Development and Validation of an LC-MS/MS Method for the Simultaneous Determination of Alprazolam, Bromazepam, Clonazepam, Diazepam and Flunitrazpam in Human Urine and Its Application to Samples from Suspected Drug Abusers
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation
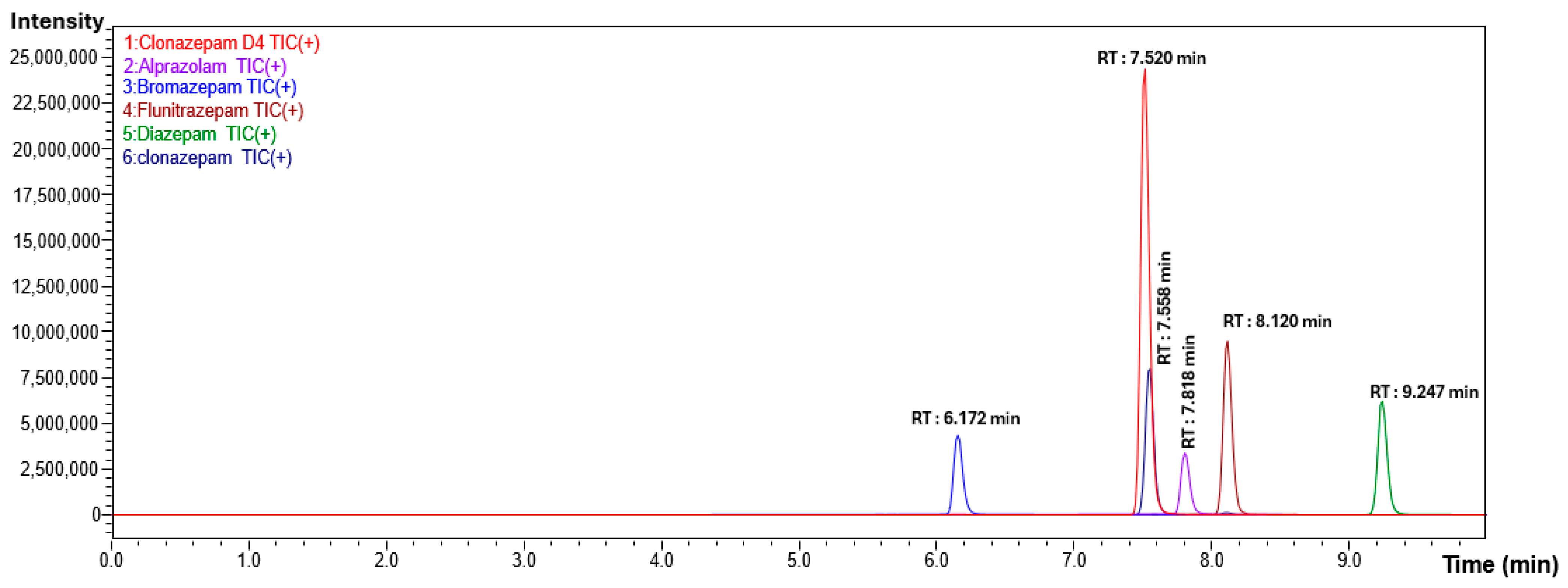
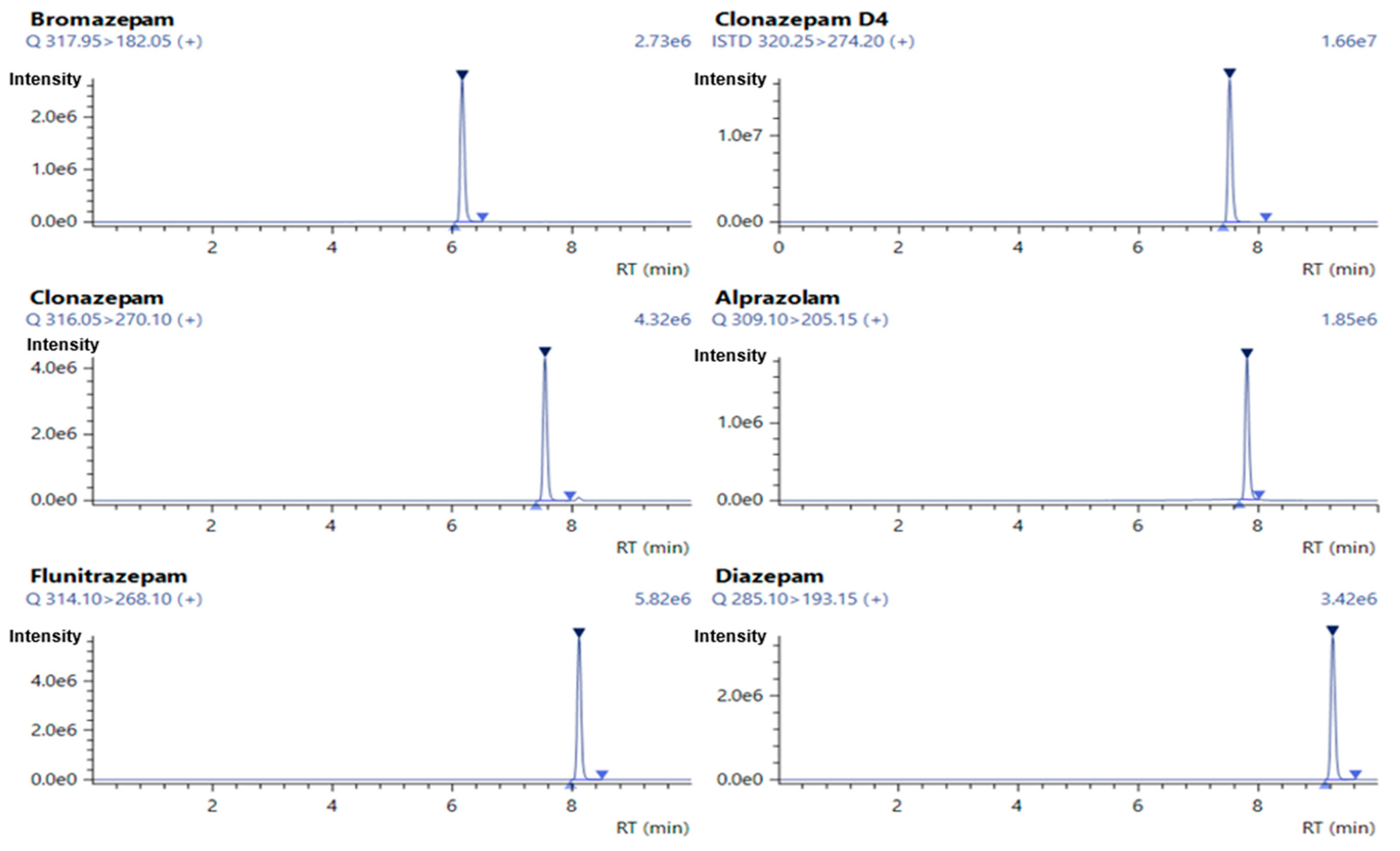
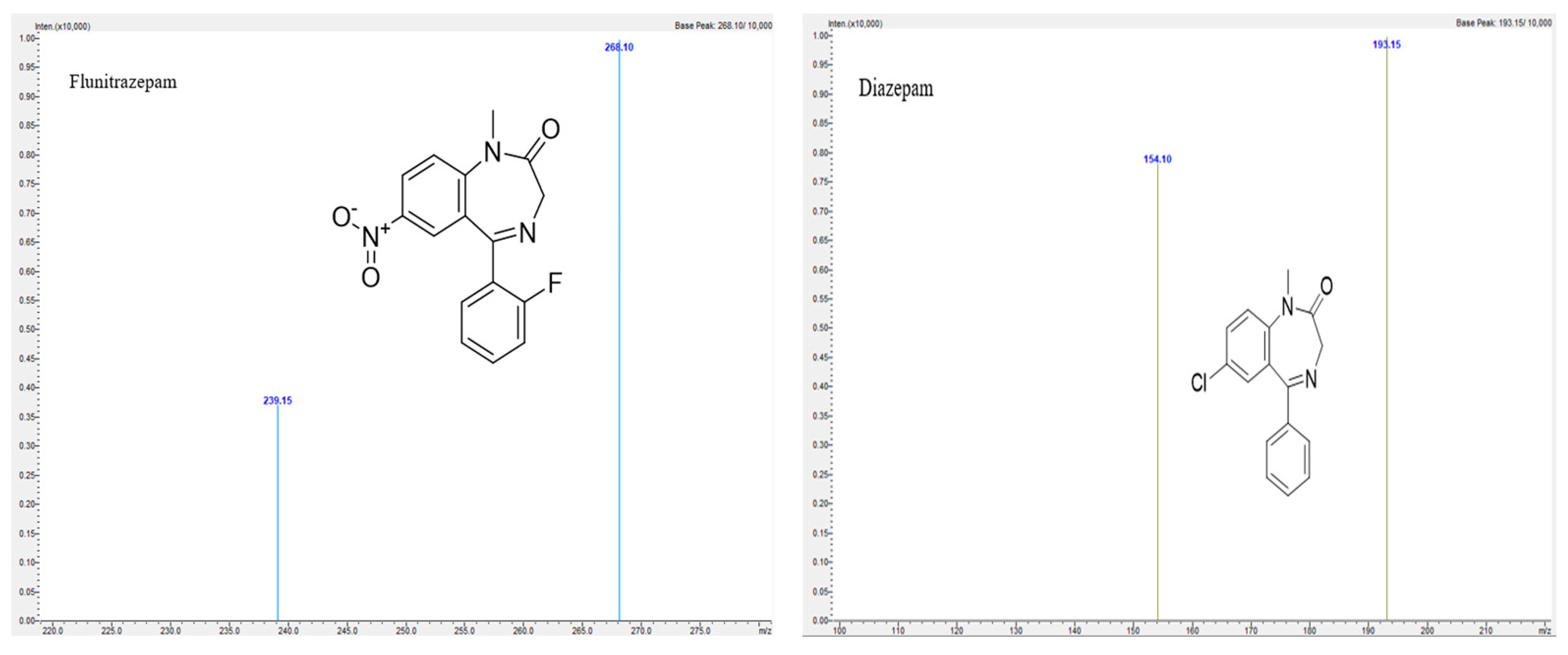
2.2.1. Specificity
2.2.2. Linearity and Calibration Curve
2.2.3. Limit of Quantification and Limit of Detection
2.2.4. Accuracy
2.2.5. Precision
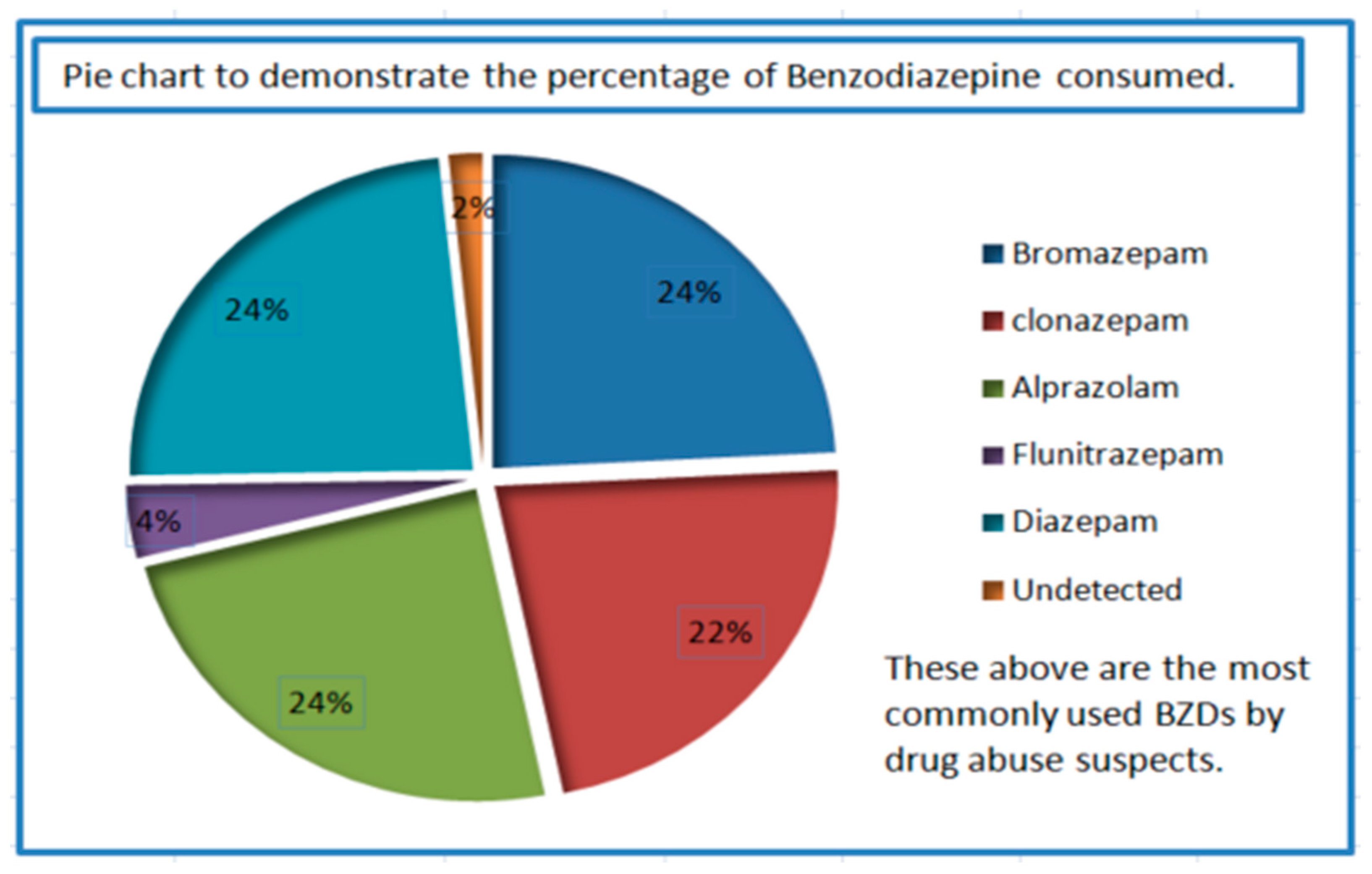
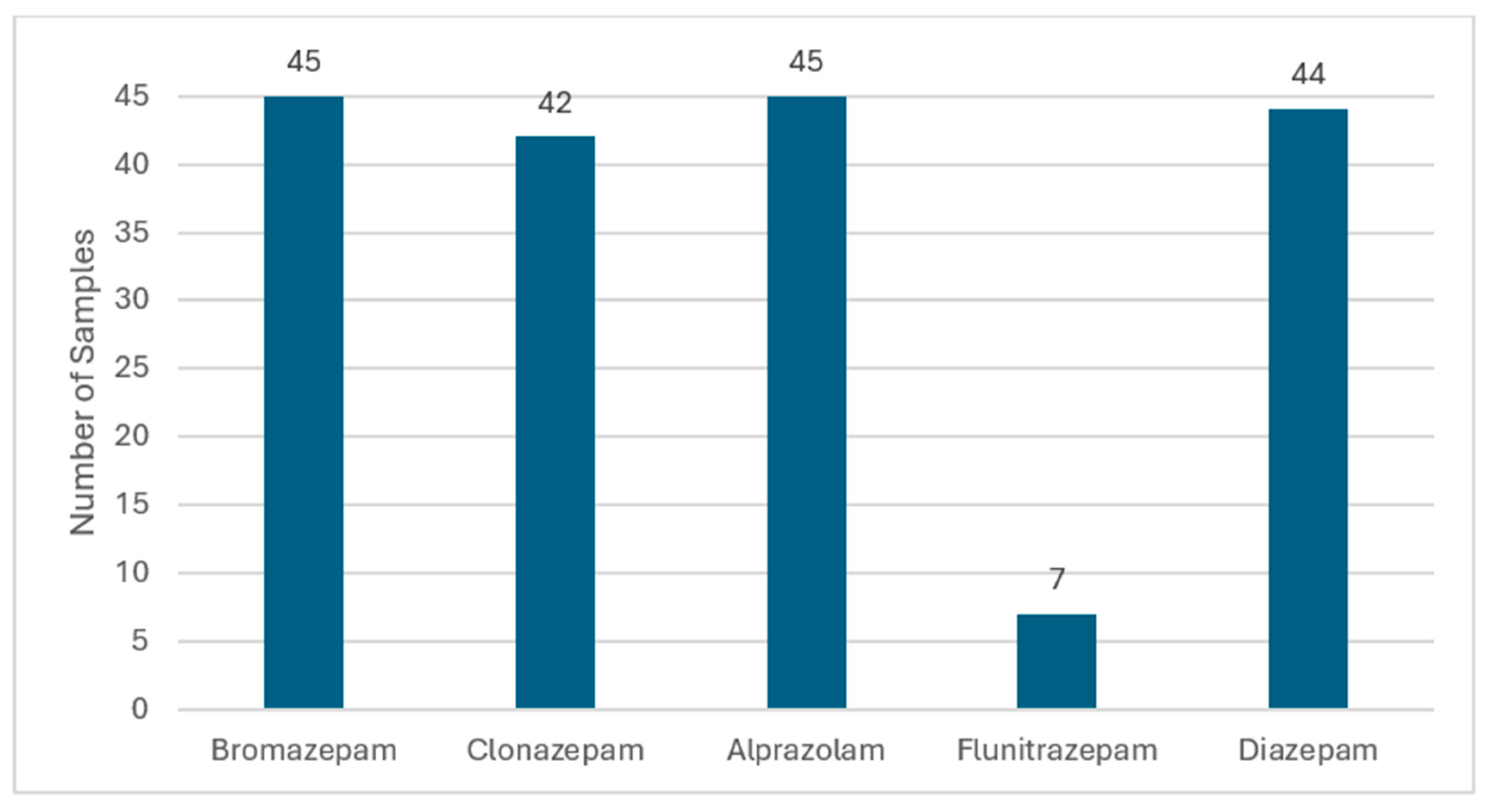
2.3. Application to Samples from Suspected Drug Abusers
3. Material and Methods
3.1. Chemicals and Reagent
3.2. Instruments and Chromatographic Conditions
3.3. Preparation of Standard Solutions and Samples
3.3.1. Preparation of Standard Solutions
3.3.2. Preparation of Control Sample from Sigma-Trix Urine Diluent
3.3.3. Preparation of Processed Quality Control Samples
3.4. Method Validation
3.4.1. Specificity
3.4.2. Linearity
3.4.3. Limit of Quantification and Detection
3.4.4. Accuracy
3.4.5. Precision
3.5. Application to Samples from Suspected Drug Abusers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sofalvi, S.; Lavins, E.S.; Kaspar, C.K.; Michel, H.M.; Mitchell-Mata, C.L.; Huestis, M.A.; Apollonio, L.G. Development and validation of an LC–MS-MS method for the detection of 40 benzodiazepines and three Z-drugs in blood and urine by solid-phase extraction. J. Anal. Toxicol. 2020, 44, 708–717. [Google Scholar] [CrossRef]
- Al-Waheeb, S.; Al-Omair, N.; Mahdi, A. Patterns of drug overdose deaths in Kuwait from 2014 to 2018. Public Health Pract. 2021, 2, 100181. [Google Scholar] [CrossRef] [PubMed]
- Al-Matrouk, A.; Al-Hasan, M.; Naqi, H.; Al-Abkal, N.; Mohammed, H.; Haider, M.; Al-Shammeri, D.; Bojbarah, H. Snapshot of narcotic drugs and psychoactive substances in Kuwait: A qualitative analysis of illicit drugs and their associated mortality in Kuwait from 2015 to 2018. BMC Public Health 2021, 21, 671. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K. Advances in drugs of abuse testing. Clin. Chim. Acta 2021, 514, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Saxon, A.J.; Calsyn, D.A.; Haver, V.M.; Delaney, C.J. Clinical evaluation and use of urine screening for drug abuse. West. J. Med. 1988, 149, 296–303. [Google Scholar]
- Liu, Z.; Short, J.; Rose, A.; Ren, S.; Contel, N.; Grossman, S.; Unger, S. The simultaneous determination of diazepam and its three metabolites in dog plasma by high-performance liquid chromatography with mass spectroscopy detection. J. Pharm. Biomed. Anal. 2001, 26, 321–330. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, J.Y.; Chae, S.I.; Kang, B.K.; An, T.G.; Shim, W.S.; Noh, Y.S.; Hwang, S.J.; Chung, E.K.; Lee, K.-T. Development of a simple and sensitive HPLC-MS/MS method for determination of diazepam in human plasma and its application to a bioequivalence study. Transl. Clin. Pharmacol. 2017, 25, 173–178. [Google Scholar] [CrossRef]
- Smink, B.E.; Brandsma, J.E.; Dijkhuizen, A.; Lusthof, K.J.; Gier, J.J.D.; Egberts, A.C.G.; Uges, D.R.A. Quantitative analysis of 33 benzodiazepines, metabolites and benzodiazepine-like substances in whole blood by liquid chromatography-(tandem) mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 811, 13–20. [Google Scholar] [CrossRef]
- Abarca, R.; Gerona, R. Development and validation of an LC-MS/MS assay for the quantitative analysis of alprazolam, α-hydroxyalprazolam and hydrocodone in dried blood spots. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2023, 1220, 123639. [Google Scholar] [CrossRef]
- Cavedal, L.E.; Mendes, F.D.; Domingues, C.C.; Patni, A.K.; Monif, T.; Reyar, S.; Pereira, A.d.S.; Mendes, G.D.; De Nucci, G. Clonazepam quantification in human plasma by high-performance liquid chromatography coupled with electrospray tandem mass spectrometry in a bioequivalence study. J. Mass Spectrom. 2007, 42, 81–88. [Google Scholar] [CrossRef]
- Kratzsch, C.; Tenberken, O.; Peters, F.T.; Weber, A.A.; Kraemer, T.; Maurer, H.H. Screening, library-assisted identification and validated quantification of 23 benzodiazepines, flumazenil, zaleplone, zolpidem and zopiclone in plasma by liquid chromatography/mass spectrometry with atmospheric pressure chemical ionization. J. Mass Spectrom. 2004, 39, 856–872. [Google Scholar] [CrossRef]
- Glover, S.J.; Allen, K.R. Measurement of benzodiazepines in urine by liquid chromatography-tandem mass spectrometry: Confirmation of samples screened by immunoassay. Ann. Clin. Biochem. 2010, 47, 111–117. [Google Scholar] [CrossRef]
- Chèze, M.; Villain, M.; Pépin, G. Determination of bromazepam, clonazepam and metabolites after a single intake in urine and hair by LC-MS/MS: Application to forensic cases of drug facilitated crimes. Forensic Sci. Int. 2004, 145, 123–130. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, J.F.; Lin, S.Y.; Chen, B.H. Simultaneous identification of abused drugs, benzodiazepines, and new psychoactive substances in urine by liquid chromatography tandem mass spectrometry. Kaohsiung J. Med. Sci. 2016, 32, 118–127. [Google Scholar] [CrossRef]
- Villain, M.; Chèze, M.; Tracqui, A.; Ludes, B.; Kintz, P. Windows of detection of zolpidem in urine and hair: Application to two drug facilitated sexual assaults. Forensic Sci. Int. 2004, 143, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P.; Villain, M.; Cirimele, V.; Pépin, G.; Ludes, B. Windows of detection of lorazepam in urine, oral fluid and hair, with a special focus on drug-facilitated crimes. Forensic Sci. Int. 2004, 145, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.J.; Coles, R.; Merrell, M.; McMillin, G.A. Quantitation of benzodiazepines in urine, serum, plasma, and meconium by LC-MS-MS. J. Anal. Toxicol. 2008, 32, 491–498. [Google Scholar] [CrossRef][Green Version]
- Jang, M.; Chang, H.; Yang, W.; Choi, H.; Kim, E.; Yu, B.H.; Oh, Y.; Chung, H. Development of an LC-MS/MS method for the simultaneous determination of 25 benzodiazepines and zolpidem in oral fluid and its application to authentic samples from regular drug users. J. Pharm. Biomed. Anal. 2013, 74, 213–222. [Google Scholar] [CrossRef]
- Arantes, A.C.F.; da Cunha, K.F.; Cardoso, M.S.; Oliveira, K.D.; Costa, J.L. Development and validation of quantitative analytical method for 50 drugs of antidepressants, benzodiazepines and opioids in oral fluid samples by liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2021, 39, 179–197. [Google Scholar] [CrossRef]
- Kronstrand, R.; Nyström, I.; Josefsson, M.; Hodgins, S. Segmental ion spray LC-MS-MS analysis of benzodiazepines in hair of psychiatric patients. J. Anal. Toxicol. 2002, 26, 479–484. [Google Scholar] [CrossRef]
- Villain, M.; Chèze, M.; Tracqui, A.; Ludes, B.; Kintz, P. Testing for zopiclone in hair application to drug-facilitated crimes. Forensic Sci. Int. 2004, 145, 117–121. [Google Scholar] [CrossRef]
- Villain, M.; Chèze, M.; Dumestre, V.; Ludes, B.; Kintz, P. Hair to document drug-facilitated crimes: Four cases involving bromazepam. J. Anal. Toxicol. 2004, 28, 516–519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banaszkiewicz, L.; Woźniak, M.K.; Kaliszan, M.; Kot-Wasik, A. Determination of benzodiazepines and Z-hypnotic drugs in whole blood samples by GC–MS/MS: Method development, validation and application. Microchem. J. 2023, 193, 109115. [Google Scholar] [CrossRef]
- Perez, E.R.; Knapp, J.A.; Horn, C.K.; Stillman, S.L.; Evans, J.E.; Arfsten, D.P. Comparison of LC-MS-MS and GC-MS analysis of benzodiazepine compounds included in the drug demand reduction urinalysis program. J. Anal. Toxicol. 2016, 40, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Negrusz, A.; Moore, C.; Deitermann, D.; Lewis, D.; Kaleciak, K.; Kronstrand, R.; Feeley, B.; Niedbala, R.S. Highly sensitive micro-plate enzyme immunoassay screening and NCI-GC-MS confirmation of flunitrazepam and its major metabolite 7-aminoflunitrazepam in hair. J. Anal. Toxicol. 1999, 23, 429–435. [Google Scholar] [CrossRef]
- Cirimele, V.; Kintz, P.; Ludes, B. Screening for forensically relevant benzodiazepines in human hair by gas chromatography-negative ion chemical ionization-mass spectrometry. J. Chromatogr. B Biomed. Appl. 1997, 700, 119–129. [Google Scholar] [CrossRef]
- Pirnay, S.; Ricordel, I.; Libong, D.; Bouchonnet, S. Sensitive method for the detection of 22 benzodiazepines by gas chromatography-ion trap tandem mass spectrometry. J. Chromatogr. A 2002, 954, 235–245. [Google Scholar] [CrossRef]
- Albishri, H.M.; Aldawsari, N.A.; Abd El-Hady, D. A Simple and Reliable Liquid Chromatographic Method for Simultaneous Determination of Five Benzodiazepine Drugs in Human Plasma. Analytica 2022, 3, 251–265. [Google Scholar] [CrossRef]
- Al-Hawasli, H.; Al-Khayat, M.A.; Al-Mardini, M.A. Development of a validated HPLC method for the separation and analysis of a Bromazepam, Medazepam and Midazolam mixture. J. Pharm. Anal. 2012, 2, 484–491. [Google Scholar] [CrossRef]
- Soltaninejad, K.; Karimi, M.; Nateghi, A.; Daraei, B. Simultaneous determination of six benzodiazepines in spiked soft drinks by high performance liquid chromatography with ultra violet detection (HPLC-UV). Iran. J. Pharm. Res. 2016, 15, 457–463. [Google Scholar]
- Bell, C.; George, C.; Kicman, A.T.; Traynor, A. Development of a rapid LC-MS/MS method for direct urinalysis of designer drugs. Drug Test. Anal. 2011, 3, 496–504. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, D.; Wang, S. Drug confirmation by mass spectrometry: Identification criteria and complicating factors. Clin. Chim. Acta 2015, 438, 119–125. [Google Scholar] [CrossRef]
- Seger, C. Usage and limitations of liquid chromatography-tandem mass spectrometry (LC-MS/MS) in clinical routine laboratories. Wien. Med. Wochenschr. 2012, 162, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Dams, R.; Huestis, M.A.; Lambert, W.E.; Murphy, C.M. Matrix effect in bio-analysis of illicit drugs with LC-MS/MS: Influence of ionization type, sample preparation, and biofluid. J. Am. Soc. Mass Spectrom. 2003, 14, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Stokvis, E.; Rosing, H.; Beijnen, J.H. Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: Necessity or not? Rapid Commun. Mass Spectrom. 2005, 19, 401–407. [Google Scholar] [CrossRef]
- Srinivas, N.R. Dodging matrix effects in liquid chromatography tandem mass spectrometric assays—Compilation of key learnings and perspectives. Biomed. Chromatogr. 2009, 23, 451–454. [Google Scholar] [CrossRef]
- Fisichella, M.; Odoardi, S.; Strano-Rossi, S. High-throughput dispersive liquid/liquid microextraction (DLLME) method for the rapid determination of drugs of abuse, benzodiazepines and other psychotropic medications in blood samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and application to forensic cases. Microchem. J. 2015, 123, 33–41. [Google Scholar] [CrossRef]
- Kohler, I.; Schappler, J.; Sierro, T.; Rudaz, S. Dispersive liquid-liquid microextraction combined with capillary electrophoresis and time-of-flight mass spectrometry for urine analysis. J. Pharm. Biomed. Anal. 2013, 73, 82–89. [Google Scholar] [CrossRef]
- Saraji, M.; Khalili Boroujeni, M.; Hajialiakbari Bidgoli, A.A. Comparison of dispersive liquid-liquid microextraction and hollow fiber liquid-liquid-liquid microextraction for the determination of fentanyl, alfentanil, and sufentanil in water and biological fluids by high-performance liquid chromatography. Anal. Bioanal. Chem. 2011, 400, 2149–2158. [Google Scholar] [CrossRef]
- Shamsipur, M.; Fattahi, N. Extraction and determination of opium alkaloids in urine samples using dispersive liquid-liquid microextraction followed by high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2978–2983. [Google Scholar] [CrossRef]
- Ahmadi-Jouibari, T.; Fattahi, N.; Shamsipur, M. Rapid extraction and determination of amphetamines in human urine samples using dispersive liquid-liquid microextraction and solidification of floating organic drop followed by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2014, 94, 145–151. [Google Scholar] [CrossRef]
- Mercieca, G.; Odoardi, S.; Cassar, M.; Strano Rossi, S. Rapid and simple procedure for the determination of cathinones, amphetamine-like stimulants and other new psychoactive substances in blood and urine by GC–MS. J. Pharm. Biomed. Anal. 2018, 149, 494–501. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2019: Trends and Developments; Publications Office of the European Union: Luxembourg, 2019; Available online: https://www.narkotik.pol.tr/kurumlar/narkotik.pol.tr/TUB%C4%B0M/Uluslar-Arasi-Yayinlar/EUROPEAN-DRUG-REPORT-2021-INGILIZCE.pdf (accessed on 17 July 2025).
- International Conference on Harmonization of Technical Requirement for Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedure: Text and Methodology Q2(R1); European Medicines Agency: Geneva, Switzerland. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r1-validation-analytical-procedures-text-and-methodology-step-5-first-version_en.pdf (accessed on 14 September 2023).
- Fraser, A.D. A 6-year experience with urine drug testing by family service agencies in Nova Scotia, Canada. Forensic Sci. Int. 2001, 121, 151–156. [Google Scholar] [CrossRef]


| Concentration in ng/mL. | Bromazepam | Clonazepam | ||||
|---|---|---|---|---|---|---|
| % Mean Recovery | Standard Deviation | % RSD | % Mean Recovery | Standard Deviation | % RSD | |
| of the Calculated Result (n = 3) | of the Calculated Result (n = 3) | |||||
| 2.0 | 118.188 | 3.968 | 3.372 | 111.044 | 7.413 | 6.676 |
| 6.0 | 100.870 | 3.367 | 3.338 | 104.750 | 5.501 | 5.252 |
| 100.0 | 112.253 | 1.484 | 1.322 | 94.961 | 1.351 | 1.422 |
| 200.0 | 107.795 | 3.692 | 3.425 | 93.734 | 1.277 | 1.363 |
| Alprazolam | Flunitrazepam | |||||
| 2.0 | 98.156 | 4.116 | 4.193 | 100.249 | 9.482 | 9.459 |
| 6.0 | 96.520 | 3.422 | 3.545 | 107.440 | 7.015 | 6.529 |
| 100.0 | 105.240 | 1.459 | 1.387 | 82.356 | 5.621 | 6.825 |
| 200.0 | 103.905 | 2.494 | 2.400 | 86.120 | 0.707 | 0.821 |
| Diazepam | ||||||
| 2.0 | 104.655 | 7.914 | 7.562 | |||
| 6.0 | 98.591 | 6.189 | 6.277 | |||
| 100.0 | 105.282 | 2.427 | 2.305 | |||
| 200.0 | 105.407 | 2.266 | 2.149 | |||
| Name of Analyte. | Concentration in ng/mL | Repeatability | Intermediate Precision |
|---|---|---|---|
| % RSD of Peak Area Ratio | |||
| Bromazepam | 100.0 | 0.82 | 1.85 |
| Clonazepam | 1.34 | 3.05 | |
| Alprazolam | 1.00 | 2.38 | |
| Flunitrazepam | 0.95 | 2.43 | |
| Diazepam | 0.82 | 1.21 | |
| Serial No. | Sample No. | Bromazepam | Clonazepam | Alprazolam | Flunitrazepam | Diazepam |
|---|---|---|---|---|---|---|
| (Average Concentration in Triplicate (ng/mL)) | ||||||
| 1 | sam46 | 5.4748 | 1.2251 | 6.0697 | - | 2.1709 |
| 2 | sam47 | 5.4886 | 2.2427 | 3.9326 | - | 3.9460 |
| 3 | sam48 | 4.9436 | 0.4650 | 4.5253 | - | 2.2870 |
| 4 | sam49 | 7.2516 | - | 5.7366 | - | 2.5102 |
| 5 | sam50 | 5.0215 | 0.6228 | 5.3686 | - | 2.0624 |
| 6 | sam 51 | 5.2696 | 0.6858 | 5.2520 | - | 3.3663 |
| 7 | sam52 | 5.0583 | 0.8532 | 4.5659 | - | 2.8131 |
| 8 | sam53 | 5.3205 | 1.0782 | 12.5619 | 2.2799 | 2.7165 |
| 9 | sam54 | 5.0251 | 1.1383 | 4.7316 | - | 2.4005 |
| 10 | sam55 | - | - | - | - | - |
| 11 | sam56 | 5.0142 | 1.2450 | 4.4569 | 3.6955 | 6.3266 |
| 12 | sam57 | 5.0805 | 0.5725 | 5.1489 | - | 4.9194 |
| 13 | sam58 | 5.3646 | 0.9026 | 5.5856 | - | 1.9269 |
| 14 | sam59 | 9.7299 | 2.6409 | 4.5594 | - | 1.4427 |
| 15 | Sam60 | 5.0183 | 1.0396 | 5.3341 | - | 1.9927 |
| 16 | sam61 | 5.1545 | 0.9852 | 5.5671 | - | 1.9981 |
| 17 | sam62 | 6.2667 | 0.9860 | 4.9319 | - | 3.4057 |
| 18 | sam63 | 6.1029 | 1.2010 | 6.2537 | - | 2.4384 |
| 19 | sam64 | 4.9545 | 9.1650 | 4.6334 | 5.4524 | 13.6228 |
| 20 | sam65 | 6.0903 | 10.0788 | 5.9130 | 6.0009 | 12.7135 |
| 21 | sam66 | 5.2878 | 0.5787 | 4.9598 | - | 9.8523 |
| 22 | sam67 | 6.7173 | 0.9566 | 4.5280 | - | 2.2346 |
| 23 | sam68 | - | - | - | - | - |
| 24 | sam 69 | 5.7848 | 1.2066 | 6.0432 | - | 12.3481 |
| 25 | Sam 70 | 5.1985 | 1.9877 | 6.4398 | 3.0140 | - |
| 26 | Sam71 | 4.9441 | -- | 5.2073 | - | 14.0141 |
| 27 | sam 72 | 6.9293 | 0.7251 | 5.9935 | 2.9429 | 3.2799 |
| 28 | sam 73 | 6.0371 | 1.9585 | 2.2617 | - | 5.8814 |
| 29 | sam74 | 4.9269 | 0.5910 | 4.4989 | - | 5.2207 |
| 30 | sam75 | 5.1447 | 1.2810 | 2.5473 | - | 7.7373 |
| 31 | sam 76 | 5.1885 | 0.6395 | 4.3771 | - | 8.4144 |
| 32 | sam77 | 5.2606 | 1.2079 | 233.7426 | - | 3.1451 |
| 34 | sam 78 | 5.0248 | 0.5873 | 4.7209 | 2.5989 | 2.3015 |
| 35 | sam79 | 5.1484 | 0.5479 | 5.2195 | - | 1.9525 |
| 36 | sam 80 | 5.2637 | 0.8489 | 5.0545 | - | 2.0228 |
| 37 | sam 81 | 5.1437 | 0.7519 | 6.1830 | - | 2.1318 |
| 38 | sam82 | 4.9497 | 0.7275 | 99.7967 | - | 2.4242 |
| 39 | sam 83 | 4.9711 | 0.6097 | 5.9817 | - | 2.2114 |
| 40 | sam 84 | 5.1864 | 0.5213 | 6.2792 | - | 2.1758 |
| 41 | sam85 | 4.9337 | 1.1259 | 2.2515 | - | 2.7399 |
| 42 | sam86 | 5.2481 | 0.8119 | 1.8735 | - | 2.7906 |
| 43 | sam 87 | - | - | - | - | - |
| 44 | Sam88 | 5.0336 | 8.3261 | 4.6716 | - | 4.0663 |
| 45 | sam 89 | 8.2721 | 2.2338 | 4.7494 | - | 11.3568 |
| 46 | sam 90 | 5.0125 | - | 5.0546 | - | 10.4125 |
| 47 | sam 91 | 5.0383 | 0.7838 | 4.5123 | - | 5.6242 |
| 48 | sam 92 | 4.9101 | 0.7944 | 178.9956 | - | 2.4911 |
| Time in Minutes | % Composition of B |
|---|---|
| 0.01 | 30 |
| 1.00 | 30 |
| 10.00 | 100 |
| 12.00 | 100 |
| 12.10 | 30 |
| 15.00 | 30 |
| Compound | Retention Time (minutes) | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (CE) |
|---|---|---|---|---|
| Bromazepam | 6.172 | 317.95 | 209.05 | −28.0 |
| 182.05 | −33.0 | |||
| Clonazepam-D4 | 7.520 | 320.25 | 274.20 | −26.0 |
| 218.10 | −39.0 | |||
| Clonazepam | 7.558 | 316.05 | 270.10 | −25.0 |
| 214.00 | −37.0 | |||
| Alprazolam | 7.818 | 309.10 | 281.15 | −26.0 |
| 205.15 | −45.0 | |||
| Flunitrazepam | 8.120 | 314.10 | 268.10 | −25.0 |
| 239.15 | −36.0 | |||
| Diazepam | 9.247 | 285.10 | 193.15 | −30.0 |
| 154.10 | −27.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, H.; Gandhi, V.; Akil, L.; Al-Tannak, N.F.; Rattray, N.J.W.; Khadra, I. Development and Validation of an LC-MS/MS Method for the Simultaneous Determination of Alprazolam, Bromazepam, Clonazepam, Diazepam and Flunitrazpam in Human Urine and Its Application to Samples from Suspected Drug Abusers. Molecules 2025, 30, 3451. https://doi.org/10.3390/molecules30173451
Kamal H, Gandhi V, Akil L, Al-Tannak NF, Rattray NJW, Khadra I. Development and Validation of an LC-MS/MS Method for the Simultaneous Determination of Alprazolam, Bromazepam, Clonazepam, Diazepam and Flunitrazpam in Human Urine and Its Application to Samples from Suspected Drug Abusers. Molecules. 2025; 30(17):3451. https://doi.org/10.3390/molecules30173451
Chicago/Turabian StyleKamal, Husein, Varun Gandhi, Lina Akil, Naser F. Al-Tannak, Nicholas J. W. Rattray, and Ibrahim Khadra. 2025. "Development and Validation of an LC-MS/MS Method for the Simultaneous Determination of Alprazolam, Bromazepam, Clonazepam, Diazepam and Flunitrazpam in Human Urine and Its Application to Samples from Suspected Drug Abusers" Molecules 30, no. 17: 3451. https://doi.org/10.3390/molecules30173451
APA StyleKamal, H., Gandhi, V., Akil, L., Al-Tannak, N. F., Rattray, N. J. W., & Khadra, I. (2025). Development and Validation of an LC-MS/MS Method for the Simultaneous Determination of Alprazolam, Bromazepam, Clonazepam, Diazepam and Flunitrazpam in Human Urine and Its Application to Samples from Suspected Drug Abusers. Molecules, 30(17), 3451. https://doi.org/10.3390/molecules30173451








