High-Value Utilization of Amaranth Residue and Waste LDPE by Co-Pyrolysis
Abstract
1. Introduction
2. Results
2.1. Results of Proximate and Ultimate Analyses
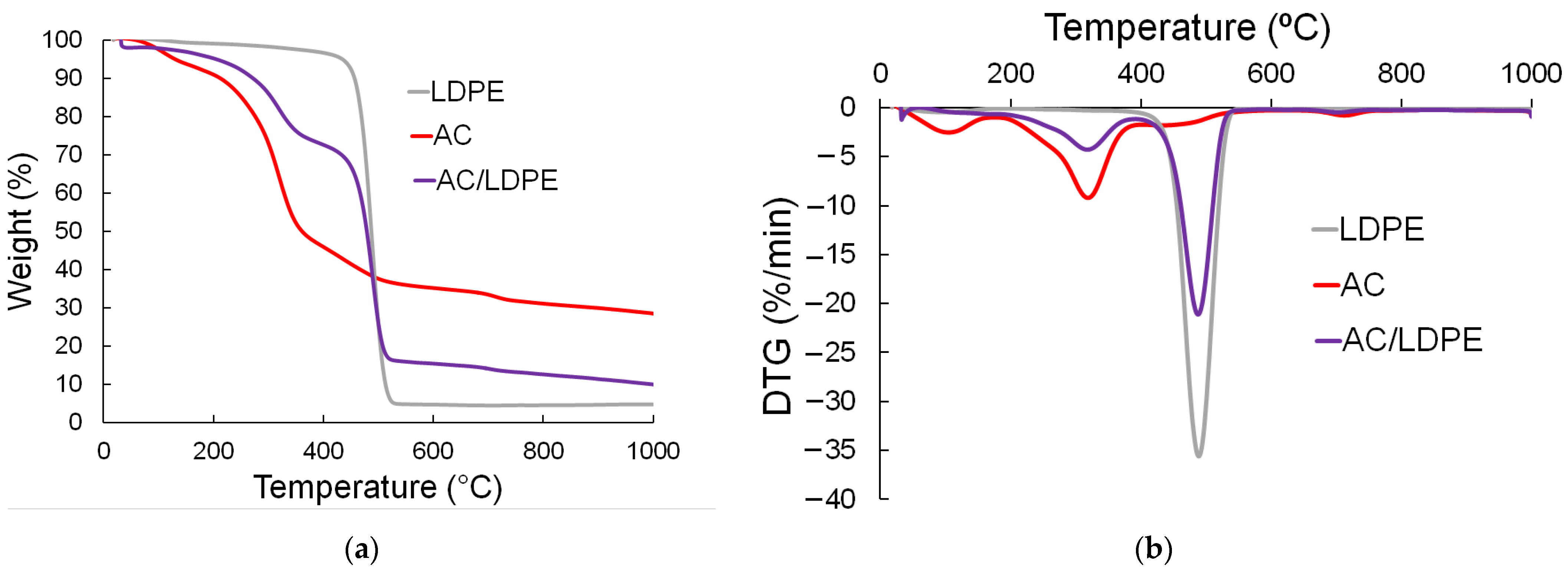
2.2. TGA Results
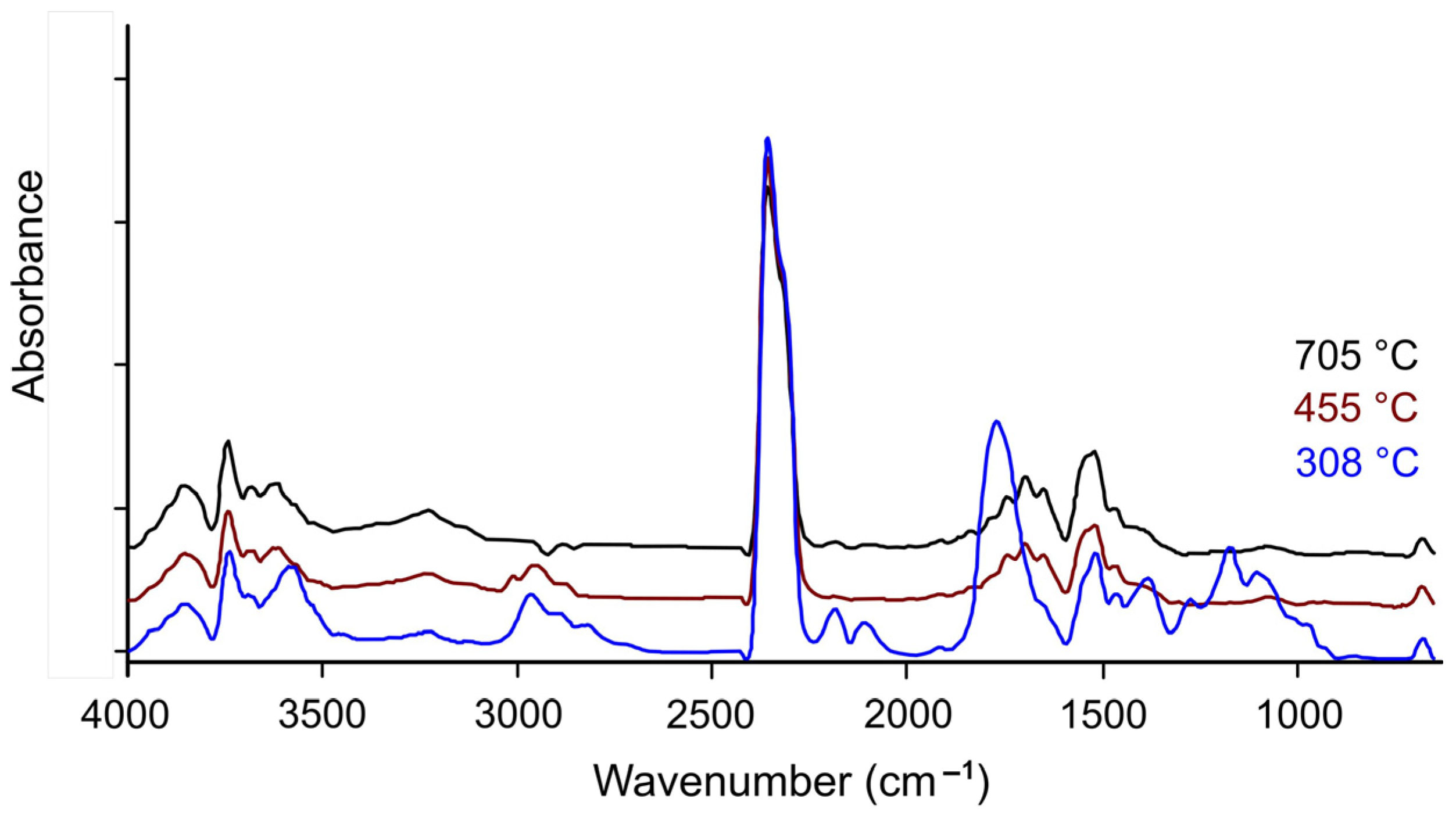
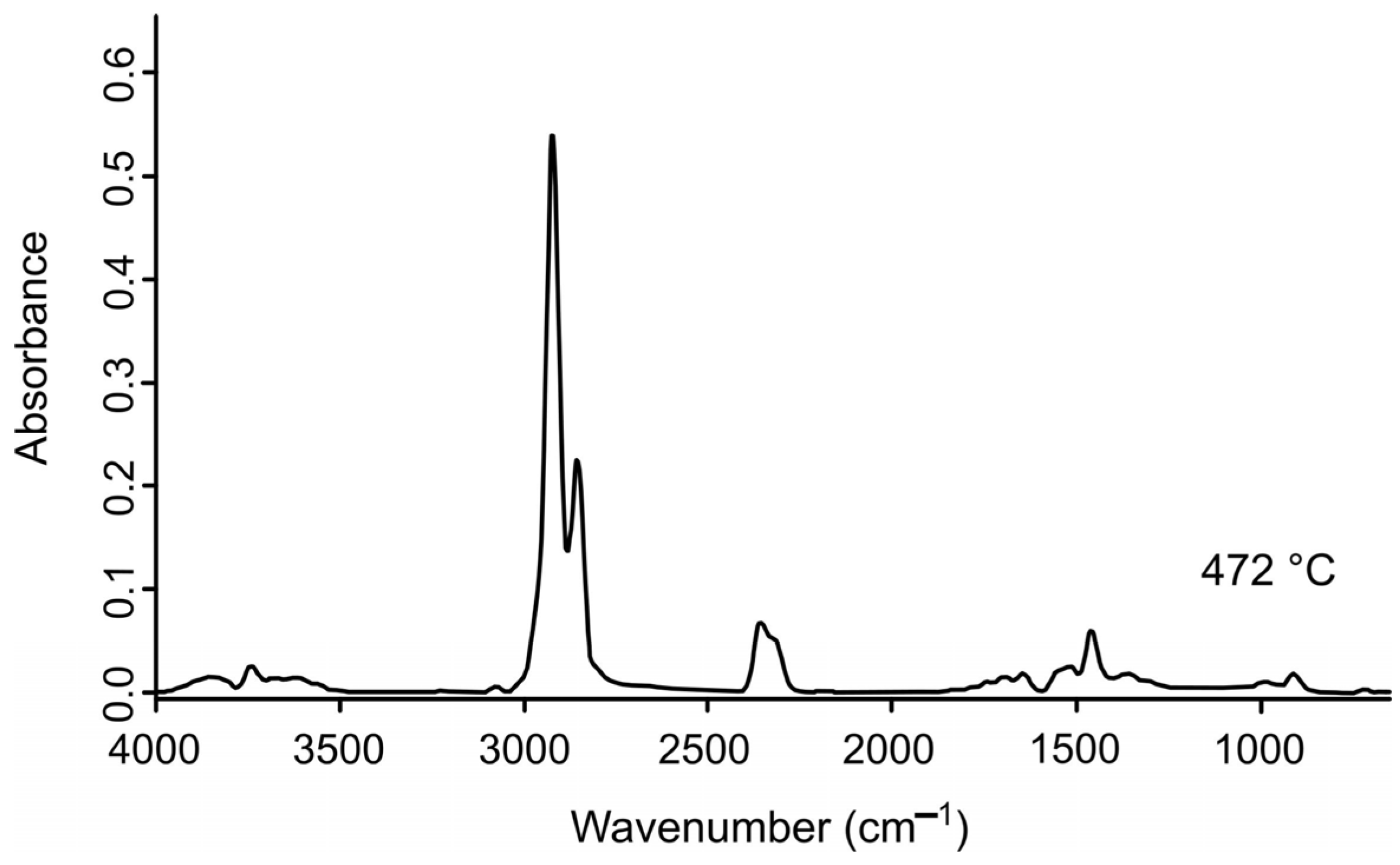
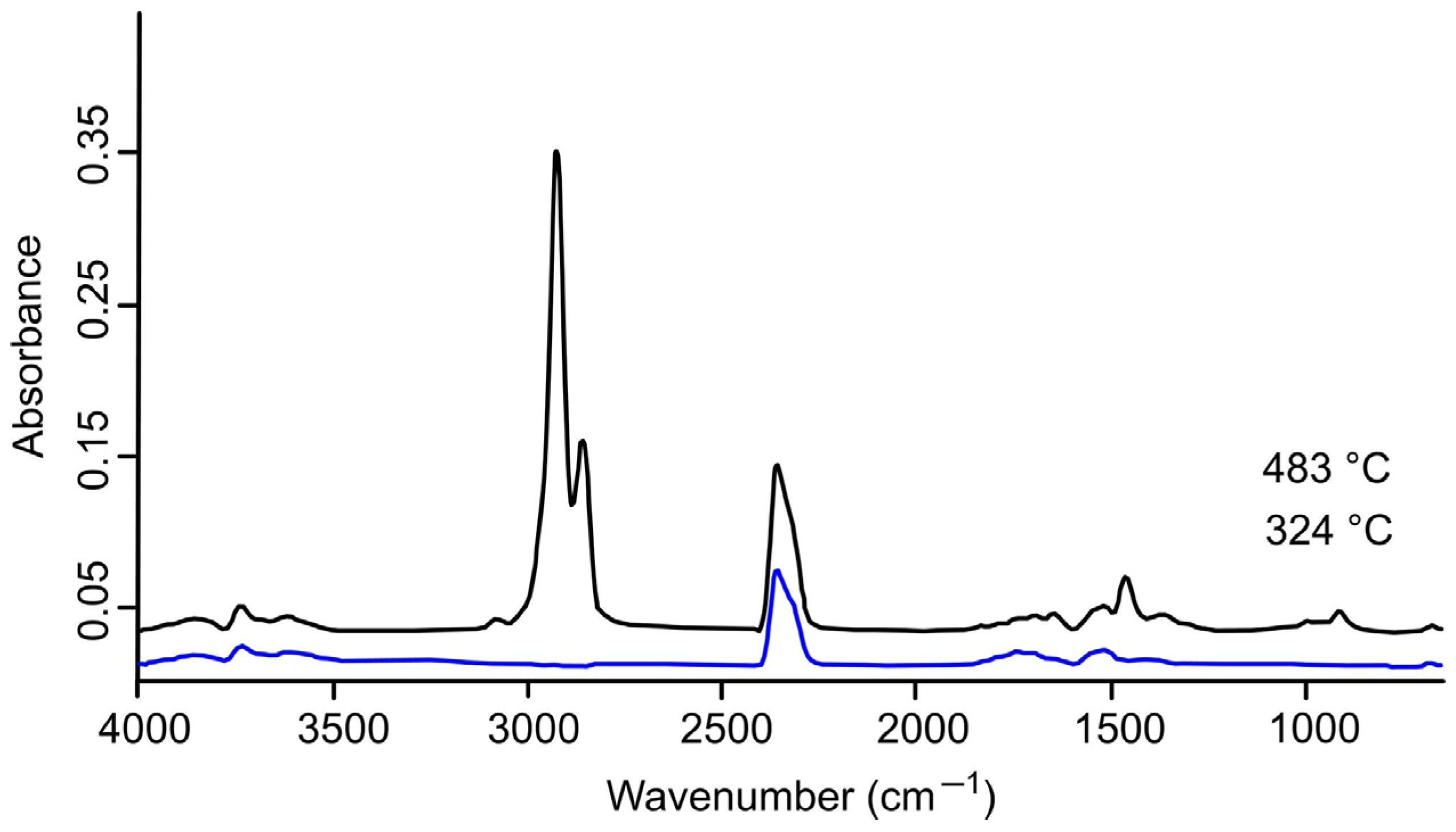
2.3. FTIR Spectrum Analysis
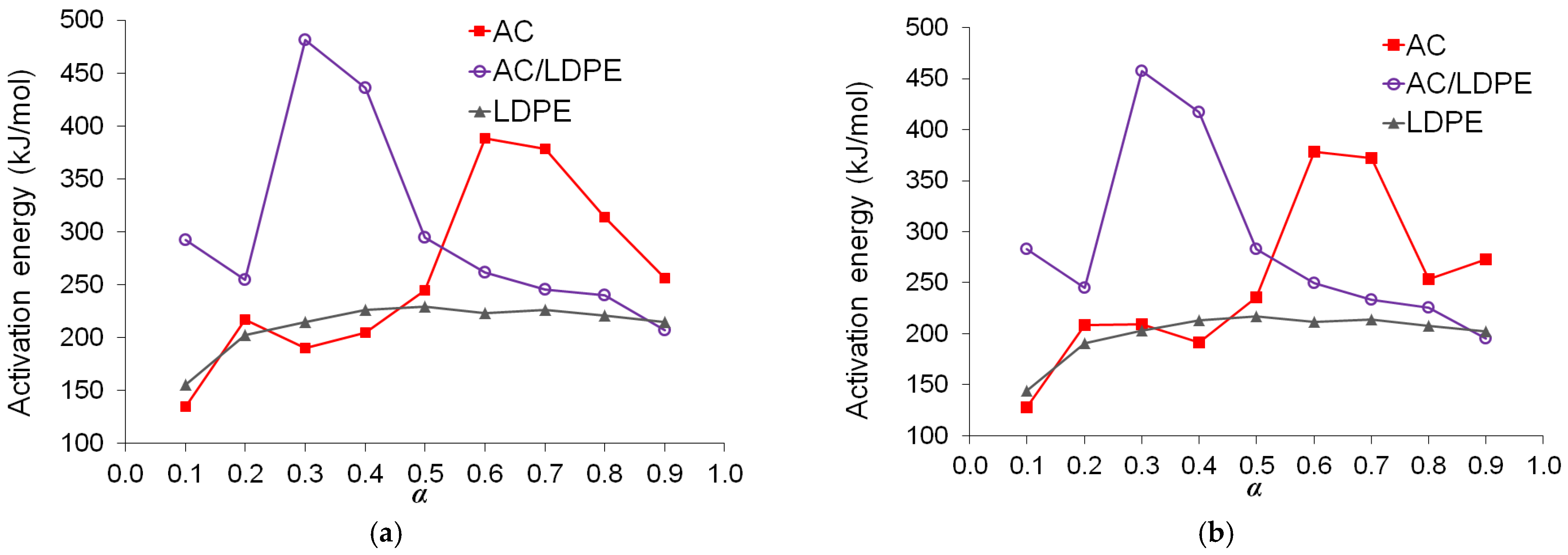
2.4. Kinetic Analysis
2.4.1. Model-Free Methods
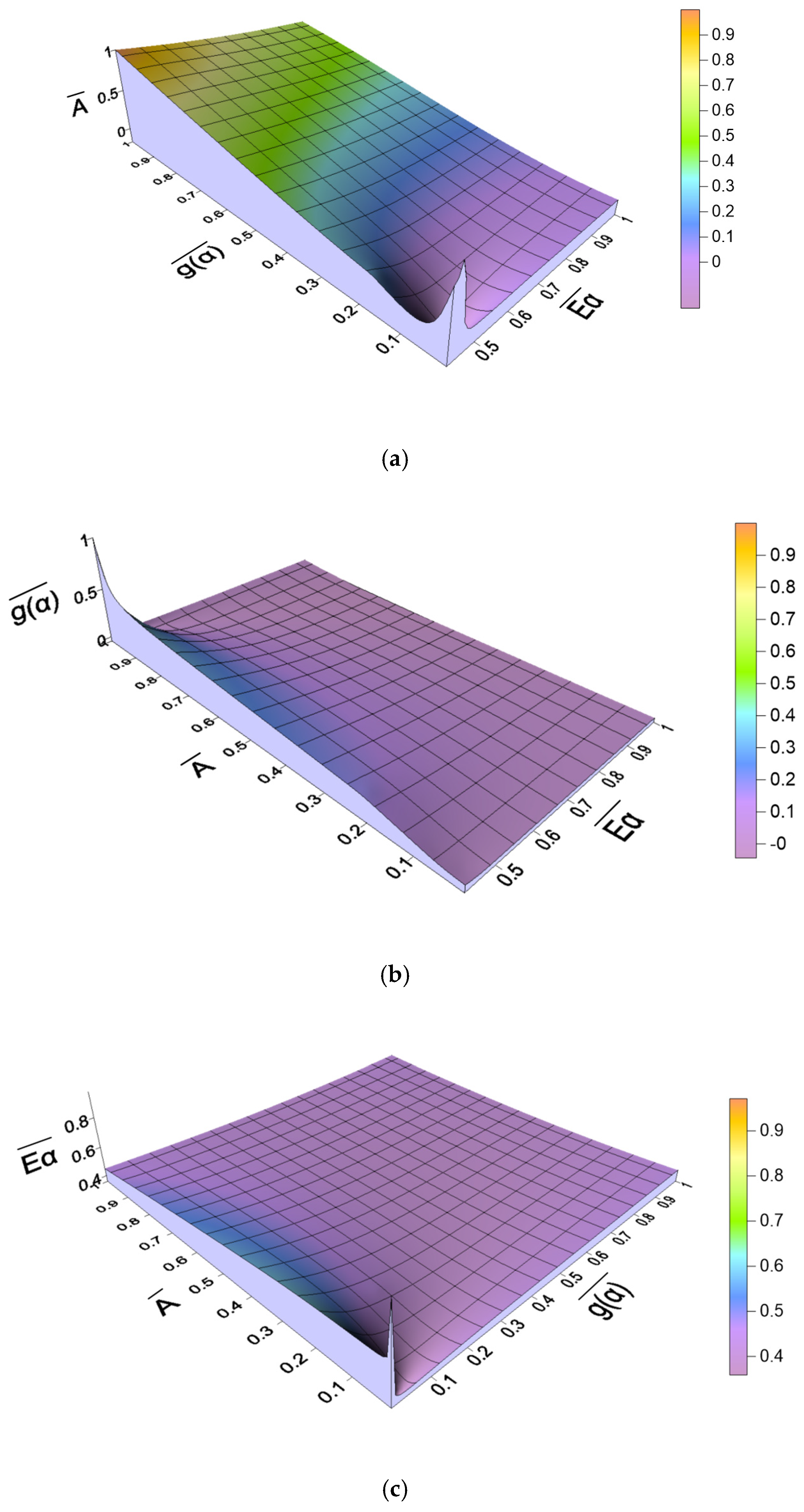
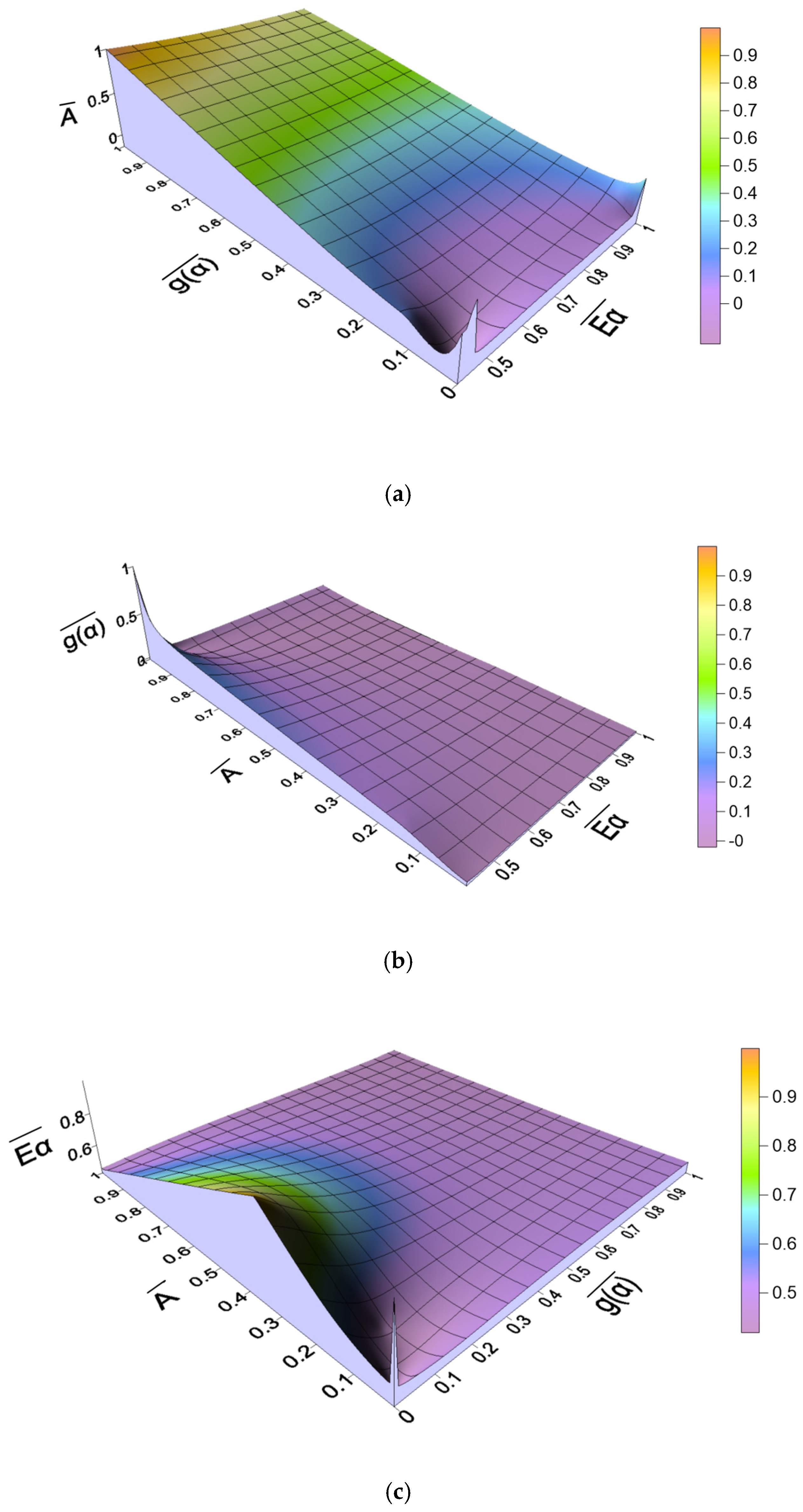
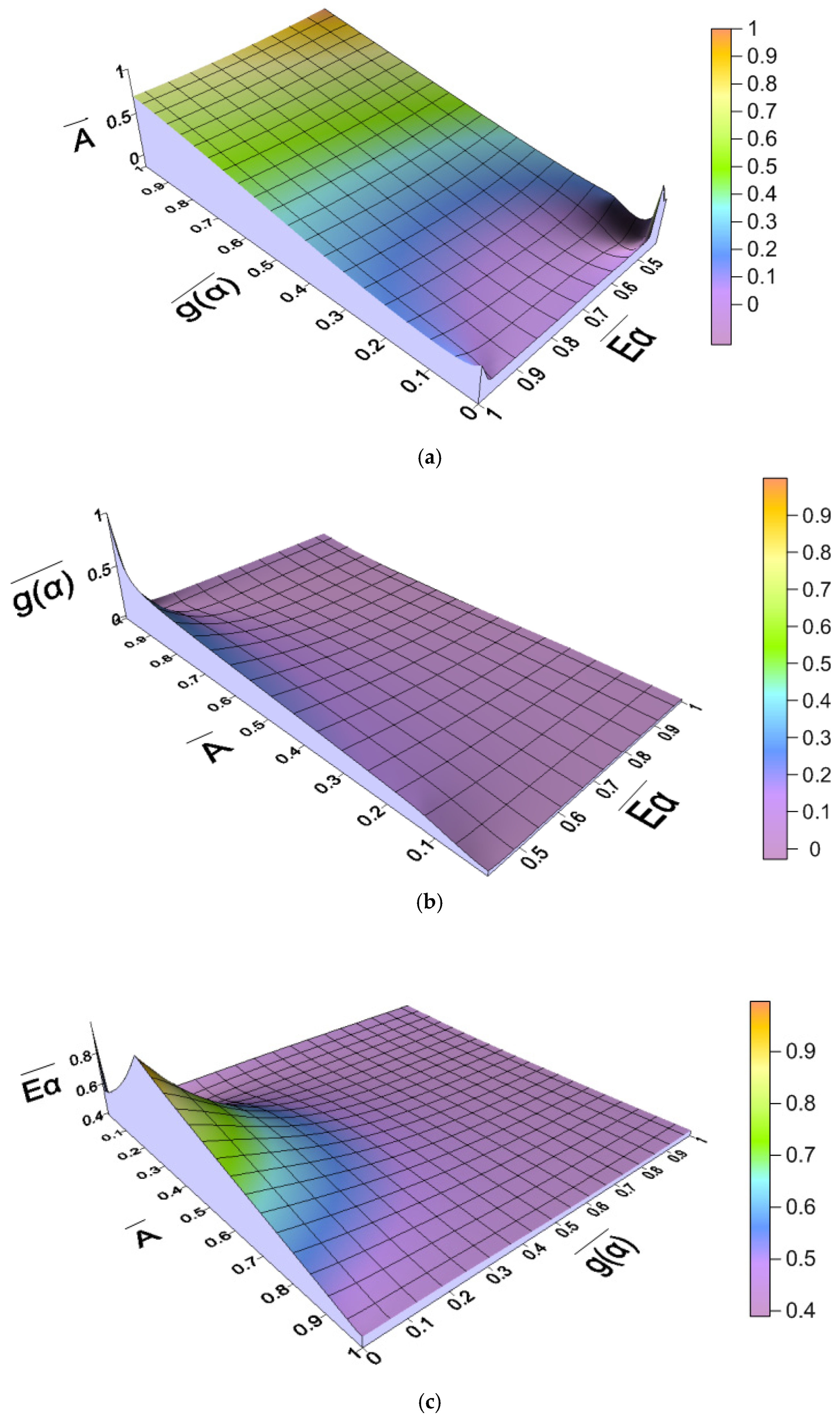
2.4.2. Relationship Between Kinetic Parameters
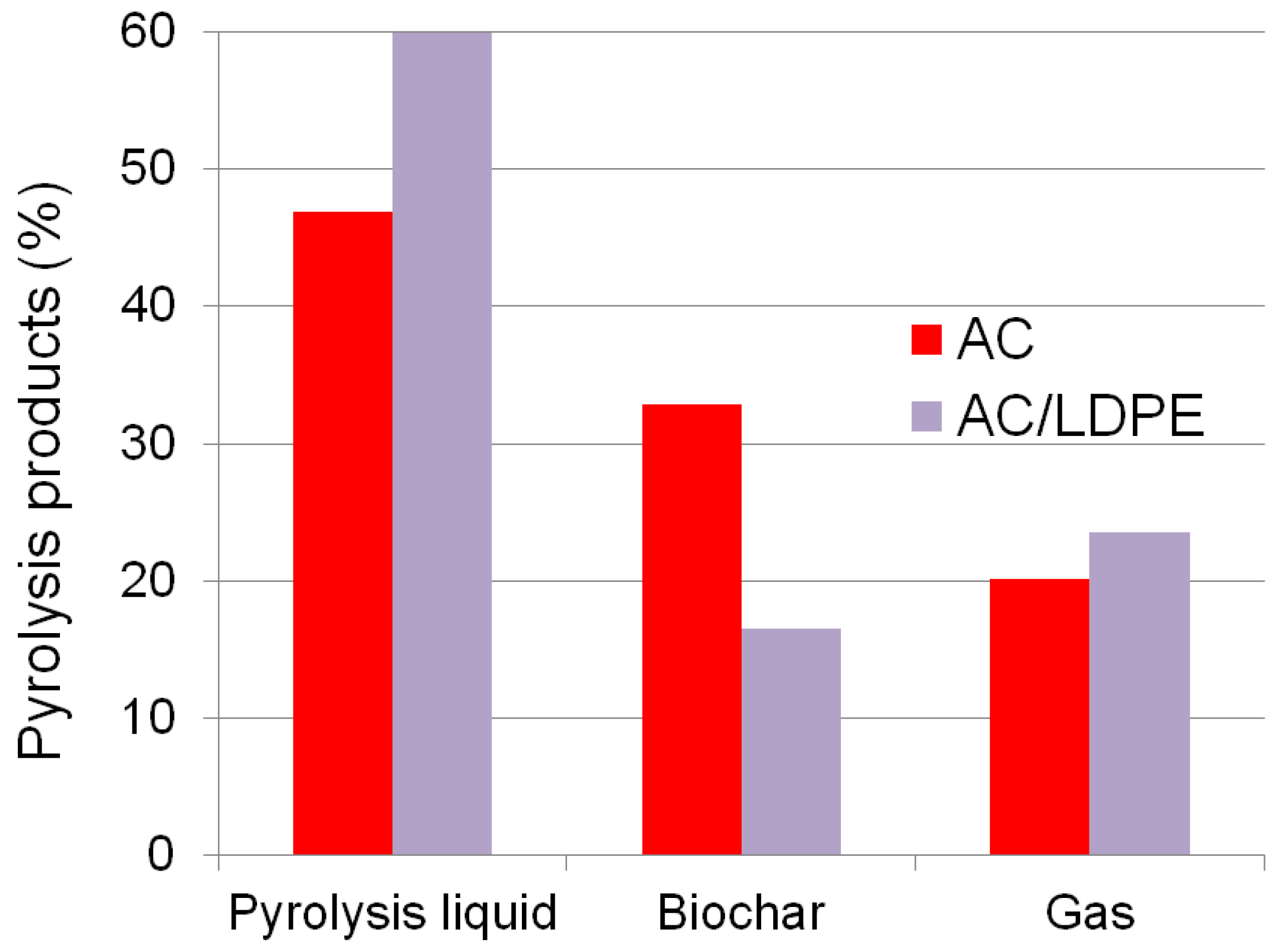
2.5. Material Balance and Pyrolysis Products
2.5.1. The Material Balance of the Process
2.5.2. Composition of Pyrolysis Liquid
2.5.3. Characteristics of Biochar
3. Discussion
4. Materials and Methods
4.1. Sample Materials
4.2. Physicochemical Characterization
4.3. Thermogravimetric Analysis
4.4. Kinetic Analysis
4.5. Experimental Pyrolysis Procedure
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

Appendix B
References
- Mykolenko, S.; Soon, W.L.; Mezzenga, R. Production and characterization of amaranth amyloid fibrils from food protein waste. Food Hydrocoll. 2024, 149, 109604. [Google Scholar] [CrossRef]
- Mukuwapasi, B.; Mavengahama, S.; Gerrano, A.S. Grain amaranth: A versatile untapped climate-smart crop for enhancing food and nutritional security. Discov. Agric. 2024, 2, 44. [Google Scholar] [CrossRef]
- Bamisaye, A.; Ige, A.R.; Adegoke, K.A.; Adegoke, I.A.; Bamidele, M.O.; Alli, Y.A.; Adeleke, O.; Idowu, M.A. Amaranthus hybridus waste solid biofuel: Comparative and machine learning studies. RSC Adv. 2024, 14, 11541–11556. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Campoy, A.H.; De León-Rodríguez, A.; Espitia-Rangel, E.; Barba-de la Rosa, A.P. Comparison of Traditional and Modern Techniques for Betalains Extraction from Amaranth Agro-Industrial Waste: The Recovery of High Value By-Products. Waste Biomass Valor. 2024, 15, 4325–4336. [Google Scholar] [CrossRef]
- Hongthong, S.; Sangsida, W.; Wongcharee, S.; Chanthakhot, A.; Aungthitipan, P.; Suwannahong, K.; Kreetachat, T.; Rioyo, J. Enhanced biochar production via co-pyrolysis of biomass residual with plastic waste after recycling process. Int. J. Chem. Eng. 2024, 2024, 1176275. [Google Scholar] [CrossRef]
- Seah, C.C.; Tan, C.H.; Arifin, N.A.; Hafriz, R.S.R.M.; Salmiaton, A.; Nomanbhay, S.; Shamsuddin, A.H. Co-pyrolysis of biomass and plastic: Circularity of wastes and comprehensive review of synergistic mechanism. Results Eng. 2023, 17, 100989. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Vibhakar, C.; Sabeenian, R.S.; Kaliappan, S.; Patil, P.Y.; Patil, P.P.; Madhu, P. Production and optimization of energy rich biofuel through co-pyrolysis by utilizing mixed agricultural residues and mixed waste plastics. Adv. Mater. Sci. Eng. 2022, 2022, 8175552. [Google Scholar] [CrossRef]
- Mohan, I.; Pandey, S.P.; Sahoo, A.; Kumar, S. Investigation of waste LDPE with Pongamia pinnata seed for sustainable resource recovery: Thermodynamics, kinetics and artificial neural network modeling for co-pyrolysis potential. Sustain. Chem. Environ. 2024, 6, 100089. [Google Scholar] [CrossRef]
- Lee, N.; Joo, J.; Lin, K.A.; Lee, J. Waste-to-fuels: Pyrolysis of low-density polyethylene waste in the presence of H-ZSM-11. Polymers 2021, 13, 1198. [Google Scholar] [CrossRef]
- Yang, J.; Rizkiana, J.; Widayatno, W.B.; Karnjanakom, S.; Kaewpanha, M.; Hao, X.; Abudula, A.; Guan, G. Fast co-pyrolysis of low density polyethylene and biomass residue for oil production. Energy Convers. Manag. 2016, 120, 422–429. [Google Scholar] [CrossRef]
- Karaeva, J.; Timofeeva, S.; Islamova, S.; Bulygina, K.; Aliev, F.; Panchenko, V.; Bolshev, V. Pyrolysis of amaranth inflorescence wastes: Bioenergy potential, biochar and hydrocarbon rich bio-oil production. Agriculture 2023, 13, 260. [Google Scholar] [CrossRef]
- Garba, M.U.; Inalegwu, A.; Musa, U.; Aboje, A.A.; Kovo, A.S.; Adeniyi, D.O. Thermogravimetric characteristic and kinetic of catalytic co-pyrolysis of biomass with low- and high-density polyethylenes. Biomass Convers. Bioref. 2018, 8, 143–150. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Prasad, M.; Somasundaram, M. Co-pyrolysis of Juliflora biomass with low-density polyethylene for bio-oil synthesis. Energy Sources Part A Recovery Util. Environ. Effects 2021, 43, 1134–1149. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; (Thanos) Bourtsalas, A.C.; Chi, Y.; Yan, J. Synergistic effects on char and oil produced by the co-pyrolysis of pine wood, polyethylene and polyvinyl chloride. Fuel 2018, 230, 359–367. [Google Scholar] [CrossRef]
- Xie, T.; Huo, L.; Yao, Z.; Zhang, X.; Liu, Z.; Jia, J.; Zhao, Y.; Zhao, L. Co-pyrolysis of biomass and polyethylene: Mechanistic insights into functional group transformations on solid matrix. Chem. Eng. J. 2024, 482, 149166. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El bouadili, A.; Nadifiyine, M.; Benchanaa, M.; Mokhlisse, A. Pyrolysis kinetics of olive residue/plastic mixtures by non-isothermal thermogravimetry. Fuel Process. Technol. 2009, 90, 722–728. [Google Scholar] [CrossRef]
- Alam, M.; Bhavanam, A.; Jana, A.; Viroja, J.S.; Peela, N.R. Co-pyrolysis of bamboo sawdust and plastic: Synergistic effects and kinetics. Renew. Energy 2020, 149, 1133–1145. [Google Scholar] [CrossRef]
- Sekyere, D.T.; Zhang, J.; Chen, Y.; Huang, Y.; Wang, M.; Wang, J.; Niwamanya, N.; Barigye, A.; Tian, Y. Production of light olefins and aromatics via catalytic co-pyrolysis of biomass and plastic. Fuel 2023, 333, 126339. [Google Scholar] [CrossRef]
- Xie, T.; Zhao, L.; Yao, Z.; Kang, K.; Jia, J.; Hu, T.; Zhang, X.; Sun, Y.; Huo, L. Co-pyrolysis of biomass and polyethylene: Insights into characteristics, kinetic and evolution paths of the reaction process. Sci. Total Environ. 2023, 897, 165443. [Google Scholar] [CrossRef] [PubMed]
- Bisen, D.; Chouhan, A.P.S.; Sarma, A.K.; Rajamohan, S.; Elumalai, P.V.; Balasubramanian, D.; Cherie, A. Thermogravimetric analysis of rice husk and low-density polyethylene co-pyrolysis: Kinetic and thermodynamic parameters. Sci. Rep. 2024, 14, 31798. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, V.S.; Dhanalakshmi, C.S.; Madhu, P.; Tamilselvam, P. Co-pyrolysis of neem wood bark and low-density polyethylene: Influence of plastic on pyrolysis product distribution and bio-oil characterization. Environ. Sci. Pollut. Res. Int. 2022, 29, 88213–88223. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, Y.; Abdelouahed, L.; Antoine, E.S.; Roland, E.H.; Bechara, T. Co-pyrolysis of plastic polymers and biomass: Effect of beech wood/plastic ratio and temperature on enhanced oil production in a tubular pyrolyzer. Renew. Energy 2023, 218, 119252. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, B.; Liu, C.; Shujaa aldeen, A. Mechanism of synergistic effects and kinetics analysis in catalytic co-pyrolysis of water hyacinth and HDPE. Energy Convers. Manag. 2021, 228, 113717. [Google Scholar] [CrossRef]
- Islamova, S.; Tartygasheva, A.; Karaeva, J.; Panchenko, V.; Litti, Y. A comprehensive study on the combustion of sunflower husk pellets by thermogravimetric and kinetic analysis, Kriging method. Agriculture 2023, 13, 840. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Khass, T.M.; Mostafa, M.E. Thermal degradation behaviour and chemical kinetic characteristics of biomass pyrolysis using TG/DTG/DTA techniques. Biomass Convers. Bioref. 2024, 14, 17779–17803. [Google Scholar] [CrossRef]
- Nawaz, A.; Razzak, S.A. Co-pyrolysis of biomass and different plastic waste to reduce hazardous waste and subsequent production of energy products: A review on advancement, synergies, and future prospects. Renew. Energy 2024, 224, 120103. [Google Scholar] [CrossRef]
- Hadey, C.; Allouch, M.; Loulidi, I.; Kali, A.; Zouhair, F.Z.; Alrashdi, A.A.; Amar, A.; Jabri, M.; Alami, M.; Lgaz, H.; et al. Investigating the impact of heating rates on the kinetic and combustion dynamics of pyrolyzed agricultural biomass. Biomass Convers. Bioref. 2024, 15, 8843–8853. [Google Scholar] [CrossRef]
- Pambudi, S.; Jongyingcharoen, J.S.; Saechua, W. Evaluation of pyrolysis characteristics and kinetic parameters from several prospected biomass residues by thermogravimetric analysis. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Sustainable Energy and Green Technology, Ho Chi Minh, Vietnam, 10–13 December 2023; Volume 1372. [Google Scholar] [CrossRef]
- Karaeva, J.; Timofeeva, S.; Gilfanov, M.; Slobozhaninova, M.; Sidorkina, O.; Luchkina, E.; Panchenko, V.; Bolshev, V. Exploring the prospective of weed Amaranthus retroflexus for biofuel production through pyrolysis. Agriculture 2023, 13, 687. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Islamova, S.I.; Gerasimov, A.V. Pyrolysis kinetics of new bioenergy feedstock from anaerobic digestate of agro-waste by thermogravimetric analysis. J. Environ. Chem. Eng. 2022, 10, 107850. [Google Scholar] [CrossRef]
- Rajan, K.P.; Mustafa, I.; Gopanna, A.; Thomas, S.P. Catalytic pyrolysis of waste low-density polyethylene (LDPE) carry bags to fuels: Experimental and exergy analyses. Recycling 2023, 8, 63. [Google Scholar] [CrossRef]
- Xuan, W.; Yan, S.; Dong, Y. Exploration of pyrolysis behaviors of waste plastics (polypropylene plastic/polyethylene plastic/polystyrene plastic): Macro-thermal kinetics and micro-pyrolysis mechanism. Processes 2023, 11, 2764. [Google Scholar] [CrossRef]
- Fonseca, F.G.; Funke, A.; Niebel, A.; Soares Dias, A.P.; Dahmen, N. Moisture content as a design and operational parameter for fast pyrolysis. J. Anal. Appl. Pyrolysis 2019, 139, 73–86. [Google Scholar] [CrossRef]
- Alvarado Flores, J.J.; Pintor Ibarra, L.F.; Mendez Zetina, F.D.; Rutiaga Quiñones, J.G.; Alcaraz Vera, J.V.; Avalos Rodriguez, M.L. Pyrolysis and physicochemical, thermokinetic and thermodynamic analyses of Ceiba aesculifolia (Kunth) britt and baker waste to evaluate its bioenergy potential. Molecules 2024, 29, 4388. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, K.; Wei, B.; Yang, H.; Jin, L.; Hu, H. Pyrolysis behavior of low-density polyethylene over HZSM-5 via rapid infrared heating. Sci. Total Environ. 2022, 806, 151287. [Google Scholar] [CrossRef]
- Hariadi, D.; Saleh, S.M.; Yamin, R.A.; Aprilia, S. Utilization of LDPE plastic waste on the quality of pyrolysis oil as an asphalt solvent alternative. Therm. Sci. Eng. Prog. 2021, 23, 100872. [Google Scholar] [CrossRef]
- Nazarloo, N.H.; Zabihi, O.; Shirvanimoghaddam, K.; Ahmadi, M.; Zamani, P.; Naebe, M. Innovative ex-situ catalyst bed integration for LDPE plastic pyrolysis: A thermodynamically closed system approach. Chem. Eng. J. 2024, 495, 153450. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Rocha, L.A.O.; Zhang, H.; Zhang, S.; Zhang, H. Insights into the char-production mechanism during co-pyrolysis of biomass and plastic wastes. Energy 2024, 312, 133642. [Google Scholar] [CrossRef]
- Fu, J.; Wu, X.; Liu, J.; Evrendilek, F.; Chen, T.; Xie, W.; Xu, W.; He, Y. Co-circularity of spent coffee grounds and polyethylene via co-pyrolysis: Characteristics, kinetics, and products. Fuel 2023, 337, 127061. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, Y.; Qiu, L.; Li, W.; Sun, R.; Zhu, M.; Yang, X. Effect of reaction conditions on energy yield of pyrolysis gas from apple tree branches. ACS Omega 2024, 9, 28028–28036. [Google Scholar] [CrossRef] [PubMed]
- Islamova, S.I.; Timofeeva, S.S.; Khamatgalimov, A.R.; Ermolaev, D.V. Kinetic analysis of the thermal decomposition of lowland and high-moor peats. Solid Fuel Chem. 2020, 54, 154–162. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Evrendilek, F.; Zhang, X.; Buyukada, M. TG-FTIR and Py-GC/MS analyses of pyrolysis behaviors and products of cattle manure in CO2 and N2 atmospheres: Kinetic, thermodynamic, and machine-learning models. Energy Convers. Manag. 2019, 195, 346–359. [Google Scholar] [CrossRef]
- Wang, B.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y.; Qiao, Y. Effects of heating rate on fast pyrolysis behavior and product distribution of Jerusalem artichoke stalk by using TG-FTIR and Py-GC/MS. Renew. Energy 2019, 132, 486–496. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Zhao, W.; Zhou, J. TG-FTIR-MS study of pyrolysis products evolving from peat. J. Anal. Appl. Pyrolysis 2016, 117, 296–309. [Google Scholar] [CrossRef]
- Baruah, D.; Mallick, D.; Kalita, P.; Moholkar, S. A Detailed study of pyrolysis kinetics of elephant grass using thermogravimetric analysis. In Proceedings of the 2nd International Conference on Energy Power and Environment (ICEPE 2018), Shillong, India, 1–2 June 2018; pp. 1–5. [Google Scholar]
- Huang, X.; Cao, J.-P.; Zhao, X.-Y.; Wang, J.-X.; Fan, X.; Zhao, Y.-P.; Wei, X.-Y. Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel 2016, 169, 93–98. [Google Scholar] [CrossRef]
- Ceylan, S.; Topçu, Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour. Technol. 2014, 156, 182–188. [Google Scholar] [CrossRef]
- Bai, X.; Wang, G.; Zhu, Z.; Cai, C.; Wang, Z.; Wang, D. Investigation of improving the yields and qualities of pyrolysis products with combination rod-milled and torrefaction pretreatment. Renew. Energy 2020, 151, 446–453. [Google Scholar] [CrossRef]
- Jerzak, W.; Bieniek, A.; Magdziarz, A. Multifaceted analysis of products from the intermediate co-pyrolysis of biomass with Tetra Pak waste. Int. J. Hydrog. Energy 2023, 48, 31. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Jewiarz, M.; Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 2019, 21, 786–800. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sanchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Variation in feedstock wood chemistry strongly influences biochar liming potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Volpi, M.P.C.; Silva, J.C.G.; Hornung, A.; Ouadi, M. Review of the Current State of Pyrolysis and Biochar Utilization in Europe: A Scientific Perspective. Clean Technol. 2024, 6, 152–175. [Google Scholar] [CrossRef]
- Ghai, H.; Sakhuja, D.; Yadav, S.; Solanki, P.; Putatunda, C.; Bhatia, R.K.; Bhatt, A.K.; Varjani, S.; Yang, Y.-H.; Bhatia, S.K.; et al. An Overview on Co-Pyrolysis of Biodegradable and Non-Biodegradable Wastes. Energies 2022, 15, 4168. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Kovalev, A.A.; Kovalev, D.A.; Gilfanov, M.F.; Grigoriev, V.S.; Litti, Y.V. Co-pyrolysis of agricultural waste and estimation of the applicability of pyrolysis in the integrated technology of biorenewable hydrogen production. Int. J. Hydrogen Energy 2022, 47, 11787–11798. [Google Scholar] [CrossRef]
- Madadi, M.; Wang, Y.; Xu, C.; Liu, P.; Wang, Y.; Xia, T.; Tu, Y.; Lin, X.; Song, B.; Yang, X.; et al. Using Amaranthus green proteins as universal biosurfactant and biosorbent for effective enzymatic degradation of diverse lignocellulose residues and efficient multiple trace metals remediation of farming lands. J. Hazard. Mater. 2021, 406, 124727. [Google Scholar] [CrossRef]
- Cheng, S.; Shu, J.; Xia, H.; Wang, S.; Zhang, L.; Peng, J.; Li, C.; Jiang, X.; Zhang, Q. Pyrolysis of Crofton weed for the production of aldehyde rich bio-oil and combustible matter rich bio-gas. Appl. Therm. Eng. 2019, 148, 1164–1170. [Google Scholar] [CrossRef]
- Machado, H.; Cristino, A.F.; Orišková, S.; Galhano dos Santos, R. Bio-Oil: The Next-Generation Source of Chemicals. Reactions 2022, 3, 118–137. [Google Scholar] [CrossRef]
- Younas, R.; Hao, S.; Zhang, L.; Zhang, S. Hydrothermal liquefaction of rice straw with NiO nanocatalyst for bio-oil production. Renew. Energy 2017, 113, 532–545. [Google Scholar] [CrossRef]
- Devi, T.E.; Parthiban, R. Hydrothermal liquefaction of Nostoc ellipsosporum biomass grown in municipal wastewater under optimized conditions for bio-oil production. Bioresour. Technol. 2020, 316, 123943. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.G.; Iwuozor, K.O.; Emenike, E.C.; Ajala, O.J.; Ogunniyi, S.; Muritala, K.B. Thermochemical co-conversion of biomass-plastic waste to biochar: A review. Green Chem. Eng. 2024, 5, 31–49. [Google Scholar] [CrossRef]
- Arifah, Z.; Jamilatun, S.; Rahayu, A.; Astuti, E.; Ardiansyah, R.S. Review: Biochar from Co-Pyrolysis of Biomass and Plastic. Indones. J. Chem. Eng. 2023, 1, 34–48. [Google Scholar] [CrossRef]
- Mostafa, M.E.; Alsulami, R.A.; Khedr, Y.M. Chemical kinetic models, reaction mechanism estimation and thermodynamic parameters for the thermochemical conversion of solid wastes: Review. J. Anal. Appl. Pyrolysis 2024, 179, 106431. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Vyazovkin, S. The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be. Thermo 2022, 2, 435–452. [Google Scholar] [CrossRef]
- Muigai, H.H.; Choudhury, B.J.; Kalita, P.; Moholkar, V.S. Co–pyrolysis of biomass blends: Characterization, kinetic and thermodynamic analysis. Biomass Bioenergy 2020, 143, 105839. [Google Scholar] [CrossRef]
- Zhong, Y.; Ding, Y.; Lu, K.; Mao, S.; Li, C. Kinetic parameters and reaction mechanism study of biomass pyrolysis by combined kinetics coupled with a heuristic optimization algorithm. Fuel 2023, 334, 126622. [Google Scholar] [CrossRef]
- Li, B.; Liu, G.; Gao, W.; Cong, H.-Y.; Bi, M.-S.; Ma, L.; Deng, J.; Shu, C.-M. Study of combustion behaviour and kinetics modelling of Chinese Gongwusu coal gangue: Model-fitting and model-free approaches. Fuel 2020, 268, 117284. [Google Scholar] [CrossRef]
- Castells, B.; Amez, I.; Medic, L.; García-Torrent, J. Torrefaction influence on combustion kinetics of Malaysian oil palm wastes. Fuel Process. Technol. 2021, 218, 106843. [Google Scholar] [CrossRef]
- Okot, D.K. Briquetting and Torrefaction of Agricultural Residues for Energy Production. Ph.D. Thesis, School of Engineering, Newcastle University, Newcastle, UK, 2019. Available online: http://theses.ncl.ac.uk/jspui/handle/10443/4716 (accessed on 17 July 2025).
- Shahbeig, H.; Nosrati, M. Pyrolysis of biological wastes for bioenergy production: Thermo-kinetic studies with machine-learning method and Py-GC/MS analysis. Fuel 2020, 269, 117238. [Google Scholar] [CrossRef]
- Klingenberg, P.; Brüll, R.; Fell, T.; Barton, B.; Soll, M.; Emans, T.; Bakker, F.; Geertz, G. Quality comparison of plastic packaging waste from different separation systems: Result enhancement with non-negative matrix factorization of FTIR spectra. Waste Manag. 2024, 178, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Perez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Perez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]

 Hydrocarbons;
Hydrocarbons;  O-containing;
O-containing;  N-containing;
N-containing;  Other;
Other;  Saturated;
Saturated;  Unsaturated;
Unsaturated;  Aromatics;
Aromatics;  Cyclics;
Cyclics;  Ethers;
Ethers;  Ketones;
Ketones;  Carboxylic acids;
Carboxylic acids;  Phenols;
Phenols;  Cyclics;
Cyclics;  Alcohols;
Alcohols;  Heterocycles;
Heterocycles;  Aldehydes;
Aldehydes;  Amides;
Amides;  Heterocycles;
Heterocycles;  Nitriles;
Nitriles;  Amines;
Amines;  Cyclics;
Cyclics;  Ketones.
Ketones.
 Hydrocarbons;
Hydrocarbons;  O-containing;
O-containing;  N-containing;
N-containing;  Other;
Other;  Saturated;
Saturated;  Unsaturated;
Unsaturated;  Aromatics;
Aromatics;  Cyclics;
Cyclics;  Ethers;
Ethers;  Ketones;
Ketones;  Carboxylic acids;
Carboxylic acids;  Phenols;
Phenols;  Cyclics;
Cyclics;  Alcohols;
Alcohols;  Heterocycles;
Heterocycles;  Aldehydes;
Aldehydes;  Amides;
Amides;  Heterocycles;
Heterocycles;  Nitriles;
Nitriles;  Amines;
Amines;  Cyclics;
Cyclics;  Ketones.
Ketones.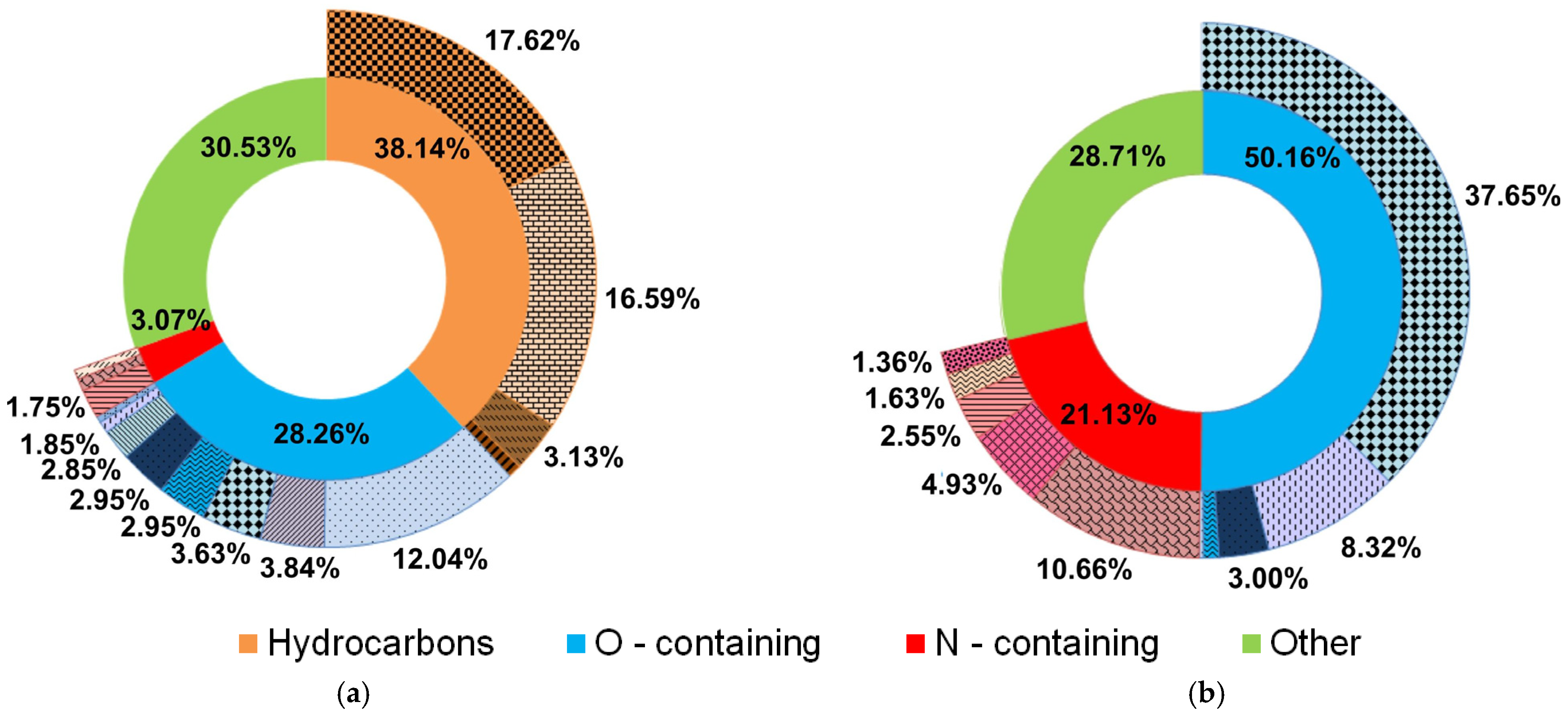

| Samples | Ultimate Analysis (wt.%, on Air Dry Basis) | Proximate Analysis (wt.%, on Air Dry Basis) | HHV (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | Moisture | Ash | Volatile Matter | Fixed Carbon | ||
| AC | 38.49 ± 0.07 | 6.09 ± 0.3 | 1.44 ± 0.5 | 40.98 ± 1.02 | 7.4 ± 0.03 | 13.0 ± 0.7 | 75.8 ± 0.9 | 11.2 ± 0.2 | 20.3 ± 0.47 |
| LDPE | 85.43 ± 0.5 | 13.52 ± 0.6 | - | 0.95 ± 0.9 | 0.0 | 0.1 ± 0.02 | 99.9 ± 0.4 | 0.0 | 45.7 ± 0.7 |
| AC/LDPE | 61.96 ± 0.9 | 9.81 ± 0.09 | 0.72 ± 0.02 | 21.01 ± 0.1 | 3.7 ± 0.07 | 6.5 ± 0.4 | 87.9 ± 1.02 | 5.6 ± 0.3 | 31.0 ± 0.42 |
| Pyrolysis Stage | Starting Temperature (°C) | Ending Temperature (°C) | Temperature Peak on DTG Curve (°C) |
|---|---|---|---|
| Drying | 25 | 190 | 95 |
| Fast decomposition | 190 | 550 | 467 |
| Slow decomposition | 550 | 1000 | - |
| Samples | Mass Loss (wt.%, on Air Dry Basis) | |||
|---|---|---|---|---|
| Drying | Fast Decomposition | Slow Decomposition | Residual Mass at 1000 °C | |
| AC | 8.3 | 64.9 | 7.8 | 27.3 |
| LDPE | 0.0 | 92.7 | 0.01 | 7.12 |
| AC/LDPE | 2.3 | 76.8 | 5.1 | 18.1 |
| β (°C/min) | Reaction Mechanism | Linear Regression Equation | g(α) | Eα (kJ/mol) | A (1/s) |
|---|---|---|---|---|---|
| 5 | F9 | y = −33.54x + 39.98 | 36,803.89 | 332.40 | 5.31 × 1027 |
| 10 | F11 | y = −25.34x + 30.08 | 848,768.02 | 300.39 | 2.23 × 1026 |
| 20 | F11 | y = −25.63x + 30.06 | 848,768.02 | 306.00 | 3.28 × 1026 |
| A (1/s) | ||||||
|---|---|---|---|---|---|---|
| 0.20 | 254.39 | 0.45 | 1.19 × 1027 | 0.99 | 8.31 | 0.00 |
| 0.25 | 564.39 | 1.00 | 2.82 × 1026 | 0.23 | 16.8 | 0.00 |
| 0.30 | 481.28 | 0.85 | 1.07 × 1024 | 0.00 | 34.4 | 0.00 |
| 0.35 | 551.35 | 0.98 | 1.85 × 1023 | 0.00 | 73.3 | 0.00 |
| 0.40 | 436.12 | 0.77 | 2.02 × 1023 | 0.00 | 164 | 0.00 |
| 0.45 | 322.35 | 0.57 | 2.97 × 1023 | 0.00 | 394 | 0.00 |
| 0.50 | 294.43 | 0.52 | 5.46 × 1023 | 0.00 | 1020 | 0.00 |
| 0.55 | 270.83 | 0.48 | 1.28 × 1024 | 0.00 | 2940 | 0.00 |
| 0.60 | 261.61 | 0.46 | 3.17 × 1024 | 0.00 | 9540 | 0.00 |
| 0.65 | 253.06 | 0.49 | 9.21 × 1024 | 0.01 | 36,200 | 0.00 |
| 0.70 | 245.40 | 0.44 | 3.30 × 1025 | 0.03 | 169,000 | 0.02 |
| 0.75 | 247.26 | 0.44 | 1.68 × 1026 | 0.14 | 1,050,000 | 0.11 |
| 0.80 | 240.13 | 0.43 | 1.21 × 1027 | 1.00 | 9,770,000 | 1.00 |
| Samples | Ultimate Analysis (wt.%, on Air Dry Basis) | Proximate Analysis (wt.%, on Air Dry Basis) | HHV, MJ/kg | |||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | Ash | Volatile Matter | Fixed Carbon | ||
| AC_biochar | 57.67 ± 1.07 | 2.30 ± 0.25 | 1.92 ± 0.6 | 10.46 ± 0.7 | 27.65 ± 0.1 | 24.6 ± 0.3 | 47.75 ± 0.8 | 18.9 ± 0.15 |
| AC/LDPE_biochar | 57.16 ± 0.9 | 1.97 ± 0.2 | 1.87 ± 0.02 | 7.66 ± 0.3 | 31.34 ± 0.06 | 19.3 ± 0.8 | 49.36 ± 0.5 | 19.7 ± 0.06 |
| Samples | Content of Macro- and Microelements (wt.%, on Air Dry Basis) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | P | Cl | S | Si | Fe | Ti | Zn | Mn | Br | Sr | |
| AC_biochar | 49.8 | 35.7 | 4.1 | 3.8 | 2.4 | 1.6 | 1.3 | 0.7 | 0.2 | 0.1 | 0.1 | 0.04 | 0.03 |
| AC/LDPE_biochar | 52.6 | 32.7 | 3.9 | 3.6 | 2.9 | 1.5 | 1.7 | 0.8 | - | 0.1 | 0.1 | 0.04 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaeva, J.; Timofeeva, S.; Islamova, S.; Slobozhaninova, M.; Oleynikova, E.; Sidorkina, O. High-Value Utilization of Amaranth Residue and Waste LDPE by Co-Pyrolysis. Molecules 2025, 30, 3471. https://doi.org/10.3390/molecules30173471
Karaeva J, Timofeeva S, Islamova S, Slobozhaninova M, Oleynikova E, Sidorkina O. High-Value Utilization of Amaranth Residue and Waste LDPE by Co-Pyrolysis. Molecules. 2025; 30(17):3471. https://doi.org/10.3390/molecules30173471
Chicago/Turabian StyleKaraeva, Julia, Svetlana Timofeeva, Svetlana Islamova, Marina Slobozhaninova, Ekaterina Oleynikova, and Olga Sidorkina. 2025. "High-Value Utilization of Amaranth Residue and Waste LDPE by Co-Pyrolysis" Molecules 30, no. 17: 3471. https://doi.org/10.3390/molecules30173471
APA StyleKaraeva, J., Timofeeva, S., Islamova, S., Slobozhaninova, M., Oleynikova, E., & Sidorkina, O. (2025). High-Value Utilization of Amaranth Residue and Waste LDPE by Co-Pyrolysis. Molecules, 30(17), 3471. https://doi.org/10.3390/molecules30173471






