Film-Forming Corrosion Inhibitor of ZnAl Layered Double Hydroxide Intercalated with Mussel Adhesive Protein
Abstract
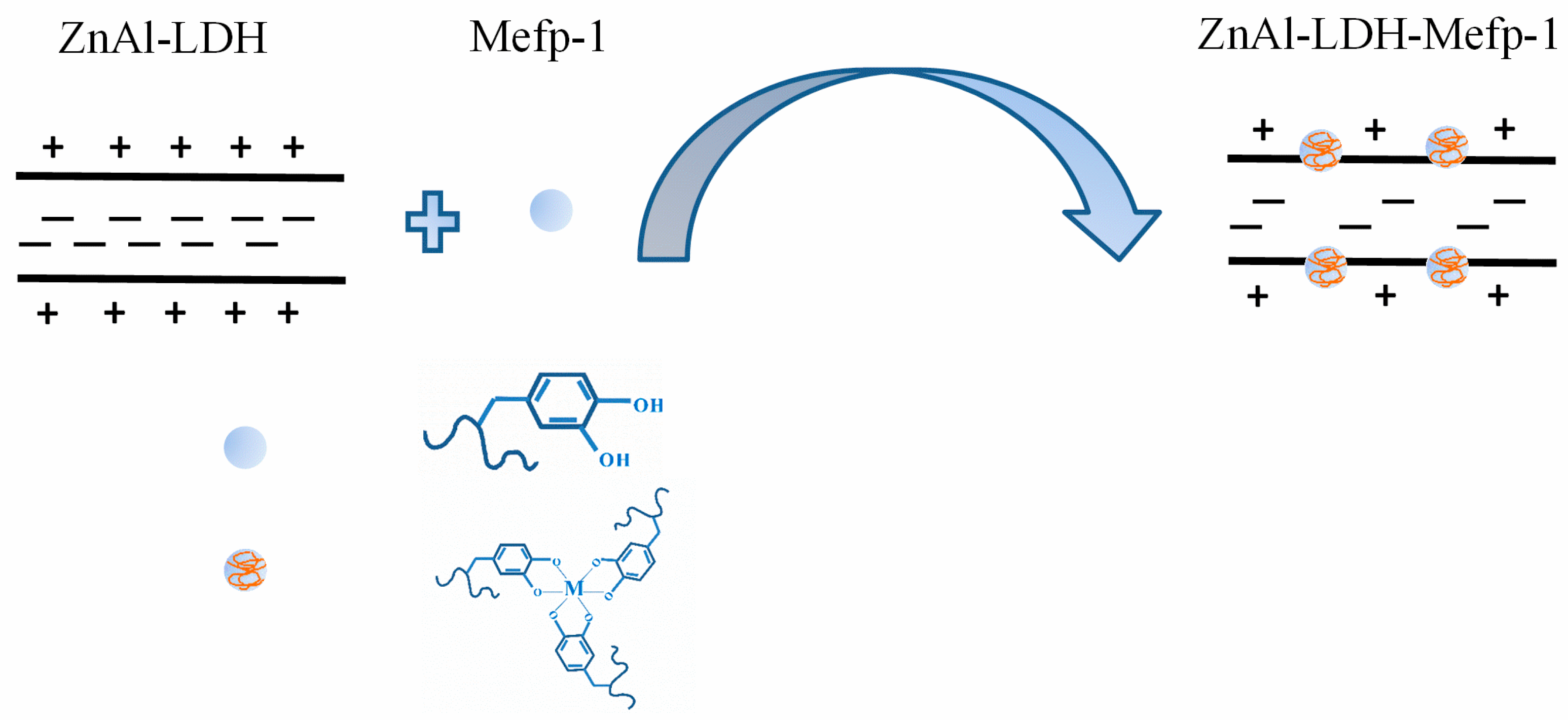
1. Introduction
2. Results and Discussion
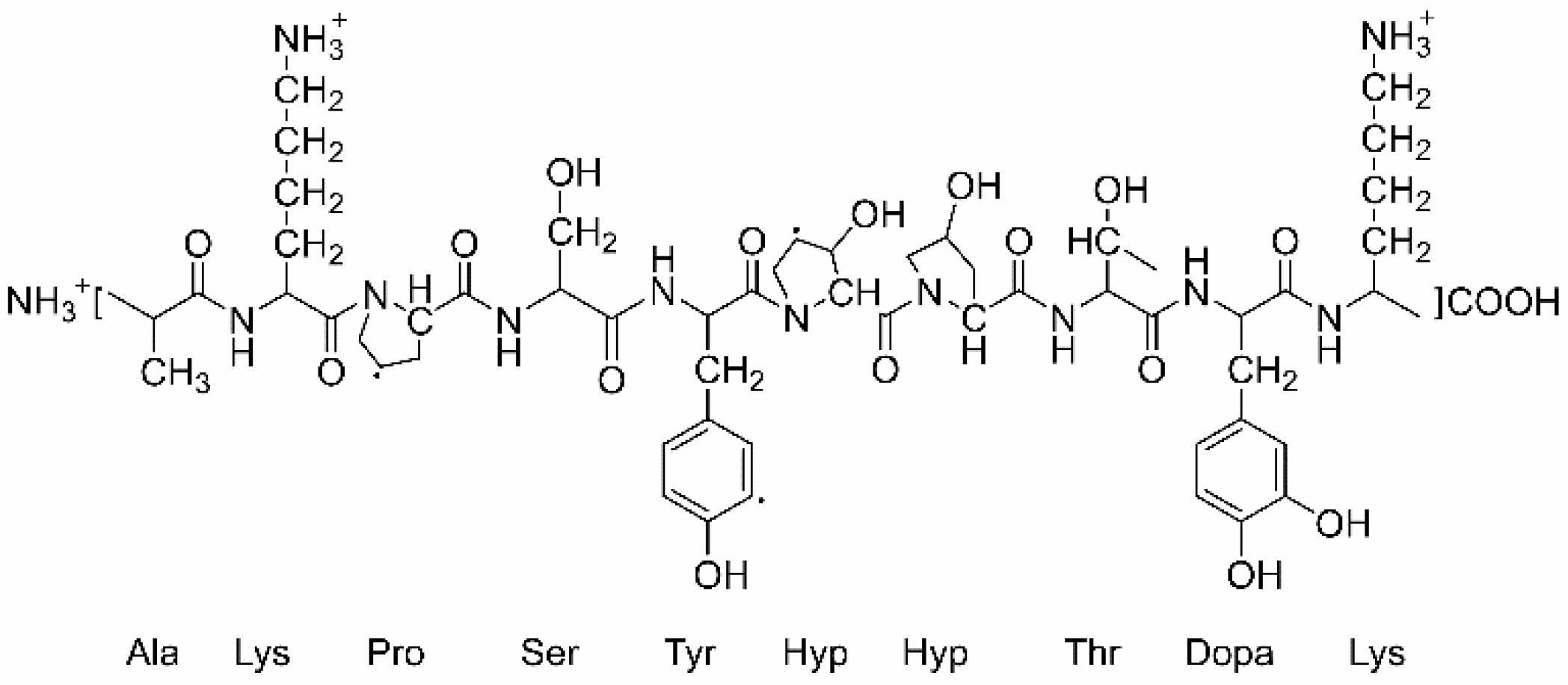
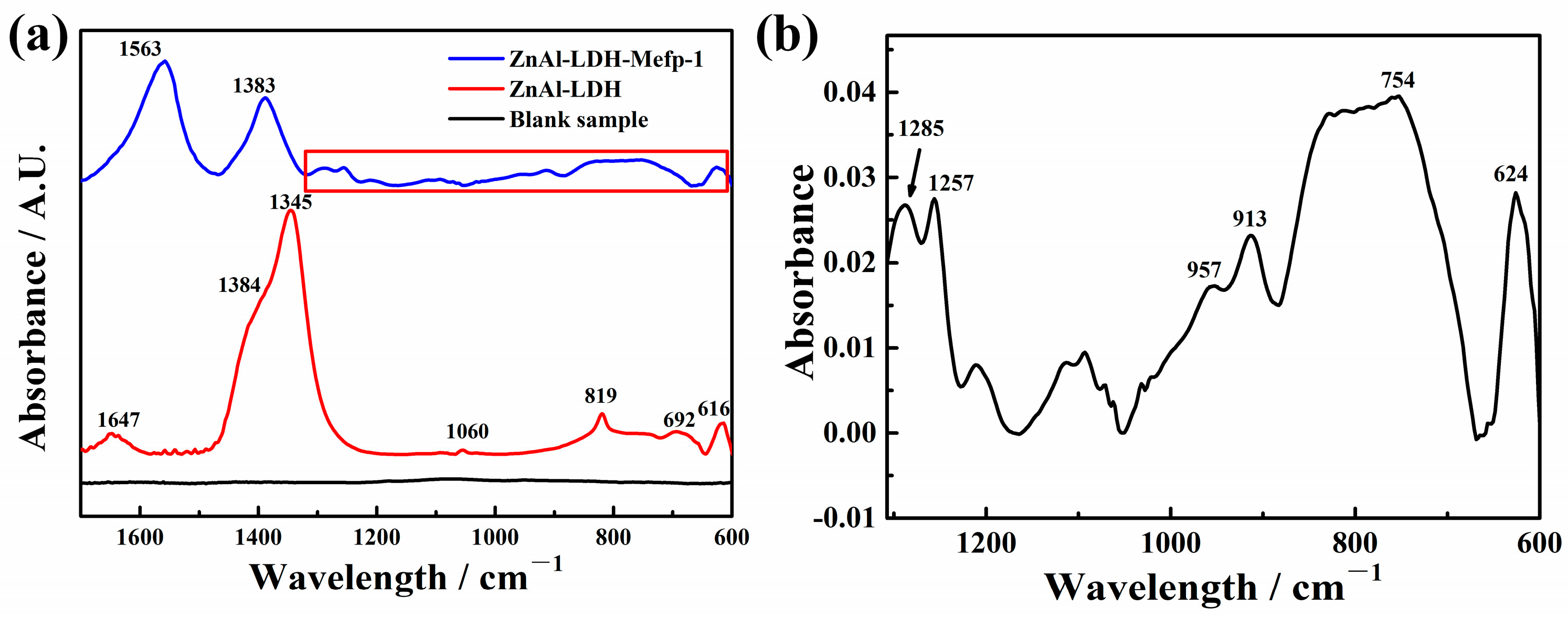
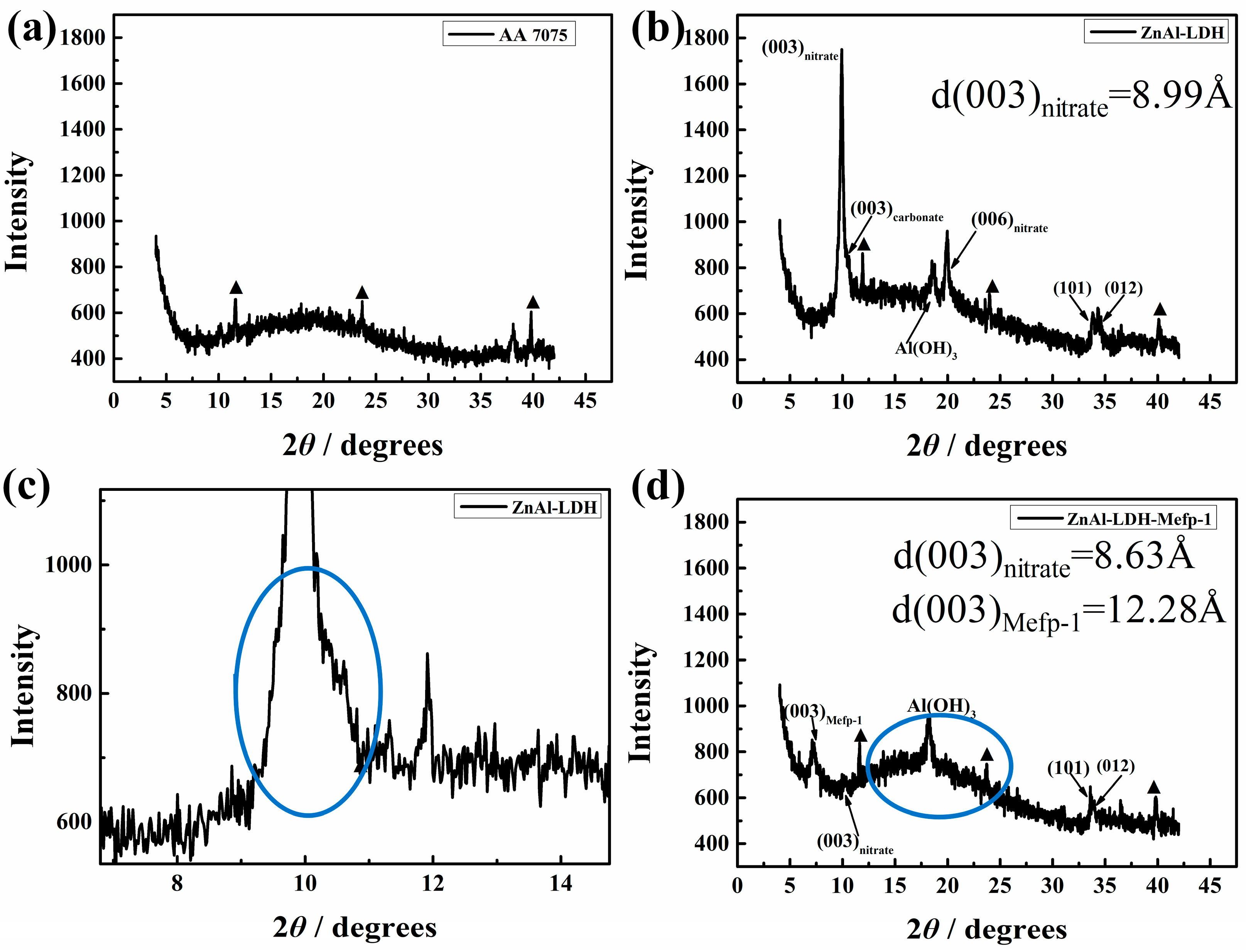
2.1. Characterizations of the LDH Films Intercalated with Mefp-1
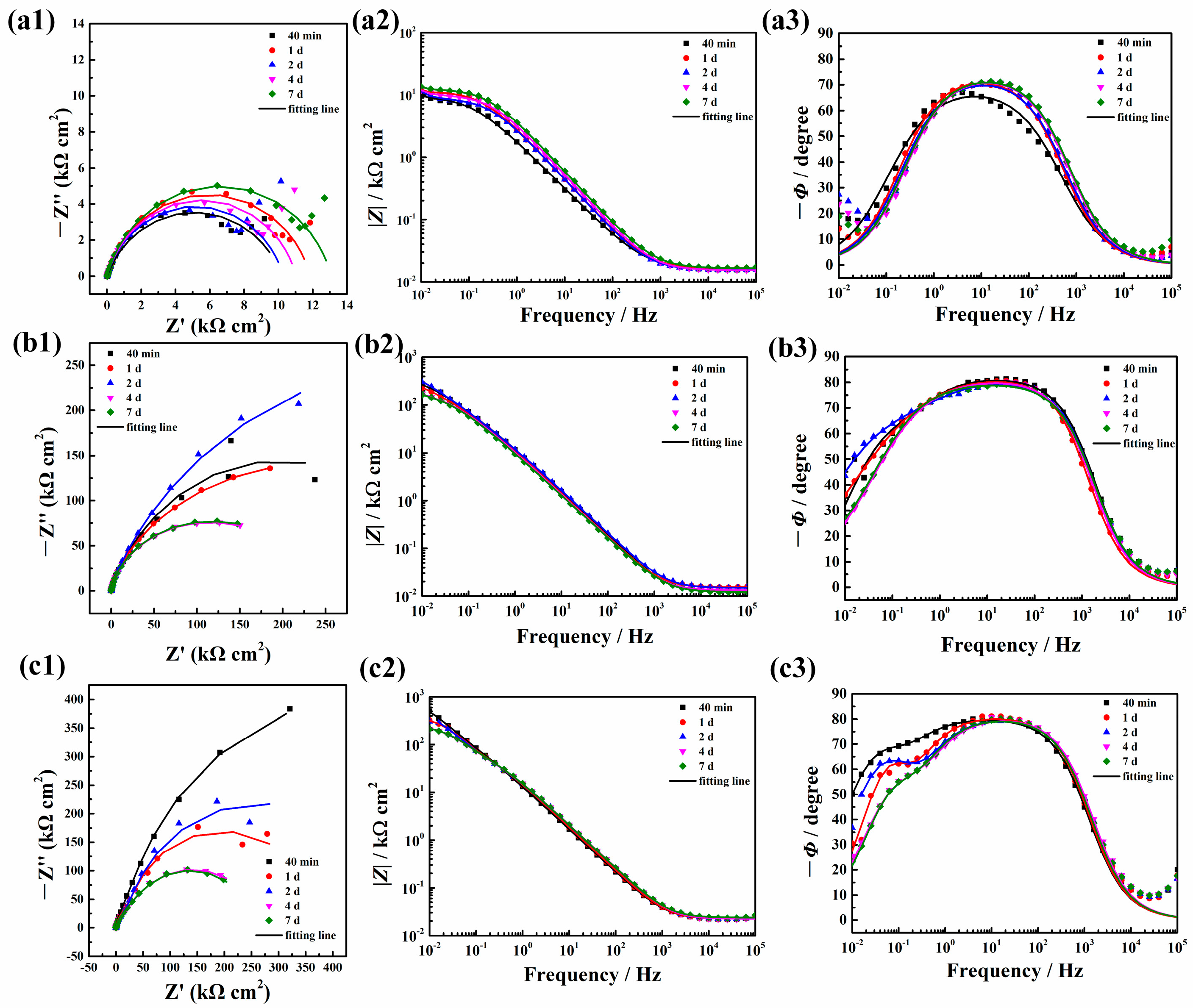
2.2. Corrosion Protective Performance of ZnAl-LDH-Mefp-1 Film on Al Substrate
3. Materials and Methods
3.1. Materials
3.2. Preparation of LDH Films on AA7075 Substrate
3.3. Characterizations of LDH Films
3.4. Electrochemical Impedance Spectra (EIS) Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAPs | Mussel adhesive proteins |
| LDH | Layered double hydroxide |
| Mefp-1 | Mytilus edulis foot protein |
| pI | Isoelectric point |
| DOPA | Dihydroxyphenylalanine |
| DOX | Doxorubicin |
| PAA | Polyacrylic acid |
| SEM | Scanning electronic microscopy |
| XRD | X-ray diffraction |
| ATR-FTIR | Attenuated total reflectance Fourier transform infrared spectroscopy |
| EDS | Energy dispersive spectroscopy |
| EIS | Electrochemical Impedance Spectroscopy |
References
- Galvão, T.L.; Neves, C.S.; Caetano, A.P.; Maia, F.; Mata, D.; Malheiro, E.; Bastos, A.C.; Salak, A.N.; Gomes, J.R.; Tedim, J.; et al. Control of crystallite and particle size in the synthesis of layered double hydroxides: Macromolecular insights and a complementary modeling tool. J. Colloid Interface Sci. 2016, 468, 86–94. [Google Scholar] [CrossRef]
- Dong, B.; Liu, W.; Zeng, L.; Chen, P.; Yang, Q.; Hong, S. Electrodeposition-hydrothermal synthesis of inhibitor anion-intercalated layered double hydroxide films for corrosion resistance on steel substrates. Constr. Build. Mater. 2023, 408, 133585. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Hou, L.; Zhang, S.; Wu, P.; Zhang, Y.; Liu, B.; Wei, Y. Enhancing the corrosion resistance of the epoxy coating using CaAl LDH intercalated with L-cysteine and its derivatives as a pigment on steel substrate. Prog. Org. Coat. 2024, 193, 108527. [Google Scholar] [CrossRef]
- Mojsilović, K.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Stojadinović, S.; Vasilić, R. Simple incorporation and calcination of Zn-Al LDH during PEO processing in near-neutral pH solutions. Appl. Surf. Sci. 2024, 677. [Google Scholar] [CrossRef]
- Santos, F.d.S.; Binder, L.; Scharnagl, N.; da Conceição, T.F. Sustainable smart coatings of chitosan and LDH loaded with natural inhibitors for corrosion protection of Mg AZ31 alloy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133639. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, R.; Wang, M.; Xu, H.; Wang, W.; Yan, Y.; Liu, B.; Han, J.; Di, Z.; Shen, H.; et al. Improving the corrosion resistance of waterborne epoxy resin coating by using silane modified Ca-Al LDH filler. Int. J. Electrochem. Sci. 2024, 19. [Google Scholar] [CrossRef]
- Chen, Y.; Shui, Z.; Chen, W.; Chen, G. Chloride binding of synthetic Ca–Al–NO3 LDHs in hardened cement paste. Constr. Build. Mater. 2015, 93, 1051–1058. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Truc, T.A.; Duong, N.T.; Vu, P.G.; Hoang, T. Preparation and characterization of nanocontainers of corrosion inhibitor based on layered double hydroxides. Appl. Clay Sci. 2012, 67–68, 18–25. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Truc, T.A.; Duong, N.T.; Pébère, N.; Olivier, M.-G. Layered double hydroxides as containers of inhibitors in organic coatings for corrosion protection of carbon steel. Prog. Org. Coat. 2012, 74, 343–348. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, D.; Li, J.; Zhang, M.; Wang, Z.; Wu, J.; Wang, C. Effect of different anion intercalations on the corrosion resistance of layered double hydroxides on the surface of LA43M. Mater. Corros. 2023, 75, 398–408. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; He, X.; Yao, W.; Wu, J.; He, Y.; Zhou, Y.; Yuan, Y.; Wang, J.; Xie, Z.; et al. Corrosion resistance study of ZIF-67/Co-Fe LDHs composite coating on the surface of AZ31 magnesium alloy micro-arc oxidation film. J. Alloy. Compd. 2024, 989, 174278. [Google Scholar] [CrossRef]
- Zhang, F.; Pan, J. Recent Development of Corrosion Protection Strategy Based on Mussel Adhesive Protein. Front. Mater. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Doraiswamy, A.; Narayan, R.; Cristescu, R.; Mihailescu, I.; Chrisey, D. Laser processing of natural mussel adhesive protein thin films. Mater. Sci. Eng. C 2007, 27, 409–413. [Google Scholar] [CrossRef]
- Fant, C.; Hedlund, J.; Höök, F.; Berglin, M.; Fridell, E.; Elwing, H. Investigation of Adsorption and Cross-Linking of a Mussel Adhesive Protein Using Attenuated Total Internal Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR). J. Adhes. 2010, 86, 25–38. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeung, D.-G.; Oh, J.-M. Boosting the anticancer activity of doxorubicin with a layered double hydroxide nanocarrier. Appl. Clay Sci. 2021, 203, 106000. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, F.; Lin, C.; Pan, J. Corrosion Protection and Self-Healing of a Nanocomposite Film of Mussel Adhesive Protein and CeO2Nanoparticles on Carbon Steel. J. Electrochem. Soc. 2016, 163, C545–C552. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Zhao, L.; Lu, W.; Zhang, F.; Evans, D.G.; Duan, X. One-Step Hydrothermal Crystallization of a Layered Double Hydroxide/Alumina Bilayer Film on Aluminum and Its Corrosion Resistance Properties. Langmuir 2009, 25, 9894–9897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, L.; Chen, H.; Xu, S.; Evans, D.G.; Duan, X. Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films on Aluminum. Angew. Chem. Int. Ed. Engl. 2008, 47, 2466–2469. [Google Scholar] [CrossRef]
- Lei, X.; Wang, L.; Zhao, X.; Chang, Z.; Jiang, M.; Yan, D.; Sun, X. Oriented CuZnAl Ternary Layered Double Hydroxide Films: In situ Hydrothermal Growth and Anticorrosion Properties. Ind. Eng. Chem. Res. 2013, 52, 17934–17940. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D. Synthesis, characterization, and controlled release anticorrosion behavior of benzoate intercalated Zn–Al layered double hydroxides. Mater. Res. Bull. 2011, 46, 1963–1968. [Google Scholar] [CrossRef]
- Zuo, J.; Wu, B.; Dong, B.; Xing, F.; Ma, J.; Wei, G. Effects of nitrite ion intercalated CaAl and MgAl layered double hydroxides on the properties of concrete mortar. Cem. Concr. Compos. 2023, 145, 105306. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, S.; Pan, X.; Zhang, C.; Du, S.; Liu, Y. Synthesis and Enhanced Corrosion Protection Performance of Reduced Graphene Oxide Nanosheet/ZnAl Layered Double Hydroxide Composite Films by Hydrothermal Continuous Flow Method. ACS Appl. Mater. Interfaces 2017, 9, 18263–18275. [Google Scholar] [CrossRef]
- Li, D.; Wang, F.; Yu, X.; Wang, J.; Liu, Q.; Yang, P.; He, Y.; Wang, Y.; Zhang, M. Anticorrosion organic coating with layered double hydroxide loaded with corrosion inhibitor of tungstate. Prog. Org. Coat. 2011, 71, 302–309. [Google Scholar] [CrossRef]
- Tackett, J.E. FT-IR characterization of metal acetates in aqueous solution. Appl. Spectrosc. 1989, 43, 483–489. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, F.; Zhou, Q. Protective behaviors of 2-mercaptobenzothiazole intercalated Zn–Al-layered double hydroxide coating. J. Coat. Technol. Res. 2014, 11, 793–803. [Google Scholar] [CrossRef]
- Alen, S.; Sajan, D.; Vijayan, N.; Chaitanya, K.; Němec, I.; Jothy, V.B. Growth, electronic absorption and vibrational spectral analysis of semiorganic nonlinear optical material potassium acid phthalate: A scaled quantum mechanical force field study. J. Mol. Struct. 2013, 1040, 155–163. [Google Scholar] [CrossRef]
- McBride, M.B.; Wesselink, L.G. Chemisorption of catechol on gibbsite, boehmite, and noncrystalline alumina surfaces. Environ. Sci. Technol. 1988, 22, 703–708. [Google Scholar] [CrossRef]
- Yu, X.; Ma, Y.; Xu, D.; Li, J.; Feng, Z.; Ouyang, Y.; Yong, Q.; Xie, Z.-H. Polydopamine-coated zeolitic imidazolate framework for enhanced anti-corrosion and self-healing capabilities of epoxy coating on magnesium alloy. Appl. Surf. Sci. 2024, 680, 161332. [Google Scholar] [CrossRef]
- Simsek, I.; Nalcacioglu, C.; Ozyurek, D. The Effects of Aging Temperature on the Corrosion and Electrical Conductivity in the AA7075 Alloy Produced by Powder Metallurgy Method. Acta Phys. Pol. A 2019, 135, 722–725. [Google Scholar] [CrossRef]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of Active Corrosion Protection via Combination of Inhibitor-Loaded Nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. Preparation of layered double hydroxide films with different orientations on the opposite sides of a glass substrate by in situ hydrothermal crystallization. Chem. Commun. 2009, 6836–6838. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, S.; Tedim, J.; Rodrigues, L.M.; Salak, A.; Zheludkevich, M.; Dick, L.F.P.; Ferreira, M.G.S. Novel Inorganic Host Layered Double Hydroxides Intercalated with Guest Organic Inhibitors for Anticorrosion Applications. ACS Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.L.; Salak, A.N.; Lisenkov, A.; Ferreira, M.G.S. Nanostructured LDH-container layer with active protection functionality. J. Mater. Chem. 2011, 21, 15464–15470. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: The effects on morphology, composition and corrosion resistance. Corros. Sci. 2013, 74, 130–138. [Google Scholar] [CrossRef]
- Cheng, C.; Nie, S.; Li, S.; Peng, H.; Yang, H.; Ma, L.; Sun, S.; Zhao, C. Biopolymer functionalized reduced graphene oxide with enhanced biocompatibility via mussel inspired coatings/anchors. J. Mater. Chem. B 2012, 1, 265–275. [Google Scholar] [CrossRef]
- Cottis, R.; Homborg, A.; Mol, J. The relationship between spectral and wavelet techniques for noise analysis. Electrochim. Acta 2015, 202, 277–287. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.; Bastos, A.; Salak, A.; Lisenkov, A.; Ferreira, M. Influence of preparation conditions of Layered Double Hydroxide conversion films on corrosion protection. Electrochim. Acta 2014, 117, 164–171. [Google Scholar] [CrossRef]
- Tedim, J.; Bastos, A.; Kallip, S.; Zheludkevich, M.; Ferreira, M. Corrosion protection of AA2024-T3 by LDH conversion films. Analysis of SVET results. Electrochim. Acta 2016, 210, 215–224. [Google Scholar] [CrossRef]
- Lin, K.; Luo, X.; Pan, X.; Zhang, C.; Liu, Y. Enhanced corrosion resistance of LiAl-layered double hydroxide (LDH) coating modified with a Schiff base salt on aluminum alloy by one step in-situ synthesis at low temperature. Appl. Surf. Sci. 2019, 463, 1085–1096. [Google Scholar] [CrossRef]
- Zeng, R.C.; Liu, Z.G.; Zhang, F.; Li, S.Q.; He, Q.K.; Cui, H.-Z.; Han, E.-H. Corrosion resistance of in-situ Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy by one-step formation. Trans. Nonferrous Met. Soc. China 2015, 25, 1917–1925. [Google Scholar] [CrossRef]
- Wu, L.; Zheng, Z.; Pan, F.; Tang, A.; Zhang, G.; Liu, L. Influence of Reaction Temperature on the Controlled Growth of Mg-Al LDH Film. Int. J. Electrochem. Sci. 2017, 12, 6352–6364. [Google Scholar] [CrossRef]
- Lin, B.; Xu, Y. Effect of [NO2—]/[Cl—] Ratio on Corrosion Behavior of Fine-grain High-strength Reinforcement in Simulated Concrete Pore Solutions. Int. J. Electrochem. Sci. 2016, 11, 3824–3842. [Google Scholar] [CrossRef]
- Chen, W.; Du, R.-G.; Ye, C.-Q.; Zhu, Y.-F.; Lin, C.-J. Study on the corrosion behavior of reinforcing steel in simulated concrete pore solutions using in situ Raman spectroscopy assisted by electrochemical techniques. Electrochim. Acta 2010, 55, 5677–5682. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Zhou, C.; Tan, H. Effect of carbonation on the electrochemical behavior of corrosion resistance low alloy steel rebars in cement extract solution. Constr. Build. Mater. 2017, 130, 193–201. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thienylmethyl)-4-amino-1,2,4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, F.; Hu, S.; Cao, Z.; Wu, Z.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies. Corros. Sci. 2013, 74, 271–282. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardas, G.; Culha, M.; Yazıcı, B.; Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Solmaz, R.; Salcı, A.; Dursun, Y.A.; Kardaş, G. A comprehensive study on the adsorption, corrosion inhibition efficiency and stability of acriflavine on mild steel in 1 M HCl solution. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131908. [Google Scholar] [CrossRef]


| Al | Rs (Ω cm2) | Rct (kΩ cm2) | CPEdl | W (kΩ cm2) | |
|---|---|---|---|---|---|
| Y0 (10−5 Ω−1 cm−2 sn) | n | ||||
| 40 min | 16.3 ± 0.6 | 11.6 ± 1.8 | 12.4 ± 0.4 | 0.78 | 27.0 ± 5.5 |
| 1 d | 16.7 ± 0.2 | 10.0 ± 1.1 | 8.1 ± 1.4 | 0.83 | 32.7 ± 18.0 |
| 2 d | 15.3 ± 0.2 | 7.9 ± 0.9 | 7.8 ± 1.2 | 0.84 | 66.3 ± 29.7 |
| 4 d | 15.7 ± 0.2 | 9.1 ± 0.8 | 6.5 ± 1.1 | 0.83 | 58.5 ± 28.6 |
| `7 d | 16.6 ± 0.2 | 11.2 ± 0.9 | 5.7 ± 0.8 | 0.83 | 48.4 ± 25.0 |
| ZnAl-LDH | Rs (Ω cm2) | RLDH (kΩ cm2) | CPELDH | Rct (kΩ cm2) | CPEdl | ||
|---|---|---|---|---|---|---|---|
| Y0 (10−5 Ω−1 cm−2 sn) | n | Y0 (10−5 Ω−1 cm−2 sn) | n | ||||
| 40 min | 14.0 ± 0.8 | 71.2 ± 9.8 | 1.4 ± 0.3 | 0.91 | 212.7 ± 63.6 | 4.1 ± 3.4 | 0.66 |
| 1 d | 14.3 ± 0.7 | 41.0 ± 14.1 | 1.7 ± 0.4 | 0.90 | 260.4 ± 182.3 | 11.2 ± 12.3 | 0.71 |
| 2 d | 13.2 ± 2.1 | 40.4 ± 13.1 | 1.5 ± 0.2 | 0.90 | 343.5 ± 292.1 | 1.9 ± 1.1 | 0.65 |
| 4 d | 15.5 ± 2.3 | 24.0 ± 6.5 | 2.1 ± 0.8 | 0.88 | 168.9 ± 60.3 | 7.4 ± 6.1 | 0.63 |
| 7 d | 14.7 ± 1.6 | 45.8 ± 19.4 | 2.4 ± 1.0 | 0.86 | 156.1 ± 95.7 | 16.3 ± 7.0 | 0.63 |
| ZnAl-LDH-Mefp-1 | Rs (Ω cm2) | RLDH (kΩ cm2) | CPELDH | Rct (kΩ cm2) | CPEdl | ||
|---|---|---|---|---|---|---|---|
| Y0 (10−5 Ω−1 cm−2 sn) | n | Y0 (10−5 Ω−1 cm−2 sn) | n | ||||
| 40 min | 19.2 ± 4.0 | 94.4 ± 73.0 | 1.2 ± 0.2 | 0.91 | 550.9 ± 276.1 | 1.0 ± 0.5 | 0.87 |
| 1 d | 19.1 ± 2.8 | 64.7 ± 23.7 | 1.3 ± 0.3 | 0.90 | 305.1 ± 103.1 | 1.0 ± 0.4 | 0.89 |
| 2 d | 18.9 ± 3.3 | 67.2 ± 26.5 | 1.4 ± 0.3 | 0.90 | 321.6 ± 149.7 | 1.4 ± 0.4 | 0.89 |
| 4 d | 19.0 ± 1.6 | 40.1 ± 25.2 | 1.3 ± 0.1 | 0.92 | 288.1 ± 90.3 | 1.4 ± 0.4 | 0.81 |
| 7 d | 19.3 ± 2.5 | 47.0 ± 24.3 | 1.4 ± 0.2 | 0.90 | 298.3 ± 125.9 | 1.4 ± 0.4 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Zheng, D.; Zhang, F.; Pan, J.; Lin, C.; Wang, J.; Huang, C. Film-Forming Corrosion Inhibitor of ZnAl Layered Double Hydroxide Intercalated with Mussel Adhesive Protein. Molecules 2025, 30, 3480. https://doi.org/10.3390/molecules30173480
Cao Y, Zheng D, Zhang F, Pan J, Lin C, Wang J, Huang C. Film-Forming Corrosion Inhibitor of ZnAl Layered Double Hydroxide Intercalated with Mussel Adhesive Protein. Molecules. 2025; 30(17):3480. https://doi.org/10.3390/molecules30173480
Chicago/Turabian StyleCao, Yanhui, Dajiang Zheng, Fan Zhang, Jinshan Pan, Changjian Lin, Jingjing Wang, and Congshu Huang. 2025. "Film-Forming Corrosion Inhibitor of ZnAl Layered Double Hydroxide Intercalated with Mussel Adhesive Protein" Molecules 30, no. 17: 3480. https://doi.org/10.3390/molecules30173480
APA StyleCao, Y., Zheng, D., Zhang, F., Pan, J., Lin, C., Wang, J., & Huang, C. (2025). Film-Forming Corrosion Inhibitor of ZnAl Layered Double Hydroxide Intercalated with Mussel Adhesive Protein. Molecules, 30(17), 3480. https://doi.org/10.3390/molecules30173480







