Design, Synthesis and Herbicidal Activity of 5-(1-Amino-4-phenoxybutylidene)barbituric Acid Derivatives Containing an Enamino Diketone Motif
Abstract
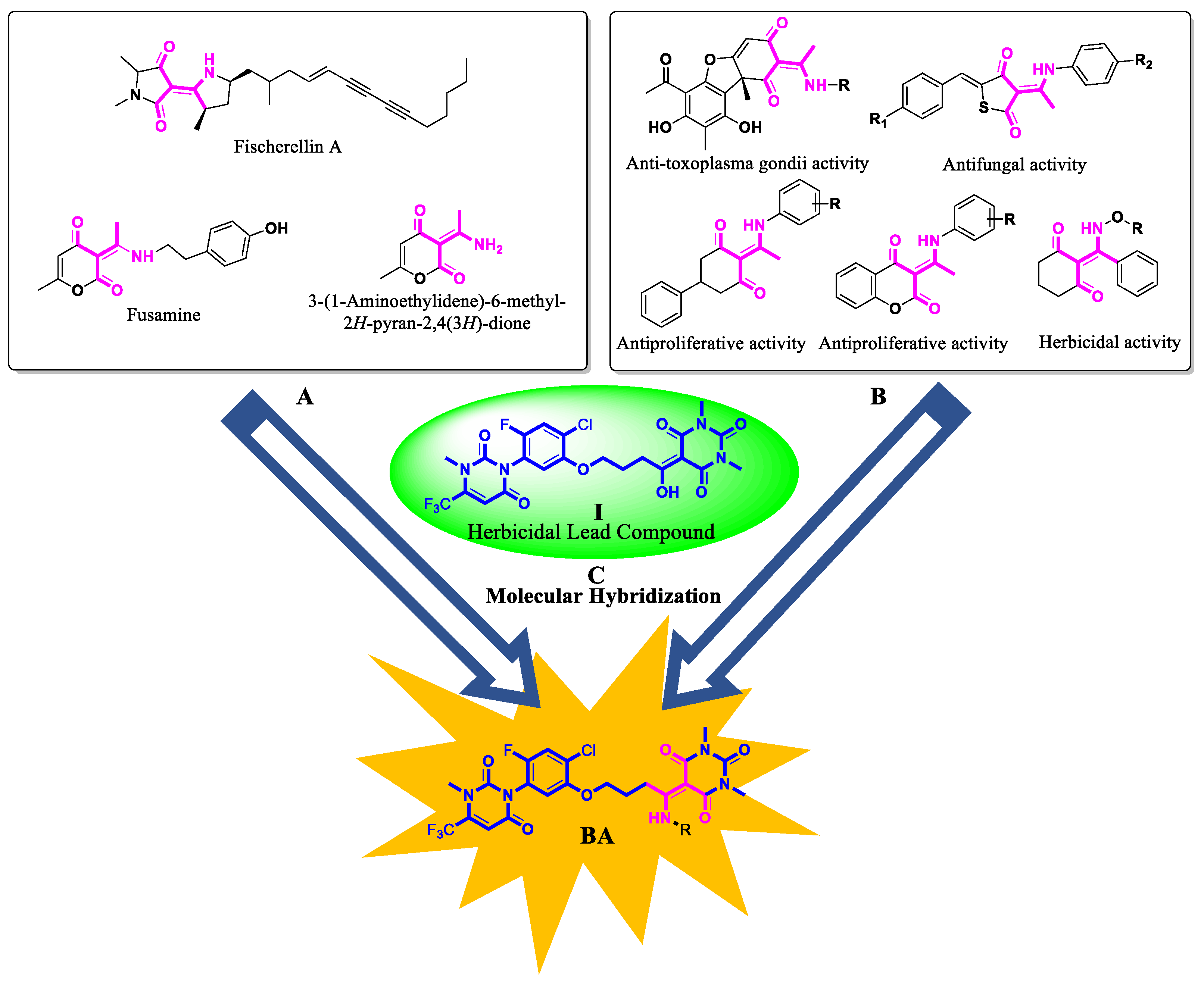
1. Introduction
2. Results and Discussion
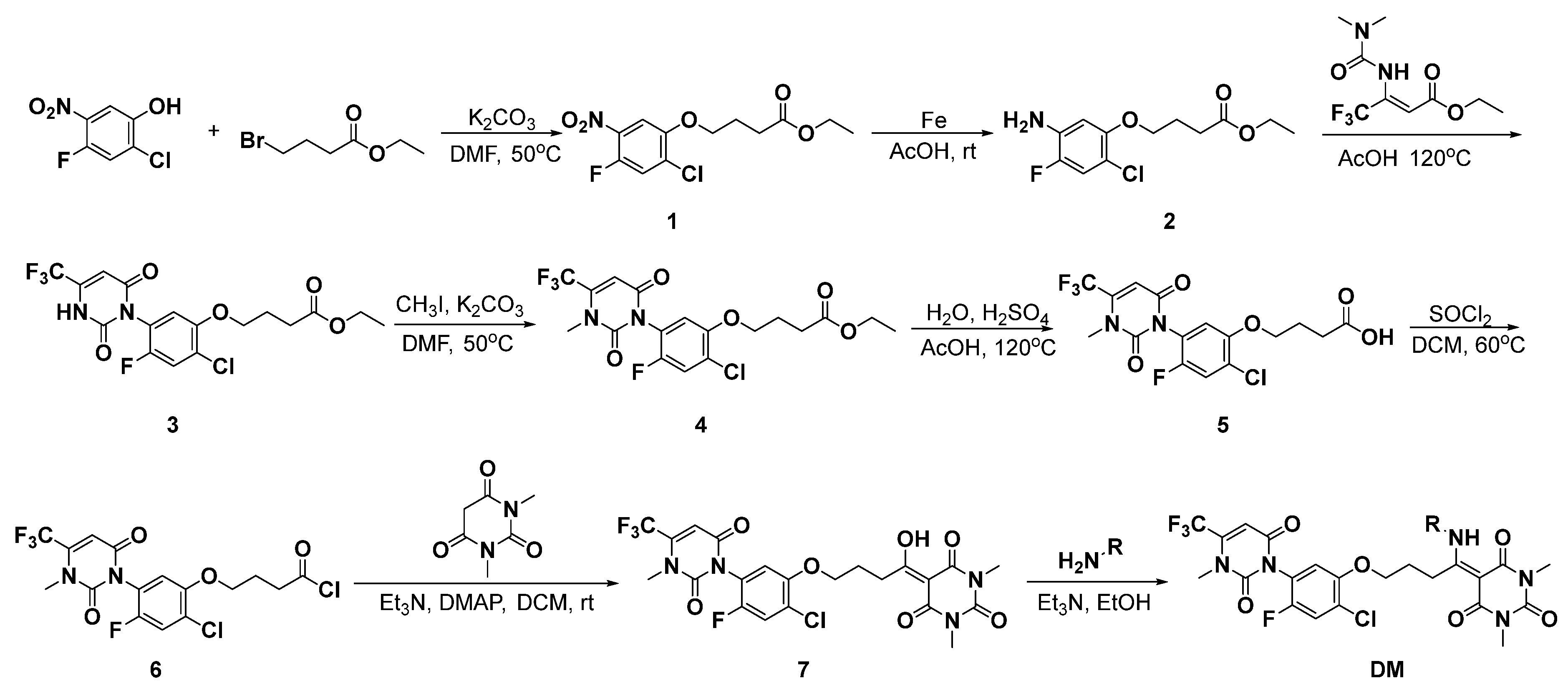
2.1. Chemistry
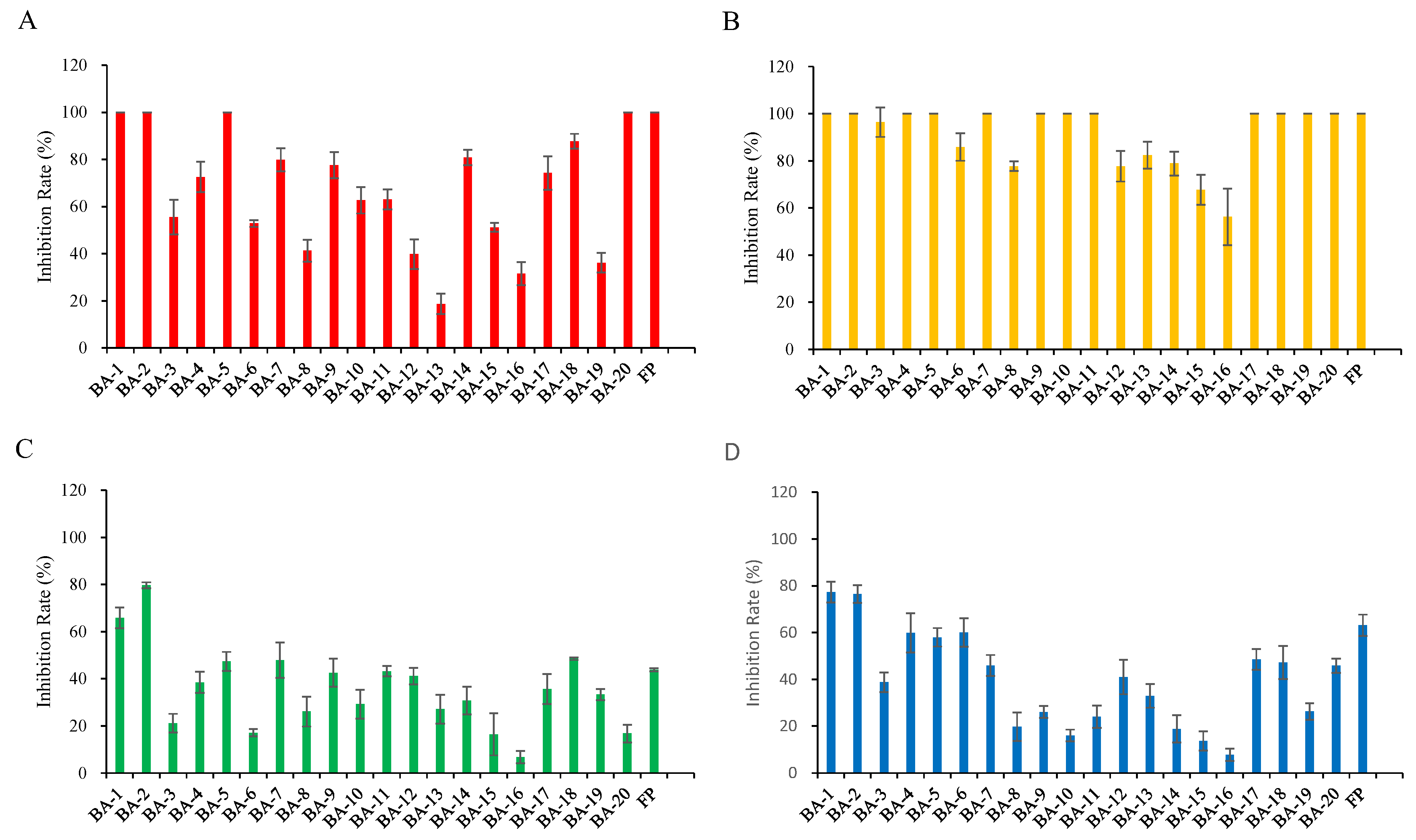
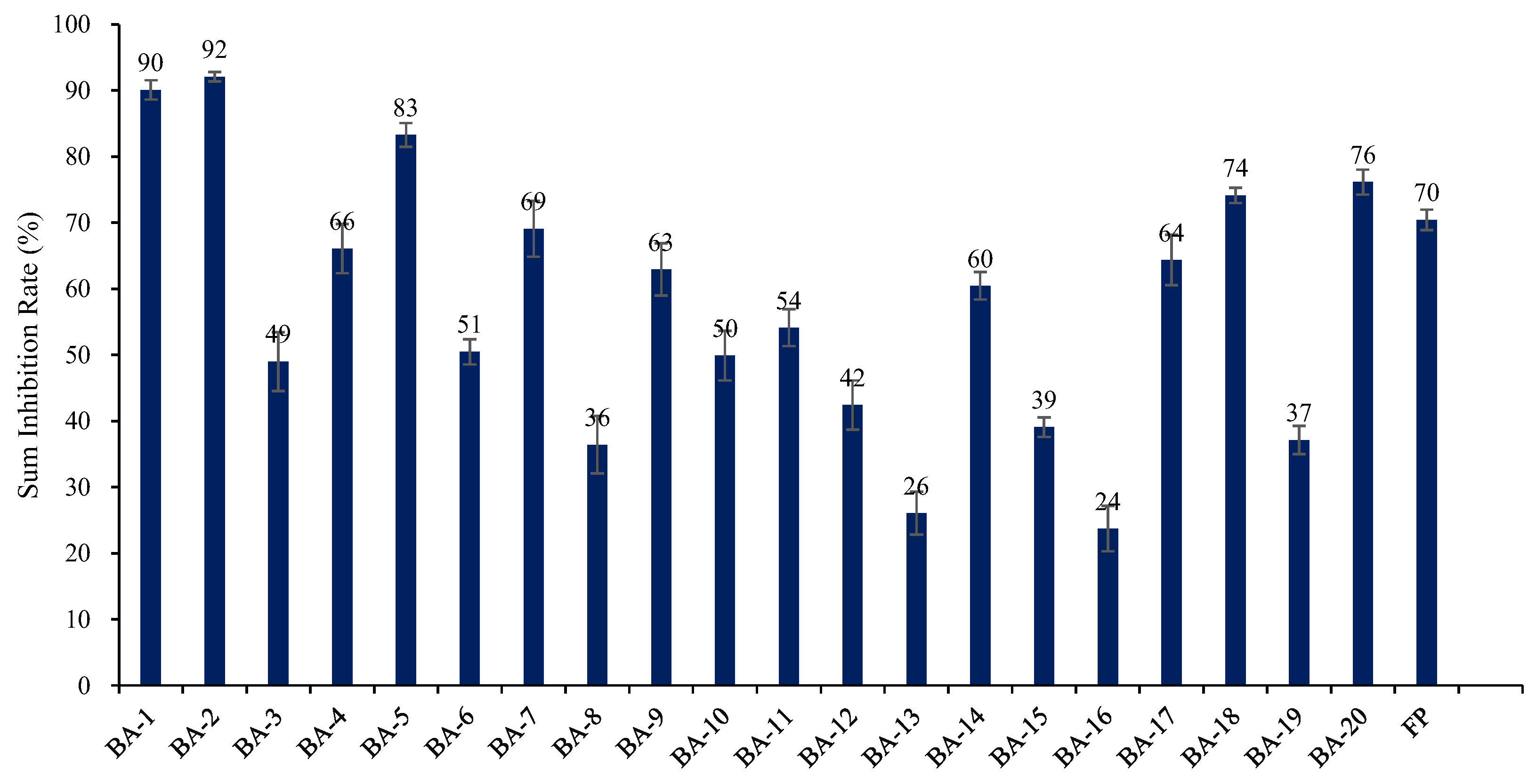
2.2. Herbicidal Activity and SAR
2.3. Herbicidal Spectrum and Crop Safety of Target Compound BA-1
2.4. Molecular Simulation Analysis of BA-1
3. Materials and Methods
3.1. Chemical Synthesis Procedures
3.1.1. Synthesis of Intermediates 1–7
3.1.2. General Procedure for the Synthesis of Target Compounds BA-1 to BA-20
3.2. Evaluation of Herbicidal Activity
3.3. Crop Safety
3.4. Molecular Simulation Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Yang, F.; Jia, W.; Jiang, Y.; Wu, X.; Song, S.; Shen, H.; Shen, J. Nanomaterials and nanotechnology in agricultural pesticide delivery: A review. Langmuir 2024, 40, 18806–18820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lv, F.; Yang, J. Pesticides toxicity, removal and detoxification in plants: A Review. Agronomy 2024, 14, 1260. [Google Scholar] [CrossRef]
- Giugliano, R.; Armenio, V.; Savio, V.; Vaccaro, E.; Ciccotelli, V.; Vivaldi, B. Monitoring of non-maximum-residue-level pesticides in animal feed: A study from 2019 to 2023. Toxics 2024, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31–34. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Yang, R.; Lv, M.; Xu, H. Synthesis of piperine analogs containing isoxazoline/pyrazoline scaffold and their pesticidal bioactivities. J. Agric. Food Chem. 2018, 66, 11254–11264. [Google Scholar] [CrossRef]
- Fang, J.P.; He, Z.Z.; Liu, T.T.; Li, J.; Dong, L.Y. A novel mutation Asp-2078-Glu in ACCase confers resistance to ACCase herbicides in barnyardgrass (Echinochloa crus-galli). Pestic. Biochem. Physiol. 2020, 168, 104634. [Google Scholar] [CrossRef]
- Wang, J.Z.; Peng, Y.J.; Chen, W.; Yu, Q.; Bai, L.Y.; Pan, L. The Ile-2041-Val mutation in the ACCase gene confers resistance to clodinafop-propargyl in American sloughgrass (Beckmannia syzigachne Steud). Pest Manag. Sci. 2021, 77, 2425–2432. [Google Scholar] [CrossRef]
- Darmency, H.; Colbach, N.; Corre, V.L. Relationship between weed dormancy and herbicide rotations: Implications in resistance evolution. Pest Manag. Sci. 2017, 73, 1994–1999. [Google Scholar] [CrossRef]
- Chio, E.H.; Li, Q.X. Pesticide research and development: General discussion and Spinosad case. J. Agric. Food Chem. 2022, 70, 8913–8919. [Google Scholar] [CrossRef]
- Han, S.B.; Wang, S.M.; Fu, S.Y.; Chen, K.; Gao, G.; Cheng, Y.N.; Liu, M.; Zhang, X.M.; Lei, K. Design, synthesis, and herbicidal activity of novel 5-acylbarbituric acid derivatives containing maleimide moieties and evaluation of their mode of action. J. Agric. Food Chem. 2025, 73, 11386–11398. [Google Scholar] [CrossRef]
- Sun, S.S.; Kou, S.; Wang, W.F.; Li, Y.Z.; Wang, Z.R.; Huo, J.Q.; An, Z.X.; Zhu, L.; Chen, L.; Zhang, J.L. Synthesis of novel propionamide-methylpyrazole carboxylates as herbicidal candidates. J. Agric. Food Chem. 2024, 72, 21401–21409. [Google Scholar] [CrossRef]
- Feng, T.; Liu, Q.; Xu, Z.Y.; Li, H.T.; Wei, W.; Shi, R.C.; Zhang, L.; Cao, Y.M.; Liu, S.Z. Design, synthesis, herbicidal activity, and structure-activity relationship study of novel 6-(5-aryl-substituted-1-pyrazolyl)-2-picolinic acid as potential herbicides. Molecules 2023, 28, 1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Zhang, R.B.; Yu, S.Y.; Liang, L.; Ismail, I.; Li, Y.H.; Xu, H.; Wen, X.; Xi, Z. Discovery of novel N-isoxazolinylphenyltriazinones as promising protoporphyrinogen IX oxidase inhibitors. J. Agric. Food Chem. 2019, 67, 12382–12392. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.M.; Wolk, C.P.; Jüttner, F. Fischerellin, a new allelochemical from the freshwater cyanobacterium Fischerella musicola. J. Phycol. 1991, 27, 686–692. [Google Scholar] [CrossRef]
- Ding, L.; Dahse, H.-M.; Hertweck, C. Cytotoxic alkaloids from Fusarium incarnatum associated with the mangrove tree Aegiceras corniculatum. J. Nat. Prod. 2012, 75, 617–621. [Google Scholar] [CrossRef]
- Avdović, E.H.; Stojković, D.L.; Jevtić, V.V.; Milenković, D.; Marković, Z.S.; Vuković, N.; Potočňák, I.; Radojević, I.D.; Čomić, L.R.; Trifunović, S.R. Preparation and antimicrobial activity of a new palladium(II) complexes with a coumarin-derived ligands. crystal structures of the 3-(1-(o-toluidino)ethylidene)-chroman-2,4-dione and 3-(1-(m-toluidino) ethylidene)-chroman-2,4-dione. Inorg. Chim. Acta 2019, 484, 52–59. [Google Scholar] [CrossRef]
- Mladenović, M.; Vuković, N.; Nićiforović, N.; Sukdolak, S.; Solujić, S. Synthesis and molecular descriptor characterization of novel 4-hydroxy-chromene-2-one derivatives as antimicrobial agents. Molecules 2009, 14, 1495–1512. [Google Scholar] [CrossRef]
- Baldwin, A.G.; Bevan, J.; Brough, D.; Ledder, R.; Freeman, S. Synthesis and antibacterial activities of enamine derivatives of dehydroacetic acid. Med. Chem. Res. 2018, 27, 884–889. [Google Scholar] [CrossRef]
- Garmaise, D.L.; Chu, D.T.W.; Bernstein, E.; Inaba, M. Synthesis and antibacterial activity of 2’-substituted chelocardin analogues. J. Med. Chem. 1979, 22, 559–564. [Google Scholar] [CrossRef]
- Bangalore, P.K.; Vagolu, S.K.; Bollikanda, R.K.; Veeragoni, D.K.; Choudante, P.C.; Misra, S.; Sriram, D.; Sridhar, B.; Kantevari, S. Usnic acid enaminone-coupled 1,2,3-triazoles as antibacterial and antitubercular agents. J. Nat. Prod. 2020, 83, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Canela, M.D.; Pérez-Pérez, M.J.; Noppen, S.; Sáez-Calvo, G.; Díaz, J.F.; Camarasa, M.J.; Liekens, S.; Priego, E.M. Novel colchicine-site binders with a cyclohexanedione scaffold identified through a ligand-based virtual screening approach. J. Med. Chem. 2014, 57, 3924–3938. [Google Scholar] [CrossRef] [PubMed]
- Ilić, D.R.; Jevtić, V.V.; Radić, G.P.; Arsikin, K.; Ristić, B.; Harhaji-Trajković, L.; Vuković, N.; Sukdolak, S.; Klisurić, O.; Trajković, V.; et al. Synthesis, characterization and cytotoxicity of a new palladium(II) complex with a coumarine-derived ligand. Eur. J. Med. Chem. 2014, 74, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Budzisz, E.; Keppler, B.K.; Giester, G.; Wozniczka, M.; Kufelnicki, A.; Nawrot, B. Synthesis, crystal structure and biological characterization of a novel Palladium(II) complex with a coumarin-derived ligand. Eur. J. Inorg. Chem. 2004, 22, 4412–4419. [Google Scholar] [CrossRef]
- Budzisz, E.; Małecka, M.; Lorenz, I.P.; Mayer, P.; Kwiecien, R.A.; Paneth, P.; Krajewska, U.; Rozalski, M. Synthesis, cytotoxic effect, and structure–activity relationship of Pd(II) complexes with coumarin derivatives. Inorg. Chem. 2006, 45, 9688–9695. [Google Scholar] [CrossRef]
- Budzisz, E.; Brzezinska, E.; Krajewska, U.; Rozalski, M. Cytotoxic effects, alkylating properties and molecular modelling of coumarin derivatives and their phosphonic analogues. Eur. J. Med. Chem. 2003, 38, 597–603. [Google Scholar] [CrossRef]
- Dias, L.C.; Demuner, A.J.; Valente, V.V.M.; Barbosa, L.C.A.; Martins, F.T.; Doriguetto, A.C.; Ellena, J. Preparation of achiral and chiral (E)-enaminopyran-2,4-diones and their phytotoxic activity. J. Agric. Food Chem. 2009, 57, 1399–1405. [Google Scholar] [CrossRef]
- Serban, A.; Watson, K.G.; Bird, G.J.; Farquharson, G.J.; Cross, L.E. Preparation of 2-[1-(Ethoxyimino)Propyl]-5-Indanylcyclohexene-1,3-Diones and Analogs as Herbicides and Plant Growth Regulators. US 4952722A, 28 August 1990. [Google Scholar]
- Liu, Y.X.; Zhao, H.P.; Wang, Z.W.; ·Li, Y.H.; Song, H.B.; Riches, H.; Beattie, D.; Gu, Y.C.; Wang, Q.M. The discovery of 3-(1-aminoethylidene)quinoline-2,4(1H,3H)-dione derivatives as novel PSII electron transport inhibitors. Mol. Divers. 2013, 17, 701–710. [Google Scholar] [CrossRef]
- Batran, R.Z.; Khedr, M.A.; Abdel Latif, N.A.; Abd El Aty, A.A.; Shehata, A.N. Synthesis, homology modeling, molecular docking, dynamics, and antifungal screening of new 4-hydroxycoumarin derivatives as potential chitinase inhibitors. J. Mol. Struct. 2019, 1180, 260–271. [Google Scholar] [CrossRef]
- Lv, P.; Chen, Y.L.; Zhao, Z.; Shi, T.Z.; Wu, X.W.; Xue, J.Y.; Li, Q.X.; Hua, R.M. Design, synthesis, and antifungal activities of 3-acyl thiotetronic acid derivatives: New fatty acid synthase inhibitors. J. Agric. Food Chem. 2018, 66, 1023–1032. [Google Scholar] [CrossRef]
- Neumann, D.M.; Cammarata, A.; Backes, G.; Palmer, G.E.; Jursic, B.S. Synthesis and antifungal activity of substituted 2,4,6-pyrimidinetrione carbaldehyde hydrazones. Bioorg. Med. Chem. 2014, 22, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Jin, C.M.; Zhang, H.M.; Jin, C.-M.; Shen, Q.K.; Quan, Z.S. Synthesis and biological evaluation of (+)-usnic acid derivatives as potential anti-toxoplasma gondii agents. J. Agric. Food Chem. 2019, 67, 9630–9642. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chen, K.; Li, N.; Wang, X.K.; Wang, S.B.; Li, P.; Hua, X.W.; Lei, K.; Ji, L.S. Discovery of 3-(1-amino-2-phenoxyethylidene)-6-methyl-2H-pyran-2,4(3H)-dione derivatives as novel herbicidal leads. Agronomy 2022, 12, 202. [Google Scholar] [CrossRef]
- Wang, C.C.; Liu, H.; Zhao, W.; Li, P.; Ji, L.S.; Liu, R.M.; Lei, K.; Xu, X.H. Synthesis and herbicidal activity of 5-(1-amino-2-phenoxyethylidene)barbituric acid derivatives. Chin. J. Org. Chem. 2021, 41, 2063–2073. [Google Scholar] [CrossRef]
- Hwang, J.-I.; Norsworthy, J.K.; Gonzalez-Torralva, F.; Piveta, L.B.; Tom Barber, L.; Butts, T.R. Cross-resistance of Barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] to aryloxyphenoxypropionate herbicides. Pest. Biochem. Physiol. 2022, 184, 105089. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Tanchuk, V.Y.; Tanin, V.O.; Vovk, I.; Poda, G. A new improved hybrid scoring function for molecular docking and scoring based on autodock and autodock vina. Chem. Biol. Drug Des. 2016, 87, 618–625. [Google Scholar] [CrossRef]
- Di, M.E.; Toti, D.; Polticelli, F. Dockingapp: A user friendly interface for facilitated docking simulations with autodock vina. J. Comput. Aid. Mol. Des. 2017, 31, 213–218. [Google Scholar]
- Chaudhari, R.; Li, Z. Pymine: A pymol plugin to integrate and visualize data for drug discovery. BMC Res. Notes 2015, 8, 517. [Google Scholar] [CrossRef]
- Lv, Y.; Li, K.; Lei, L.; Yu, Z.W.; Wu, R.Z.; Chen, A.K.; Tian, R.X.; Deng, Y.X.; Tang, L.F.; Fan, Z.J. Design, synthesis, and assessment of fungicidal activity of active substructure 1,2,4-triazole containing coumarin. J. Agric. Food Chem. 2024, 72, 27075–27083. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]




| Comp. | Dosage (g ha−1) | Inhibition Rate (%) | SIR | |||
|---|---|---|---|---|---|---|
| B. campestris | A. tricolor | E. crus-galli | D. sanguinalis | |||
| BA-1 | 75 | 100 | 100 | 57 ± 5 | 62 ± 2 | 87 ± 1 |
| 37.5 | 94 ± 3 | 100 | 23 ± 4 | 16 ± 2 | 72 ± 3 | |
| 18.8 | 84 ± 2 | 100 | 18 ± 4 | 11 ± 4 | 64 ± 2 | |
| BA-2 | 75 | 92 ± 2 | 100 | 62 ± 8 | 44 ± 4 | 78 ± 2 |
| 37.5 | 80 ± 5 | 100 | 51 ± 7 | 0 ± 0 | 66 ± 5 | |
| 18.8 | 58 ± 3 | 100 | 42 ± 5 | 0 ± 2 | 51 ± 4 | |
| BA-5 | 75 | 100 | 100 | 15 ± 1 | 35 ± 2 | 77 ± 1 |
| 37.5 | 79 ± 3 | 100 | 11 ± 5 | 8 ± 6 | 59 ± 1 | |
| 18.8 | 40 ± 7 | 100 | 2 ± 6 | 0 ± 0 | 32 ± 6 | |
| BA-18 | 75 | 71 ± 8 | 100 | 42 ± 6 | 29 ± 10 | 63 ± 4 |
| 37.5 | 70 ± 1 | 100 | 28 ± 9 | 6 ± 1 | 57 ± 3 | |
| 18.8 | 63 ± 1 | 100 | 21 ± 5 | 0 ± 4 | 50 ± 1 | |
| BA-20 | 75 | 74 ± 2 | 100 | 18 ± 13 | 2 ± 2 | 56 ± 3 |
| 37.5 | 61 ± 3 | 72 ± 3 | 7 ± 6 | 0 ± 5 | 45 ± 3 | |
| 18.8 | 54 ± 4 | 56 ± 4 | 0 ± 3 | 0 ± 0 | 39 ± 1 | |
| FP | 75 | 88 ± 4 | 100 | 52 ± 2 | 48 ± 3 | 77 ± 3 |
| 37.5 | 53 ± 4 | 100 | 50 ± 3 | 42 ± 3 | 54 ± 4 | |
| 18.8 | 37 ± 6 | 90 ± 3 | 51 ± 6 | 13 ± 4 | 40 ± 2 | |
| Comp. | Injury (%) | |||
|---|---|---|---|---|
| T. aestivum | Z. mays | G. hirsutum | G. max | |
| BA-1 | 4 ± 2 | 2 ± 2 | 44 ± 2 | 47 ± 3 |
| FP | 5 ± 2 | 3 ± 1 | 33 ± 4 | 14 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wang, S.; Fu, S.; Zhang, Y.; Gao, W.; Liu, J.; Liu, R.; Lei, K. Design, Synthesis and Herbicidal Activity of 5-(1-Amino-4-phenoxybutylidene)barbituric Acid Derivatives Containing an Enamino Diketone Motif. Molecules 2025, 30, 3445. https://doi.org/10.3390/molecules30163445
Chen K, Wang S, Fu S, Zhang Y, Gao W, Liu J, Liu R, Lei K. Design, Synthesis and Herbicidal Activity of 5-(1-Amino-4-phenoxybutylidene)barbituric Acid Derivatives Containing an Enamino Diketone Motif. Molecules. 2025; 30(16):3445. https://doi.org/10.3390/molecules30163445
Chicago/Turabian StyleChen, Ke, Shumin Wang, Shuyue Fu, Yuxiao Zhang, Wei Gao, Jin Liu, Rui Liu, and Kang Lei. 2025. "Design, Synthesis and Herbicidal Activity of 5-(1-Amino-4-phenoxybutylidene)barbituric Acid Derivatives Containing an Enamino Diketone Motif" Molecules 30, no. 16: 3445. https://doi.org/10.3390/molecules30163445
APA StyleChen, K., Wang, S., Fu, S., Zhang, Y., Gao, W., Liu, J., Liu, R., & Lei, K. (2025). Design, Synthesis and Herbicidal Activity of 5-(1-Amino-4-phenoxybutylidene)barbituric Acid Derivatives Containing an Enamino Diketone Motif. Molecules, 30(16), 3445. https://doi.org/10.3390/molecules30163445







