Synergistic Effects of Injectable Platelet-Rich Fibrin and Bioactive Peptides on Dermal Fibroblast Viability and Extracellular Matrix Gene Expression: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Crystal Violet Assay
2.2. MTT Assay
2.3. RealTime-Glo™ Cell Viability Assay
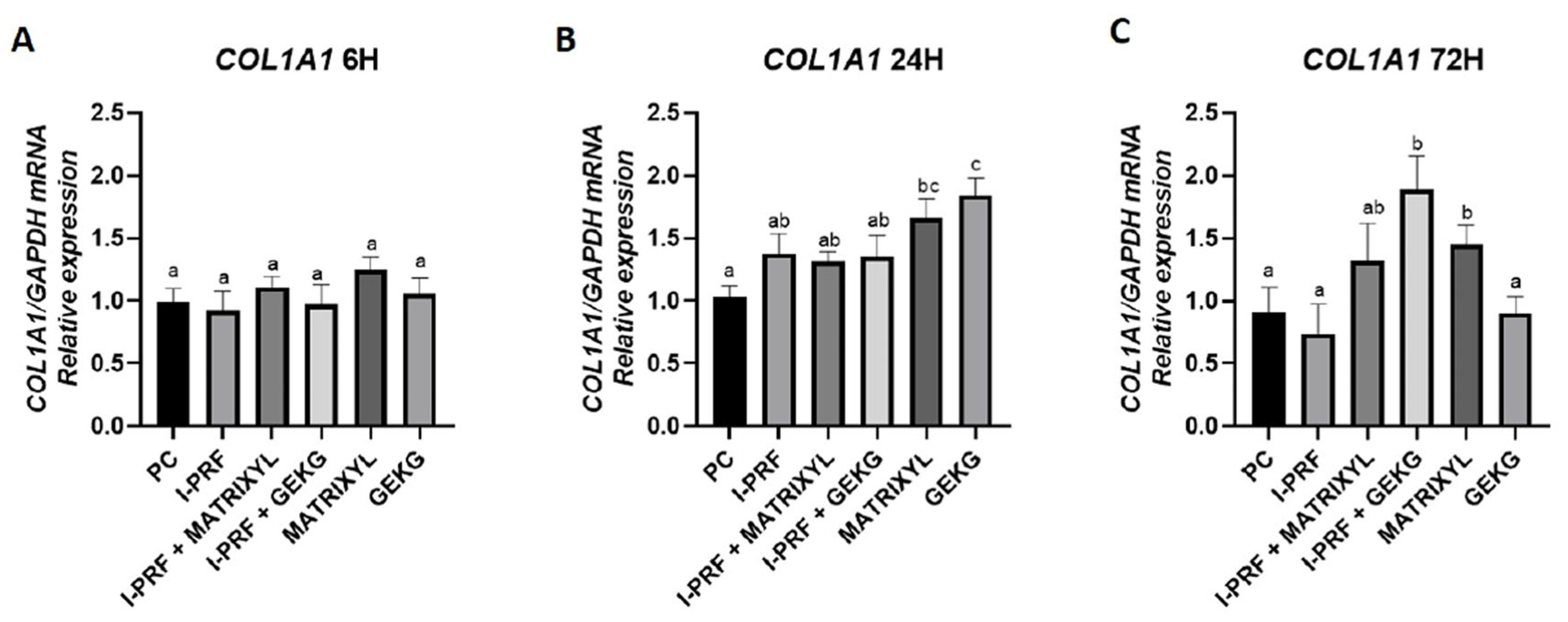
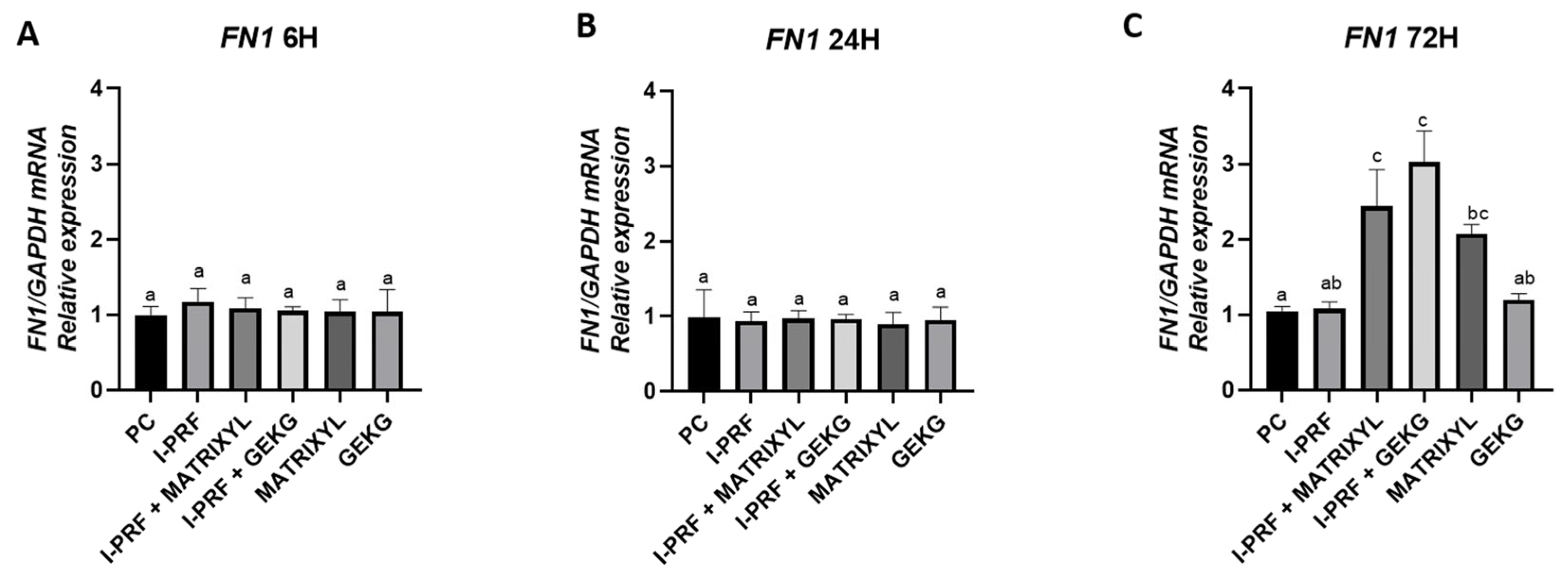
2.4. RT-qPCR: Gene Expression of ECM Markers
2.4.1. COL1A1
2.4.2. FN1
2.4.3. HAS1
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Cell Culture
4.3. Preparation of Biostimulatory Agents
4.4. Experimental Design and Cell Culture
- PC: positive control (DMEM + 10% FBS + 1% penicillin/streptomycin);
- G1: i-PRF;
- G2: i-PRF + Matrixyl (10 ppm);
- G3: i-PRF + GEKG (10 ppm);
- G4: Matrixyl (10 ppm);
- G5: GEKG (10 ppm).
4.5. Cell Viability and Proliferation
4.6. Gene Expression Assays
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| I-PRF | Injectable Platelet-Rich Fibrin |
| GEKG | Tetrapeptide-21 |
| COL1A1 | Collagen Type I Alpha-1 |
| FN1 | Fibronectin 1 |
| HAS 1 | Hyaluronan Synthase 1 |
| PRF | Platelet-Rich Fibrin |
| FBH | Human Dermal Fibroblasts |
| ECM | Extracellular Matrix |
| PDGF | Platelet-derived Growth Factor |
| TGF- | Transforming Growth Factor-beta |
| VEGF | Vascular Endothelial Growth Factor |
| FBS | Fetal Bovine Serum |
| DMEM/F-12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| DMSO | Dimethylsulfoxide |
| FBH CK 001 | Primary Human Dermal Fibroblasts |
References
- Swift, A.; Liew, S.; Weinkle, S.; Garcia, J.K.; Silberberg, M.B. The Facial Aging Process from the “inside Out”. Aesthetic Surg. J. 2021, 41, 1107–1119. [Google Scholar] [CrossRef]
- Li, K.; Meng, F.; Li, Y.R.; Tian, Y.; Chen, H.; Jia, Q.; Cai, H.; Jiang, H.B. Application of Nonsurgical Modalities in Improving Facial Aging. Int. J. Dent. 2022, 2022, 8332631. [Google Scholar] [CrossRef] [PubMed]
- De Melo, F.; Nicolau, P.; Piovano, L.; Lin, S.L.; Baptista-Fernandes, T.; King, M.I.; Camporese, A.; Hong, K.; Khattar, M.M.; Christen, M.O. Recommendations for Volume Augmentation and Rejuvenation of the Face and Hands with the New Generation Polycaprolactone-Based Collagen Stimulator (Ellansé®). Clin. Cosmet. Investig. Dermatol. 2017, 10, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Goldie, K.; Chernoff, G.; Corduff, N.; Davies, O.; van Loghem, J.; Viscomi, B. Consensus Agreements on Regenerative Aesthetics: A Focus on Regenerative Biostimulation With Calcium Hydroxylapatite. Dermatol. Surg. 2024, 50, S172–S176. [Google Scholar] [CrossRef]
- Signori, R.; Barbosa, A.d.P.; Cezar-dos-Santos, F.; Carbone, A.C.; Ventura, S.; Nobre, B.B.d.S.; Neves, M.L.B.B.; Câmara-Souza, M.B.; Poluha, R.L.; De la Torre Canales, G. Efficacy and Safety of Poly-l-Lactic Acid in Facial Aesthetics: A Systematic Review. Polymers 2024, 16, 2564. [Google Scholar] [CrossRef]
- Davies, C.; Miron, R.J. Autolougous Platelet Concentrates in Esthetic Medicine. Periodontol. 2000 2025, 97, 363–419. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Levy, F.M. Autologous Platelet Concentrates for Facial Rejuvenation. J. Appl. Oral Sci. 2022, 30, e20220020. [Google Scholar] [CrossRef]
- Nacopoulos, C.; Vesala, A.M. Lower Facial Regeneration with a Combination of Platelet-Rich Fibrin Liquid Matrices Based on the Low Speed Centrifugation Concept-Cleopatra Technique. J. Cosmet. Dermatol. 2020, 19, 185–189. [Google Scholar] [CrossRef]
- Hassan, H.; Quinlan, D.J.; Ghanem, A. Injectable Platelet-Rich Fibrin for Facial Rejuvenation: A Prospective, Single-Center Study. J. Cosmet. Dermatol. 2020, 19, 3213–3221. [Google Scholar] [CrossRef]
- El Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of Relative Centrifugal Forces Increases Growth Factor Release within Solid Platelet-Rich-Fibrin (PRF)-Based Matrices: A Proof of Concept of LSCC (Low Speed Centrifugation Concept). Eur. J. Trauma Emerg. Surg. 2019, 45, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Zhang, Y.; Miron, R.J. Fluid Platelet-Rich Fibrin Stimulates Greater Dermal Skin Fibroblast Cell Migration, Proliferation, and Collagen Synthesis When Compared to Platelet-Rich Plasma. J. Cosmet. Dermatol. 2019, 18, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Chai, J.; Zheng, S.; Feng, M.; Sculean, A.; Zhang, Y. A Novel Method for Evaluating and Quantifying Cell Types in Platelet Rich Fibrin and an Introduction to Horizontal Centrifugation. J. Biomed. Mater. Res. Part A 2019, 107, 2257–2271. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Optimization of Platelet-Rich Fibrin. Periodontol. 2000 2024, 94, 79–91. [Google Scholar] [CrossRef]
- Aldag, C.; Teixeira, D.N.; Leventhal, P.S. Skin Rejuvenation Using Cosmetic Products Containing Growth Factors, Cytokines, and Matrikines: A Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef]
- Neubert, R.H.H.; Sommer, E.; Schölzel, M.; Tuchscherer, B.; Mrestani, Y.; Wohlrab, J. Dermal Peptide Delivery Using Enhancer Moleculs and Colloidal Carrier Systems. Part II: Tetrapeptide PKEK. Eur. J. Pharm. Biopharm. 2018, 124, 28–33. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative Release of Growth Factors from PRP, PRF, and Advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, 343–346. [Google Scholar] [CrossRef]
- Farwick, M.; Grether-Beck, S.; Marini, A.; Maczkiewitz, U.; Lange, J.; Köhler, T.; Lersch, P.; Falla, T.; Felsner, I.; Brenden, H.; et al. Bioactive Tetrapeptide GEKG Boosts Extracellular Matrix Formation: In Vitro and in Vivo Molecular and Clinical Proof. Exp. Dermatol. 2011, 20, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. Platelet-Rich Plasma for Managing Pain and Inflammation in Osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-Rich Plasma: From Basic Science to Clinical Applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Orive, G. Potential of Endogenous Regenerative Technology for in Situ Regenerative Medicine. Adv. Drug Deliv. Rev. 2010, 62, 741–752. [Google Scholar] [CrossRef]
- Robinson, L.R.; Fitzgerald, N.C.; Doughty, D.G.; Dawes, N.C.; Berge, C.A.; Bissett, D.L. Topical Palmitoyl Pentapeptide Provides Improvement in Photoaged Human Facial Skin. Int. J. Cosmet. Sci. 2005, 27, 155–160. [Google Scholar] [CrossRef]
- Harris, S.M.; Falla, T.J.; Zhang, L. Peptide Fragments for Inducing Synthesis of Extracellular Matrix Proteins. WO Patent 2007146269A2, 21 December 2007. [Google Scholar]
- Farwick, M.; Lersch, P. Personal Care and Cosmetic Composition Containing Tetrapeptides with Motifs GX1X2G, PX1X2P, or PX1X2K. WO Patent 2009068351, 4 June 2009. [Google Scholar]
- Mine, S.; Fortunel, N.O.; Pageon, H.; Asselineau, D. Aging Alters Functionally Human Dermal Papillary Fibroblasts but Not Reticular Fibroblasts: A New View of Skin Morphogenesis and Aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin Tissue Engineering—In Vivo and in Vitro Applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Miron, R.J.; Pikos, M.A.; Estrin, N.E.; Kobayashi-Fujioka, M.; Espinoza, A.R.; Basma, H.; Zhang, Y. Extended platelet-rich fibrin. Periodontol. 2000 2024, 94, 114–130. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable Platelet Rich Fibrin (i-PRF): Opportunities in Regenerative Dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Improved Growth Factor Delivery and Cellular Activity Using Concentrated Platelet-Rich Fibrin (C-PRF) When Compared with Traditional Injectable (i-PRF) Protocols. Clin. Oral Investig. 2020, 24, 4373–4383. [Google Scholar] [CrossRef]
- Miron, R.J. Understanding Platelet-Rich Fibrin; Quintessence Publishing: Chicago, IL, USA, 2021. [Google Scholar]
- Pagnan, A.L.; Pessoa, A.S.; Tokuhara, C.K.; Fakhoury, V.S.; Oliveira, G.S.N.; Sanches, M.L.R.; Inacio, K.K.; Ximenes, V.F.; Oliveira, R.C. Anti-Tumour Potential and Selectivity of Caffeic Acid Phenethyl Ester in Osteosarcoma Cells. Tissue Cell 2022, 74, 101705. [Google Scholar] [CrossRef]
- De Oliveira, F.A.; Matos, A.A.; Matsuda, S.S.; Buzalaf, M.A.R.; Bagnato, V.S.; Machado, M.A.d.A.M.; Damante, C.A.; de Oliveira, R.C.; Peres-Buzalaf, C. Low Level Laser Therapy Modulates Viability, Alkaline Phosphatase and Matrix Metalloproteinase-2 Activities of Osteoblasts. J. Photochem. Photobiol. B Biol. 2017, 169, 35–40. [Google Scholar] [CrossRef]
- Pessoa, A.d.S.; Tokuhara, C.K.; Fakhoury, V.S.; Pagnan, A.L.; de Oliveira, G.S.N.; Sanches, M.L.R.; Inacio, K.K.; Costa, B.C.; Ximenes, V.F.; de Oliveira, R.C. The Dimerization of Methyl Vanillate Improves Its Effect against Breast Cancer Cells via Pro-Oxidant Effect. Chem. Biol. Interact. 2022, 361, 109962. [Google Scholar] [CrossRef]
- Inacio, K.K.; Pessoa, A.d.S.; Tokuhara, C.K.; Pagnan, A.L.; Sanches, M.L.R.; Fakhoury, V.S.; de Oliveira, G.S.N.; de Oliveira, F.A.; Ximenes, V.F.; de Oliveira, R.C. Menadione and Protocatechuic Acid: A Drug Combination with Antitumor Effects in Murine Osteosarcoma Cells. Arch. Biochem. Biophys. 2024, 751, 109840. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.F.; Morandini, A.C.; Dionísio, T.J.; Faria, F.A.; Lima, M.C.; Figueiredo, C.M.; Colombini-Ishikiriama, B.L.; Sipert, C.R.; Maciel, R.P.; Akashi, A.P.; et al. Functional Local Renin-Angiotensin System in Human and Rat Periodontal Tissue. PLoS ONE 2015, 10, e0134601. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paccola, A.G.L.; Santos, T.M.C.d.; Minelo, M.C.; Garbieri, T.F.; Sanches, M.L.R.; Dionísio, T.J.; Oliveira, R.C.d.; Santos, C.F.; Buzalaf, M.A.R. Synergistic Effects of Injectable Platelet-Rich Fibrin and Bioactive Peptides on Dermal Fibroblast Viability and Extracellular Matrix Gene Expression: An In Vitro Study. Molecules 2025, 30, 3415. https://doi.org/10.3390/molecules30163415
Paccola AGL, Santos TMCd, Minelo MC, Garbieri TF, Sanches MLR, Dionísio TJ, Oliveira RCd, Santos CF, Buzalaf MAR. Synergistic Effects of Injectable Platelet-Rich Fibrin and Bioactive Peptides on Dermal Fibroblast Viability and Extracellular Matrix Gene Expression: An In Vitro Study. Molecules. 2025; 30(16):3415. https://doi.org/10.3390/molecules30163415
Chicago/Turabian StylePaccola, Ana Giulia Lenci, Thomas Marcelino Couto dos Santos, Maria Clara Minelo, Thais Francini Garbieri, Mariana Liessa Rovis Sanches, Thiago José Dionísio, Rodrigo Cardoso de Oliveira, Carlos Ferreira Santos, and Marília Afonso Rabelo Buzalaf. 2025. "Synergistic Effects of Injectable Platelet-Rich Fibrin and Bioactive Peptides on Dermal Fibroblast Viability and Extracellular Matrix Gene Expression: An In Vitro Study" Molecules 30, no. 16: 3415. https://doi.org/10.3390/molecules30163415
APA StylePaccola, A. G. L., Santos, T. M. C. d., Minelo, M. C., Garbieri, T. F., Sanches, M. L. R., Dionísio, T. J., Oliveira, R. C. d., Santos, C. F., & Buzalaf, M. A. R. (2025). Synergistic Effects of Injectable Platelet-Rich Fibrin and Bioactive Peptides on Dermal Fibroblast Viability and Extracellular Matrix Gene Expression: An In Vitro Study. Molecules, 30(16), 3415. https://doi.org/10.3390/molecules30163415







