Nickel-Catalyzed Cyanation of Aryl Halides
Abstract
1. Introduction
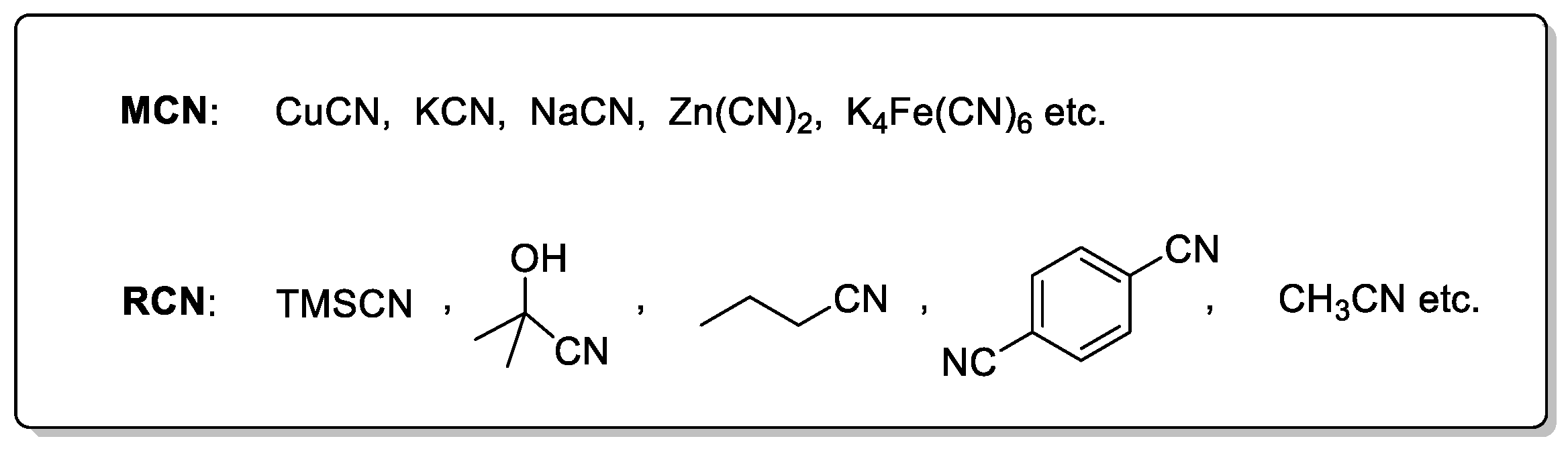
2. Metal Cyanide Sources
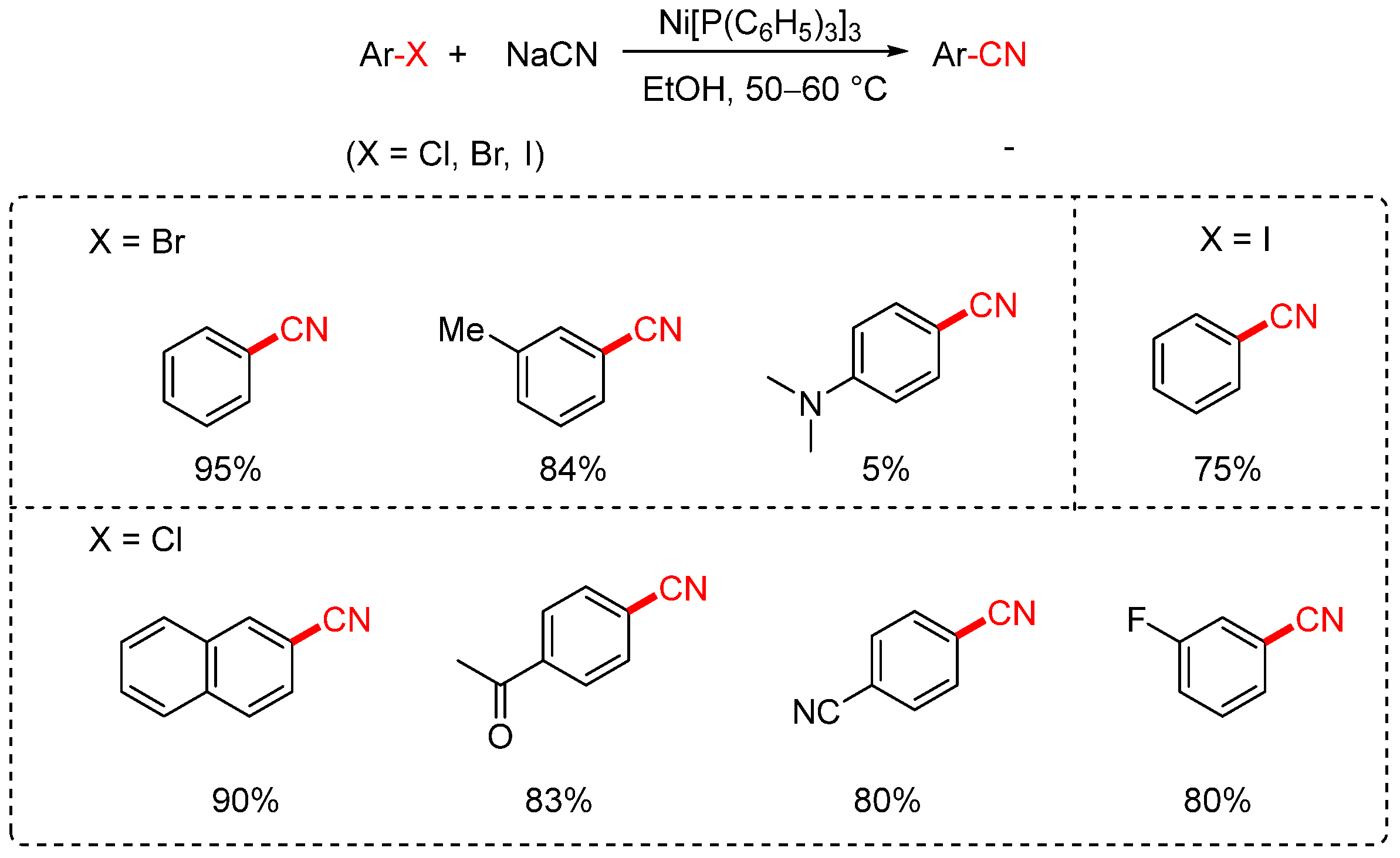
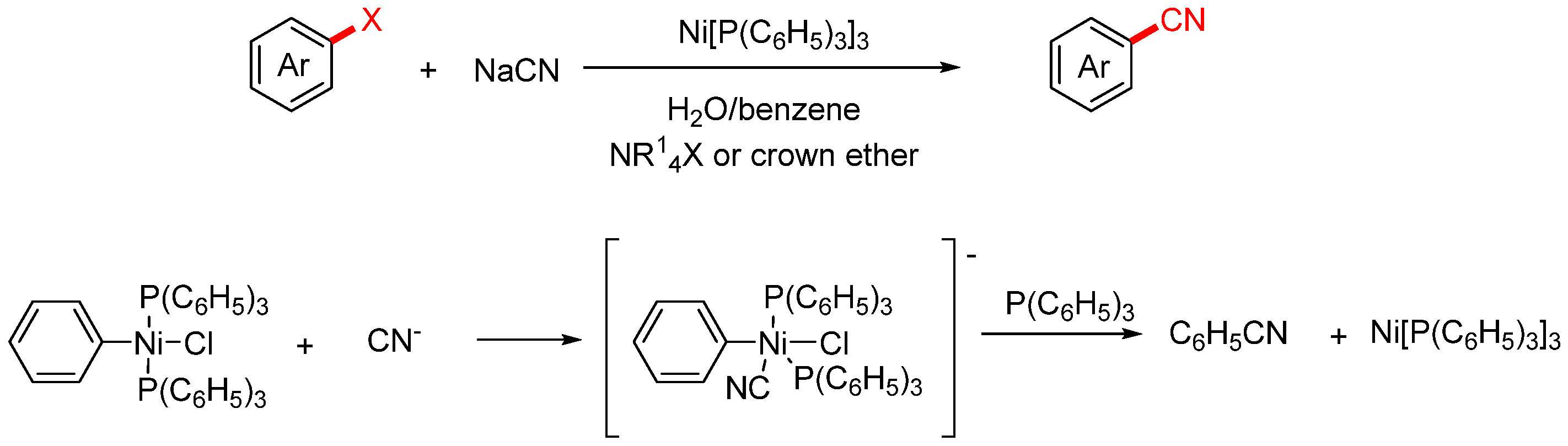
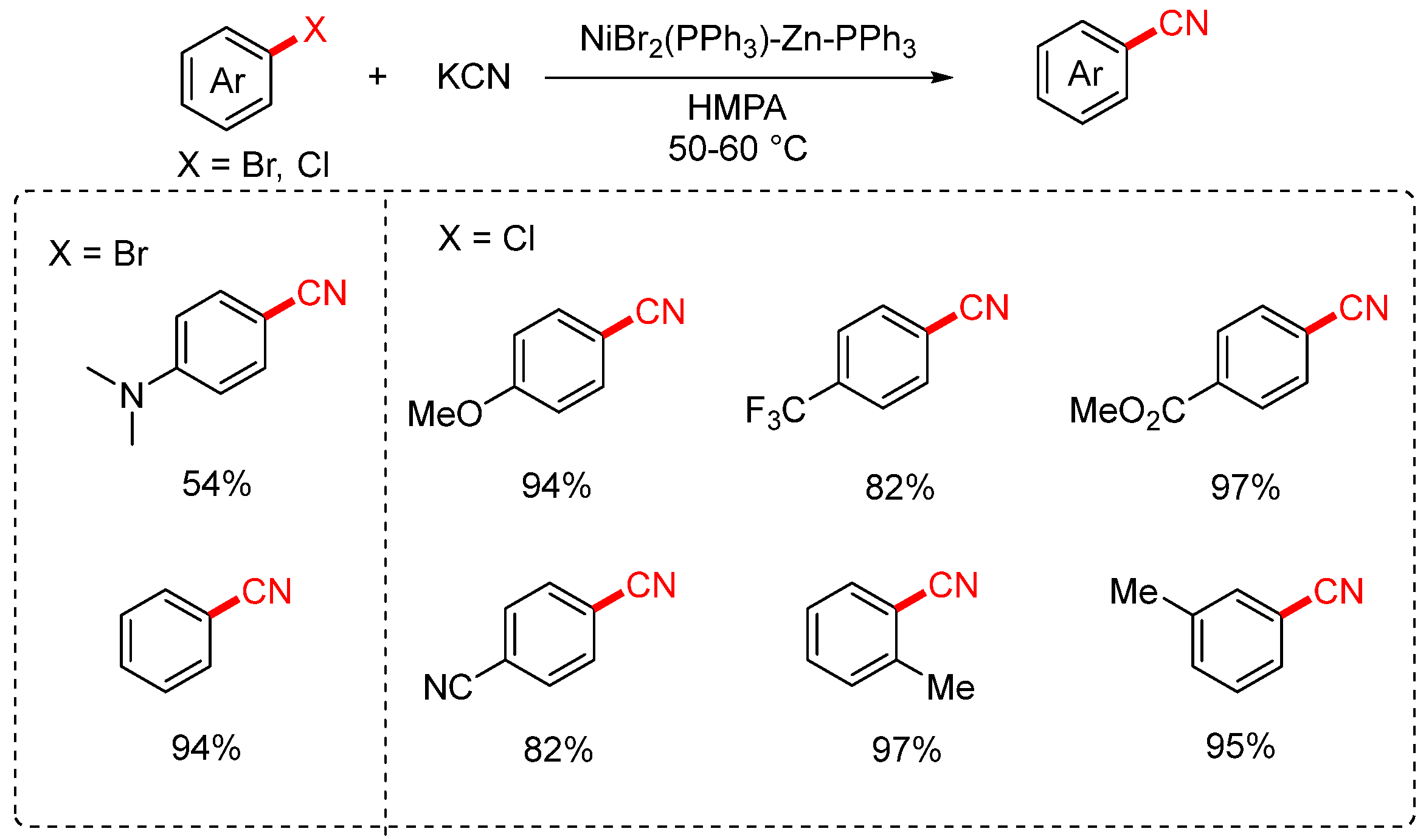
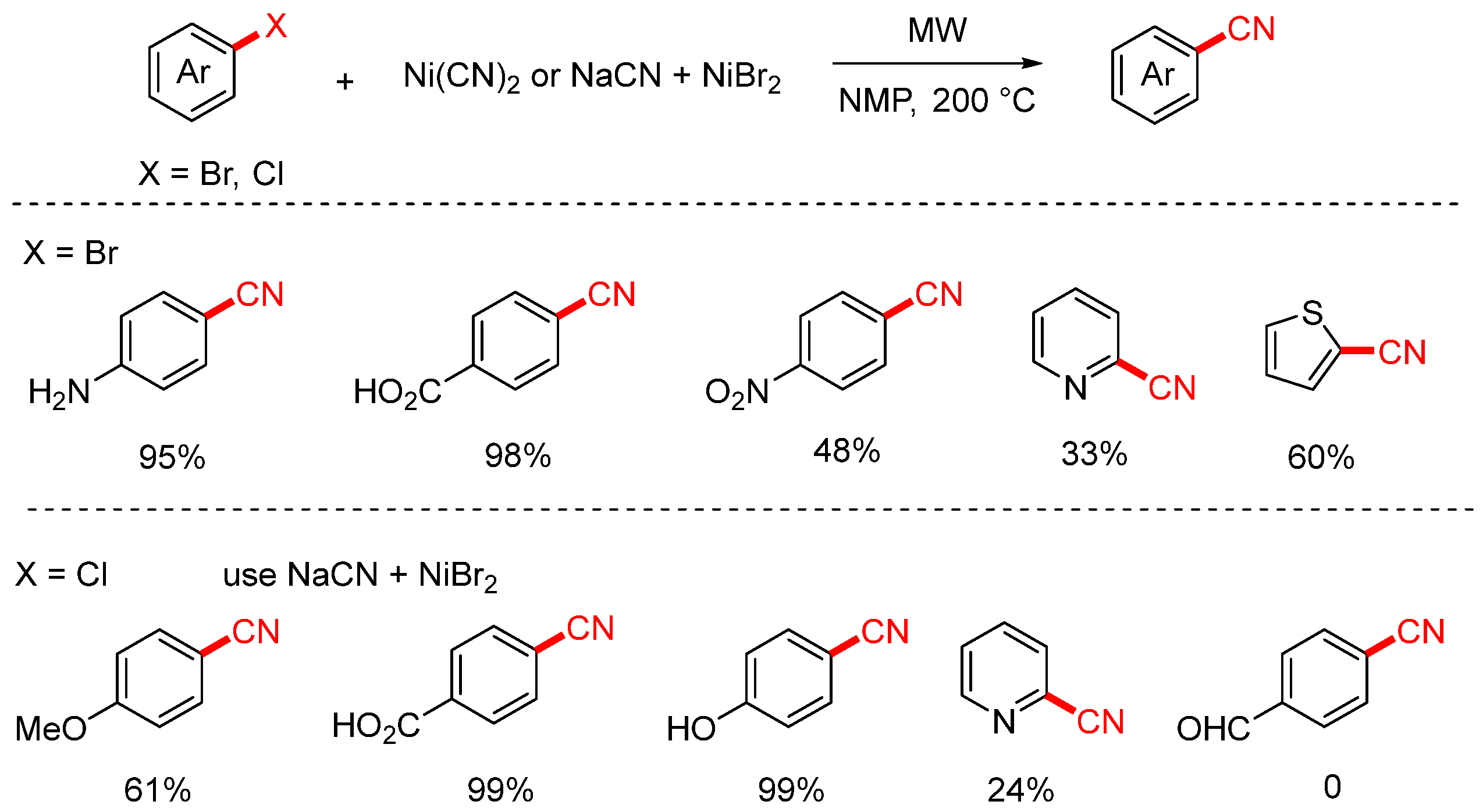
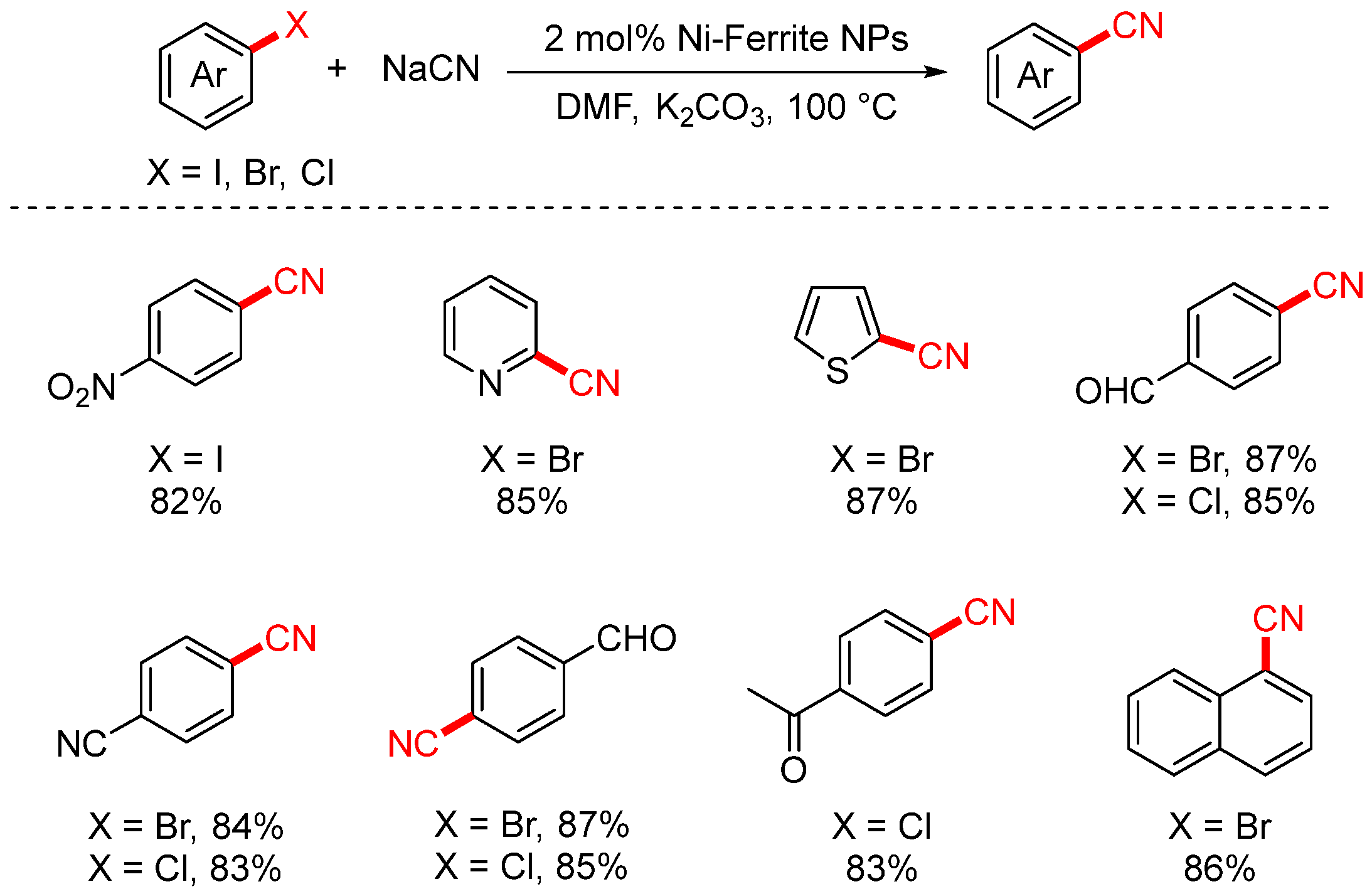
2.1. NaCN and KCN
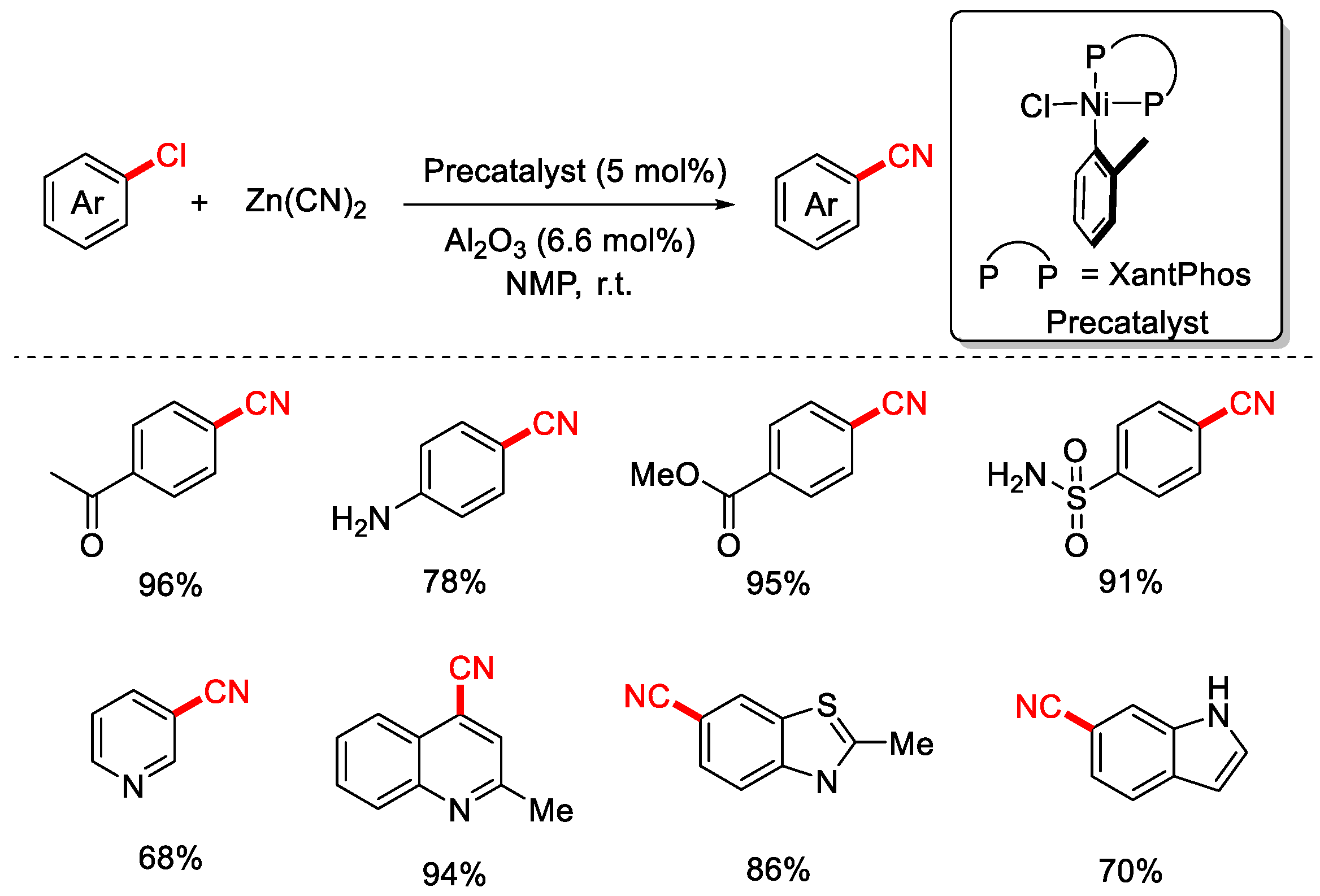
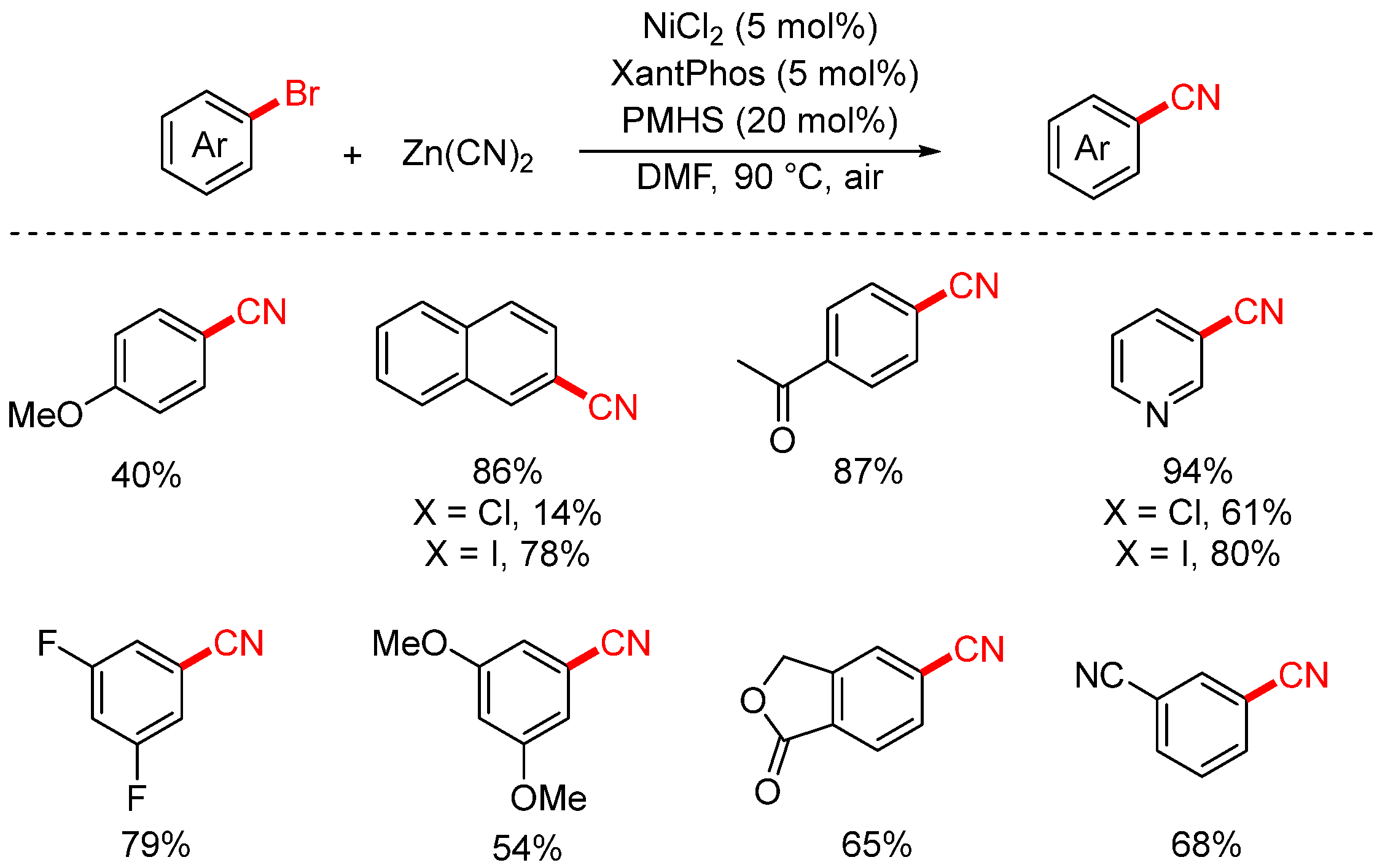
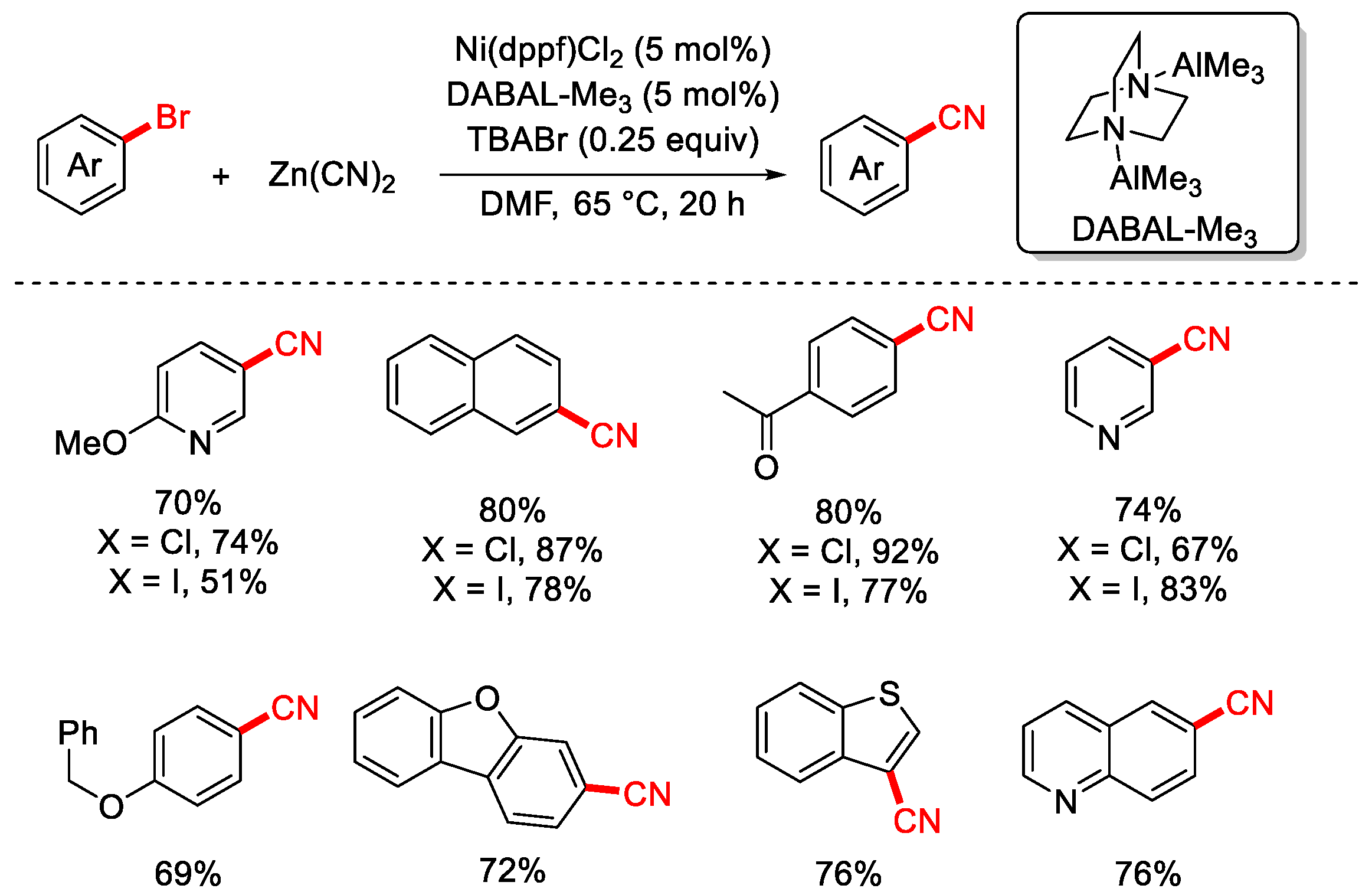
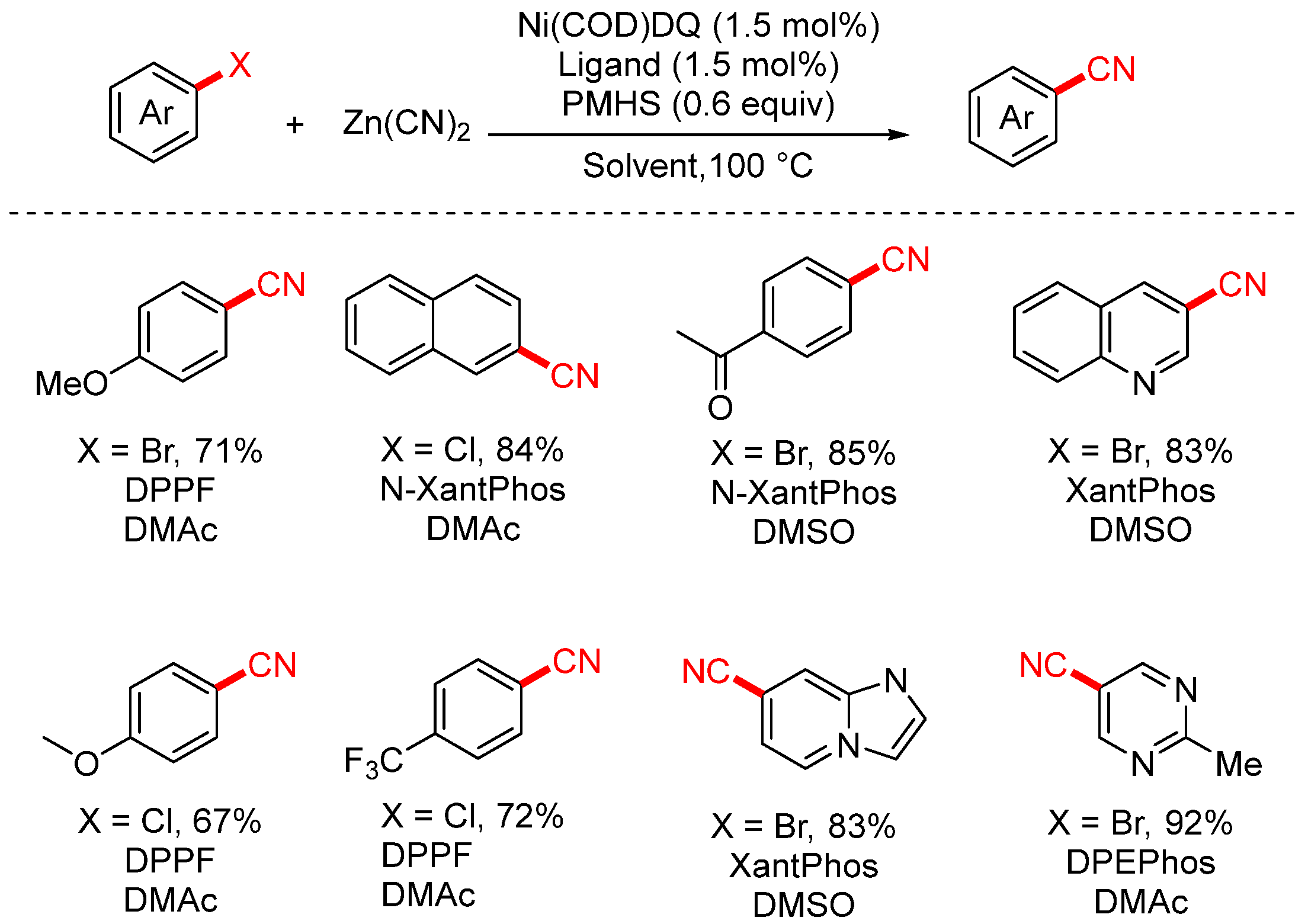
2.2. Zn(CN)2
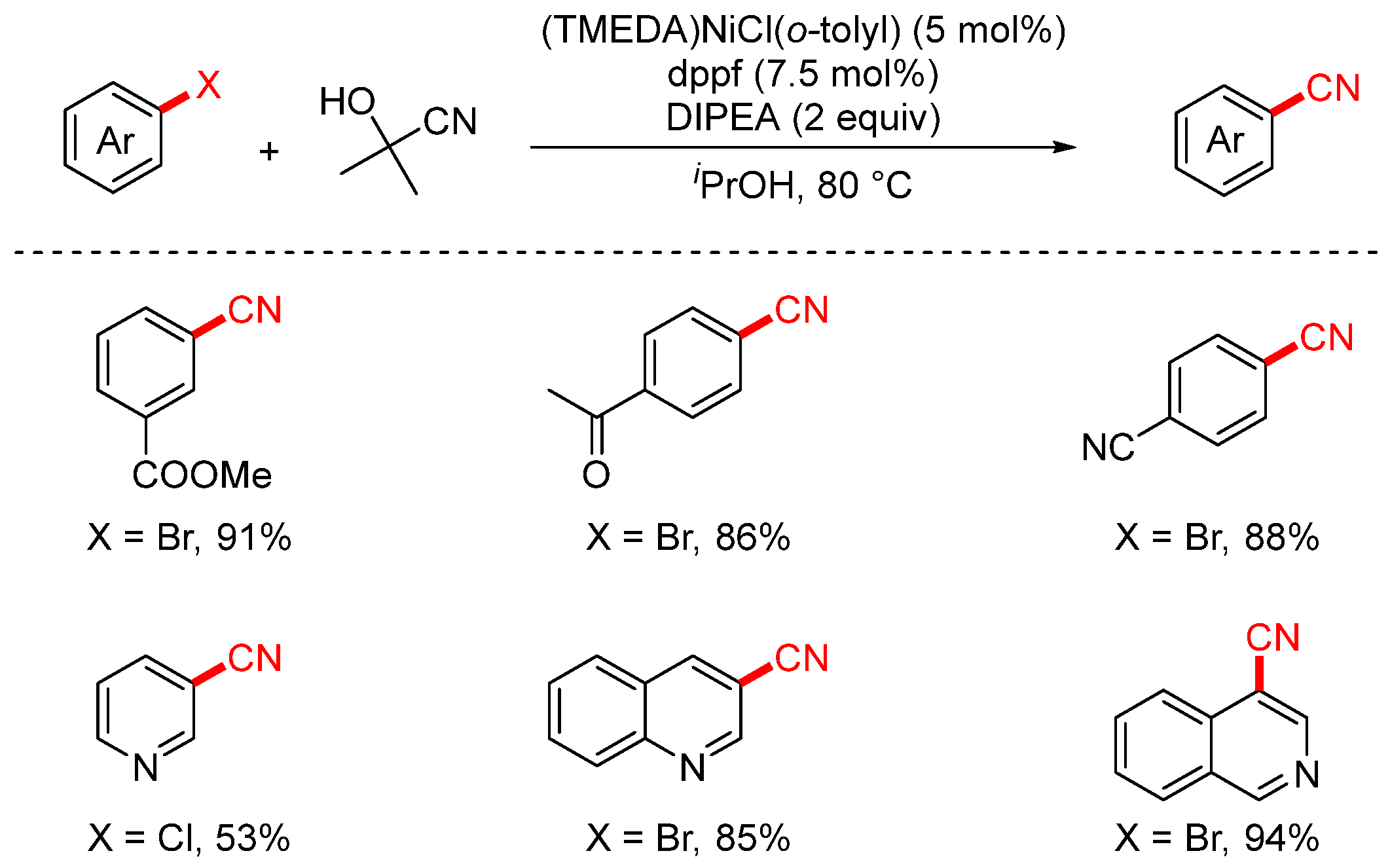
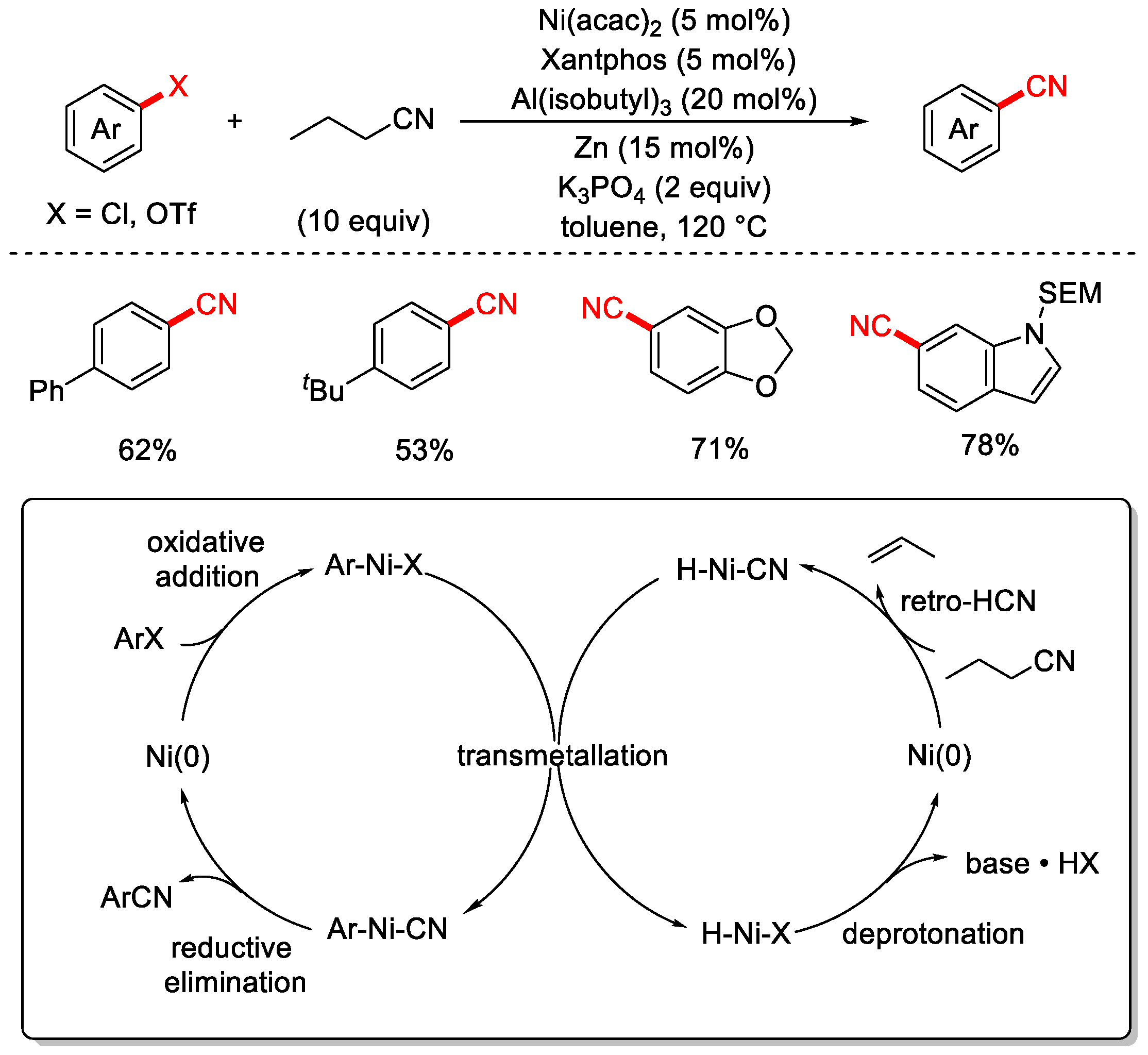
3. Non-Metal or Organic Cyanide Sources
3.1. Cyanating Reagents
3.2. Cyanating Solvents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ar–X | Aryl halide |
| Ar–CN | Aryl nitrile |
| TMSCN | Trimethylsilyl cyanide |
| DMF | N,N-Dimethylformamide |
| HMPA | Hexamethylphosphoramide |
| PMHS | Polymethylhydrosiloxane |
| DABAL-Me3 | Tris(dimethylaluminum)aluminate |
| TBABr | Tetrabutylammonium bromide |
| COD | 1,5-Cyclooctadiene |
| DQ | 1,4-Benzoquinone |
| MPMN | 2-methyl-2-phenyl malononitrile |
| DMAc | N,N-Dimethylacetamide |
| TFAA | Trifluoroacetic anhydride |
| Triphos | Tris[2-(diphenylphosphino)ethyl]phosphine |
| PC | Photocatalyst |
| SET | Single-electron transfer |
| RE | Reductive elimination |
| LED | Light-emitting diode |
| dtbbpy | 4,4′-Di-tert-butyl-2,2′-bipyridine |
| TMSB | Trimethylsilyl bromide |
| OMe-BBN | Methoxy-substituted borabicyclo[3.3.1]nonane |
References
- Kleemann, A.; Engel, J. Pharmaceutical Substances: Synthesis, Patents, Applications, 4th ed.; Thieme: Stuttgart, Germany, 2001. [Google Scholar]
- Larock, R. Comprehensive Organic Transformations: A Guide to Functional Group Preparations; Wiley-VCH: Weinheim, Germany, 1989; pp. 819–995. [Google Scholar]
- Fleming, F.G. Nitrile-containing natural products. Nat. Prod. Rep. 1999, 16, 597–606. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, X.; Yu, Z.; Song, C.; Du, Y. Nitrile Containing Pharmaceuticals: Target, Mechanism of Action, and Their SAR Studies. RSC Med. Chem. 2021, 12, 1650–1671. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P.J. Isocyanides and Cyanides as Natural Products. Acc. Chem. Res. 1992, 25, 433–439. [Google Scholar] [CrossRef]
- Jones, L.H.; Summerhill, N.W.; Swain, N.A.; Mills, J.E. Aromatic chloride to nitrile transformation: Medicinal and synthetic chemistry. Med. Chem. Commun. 2010, 1, 309–318. [Google Scholar] [CrossRef]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Singhal, N. Facile and highly selective conversion of nitriles to amides via indirect acid-catalyzed hydration using TFA or AcOH− H2SO4. J. Org. Chem. 2005, 70, 1926–1929. [Google Scholar] [CrossRef]
- Muller, J.; Wurtele, C.; Walter, O.; Schindler, S. Transformation of nitrile to cyanide and aldehyde using a cobalt (II) complex and dioxygen. Angew. Chem. Int. Ed. 2007, 46, 7775–7777. [Google Scholar] [CrossRef]
- Fang, W.-Y.; Wang, S.-M.; Zhang, Z.-W.; Qin, H.-L. Clickable transformation of nitriles (RCN) to oxazolyl sulfonyl fluoride warheads. Org. Lett. 2020, 22, 8904–8909. [Google Scholar] [CrossRef]
- Zhang, S.; del Pozo, J.; Romiti, F.; Mu, Y.; Torker, S.; Hoveyda, A.H. Delayed Catalyst Function Enables Direct Enantioselective Conversion of Nitriles to NH2-amines. Science 2019, 364, 45–51. [Google Scholar] [CrossRef]
- Liskey, C.W.; Liao, X.; Hartwig, J.F. Cyanation of Arenes via Iridium-Catalyzed Borylation. J. Am. Chem. Soc. 2010, 132, 11389–11391. [Google Scholar] [CrossRef]
- Rosenmund, K.W.; Struck, E. Das am Ringkohlenstoff gebundene Halogen und sein Ersatz durch andere Substituenten. I. Mitteilung: Ersatz des Halogens durch die Carboxylgruppe. Ber. Dtsch. Chem. Ges. 1919, 52, 1749. [Google Scholar] [CrossRef]
- Koelsch, C.F.; Whitney, A.G. The Rosemund-von Braun Nitrile Synthesis. J. Org. Chem. 1941, 6, 795–803. [Google Scholar] [CrossRef]
- Sandmeyer, T. Ueberfuhrung der drei nitraniline in die nitrobenzoesauren. Berichte der deutschen chemischen Gesellschaft. Chem. Ber. 1885, 18, 1492. [Google Scholar] [CrossRef]
- Sandmeyer, T. Uder die Ersetzung der Amid-gruppe durch Chlor, Brom und Cyan in den aromatischen Substanzen. Chem. Ber. 1884, 17, 2650. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Q.; Li, J. Sandmeyer cyanation of arenediazonium tetrafluoroborate using acetonitrile as a cyanide source. Org. Chem. Front. 2015, 2, 231–235. [Google Scholar] [CrossRef]
- Stevenson, A. Ammonolysis. J. Ind. Eng. Chem. 1949, 41, 1846–1851. [Google Scholar] [CrossRef]
- Kubota, H.; Rice, K.C. Palladium-Catalyzed Cyanation of Hindered, Electron-Rich Aryl Triflates by Zinc Cyanide. Tetrahedron Lett. 1998, 39, 2907–2910. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Cai, C. Palladium-Catalyzed Cyanation of Aryl Triflates. Synth. Commun. 2008, 38, 2753–2760. [Google Scholar] [CrossRef]
- Yeung, P.Y.; So, C.M.; Lau, C.P.; Kwong, F.Y. A Mild and Efficient Palladium-Catalyzed Cyanation of Aryl Mesylates in Water or tBuOH/Water. Angew. Chem. Int. Ed. 2010, 49, 8918–8922. [Google Scholar] [CrossRef]
- Takise, R.; Itami, K.; Yamaguchi, J. Cyanation of Phenol Derivatives with Aminoacetonitriles by Nickel Catalysis. Org. Lett. 2016, 18, 4428–4431. [Google Scholar] [CrossRef]
- Heravi, M.; Panahi, F.; Iranpoor, N. Nickel-Catalyzed Deoxycyanation of Activated Phenols via Cyanurate Intermediates with Zn(CN)2: A Route to Aryl Nitriles. Org. Lett. 2018, 20, 2753–2756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Fang, W.Y.; Rakesh, K.P.; Qin, H.L. Pd-Catalyzed one-pot dehydroxylative coupling of phenols with K4[Fe(CN)6] mediated by SO2F2: A practical method for the direct conversion of phenols to aryl nitriles. Org. Chem. Front. 2018, 5, 1835–1839. [Google Scholar] [CrossRef]
- Gan, Y.; Wang., G.; Xie, X.; Liu, Y.H. Nickel-Catalyzed Cyanation of Phenol Derivatives with Zn(CN)2 Involving C–O Bond Cleavage. J. Org. Chem. 2018, 83, 14036–14048. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hao, X.; Goodhue, C.E.; Yu, J.Q. Cu(II)-Catalyzed Functionalizations of Aryl C-H Bonds Using O2 as an Oxidant. J. Am. Chem. Soc. 2006, 128, 6790–6791. [Google Scholar] [CrossRef]
- Jia, X.F.; Yang, D.P.; Zhang, S.H.; Cheng, J. Chelation-Assisted Palladium-Catalyzed Direct Cyanation of 2-Arylpyridine C−H Bonds. Org. Lett. 2009, 11, 4716–4719. [Google Scholar] [CrossRef]
- Jin, J.S.; Wen, Q.D.; Lu, P.; Wang, Y.G. Copper-catalyzed cyanation of arenes using benzyl nitrile as a cyanide anion surrogate. Chem. Commun. 2012, 48, 9933–9935. [Google Scholar] [CrossRef]
- Xu, H.; Liu, P.T.; Li, Y.H.; Han, F.S. Copper-Mediated Direct Aryl C–H Cyanation with Azobisisobutyronitrile via a Free-Radical Pathway. Org. Lett. 2013, 15, 3354–3357. [Google Scholar] [CrossRef]
- Pan, C.D.; Jin, H.M.; Xu, P.; Liu, X.; Cheng, Y.X.; Zhu, C.J. Copper-Mediated Direct C2-Cyanation of Indoles Using Acetonitrile as the Cyanide Source. J. Org. Chem. 2013, 78, 9494–9498. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.H.; Zhang, B.; Sun, Y.; Wang, L.; Chen, J.B.; Cheng, J. Copper-mediated C3-cyanation of indoles by the combination of amine and ammonium. Chem. Comm. 2014, 50, 2315–2317. [Google Scholar] [CrossRef]
- Yan, Y.P.; Yuan, Y.Z.; Jiao, N. Cu-mediated C–H cyanation of arenes using N,N-dimethylformamide (DMF) as the “CN” source. Org. Chem. Front. 2014, 1, 1176–1179. [Google Scholar] [CrossRef]
- Delcaillau, T.; Boehm, P.; Morandi, B. Nickel-Catalyzed Reversible Functional Group Metathesis between Aryl Nitriles and Aryl Thioethers. J. Am. Chem. Soc. 2021, 143, 3723–3728. [Google Scholar] [CrossRef]
- Long, Y.; Zheng, Y.L.; Xia, Y.; Qu, L.; Yang, Y.H.; Xiang, H.F.; Zhou, X.G. Nickel-Catalyzed Synthesis of an Aryl Nitrile via Aryl Exchange between an Aromatic Amide and a Simple Nitrile. ACS Catal. 2022, 12, 4688–4695. [Google Scholar] [CrossRef]
- Cassar, L.; Ferrara, S.; Foá, M. Nickelcatalyzed cyanation of aromatic halides. Adv. Chem. Ser. 1974, 132, 252. [Google Scholar]
- Cassar, L.; Foá, M.; Montanari, F.; Marinelli, G.P. Phasetransfer catalysis in the nickel-catalyzed cyanation of aryl halides. J. Organomet. Chem. 1979, 173, 335. [Google Scholar] [CrossRef]
- Okano, T.; Iwahara, M.; Kiji, J. Catalytic cyanation of aryl halides with NaCN in the presence of crowned phosphine complexes of palladium under solid-liquid twophase conditions. Synlett 1998, 1998, 243. [Google Scholar] [CrossRef]
- Dalton, J.R.; Regen, S.L. Alumina assisted aryl cyanation. J. Org. Chem. 1979, 44, 4443. [Google Scholar] [CrossRef]
- Zanon, J.; Klapars, A.; Buchwald, S.L. Copper-catalyzed domino halide exchange-cyanation of aryl bromides. J. Am. Chem. Soc. 2003, 125, 2890. [Google Scholar] [CrossRef]
- Ushkov, A.V.; Grushin, V.V. Rational Catalysis Design on the Basis of Mechanistic Understanding: Highly Efficient Pd-Catalyzed Cyanation of Aryl Bromides with NaCN in Recyclable Solvents. J. Am. Chem. Soc. 2011, 133, 10999–11005. [Google Scholar] [CrossRef]
- Takagi, K.; Okamoto, T.; Sakakibara, Y.; Oka, S. Palladium(II) catalyzed synthesis of aryl cyanides from aryl halides. Chem. Lett. 1973, 2, 471. [Google Scholar] [CrossRef]
- Akita, Y.; Shimazaki, M.; Ohta, A. Einfuhrung der Cyano-Gruppe in Pyrazine. Synthesis 1981, 1981, 974. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Okuda, F.; Shimobayashi, A.; Kirino, K.; Sakai, M.; Uchino, N.; Takagi, K. The cyanation of aromatic halides catalyzed by Nickel(0) complexes generated in situ. I. General scope and limitations. Bull. Chem. Soc. Jpn. 1988, 61, 1985. [Google Scholar] [CrossRef]
- Anderson, B.A.; Bell, E.C.; Ginah, F.O.; Harn, N.K.; Pagh, L.M.; Wepsiec, J.P. Cooperative catalyst effects in palladium-mediated cyanation reactions of aryl halides and triflates. J. Org. Chem. 1998, 63, 8224. [Google Scholar] [CrossRef]
- Sundermeier, M.; Zapf, A.; Beller, M.; Sans, J. A new palladium catalyst system for the cyanation of aryl chlorides. Tetrahedron Lett. 2001, 42, 6707. [Google Scholar] [CrossRef]
- Sundermeier, M.; Zapf, A.; Mutyala, S.; Baumann, W.; Sans, J.; Weiss, S.; Beller, M. Progress in the palladium-catalyzed cyanation of aryl chlorides. Chem. A Eur. J. 2003, 9, 1828. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Williams, J.M. Palladium catalyzed cyanation of aryl bromides promoted by low-level organotin compounds. Org. Lett. 2004, 6, 2837. [Google Scholar] [CrossRef] [PubMed]
- Cristau, H.-J.; Ouali, A.; Spindler, J.-F.; Taillefer, M. Mild and Efficient Copper-Catalyzed Cyanation of Aryl Iodides and Bromides. Chem. A Eur. J. 2005, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- Tschaen, D.; Desmond, M.R.; King, A.O.; Fortin, M.C.; Pipik, B.; King, S.; Verhoeven, T.R. An improved procedure for aromatic cyanation. Synth. Commun. 1994, 24, 887. [Google Scholar] [CrossRef]
- Maligres, P.E.; Waters, M.S.; Fleitz, F.; Askin, D. A highly catalytic robust palladium catalyzed cyanation of aryl bromides. Tetrahedron Lett. 1999, 40, 8193. [Google Scholar] [CrossRef]
- Alterman, M.; Hallberg, A.J. Fast microwave-assisted preparation of aryl and vinyl nitriles and the corresponding tetrazoles from organo-halides. J. Org. Chem. 2000, 65, 7984. [Google Scholar] [CrossRef]
- Jin, F.; Confalone, P.N. Palladium-catalyzed cyanation reactions of aryl chlorides. Tetrahedron Lett. 2000, 41, 3271–3273. [Google Scholar] [CrossRef]
- Chidambaram, R. A robust palladium-catalyzed cyanation procedure: Beneficial effect of zinc acetate. Tetrahedron Lett. 2004, 45, 1441–1444. [Google Scholar] [CrossRef]
- Shevlin, M. Sulfate additives generate robust and highly active palladium catalysts for the cyanation of aryl chlorides. Tetrahedron Lett. 2010, 51, 4833–4836. [Google Scholar] [CrossRef]
- Cohen, D.T.; Buchwald, S.L. Mild Palladium-Catalyzed Cyanation of (Hetero)aryl Halides and Triflates in Aqueous Media. Org. Lett. 2015, 17, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Schareina, T.; Zapf, A.; Beller, M. Improving palladium-catalyzed cyanation of aryl halides: Development of a state-of-the-art methodology using potassium hexacyanoferrate(II) as cyanating agent. J. Organomet. Chem. 2004, 689, 4576–4583. [Google Scholar] [CrossRef]
- Schareina, T.; Jackstell, R.; Schulz, T.; Zapf, A.; Cotte, A.; Gotta, M.; Beller, M. Increasing the Scope of Palladium-Catalyzed Cyanations of Aryl Chlorides. Adv. Synth. Catal. 2009, 351, 643–648. [Google Scholar] [CrossRef]
- Chatani, N.; Hanafusa, T. Palladium-catalyzed cyanation of aryl halides by trimethylsilyl cyanide. J. Org. Chem. 1986, 51, 4714. [Google Scholar] [CrossRef]
- Sundermeier, M.; Mutyala, M.; Zapf, A.; Spannenberg, A.; Beller, M. A convenient and efficient procedure for the palladium-catalyzed cyanation of aryl halides using trimethylsilylcyanide. J. Organomet. Chem. 2003, 684, 50–55. [Google Scholar] [CrossRef]
- Sundermeier, M.; Zapf, A.; Beller, M. A Convenient Procedure for the Palladium-Catalyzed Cyanation of Aryl Halides. Angew. Chem. Int. Ed. 2003, 42, 1661–1664. [Google Scholar] [CrossRef]
- Wen, Q.D.; Jin, J.S.; Hu, B.B.; Lu, P.; Wang, Y.G. Palladium-catalyzed cyanide metathesis: Utilization of benzyl cyanide as an operator-benign reagent for aryl halide cyanations. RSC Adv. 2012, 2, 6167–6169. [Google Scholar] [CrossRef]
- Song, R.J.; Wu, J.C.; Liu, Y.; Deng, G.B.; Wu, C.Y.; Wei, W.T.; Li, J.H. Copper-Catalyzed Oxidative Cyanation of Aryl Halides with Nitriles Involving Carbon–Carbon Cleavage. Synlett 2012, 23, 2491–2496. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, M.; Lu, W.; Li, L.; Shen, Z. Acetonitrile as a Cyanating Reagent: Cu-Catalyzed Cyanation of Arenes. Org. Lett. 2015, 17, 2602. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shen, Z. Copper-Catalyzed Acyloxycyanation of Alkynes with Acetonitrile: Regioselective Construction of Cyclic Acrylonitriles by 6-endo or 5-exo Cyclization. Adv. Syn. Catal. 2017, 359, 3515. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Ren, X.Y.; Chen, J.B.; Hu, M.L.; Cheng, J. Copper-Mediated Cyanation of Aryl Halide with the Combined Cyanide Source. Org. Lett. 2011, 13, 5004–5007. [Google Scholar] [CrossRef] [PubMed]
- Anbarasan, P.; Schareina, T.; Beller, M. Recent developments and perspectives in palladium-catalyzed cyanation of aryl halides: Synthesis of benzonitriles. Chem. Soc. Rev. 2011, 40, 5049–5067. [Google Scholar] [CrossRef]
- Neetha, M.; Afsina, C.M.A.; Aneeja, T.; Anilkumar, G. Recent advances and prospects in the palladium-catalyzed cyanation of aryl halides. RSC Adv. 2020, 10, 33683–33699. [Google Scholar] [CrossRef]
- Pimparkar, S.; Koodan, A.; Maiti, S.; Ahmed, N.S.; Mostafa, M.; Maiti, D. C–CN bond formation: An overview of diverse strategies. Chem. Commun. 2021, 57, 2210–2232. [Google Scholar] [CrossRef]
- Kanchana, U.S.; Mathew, T.V.; Anilkumar, G. Recent advances and prospects in the nickel-catalyzed cyanation. J. Organomet. Chem. 2020, 920, 121337. [Google Scholar] [CrossRef]
- Cassar, L. A new nickel-catalyzed synthesis of aromatic nitriles. J. Organomet. Chem. 1973, 54, C57. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Ido, Y.; Sasaki, K.; Sakai, M.; Uchino, N. The Cyanation of Aromatic Halides Catalyzed by Nickel(0) Complexes Generated in Situ. II. The Cyanation of Heteroaromatic Halides. Bull. Chem. Soc. Jpn. 1993, 66, 2776. [Google Scholar] [CrossRef]
- Arvela, R.K.; Leadbeater, N.E. Rapid, Easy Cyanation of Aryl Bromides and Chlorides Using Nickel Salts in Conjunction with Microwave Promotion. J. Org. Chem. 2003, 68, 9122. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Tavakoli, G.; Rezvani, H.R. Highly active recyclable heterogeneous nanonickel ferrite catalyst for cyanation of aryl and heteroaryl halides. Appl. Organomet. Chem. 2014, 28, 750. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, A.; Chen, H.; Liu, Y. General and Mild Nickel-catalyzed Cyanation of Aryl/Heteroaryl Chlorides with Zn(CN)2: Key Roles of DMAP. Org. Lett. 2017, 19, 2118–2121. [Google Scholar] [CrossRef]
- Beattie, D.D.; Schareina, T.; Beller, M. A Room Temperature Cyanation of (Hetero)Aromatic Chlorides by an Air Stable Nickel(II) XantPhos Precatalyst and Zn(CN)2. Org. Biomol. Chem. 2017, 15, 4291–4294. [Google Scholar] [CrossRef] [PubMed]
- Hethcox, J.C.; Stifri, R.J. Air-Tolerant Nickel-Catalyzed Cyanation of (Hetero)aryl Halides Enabled by Polymethylhydrosiloxane, a Green Reductant. J. Org. Chem. 2022, 87, 4951–4954. [Google Scholar] [CrossRef] [PubMed]
- Camacho, G.D.; Hethcox, J.C. Nickel-Catalyzed Cyanation of (Hetero)aryl Bromides Using DABAL-Me3 as a Soluble Reductant. Org. Lett. 2022, 24, 8397–8400. [Google Scholar] [CrossRef] [PubMed]
- Porte, V.; Kollmus, P.; Serrano, E.; Santagostino, M. High-throughput Experimentation Enables the Development of a Nickel-catalyzed Cyanation Platform for (Hetero)aryl Halides. Chem. Eur. J. 2025, 31, e202403795. [Google Scholar] [CrossRef]
- Burg, F.; Egger, J.; Deutsch, J.; Guimond, N. A Homogeneous Method for the Conveniently Scalable Palladium- and Nickel-catalyzed Cyanation of Aryl Halides. Org. Process Res. Dev. 2016, 20, 1540–1545. [Google Scholar] [CrossRef]
- Yu, P.; Morandi, B. Nickel-catalyzed Cyanation of Aryl Chlorides and Triflates Using Butyronitrile: Merging Retro-hydrocyanation with Cross-coupling. Angew. Chem. Int. Ed. 2017, 56, 15693–15697. [Google Scholar] [CrossRef]
- Mills, L.R.; Graham, J.M.; Patel, P.; Rousseaux, S.A.L. Ni catalyzed Reductive Cyanation of Aryl Halides and Phenol Derivatives via Transnitrilation. J. Am. Chem. Soc. 2019, 141, 19257–19262. [Google Scholar] [CrossRef]
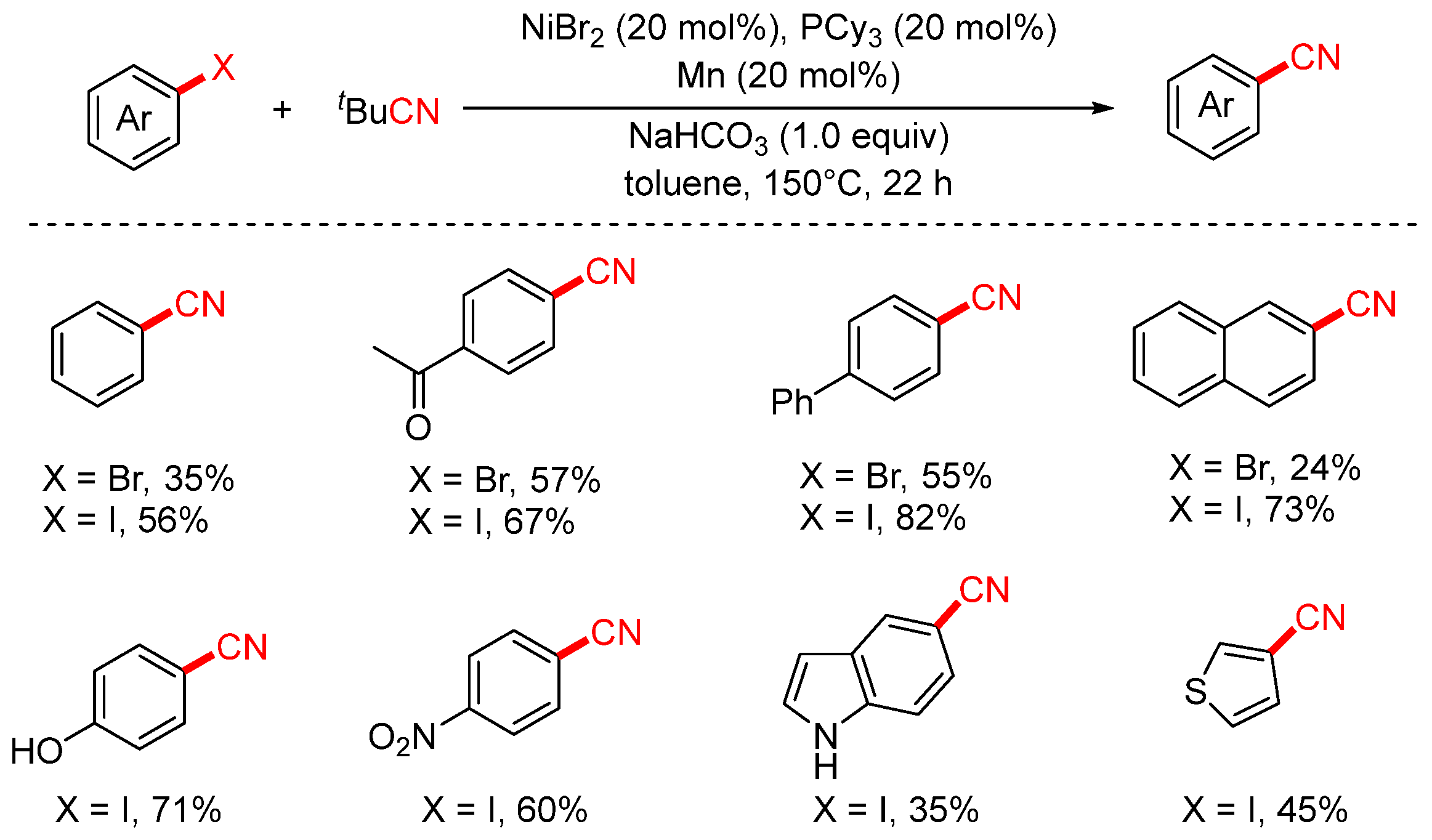
- Chen, H.; Sun, S.; Liu, Y.A.; Liao, X. Nickel-catalyzed Cyanation of Aryl Halides and Hydrocyanation of Alkynes via C−CN Bond Cleavage and Cyano Transfer. ACS Catal. 2020, 10, 1397–1405. [Google Scholar] [CrossRef]
- Dong, Y.N.; Yang, P.J.; Zhao, S.Z.; Li, Y.H. Reductive cyanation of organic chlorides using CO2 and NH3 via Triphos–Ni(I) species. Nat. Commun. 2020, 11, 4096. [Google Scholar] [CrossRef] [PubMed]
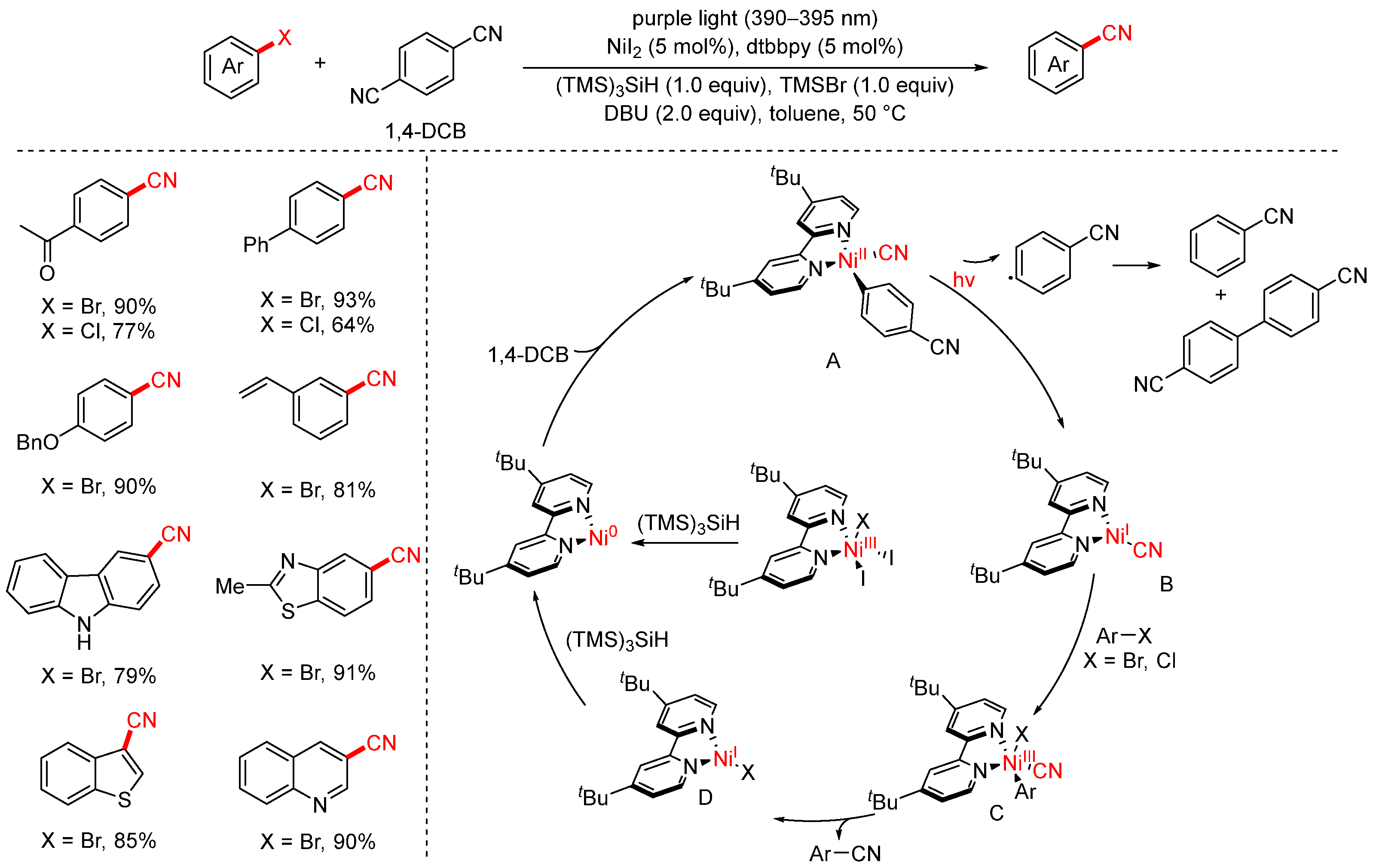
- Jia, Y.; Liu, Y.Y.; Lu, L.Q.; Liu, S.H.; Zhou, H.B.; Lan, Y.; Xiao, W.J. Photoredox Catalysis Unlocks the Nickel-catalyzed Cyanation of Aryl Halides under Benign Conditions. CCS Chem. 2021, 3, 2032–2041. [Google Scholar] [CrossRef]
- Yan, Y.G.; Sun, J.J.; Li, G.; Yang, L.; Zhang, W.; Cao, R.; Wang, C.; Xiao, J.L.; Xue, D. Photochemically Enabled, Ni-Catalyzed Cyanation of Aryl Halides. Org. Lett. 2022, 24, 2271–2275. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, Z.G.; Hu, Y.Y.; Liu, Y.K.; Jin, H.W.; Zhou, B.W. Nickel-catalyzed cyanation reaction of aryl/alkenyl halides with alkyl isocyanides. Org. Biomol. Chem. 2022, 20, 8049–8053. [Google Scholar] [CrossRef]
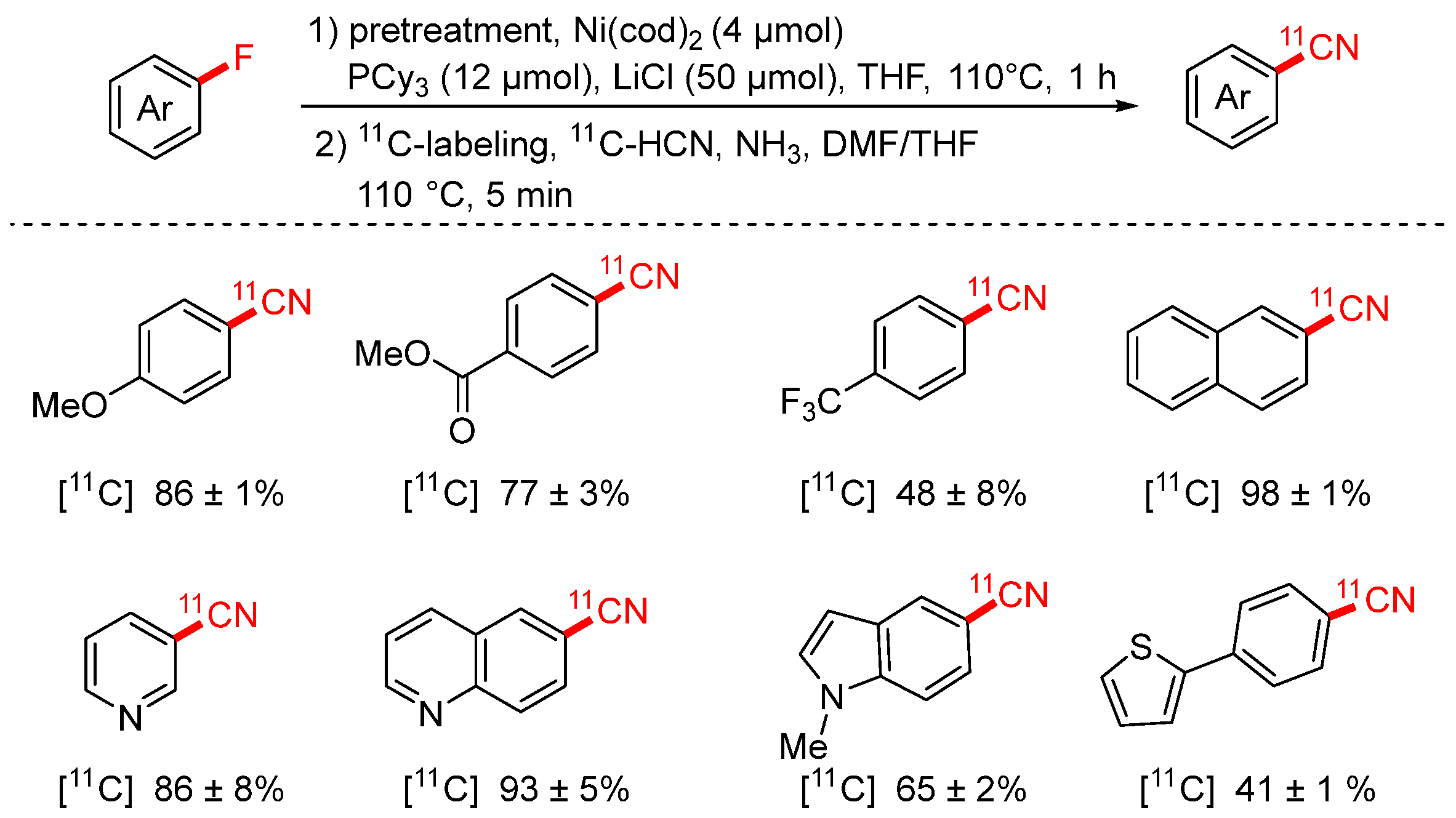
- Zhang, Z.; Niwa, T.; Watanabe, K.; Hosoya, T. 11C-Cyanation of Aryl Fluorides via Nickel and Lithium Chloride-Mediated C-F Bond Activation. Angew. Chem. Int. Ed. 2023, 62, e202302956. [Google Scholar] [CrossRef]
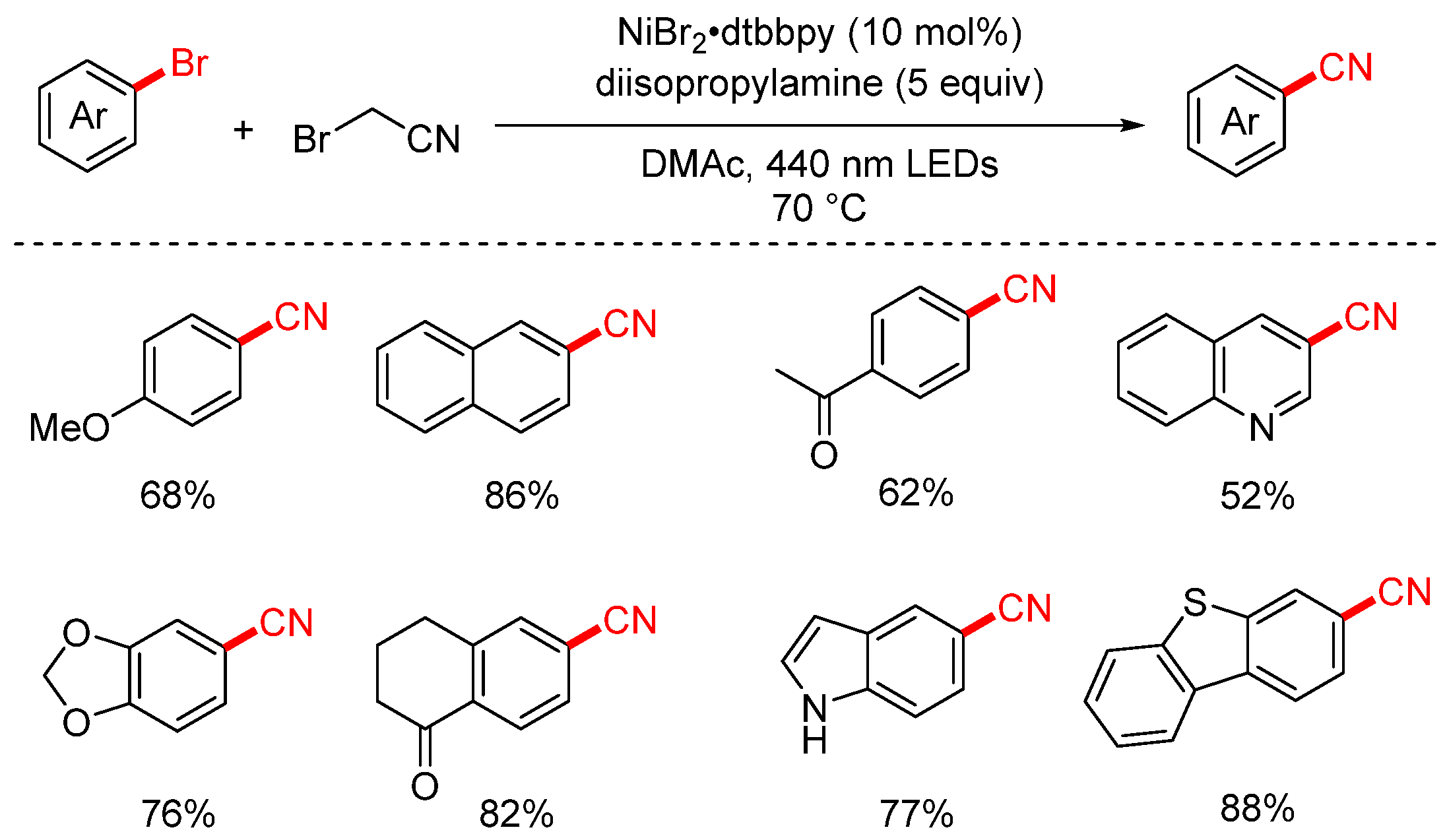
- Lee, S.C.; Zhu, C.; Huang, K.; Bau, J.A.; Jia, J.Q.; Yue, H.F.; Rueping, M. Photoinduced Nickel-Catalyzed Demethylative Cyanation and Decarboxylative Cyanomethylation of Aryl Halides. ACS Catal. 2023, 13, 16279–16285. [Google Scholar] [CrossRef]
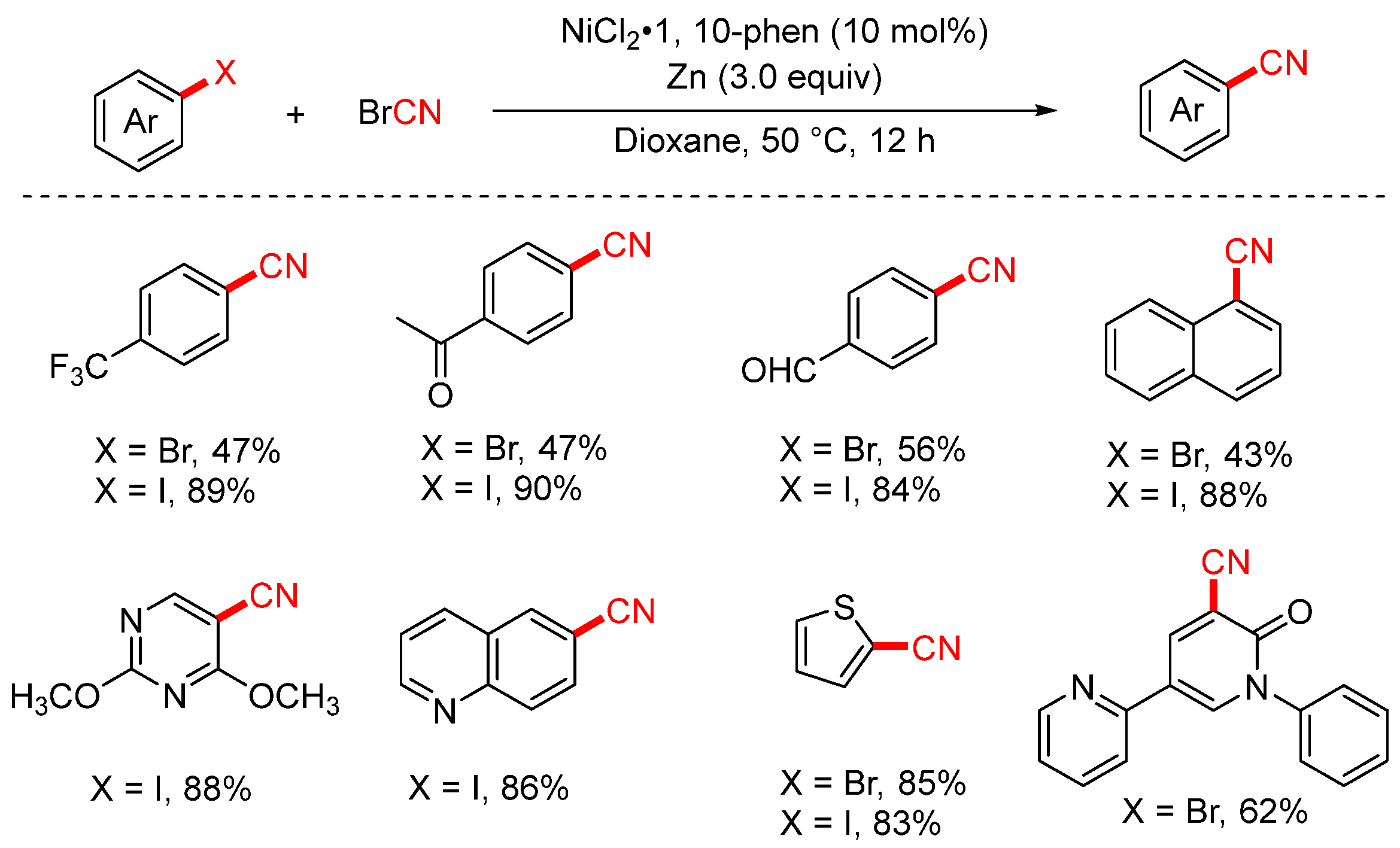
- Wu, Y.J.; Ma, C.; Bilal, M.; Liang, Y.F. Nickel-Catalyzed Reductive Cyanation of Aryl Halides and Epoxides with Cyanogen Bromide. Molecules 2024, 29, 6016. [Google Scholar] [CrossRef]
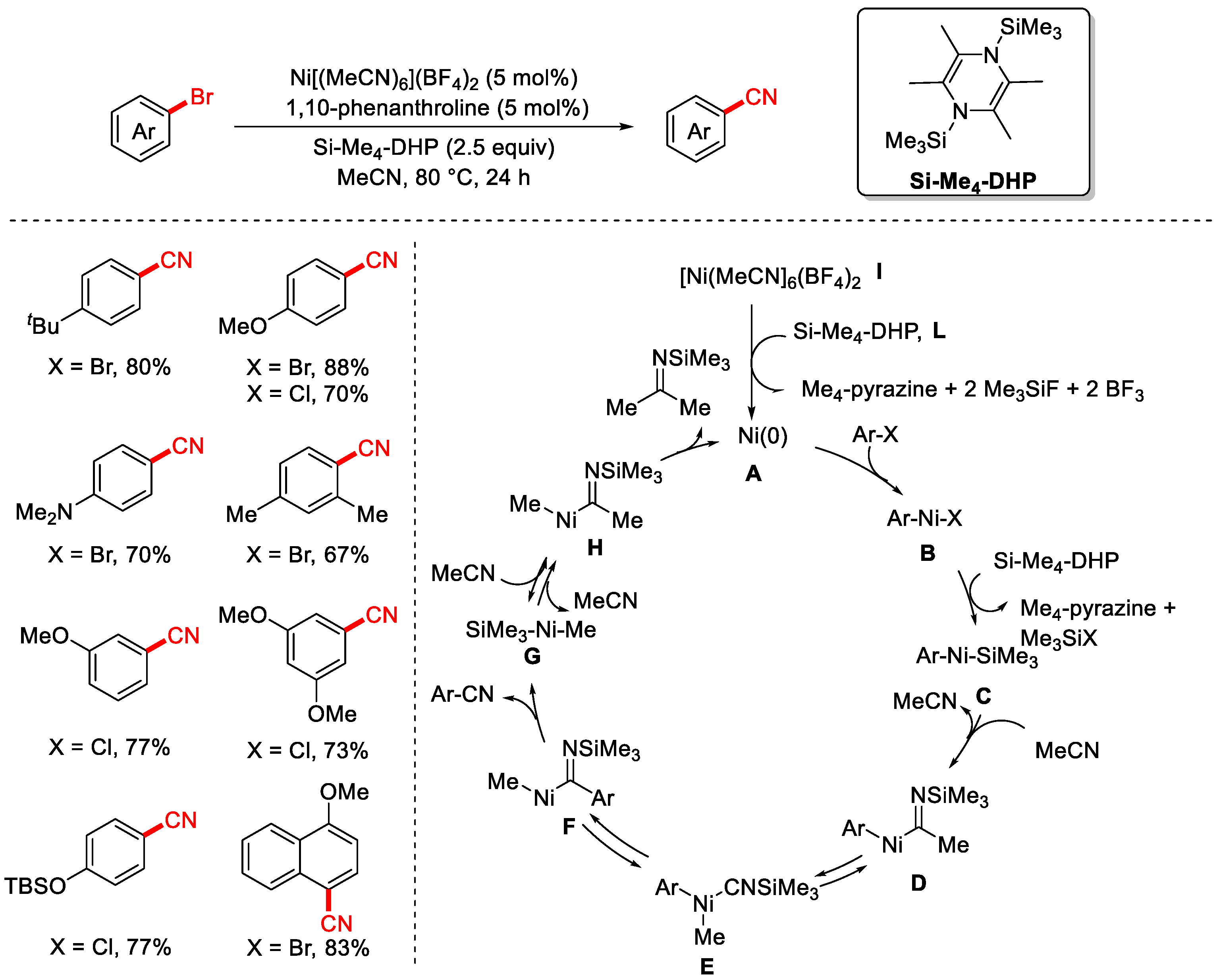
- Ueda, Y.; Tsujimoto, N.; Yurino, T.; Tsurugi, H.; Mashima, K. Nickel-catalyzed Cyanation of Aryl Halides and Triflates Using Acetonitrile via C−CN Bond Cleavage Assisted by 1,4-Bis(trimethylsilyl)-2,3,5,6-tetramethyl-1,4-Dihydropyrazine. Chem. Sci. 2019, 10, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Zeng, B.; Qasim, R.; Zheng, D.H.; Zhang, Q.; Jin, Y.; Wang, Q.F.; Meguellati, K. Ni-Catalyzed Cyanation of (Hetero)aryl Halides with Acetonitrile as Cyano Source. ACS Catal. 2024, 14, 2350–2357. [Google Scholar] [CrossRef]


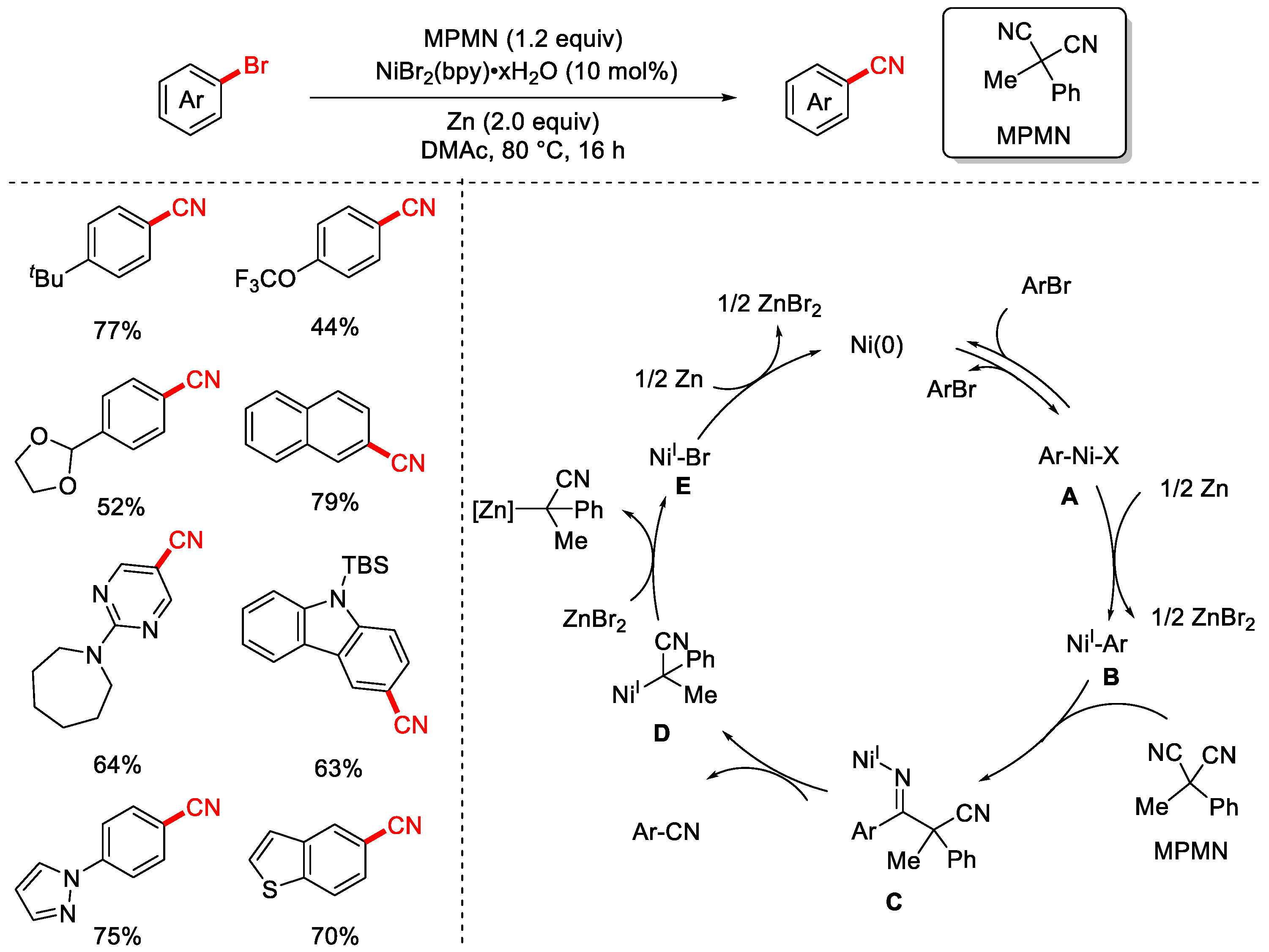
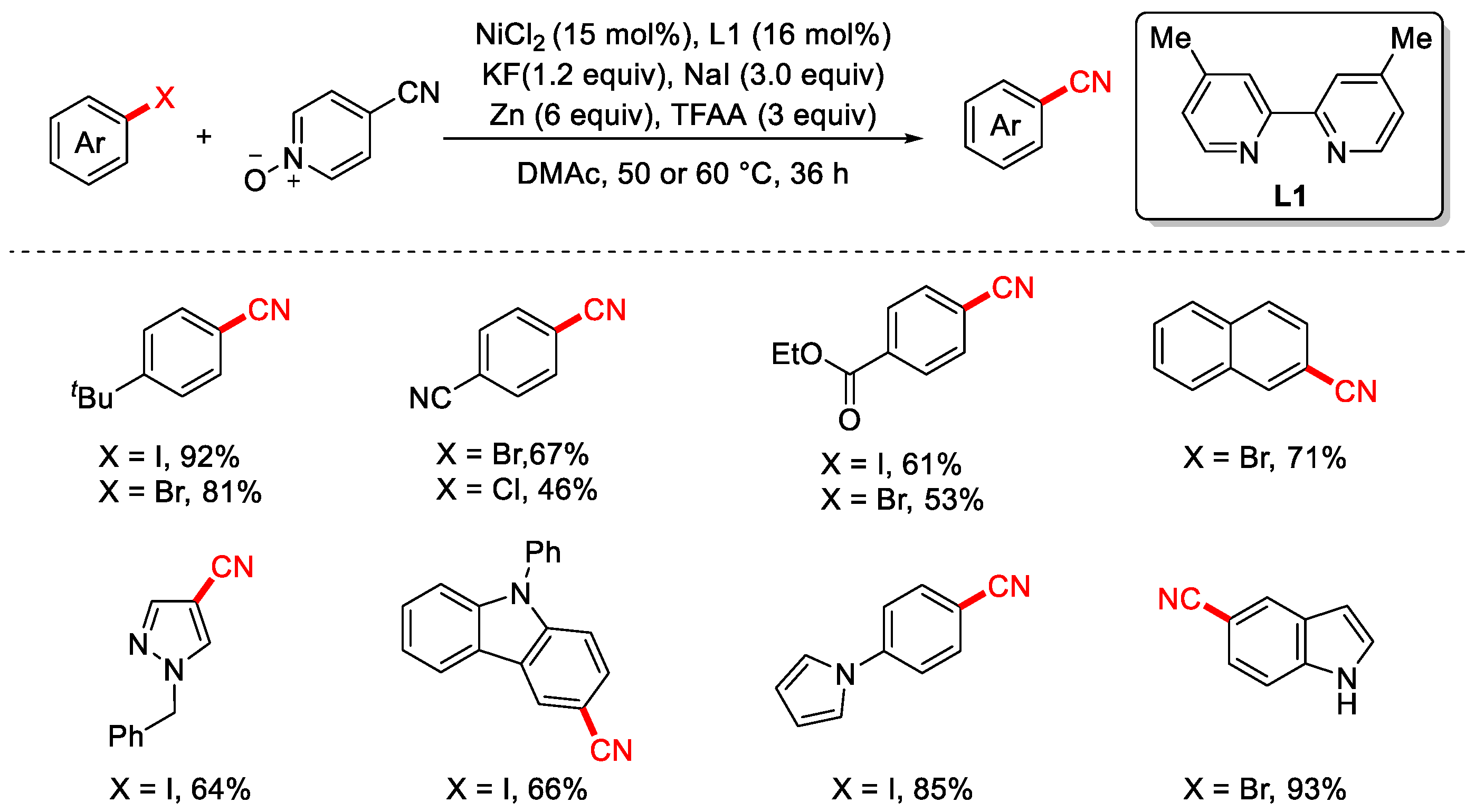

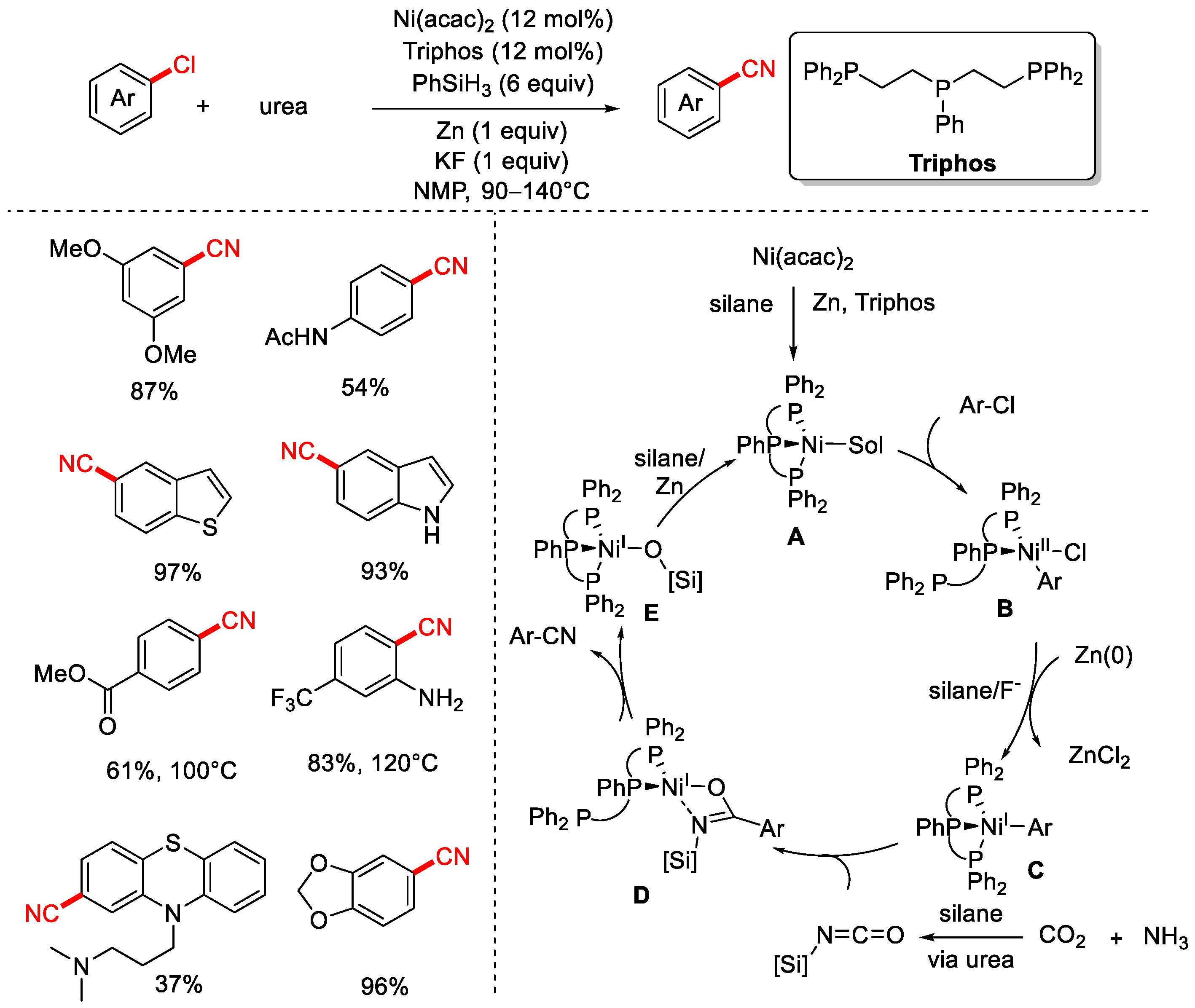
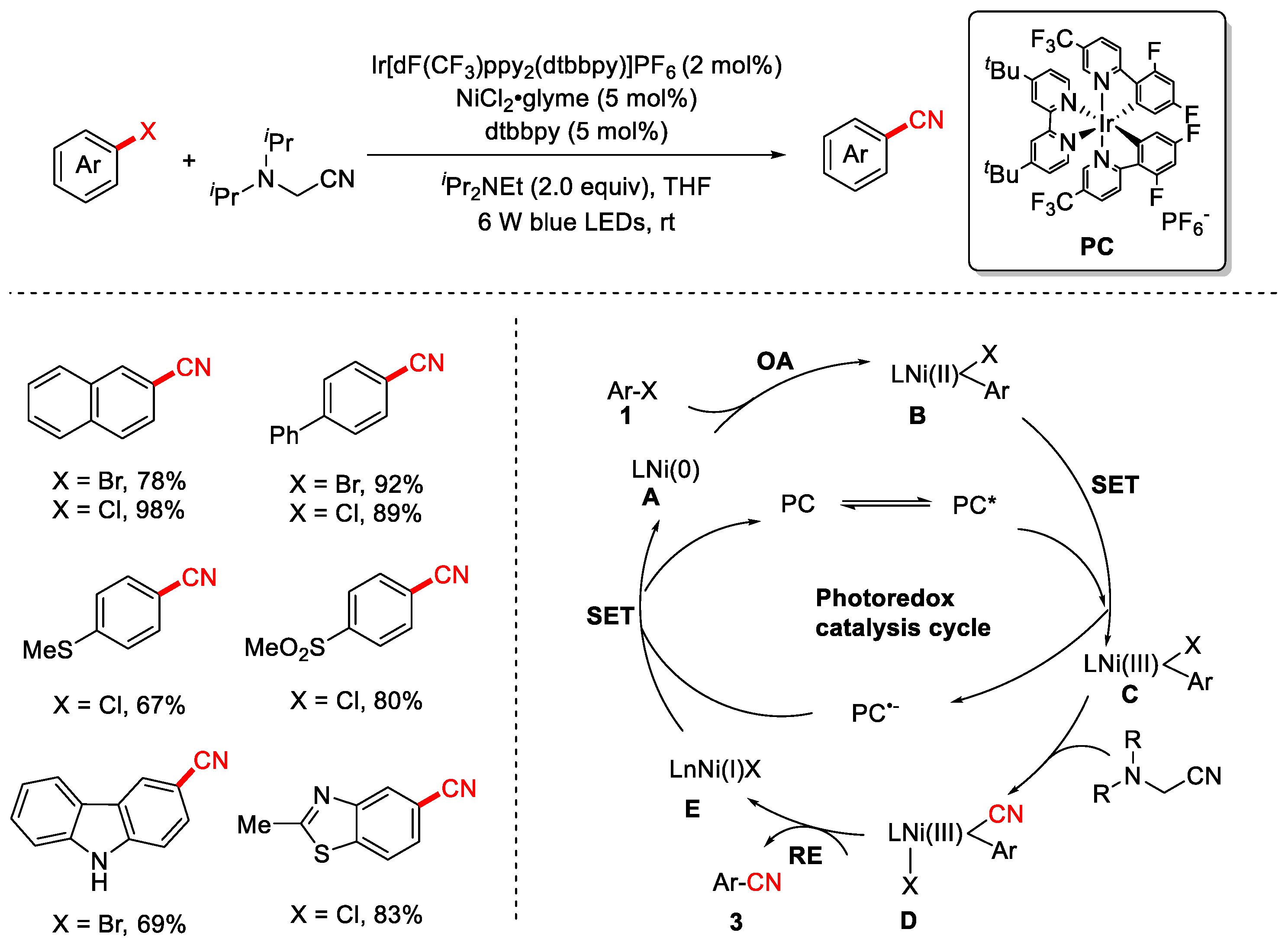

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Huo, C.; Zhou, D.; Zhang, J.; Kong, H.; Ren, W.; Qu, F.; Liu, T.; Chen, H.; Wang, X. Nickel-Catalyzed Cyanation of Aryl Halides. Molecules 2025, 30, 3440. https://doi.org/10.3390/molecules30163440
Ma Z, Huo C, Zhou D, Zhang J, Kong H, Ren W, Qu F, Liu T, Chen H, Wang X. Nickel-Catalyzed Cyanation of Aryl Halides. Molecules. 2025; 30(16):3440. https://doi.org/10.3390/molecules30163440
Chicago/Turabian StyleMa, Zhenqiang, Cuimeng Huo, Duo Zhou, Jingyi Zhang, Hongjun Kong, Wenke Ren, Fengbo Qu, Tingting Liu, Hui Chen, and Xilong Wang. 2025. "Nickel-Catalyzed Cyanation of Aryl Halides" Molecules 30, no. 16: 3440. https://doi.org/10.3390/molecules30163440
APA StyleMa, Z., Huo, C., Zhou, D., Zhang, J., Kong, H., Ren, W., Qu, F., Liu, T., Chen, H., & Wang, X. (2025). Nickel-Catalyzed Cyanation of Aryl Halides. Molecules, 30(16), 3440. https://doi.org/10.3390/molecules30163440





