marei, H.E.; Zonfrillo, M.; Casalbore, P.; Felsani, A.; Giannetti, S.; Trevisi, G.; Althani, A.; Mangiola, A. The interference of Notch1 target Hes1 affects cell growth, differentiation and invasiveness of GBM stem cells through modulation of multiple oncogenic targets. Oncotarget 2017, 8, 17873–17886. [Google Scholar] [CrossRef]
- Raghu, H.; Gondi, C.S.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch-1 receptor. Mol. Cancer 2011, 10, 130. [Google Scholar] [CrossRef]
- Wei, L.; Pan, M.; Jiang, Q.; Hu, B.; Zhao, J.; Zou, C.; Chen, L.; Tang, C.; Zou, D. Eukaryotic initiation factor 4A-3 promotes Glioblastoma growth and invasion through the Notch1-dependent pathway. BMC Cancer 2023, 23, 550. [Google Scholar] [CrossRef]
- Mischel, P.S.; Cloughesy, T.F. Targeted molecular therapy of GBM. Brain Pathol. 2003, 13, 52–61. [Google Scholar] [CrossRef]
- Shih, A.H.; Holland, E.C. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006, 232, 139–147. [Google Scholar] [CrossRef]
- Heldin, C.H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Hovinga, K.E.; Shimizu, F.; Wang, R.; Panagiotakos, G.; Van Der Heijden, M.; Moayedpardazi, H.; Correia, A.S.; Soulet, D.; Major, T.; Menon, J.; et al. Inhibition of Notch signaling in Glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells 2010, 28, 1019–1029. [Google Scholar] [CrossRef]
- Cantanhede, I.G.; de Oliveira, J.R.M. PDGF family expression in Glioblastoma multiforme: Data compilation from Ivy GBM Atlas Project database. Sci. Rep. 2017, 7, 15271. [Google Scholar] [CrossRef]
- Westermark, B. Platelet-derived growth factor in Glioblastoma—Driver or biomarker? Upsala, J. Med. Sci. 2014, 119, 298–305. [Google Scholar] [CrossRef]
- Popescu, A.M.; Alexandru, O.; Brindusa, C.; Purcaru, S.O.; Tache, D.E.; Tataranu, L.G.; Taisescu, C.; Dricu, A. Targeting the VEGF and PDGF signaling pathway in GBM treatment. Int. J. Clin. Exp. Pathol. 2015, 8, 7825–7837. [Google Scholar] [PubMed]
- Cenciarelli, C.; Marei, H.E.; Zonfrillo, M.; Pierimarchi, P.; Paldino, E.; Casalbore, P.; Felsani, A.; Vescovi, A.L.; Maira, G.; Mangiola, A. PDGF receptor alpha inhibition induces apoptosis in Glioblastoma cancer stem cells refractory to anti-Notch and anti-EGFR treatment. Mol. Cancer 2014, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, S.; Salib, S.; Balasubramaniam, M.; Aboud, O. Epidermal growth factor receptor inhibitors in Glioblastoma: Current status and future possibilities. Int. J. Mol. Sci. 2024, 25, 2316. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Dei Cas, M.; Tringali, C.; Compostella, F.; Paroni, R.; Giussani, P. Ceramide is involved in temozolomide resistance in human Glioblastoma U87MG overexpressing EGFR. Int. J. Mol. Sci. 2023, 24, 15394. [Google Scholar] [CrossRef]
- Doan, N.B.; Nguyen, H.S.; Al-Gizawiy, M.M.; Mueller, W.M.; Sabbadini, R.A.; Rand, S.D.; Connelly, J.M.; Chitambar, C.R.; Schmainda, K.M.; Mirza, S.P. Acid ceramidase confers radioresistance to Glioblastoma cells. Oncol. Rep. 2017, 38, 1932–1940. [Google Scholar] [CrossRef]
- Nguyen, H.S.; Awad, A.J.; Shabani, S.; Doan, N. Molecular targeting of acid ceramidase inGlioblastoma: A review of its role, potential treatment, and challenges. Pharmaceutics 2018, 10, 45. [Google Scholar] [CrossRef]
- Doan, N.B.; Alhajala, H.; Al-Gizawiy, M.M.; Mueller, W.M.; Rand, S.D.; Connelly, J.M.; Cochran, E.J.; Chitambar, C.R.; Clark, P.; Kuo, J.; et al. Acid ceramidase and its inhibitors: A de novo drug target and a new class of drugs for killing Glioblastoma cancer stem cells with high efficiency. Oncotarget 2017, 8, 112662–112674. [Google Scholar] [CrossRef]
- Doan, N.B.; Nguyen, H.S.; Montoure, A.; Al-Gizawiy, M.M.; Mueller, W.M.; Kurpad, S.; Rand, S.D.; Connelly, J.M.; Chitambar, C.R.; Schmainda, K.M.; et al. Acid ceramidase is a novel drug target for pediatric brain tumors. Oncotarget 2017, 8, 24753–24761. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood–brain/brain tumor barriers for Glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood–brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Neuwelt, E.; Abbott, N.J.; Abrey, L.; Banks, W.A.; Blakley, B.; Davis, T.; Engelhardt, B.; Grammas, P.; Nedergaard, M.; Nutt, J.; et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008, 7, 84–96. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Keaney, J.; Campbell, M. The dynamic blood–brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F.; Stupp, R.; Homicsko, K. Standards of care and novel approaches in the management of Glioblastoma multiforme. Chin. J. Cancer 2014, 33, 32–39. [Google Scholar] [CrossRef]
- Barani, I.J.; Larson, D.A. Radiation therapy of Glioblastoma. Curr. Underst. Treat. Gliomas 2015, 163, 49–73. [Google Scholar]
- Schneider, S.W.; Ludwig, T.; Tatenhorst, L.; Braune, S.; Oberleithner, H.; Senner, V.; Paulus, W. Glioblastoma cells release factors that disrupt blood-brain barrier features. Acta Neuropathol. 2004, 107, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, C.L.; Liu, C.M. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des. Dev. Ther. 2015, 9, 2089–2100. [Google Scholar] [CrossRef]
- Lemée, J.M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in Glioblastoma: Don’t forget the peritumoral brain zone. Neuro-oncology 2015, 17, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat GBM. Neuro-oncology 2016, 18, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: Results of a prospective pilot study. Neuro-oncology 2013, 15, 1058–1067. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; Spohr, T.C.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L. The targeted delivery of cancer drugs across the blood-brain barrier: Chemical modifications of drugs or drug-nanoparticles? Drug Discovery Today 2008, 13, 1099–1106. [Google Scholar] [CrossRef]
- Liu, H.-J.; Xu, P. Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor. Adv. Drug Deliv. Rev. 2022, 191, 114619. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.Y.; Green, J.J. Therapeutic nanomedicine for brain cancer. Ther. Deliv. 2013, 4, 687–704. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for Glioblastoma multiforme: An update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Beier, D.; Schulz, J.B.; Beier, C.P. Chemoresistance of Glioblastoma cancer stem cells—Much more complex than expected. Mol. Cancer 2011, 10, 128. [Google Scholar] [CrossRef]
- Sundar, S.J.; Hsieh, J.K.; Manjila, S.; Lathia, J.D.; Sloan, A. The role of cancer stem cells in Glioblastoma. Neurosurg. Focus 2014, 37, E6. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in GBM in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for GBM. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Aldape, K.; Zadeh, G.; Mansouri, S.; Reifenberger, G.; von Deimling, A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The brain tumor microenvironment. Glia 2011, 59, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Vecchione, L.; Siena, S.; Tejpar, S.; Bardelli, A. Targeted therapies: How personal should we go? Nat. Rev. Clin. Oncol. 2012, 9, 87–97. [Google Scholar] [CrossRef]
- Martini, M.; Vecchione, L.; Siena, S.; Tejpar, S.; Bardelli, A. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Vasconcelos, M.H.; Caires, H.R.; Ābols, A.; Xavier, C.P.; Linē, A. Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist. Updates 2019, 47, 100647. [Google Scholar] [CrossRef]
- Bach, D.H.; Hong, J.Y.; Park, H.J.; Lee, S.K. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int. J. Cancer 2017, 141, 220–230. [Google Scholar] [CrossRef]
- Yekula, A.; Yekula, A.; Muralidharan, K.; Kang, K.; Carter, B.S.; Balaj, L. Extracellular vesicles in Glioblastoma tumor microenvironment. Front. Immunol. 2020, 10, 3137. [Google Scholar] [CrossRef]
- Samuel, P.; Fabbri, M.; Carter, D.R.F. Mechanisms of drug resistance in cancer: The role of extracellular vesicles. Proteomics 2017, 17, 1600375. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered extracellular vesicles for cancer therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef]
- Debinski, W.; Tatter, S.B. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev. Neurother. 2009, 9, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.; Wadajkar, A.S.; Roberts, N.B.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Nam, L.; Coll, C.; Erthal, L.C.S.; De La Torre, C.; Serrano, D.; Martínez-Máñez, R.; Santos-Martínez, M.J.; Ruiz-Hernández, E. Drug delivery nanosystems for the localized treatment of Glioblastoma multiforme. Materials 2018, 11, 779. [Google Scholar] [CrossRef]
- Vigani, B.; Valentino, C.; Sandri, G.; Listro, R.; Fagiani, F.; Collina, S.; Lanni, C.; Bonferoni, M.; Caramella, C.; Rossi, S.; et al. A composite nanosystem as a potential tool for the local treatment of Glioblastoma: Chitosan-coated solid lipid nanoparticles embedded in electrospun nanofibers. Polymers 2021, 13, 1371. [Google Scholar] [CrossRef]
- Straehla, J.P.; Warren, K.E. Pharmacokinetic principles and their application to central nervous system tumors. Pharmaceutics 2020, 12, 948. [Google Scholar] [CrossRef]
- Jones, A.R.; Shusta, E.V. Blood–brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef]
- Stanimirovic, D.B.; Sandhu, J.K.; Costain, W.J. Emerging technologies for delivery of biotherapeutics and gene therapy across the blood–brain barrier. BioDrugs 2018, 32, 547–559. [Google Scholar] [CrossRef]
- Elliott, R.; He, M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), S5. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, N.D.; Muldoon, L.L.; Culp, A.Y.; Neuwelt, E.A. Delivery of chemotherapeutics across the blood-brain barrier: Challenges and advances. Adv. Pharmacol. 2014, 71, 203–243. [Google Scholar] [PubMed]
- Chen, K.-T.; Chai, W.-Y.; Lin, Y.-J.; Lin, C.-J.; Chen, P.-Y.; Tsai, H.-C.; Huang, C.-Y.; Kuo, J.S.; Liu, H.-L.; Wei, K.-C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-drug conjugates and their targets in advanced cancer therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- Régina, A.; Demeule, M.; Ché, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.-P. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Central Nervous System Cancers; Version 1.2015; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2015. [Google Scholar] [CrossRef]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef]
- Mukherjee, D.; Quiñones-Hinojosa, A. Impact of extent of resection on outcomes in patients with high-grade gliomas. In Tumors of the Central Nervous System; Hayat, M.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 173–179. [Google Scholar]
- Zhao, S.; Wu, J.; Wang, C.; Liu, H.; Dong, X.; Shi, C.; Li, H. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid–induced porphyrins: A systematic review and meta-analysis of prospective studies. PLoS ONE 2013, 8, e63682. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Ellor, S.V.; Pagano-Young, T.A.; Avgeropoulos, N.G. Glioblastoma: Background, standard treatment paradigms, and supportive care considerations. J. Law Med. Ethics 2014, 42, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Walid, M.S. Prognostic factors for long-term survival after Glioblastoma. Perm. J. 2008, 12, 45–48. [Google Scholar] [CrossRef]
- Roth, P.; Gramatzki, D.; Weller, M. Management of elderly patients with Glioblastoma. Curr. Neurol. Neurosci. Rep. 2017, 17, 35. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of Glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Fu, P.; He, Y.S.; Huang, Q.; Ding, T.; Cen, Y.C.; Zhao, H.Y.; Wei, X. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed GBM. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Hanna, C.; Lawrie, T.A.; Rogozińska, E.; Kernohan, A.; Jefferies, S.; Bulbeck, H.; Ali, U.M.; Robinson, T.; Grant, R. Treatment of newly diagnosed Glioblastoma in the elderly: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 3, CD013261. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Baborie, A.; Alam, F.; Joyce, K.; Moxham, M.; Sibson, R.; Crooks, D.; Husband, D.; Shenoy, A.; Brodbelt, A.; et al. Walker CExtent of MGMTpromoter methylation correlates with outcome in Glioblastomas given temozolomide radiotherapy Br, J. Cancer 2009, 101, 124–131. [Google Scholar] [CrossRef]
- Halperin, E.C.; Wazer, D.E.; Perez, C.A.; Brady, L.W. Perez and Brady’s: Principles and Practice of Radiation Oncology, 7th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018. [Google Scholar]
- Jayasinghe, M.K.; Tan, M.; Peng, B.; Yang, Y.; Sethi, G.; Pirisinu, M.; Le, M.T. New approaches in extracellular vesicle engineering for improving the efficacy of anti-cancer therapies. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 62–78. [Google Scholar]
- Hernández-Pedro, N.Y.; Rangel-López, E.; Magaña-Maldonado, R.; de la Cruz, V.P.; Santamaría del Angel, A.; Pineda, B.; Sotelo, J. Application of nanoparticles on diagnosis and therapy in gliomas. BioMed Res. Int. 2013, 2013, 676241. [Google Scholar] [CrossRef]
- Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology meets oncology: Nanomaterials in brain cancer research, diagnosis and therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, J.; Sun, X. Reactive oxygen species-based nanomaterials for cancer therapy. Front. Chem. 2021, 9, 650587. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer nanotechnology: A new revolution for cancer diagnosis and therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Almanghadim, H.G.; Nourollahzadeh, Z.; Khademi, N.S.; Tezerjani, M.D.; Sehrig, F.Z.; Estelami, N.; Shirvaliloo, M.; Sheervalilou, R.; Sargazi, S. Application of nanoparticles in cancer therapy with an emphasis on cell cycle. Cell Biol. Int. 2021, 45, 1989–1998. [Google Scholar] [CrossRef]
- Shahraki, K.; Boroumand, P.G.; Lotfi, H.; Radnia, F.; Shahriari, H.; Sargazi, S.; Mortazavi, S.S.; Shirvaliloo, M.; Shirvalilou, S.; Sheervalilou, R. An update in the applications of exosomes in cancer theranostics: From research to clinical trials. J. Cancer Res. Clin. Oncol. 2023, 149, 8087–8116. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.; Hong, S.; Farokhzad, O.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–759. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Appeldoorn, C.C.; Rip, J.; Dorland, R.; van der Pol, S.M.; Kooij, G.; de Vries, H.E.; Reijerkerk, A. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J. Control. Release 2012, 164, 364–369. [Google Scholar] [CrossRef]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef]
- Telrandhe, R. Nanotechnology for cancer therapy: Recent developments. Eur. J. Pharm. Med. Res. 2016, 3, 284–294. [Google Scholar]
- Rutka, J.T.; Kim, B.; Etame, A.; Diaz, R.J. Nanosurgical resection of malignant brain tumors: Beyond the cutting edge. ACS Nano 2014, 8, 9716–9722. [Google Scholar] [CrossRef]
- Martin, F.; Melnik, K.; West, T.; Shapiro, J.; Cohen, M.; Boiarski, A.; Ferrari, M. Acute toxicity of intravenously administered microfabricated silicon dioxide drug delivery particles in mice: Preliminary findings. Drugs R&D 2005, 6, 71–81. [Google Scholar]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef]
- Pan, B.; Cui, D.; Sheng, Y.; Ozkan, C.; Gao, F.; He, R.; Li, Q.; Xu, P.; Huang, T. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 2007, 67, 8156–8163. [Google Scholar] [CrossRef]
- Mohanraj, V.; Chen, Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Yan, F.; Xu, H.; Anker, J.; Kopelman, R.; Ross, B.; Rehemtulla, A.; Reddy, R. Synthesis and characterization of silica-embedded iron oxide nanoparticles for magnetic resonance imaging. J. Nanosci. Nanotechnol. 2004, 4, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The clinical translation of organic nanomaterials for cancer therapy: A focus on polymeric nanoparticles, micelles, liposomes and exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Zhang, L.; Lei, J. Multifunctional metal–organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50, 1188–1218. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers 2018, 10, 1133. [Google Scholar] [CrossRef]
- Xiao, W.; Ehsanipour, A.; Sohrabi, A.; Seidlits, S.K. Hyaluronic-acid based hydrogels for 3-dimensional culture of patient-derived GBM cells. J. Vis. Exp. 2018, 138, e58176. [Google Scholar]
- Lapcík, L., Jr.; Lapcík, L.; De Smedt, S.; Demeester, J.; Chabrecek, P. Hyaluronan: Preparation, structure, properties, and applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Wei, Y.; Cong, F. Hyaluronan-inorganic nanohybrid materials for biomedical applications. Biomacromolecules 2017, 18, 1677–1696. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Rigas, B.; Constantinides, P.P. Nanodelivery strategies in cancer chemotherapy: Biological rationale and pharmaceutical perspectives. Nanomedicine 2012, 7, 1577–1590. [Google Scholar] [CrossRef]
- Fei, W.; Zhang, M.; Fan, X.; Ye, Y.; Zhao, M.; Zheng, C.; Li, Y.; Zheng, X. Engineering of bioactive metal sulfide nanomaterials for cancer therapy. J. Nanobiotechnol. 2021, 19, 93. [Google Scholar] [CrossRef]
- Wiwatchaitawee, K.; Quarterman, J.C.; Geary, S.M.; Salem, A.K. Enhancement of therapies for Glioblastoma (GBM) using nanoparticle-based delivery systems. AAPS PharmSciTech 2021, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted nanotechnology in Glioblastoma multiforme. Front. Pharmacol. 2017, 8, 166. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Gao, P.; Sun, Y.; Duan, Y.-R. Development of targeted therapies in treatment of Glioblastoma. Cancer Biol. Med. 2015, 12, 223. [Google Scholar]
- Jurj, A.; Braicu, C.; Pop, L.-A.; Tomuleasa, C.; Gherman, C.D.; Berindan-Neagoe, I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Dev. Ther. 2017, 11, 2871. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 2259–2266. [Google Scholar] [CrossRef]
- Gref, R.; Domb, A.; Quellec, P.; Blunk, T.; Müller, R.; Verbavatz, J.-M.; Langer, R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 1995, 16, 215–233. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, M.H.; Najda, A.; Albadrani, G.M.; Sayed, A.A. Natural bioactive molecules: An alternative approach to the treatment and control of Glioblastoma multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Kumar, A.; Badde, S.; Kamble, R.; Pokharkar, V. Development and characterization of liposomal drug delivery system for Nimesulide. Int. J. Pharm. Pharm. Sci. 2010, 2, 87–89. [Google Scholar]
- Patidar, A.; Thakur, D.S.; Kumar, P.; Verma, J. A review on novel lipid based nanocarriers. Int. J. Pharm. Pharm. Sci. 2010, 2, 30–35. [Google Scholar]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Faria, V.; Quintas, J.P.; Almeida, A.J. Targeted delivery of lipid nanoparticles by means of surface chemical modification. Curr. Org. Chem. 2017, 21, 2360–2375. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Mendes, M.; Miranda, A.; Cova, T.; Gonçalves, L.; Almeida, A.J.; Sousa, J.J.; do Vale, M.L.C.; Marques, E.F.; Pais, A.; Vitorino, C. Modeling of ultra-small lipid nanoparticle surface charge for targeting Glioblastoma. Eur. J. Pharm. Sci. 2018, 117, 255–269. [Google Scholar] [CrossRef]
- Lu, W. Adsorptive-mediated brain delivery systems. Curr. Pharm. Biotechnol. 2012, 13, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J. Control. Release 2003, 91, 75–83. [Google Scholar] [CrossRef]
- Tang, J.; Ji, H.; Ren, J.; Li, M.; Zheng, N.; Wu, L. Solid lipid nanoparticles with TPGS and Brij 78: A co-delivery vehicle of curcumin and piperine for reversing P-glycoprotein-mediated multidrug resistance in vitro. Oncol. Lett. 2017, 13, 389–395. [Google Scholar] [CrossRef]
- Wang, S.-W.; Monagle, J.; McNulty, C.; Putnam, D.; Chen, H. Determination of P-glycoprotein inhibition by excipients and their combinations using an integrated high-throughput process. J. Pharm. Sci. 2004, 93, 2755–2767. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Pandey, R.S.; Jain, V.; Katare, O.P.; Chandra, R.; Katyal, A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in Glioblastoma cells. Nanomedicine 2013, 9, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Venishetty, V.K.; Komuravelli, R.; Kuncha, M.; Sistla, R.; Diwan, P.V. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomedicine 2013, 9, 111–121. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Tekade, R.K.; Chougule, M.B. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: Current progress and advances. J. Control. Release 2014, 194, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor microenvironment targeted nanotherapy. Front. Pharmacol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Liang, C.-T. Inhibition of human brain malignant Glioblastoma cells using carmustine-loaded catanionic solid lipid nanoparticles with surface anti-epithelial growth factor receptor. Biomaterials 2011, 32, 3340–3350. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lee, I.-H. Delivery of doxorubicin to Glioblastoma multiforme in vitro using solid lipid nanoparticles with surface aprotinin and melanotransferrin antibody for enhanced chemotherapy. J. Taiwan Inst. Chem. Eng. 2016, 61, 32–45. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef]
- Chou, L.Y.T.; Ming, K.; Chan, W.C.W. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef]
- Singh, I.; Swami, R.; Pooja, D.; Jeengar, M.K.; Khan, W.; Sistla, R. Lactoferrin bioconjugated solid lipid nanoparticles: A new drug delivery system for potential brain targeting. J. Drug Target. 2016, 24, 212–223. [Google Scholar] [CrossRef]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing Glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef]
- Shen, H.; Shi, S.; Zhang, Z.; Gong, T.; Sun, X. Coating solid lipid nanoparticles with hyaluronic acid enhances antitumor activity against melanoma stem-like cells. Theranostics 2015, 5, 755–771. [Google Scholar] [CrossRef]
- Tran, T.H.; Choi, J.Y.; Ramasamy, T.; Truong, D.H.; Nguyen, C.N.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells. Carbohydr. Polym. 2014, 114, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Srivastava, S.K.; Chaudhuri, T.K.; Upadhyay, G. Multifaceted role of matrix metalloproteinases (MMPs). Front. Mol. Biosci. 2015, 2, 22. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Wu, P.-C.; Tsai, T.-H.; Fang, Y.-P.; Tsai, Y.-H.; Cheng, T.-C.; Huang, C.-C.; Huang, M.-Y.; Chen, F.-M.; Hsieh, Y.-C.; et al. Development of pH-sensitive cationic pegylated solid lipid nanoparticles for selective cancer-targeted therapy. J. Biomed. Nanotechnol. 2017, 13, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; Schipper, N.G.; Verhoef, J.C.; Merkus, F.W.H. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Charlton, S.; Jones, N.S.; Davis, S.S.; Illum, L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: Effect of polymer type and nasal delivery device. Eur. J. Pharm. Sci. 2007, 30, 295–302. [Google Scholar] [CrossRef]
- Haffejee, N.; Du Plessis, J.; Müller, D.G.; Schultz, C.; Kotzé, A.F.; Goosen, C. Intranasal toxicity of selected absorption enhancers. Pharmazie 2001, 56, 882–888. [Google Scholar]
- Bies, C.; Lehr, C.-M.; Woodley, J.F. Lectin-mediated drug targeting: History and applications. Adv. Drug Deliv. Rev. 2004, 56, 425–435. [Google Scholar] [CrossRef]
- Fu, T.; Burbage, C.; Tagge, E.P.; Brothers, T.; Willingham, M.C.; Frankel, A.E. Ricin toxin contains three lectin sites which contribute to its in vivo toxicity. Int. J. Immunopharmacol. 1996, 18, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Solid lipid nanoparticles and nanostructured lipid carriers as smart drug delivery systems in the treatment of Glioblastoma multiforme. Pharmaceutics 2020, 12, 860. [Google Scholar] [CrossRef]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef]
- Sheervalilou, R.; Shirvaliloo, M.; Sargazi, S.; Ghaznavi, H. Recent advances in iron oxide nanoparticles for brain cancer theranostics: From in vitro to clinical applications. Expert Opin. Drug Deliv. 2021, 18, 949–977. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic nanoparticles in cancer therapy and diagnosis. Adv. Healthc. Mater. 2020, 9, 1901058. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Urbantat, R.M.; Jelgersma, C.; Brandenburg, S.; Nieminen-Kelhä, M.; Kremenetskaia, I.; Zollfrank, J.; Mueller, S.; Rubarth, K.; Koch, A.; Vajkoczy, P. Tumor-associated microglia/macrophages as a predictor for survival in Glioblastoma and temozolomide-induced changes in CXCR2 signaling with new resistance overcoming strategy by combination therapy. Int. J. Mol. Sci. 2021, 22, 11180. [Google Scholar] [CrossRef]
- Mukherjee, S.; Liang, L.; Veiseh, O. Recent advancements of magnetic nanomaterials in cancer therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef]
- Pourgholi, F.; Farhad, J.-N.; Kafil, H.S.; Yousefi, M. Nanoparticles: Novel vehicles in treatment of Glioblastoma. Biomed. Pharmacother. 2016, 77, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Anarjan, N.; Vaghari, H.; Sayyar, Z.; Berenjian, A. A biotechnological perspective on the application of iron oxide nanoparticles. Nano Res. 2016, 9, 2203–2225. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Li, F.; Lu, J.; Kong, X.; Hyeon, T.; Ling, D. Dynamic nanoparticle assemblies for biomedical applications. Adv. Mater. 2017, 29, 1605897. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Bruschi, M.L.; de Toledo, L.A.S. Pharmaceutical applications of iron-oxide magnetic nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef]
- Alexiou, C.; Schmid, R.J.; Jurgons, R.; Kremer, M.; Wanner, G.; Bergemann, C.; Huenges, E.; Nawroth, T.; Arnold, W.; Parak, F.G. Targeting cancer cells: Magnetic nanoparticles as drug carriers. Eur. Biophys. J. 2006, 35, 446–450. [Google Scholar] [CrossRef]
- Abadi, B.; Yazdanpanah, N.; Nokhodchi, A.; Rezaei, N. Smart biomaterials to enhance the efficiency of immunotherapy in GBM: State of the art and future perspectives. Adv. Drug Deliv. Rev. 2021, 179, 114035. [Google Scholar] [CrossRef]
- Ciccarese, F.; Raimondi, V.; Sharova, E.; Silic-Benussi, M.; Ciminale, V. Nanoparticles as tools to target redox homeostasis in cancer cells. Antioxidants 2020, 9, 211. [Google Scholar] [CrossRef]
- Jin, J.; Ovais, M.; Chen, C. Stimulus-responsive gold nanotheranostic platforms for targeting the tumor microenvironment. Nano Today 2018, 22, 83–99. [Google Scholar] [CrossRef]
- Aryal, S.; Bisht, G. New paradigm for a targeted cancer therapeutic approach: A short review on potential synergy of gold nanoparticles and cold atmospheric plasma. Biomedicines 2017, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Y.; Qutaish, H.; Kim, J.H.; Huang, X.-F.; Alvi, S.; Konstantinov, K. Microenvironmental behaviour of nanotheranostic systems for controlled oxidative stress and cancer treatment. Nanomaterials 2022, 12, 2462. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S.; Liang, J.; Wang, R.; Zhong, Y.; Shi, Y. Aptamer-conjugated gold nanoparticles targeting epidermal growth factor receptor variant III for the treatment of Glioblastoma. Int. J. Nanomed. 2020, 15, 1363. [Google Scholar] [CrossRef] [PubMed]
- Saleem, J.; Wang, L.; Chen, C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv. Healthc. Mater. 2018, 7, 1800525. [Google Scholar] [CrossRef]
- Leite, M.L.; da Cunha, N.B.; Costa, F.F. Antimicrobial peptides, nanotechnology, and natural metabolites as novel approaches for cancer treatment. Pharmacol. Ther. 2018, 183, 160–176. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Kiseleva, R.; Vertegel, A.; Ray, S.K. Carbon nanomaterials for drug delivery and cancer therapy. J. Nanosci. Nanotechnol. 2015, 15, 5501–5511. [Google Scholar] [CrossRef]
- Benos, L.; Spyrou, L.A.; Sarris, I.E. Development of a new theoretical model for blood-CNTs effective thermal conductivity pertaining to hyperthermia therapy of Glioblastoma multiforme. Comput. Methods Programs Biomed. 2019, 172, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’Ascenzo, M.; Primiano, A.; Gervasoni, J.; De Maio, F.; De Spirito, M.; Papi, M. Enhanced chemotherapy for Glioblastoma multiforme mediated by functionalized graphene quantum dots. Materials 2020, 13, 4139. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Friggeri, G.; Augello, A.; De Spirito, M.; Papi, M. Carboxylated graphene quantum dots-mediated photothermal therapy enhances drug-membrane permeability, ROS production, and the immune system recruitment on 3D Glioblastoma models. Cancer Nanotechnol. 2023, 14, 13. [Google Scholar] [CrossRef]
- Perini, G.; Rosa, E.; Friggeri, G.; Di Pietro, L.; Barba, M.; Parolini, O.; Ciasca, G.; Moriconi, C.; Papi, M.; De Spirito, M. INSIDIA 2.0 high-throughput analysis of 3D cancer models: Multiparametric quantification of graphene quantum dots photothermal therapy for Glioblastoma and pancreatic cancer. Int. J. Mol. Sci. 2022, 23, 3217. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Fu, Q.; Ye, J.; Su, L.; Ge, X.; Chen, L.; Song, J.; Yang, H. Neodymium (3+)-coordinated black phosphorus quantum dots with retrievable NIR/X-ray optoelectronic switching effect for anti-Glioblastoma. Small 2022, 18, 2105160. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Wahab, R.; Bhartiya, P.; Linh, N.N.; Khan, F.; Al-Khedhairy, A.A.; Choi, E.H. Cold atmospheric plasma and gold quantum dots exert dual cytotoxicity mediated by the cell receptor-activated apoptotic pathway in Glioblastoma cells. Cancers 2020, 12, 457. [Google Scholar] [CrossRef]
- Miranda, A.; Blanco-Prieto, M.J.; Sousa, J.; Pais, A.; Vitorino, C. Breaching barriers in Glioblastoma. Part II: Targeted drug delivery and lipid nanoparticles. Int. J. Pharm. 2017, 531, 389–410. [Google Scholar] [CrossRef]
- Abbasi, M.; Boka, D.A.; DeLoit, H. Nanomaterial-enhanced microneedles: Emerging therapies for diabetes and obesity. Pharmaceutics 2024, 16, 1344. [Google Scholar] [CrossRef] [PubMed]
- Beirampour, N.; Bustos-Salgado, P.; Garrós, N.; Mohammadi-Meyabadi, R.; Domènech, Ò.; Suñer-Carbó, J.; Rodríguez-Lagunas, M.J.; Kapravelou, G.; Montes, M.J.; Calpena, A.; et al. Formulation of polymeric nanoparticles loading baricitinib as a topical approach in ocular application. Pharmaceutics 2024, 16, 1092. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.M.; Rabkin, S.D. Current status of gene therapy for brain tumors. Transl. Res. 2013, 161, 339–354. [Google Scholar] [CrossRef]
- Kumari, S.; Gupta, R.; Ambasta, R.K.; Kumar, P. Emerging trends in post-translational modification: Shedding light on Glioblastoma multiforme. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188999. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Imeh, A.E.; Li, W. Polymeric nanoparticles—Promising carriers for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1024143. [Google Scholar] [CrossRef]
- Ferraro, C.; Dattilo, M.; Patitucci, F.; Prete, S.; Scopelliti, G.; Parisi, O.I.; Puoci, F. Exploring protein-based carriers in drug delivery: A review. Pharmaceutics 2024, 16, 1172. [Google Scholar] [CrossRef]
- Mora-Cabello, R.; Fuentes-Ríos, D.; Gago, L.; Cabeza, L.; Moscoso, A.; Melguizo, C.; Prados, J.; Sarabia, F.; López-Romero, J.M. Magnetic nanoparticles with on-site azide and alkyne functionalized polymer coating in a single step through a solvothermal process. Pharmaceutics 2024, 16, 1226. [Google Scholar] [CrossRef]
- Shishlyannikov, S.M.; Zubkov, I.N.; Vysochinskaya, V.V.; Gavrilova, N.V.; Dobrovolskaya, O.A.; Elpaeva, E.A.; Maslov, M.A.; Vasin, A. Stable polymer-lipid hybrid nanoparticles based on mcl-polyhydroxyalkanoate and cationic liposomes for mRNA delivery. Pharmaceutics 2024, 16, 1305. [Google Scholar] [CrossRef]
- Sun, C.; Fang, C.; Stephen, Z.; Veiseh, O.; Hansen, S.; Lee, D.; Ellenbogen, R.G.; Olson, J.; Zhang, M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine 2008, 3, 495–505. [Google Scholar] [CrossRef]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. Semin. Cancer Biol. 2021, 69, 52–68. [Google Scholar] [CrossRef]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Targeted polymeric nanoparticle for anthracycline delivery in hypoxia-induced drug resistance in metastatic breast cancer cells. Anticancer Drugs 2021, 32, 745–754. [Google Scholar] [CrossRef]
- Dugas, T.R.; Brewer, G.; Longwell, M.; Fradella, T.; Braun, J.; Astete, C.E.; Jennings, M.H.; Sabliov, C.M. Nanoentrapped polyphenol coating for sustained drug release from a balloon catheter. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.I.R.; do Carmo Neto, J.R.; Rocha, V.L.; Machado, J.R.; Amaral, A.C.; Miguel, M.P. A revision of polymeric nanoparticles as a strategy to improve the biological activity of melatonin. Curr. Med. Chem. 2023, 30, 3315–3334. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Kydd, J.; Piel, B.; Rai, P. Targeting cancer using polymeric nanoparticle mediated combination chemotherapy. Int. J. Nanomed. Nanosurg. 2016, 2, 10–16966. [Google Scholar]
- Geszke-Moritz, M.; Moritz, M. Biodegradable polymeric nanoparticle-based drug delivery systems: Comprehensive overview, perspectives and challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Heon Lee, I.; Palombo, M.S.; Zhang, X.; Szekely, Z.; Sinko, P.J. Design and evaluation of a CXCR4 targeting peptide 4DV3 as an HIV entry inhibitor and a ligand for targeted drug delivery. Eur. J. Pharm. Biopharm. 2019, 138, 11–22. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef]
- Ho, K.S.; Aman, A.M.; Al-awar, R.S.; Shoichet, M.S. Amphiphilic micelles of poly(2-methyl-2-carboxytrimethylene carbonate-co-D,L-lactide)-graft-poly(ethylene glycol) for anti-cancer drug delivery to solid tumours. Biomaterials 2012, 33, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of Glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Mughees, M.; Mangangcha, I.R.; Swami, S.; Wajid, S. Secretome profiling of Artemisia absinthium extract-loaded polymeric nanoparticle-treated MCF-7 and MDA-MB-231 revealed perturbation in microtubule assembly and cell migration. Front. Oncol. 2023, 13, 1209168. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Q.; Mo, J.; Dai, H. Drug-loaded polymeric nanoparticles for cancer stem cell targeting. Front. Pharmacol. 2017, 8, 51. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, D.; Lei, H.; Liu, J.; Lei, J.; Wang, L.; Ma, X. Development of novel lignin-based targeted polymeric nanoparticle platform for efficient delivery of anticancer drugs. ACS Biomater. Sci. Eng. 2018, 4, 1730–1737. [Google Scholar] [CrossRef]
- Marshall, S.K.; Angsantikul, P.; Pang, Z.; Nasongkla, N.; Hussen, R.S.D.; Thamphiwatana, S.D. Biomimetic targeted theranostic nanoparticles for breast cancer treatment. Molecules 2022, 27, 6473. [Google Scholar] [CrossRef]
- Muhtadi, W.K.; Novitasari, L.; Danarti, R.; Martien, R. Development of polymeric nanoparticle gel prepared with the combination of ionic pre-gelation and polyelectrolyte complexation as a novel drug delivery of timolol maleate. Drug Dev. Ind. Pharm. 2020, 46, 1844–1852. [Google Scholar] [CrossRef]
- Nance, E.; Timbie, K.; Miller, G.W.; Song, J.; Louttit, C.; Klibanov, A.L.; Shih, T.Y.; Swaminathan, G.; Tamargo, R.J.; Woodworth, G.F.; et al. Non-invasive delivery of stealth brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J. Control. Release 2014, 189, 123–132. [Google Scholar] [CrossRef]
- Naser, S.S.; Gupta, A.; Choudhury, A.; Yadav, A.; Sinha, A.; Kirti, A.; Singh, D.; Kujawska, M.; Kaushik, N.K.; Ghosh, A.; et al. Biophysical translational paradigm of polymeric nanoparticle: Embarked advancement to brain tumor therapy. Biomed. Pharmacother. 2024, 179, 117372. [Google Scholar] [CrossRef]
- Niza, E.; Ocaña, A.; Castro-Osma, J.A.; Bravo, I.; Alonso-Moreno, C. Polyester polymeric nanoparticles as platforms in the development of novel nanomedicines for cancer treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef]
- Nozohouri, S.; Salehi, R.; Ghanbarzadeh, S.; Adibkia, K.; Hamishehkar, H. A multilayer hollow nanocarrier for pulmonary co-drug delivery of methotrexate and doxorubicin in the form of dry powder inhalation formulation. Mater Sci. Eng. C Mater Biol. Appl. 2019, 99, 752–761. [Google Scholar] [CrossRef]
- Ou, B.S.; Baillet, J.; Picece, V.C.T.M.; Gale, E.C.; Powell, A.E.; Saouaf, O.M.; Yan, J.; Nejatfard, A.; Lopez Hernandez, H.; Appel, E.A. Nanoparticle-conjugated toll-like receptor 9 agonists improve the potency, durability, and breadth of COVID-19 vaccines. ACS Nano 2024, 18, 3214–3233. [Google Scholar] [CrossRef]
- Pillai, S.C.; Borah, A.; Jindal, A.; Jacob, E.M.; Yamamoto, Y.; Kumar, D.S. BioPerine encapsulated nanoformulation for overcoming drug-resistant breast cancers. Asian J. Pharm. Sci. 2020, 15, 701–712. [Google Scholar] [CrossRef]
- Raman, S.; Mahmood, S.; Hilles, A.R.; Javed, M.N.; Azmana, M.; Al-Japairai, K.A.S. Polymeric nanoparticles for brain drug delivery—A review. Curr. Drug Metab. 2020, 21, 649–660. [Google Scholar] [CrossRef]
- Ramírez-García, P.D.; Retamal, J.S.; Shenoy, P.; Imlach, W.; Sykes, M.; Truong, N.; Constandil, L.; Pelissier, T.; Nowell, C.J.; Khor, S.Y.; et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 2019, 14, 1150–1159. [Google Scholar] [CrossRef]
- Sabit, H.; Abdel-Hakeem, M.; Shoala, T.; Abdel-Ghany, S.; Abdel-Latif, M.M.; Almulhim, J.; Mansy, M. Nanocarriers: A reliable tool for the delivery of anticancer drugs. Pharmaceutics 2022, 14, 1566. [Google Scholar] [CrossRef]
- Cheng, X.; Li, D.; Sun, M.; He, L.; Zheng, Y.; Wang, X.; Qamar, Z.; Qizilbash, F.F.; Annu, M.S.; Alhakamy, N.A.; et al. Co-delivery of DOX and PDTC by pH-sensitive nanoparticles to overcome multidrug resistance in breast cancer. Colloids Surf. B Biointerfaces 2019, 181, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, A.; Qamar, Z.; Qizilbash, F.F.; Annu, M.S.; Alhakamy, N.A.; Baboota, S.; Ali, J. Polymeric nanoparticles: Exploring the current drug development and therapeutic insight of breast cancer treatment and recommendations. Polymers 2021, 13, 4400. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zong, H.; Kopelman, R. Click conjugation of peptide to hydrogel nanoparticles for tumor-targeted drug delivery. Biomacromolecules 2014, 15, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Q.; Dong, X.; Xu, M.; Yang, J.; Yi, X.; Chen, B.; Dong, X.; Wang, Y.; Lou, X.; et al. Biocompatible AIEgen/p-glycoprotein siRNA@reduction-sensitive paclitaxel polymeric prodrug nanoparticles for overcoming chemotherapy resistance in ovarian cancer. Theranostics 2021, 11, 3710–3724. [Google Scholar] [CrossRef]
- Xu, B.; Zeng, F.; Deng, J.; Yao, L.; Liu, S.; Hou, H.; Huang, Y.; Zhu, H.; Wu, S.; Li, Q.; et al. A homologous and molecular dual-targeted biomimetic nanocarrier for EGFR-related non-small cell lung cancer therapy. Bioact. Mater. 2023, 27, 337–347. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Z.; Wang, H.; Chang, Y.; Xu, J.F.; Zhang, X. A polymeric nanoparticle to co-deliver mitochondria-targeting peptides and Pt(IV) prodrug: Toward high loading efficiency and combination efficacy. Angew. Chem. Int. Ed. 2024, 63, e202402291. [Google Scholar] [CrossRef]
- Zubris, K.A.; Colson, Y.L.; Grinstaff, M.W. Hydrogels as intracellular depots for drug delivery. Mol. Pharm. 2012, 9, 196–200. [Google Scholar] [CrossRef]
- Zu, M.; Ma, Y.; Cannup, B.; Xie, D.; Jung, Y.; Zhang, J.; Yang, C.; Gao, F.; Merlin, D.; Xiao, B. Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv. Drug Deliv. Rev. 2021, 176, 113887. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of polymeric nanoparticles for blood-brain barrier transfer-strategies and challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.W.K.; Deák, Á.; Csanády, M., Jr.; Szemerédi, N.; Szabó, D.; Turcsányi, Á.; Ungor, D.; Spengler, G.; Rovó, L.; Janovák, L. pH-triggered hydrogel nanoparticles for efficient anticancer drug delivery and bioimaging applications. Pharmaceutics 2024, 16, 931. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.P.D.; Martins, N.O.; Dos Santos, C.R.; Damas, E.B.O.; Araujo, P.L.; Silva, G.O.; Joanitti, G.A.; Carneiro, M.L.B. Annatto (bixa orellana)-based nanostructures for biomedical applications—A systematic review. Pharmaceutics 2024, 16, 1275. [Google Scholar] [CrossRef]
- Gandhi, S.; Shastri, D.H.; Shah, J.; Nair, A.B.; Jacob, S. Nasal delivery to the brain: Harnessing nanoparticles for effective drug transport. Pharmaceutics 2024, 16, 481. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Deshmukh, R.; Shukla, V.P.; Khunt, D.; Prajapati, B.G.; Rashid, S.; Ali, N.; Elossaily, G.M.; Suryawanshi, V.K.; Kumar, A. Recent advancements in gallic acid-based drug delivery: Applications, clinical trials, and future directions. Pharmaceutics 2024, 16, 1202. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, G.; Cho, S.B.; Im, H.J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of GBM multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Gawel, A.M.; Betkowska, A.; Gajda, E.; Godlewska, M.; Gawel, D. Current non-metal nanoparticle-based therapeutic approaches for GBM treatment. Biomedicines 2024, 12, 1822. [Google Scholar] [CrossRef]
- Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic therapy for GBM: Illuminating the path toward clinical applicability. Cancers 2023, 15, 3427. [Google Scholar] [CrossRef]
- Caverzan, M.D.; Ibarra, L.E. Advancing Glioblastoma treatment through iron metabolism: A focus on TfR1 and ferroptosis innovations. Int. J. Biol. Macromol. 2024, 278 Pt 2, 134777. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chavarro, V.S.; Gerstl, J.V.E.; Blitz, S.E.; Spanehl, L.; Dubinski, D.; Valdes, P.A.; Tran, L.N.; Gupta, S.; Esposito, L.; et al. Recurrent Glioblastoma—Molecular underpinnings and evolving treatment paradigms. Int. J. Mol. Sci. 2024, 25, 6733. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Sekar, T.V.; Ananta, J.S.; Devulapally, R.; Afjei, R.; Babikir, H.A.; Paulmurugan, R.; Massoud, T.F. Targeted nanoparticle delivery of therapeutic antisense microRNAs presensitizes Glioblastoma cells to lower effective doses of temozolomide in vitro and in a mouse model. Oncotarget 2018, 9, 21478–21494. [Google Scholar] [CrossRef][Green Version]
- Cruz, N.; Herculano-Carvalho, M.; Roque, D.; Faria, C.C.; Cascão, R.; Ferreira, H.A.; Reis, C.P.; Matela, N. Highlighted advances in therapies for difficult-to-treat brain tumours such as Glioblastoma. Pharmaceutics 2023, 15, 928. [Google Scholar] [CrossRef] [PubMed]
- Sandbhor, P.; Goda, J.; Mohanty, B.; Gera, P.; Yadav, S.; Chekuri, G.; Chaudhari, P.; Dutt, S.; Banerjee, R. Targeted nano-delivery of chemotherapy via intranasal route suppresses in vivo Glioblastoma growth and prolongs survival in the intracranial mouse model. Drug Deliv. Transl. Res. 2023, 13, 608–626. [Google Scholar] [CrossRef]
- Ruiz-Garcia, H.; Ramirez-Loera, C.; Malouff, T.D.; Seneviratne, D.S.; Palmer, J.D.; Trifiletti, D.M. Novel strategies for nanoparticle-based radiosensitization in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 9673. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J.; Lou, L.; Qi, X.; Zhao, L.; Fan, B.; Sun, G.; Lv, Z.; Fan, Z.; Jiao, B.; et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of Glioblastoma. Bioeng. Transl. Med. 2020, 6, e10203. [Google Scholar] [CrossRef]
- Kim, B.D.; Mondal, S.K.; Kenyon, E.; Chen, M.; Mallett, C.L.; deCarvalho, A.C.; Medarova, Z.; Moore, A. Nanoparticle delivery of an oligonucleotide payload in a Glioblastoma multiforme animal model. J. Vis. Exp. 2024, e66986. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Lin, Y.L.; Luh, F.; Yen, Y.; Chen, R.M. Preclinical effects of CRLX101, an investigational camptothecin-containing nanoparticle drug conjugate, on treating Glioblastoma multiforme via apoptosis and antiangiogenesis. Oncotarget 2016, 7, 42408–42421. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference–based spherical nucleic acids in patients with recurrent Glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Rocchi, P.; Brown, N.; Carmès, L.; Guthier, R.; Iyer, M.; Seban, L.; Morris, T.; Bennett, S.; Lavelle, M.; et al. Tuning ultrasmall theranostic nanoparticles for MRI contrast and radiation dose amplification. Theranostics 2023, 13, 4711–4729. [Google Scholar] [CrossRef]
- Smolarska, A.; Pruszynska, I.; Wasylko, W.; Godlewska, K.; Markowska, M.; Rybak, A.; Botther, J.; Kucharzewska, P.; Nowakowska, J.; Szeliga, J.; et al. Targeted therapies for Glioblastoma treatment. J. Physiol. Pharmacol. 2023, 74, 251–261. [Google Scholar] [CrossRef]
- Nan, J.; Yang, W.; Xie, Y.; Yu, M.; Chen, Y.; Zhang, J. Emerging Nano-Immunotherapeutic Approaches to Glioma. Small Struct. 2023, 4, 2300016. [Google Scholar] [CrossRef]
- Beola, L.; Iturrioz-Rodriguez, N.; Pucci, C.; Bertorelli, R.; Ciofani, G. Drug-Loaded Lipid Magnetic Nanoparticles for Combined Local Hyperthermia and Chemotherapy against Glioblastoma Multiforme. ACS Nano 2023, 17, 18441–18455. [Google Scholar] [CrossRef]
- Dhiman, A.; Shah, Y.; Rana, D. Comprehensive review on Glioblastoma: Nanotechnology, immunotherapy and combined therapeutic approaches. RSC Pharm. 2025, 2, 207–234. [Google Scholar] [CrossRef]
- Alfonso-Triguero, P.; Lorenzo, J.; Candiota, A.P.; Arús, C.; Ruiz-Molina, D.; Novio, F. Platinum-Based Nanoformulations for Glioblastoma Treatment: The Resurgence of Platinum Drugs? Nanomaterials 2023, 13, 1619. [Google Scholar] [CrossRef]
- Liu, D.; Dai, X.; Tao, Z.; Zhou, H.; Hong, W.; Qian, H.; Cheng, H.; Wang, X. Advances in blood–brain barrier-crossing nanomedicine for anti-glioma. Cancer Nano 2023, 14, 58. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, N.; Qu, S.; Wang, H.; Li, J. Advances in nanotechnology for the treatment of GBM. Front. Neurosci. 2023, 17, 1180943. [Google Scholar] [CrossRef]
- Hartshorn, C.M.; Bradbury, M.S.; Lanza, G.M.; Nel, A.E.; Rao, J.; Wang, A.Z.; Wiesner, U.B.; Yang, L.; Grodzinski, P. Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano 2018, 12, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, Z.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Nanotherapeutics for antimetastatic treatment. Trends Cancer 2020, 6, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Yasri, S.; Wiwanitkit, V. Nanotechnology in oncology: A concern on its unwanted effects and ethics. J. Med. Soc. 2018, 32, 81. [Google Scholar] [CrossRef]
- Tinkle, S.S. Nanotechnology: Collaborative opportunities for ecotoxicology and environmental health. Environ. Toxicol. Chem. 2008, 27, 1823. [Google Scholar] [CrossRef]
- Farooq Mana, G.E.; Parekh, U.A.; Naeem, F.; Abid, S.F.; Khan, M.H.; Zahra, S.G.; Sarkar, H.P.; Chaurasia, B. A Systematic Review of Nanomedicine in Glioblastoma Treatment: Clinical Efficacy, Safety, and Future Directions. Brain Sci. 2023, 13, 1727. [Google Scholar] [CrossRef] [PubMed]

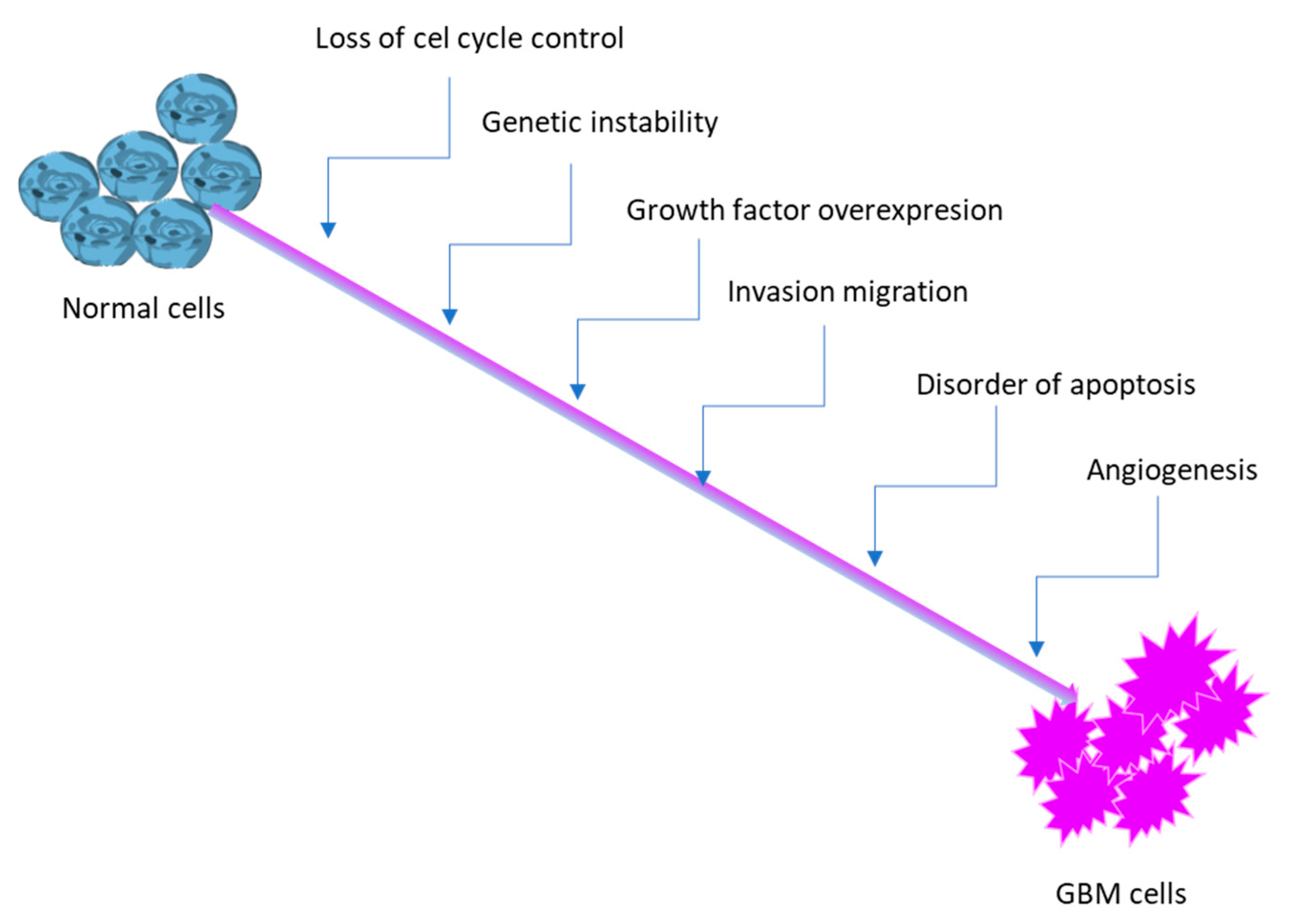
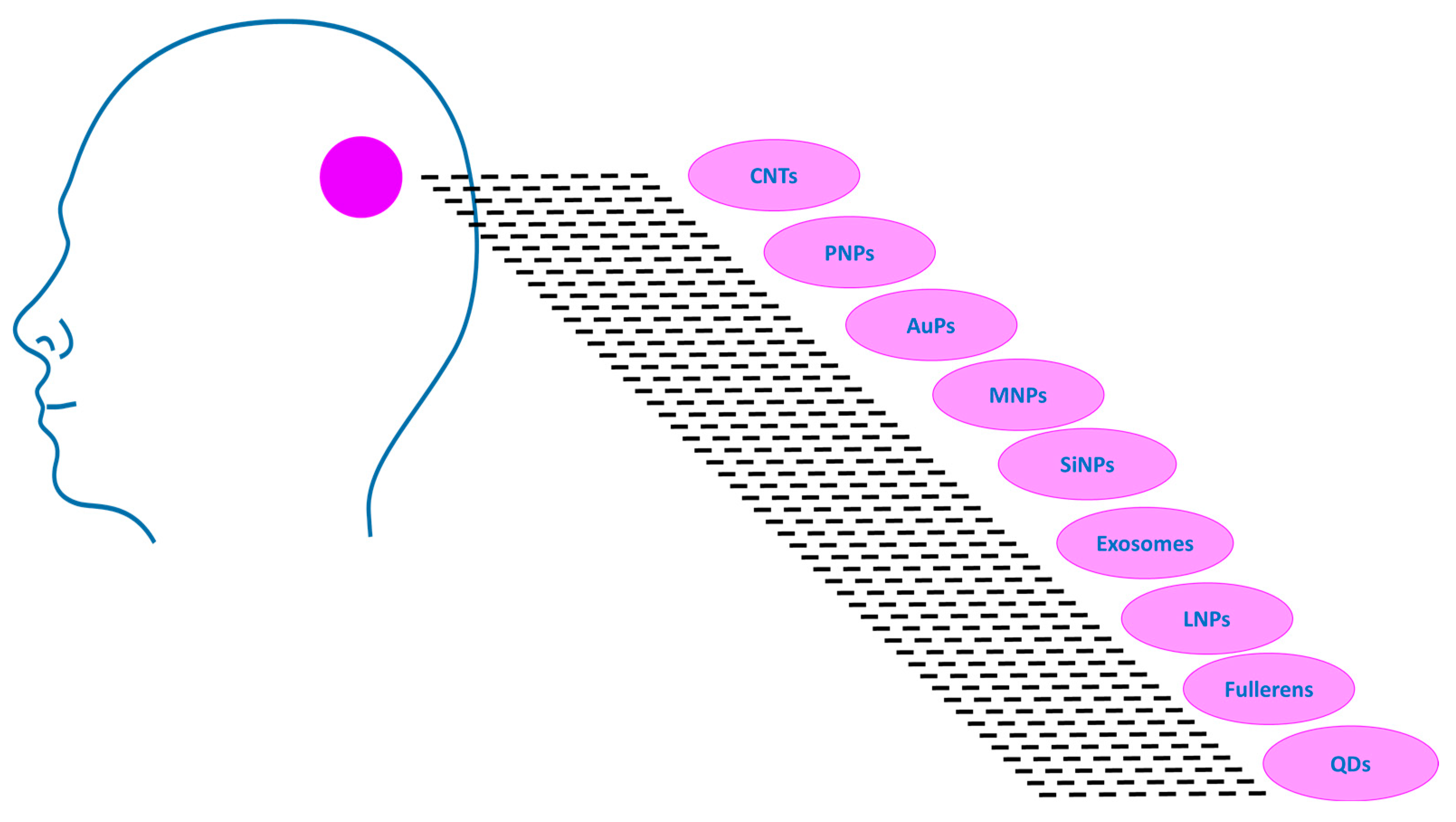
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Rudy, I.; Pięta, K.; Aebisher, D. Nano-Based Technology in Glioblastoma. Molecules 2025, 30, 3485. https://doi.org/10.3390/molecules30173485
Bartusik-Aebisher D, Rudy I, Pięta K, Aebisher D. Nano-Based Technology in Glioblastoma. Molecules. 2025; 30(17):3485. https://doi.org/10.3390/molecules30173485
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Izabela Rudy, Karolina Pięta, and David Aebisher. 2025. "Nano-Based Technology in Glioblastoma" Molecules 30, no. 17: 3485. https://doi.org/10.3390/molecules30173485
APA StyleBartusik-Aebisher, D., Rudy, I., Pięta, K., & Aebisher, D. (2025). Nano-Based Technology in Glioblastoma. Molecules, 30(17), 3485. https://doi.org/10.3390/molecules30173485






