8-OXO-Cordycepin Is Not a Suitable Substrate for Adenosine Deaminase-Preliminary Experimental and Theoretical Studies
Abstract
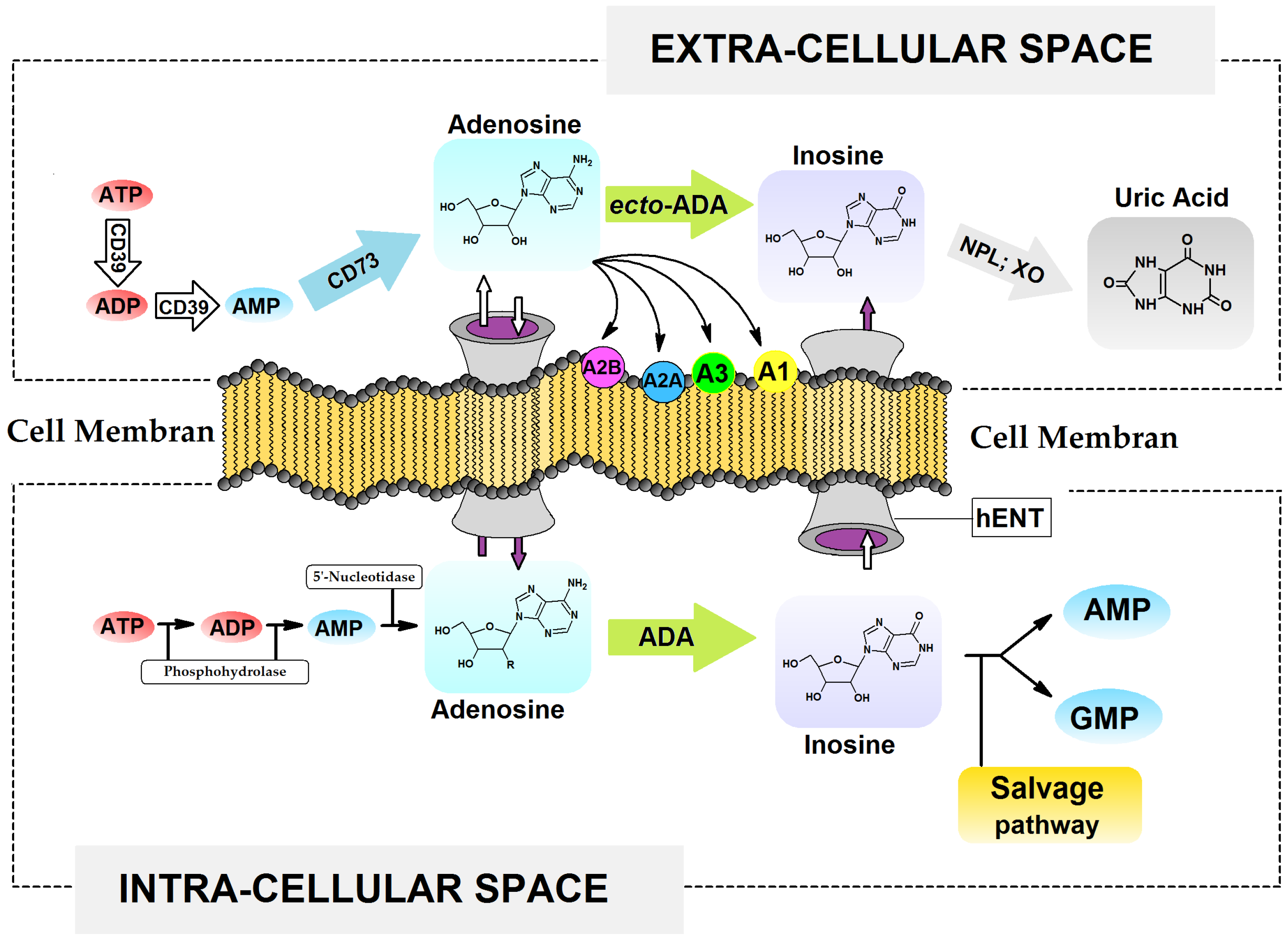
1. Introduction
2. Results
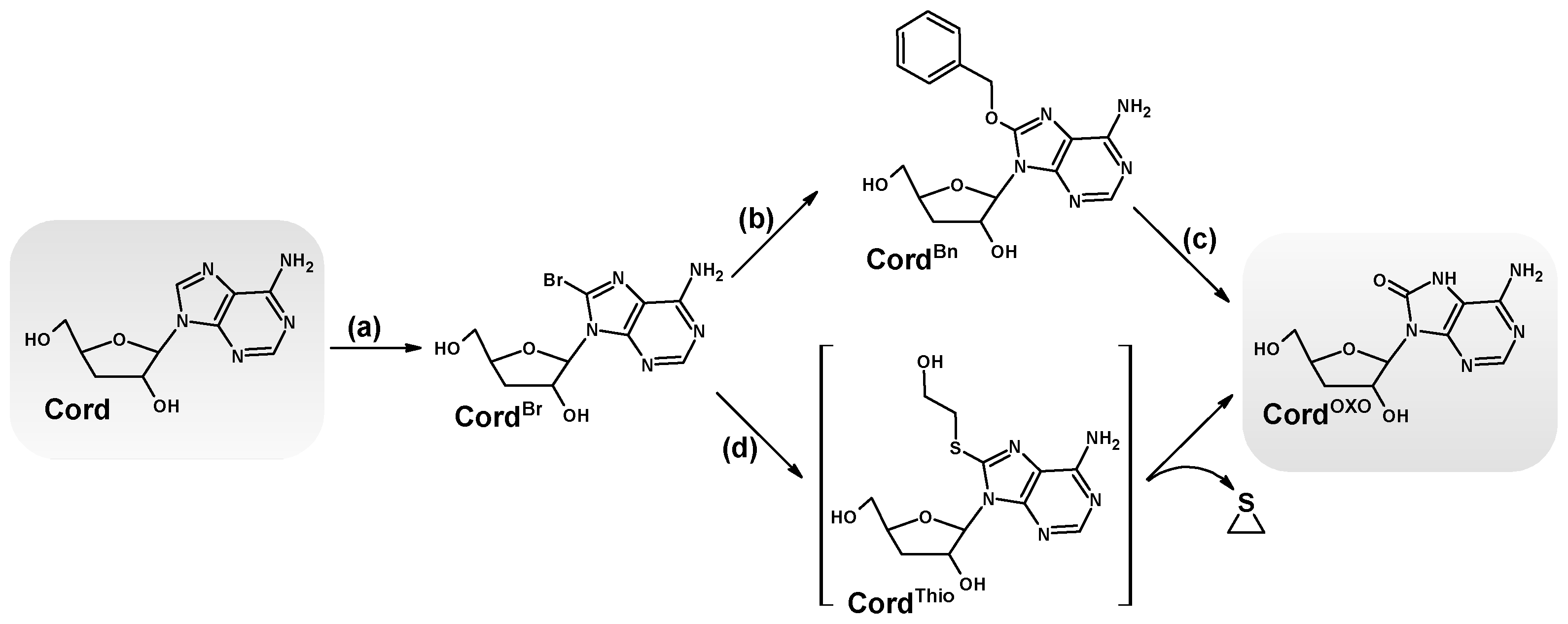
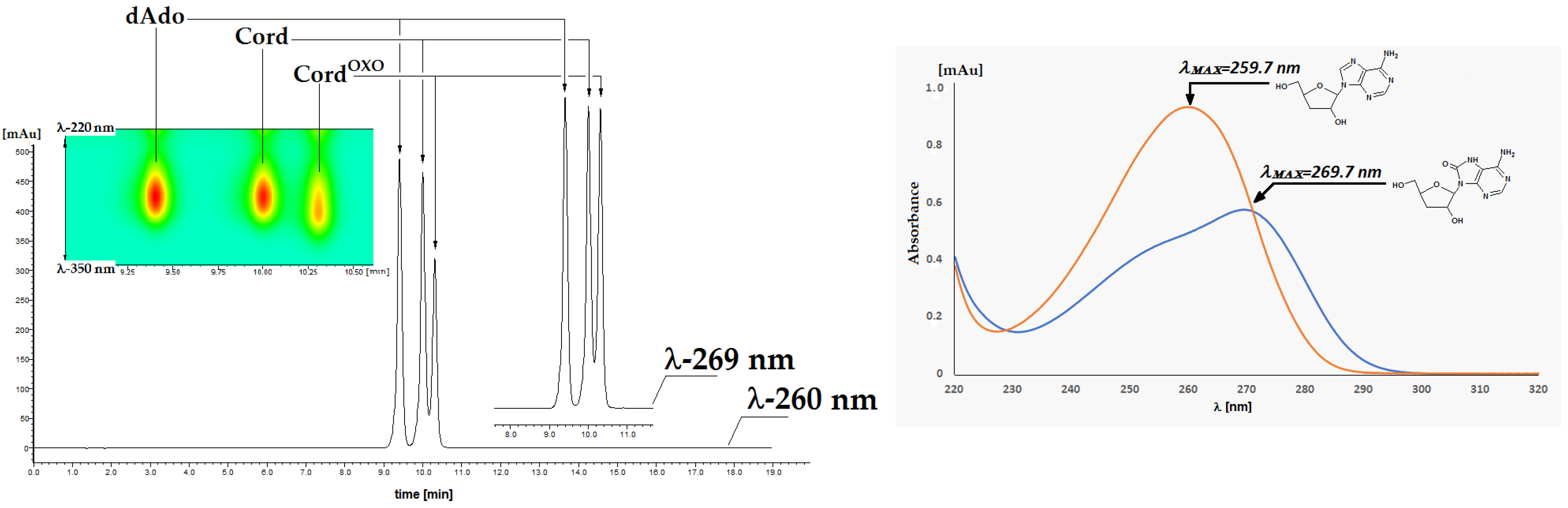
2.1. Chemical Synthesis of 7,8-Dihydro-8-Oxo-3′-Deoxyadenosine and Its Characterisation
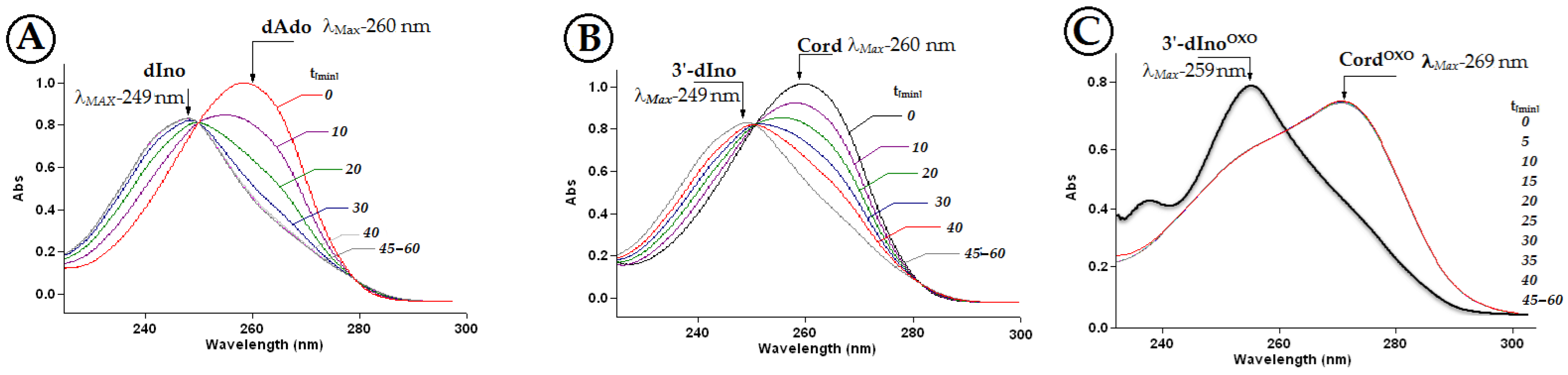
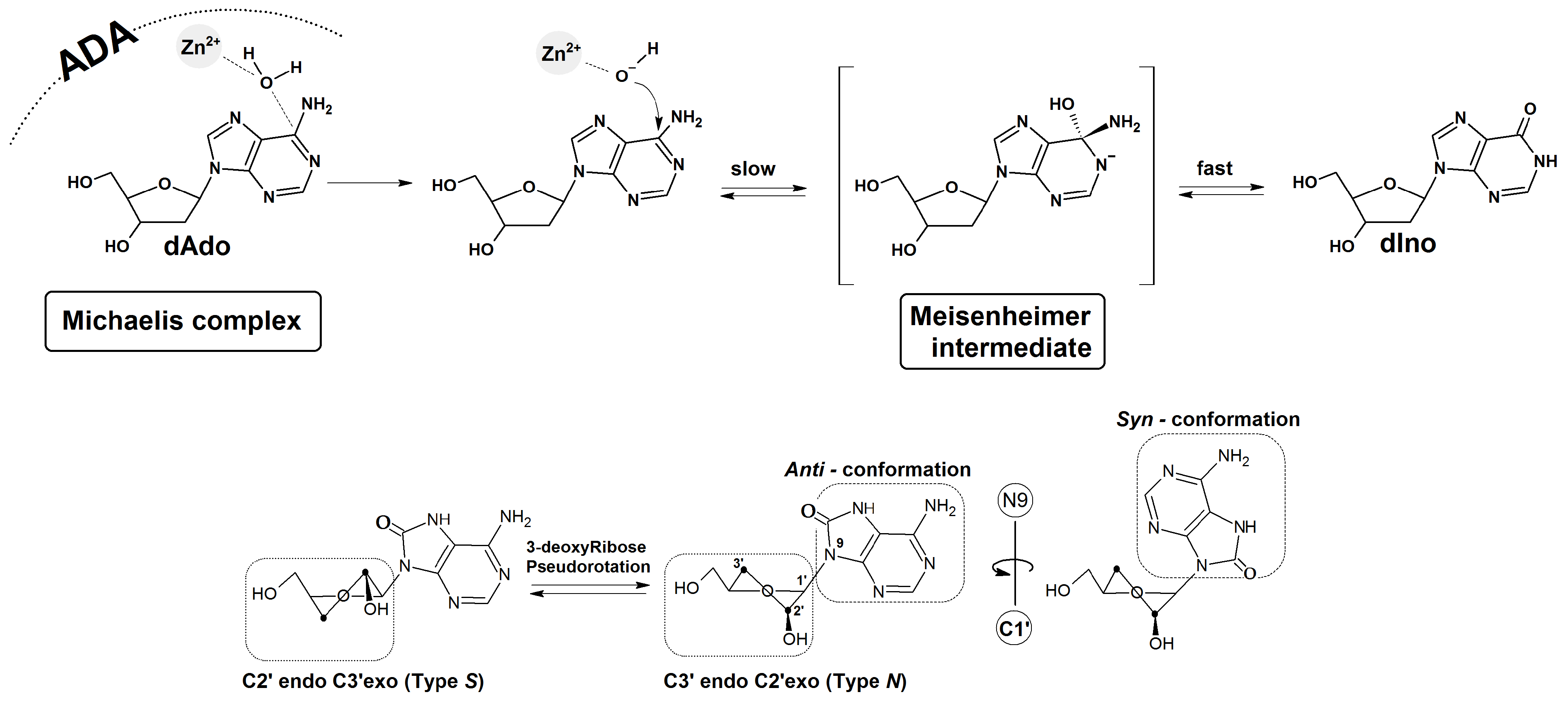
2.2. The Stability of 7,8-Dihydro-8-Oxo-3′-Deoxyadenosine in the Presence of Adenosine Deaminase
2.3. DFTB Structural Investigation of Adenosine Deaminase and dAdo, Cord and CordOXO Settled in the Active Enzyme Centre
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Spectroscopic and Elementary Analysis
4.3. Procedure for 8-Bromo-3′-Deoxyadenosine Synthesis
4.4. Procedure A for 7,8-Dihydro-8-Oxo-3′-Deoxyadenosine Synthesis
4.5. Procedure B for 7,8-Dihydro-8-Oxo-3′-Deoxyadenosine Synthesis
4.6. General Procedure for Adenosine Deaminase Activity—UV Assay
4.7. General Procedure for Adenosine Deaminase Activity—RP HPLC Assay
4.8. Geometry Optimisation
5. Conclusions
6. Further Perspectives and Remarks
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yegutkin, G.G. Enzymes Involved in Metabolism of Extracellular Nucleotides and Nucleosides: Functional Implications and Measurement of Activities. Crit. Rev. Biochem. Mol. Biol. 2014, 9238, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Nucleotide- and Nucleoside-Converting Ectoenzymes: Important Modulators of Purinergic Signalling Cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, B.P.; Pickkers, P.; Deussen, A.; Rongen, G.A.; van den Broek, P.; van der Hoeven, J.G.; Smits, P.; Riksen, N.P. Measurement of the Endogenous Adenosine Concentration in Humans In Vivo: Methodological Considerations. Curr. Drug Metab. 2008, 9, 679–685. [Google Scholar] [CrossRef]
- Idzko, M.; Ferrari, D.; Riegel, A.; Eltzschig, H.K. Extracellular Nucleotide and Nucleoside Signaling in Vascular and Blood Disease. Blood 2014, 124, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Kim, D.; Kesavan, R.; Brown, H.; Dey, T. De Novo and Salvage Purine Synthesis Pathways across Tissues and Tumors. Cell 2025, 187, 3602–3618.e20. [Google Scholar] [CrossRef]
- Wu, H.; Gong, Y.; Ji, P.; Xie, Y.; Jiang, Y.Z.; Liu, G. Targeting Nucleotide Metabolism: A Promising Approach to Enhance Cancer Immunotherapy. J. Hematol. Oncol. 2022, 15, 45. [Google Scholar] [CrossRef]
- Venugopala, K.N. Current Understanding of the Role of Adenosine Receptors in Cancer. Molecules 2024, 29, 3501. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019, 13, 24. [Google Scholar] [CrossRef]
- Sun, X.; Setrerrahmane, S.; Li, C.; Hu, J.; Xu, H. Nucleic Acid Drugs: Recent Progress and Future Perspectives. Signal Transduct. Target. Ther. 2024, 9, 316. [Google Scholar] [CrossRef]
- Boffetta, P.; Hall, C.B.; Todd, A.C.; Goldfarb, D.G.; Schymura, M.J.; Li, J.; Cone, J.E.; Zeig-Owens, R. Cancer Risk among World Trade Center Rescue and Recovery Workers: A Review. CA Cancer J. Clin. 2022, 72, 308–314. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.S. Cordycepin, a Metabolic Product Isolated from Cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar] [CrossRef]
- Yu, G.; Peng, J.; Li, L.; Yu, W.; He, B.; Xie, B. The Role and Mechanisms of Cordycepin in Inhibiting Cancer Cells. Braz. J. Med. Biol. Res. 2024, 57, e13889. [Google Scholar] [CrossRef]
- Li, G.; Nakagome, I.; Hirono, S.; Itoh, T.; Fujiwara, R. Inhibition of Adenosine Deaminase (ADA)—Mediated Metabolism of Cordycepin by Natural Substances. Pharmacol. Res. Perspect. 2015, 3, e00121. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Lin, L.-C.; Tsai, T.-H. Pharmacokinetics of Adenosine and Cordycepin, a Bioactive Constituent of Cordyceps sinensis in Rat. J. Agric. Food Chem. 2010, 58, 4638–4643. [Google Scholar] [CrossRef]
- Franco, R.; Casadó, V.; Ciruela, F.; Saura, C.; Mallol, J.; Canela, E.I.; Lluis, C. Cell Surface Adenosine Deaminase: Much More than an Ectoenzyme. Prog. Neurobiol. 1997, 52, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Cristalli, G.; Costanzi, S.; Lambertucci, C.; Lupidi, G.; Vittori, S.; Volpini, R.; Camaioni, E. Adenosine Deaminase: Functional Implications and Different Classes of Inhibitors. Med. Res. Rev. 2001, 21, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, S.; Malling, H.; Klenow, H. Isolation of 3′-deoxyadenosine (cordycepin) from the liquid medium of Cordyceps militaris (L. ex Fr.) Link. Biochim. Biophys. Acta 1964, 95, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, M.P.; Ferrua, F.; Castagnaro, L.; Rolfe, K.; De Boever, E.; Reinhardt, R.R.; Appleby, J.; Roncarolo, M.G.; Aiuti, A. Gene Therapy for Adenosine Deaminase Deficiency: A Comprehensive Evaluation of Short- and Medium-Term Safety. Mol. Ther. 2018, 26, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Parekh, S.J.; Bishop, M.B. Hairy-Cell Leukemia: Induction of Complete Remission with pentostatin (2′-deoxycoformycin). J. Clin. Oncol. 2019, 2, 1336–1342. [Google Scholar] [CrossRef]
- Leach, J.W.; Pham, T.; Diamandidis, D.; George, J.N. Thrombotic Thrombocytopenic Purpura—Hemolytic Uremic Syndrome (TTP-HUS) Following Treatment with Deoxycoformycin in a Patient with Cutaneous T-Cell Lymphoma (Sezary Syndrome): A Case Report. Am. J. Hematol. 1999, 270, 268–270. [Google Scholar] [CrossRef]
- KruKruchinin, A.A.; Kamzeeva, P.N.; Zharkov, D.O.; Aralov, A.V.; Makarova, A.V. 8-Oxoadenine: A «New» Player of the Oxidative Stress in Mammals? Int. J. Mol. Sci. 2024, 25, 1342. [Google Scholar] [CrossRef] [PubMed]
- Bodepudi, V.; Shibutani, S.; Johnson, F. Synthesis of 2′-deoxy-7,8-dihydro-8-oxoguanosine and 2′-deoxy-7,8-dihydro-8-oxoadenosine and their incorporation into oligomeric DNA. Chem. Res. Toxicol. 1992, 5, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Navacchia, M.L.; Postigo, A. A Facile One—Pot Synthesis of 8-Oxo-7,8-Dihydro-(2′-deoxy) Adenosine in Water. Tetrahedron Lett. 2006, 47, 711–714. [Google Scholar] [CrossRef]
- Zheng, Y.; Haratipour, P.; Kashemirov, B.A.; Mckenna, C.E. Synthesis of 8-oxo-dGTP and its β,γ-CH2-, β,γ-CHF-, and β,γ-CF2- analogues. Tetrahedron Lett. 2021, 67, 152890. [Google Scholar] [CrossRef]
- Changeux, J. Golden Anniversary of the Nicotinic Receptor. Neuron 2020, 107, 14–16. [Google Scholar] [CrossRef]
- Blackburn, M.R.; Kellems, R.E. Adenosine Deaminase Deficiency: Metabolic Basis of Immune Deficiency and Pulmonary Inflammation. Adv. Immunol. 2005, 86, 1–41. [Google Scholar] [CrossRef]
- Wang, Z.; Quiocho, F.A. Complexes of Adenosine Deaminase with Two Potent Inhibitors: X-Ray Structures in Four Independent Molecules at PH of Maximum Activity. Biochemistry 1998, 2960, 8314–8324. [Google Scholar] [CrossRef]
- Lennox, A.J.J. Meisenheimer Complexes in S N Ar Reactions: Intermediates or Transition States? Angew. Chem. Int. Ed. Engl. 2018, 57, 14686–14688. [Google Scholar] [CrossRef]
- Gabrielli, B.; Chau, Y.Q.; Giles, N.; Harding, A.; Stevens, F.; Beamish, H. Caffeine Promotes Apoptosis in Mitotic Spindle Checkpoint-Arrested Cells. J. Biol. Chem. 2007, 282, 6954–6964. [Google Scholar] [CrossRef]
- Seabra, G.D.M.; Walker, R.C.; Elstner, M.; Case, D.A.; Adrian, E. Simulations within the Amber Molecular Dynamics Package. J. Phys. Chem. A 2011, 111, 5655–5664. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Generalized Born Solvation Model SM12. J. Chem. Theory Comput. 2013, 9, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. The Electronic Properties of Cordycepin in the Adenine Nucleoside Landscape: A Theoretical Approach. Molecules 2025, 30, 2289. [Google Scholar] [CrossRef]
- Schmidt, G. Über Fermentative Desaminierung Im Muskel. Biol. Chem. 1928, 179, 243–282. [Google Scholar] [CrossRef]
- Whitmore, K.V.; Gaspar, H.B. Adenosine Deaminase Deficiency—More Than Just an Immunodeficiency. Front. Immunol. 2016, 7, 314. [Google Scholar] [CrossRef]
- Dvorak, C.C.; Haddad, E.; Heimall, J.; Dunn, E.; Buckley, R.H.; Kohn, D.B.; Cowan, M.J.; Pai, S.; Griffith, L.M.; Cuvelier, G.D.E.; et al. Translational and Clinical Immunology The Diagnosis of Severe Combined Immunodeficiency (SCID): The Primary Immune Deficiency Treatment Consortium (PIDTC) 2022 Definitions. J. Allergy Clin. Immunol. 2022, 151, 539–546. [Google Scholar] [CrossRef]
- Bagnara, A.S.; McDonald, L.E.; Slade, H.M. Deoxyadenosine Toxicity in an Adenosine Deaminase-Inhibited Human CCRF-CEM T-Lymphoblastoid Cell Line Causes Cell Swelling. Biochim. Biophys. Acta 1992, 1180, 163–172. [Google Scholar] [CrossRef]
- Williams, S.P.; Kuyper, L.F.; Pearce, K.H. Recent Applications of Protein Crystallography and Structure-Guided Drug Design. Curr. Opin. Chem. Biol. 2005, 8, 371–380. [Google Scholar] [CrossRef]
- Shelton, J.; Lu, X.; Hollenbaugh, J.A.; Cho, J.H.; Amblard, F.; Schinazi, R.F. Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs. Chem. Rev. 2016, 116, 14379–14455. [Google Scholar] [CrossRef]
- Noji, T.; Karasawa, A.; Kusaka, H. Adenosine Uptake Inhibitors. Eur. J. Pharmacol. 2004, 495, 1–16. [Google Scholar] [CrossRef]
- Kraupp, M.; Marz, R. Nucleobase and Nucleoside Transport in Mammalian Cells. Wien. Klin. Wochenschr. 1995, 107, 677–680. [Google Scholar] [PubMed]
- Kane, B.J.; Kuhn, G.J.; Roush, M.K. Pentostatin: Anadenosine Deaminase Inhibitor Forthetreatment Ofhairy Cell Leukemia. Ann. Pharmacother. 1992, 26, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.I.; Fujimoto, M.; Hara, H.; Sugimoto, N. Nucleic Acid Duplex Stability: Influence of Base Composition on Cation Effects. Nucleic Acids Res. 1999, 27, 2957–2965. [Google Scholar] [CrossRef] [PubMed]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Suhai, S.; Seifert, G. Self-Consistent-Charge Density-Functional Tight-Binding Method for Simulations of Complex Materials Properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Gaus, M.; Goez, A.; Elstner, M. Parametrization and Benchmark of DFTB3 for Organic Molecules. J. Chem. Theory Comput. 2012, 9, 338–354. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]

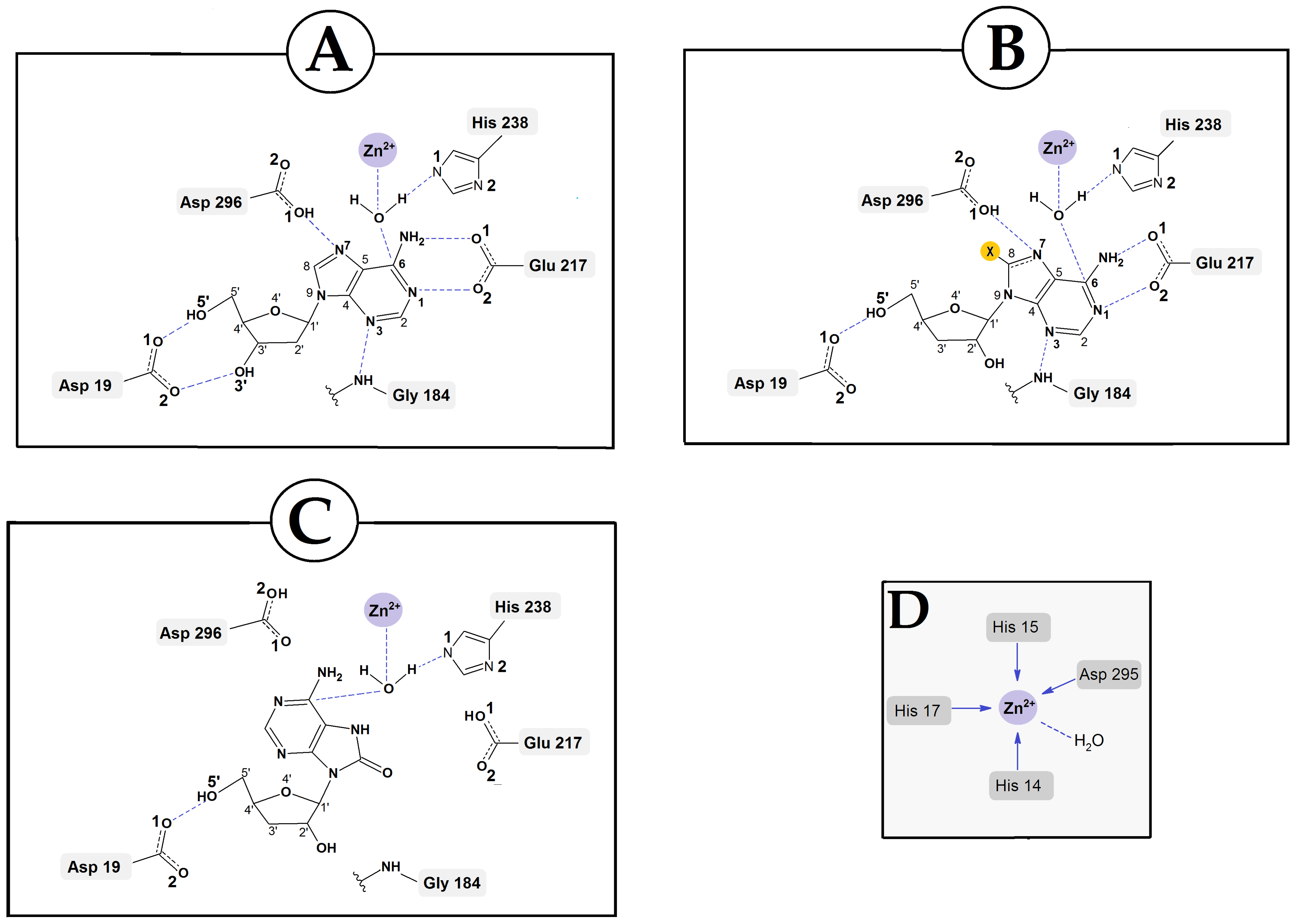
| Protein ADA | Atom Number | Ligand | ||||
|---|---|---|---|---|---|---|
| 2′-dCof | dAdo | Cord | antiCordOXO | synCordOXO | ||
| Asp 19 O1 | O5′ | 2.80 */2.80 | 2.95 | 2.90 | 2.97 | 3.34 |
| Asp 19 O2 | O3′ | 2.89 */2.88 | 3.10 | ---- | ---- | ---- |
| Gly 184 N | N3 | 3.29 */3.30 | 3.45 | 3.40 | 3.40 | 6.60 |
| Glu 217 O1 | N6 | ---- | 3.76 | 3.70 | 3.74 | 6.53 |
| Glu 217 O2 | N1 | 2.79 */2.79 | 3.14 | 3.15 | 3.15 | 8.74 |
| His 238 N1 | O (H2O) | 3.25 */3.25 | 2.84 | 2.84 | 2.84 | 2.86 |
| Asp 296 O1 | N7 | 2.76 */2.76 | 3.15 | 3.20 | 3.23 | 5.48 |
| Zn2+ | O (H2O) | 1.84 */1.83 | 2.12 | 2.11 | 2.11 | 2.15 |
| O (H2O) | C6 (8 *) | 1.60 */1.60 | 2.73 | 2.76 | 2.72 | 4.03 |
| His 14 N | Zn2+ | 2.67 */2.67 | 2.04 | 2.96 | 2.04 | 2.05 |
| His 15 N | 2.78 */2.78 | 2.02 | 3.34 | 2.03 | 2.07 | |
| His 17 N | 2.73 */2.73 | 1.98 | 1.96 | 1.99 | 2.00 | |
| Asp 295 O | 2.38 */2.39 | 2.81 | 2.83 | 2.84 | 2.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowski, B.T. 8-OXO-Cordycepin Is Not a Suitable Substrate for Adenosine Deaminase-Preliminary Experimental and Theoretical Studies. Molecules 2025, 30, 3377. https://doi.org/10.3390/molecules30163377
Karwowski BT. 8-OXO-Cordycepin Is Not a Suitable Substrate for Adenosine Deaminase-Preliminary Experimental and Theoretical Studies. Molecules. 2025; 30(16):3377. https://doi.org/10.3390/molecules30163377
Chicago/Turabian StyleKarwowski, Boleslaw T. 2025. "8-OXO-Cordycepin Is Not a Suitable Substrate for Adenosine Deaminase-Preliminary Experimental and Theoretical Studies" Molecules 30, no. 16: 3377. https://doi.org/10.3390/molecules30163377
APA StyleKarwowski, B. T. (2025). 8-OXO-Cordycepin Is Not a Suitable Substrate for Adenosine Deaminase-Preliminary Experimental and Theoretical Studies. Molecules, 30(16), 3377. https://doi.org/10.3390/molecules30163377







